An Epistatic Interaction between Pnpla2 and Lipe Reveals New Pathways of Adipose Tissue Lipolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. TG Hydrolase Activity Assay
2.3. Transacylation Assay
2.4. Determination of TG and DG Contents of WAT
2.5. Purification of HSL Protein
2.6. Western Blot
2.7. Statistical Analysis
3. Results
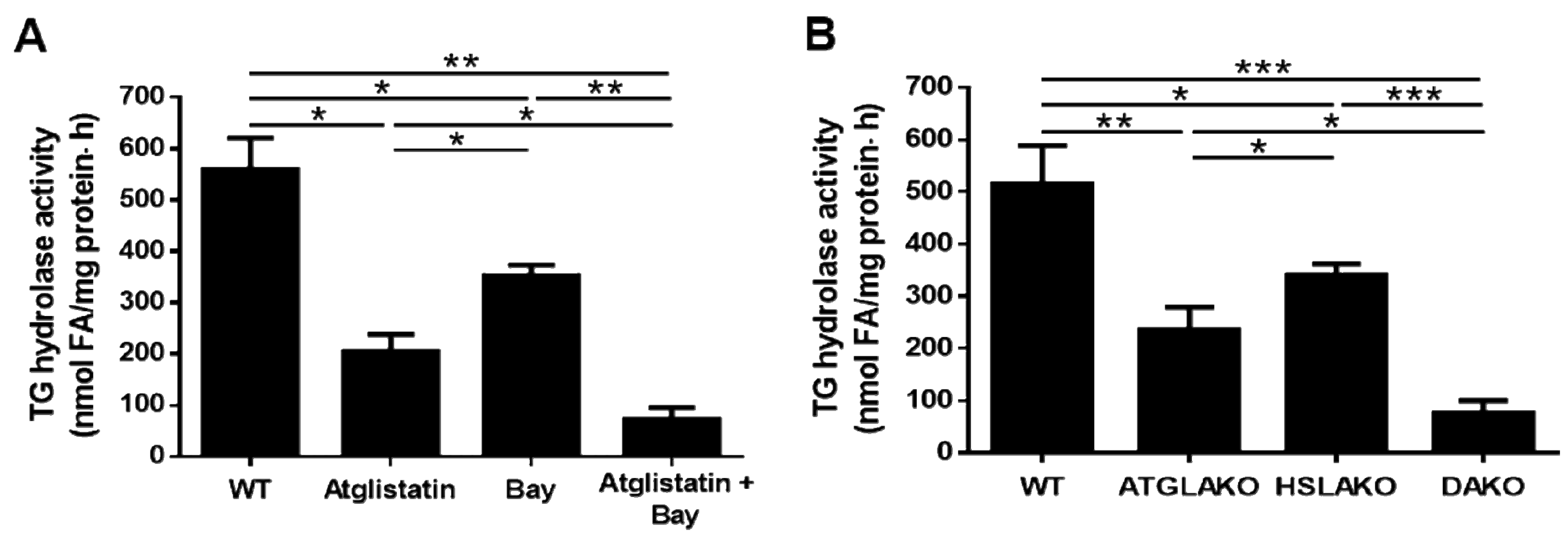
3.1. Adipose HSL Has TG Hydrolase Activity both in the Presence and Absence of ATGL
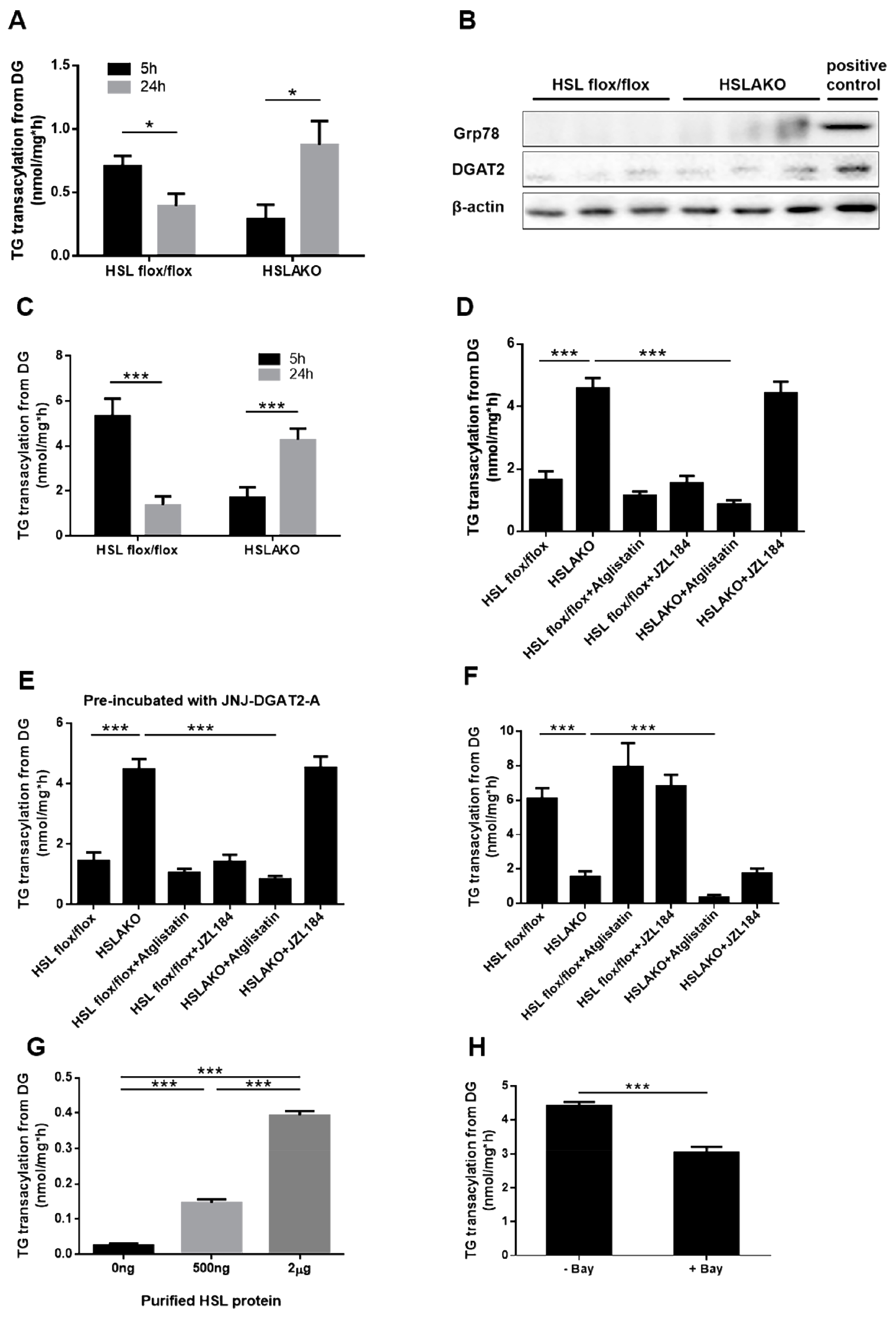
3.2. Adipose ATGL Functions as a Transacylase in the Absence of HSL in 24-h Fasted Mice
3.3. Adipose HSL Shows Transacylase Activity in 5-h Fasted Mice
3.4. Transacylation Activity of HSL Is Phosphorylation Independent
3.5. Higher Levels of ATGL-Mediated Transacylation in 24-h Fasted Than in 5-h Fasted HSL-Deficient Adipose Tissues Relate both to Substrate Availability and to ATGL Level
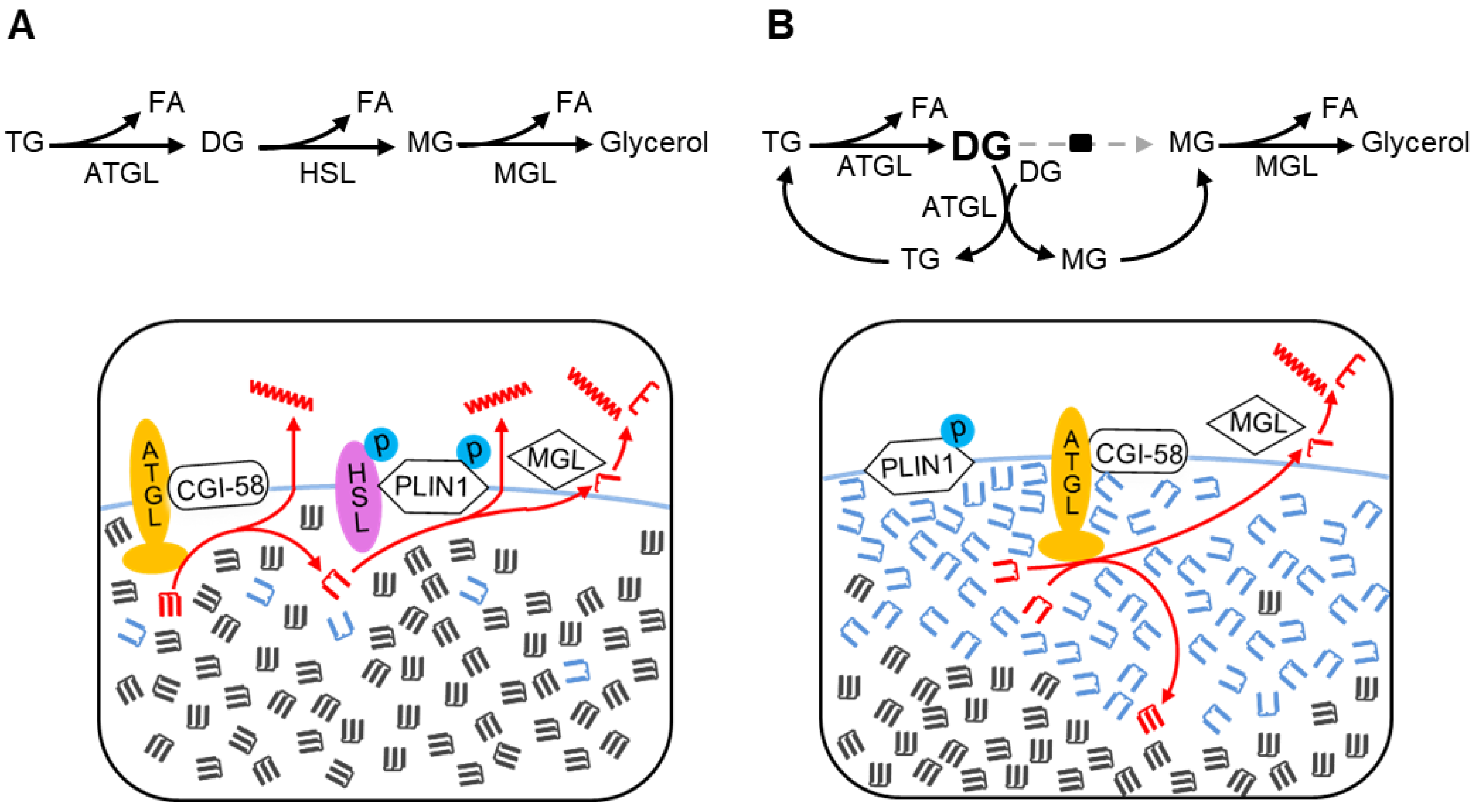
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zechner, R.; Madeo, F.; Kratky, D. Cytosolic lipolysis and lipophagy: Two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Cai, G.H.; Yang, H.; Wang, S.P.; Mitchell, G.A.; Wu, J.W. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017, 13, e1007110. [Google Scholar] [CrossRef]
- Wu, J.W.; Preuss, C.; Wang, S.P.; Yang, H.; Ji, B.; Carter, G.W.; Gladdy, R.; Andelfinger, G.; Mitchell, G.A. Epistatic interaction between the lipase-encoding genes pnpla2 and lipe causes liposarcoma in mice. PLoS Genet. 2017, 13, e1006716. [Google Scholar] [CrossRef]
- Wu, J.W.; Wang, S.P.; Alvarez, F.; Casavant, S.; Gauthier, N.; Abed, L.; Soni, K.G.; Yang, G.; Mitchell, G.A. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 2011, 54, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Halldin, M.U.; Forslund, A.; von Dobeln, U.; Eklund, C.; Gustafsson, J. Increased lipolysis in lchad deficiency. J. Inherit. Metab Dis. 2007, 30, 39–46. [Google Scholar] [CrossRef]
- Spiekerkoetter, U.; Lindner, M.; Santer, R.; Grotzke, M.; Baumgartner, M.R.; Boehles, H.; Das, A.; Haase, C.; Hennermann, J.B.; Karall, D.; et al. Treatment recommendations in long-chain fatty acid oxidation defects: Consensus from a workshop. J. Inherit. Metab. Dis. 2009, 32, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Yang, H.; Wang, S.P.; Soni, K.G.; Brunel-Guitton, C.; Mitchell, G.A. Inborn errors of cytoplasmic triglyceride metabolism. J. Inherit. Metab. Dis. 2015, 38, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Leen, W.G.; Taher, M.; Verbeek, M.M.; Kamsteeg, E.J.; van de Warrenburg, B.P.; Willemsen, M.A. Glut1 deficiency syndrome into adulthood: A follow-up study. J. Neurol. 2014, 261, 589–599. [Google Scholar] [CrossRef]
- Daci, A.; Bozalija, A.; Jashari, F.; Krasniqi, S. Individualizing treatment approaches for epileptic patients with glucose transporter type1 (glut-1) deficiency. Int. J. Mol. Sci. 2018, 19, 122. [Google Scholar] [CrossRef]
- Crenn, P.; Maillot, F. [dietary advice for treatment of inborn errors of metabolism in adult neurology: Principes and limitations]. Rev. Neurol. 2007, 163, 936–941. [Google Scholar] [CrossRef]
- Stenlid, M.H.; Ahlsson, F.; Forslund, A.; von Dobeln, U.; Gustafsson, J. Energy substrate metabolism in pyruvate dehydrogenase complex deficiency. J. Pediatr. Endocrinol. Metab. 2014, 27, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Attane, C.; Peyot, M.L.; Lussier, R.; Poursharifi, P.; Zhao, S.; Zhang, D.; Morin, J.; Pineda, M.; Wang, S.; Dumortier, O.; et al. A beta cell atgl-lipolysis/adipose tissue axis controls energy homeostasis and body weight via insulin secretion in mice. Diabetologia 2016, 59, 2654–2663. [Google Scholar] [CrossRef]
- Schreiber, R.; Diwoky, C.; Schoiswohl, G.; Feiler, U.; Wongsiriroj, N.; Abdellatif, M.; Kolb, D.; Hoeks, J.; Kershaw, E.E.; Sedej, S.; et al. Cold-induced thermogenesis depends on atgl-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab. 2017, 26, 753–763.e7. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Garcia-Macia, M.; Sahu, S.; Athonvarangkul, D.; Liebling, E.; Merlo, P.; Cecconi, F.; Schwartz, G.J.; Singh, R. Autophagy in the cns and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab. 2016, 23, 113–127. [Google Scholar] [CrossRef]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Belfrage, P.; Jergil, B.; Stralfors, P.; Tornqvist, H. Hormone-sensitive lipase of rat adipose tissue: Identification and some properties of the enzyme protein. FEBS Lett. 1977, 75, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Tornqvist, H.; Belfrage, P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J. Biol. Chem. 1976, 251, 813–819. [Google Scholar]
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Zimmermann, R.; Hayn, M.; Theussl, C.; Waeg, G.; Wagner, E.; Sattler, W.; Magin, T.M.; Wagner, E.F.; Zechner, R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002, 277, 4806–4815. [Google Scholar] [CrossRef] [PubMed]
- Taschler, U.; Radner, F.P.; Heier, C.; Schreiber, R.; Schweiger, M.; Schoiswohl, G.; Preiss-Landl, K.; Jaeger, D.; Reiter, B.; Koefeler, H.C.; et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J. Biol. Chem. 2011, 286, 17467–17477. [Google Scholar] [CrossRef] [PubMed]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. Fat signals--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef]
- Wu, J.W.; Wang, S.P.; Casavant, S.; Moreau, A.; Yang, G.S.; Mitchell, G.A. Fasting energy homeostasis in mice with adipose deficiency of desnutrin/adipose triglyceride lipase. Endocrinology 2012, 153, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Osterlund, T. Hormone-sensitive lipase and neutral cholesteryl ester lipase. Methods Mol. Biol. 1999, 109, 109–121. [Google Scholar] [PubMed]
- Mayer, N.; Schweiger, M.; Romauch, M.; Grabner, G.F.; Eichmann, T.O.; Fuchs, E.; Ivkovic, J.; Heier, C.; Mrak, I.; Lass, A.; et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat. Chem. Biol. 2013, 9, 785–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claus, T.H.; Lowe, D.B.; Liang, Y.; Salhanick, A.I.; Lubeski, C.K.; Yang, L.; Lemoine, L.; Zhu, J.; Clairmont, K.B. Specific inhibition of hormone-sensitive lipase improves lipid profile while reducing plasma glucose. J. Pharmacol. Exp. Ther. 2005, 315, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.B.; Magnuson, S.; Qi, N.; Campbell, A.M.; Cook, J.; Hong, Z.; Wang, M.; Rodriguez, M.; Achebe, F.; Kluender, H.; et al. In vitro sar of (5-(2h)-isoxazolonyl) ureas, potent inhibitors of hormone-sensitive lipase. Bioorg. Med. Chem. Lett. 2004, 14, 3155–3159. [Google Scholar] [CrossRef]
- Lehner, R.; Kuksis, A. Triacylglycerol synthesis by an sn-1,2(2,3)-diacylglycerol transacylase from rat intestinal microsomes. J. Biol. Chem. 1993, 268, 8781–8786. [Google Scholar] [PubMed]
- Buhman, K.K.; Smith, S.J.; Stone, S.J.; Repa, J.J.; Wong, J.S.; Knapp, F.F., Jr.; Burri, B.J.; Hamilton, R.L.; Abumrad, N.A.; Farese, R.V., Jr. Dgat1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J. Biol. Chem. 2002, 277, 25474–25479. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M.; Mancuso, D.J.; Yan, W.; Sims, H.F.; Gibson, B.; Gross, R.W. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase a2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 2004, 279, 48968–48975. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Yang, L.; Na, H.; Zhang, P.; Zhang, H.; Wang, Y.; Chen, Y.; Yu, J.; Huo, C.; et al. Isolating lipid droplets from multiple species. Nat. Protoc. 2013, 8, 43–51. [Google Scholar] [CrossRef]
- Soni, K.G.; Lehner, R.; Metalnikov, P.; O’Donnell, P.; Semache, M.; Gao, W.; Ashman, K.; Pshezhetsky, A.V.; Mitchell, G.A. Carboxylesterase 3 (ec 3.1.1.1) is a major adipocyte lipase. J. Biol. Chem. 2004, 279, 40683–40689. [Google Scholar] [CrossRef] [PubMed]
- Irshad, Z.; Dimitri, F.; Christian, M.; Zammit, V.A. Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J. Lipid Res. 2017, 58, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Thayer, S.A. Monoacylglycerol lipase inhibitor jzl184 prevents hiv-1 gp120-induced synapse loss by altering endocannabinoid signaling. Neuropharmacology 2018, 128, 269–281. [Google Scholar] [CrossRef]
- Pan, B.; Wang, W.; Long, J.Z.; Sun, D.; Hillard, C.J.; Cravatt, B.F.; Liu, Q.S. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (jzl184) enhances retrograde endocannabinoid signaling. J. Pharmacol. Exp. Ther. 2009, 331, 591–597. [Google Scholar] [CrossRef]
- Fortier, M.; Soni, K.; Laurin, N.; Wang, S.P.; Mauriege, P.; Jirik, F.R.; Mitchell, G.A. Human hormone-sensitive lipase (hsl): Expression in white fat corrects the white adipose phenotype of hsl-deficient mice. J. Lipid Res. 2005, 46, 1860–1867. [Google Scholar] [CrossRef]
- Wang, S.P.; Wu, J.W.; Bourdages, H.; Lefebvre, J.F.; Casavant, S.; Leavitt, B.R.; Labuda, D.; Trasler, J.; Smith, C.E.; Hermo, L.; et al. The catalytic function of hormone-sensitive lipase is essential for fertility in male mice. Endocrinology 2014, 155, 3047–3053. [Google Scholar] [CrossRef]
- Wang, S.P.; Chung, S.; Soni, K.; Bourdages, H.; Hermo, L.; Trasler, J.; Mitchell, G.A. Expression of human hormone-sensitive lipase (hsl) in postmeiotic germ cells confers normal fertility to hsl-deficient mice. Endocrinology 2004, 145, 5688–5693. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Ben Ali, Y.; Abdelkafi, S.; Mendoza, L.D.; Leclaire, J.; Fotiadu, F.; Buono, G.; Carriere, F.; Abousalham, A. In vitro stereoselective hydrolysis of diacylglycerols by hormone-sensitive lipase. Biochim. Biophys. Acta 2010, 1801, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Siloto, R.M.; Lehner, R.; Stone, S.J.; Weselake, R.J. Acyl-coa:Diacylglycerol acyltransferase: Molecular biology, biochemistry and biotechnology. Prog. Lipid Res. 2012, 51, 350–377. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids. Dgat enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef]
- Anthonsen, M.W.; Ronnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [CrossRef]
- Bell, R.M.; Coleman, R.A. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 1980, 49, 459–487. [Google Scholar] [CrossRef]
- Albert, J.S.; Yerges-Armstrong, L.M.; Horenstein, R.B.; Pollin, T.I.; Sreenivasan, U.T.; Chai, S.; Blaner, W.S.; Snitker, S.; O’Connell, J.R.; Gong, D.W.; et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N. Engl. J. Med. 2014, 370, 2307–2315. [Google Scholar] [CrossRef]
- Osuga, J.; Ishibashi, S.; Oka, T.; Yagyu, H.; Tozawa, R.; Fujimoto, A.; Shionoiri, F.; Yahagi, N.; Kraemer, F.B.; Tsutsumi, O.; et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.P.; Laurin, N.; Himms-Hagen, J.; Rudnicki, M.A.; Levy, E.; Robert, M.F.; Pan, L.; Oligny, L.; Mitchell, G.A. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes. Res. 2001, 9, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef]
- Rohm, M.; Sommerfeld, A.; Strzoda, D.; Jones, A.; Sijmonsma, T.P.; Rudofsky, G.; Wolfrum, C.; Sticht, C.; Gretz, N.; Zeyda, M.; et al. Transcriptional cofactor tblr1 controls lipid mobilization in white adipose tissue. Cell Metab. 2013, 17, 575–585. [Google Scholar] [CrossRef]
- Heine, M.; Fischer, A.W.; Schlein, C.; Jung, C.; Straub, L.G.; Gottschling, K.; Mangels, N.; Yuan, Y.; Nilsson, S.K.; Liebscher, G.; et al. Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated brown adipose tissue in mice. Cell Metab. 2018, 28, 644–655.e4. [Google Scholar] [CrossRef]
- Gao, H.; Mejhert, N.; Fretz, J.A.; Arner, E.; Lorente-Cebrian, S.; Ehrlund, A.; Dahlman-Wright, K.; Gong, X.; Stromblad, S.; Douagi, I.; et al. Early b cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab. 2014, 19, 981–992. [Google Scholar] [CrossRef]
- Koliwad, S.K.; Streeper, R.S.; Monetti, M.; Cornelissen, I.; Chan, L.; Terayama, K.; Naylor, S.; Rao, M.; Hubbard, B.; Farese, R.V., Jr. Dgat1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J. Clin. Investig. 2010, 120, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Ma, Y.; Chanturiya, T.; Cao, Q.; Wang, Y.; Kadegowda, A.K.G.; Jackson, R.; Rumore, D.; Xue, B.; Shi, H.; et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab. 2017, 26, 764–777.e5. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Seol, H.S.; Kamada, T. Tissue distribution of lipase genes related to triglyceride metabolism in laying hens (gallus gallus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 155, 62–66. [Google Scholar] [CrossRef]
- Saneyasu, T.; Shiragaki, M.; Kurachi, K.; Kamisoyama, H.; Honda, K. Effects of short-term refeeding on the expression of genes involved in lipid metabolism in chicks (gallus gallus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 166, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, C.C.; Yang, H.; Soni, K.G.; Wang, S.P.; Mitchell, G.A.; Wu, J.W. An Epistatic Interaction between Pnpla2 and Lipe Reveals New Pathways of Adipose Tissue Lipolysis. Cells 2019, 8, 395. https://doi.org/10.3390/cells8050395
Zhang X, Zhang CC, Yang H, Soni KG, Wang SP, Mitchell GA, Wu JW. An Epistatic Interaction between Pnpla2 and Lipe Reveals New Pathways of Adipose Tissue Lipolysis. Cells. 2019; 8(5):395. https://doi.org/10.3390/cells8050395
Chicago/Turabian StyleZhang, Xiao, Cong Cong Zhang, Hao Yang, Krishnakant G. Soni, Shu Pei Wang, Grant A. Mitchell, and Jiang Wei Wu. 2019. "An Epistatic Interaction between Pnpla2 and Lipe Reveals New Pathways of Adipose Tissue Lipolysis" Cells 8, no. 5: 395. https://doi.org/10.3390/cells8050395
APA StyleZhang, X., Zhang, C. C., Yang, H., Soni, K. G., Wang, S. P., Mitchell, G. A., & Wu, J. W. (2019). An Epistatic Interaction between Pnpla2 and Lipe Reveals New Pathways of Adipose Tissue Lipolysis. Cells, 8(5), 395. https://doi.org/10.3390/cells8050395



