Motor Endplate—Anatomical, Functional, and Molecular Concepts in the Historical Perspective
Abstract
“These were the happy days, for which the current generation might envy us, when the first good, handy, and affordable microscopes came from the workshops of Plössl in Vienna and Pistor and Schick in Berlin, the happy days, when it was still possible to make fundamental discoveries by simply scraping with the blade of a scalpel or the fingernails on an animal’s membrane.”Jacob Henle, Orbituary for Theodor Schwann (1882)
1. Introduction
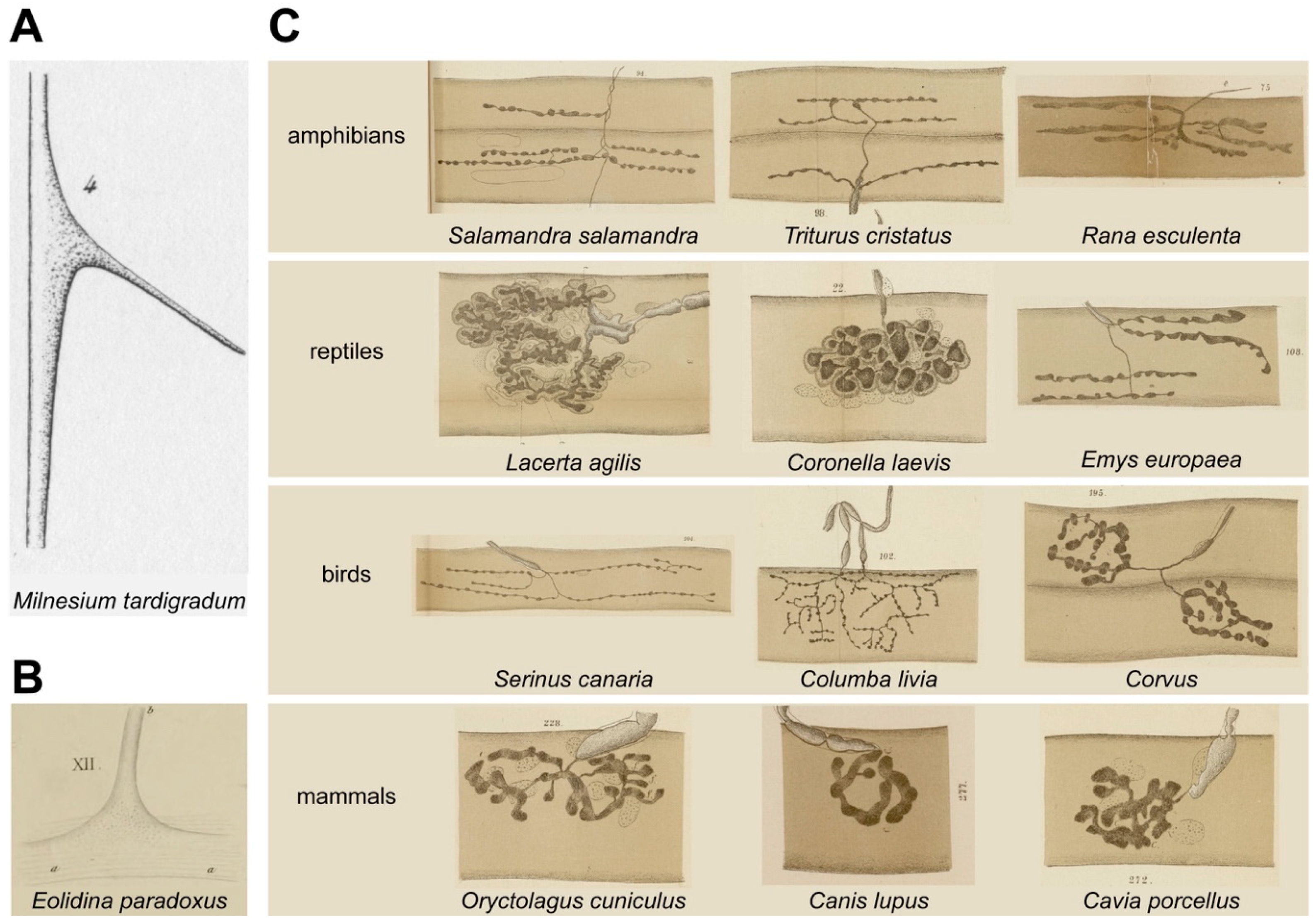
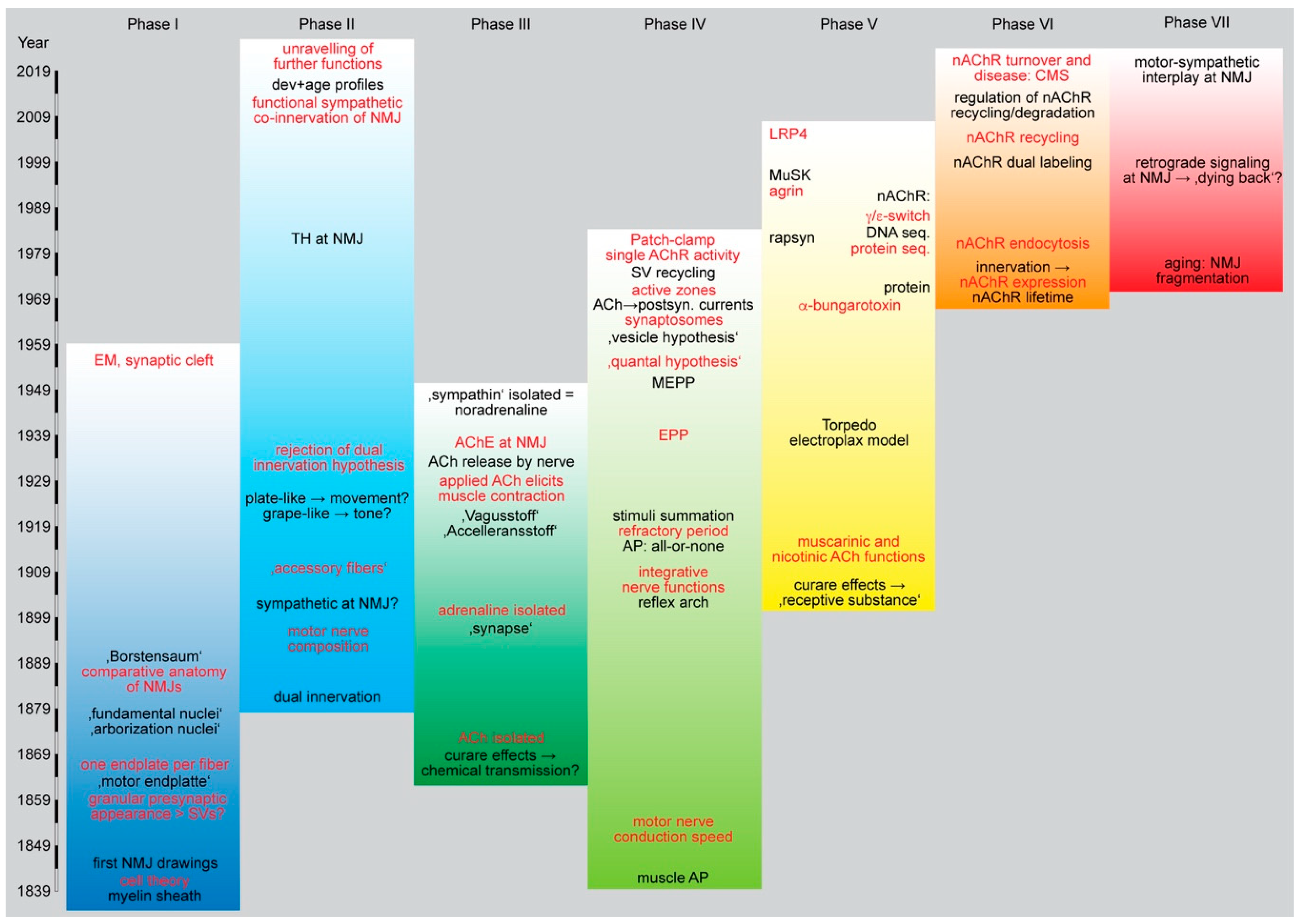
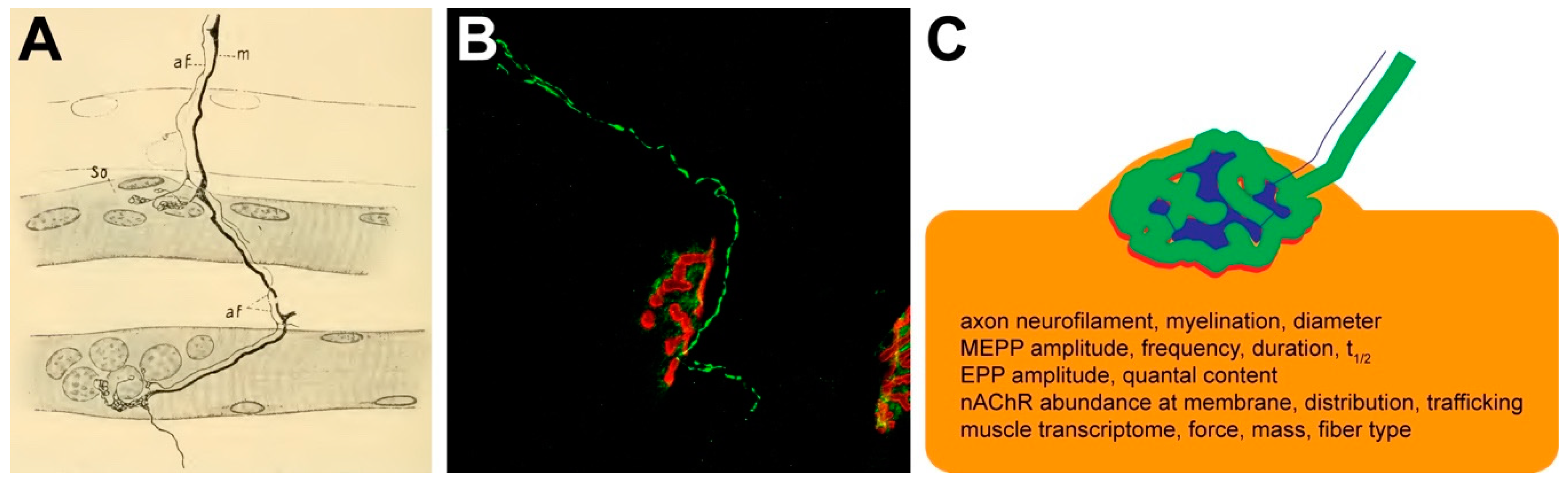
2. Phase I: Discovery of the NMJ
3. Phase II: Small vs. Myelinated Fibers, Plastic Tone vs. Voluntary Movement
4. Phase III: ACh and NE—Neurotransmitters at the NMJ
5. Phase IV: MEPP, EPP, and AP—Conversion of Chemical Signals into Electrical Activity
6. Phase V: Nerve—Muscle Signaling—Feed Forward
7. Phase VI: Postsynaptic Apparatus—nAChR Turnover
8. Phase VII: The Future of NMJ Research
Funding
Acknowledgments
Conflicts of Interest
References
- Galenus, A. Book I. In De Placitis Hippocratis Et Platonis; 162AD; pp. I 9.1–9.2.
- Goss, C.M. On movement of muscles by galen of pergamon. Am. J. Anat. 1968, 123, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Schwann, T. Mikroskopische Untersuchungen über die Uebereinstimmung in der Struktur und dem Wachstum der Thiere und Pflanzen mit 4 Kupfertafeln; Verlag der Sander’schen Buchhandlung: Berlin, Germany, 1839. [Google Scholar]
- Doyère, L.M.F. Memoire sur les Tardigrades. Ann. des Sci. Nat. Zool. S II 1840, 14, 269–362. [Google Scholar]
- Quatrefages de Bréau, J.L.A. De Mémoire sur l’Eolidine paradoxale (Eolidina paradoxum Nob.). Ann. Sci. Nat. Zool. S. II 1843, 19, 274–312. [Google Scholar]
- Kühne, W. Neue Untersuchungen über motorische Nervenendigungen. Z. Biol. 1886, 23, 1–148. [Google Scholar]
- Bock, O. Cajal, Golgi, Nansen, Schäfer and the Neuron Doctrine. Endeavour 2013, 37, 228–234. [Google Scholar] [CrossRef]
- Palade, G.E.; Palay, S.L. Electron microscope observations of interneural and and neurmuscular synapses. Anat. Rec. 1954, 118, 335. [Google Scholar]
- Reger, J.F. Electron microscopy of the motor end-plate in rat intercostal muscle. Anat. Rec. 1955, 122, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Andersson-Cedergren, E. Ultrastructure of motor endplate and sarcoplasmic components of mouse skeletal muscle fiber. J. Ultrastruct. Res. Suppl. 1959, 1, 5–181. [Google Scholar] [CrossRef]
- Krause, W. Über die Endigung der Muskelnerven. Zweiter Artikel. Zeitsch. Ration. Med. 1863, 20, 1–18. [Google Scholar]
- Kramer, B.; Hattingh, J.; Teixeira, M.; Wolf, D.; Raath, J.P. The neuromuscular junction in the African elephant Loxodonta africana and African buffalo Syncerus caffer. S. Afr. Tydskr. Natuurnav. 1995, 25, 14–16. [Google Scholar]
- Zenker, W.; Snobl, D.; Boetschi, R. Multifocal innervation and muscle length. A morphological study on the role of myo-myonal junctions, fiber branching and multiple innervation in muscles of different size and shape. Anat. Embryol. 1990, 182, 273–283. [Google Scholar] [PubMed]
- Rouget, C.M.B. Sur la terminaison des nerfs moteurs dans les muscles. Verhand. Schweiz. Nat. Gesell. 1863, 47, 148–155. [Google Scholar]
- Rouget, C.M.B. Note sur la terminaison des nerfs moteurs dans les muscles. Comp. Rend. Hebd. Séances Acad. Sci. 1866, 62, 1377–1381. [Google Scholar]
- Ranvier, L.-A. Traité Technique d’histologie; Libraire F. Savy: Paris, France, 1878. [Google Scholar]
- Golgi, C. Sui Nervi dei Tendini. Mem. Accad. Sci. Torino. Ser. II 1880, 32, 359–385. [Google Scholar]
- Bremer, L. Ueber die Endigungen der markhaltigen und marklosen Nerven im quergestreiften Muskel. Arch. Mikr. Anat. 1882, 21, 165–201. [Google Scholar] [CrossRef]
- Sherrington, C.S. On the Anatomical Constitution of Nerves of Skeletal Muscles; with Remarks on Recurrent Fibres in the Ventral Spinal Nerve-Root. J. Physiol. 1894, 17, 211–258. [Google Scholar] [CrossRef]
- Kühne, W. Die Muskelspindeln: Ein Beitrag zur Lehre von der Entwicklung der Muskeln und Nervenfasern. Arch. Pathol. Anat. Physiol. Clin. Med. 1863, 28, 528–538. [Google Scholar] [CrossRef]
- Remak, R. Ueber die Structur des Nervensystems. Neue Notizen Gebiete Nat. Heilkd. 1838, 6, 173–175. [Google Scholar]
- Gaskell, W.H. The Involuntary Nervous System; Longmans, Green and Co.: London, UK; New York, NY, USA; Bombay, India; Calcutta, India; Madras, India, 1916. [Google Scholar]
- Langley, J.N. The Autonomic Nervous System. Part I; W. Heffer & Sons Ltd.: Cambridge, MA, USA, 1921. [Google Scholar]
- Stöhr, P.J. Mikroskopische Anatomie des Vegetativen Nervensystems; Verlag von Julius Springer: Berlin, Germany, 1928. [Google Scholar]
- Lombard, J.H.; Cowley, A.W., Jr. Neural Control of Blood Vessels. In Primer on the Autonomic Nervous System; Robertson, D., Biaggioni, I., Burnstock, G., Low, P.A., Paton, J.F.R., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2012; pp. 187–192. [Google Scholar]
- Perroncito, A. Etudes ultérieures sur la terminaison des nerfs dans les muscles à fibres striées. Arch. Ital. Biol. 1902, 38, 393. [Google Scholar]
- Boeke, J. Die motorische Endplatte bei den höheren Vertebraten, ihre Entwickelung, Form und Zusammenhang mit der Muskelfaser. Anat. Anz. 1909, 35, 240–256. [Google Scholar]
- Boeke, J. Ueber eine aus marklosen Fasern hervorgehende zweite Art von hypolemmalen Nervenendplatten bei den quergestreiften Muskelfasern der Vertebraten. Anat. Anz. 1909, 35, 481–484. [Google Scholar]
- Boeke, J. Die doppelte (motorische und sympathische) efferente Innervation der quergestreiften Muskelfasern. Anat. Anz. 1913, 44, 343–356. [Google Scholar]
- Agduhr, E. Sympathetic innervation of the muscles of the extremities. A histo-experimental study. Verhand. Akad. Wetensch. 1920, 20, 1–34. [Google Scholar]
- Hines, M. Studies on the Innervation of Skeletal Muscle. Am. J. Anat. 1931, 47, 1–53. [Google Scholar] [CrossRef]
- Kulchitsky, N. Nerve Endings in the Muscles of the Frog. J. Anat. 1924, 59, 1–17. [Google Scholar]
- Hunter, J.I. The Sympathetic Innervation of Striated Muscle. Br. Med. J. 1925, 197–201. [Google Scholar] [CrossRef][Green Version]
- Hinsey, J.C. The Innervation of Skeletal Muscle. Physiol. Rev. 1934, 14, 514–585. [Google Scholar] [CrossRef]
- Hines, M. Studies in the Innervation of Skeletal Muscle. J. Comp. Neurol. 1932, 56, 105–133. [Google Scholar] [CrossRef]
- Wilkinson, H.J. Further Experimental Studies on the Innervation of Striated Muscle. J. Comp. Neurol. 1934, 59, 221–238. [Google Scholar] [CrossRef]
- Tower, S.S. Further Study of the Sympathetic Innervation to Skeletal Muscle: Anatomical Considerations. J. Comp. Neurol. 1931, 53, 177–203. [Google Scholar] [CrossRef]
- Boeke, J.; Dusser de Barenne, J. De sympathische innervatie van de dwarsgestreepte spieren bij de gewehrvehde dieren. Proc. Akad. Wetensch. 1919, 21, 1227. [Google Scholar]
- Chan-Palay, V.; Engel, A.G.; Palay, S.L.; Wu, J.Y. Synthesizing enzymes for four neuroactive substances in motor neurons and neuromuscular junctions: Light and electron microscopic immunocytochemistry. Proc. Natl. Acad. Sci. USA 1982, 79, 6717–6721. [Google Scholar] [CrossRef]
- Chan-Palay, V.; Engel, A.G.; Wu, J.Y.; Palay, S.L. Coexistence in human and primate neuromuscular junctions of enzymes synthesizing acetylcholine, catecholamine, taurine, and gamma-aminobutyric acid. Proc. Natl. Acad. Sci. USA 1982, 79, 7027–7030. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Saito, M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc. R. Soc. B Biol. Sci. 1981, 212, 317–332. [Google Scholar]
- Falck, B.; Torp, A. New evidence for the localization of noradrenalin in the adrenergic nerve terminals. Med. Exp. Int. J. Exp. Med. 1962, 6, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Falck, B.; Hillarp, N.A.; Thieme, G.; Torp, A. Fluorescence of catechol amines and related compounds condensed with formaldehyde. Brain Res. Bull. 1982, 9, xi–xv. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta. Physiol. Scand. Suppl. 1964, 232, 1–55. [Google Scholar]
- Rudolf, R.; Khan, M.M.; Lustrino, D.; Labeit, S.; Kettelhut, Í.C.; Navegantes, L.C.C. Alterations of cAMP-dependent signaling in dystrophic skeletal muscle. Front. Physiol. 2013, 4, 290. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Lustrino, D.; Silveira, W.A.; Wild, F.; Straka, T.; Issop, Y.; O’Connor, E.; Cox, D.; Reischl, M.; Marquardt, T.; et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 746–750. [Google Scholar] [CrossRef]
- Rodrigues, A.C.Z.; Messi, M.L.; Wang, Z.-M.; Abba, M.C.; Pereyra, A.; Birbrair, A.; Zhang, T.; O’Meara, M.; Kwan, P.; Lopez, E.I.S.; et al. The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta. Physiol. 2018, 225, e13195. [Google Scholar] [CrossRef]
- Rodrigues, A.Z.C.; Wang, Z.-M.; Messi, M.L.; Delbono, O. Sympathomimetics regulate neuromuscular junction transmission through TRPV1, P/Q- and N-type Ca2+ channels. Mol. Cell. Neurosci. 2019, 95, 59–70. [Google Scholar] [CrossRef]
- Gomes, A.V.; Waddell, D.S.; Siu, R.; Stein, M.; Dewey, S.; Furlow, J.D.; Bodine, S.C. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J. 2012, 26, 2986–2999. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Centner, T.; Yano, J.; Kimura, E.; McElhinny, A.S.; Pelin, K.; Witt, C.C.; Bang, M.L.; Trombitas, K.; Granzier, H.; Gregorio, C.C.; Sorimachi, H.; Labeit, S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J. Mol. Biol. 2001, 306, 717–726. [Google Scholar] [CrossRef]
- Khan, M.M.; Strack, S.; Wild, F.; Hanashima, A.; Gasch, A.; Brohm, K.; Reischl, M.; Carnio, S.; Labeit, D.; Sandri, M.; Labeit, S.; Rudolf, R. Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy 2014, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wild, F.; Khan, M.M.; Straka, T.; Rudolf, R. Progress of endocytic CHRN to autophagic degradation is regulated by RAB5-GTPase and T145 phosphorylation of SH3GLB1 at mouse neuromuscular junctions in vivo. Autophagy 2016, 12, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Straka, T.; Vita, V.; Prokshi, K.; Hörner, S.J.; Khan, M.M.; Pirazzini, M.; Williams, M.P.I.; Hafner, M.; Zaglia, T.; Rudolf, R. Postnatal Development and Distribution of Sympathetic Innervation in Mouse Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 1935. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Warwick, A.K.; Satoh, A.; Santosa, K.B.; Imai, S.; Jablonka-Shariff, A. Hypothalamic Sirt1 protects terminal Schwann cells and neuromuscular junctions from age-related morphological changes. Aging Cell 2018, e12776. [Google Scholar] [CrossRef]
- Bernard, C. Leçons sur les Effets des Substances Toxiques et Médicamenteuses; J.B. Baillière et Fils: Paris, France, 1857. [Google Scholar]
- Vulpian, E.F.A. Leçons sur la Physiologie Générale et Comparée du Système Nerveux; Germer Baillière: Paris, France; London, UK; New York, NY, USA, 1866. [Google Scholar]
- Cousin, M.-T. L’apport des scientifiques français à l’anesthésiologie. Les curares et la jonction neuromusculaire. Hist. Sci. Med. 2000, 24, 219–230. [Google Scholar]
- du Bois-Reymond, E.H. Experimentalkritik der Entladungshypothese über die Wirkung von Nerv auf Muskel. In Gesammelte Abhandlungen zur Allgemeinen Muskel-und Nervenphysik; Veit und Comp.: Leipzig, Germany, 1877; pp. 698–736. [Google Scholar]
- Foster, M.A.; Sherrington, C.S. Section II. The Structure of the Spinal Cord. In A Textbook of Physiology; Macmillan: London, UK, 1897; pp. 922–972. [Google Scholar]
- Sherrington, C.S. The Integrative Action of the Nervous System; Oxford Univ. Press: London, UK, 1906. [Google Scholar]
- Oliver, G.; Schäfer, E.A. The Physiological Effects of Extracts of the Suprarenal Capsules. J. Physiol. 1895, 18, 230–276. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.J. Ueber den blutdruckerregenden Bestandtheil der Nebenniere, das Epinephrin. Zeitsch. Physiol. Chem. 1900, 28, 318–362. [Google Scholar] [CrossRef]
- Takamine, J. Adrenalin, the active principle of the suprarenal glands, and its mode of preparation. Am. J. Pharm. 1901, 73, 523–531. [Google Scholar]
- Aldrich, T.B. A preliminary report on the active principle of the suprarenal gland. Am. J. Physiol. 1901, 5, 457–461. [Google Scholar] [CrossRef]
- Von Euler, U.S. Sympathin in adrenergic nerve fibres. J. Physiol. 1946, 105, 26. [Google Scholar] [PubMed]
- Von Euler, U.S. The presence of a sympathomimetic substance in extracts of mammalian heart. J. Physiol. 1946, 105, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Von Euler, U.S. A substance with sympathin E properties in spleen extracts. Nature 1946, 157, 369. [Google Scholar] [CrossRef] [PubMed]
- Baeyer, A. Synthese des Neurins. Justus Liebigs Ann. Chem. 1866, 140, 306–313. [Google Scholar]
- Baeyer, A. Ueber das Neurin. Justus Liebigs Ann. Chem. 1867, 142, 322–326. [Google Scholar] [CrossRef]
- Hunt, R.; De M. Taveau, R. On the Physiological Action of Certain Cholin Derivatives and New Methods for Detecting Cholin. Br. Med. J. 1906, 2, 1788–1791. [Google Scholar]
- Loewi, O. Über humorale Übertragbarkeit der Herznervenwirkung. Pflüg. Arch. 1921, 189, 201–213. [Google Scholar] [CrossRef]
- Hess, W.R. Ueber die Wirkung von Acetylcholin auf den Skelettmuskel. Q. J. Exp. Physiol. 1923, 13, 144–145. [Google Scholar]
- Dale, S.H.H.; Feldberg, S.W. Chemical transmission at motor nerve endings in voluntary muscle? J. Phys. J. Physiol. 1934, 81, 39. [Google Scholar]
- Dale, S.H.H.; Feldberg, S.W.; Vogt, M.L. Release of Acetylcholine at Voluntary Motor Nerve Endings. J. Physiol. 1936, 86, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Stedman, E.; Stedman, E.; Easson, L.H. Choline-esterase. An enzyme present in the blood-serum of the horse. Biochem. J. 1932, 26, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Marnay, A.; Nachmansohn, D. Cholinesterase in voluntary frog’s muscle. J. Physiol. 1937, 89, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nachmansohn, D. Electricity Elicited by an Organic Chemical Process. Science 1940, 91, 405–406. [Google Scholar] [CrossRef]
- Cartaud, J.; Cartaud, A.; Kordeli, E.; Ludosky, M.A.; Marchand, S.; Stetzkowski-Marden, F. TheTorpedo electrocyte: A model system to study membrane-cytoskeleton interactions at the postsynaptic membrane. Microsc. Res. Tech. 2000, 49, 73–83. [Google Scholar] [CrossRef]
- Eccles, J.C.; O’Connor, W.J. Responses which Nerve Impulses Evoke in Mammalian Striated Muscles. J. Physiol. 1939, 97, 44–102. [Google Scholar] [CrossRef] [PubMed]
- Moruzzi, G. The electrophysiological work of Carlo Matteucci 1964. Brain Res. Bull. 1996, 40, 69–91. [Google Scholar] [CrossRef]
- Du Bois-Reymond, E.H. Vorläufiger Abriss einer Untersuchung über den sogenannten Froschstrom und über die elektromotorischen Fische. Poggendorff’s Ann. Phys. Chem. 1843, 53, 1–30. [Google Scholar] [CrossRef]
- Helmholtz, H. Von Messungen über Fortpflanzungsgeschwindigkeit der Reizung in den Nerven. Arch. Anat. Physiol. Wissenschaft. Med. 1852, 199–216. [Google Scholar]
- Adrian, E.D. On the summation of propagated disturbances in nerve and muscle. J. Physiol. 1912, 44, 68–124. [Google Scholar] [CrossRef] [PubMed]
- Adrian, E.D. The all-or-none principle in nerve. J. Physiol. 1914, 47, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Thatcher, C. Amplification of Action Currents with the Electron Tube in Recording with the String Galvanometer. Am J. Physiol. 1920, 52, 409–471. [Google Scholar] [CrossRef]
- Gasser, H.S.; Newcomer, H.S. Physiological Action Currents in the Phrenic Nerve. An Application of the Thermionic Vacuum Tube to Nerve Physiology. Am. J. Physiol. 1921, 57, 1–26. [Google Scholar] [CrossRef]
- Gasser, H.S.; Erlanger, J. A Study of the Action Currents of Nerve with the Cathode Ray Oscillograph. Am. J. Physiol. 1922, 62, 496–524. [Google Scholar] [CrossRef]
- Göpfert, H.; Schäfer, H. Über den direkt und indirekt erregten Aktionsstrom und die Funktion der motorischen Endplatte. Pflüg. Arch. 1938, 239, 597–619. [Google Scholar] [CrossRef]
- Eccles, J.C.; Katz, B.; Kuffler, S.W. Nature of the “endplate potential” in curarized muscle. J. Neurophysiol. 1941, 4, 362–387. [Google Scholar] [CrossRef]
- Katz, B. Impedance changes in frog’s muscle associated with electrotonic and “endplate” potentials. J. Neurophysiol. 1942, 5, 169–184. [Google Scholar] [CrossRef]
- Eccles, J.C.; Katz, B.; Kuffler, S.W. Effect of eserine on neuromuscular transmission. J. Neurophysiol. 1942, 5, 211–230. [Google Scholar] [CrossRef]
- Fatt, P.; Katz, B. Spontaneous Subthreshold Activity at Motor Nerve Endings. J. Physiol. 1952, 117, 109–128. [Google Scholar] [PubMed]
- Del Castillo, J.; Katz, B. Quantal Components of the End-Plate Potential. J. Physiol. 1954, 124, 560–573. [Google Scholar] [CrossRef]
- Birks, R.; Huxley, H.E.; Katz, B. The Fine Structure of the Neuromuscular Junction of the Frog. J. Physiol. 1960, 150, 134–144. [Google Scholar] [CrossRef]
- Whittaker, V.P.; Michaelson, I.A.; Kirkland, R.J. The separation of synaptic vesicles from nerve-ending particles (“synaptosomes”). Biochem. J. 1964, 90, 293–303. [Google Scholar] [CrossRef]
- Whittaker, V.P.; Sheridan, M.N. The Morphology and Acetylcholine Content of Isolated Cerebral Cortical Synaptic Vesicles. J. Neurochem. 1965, 12, 363–372. [Google Scholar] [CrossRef]
- Sheridan, M.N.; Whittaker, V.P.; Israël, M. The subcellular fractionation of the electric organ of Torpedo. Z. Zellforsch. Mikrosk. Anat. 1966, 74, 293–307. [Google Scholar] [CrossRef]
- Couteaux, R.; Pécot-Dechavassine, M. Vésicules synaptiques et poches au niveau des “zones actives” de la jonction neuromusculaire. Compt. Rend. Hebd. Séances Acad. Sci. 1970, 271, 2346–2349. [Google Scholar]
- Heuser, J.E.; Reese, T.S.; Landis, D.M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J. Neurocytol. 1974, 3, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Heuser, J.E.; Reese, T.S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 1973, 57, 315–344. [Google Scholar] [CrossRef]
- Südhof, T.C.; Jahn, R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron 1991, 6, 665–677. [Google Scholar] [CrossRef]
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef]
- Harlow, M.; Ress, D.; Koster, A.; Marshall, R.M.; Schwarz, M.; McMahan, U.J. Dissection of active zones at the neuromuscular junction by EM tomography. J. Physiol. Paris 1998, 92, 75–78. [Google Scholar] [CrossRef]
- Harlow, M.L.; Ress, D.; Stoschek, A.; Marshall, R.M.; McMahan, U.J. The architecture of active zone material at the frog’s neuromuscular junction. Nature 2001, 409, 479–484. [Google Scholar] [CrossRef]
- Szule, J.A.; Jung, J.H.; McMahan, U.J. The structure and function of “active zone material” at synapses. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140189. [Google Scholar] [CrossRef]
- Takeuchi, N. Some properties of conductance changes at the end-plate membrane during the action of acetylcholine. J. Physiol. 1963, 167, 128–140. [Google Scholar] [CrossRef]
- Katz, B.; Miledi, R. Membrane noise produced by acetylcholine. Nature 1970, 226, 962–963. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, J.; Katz, B. On the localization of acetylcholine receptors. J. Physiol. 1955, 128, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Miledi, R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J. Physiol. 1960, 151, 24–30. [Google Scholar] [PubMed]
- Neher, E.; Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 1976, 260, 799–802. [Google Scholar] [CrossRef]
- Sakmann, B.; Neher, E. Patch Clamp Techniques for Studying Ionic Channels in Excitable Membranes. Annu. Rev. Physiol. 1984, 46, 455–472. [Google Scholar] [CrossRef]
- Langley, J.N. On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J. Physiol. 1905, 33, 374–413. [Google Scholar] [CrossRef]
- Dale, S.H.H. The Action of Certain Esters and Ethers of Choline, and their Relation to Muscarine. J. Pharmacol. Exp. Ther. 1914, 6, 147–190. [Google Scholar]
- Kruse, A.C.; Kobilka, B.K.; Gautam, D.; Sexton, P.M.; Christopoulos, A.; Wess, J. Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug Discov. 2014, 13, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Nadal, L.; Garcia, N.; Hurtado, E.; Simó, A.; Tomàs, M.; Lanuza, M.A.; Santafé, M.; Tomàs, J. Presynaptic muscarinic acetylcholine autoreceptors (M1, M2 and M4 subtypes), adenosine receptors (A1 and A2A) and tropomyosin-related kinase B receptor (TrkB) modulate the developmental synapse elimination process at the neuromuscular junction. Mol. Brain 2016, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Tomàs, J.; Garcia, N.; Lanuza, M.A.; Santafé, M.M.; Tomàs, M.; Nadal, L.; Hurtado, E.; Simó, A.; Cilleros, V. Presynaptic Membrane Receptors Modulate ACh Release, Axonal Competition and Synapse Elimination during Neuromuscular Junction Development. Front. Mol. Neurosci. 2017, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Claude, A. Fractionation of mammalian liver cells by differential centrifugation; experimental procedures and results. J. Exp. Med. 1946, 84, 61–89. [Google Scholar] [CrossRef] [PubMed]
- Claude, A. Fractionation of mammalian liver cells by differential centrifugation; problems, methods, and preparation of extract. J. Exp. Med. 1946, 84, 51–59. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. Tissue fractionation. Past and present. J. Cell Biol. 1971, 50, 20d–55d. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P.; Kasai, M.; Lee, C.Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Natl. Acad. Sci. USA 1970, 67, 1241–1247. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.C.; Chen, Y.M. Reversibility of neuromuscular blockade by neurotoxins from elapid and sea snake venoms. Taiwan Yi Xue Hui Za Zhi 1972, 71, 344–349. [Google Scholar] [PubMed]
- Chang, C.C.; Huang, M.C. Turnover of junctional and extrajunctional acetylcholine receptors of the rat diaphragm. Nature 1975, 253, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Nachmansohn, D.; Coates, C.W.; Cox, R.T. Electric Potential and Activity of Choline Esterase in the Electric Organ of Electrophorus Electricus (Linnaeus). J. Gen. Physiol. 1941, 25, 75–88. [Google Scholar] [CrossRef]
- Feldberg, W.; Fessard, A. The cholinergic nature of the nerves to the electric organ of the Torpedo (Torpedo marmorata). J. Physiol. 1942, 101, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P.; Gautron, J.; Israël, M.; Podleski, T. [Separation of excitable membranes from the electric organ of Electrophorus electricus]. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 1969, 269, 1788–1791. [Google Scholar] [PubMed]
- Weill, C.L.; McNamee, M.G.; Karlin, A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem. Biophys. Res. Commun. 1974, 61, 997–1003. [Google Scholar] [CrossRef]
- Raftery, M.A.; Vandlen, R.; Michaelson, D.; Bode, J.; Moody, T.; Chao, Y.; Reed, K.; Deutsch, J.; Duguid, J. The biochemistry of an acetylcholine receptor. J. Supramol. Struct. 1974, 2, 582–592. [Google Scholar] [CrossRef]
- Noda, M.; Takahashi, H.; Tanabe, T.; Toyosato, M.; Furutani, Y.; Hirose, T.; Asai, M.; Inayama, S.; Miyata, T.; Numa, S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature 1982, 299, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Takahashi, H.; Tanabe, T.; Toyosato, M.; Kikyotani, S.; Hirose, T.; Asai, M.; Takashima, H.; Inayama, S.; Miyata, T.; et al. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature 1983, 301, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Noda, M.; Furutani, Y.; Takahashi, H.; Notake, M.; Shimizu, S.; Kayano, T.; Tanabe, T.; Tanaka, K.; Hirose, T. Primary structure of gamma subunit precursor of calf-muscle acetylcholine receptor deduced from the cDNA sequence. Eur. J. Biochem. 1984, 143, 109–115. [Google Scholar] [CrossRef]
- Tanabe, T.; Noda, M.; Furutani, Y.; Takai, T.; Takahashi, H.; Tanaka, K.; Hirose, T.; Inayama, S.; Numa, S. Primary structure of beta subunit precursor of calf muscle acetylcholine receptor deduced from cDNA sequence. Eur. J. Biochem. 1984, 144, 11–17. [Google Scholar] [CrossRef]
- Kubo, T.; Noda, M.; Takai, T.; Tanabe, T.; Kayano, T.; Shimizu, S.; Tanaka, K.; Takahashi, H.; Hirose, T.; Inayama, S. Primary structure of delta subunit precursor of calf muscle acetylcholine receptor deduced from cDNA sequence. Eur. J. Biochem. 1985, 149, 5–13. [Google Scholar] [CrossRef]
- Cartaud, J.; Benedetti, E.L.; Cohen, J.B.; Meunier, J.C.; Changeux, J.P. Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata. FEBS Lett. 1973, 33, 109–113. [Google Scholar] [CrossRef]
- Meunier, J.C.; Sugiyama, H.; Cartaud, J.; Sealock, R.; Changeux, J.P. Functional properties of the purified cholinergic receptor protein from Electrophorus electricus. Brain Res. 1973, 62, 307–315. [Google Scholar] [CrossRef]
- Unwin, N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Q. Rev. Biophys. 2013, 46, 283–322. [Google Scholar] [CrossRef] [PubMed]
- Mishina, M.; Takai, T.; Imoto, K.; Noda, M.; Takahashi, T.; Numa, S.; Methfessel, C.; Sakmann, B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 1986, 321, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Witzemann, V.; Barg, B.; Nishikawa, Y.; Sakmann, B.; Numa, S. Differential regulation of muscle acetylcholine receptor gamma- and epsilon-subunit mRNAs. FEBS Lett. 1987, 223, 104–112. [Google Scholar] [CrossRef]
- Schaeffer, L.; Duclert, N.; Huchet-Dymanus, M.; Changeux, J.P. Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 1998, 17, 3078–3090. [Google Scholar] [CrossRef]
- Schaeffer, L.; de Kerchove d’Exaerde, A.; Changeux, J.P. Targeting transcription to the neuromuscular synapse. Neuron 2001, 31, 15–22. [Google Scholar] [CrossRef]
- Porter, S.; Froehner, S.C. Characterization and localization of the Mr = 43,000 proteins associated with acetylcholine receptor-rich membranes. J. Biol. Chem. 1983, 258, 10034–10040. [Google Scholar]
- Frail, D.E.; McLaughlin, L.L.; Mudd, J.; Merlie, J.P. Identification of the mouse muscle 43,000-dalton acetylcholine receptor-associated protein (RAPsyn) by cDNA cloning. J. Biol. Chem. 1988, 263, 15602–15607. [Google Scholar]
- Tsim, K.W.; Ruegg, M.A.; Escher, G.; Kroger, S.; McMahan, U.J. cDNA that encodes active agrin. Neuron 1992, 8, 677–689. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Bowen, D.C.; Valenzuela, D.M.; Simmons, M.V.; Poueymirou, W.T.; Thomas, S.; Kinetz, E.; Compton, D.L.; Rojas, E.; Park, J.S.; et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996, 85, 501–512. [Google Scholar] [CrossRef]
- Weatherbee, S.D.; Anderson, K.V.; Niswander, L.A. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 2006, 133, 4993–5000. [Google Scholar] [CrossRef]
- Tintignac, L.A.; Brenner, H.-R.; Rüegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef]
- Li, L.; Xiong, W.-C.; Mei, L. Neuromuscular Junction Formation, Aging, and Disorders. Annu. Rev. Physiol. 2018, 80, 159–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y. Chemistry and Pharmacology of Polypeptide Toxins in Snake Venoms. Annu. Rev. Pharmacol. 1972, 12, 265–286. [Google Scholar] [CrossRef]
- Lentz, T.L.; Mazurkiewicz, J.E.; Rosenthal, J. Cytochemical localization of acetylcholine receptors at the neuromuscular junction by means of horseradish peroxidase-labeled alpha-bungarotoxin. Brain Res. 1977, 132, 423–442. [Google Scholar] [CrossRef]
- Anderson, M.J.; Cohen, M.W. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J. Physiol. 1974, 237, 385–400. [Google Scholar] [CrossRef]
- Axelrod, D.; Ravdin, P.; Koppel, D.E.; Schlessinger, J.; Webb, W.W.; Elson, E.L.; Podleski, T.R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc. Natl. Acad. Sci. USA 1976, 73, 4594–4598. [Google Scholar] [CrossRef]
- Fambrough, D.M. Control of acetylcholine receptors in skeletal muscle. Physiol. Rev. 1979, 59, 165–227. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.K.; Hall, Z.W. Loss of alpha-bungarotoxin from junctional and extrajunctional acetylcholine receptors in rat diaphragm muscle in vivo and in organ culture. J. Physiol. 1975, 252, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Merlie, J.P.; Changeux, J.P.; Gros, F. Acetylcholine receptor degradation measured by pulse chase labelling. Nature 1976, 264, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Levitt, T.A.; Loring, R.H.; Salpeter, M.M. Neuronal control of acetylcholine receptor turnover rate at a vertebrate neuromuscular junction. Science 1980, 210, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Levitt, T.A.; Salpeter, M.M. Denervated endplates have a dual population of junctional acetylcholine receptors. Nature 1981, 291, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.F.; Drachman, D.B. Denervation accelerates the degradation of junctional acetylcholine receptors. Exp. Neurol. 1981, 73, 390–396. [Google Scholar] [CrossRef]
- Stanley, E.F.; Drachman, D.B. Rapid degradation of “new” acetylcholine receptors at neuromuscular junctions. Science 1983, 222, 67–69. [Google Scholar] [CrossRef]
- Andreose, J.S.; Xu, R.; Lomo, T.; Salpeter, M.M.; Fumagalli, G. Degradation of two AChR populations at rat neuromuscular junctions: Regulation in vivo by electrical stimulation. J. Neurosci. 1993, 13, 3433–3438. [Google Scholar] [CrossRef]
- Xu, R.; Salpeter, M.M. Acetylcholine receptors in innervated muscles of dystrophic mdx mice degrade as after denervation. J. Neurosci. 1997, 17, 8194–8200. [Google Scholar] [CrossRef]
- Strack, S.; Petersen, Y.; Wagner, A.; Röder, I.V.; Albrizio, M.; Reischl, M.; Wacker, I.U.; Wilhelm, C.; Rudolf, R. A novel labeling approach identifies three stability levels of acetylcholine receptors in the mouse neuromuscular junction in vivo. PLoS ONE 2011, 6, e20524. [Google Scholar] [CrossRef]
- Strack, S.; Khan, M.M.; Wild, F.; Rall, A.; Rudolf, R. Turnover of acetylcholine receptors at the endplate revisited: Novel insights into nerve-dependent behavior. J. Muscle Res. Cell Motil. 2015, 36, 517–524. [Google Scholar] [CrossRef]
- Akaaboune, M.; Culican, S.M.; Turney, S.G.; Lichtman, J.W. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science 1999, 286, 503–507. [Google Scholar] [CrossRef]
- Yampolsky, P.; Pacifici, P.G.; Witzemann, V. Differential muscle-driven synaptic remodeling in the neuromuscular junction after denervation. Eur. J. Neurosci. 2010, 31, 646–658. [Google Scholar] [CrossRef]
- Fumagalli, G.; Engel, A.G.; Lindstrom, J. Ultrastructural aspects of acetylcholine receptor turnover at the normal end-plate and in autoimmune myasthenia gravis. J. Neuropathol. Exp. Neurol. 1982, 41, 567–579. [Google Scholar] [CrossRef]
- Engel, A.G.; Fumagalli, G. Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Found. Symp. 1982, 90, 197–224. [Google Scholar]
- Bruneau, E.; Sutter, D.; Hume, R.I.; Akaaboune, M. Identification of nicotinic acetylcholine receptor recycling and its role in maintaining receptor density at the neuromuscular junction in vivo. J. Neurosci. 2005, 25, 9949–9959. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, R.; Khan, M.M.; Labeit, S.; Deschenes, M.R. Degeneration of Neuromuscular Junction in Age and Dystrophy. Front. Aging Neurosci. 2014, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, R.; Deschenes, M.R.; Sandri, M. Neuromuscular junction degeneration in muscle wasting. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 177–181. [Google Scholar] [CrossRef]
- Röder, I.V.; Petersen, Y.; Choi, K.R.; Witzemann, V.; Hammer, J.A., 3rd; Rudolf, R. Role of Myosin Va in the plasticity of the vertebrate neuromuscular junction in vivo. PLoS ONE 2008, 3, e3871. [Google Scholar] [CrossRef]
- Röder, I.V.; Choi, K.-R.R.; Reischl, M.; Petersen, Y.; Diefenbacher, M.E.; Zaccolo, M.; Pozzan, T.; Rudolf, R. Myosin Va cooperates with PKA RIalpha to mediate maintenance of the endplate in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-R.R.; Berrera, M.; Reischl, M.; Strack, S.; Albrizio, M.; Roder, I.V.; Wagner, A.; Petersen, Y.; Hafner, M.; Zaccolo, M.; et al. Rapsyn mediates subsynaptic anchoring of PKA type I and stabilisation of acetylcholine receptor in vivo. J. Cell Sci. 2012, 125, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pena y Valenzuela, I.; Pires-Oliveira, M.; Akaaboune, M. PKC and PKA Regulate AChR Dynamics at the Neuromuscular Junction of Living Mice. PLoS ONE 2013, 8, e81311. [Google Scholar] [CrossRef]
- Yampolsky, P.; Pacifici, P.G.; Lomb, L.; Giese, G.; Rudolf, R.; Röder, I.V.; Witzemann, V. Time lapse in vivo visualization of developmental stabilization of synaptic receptors at neuromuscular junctions. J. Biol. Chem. 2010, 285, 34589–34596. [Google Scholar] [CrossRef] [PubMed]
- Witzemann, V.; Barg, B.; Criado, M.; Stein, E.; Sakmann, B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett. 1989, 242, 419–424. [Google Scholar] [CrossRef]
- Balice-Gordon, R.J.; Lichtman, J.W. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J. Neurosci. 1993, 13, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Balice-Gordon, R.J.; Chua, C.K.; Nelson, C.C.; Lichtman, J.W. Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron 1993, 11, 801–815. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, W.J.; Harlow, M.L. Schwann cells participate in synapse elimination at the developing neuromuscular junction. Curr. Opin. Neurobiol. 2017, 47, 176–181. [Google Scholar] [CrossRef]
- Slater, C.R. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev. Biol. 1982, 94, 11–22. [Google Scholar] [CrossRef]
- Marques, M.J.; Conchello, J.A.; Lichtman, J.W. From plaque to pretzel: Fold formation and acetylcholine receptor loss at the developing neuromuscular junction. J. Neurosci. 2000, 20, 3663–3675. [Google Scholar] [CrossRef]
- Bernadzki, K.M.; Rojek, K.O.; Prószyński, T.J. Podosomes in muscle cells and their role in the remodeling of neuromuscular postsynaptic machinery. Eur. J. Cell Biol. 2014, 93, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Mouslim, C.; Aittaleb, M.; Hume, R.I.; Akaaboune, M. A role for the calmodulin kinase II-related anchoring protein (alphakap) in maintaining the stability of nicotinic acetylcholine receptors. J. Neurosci. 2012, 32, 5177–5185. [Google Scholar] [CrossRef]
- Rudolf, R.; Bogomolovas, J.; Strack, S.; Choi, K.-R.; Khan, M.M.; Wagner, A.; Brohm, K.; Hanashima, A.; Gasch, A.; Labeit, D.; Labeit, S. Regulation of nicotinic acetylcholine receptor turnover by MuRF1 connects muscle activity to endo/lysosomal and atrophy pathways. Age 2013, 35, 1663–1674. [Google Scholar] [CrossRef]
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Loefler, S.; Kern, H.; et al. Autophagy Impairment in Muscle Induces Neuromuscular Junction Degeneration and Precocious Aging. Cell Rep. 2014, 8, 1509–1521. [Google Scholar] [CrossRef]
- Wild, F.; Khan, M.M.; Rudolf, R. Evidence for the subsynaptic zone as a preferential site for CHRN recycling at neuromuscular junctions. Small GTPases 2017, 1–8. [Google Scholar] [CrossRef]
- Legay, C.; Mei, L. Moving forward with the neuromuscular junction. J. Neurochem. 2017, 142 (Suppl. 2), 59–63. [Google Scholar] [CrossRef]
- O’Connor, E.; Töpf, A.; Zahedi, R.P.; Spendiff, S.; Cox, D.; Roos, A.; Lochmüller, H. Clinical and research strategies for limb-girdle congenital myasthenic syndromes. Ann. N. Y. Acad. Sci. 2018, 1412, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.G. Congenital Myasthenic Syndromes in 2018. Curr. Neurol. Neurosci. Rep. 2018, 18, 46. [Google Scholar] [CrossRef]
- Gutmann, E.; Hanzlikova, V. Fast and slow motor units in ageing. Gerontology 1976, 22, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Valdez, G.; Tapia, J.C.; Kang, H.; Clemenson, G.D., Jr.; Gage, F.H.; Lichtman, J.W.; Sanes, J.R. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA 2010, 107, 14863–14868. [Google Scholar] [CrossRef]
- Li, Y.; Lee, Y.; Thompson, W.J. Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse. J. Neurosci. 2011, 31, 14910–14919. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Lu, Y.; Sathyamurthy, A.; Bowman, A.; Shen, C.; Li, L.; Xiong, W.; Mei, L. LRP4 is critical for neuromuscular junction maintenance. J. Neurosci. 2014, 34, 13892–13905. [Google Scholar] [CrossRef]
- Hettwer, S.; Lin, S.; Kucsera, S.; Haubitz, M.; Oliveri, F.; Fariello, R.G.; Ruegg, M.A.; Vrijbloed, J.W. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS ONE 2014, 9, e88739. [Google Scholar] [CrossRef]
- Hettwer, S.; Dahinden, P.; Kucsera, S.; Farina, C.; Ahmed, S.; Fariello, R.; Drey, M.; Sieber, C.C.; Vrijbloed, J.W. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp. Gerontol. 2013, 48, 69–75. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Taetzsch, T.; Valdez, G. NMJ maintenance and repair in aging. Curr. Opin. Physiol. 2018, 4, 57–64. [Google Scholar] [CrossRef]
- Schwander, M.; Shirasaki, R.; Pfaff, S.L.; Müller, U. Beta1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. J. Neurosci. 2004, 24, 8181–8191. [Google Scholar] [CrossRef]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, N.; Kim, N.; Burden, S.J. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature 2012, 489, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, Y.; Shen, C.; Patel, N.; Gan, L.; Xiong, W.C.; Mei, L. Distinct roles of muscle and motoneuron LRP4 in neuromuscular junction formation. Neuron 2012, 75, 94–107. [Google Scholar] [CrossRef]
- Fox, M.A.; Sanes, J.R.; Borza, D.-B.; Eswarakumar, V.P.; Fässler, R.; Hudson, B.G.; John, S.W.M.; Ninomiya, Y.; Pedchenko, V.; Pfaff, S.L.; et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 2007, 129, 179–193. [Google Scholar] [CrossRef]
- Latvanlehto, A.; Fox, M.A.; Sormunen, R.; Tu, H.; Oikarainen, T.; Koski, A.; Naumenko, N.; Shakirzyanova, A.; Kallio, M.; Ilves, M.; Giniatullin, R.; Sanes, J.R.; Pihlajaniemi, T. Muscle-derived collagen XIII regulates maturation of the skeletal neuromuscular junction. J. Neurosci. 2010, 30, 12230–12241. [Google Scholar] [CrossRef] [PubMed]
- Noakes, P.G.; Gautam, M.; Mudd, J.; Sanes, J.R.; Merlie, J.P. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature 1995, 374, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, H.; Sanes, J.R.; Carlson, S.S. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature 2004, 432, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Barik, A.; Lu, Y.; Shen, C.; Bowman, A.; Li, L.; Sathyamurthy, A.; Lin, T.W.; Xiong, W.-C.; Mei, L. Slit2 as a β-catenin/Ctnnb1-dependent retrograde signal for presynaptic differentiation. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Moloney, E.B.; de Winter, F.; Verhaagen, J. ALS as a distal axonopathy: Molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front. Neurosci. 2014, 8, 252. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Martini, M.; Scicchitano, B.M.; Romanello, V.; Boncompagni, S.; Nicoletti, C.; Pietrangelo, L.; De Panfilis, S.; Catizone, A.; Bouchè, M.; et al. Muscle Expression of SOD1 G93A Triggers the Dismantlement of Neuromuscular Junction via PKC-Theta. Antioxid. Redox Signal. 2018, 28, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Martin, L.J. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum. Mol. Genet. 2010, 19, 2284–2302. [Google Scholar] [CrossRef]
- Court, F.A.; Gillingwater, T.H.; Melrose, S.; Sherman, D.L.; Greenshields, K.N.; Morton, A.J.; Harris, J.B.; Willison, H.J.; Ribchester, R.R. Identity, developmental restriction and reactivity of extralaminar cells capping mammalian neuromuscular junctions. J. Cell Sci. 2008, 121, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolf, R.; Khan, M.M.; Witzemann, V. Motor Endplate—Anatomical, Functional, and Molecular Concepts in the Historical Perspective. Cells 2019, 8, 387. https://doi.org/10.3390/cells8050387
Rudolf R, Khan MM, Witzemann V. Motor Endplate—Anatomical, Functional, and Molecular Concepts in the Historical Perspective. Cells. 2019; 8(5):387. https://doi.org/10.3390/cells8050387
Chicago/Turabian StyleRudolf, Rüdiger, Muzamil Majid Khan, and Veit Witzemann. 2019. "Motor Endplate—Anatomical, Functional, and Molecular Concepts in the Historical Perspective" Cells 8, no. 5: 387. https://doi.org/10.3390/cells8050387
APA StyleRudolf, R., Khan, M. M., & Witzemann, V. (2019). Motor Endplate—Anatomical, Functional, and Molecular Concepts in the Historical Perspective. Cells, 8(5), 387. https://doi.org/10.3390/cells8050387





