Abstract
Cell-derived extracellular vesicles (EVs) are newly uncovered messengers for intercellular communication. They are released by almost all cell types in the three kingdoms, Archeabacteria, Bacteria and Eukaryotes. They are known to mediate important biological functions and to be increasingly involved in cell physiology and in many human diseases, especially in oncology. The aim of this review is to recapitulate the current knowledge about EVs and to summarize our pioneering work about Dictyostelium discoideum EVs. However, many challenges remain unsolved in the EV research field, before any EV application for theranostics (diagnosis, prognosis, and therapy) of human cancers, can be efficiently implemented in the clinics. Dictyostelium might be an outstanding eukaryotic cell model for deciphering the utmost challenging problem of EV heterogeneity, and for unraveling the still mostly unknown mechanisms of their specific functions as mediators of intercellular communication.
1. Introduction
After a brief presentation of the extracellular vesicles (EVs) and of the eukaryotic microorganism Dictyostelium, an overview will be given about the properties of EVs and their involvement in human health and disease. Then, our pionnering work about Dictyostelium EVs will be presented in addition to the assets of Dictyostelium, as a model for studying the mammalian EVs will be discussed.
1.1. Presentation of the Extracellular Vesicles
Cell theory was officially formulated in 1838–1839, stating that the cell is the basic component of living organisms [1]. So the cell emerged slowly to birth, as the ultimate unit of life from the seventienth to the ninetienth century. During this time, the cell was perceived as a more complex factory, regulating its multiple biological functions by means of its many macromolecular components. As a consequence, the DNA was attributed to the major director role, orchestrating all the other components in a different set of pathways. Until recently, each cell was delimited by a membrane aimed to protect its precious content from any harmful external invasion and the extracellular medium was mostly devoted to a garbage disposal; even if a few cell-derived proteins, such as proteases or hormones already had specific intercellular functions.
One important «earthquake» in cell biology arrived insidiously, initiated by the observation that plasma contains a subcellular factor that promotes the clotting of blood [2]; two decades later, Wolf showed that this subcellular factor consists of vesicles of platelet origin, named “platelet dust” [3]. Thus, the platelet plasma membrane was no longer an impermeable border, and the cell extended its field outside the cell factory. In 1981, Trams et al. reported the exfoliation of membrane ecto-enzymes in the form of microvesicles [4]. Beside these pionneer observations, two Canadian teams worked on the maturation of sheep reticulocytes into erythrocytes over a number of years, and showed that the obsolete protein transferrin was transported outside the cells by a means of extracellular vesicles, called “exosomes”. This was also observed for the maturation of human reticulocytes, and the exosome-mediated release of obsolete cellular proteins was suggested as a general mechanism [5]. In 1999, Heijnen et al. observed that activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules [6]. After the first 2005 Exosome Meeting in Canada, C. Théry and G. Raposo organised the second International Exosome Workshop (IWE) [7]. Founding of an International Society, ISEV, was decided and devoted to the study of all the Extracellular Vesicles—not limited to Exosomes—with a dedicated Journal of Extracellular Vesicles, JEV, and a yearly International Congress. This was achieved during the first 2012 ISEV Meeting with about 400 participants [8,9], whereas, the 2018 ISEV meeting gathered about 1100 participants [10]. Before ISEV, another International Society on Thrombosis and Haemostasis, ISTH, was founded in 1969, more centered on Microvesicles in Health and Disease [11], but now with many aims in common. Both Societies joined for the first time at the Educational Day before the 2016 ISEV Meeting.
1.2. Presentation of Dictyostelium
Dictyostelium discoideum was discovered in 1935 by Raper in a North Carolina (USA) forest [12], and has been widely studied ever since. For simplification, Dictyostelium is further used for Dictyostelium discoideum in this review. Dictyostelium is a eukaryotic amoeba at the border of the vegetal and animal kingdoms, which appeared in evolution about one billion years ago, long before mankind. In the wild, it grows on bacteria and cell divides by mitosis, but in the lab it can also grow in an axenic medium without any calf serum [13], or even in a completely defined medium [14,15] and cell divide also by mitosis. The individual growing cells are analogous to human leukocytes, with regard to their size (about 10 µm in diameter) and motility, and to macrophages with regard to their capacity for phagocytosis. In conditions of complete starvation, these Dictyostelium, “animal-like” cells, first experience a primitive multicellular aggregation, followed by a simple differentiation into two main “vegetal-like” cells, stalk cells, and spores. Aggregation tests in (3.5 cm in diameter) tissue culture Petri dishes with 2 × 106 adhering cells in 1 mL KK2 buffer depict cAMP-driven chemotaxis, with cell elongation within about 6 h of starvation, and further formation of nice aggregation figures, either in stars or in spirals. When being at an air-interface in (4.1 cm in diameter) differentiation tests, each complete aggregate gives rise in about 24 h from initation of starvation, to a visible so-called fruiting body, inholding (1/3) stalk cells, programmed to death and organised into a stalk, bearing a small balloon, including the (2/3) spores, programmed to further life by germination into new amoebae, when recovering normal nutrition conditions [16]. Thus, for this primitive eukaryotic species, growth and differenciation are well separated biological processes, and complete starvation induces the transformation of individual cells (about 10 µm in diameter) into a visible fruiting body (a few tenth of mm high). A. Einstein, watching J. T. Bonner’s 1940 video about this slime mold development, in Princeton (USA), was impressed by this amazing microorganism [17].
Besides its quite noticeable lifestyle, Dictyostelium possess many other assets. Its small (3.4 × 107 bp) genomic DNA has been completely sequenced [18], and covers six chromosomes, with a 90% efficient transcription into about 12,500 genes. By comparison, the human (about 109 bp) genomic DNA is 10% transcribed, with only about twice as many genes as Dictyostelium, which is devoted with some genes analogous to some important human genes. Dictyostelium cells also harbor mitochondria with a fully sequenced genome [19], and plasmids. More details about Dictyostelium can be found in the well documented website (https//www.dictybase.org), and an increasing number of specific strains and plasmids can be ordered from the Dictyostelium Stock center.
Dictyostelium has been chosen in 1999 by the NIH (USA), as a new non-mammalian model organism for biomedical research. In 2011, R. Escalante gathered the works from many labs to present Dictyostelium as a model for human disease [20]. As stated by S. Bozzaro [21]: “This model organism has been particularly useful for the study of cell motility, chemotaxis, phagocytosis, endocytic vesicle traffic, cell adhesion, pattern formation, caspase-independent cell death, and, more recently, autophagy and social evolution. It has proven to be a powerful genetic and cellular model for investigating host–pathogen interactions and microbial infections, for mitochondrial diseases, and for pharmacogenetic studies. The D. discoideum genome harbors several homologs of human genes responsible for a variety of diseases, including Chediak-Higashi syndrome, lissencephaly, mucolipidosis, Huntington disease, IBMPFD—that can affect the muscles, bones, and brain—and Shwachman-Diamond syndrome. The study of some of these genes has provided new insights on the mechanism of action of the encoded proteins and, in some cases, on the defect underlying the disease”.
2. Overview of the Extracellular Vesicles
Here are recapitulated the main EVs characteristics and reported biological functions, with no details about the few already elucidated mechanisms, which have to be searched in more specialized reviews.
2.1. Definition and Characteristics of the Extracellular Vesicles
These days, the EV field is extraordinarily complex, due to the huge diversity of their observations. After the pioneering work of Wolf on platelets [3], Apoptotic bodies, with a size up to 5 µm, released by cells dying by apoptosis [22] were the first EVs to be observed. Microvesicles or Ectosomes, previously named Microparticles, originated mainly from human body fluids, such as blood, plasma and urine, and were generally observed in a clinical environment. With a size between 100 nm and 1 µm, they were rather easy to prepare by low differential centrifugation, and to be characterised by their membrane antigens, mostly by using specific antibodies and normal fluorescence flow cytometers, at least above their 300 nm resolution threshold. These two EV classes shared a phosphatidylserine (PS) transfer from the inner to the outer lipidic bilayer, and a common biogenesis, corresponding to the shedding of pieces of the cell plasma membrane (PM), and embedding different macromolecular cargoes. Exosomes, and Exosome-like EVs, such as Prostasomes, were smaller, with a size between 40 and 150 nm, and were first mostly prepared by differential centrifugation, ending with two final steps of ultracentrifugation at 100,000g [23]; they were mostly characterised by western blots and proteomics in a biological environment. Their biogenesis were linked to endocytic processes through the cells until their accumulation into multivesicular bodies (MVBs), partly fusionning with the PM for the release of their inner vesicles outside the cells as EVs. More recently, the EV family increased with the appearance of Oncosomes, shed from the PM of some—not only tumor—cells, with a size up to 10 µm [24,25,26,27], therefore the EV family is always increasing.
All EV main classes differ first by their size, 40–150 nm for the exosomes, 100 nm–1 µm for the microvesicles, up to 5 µm for the apoptotic bodies and up to 10 µm for the oncosomes. However, these different EVs cannot be confidently discriminated by size, due to their partial overlapping. EVs differ also by their biogenesis: microvesicles and oncosomes originate from the shedding of pieces of plasma membrane; apoptotic bodies originate from a lesser known ultimate cell reconditioning, whereas exosomes experience an intracellular traffic through the well-known endocytic pathways. Besides differences in size and biogenesis, the most important characteristics of the different EVs are their respective cargoes, with defined contents of molecular components (proteins, lipids, nucleic acids and metabolites), giving them different densities. The known EV macromolecular compounds have been classified in three databases [28,29,30,31]. However, EVs can neither be discriminated by their quite different cargoes, due to the absence of some true class-specific biomarkers. Almost all cells, whatever their kingdom, Archeabacteria, Bacteria or Eukaryotes, are physiologically releasing EVs [32,33], suggesting that this might also be the case for the Last Universal Common Ancestor (LUCA) [34], which is the most recent organism from which all modern cells derive, and that EV release might indeed be of the utmost importance for cell biology. This is also the case for the different cells of the human body and the various body fluids, although with varying amounts. Therefore, the EV landscape seems to be a “continuum” of different EVs, more or less well classified into four main EV populations, as were the spectral lines of the H atom before Niels Bohr’s atomic theory. Many reviews have been devoted to the classification of EVs [35,36,37]. In the absence of a general consensus about EV nomenclature [38], the International Society ISEV advocates the general use of EVs, whatever the EV class used in the current scientific papers.
On the other hand, EVs are endowed with important biological functions, which will be summarized below. Moreover, EV concentrations are generally increasing in many diseases with specific changes of their cargoes, especially in human cancers. Therefore, tumor cell-derived EVs might be promising as biomarkers and even as therapeutic agents for drug delivery. These points of interest will also be detailed below. After a rather slow and messy emergence until 2012, the EV field is now experiencing a tremendous increase of interest in both biology and medicine, as shown by the fast growing EV publication rate [39].
2.2. Extracellular Vesicles and Intercellular Communication
Besides their characterization by size, biogenesis and cargo contents, the EV biological properties, although still mostly unknown, have been progressively discovered [40,41]. One of the earliest observations was the externalisation of the transferrin receptor from sheep reticulocytes in vitro [42]. It was later suggested as a general exsosomal process for shedding membrane proteins [5]. Already in 1996, it was mentioned that B-lymphocytes secrete antigen-presenting vesicles [43]. The roles of membrane vesicles and exosomes in immune responses were further elucidated [44,45,46]. Microvesicles also participate in important biological processes, such as the surface–membrane traffic and the horizontal transfer of proteins and RNAs among neighbouring cells. In 2006, whereas the horizontal transfer of DNA by the uptake of apoptotic bodies was already a known process [47], J. Ratajczak et al. stressed that membrane-derived vesicles were important mediators of cell-to-cell communication [48], and they brought evidence for the horizontal transfer of mRNA and protein delivery by embryonic stem cell-derived microvesicles [49]. Valadi et al. reported a novel mechanism of genetic exchange between cells, mediated by the exosome transfer of mRNA and miRNA [50]. Among other beneficial influences of exosomes, one can mention their communication of protective messages during oxidative stress [51]. Nowadays, the EV active participation in intercellular communication is convincingly claimed [35,48,52,53,54,55]. As stated by Camussi et al. [56] “even though the exact physiological role of EVs remains to be elucidated, it is becoming clear that they may transfer proteins, receptors, bioactive lipids, messenger ribonucleic acid (mRNA), and micro-RNA (miRNA) from the cell of origin to the recipient cell, which may modify their phenotype and functions”. The physiological roles of exosomes is probably important for monitoring body homeostasis during health, but is less well-studied than their pathological roles in many human diseases. The exosome-like vesicles prostasomes, originating from the prostate, represent an exception, as their influence in normal human reproduction was one of the earliest works of interest about EVs [57]. During normal pregnancy, placental vesicles have been shown to have a wide range of functional activities, transferring a variety of bioactive molecules into the maternal circulation [58]. Recently, EVs have also been implied in senescence and aging [59]. However, the EV-mediated intercellular communication is a “double-edge sword”, as cells can release prions in association with exosomes [60], and exosomes can also mediate the functional delivery of viral miRNA [61], whereas microvesicles too can contribute to viral infection [62].
2.3. Extracellular Vesicles and Human Diseases
Figure 1 (taken from [63]) shows the complexity of the cell-derived EVs. This tissue-specific EV classification points out their interest in the medical field. As stressed more recently [27], only large oncosomes are released by some tumor cells, whereas oncosomes might also be releasd by non-tumor cells. The possibility of using EVs as biomarkers and even therapeutics in many human diseases has sustained the increasing interest for EV research [39]. This approach was first experimented with the microvesicles, as detailed in [11]. Shedding vesicles play a role in inflammation and thrombosis [64], in vascular diseases [65] and in pre-eclampsia versus normal pregnancy [66]. Pregnancy affords a unique opportunity for a comparative EV study in normal physiology and disease [58]. In addition, microvesicles have important physiological roles in coagulation in vivo, by mediating the coordinate contribution of platelets, macrophages, and neutrophils [67]. Endothelial-derived microparticles are said to be biological conveyors at the crossroad of inflammation, thrombosis, and angiogenesis [68]. EVs have deleterious effects (pro-inflammatory, pro-angiogenic, pro-thrombic, vascular dysfonction, and pro-apoptotic), as well as beneficial effects (anti-inflammatory, post-ischemic angiogenesis, and anti-apoptotic), in cardiovascular pathologies, depending on the molecules they carry [69], (p. 397). Epigenetic changes induced by EVs have been particularly studied in the context of immunology, cancer and stem cell biology [56].
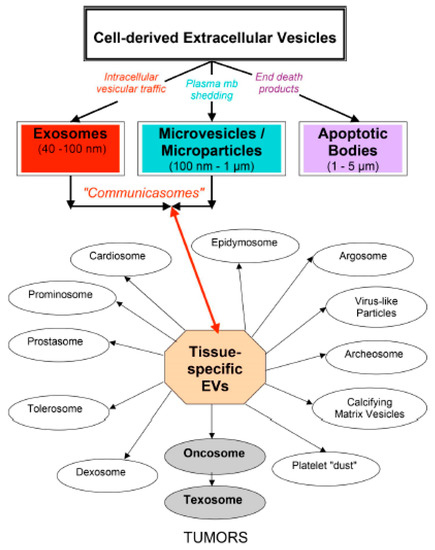
Figure 1.
Complexity of eukaryotic EVs. Following their respective biogenesis, three main classes of cell-derived EVs are now recognized: exosomes, microvesicles/microparticles and apoptotic bodies. A tissue-specific EV classification is also shown to point out their interest for the medical field.
Table 1 shows the suggested topics for abstract classifications of ISEV 2018 [10], stressing the current huge human medical involvement of EVs. Presently, EVs are involved in the immune system, in cardiovascular diseases and vascular disorders, in reproduction and pregnancy, and in the nervous system (blood-brain-barrier). They are also involved in tissue injury, repair and remodeling; in viral, bacterial, fungal, and parasitic infections; in acute and chronic inflammatory disorders; in stem cells and in cancer, especially in tumor immunology, angiogenesis, and metastasis; as well as in neurodegenerative diseases [70].

Table 1.
Topics for Abstracts Classification of ISEV 2018.
2.4. Extracellular Vesicles and Cancer
Cancer is by far the most studied human disease under the EV light. However, no unique cancer-specific pathway has yet emerged. Each of the most common human cancers behaves like a specific illness and develops its own panel of various tumor cells-derived EVs, with specific compositions and timely influences on the tumoral near or distant environment. Many papers are devoted to EVs with a given specific human cancer, but are out of the scope of this review. Each main EV class can be an actor implied in cancerogenesis, but its relative importance, compared with the ones of the other EVs classes can vary for different tumors. Microvesicles have important pathological roles as mediators of intercellular communication in cancer [71] and in tumor progression by novel microenvironment modulators [72], facilitating the spreading and release of cancer cells to generate metastases [67].
The general properties inventoried for tumor EVs are related either to the transport and intercellular transfer of active compounds, such as oncogenes, functional miRNAs, tumor suppressor proteins, and antitumoral drug resistance proteins, or to biological functions, dealing with modified immunological properties and angiogenesis, or specific organ-targeted metastasis. Many papers or recent reviews summarize these observed properties of tumor EVs [25,26,63,73,74,75,76,77,78,79,80,81].
2.5. Extracellular Vesicles, Drug Delivery, and Cancer Therapy
The more achieved EV therapy corresponds to the early use of dendritic cell-derived exosomes, as a novel cell-free vaccine for the eradication of murine tumors [82], which is now efficient as an immunotherapy treatment for some human tumors.
In 2013, Ohno et al. overviewed the potential roles of exosomes and microvesicles with respect to clinical diagnosis and disease pathogenesis [83]. Beside their great potential as diagnostic and pronostic biomarkers, EVs are promising drug delivery systems, following “the Trojan exosome hypothesis”, according to which exosomes might be good candidates for crossing the rather impervious cell biological barriers for drug delivery [84]. This has been applied to the drug delivery of RNAi [85] and siRNA [86]. Although also being Trojan horses for viral infections [87], EVs are now considered unique intercellular delivery vehicles [88]. Potential applications of EVs were suggested in cancer diagnosis, prognosis, and epidemiology [89]. Basic and clinical scientists joined to summarize recent developments and the current knowledge of EV-based therapies. Strategies to promote the therapeutic application of EVs in future clinical studies were addressed [90]. This has been recently actualized for the development of best practice models for EV therapies [39,91], which might bring future improvements in cancer care [92].
2.6. Challenges Faced for Therapeutic Use of Extracellular Vesicles
EVs are quite appealing for future theranostics (diagnosis, prognosis, and therapy) of human diseases, and especially cancer. However, no efficient clinical use is possible yet, due mainly to some challenging unsolved problems, i.e., the absence of the consensus in regard to the EV nomenclature [38], the absence of standardisation of the EV measurements at a large scale [93], and the crucial unsolved problem of EV heterogeneity [94,95,96,97]. Besides the intrisic EV heterogeneity, heterogeneity is ever present in the human body. Many different types of healthy human cells have the potential to secrete EVs into bodily fluids, with the possible increase and modification, and contribution from sick cells; futhermore, an important microbiome (10 times more bacteria than human cells) brings its own capacity to externalize EVs, due to the universal process of EV secretion [33]. The general estimation of about 100 times more viruses than human cells, their size analogy with exosomes and the recent observation of SVF-derived gesicles [98] suggest that viruses too, might contribute to an increase in the so-called “mammalian EVs”.
The EV field is complex not only by definition, as detailed above, but also greatly depends on the methods used for their preparation—of which the numbers are increasing with time. Beside the long used unique centrifugation protocols, there are some new filtration processes and some available commercial kits, based on EVs precipitation [97,99]. When working with body fluids, pre-analytical conditions before EVs preparation are also quite important. The standardisation of EV measurements is an important challenge to solve a wide inter-organisation comparison of the different EV measurements, especially in clinical set-ups. Recently, the technical challenges for working with EVs were discussed, and some possible options to overcome them were suggested [93,99]. In 2014, ISEV provided researchers with a minimal set of experimental requirements for the definition of EVs and their functions [100], which was further updated [101,102]. The deciphering of EVs into specific subpopulations, linked to their specific biological functions, remains one of the biggest challenges to solve, before using the great potentialities of EVs in the theranostics of many human diseases, including cancer.
It is to be noticed that up to now, many EV studies have been performed directly at the clinical level in human body fluids, or in vitro on different human disease-related cell lines. A simple eukaryotic EV model is urgently needed, to help solve some of the remaining challenges, which are too complex to be worked out directly at the human level. Dictyostelium might be such an appealing eukaryotic EV model, as argumented in the next part of this paper.
3. Dictyostelium as a Model for Studying Mammalian Extracellular Vesicles
3.1. Discovery of Dictyostelium Extracellular Vesicles by Serendipity
Dictyostelium EVs were unknown until our observation in 1998 [103]. This was the result of many years of unprogrammed research, favored by serendipity, as outlined below.
After a PhD in physics, I met Dictyostelium during a 1977 sabbatical at the University of British Columbia (UBC, Vancouver, Canada). Following a method, said to discriminate the rigidity of the cell plasma membrane of leukemic cells from the one of the normal cells [104,105], I used a newly achieved UBC home-made fluorescence polarization set-up with an analogic photomultiplier detection with Dictyostelium cells [106]. When coming back to the Curie’s lab in Paris, I decided to switch my research towards biology with Dictyostelium. I was helped greatly by P. Brachet (Pasteur Institute, Paris) for introducing this wonderful microorganism into a physics environment. I was very lucky to manage working with it, as a CNRS scientist until 2003, and, then until 2013, as an Honorary UPMC Research Director.
Our first goal was to build an automated set-up for fluorescence polarization measurements, but with the photon counting technology, previously elaborated in our team. We managed to measure a higher plasma membrane rigidity for Dictyostelium cells during early development, compared to one of the growing cells [107]. But we also observed a quite unexpected release of fluorescent compounds into the KK2 phosphate starvation buffer, accompanying early aggregation of the cells in suspension [108]. This was the beginning of a fruitful investigation headed by R. Klein, who deciphered the GTP catabolism, giving rise to the pteridine pathway, and ending with the formation of a specific pterin, named Dictyopterin [109].
We improved our set-up in order to measure the known spontaneous oscillations of aggregation-competent cells in suspension [110]. In parallel, I remained fascinated by watching via light microscopy, and taking pictures of Dictyostelium growing cells, and of cells further starvation-induced into aggregation and differentiation, after incubation of the cells with many different compounds, or without comparison. The growth of axenic Dictyostelium cells was also measured in a complete defined medium [14], or in the same medium without 5 × 10−7 M folic acid, and their respective developments were compared.
When becoming more familiar with Dictyostelium, I asked the first “funny” question: Why do Dictyostelium cells never get cancer? Dictyostelium, strain (Ax-2) growing cells were incubated with either the main carcinogenic compound of tobacco smoke, benzo (a) pyrene, B(a)P, or with its non-carcinogenic isomer benzo (e) pyrene, B(e)P. The current theory was that only B(a)P was metabolized into a diol-epoxide compound, which initiated the tumoral process. But with Dictyostelium cells, we noticed that the shape recognition of the two BP isomers occurred before any metabolization: only the fluorescent harmful B(a) P was released into the KK2 starvation medium. This was presented at the thirteenth International Symposium on Polynuclear Aromatic Hydrocarbons [111], but was futher rejected for publication (“nothing to do with cancer” for cancer journals/”too much dealing with cancer” for biological journals). As M. Gottesman was then deciphering the P-glycoprotein (P-gp)-mediated multidrug resistance [112,113,114], I asked whether such an ABC transporter might exist in Dictyostelium cells and explain their already recognized high resistance against many structurally different xenobiotics. By the help of a medical collaboration with A.-M. Faussat, using a mouse antibody against P-gp, we showed that, in fact, Dictyostelium cells harbor a P-gp with the same 170 kDa mass as the human P-gp [115]. However, by means of another collaboration with G. Lizard in Dijon, we showed, by using flow cytometry, that this P-gp was non-functional [116]. At the same time, the multidrug landscape became more complex with the appearance, beside the P-gp, of the multidrug resistance protein (MRP), and of the lung resistance protein (LRP), acting at the nuclear membrane level, so we have temporarily left this research field.
In order to get a homogeneous cell population in a given state of the cell cycle, before initiating starvation-induced development, we reproduced a simple method for synchronizing growing Dictyostelium cells [117]. We needed to control the cell DNA amount in a timely manner, therefore we chose the widely used DNA-specific Hoechst 33,342 (HO342) vital stain for labeling aliquots of the synchronized growing cells, as a function of time. Unexpectedly, Dictyostelium cells were completely resistant to HO342 vital staining, and when watching the cells once more with a light fluorescence microscope, we noticed that the cells were surrounded with numerous fluorescent particles. These particles turned out to be detoxyfing vesicles, inholding HO342, which was thus prevented to reach its nuclear DNA target. Here, was the long-searched resistance mechanism of Dictyostelium cells. Morover, the control experiment without HO342 labeling showed that extracellular vesicles were present, not only as a detoxyfing mechanism, but as a common physiological process [103]. This was the beginning of the fruitful EV story of Dictyostelium cells.
3.2. Story of the Dictyostelium Extracellular Vesicles (1998–2013)
Dictyostelium cells vitally stained with the DNA-specific dye, HO342, released fluorescent material in their culture medium. By means of lipid analysis and electron microscopy, we demonstrated the vesicular nature of this material, which turned out to be organelles of about 100 to 300 nm, surrounded by a lipid bilayer envelope. Furthermore, we observed that proteins and nucleic acids, both DNA and RNA, associated with these extracellular vesicles, independently of HO342 vital staining. The main vesicular DNA component exhibited a size > 21 kb, and its association with vesicles in physiological growth conditions, and not concomitant with any programmed cell death, suggested a possible involvement of these EVs in a more general intercellular mechanism, than the newly observed cellular resistance to vital HO342 DNA-staining [103].
The second important observation was that the HO342-transporting Dictyostelium EVs were able to completely overcome the natural resistance of Dictyostelium cells to HO342 vital staining and to transport the dye into their DNA nuclear target. This was true not only for Dictyostelium cells, but also for human leukemic resistant cells, K562r, as shown in a fluorescence study [118]. This gave rise to an European patent, extended to the USA and Canada, advocating for the use of Dictyostelium EVs for tranferring a molecule of interest to an eukaryotic cell [119]. This EV property was also tested with hypericin (Hyp), which is used for photodynamic therapy in some cancers, and is a very hydrophobic molecule, quite different from HO342. Here again, Dictyostelium detoxifying EVs, inholding Hyp, were able to transfer the drug to its known target, i.e., the Golgi, into living Hela cells [120]. Co-internalization of magnetic nanoparticles and fluorescent dextran in Dictyostelium cells demonstrated the possibility to design multifunctional biovesicles, carrying both magnetic agents and therapeutic molecules, for targeting a tumor area by means of magnetic attraction [121]. The Dictyostelium cells derived EV strategy for drug delivery has been further detailed [122]. In parallel, the new EV-mediated detoxifying mechanism, discovered with Dictyostelium, was presented at the International Exosome Workshop (IWE) [7], and further suggested to be involved as a new multidrug resistance mechanism, at work during the failure of antitumoral treatment by chemotherapy [123].
Our whole study about Dictyostelium EVs was summarized in a poster for the first 2012 ISEV Meeting [9]. The assets of the non-pathogenic micro-organism Dictyostelium discoideum have been stressed, in order to promote it as an interesting model for the study of eucaryotic EVs [124]. Dictyostelium EVs were also used to elaborate a new EV characterization method, Raman Tweezers Microspectroscopy (RTM), with a home-made set-up, in order to measure the global molecular compostion of a single (or a few) EV(s), without any labeling. The global molecular compositions of Dictyostelim EVs, either derived from growing cells or from aggregating cells were found to be quite different [125]. A thorough study of this technology has recently been published [126], which will potentially be useful for the molecular characterization of single (or a few) EV(s) in pre-defined EV subpopulations.
3.3. Dictyostelium Could be an Outstanding Eukaryotic Model for Studying Mammalian Extracellular Vesicles
All the intracellular and extracellular vesicles present a characteristic lipid composition and organization that governs their formation, targeting, and function. The liquid crystalline structure of lipids plays an essential role in their function as biological nanovehicles of information [127].
One asset of Dictyostelium lies in its easy manipulation in conditioned media experiments. Dictyostelium cells are able to grow in agitated suspensions or as adhering cells. In simple tissue culture flasks, one can easily control the whole Dictyostelium life cycle, i.e., the growth, as well as starvation-induced aggregation and differentiation into fruiting bodies. The corresponding conditioned media of this unique eukaryotic both in vitro and in vivo cell model could be easily collected. With the current progress for EV preparation and characterization, it would be possible to address the important challenge of EV heterogeneity (exosomes, microvesicles, apoptotic bodies, oncosomes, and their respective subpopulations) in this simple cell model. The further easy design of conditioned media experiments might offer the possibility to study the respective biological functions of these different pre-defined EV subpopulations.
Another suggestion would be to reconsider our experiment about the inhibition of multicellular development, which switches the cell death of Dictyostelium towards mammalian-like unicellular apoptosis [128], by questioning the influence of EVs. Briefly, a conditioned medium was obtained by first starving a 4 × 107 c/mL Dictyostelium cell population in agitated suspension during 22h in a KK2 phosphate buffer (pH 6.8). After getting rid of the cells by centrifugation, the conditioned medium was obtained and named t22, linked to the time of cell starvation in suspension. When performing the usual aggregation test with 2 × 106 c/mL, new growing Dictyostelium cells in 1 mL of this t22 conditioned medium, their aggregation was completely blocked and all the cells were induced to an apoptotic death, with many characteristics of human apoptosis, including the mitochondrial release of an apoptosis-inducing factor [129]. A t8 conditioned medium, obtained after only 8 hours of starvation did impair the aggregation of new cells, but without further inducing cell apoptosis. EVs were observed in the t22 conditioned medium, but not studied at that time. One can now wonder whether EVs were involved in these observed biological effects, what was the EV differences between the two (t8/t22) conditioned media, and whether a late-appearing EV subpopulation might be able to induce Dictyostelium cells into a human-like apoptotic death.
The significance of Dictyostelium for deciphering EV biological functions was nicely demonstrated recently [130]. Despite its one billion year ancestral position in evolution, Dictyostelium was brought to scientific discovery only in 1935 [12]. Its splendid patterns of starvation-induced aggregation remained for more than three decades under the mysterious Acrasiale power, then the orchestrating role of c-AMP was discovered in J. T. Bonner’s lab in 1969 [131]. Many labs were further involved in deciphering the c-AMP mechanism, but nearly five decades passed before EVs entered into this chemotaxis process [130,132]. C. A. Parent et al. have nicely shown that a polarized Dictyostelium cell migrating towards an agregation center expells EVs from its rear part, and that these EVs contain all the machinery (Adenylate Cyclase and ATP) to self-assume the c-AMP biosynthesis. Moreover, among the 68 Dictyostelium cell pumps, 13 remain associated with the EVs and one is specially devoted to quantitatively expell the newly formed c -AMP for attracting the other following Dictyostelium cells. The elucidation of the chemotaxis mechanism in Dictyostelium cells might help to clarify the exosome-promoted chemotaxis of cancer cells [133]. To my knowledge, it is only the second observation, beside the one concerning the EV-maturation of miRNAs [134], showing that EVs are not only conveyers of important macromolecular components, involved in their now widely recognized functions in intercellular communication, but that they also can act as cell-independent autonomous biological entities. This shows an appealing future for Dictyostelium EVs, and the long accumulated knowledge about this eucaryotic cell model (www.dictybase.org) might help to further elucidate this quite new EV biological function.
4. Conclusions
For the past decade, cell biology has entered “a new galaxy” by extending its research field beyond the cell plasma membrane and discovering the huge power of extracellular vesicles in intercellular communication. However, this extracellular vesicle biology is only in its infancy, and many challenging questions remain to be solved, before efficiently using EVs for the theranostics of human diseases, including cancer.
Contrary to the about 10 years working time for developing an efficient pharmaceutical compound, which are dealing with in vitro and in vivo tests before reaching the clinics, the current EV in vivo experiments are still scarce. All the cell lines linked to a given cancer type f. ex. are interesting tools for searching specific biomarkers, but are relatively too simple to catch an important cancer-specific mechanism. On the opposite, EV clinical research on human bio fluids, being highly complex, is like “looking for a needle in a haystack”. The lack of simple eukaryotic models for deciphering the EV-mediated biological functions greatly hampers the current knowledge about EV-mediated functions in human health and disease. Dictyostelium discoideum, which was recognised in 1999 by the National Institutes of Health (NIH, USA), as a new interesting model for biomedical research, might help to bridge the current gap of the human EV knowledge between in vitro and clinical research.
Funding
This research received no external funding.
Acknowledgments
The author acknowledges all the collaborations, which participated to the fruitful story of Dictyostelium extracellular vesicles.
Conflicts of Interest
The author declares none.
References
- Mazzarello, P. A unifying concept: The history of cell theory. Nat. Cell Biol. 1999, 1, E13–E15. [Google Scholar] [CrossRef] [PubMed]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [PubMed]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: Formation of exosomes as a mechanism for shedding membrane proteins. Biochem. Cell Biol. 1992, 70, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [PubMed]
- International Workshop on Exosomes (IWE) 2011 (January 19th–22nd 2011, Institut Curie, 75005 Paris, France). Available online: http://exocarta.org/IWE2011Program.pdf (accessed on 20 January 2019).
- Araldi, E.; Kramer-Albers, E.M.; Hoen, E.N.; Peinado, H.; Psonka-Antonczyk, K.M.; Rao, P.; van Niel, G.; Yanez-Mo, M.; Nazarenko, I. International Society for Extracellular Vesicles: First annual meeting, April 17–21, 2012: ISEV-2012. J. Extracell. Vesicles 2012, 1, 19995. [Google Scholar] [CrossRef] [PubMed]
- Scientific Program 2012 ISEV meeting Wednesday 18th April. Available online: http://dx.doi.org/10.3402/jev.v1i0.18182 (accessed on 20 January 2019).
- ISEV. ISEV 2018 abstract book. J. Extracell. Vesicles 2018, 7 (Suppl. S1), 1461450. [Google Scholar] [CrossRef]
- Harrison, P.; Gardiner, C.; Sargent, I.L. Extracellular Vesicles in Health and Disease; CRC Press Taylor & Francis, Pan Stanford Publishing: Boca Raton, FL, USA, 2014; p. 454. [Google Scholar]
- Raper, K. A new species of slime mold from decaying forest leaves. J. Agric. Res. 1935, 50, 136–147. [Google Scholar]
- Watts, D.J.; Ashworth, J.M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J. 1970, 119, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.; Kessin, R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 1977, 74, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Sussman, M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987, 28, 9–29. [Google Scholar] [PubMed]
- Loomis, W. Dictyostelium Discoideum: A Developmental System; Academic Press, Inc.: New York, NY, USA, 1975; 214p. [Google Scholar]
- Sunderland, M.E. Slime Mold Video. Embryo Project Encyclopedia (2008-05-02). Available online: http://embryo.asu.edu/handle/10776/1775 (accessed on 20 January 2019).
- Eichinger, L.; Pachebat, J.A.; Glöckner, G.; Rajandream, M.A.; Sucgang, R.; Berriman, M.; Song, J.; Olsen, R.; Szafranski, K.; Xu, Q.; et al. The genome of the social amoeba Dictyostelium discoideum. Nature 2005, 435, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Yoshino, R.; Angata, K.; Iwamoto, M.; Pi, M.; Kuroe, K.; Matsuo, K.; Morio, T.; Urushihara, H.; Yanagisawa, K.; et al. The mitochondrial DNA of Dictyostelium discoideum: Complete sequence, gene content and genome organization. Mol. Gen. Genet. 2000, 263, 514–519. [Google Scholar] [CrossRef]
- Escalante, R. Dictyostelium as a model for human disease. Semin. Cell Dev. Biol. 2011, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Bozzaro, S. The Model Organism Dictyostelium discoideum. In Dictyostelium Discoideum Protocols. Methods in Molecular Biology; Eichinger, L., Rivero, F., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 17–37. [Google Scholar]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30. [Google Scholar] [CrossRef] [PubMed]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Minciacchi, V.R.; de Candia, P.; Yang, J.; Posadas, E.; Kim, H.; Griffiths, D.; Bhowmick, N.; Chung, L.W.; Gandellini, P.; et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 2013, 12, 3526–3536. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Meehan, B.; Rak, J.; Di Vizio, D. Oncosomes—Large and small: What are they, where they came from? J. Extracell. Vesicles 2016, 5, 33109. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kang, B.; Kim, O.Y.; Choi, D.S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, J.; Kim, S.R.; Choi, D.S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A community web portal for extracellular vesicles research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Wildman, D.E. Extracellular Vesicles and the Promise of Continuous Liquid Biopsies. J. Pathol. Transl. Med. 2018, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles (EVs) in the three domains of life and beyond. FEMS Microbiol. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Forterre, P. Origin of life: LUCA and extracellular membrane vesicles (EMVs). Int. J. Astrobiol. 2016, 15, 7–15. [Google Scholar] [CrossRef]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharm. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hochberg, F.H.; Jones, P.S. Extracellular vesicles: The growth as diagnostics and therapeutics; a survey. J. Extracell. Vesicles 2018, 7, 1438720. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Dorronsoro, A.; Booker, C.N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Investig. 2016, 126, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, L.; Szeles, A.; Rajnavolgyi, E.; Folkman, J.; Klein, G.; Ernberg, I.; Falk, K.I. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood 1999, 93, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Eldh, M.; Ekstrom, K.; Valadi, H.; Sjostrand, M.; Olsson, B.; Jernas, M.; Lotvall, J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Pap, E.; Pallinger, E.; Pasztoi, M.; Falus, A. Highlights of a new type of intercellular communication: Microvesicle-based information transfer. Inflamm. Res. 2009, 58, 1–8. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Camussi, G.; Collino, F.; Deregibus, M. Extracellular Vesicle-Mediated Epigenetic Reprogramming of Cells. In Extracellular Vesicles in Health and Disease; Harrison, P., Gardiner, C., Sargent, I.L., Eds.; CRC Press Taylor & Francis, Pan Stanford Publishing: Boca Raton, FL, USA, 2014; pp. 79–106. [Google Scholar]
- Ronquist, G.; Brody, I. The prostasome: Its secretion and function in man. Biochim. Biophys. Acta 1985, 822, 203–218. [Google Scholar] [CrossRef]
- Sargent, I.; Dragovic, R.; Tannetta, D.; Redman, C. Extracellular Vesicles in Normal Pregnancy and Pre-Eclampsia. In Extracellular Vesicles in Health and Disease; Harrison, P., Gardiner, C., Sargent, I.L., Eds.; CRC Press Taylor & Francis, Pan Stanford Publishing: Boca Raton, FL, USA, 2014; pp. 357–390. [Google Scholar]
- Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 2018, 17, e12734. [Google Scholar] [CrossRef]
- Fevrier, B.; Vilette, D.; Archer, F.; Loew, D.; Faigle, W.; Vidal, M.; Laude, H.; Raposo, G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 2004, 101, 9683–9688. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G., Jr.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef] [PubMed]
- Tatischeff, I. Cell-derived Extracellular Vesicles Open New Perspectives for Cancer Research. Cancer Res. Front. 2015, 208–224. [Google Scholar] [CrossRef]
- Ardoin, S.P.; Shanahan, J.C.; Pisetsky, D.S. The role of microparticles in inflammation and thrombosis. Scand. J. Immunol. 2007, 66, 159–165. [Google Scholar] [CrossRef] [PubMed]
- George, F.D. Microparticles in vascular diseases. Thromb. Res. 2008, 122 (Suppl. S1), S55–S59. [Google Scholar] [CrossRef]
- Biro, E.; Lok, C.A.; Hack, C.E.; van der Post, J.A.; Schaap, M.C.; Sturk, A.; Nieuwland, R. Cell-derived microparticles and complement activation in preeclampsia versus normal pregnancy. Placenta 2007, 28, 928–935. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- Leroyer, A.S.; Anfosso, F.; Lacroix, R.; Sabatier, F.; Simoncini, S.; Njock, S.M.; Jourde, N.; Brunet, P.; Camoin-Jau, L.; Sampol, J.; et al. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb. Haemost. 2010, 104, 456–463. [Google Scholar] [CrossRef]
- Vion, A.-C.; Boulanger, C. Extracellular Vesicles in Cardiovascular Disease. In Extracellular Vesicles in Health and Disease; Harrison, P., Gardiner, C., Sargent, I.L., Eds.; CRC Press Taylor & Francis, Pan Stanford Publishing: Boca Raton, FL, USA, 2014; pp. 391–418. [Google Scholar]
- Thompson, A.G.; Gray, E.; Heman-Ackah, S.M.; Mager, I.; Talbot, K.; Andaloussi, S.E.; Wood, M.J.; Turner, M.R. Extracellular vesicles in neurodegenerative disease—Pathogenesis to biomarkers. Nat. Rev. Neurol. 2016, 12, 346–357. [Google Scholar] [CrossRef]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as mediators of intercellular communication in cancer—The emerging science of cellular ‘debris’. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [PubMed]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-derived microvesicles: Shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.J.; Cavallini, L.; Ciardiello, C.; Reis Sobreiro, M.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327–11341. [Google Scholar] [CrossRef] [PubMed]
- Penfornis, P.; Vallabhaneni, K.C.; Whitt, J.; Pochampally, R. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int. J. Cancer 2016, 138, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yoshioka, Y.; Yamamoto, Y.; Ochiya, T. How cancer cells dictate their microenvironment: Present roles of extracellular vesicles. Cell Mol. Life Sci. 2017, 74, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gao, Y.; Li, N.; Shao, F.; Wang, C.; Wang, P.; Yang, Z.; Li, R.; He, J. Exosomes: New players in cancer. Oncol. Rep. 2017, 38, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.U.; Prieto-Vila, M.; Hironaka, A.; Ochiya, T. The role of extracellular vesicle microRNAs in cancer biology. Clin. Chem. Lab. Med. 2017, 55, 648–656. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Ruger, R. The Multiple Roles of Exosomes in Metastasis. Cancer Genom. Proteom. 2017, 14, 1–15. [Google Scholar] [CrossRef]
- Jabalee, J.; Towle, R.; Garnis, C. The Role of Extracellular Vesicles in Cancer: Cargo, Function, and Therapeutic Implications. Cells 2018, 7, 93. [Google Scholar] [CrossRef]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Ishikawa, A.; Kuroda, M. Roles of exosomes and microvesicles in disease pathogenesis. Adv. Drug Deliv. Rev. 2013, 65, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef] [PubMed]
- Lakhal, S.; Wood, M.J. Exosome nanotechnology: An emerging paradigm shift in drug delivery: Exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays 2011, 33, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Schlee, M.; Coch, C.; Hartmann, G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011, 29, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Altan-Bonnet, N. Extracellular vesicles are the Trojan horses of viral infection. Curr. Opin. Microbiol. 2016, 32, 77–81. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Verma, M.; Lam, T.K.; Hebert, E.; Divi, R.L. Extracellular vesicles: Potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin. Pathol. 2015, 15, 6. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Lasser, C.; Jang, S.C.; Lotvall, J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol. Asp. Med. 2018, 60, 1–14. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Thery, C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos. Trans. R. Soc. Lond B Biol. Sci. 2018, 373, 20160479. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabanas, C.; Mager, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Breakfield, X.O.; Frederickson, R.M.; Simpson, R.J. Gesicles: Microvesicle “Cookies” for Transient Information Transfer Beetween Cells. Mol. Ther. 2011, 19, 1574–1576. [Google Scholar] [CrossRef]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; van Royen, M.E. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzas, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V.; et al. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef] [PubMed]
- Tatischeff, I.; Bomsel, M.; de Paillerets, C.; Durand, H.; Geny, B.; Segretain, D.; Turpin, E.; Alfsen, A. Dictyostelium discoideum cells shed vesicles with associated DNA and vital stain Hoechst 33342. Cell Mol Life Sci. 1998, 54, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Inbar, M.; Shinitzky, M.; Sachs, L. Microviscosity in the surface membrane lipid layer of intact normal lymphocytes and leukemic cells. FEBS Lett. 1974, 38, 268–270. [Google Scholar] [CrossRef]
- Shinitzky, M.; Inbar, M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J. Mol. Biol. 1974, 85, 603–615. [Google Scholar] [CrossRef]
- Herring, F.G.; Tatischeff, I.; Weeks, G. The fluidity of plasma membranes of Dictyostelium discoideum. The effects of polyunsaturated fatty acid incorporation assessed by fluorescence depolarization and electron paramagnetic resonance. Biochim. Biophys. Acta 1980, 602, 1–9. [Google Scholar] [CrossRef]
- Thiery, R.; Klein, R.; Tatischeff, I. Increase of DPH fluorescence polarization during development of Dictyostelium discoideum cells. FEBS Lett. 1987, 223, 381–386. [Google Scholar] [CrossRef]
- Tatischeff, I.; Klein, R.; Tham, G. Fluorescent products secreted by Dictyostelium cells which are able to aggregate. FEBS Lett. 1982, 138, 265–269. [Google Scholar] [CrossRef]
- Klein, R.; Thiery, R.; Tatischeff, I. Dictyopterin, 6-(D-threo-1,2-dihydroxypropyl)-pterin, a new natural isomer of L-biopterin. Isolation from vegetative cells of Dictyostelium discoideum and identification. Eur. J. Biochem. 1990, 187, 665–669. [Google Scholar] [CrossRef]
- Thiery, R.; Klein, R.; Tatischeff, I. Phorbol 12-myristate 13-acetate modulates the cAMP-induced light-scattering response of a Dictyostelium discoideum cell population. FEBS Lett. 1988, 241, 149–153. [Google Scholar] [CrossRef]
- Tatischeff, I.; Lavialle, F.; De Paillerets, C.; Weintraub, H.; Tham, G.; Alfsen, A. Carcinogenic benzo(a)pyrene and non-carcinogenic benzo(e)pyrene discriminated by Dictyostelium discoideum cells through internalization. In Polycyclic Aromatic Compounds. Synthesis, Properties, Analytical Measurements, Occurrence and Biological Effects; PAH XIII; Garrigues, L.M., Ed.; Gordon and Breach Science Pub.: Amsterdam, The Netherlands, 1991; pp. 695–702. [Google Scholar]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Ling, V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006, 580, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Pastan, I.; Ambudkar, S.V. P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 1996, 6, 610–617. [Google Scholar] [CrossRef]
- Tatischeff, I.; Lavialle, F. Immunological evidence of a P-glycoprotein in the microorganism Dictyostelium. C. R. Acad. Sci. III 1993, 316, 560–563. [Google Scholar] [PubMed]
- Tatischeff, I.; Lizard, G.; Roignot, P.; Lavialle, F. Dictyostelium a new eukaryotic model for analyzing cell resistance. Biol. Cell 1993, 79, 277. [Google Scholar] [CrossRef]
- Weijer, C.J.; Duschl, G.; David, C.N. A revision of the Dictyostelium discoideum cell cycle. J. Cell Sci. 1984, 70, 111–131. [Google Scholar] [PubMed]
- Tatischeff, I.; Lavialle, F.; Pigaglio-Deshayes, S.; Péchoux-Longin, C.; Chinsky, L.; Alfsen, A. Dictyostelium extracellular vesicles containing Hoechst 33342 transfer the dye into the nuclei of living cells: A fluorescence study. J. Fluoresc. 2008, 18, 319–328. [Google Scholar] [CrossRef]
- Tatischeff, I.; Alfsen, A.; Lavialle, F. Extracellular Vesicles from Non-Pathogenic Amobae Useful as Vehicle for Transferring a Molecule of Interest to an Eukaryotic Cell. (DRITT-UPMC) 2003-2012: European Priority No. 03 291 752. European Patent (Danemark, Deutschland, France, Great Britain, Italy, Netherland, Spain) USA and Canada Patents. U.S. Patent 7,722,855, 25 May 2010. [Google Scholar]
- Lavialle, F.; Deshayes, S.; Gonnet, F.; Larquet, E.; Kruglik, S.G.; Boisset, N.; Daniel, R.; Alfsen, A.; Tatischeff, I. Nanovesicles released by Dictyostelium cells: A potential carrier for drug delivery. Int. J. Pharm. 2009, 380, 206–215. [Google Scholar] [CrossRef]
- Wilhelm, C.; Lavialle, F.; Pechoux, C.; Tatischeff, I.; Gazeau, F. Intracellular trafficking of magnetic nanoparticles to design multifunctional biovesicles. Small 2008, 4, 577–582. [Google Scholar] [CrossRef]
- Tatischeff, I.; Alfsen, A. A New Biological Strategy for Drug Delivery: Eucaryotic Cell-Derived Nanovesicles. J. Biomater. Nanobiotechnol. 2011, 2, 494–499. [Google Scholar] [CrossRef]
- Tatischeff, I. Cell-derived microvesicles and antitumoral multidrug resistance. C. R. Biol. 2012, 335, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Tatischeff, I. Assets of the non-pathogenic microorganism Dictyostelium discoideum as a model for the study of eukaryotic extracellular vesicles. F1000Res 2013, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Tatischeff, I.; Larquet, E.; Falcon-Perez, J.M.; Turpin, P.Y.; Kruglik, S.G. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J. Extracell. Vesicles 2012, 1, 19179. [Google Scholar] [CrossRef] [PubMed]
- Kruglik, S.G.; Royo, F.; Guigner, J.M.; Palomo, L.; Seksek, O.; Turpin, P.Y.; Tatischeff, I.; Falcón-Pérez, J.M. Raman tweezers microspectroscopy of circa 100 nm extracellular vesicles. Nanoscale 2019, 11, 1661–1679. [Google Scholar] [CrossRef] [PubMed]
- Alfsen, A.; Tatischeff, I. The Lipid Bilayer of Biological Vesicles: A Liquid-Crystalline Material as Nanovehicles of Information. J. Biomater. Nanobiotechnol. 2014, 5, 105–115. [Google Scholar] [CrossRef]
- Tatischeff, I.; Petit, P.X.; Grodet, A.; Tissier, J.P.; Duband-Goulet, I.; Ameisen, J.C. Inhibition of multicellular development switches cell death of Dictyostelium discoideum towards mammalian-like unicellular apoptosis. Eur. J. Cell Biol. 2001, 80, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Tatischeff, I.; Estaquier, J.; Girard, M.; Sureau, F.; Tissier, J.P.; Grodet, A.; Dellinger, M.; Traincard, F.; Kahn, A.; et al. On the evolutionary conservation of the cell death pathway: Mitochondrial release of an apoptosis-inducing factor during Dictyostelium discoideum cell death. Mol. Biol. Cell 2001, 12, 3016–3030. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, P.W.; Majumdar, R.; Jenkins, L.M.; Senoo, H.; Wang, W.; Ammu, S.; Chen, S.; Narayan, K.; Iijima, M.; Parent, C.A. Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. J. Cell Biol. 2018, 217, 2891–2910. [Google Scholar] [CrossRef]
- Bonner, J.T.; Barkley, D.S.; Hall, E.M.; Konijn, T.M.; Mason, J.W.; O’Keefe, G., 3rd; Wolfe, P.B. Acrasin, Acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev. Biol. 1969, 20, 72–87. [Google Scholar] [CrossRef]
- Sung, B.H.; Weaver, A.M. Directed migration: Cells navigate by extracellular vesicles. J. Cell Biol. 2018, 217, 2613–2614. [Google Scholar] [CrossRef]
- Sung, B.H.; Weaver, A.M. Exosome secretion promotes chemotaxis of cancer cells. Cell Adhes. Migr. 2017, 11, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).