Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Groups
2.3. Diagnosis of PD, the Hoehn and Yahr Scale, and MDS-UPDRS 3 Scores
2.4. Fine Motor Tests
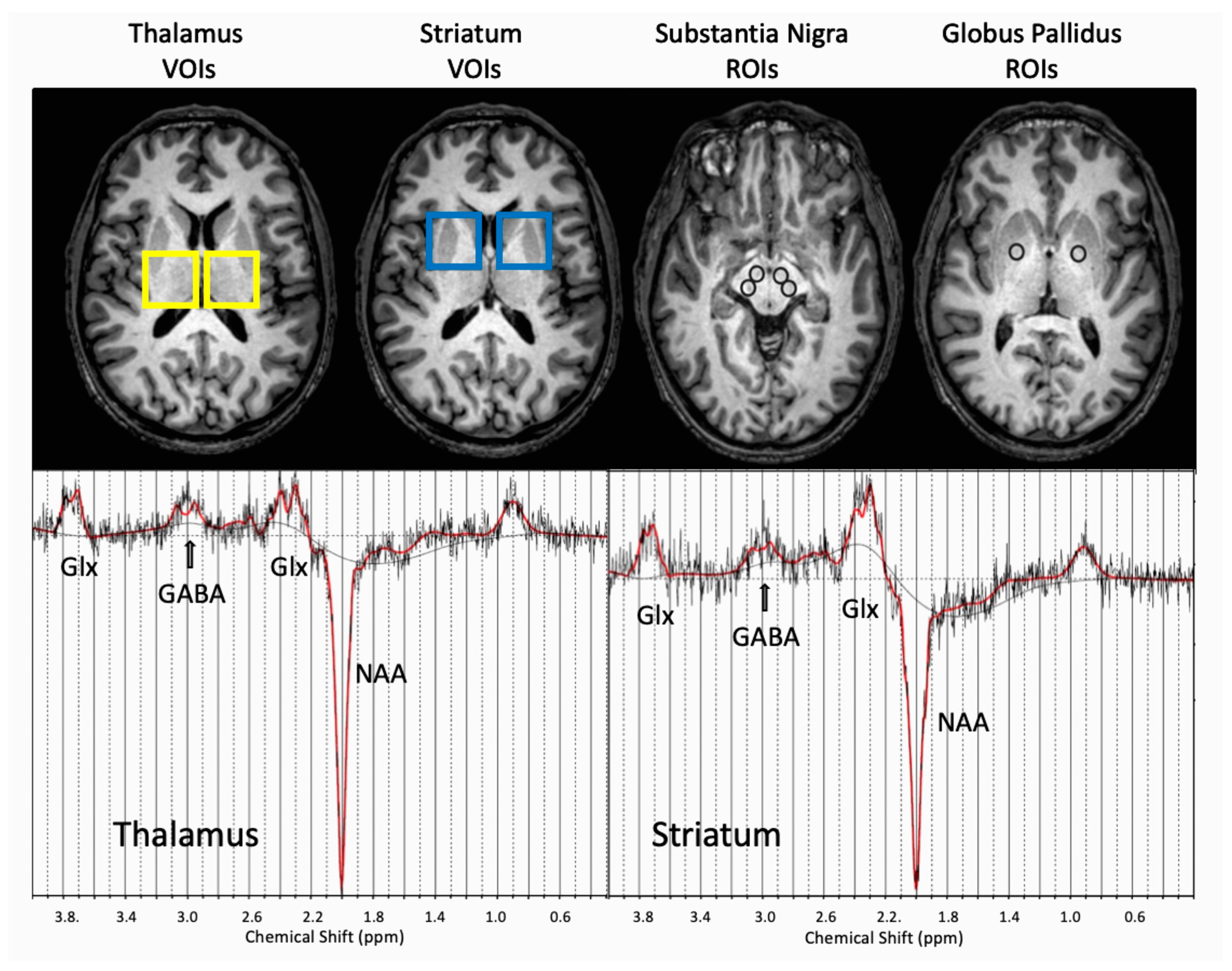
2.5. MRI and MRS Data Acquisition and Processing
2.6. Statistics
3. Results
3.1. Demographics and Clinical Data of the Study Population
3.2. Distribution of Brain Iron and Neurometabolites
3.3. Correlations Between Neuroimaging Data and Motor Dysfunctions
3.4. Predictors of Impaired Fine Motor Functions
3.5. Predictors of Gross-Motor Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Moualla, D.; Brown, D.R. Alpha-synuclein is a cellular ferrireductase. PLoS ONE 2011, 6, e15814. [Google Scholar] [CrossRef]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Muñoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A. Iron metabolism in the CNS: Implications for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.; Dydak, U.; Dharmadhikari, S.; Lotz, A.; Lehnert, M.; Quetscher, C.; Stewig, C.; Glaubitz, B.; Schmidt-Wilcke, T.; Edmondson, D.; et al. Association of exposure to manganese and iron with striatal and thalamic GABA and other neurometabolites—Neuroimaging results from the WELDOX II study. Neurotoxicology 2018, 64, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Langkammer, C.; Pirpamer, L.; Seiler, S.; Deistung, A.; Schweser, F.; Franthal, S.; Homayoon, N.; Katschnig-Winter, P.; Koegl-Wallner, M.; Pendl, T.; et al. Quantitative Susceptibility Mapping in Parkinson’s Disease. PLoS ONE 2016, 11, e0162460. [Google Scholar] [CrossRef]
- Jin, L.; Wang, J.; Jin, H.; Fei, G.; Zhang, Y.; Chen, W.; Zhao, L.; Zhao, N.; Sun, X.; Zeng, M.; et al. Nigral iron deposition occurs across motor phenotypes of Parkinson’s disease. Eur. J. Neurol. 2012, 19, 969–976. [Google Scholar] [CrossRef]
- Ulla, M.; Bonny, J.M.; Ouchchane, L.; Rieu, I.; Claise, B.; Durif, F. Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PLoS ONE 2013, 8, e57904. [Google Scholar] [CrossRef]
- Guan, X.; Xu, X.; Zhang, M. Region-Specific Iron Measured by MRI as a Biomarker for Parkinson’s Disease. Neurosci. Bull. 2017, 33, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Kastman, E.K.; Willette, A.A.; Coe, C.L.; Bendlin, B.B.; Kosmatka, K.J.; McLaren, D.G.; Xu, G.; Canu, E.; Field, A.S.; Alexander, A.L.; et al. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J. Neurosci. 2012, 32, 11897–11904. [Google Scholar] [CrossRef]
- Pesch, B.; Casjens, S.; Weiss, T.; Kendzia, B.; Arendt, M.; Eisele, L.; Behrens, T.; Ulrich, N.; Pundt, N.; Marr, A.; et al. Occupational Exposure to Manganese and Fine Motor Skills in Elderly Men: Results from the Heinz Nixdorf Recall Study. Ann. Work Expo. Health 2017, 61, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Cao, C.-J.; Zuo, L.-J.; Chen, Z.-J.; Lian, T.-H.; Wang, F.; Hu, Y.; Piao, Y.-S.; Li, L.-X.; Guo, P.; et al. Clinical features and dysfunctions of iron metabolism in Parkinson disease patients with hyper echogenicity in substantia nigra: A cross-sectional study. BMC Neurol. 2018, 18, 9. [Google Scholar] [CrossRef]
- Ciurleo, R.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Magnetic resonance spectroscopy: An in vivo molecular imaging biomarker for Parkinson’s disease? BioMed. Res. Int. 2014, 2014, 519816. [Google Scholar] [CrossRef]
- Dydak, U.; Edmondson, D.; Zauber, S.E. MRS of Parkinsonian Disorders. In Magnetic Resonance Spectroscopy of Degenerative Brain Diseases; Oz, G., Ed.; Springer: Basel, Switzerland, 2016; pp. 71–102. [Google Scholar]
- Mullins, P.G.; McGonigle, D.J.; O’Gorman, R.L.; Puts, N.A.J.; Vidyasagar, R.; Evans, C.J.; Edden, R.A.E. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage 2014, 86, 43–52. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman Tuura, R.L.; Baumann, C.R.; Baumann-Vogel, H. Beyond Dopamine: GABA, Glutamate, and the Axial Symptoms of Parkinson Disease. Front. Neurol. 2018, 9, 806. [Google Scholar] [CrossRef]
- Long, Z.; Li, X.-R.; Xu, J.; Edden, R.A.E.; Qin, W.-P.; Long, L.-L.; Murdoch, J.B.; Zheng, W.; Jiang, Y.-M.; Dydak, U. Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers. PLoS ONE 2014, 9, e88220. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Dydak, U.; Lotz, A.; Casjens, S.; Quetscher, C.; Lehnert, M.; Abramowski, J.; Stewig, C.; Yeh, C.-L.; Weiss, T.; et al. Association of exposure to manganese and iron with relaxation rates R1 and R2*-magnetic resonance imaging results from the WELDOX II study. Neurotoxicology 2018, 64, 68–77. [Google Scholar] [CrossRef]
- Van Thriel, C.; Quetscher, C.; Pesch, B.; Lotz, A.; Lehnert, M.; Casjens, S.; Weiss, T.; van Gelder, R.; Plitzke, K.; Brüning, T.; et al. Are multitasking abilities impaired in welders exposed to manganese? Translating cognitive neuroscience to neurotoxicology. Arch. Toxicol. 2017, 91, 2865–2877. [Google Scholar] [CrossRef]
- Casjens, S.; Henry, J.; Rihs, H.-P.; Lehnert, M.; Raulf-Heimsoth, M.; Welge, P.; Lotz, A.; van Gelder, R.; Hahn, J.-U.; Stiegler, H.; et al. Influence of welding fume on systemic iron status. Ann. Occup. Hyg. 2014, 58, 1143–1154. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Kraus, P.H.; Hoffmann, A. Spiralometry: Computerized assessment of tremor amplitude on the basis of spiral drawing. Mov. Disord. 2010, 25, 2164–2170. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef]
- Schwarz, S.T.; Mougin, O.; Xing, Y.; Blazejewska, A.; Bajaj, N.; Auer, D.P.; Gowland, P. Parkinson’s disease related signal change in the nigrosomes 1-5 and the substantia nigra using T2* weighted 7T MRI. NeuroImage 2018, 19, 683–689. [Google Scholar] [CrossRef]
- Schenker, C.; Meier, D.; Wichmann, W.; Boesiger, P.; Valavanis, A. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology 1993, 35, 119–124. [Google Scholar] [CrossRef]
- Hallgren, B.; Sourander, P. The effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958, 3, 41–51. [Google Scholar] [CrossRef]
- Xiao, Y.; Luo, M.; Luo, H.; Wang, J. Creatine for Parkinson’s disease. Cochrane Database Syst. Rev. 2014, CD009646. [Google Scholar] [CrossRef]
- Gong, T.; Xiang, Y.; Saleh, M.G.; Gao, F.; Chen, W.; Edden, R.A.E.; Wang, G. Inhibitory motor dysfunction in parkinson’s disease subtypes. J. Magn. Reson. Imaging 2018, 47, 1610–1615. [Google Scholar] [CrossRef]
- Růžička, E.; Krupička, R.; Zárubová, K.; Rusz, J.; Jech, R.; Szabó, Z. Tests of manual dexterity and speed in Parkinson’s disease: Not all measure the same. Parkinsonism Relat. Disord. 2016, 28, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Guerra, A.; Paparella, G.; Giordo, L.; Alunni Fegatelli, D.; Vestri, A.R.; Rothwell, J.C.; Berardelli, A. Neurophysiological correlates of bradykinesia in Parkinson’s disease. Brain 2018. [Google Scholar] [CrossRef] [PubMed]
- Angelie, E.; Bonmartin, A.; Boudraa, A.; Gonnaud, P.M.; Mallet, J.J.; Sappey-Marinier, D. Regional differences and metabolic changes in normal aging of the human brain: Proton MR spectroscopic imaging study. AJNR Am. J. Neuroradiol. 2001, 22, 119–127. [Google Scholar] [PubMed]
- Govindpani, K.; Calvo-Flores Guzmán, B.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Towards a Better Understanding of GABAergic Remodeling in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1813. [Google Scholar] [CrossRef] [PubMed]
- Sibson, N.R.; Dhankhar, A.; Mason, G.F.; Rothman, D.L.; Behar, K.L.; Shulman, R.G. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. USA 1998, 95, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Galvan, A.; Wichmann, T. GABAergic circuits in the basal ganglia and movement disorders. Progress Brain Res. 2007, 160, 287–312. [Google Scholar] [CrossRef]
- Kish, S.; Rajput, A.; Gilbert, J.; Rozdilsky, B.; Chang, L.J.; Shannak, K.; Hornykiewicz, O. GABA-dopamine relationship in Parkinson’s disease striatum. Adv. Neurol. 1987, 45, 75–77. [Google Scholar] [PubMed]
- Zecca, L.; Stroppolo, A.; Gatti, A.; Tampellini, D.; Toscani, M.; Gallorini, M.; Giaveri, G.; Arosio, P.; Santambrogio, P.; Fariello, R.G.; et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. USA 2004, 101, 9843–9848. [Google Scholar] [CrossRef]
- Hornykiewicz, O. Chemical neuroanatomy of the basal ganglia--normal and in Parkinson’s disease. J. Chem. Neuroanat. 2001, 22, 3–12. [Google Scholar] [CrossRef]
- Emir, U.E.; Tuite, P.J.; Öz, G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLoS ONE 2012, 7, e30918. [Google Scholar] [CrossRef]
- Dydak, U.; Dharmadhikari, S.; Snyder, S.; Zauber, S.E. Increased thalamic GABA levels correlate with Parkinson disease severity. In Proceedings of the AD/PD Conference, Nice, France, 18–21 March 2015. [Google Scholar]
- Dydak, U.; Jiang, Y.M.; Long, L.L.; Zhu, H.; Chen, J.; Li, W.M.; Edden, R.A.; Hu, S.; Fu, X.; Long, Z.; et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ. Health Perspect. 2011, 119, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.E.; Ward, E.J.; Yeh, C.-L.; Snyder, S.; Long, Z.; Gokalp Yavuz, F.; Zauber, S.E.; Dydak, U. Thalamic GABA levels and occupational manganese neurotoxicity: Association with exposure levels and brain MRI. Neurotoxicology 2018, 64, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Oz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef]
- Cheong, I.; Deelchand, D.K.; Eberly, L.E.; Marjańska, M.; Manousakis, G.; Guliani, G.; Walk, D.; Öz, G. Neurochemical correlates of functional decline in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Oz, G.; Hutter, D.; Tkác, I.; Clark, H.B.; Gross, M.D.; Jiang, H.; Eberly, L.E.; Bushara, K.O.; Gomez, C.M. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov. Disord. 2010, 25, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Géloën, A.; Soulage, C.O. Abnormalities in myo-inositol metabolism associated with type 2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Brit. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef]
- Long, Z.; Jiang, Y.-M.; Li, X.-R.; Fadel, W.; Xu, J.; Yeh, C.-L.; Long, L.-L.; Luo, H.-L.; Harezlak, J.; Murdoch, J.B.; et al. Vulnerability of welders to manganese exposure—A neuroimaging study. Neurotoxicology 2014, 45, 285–292. [Google Scholar] [CrossRef]
- Abbas, M.M.; Xu, Z.; Tan, L.C.S. Epidemiology of Parkinson’s Disease-East Versus West. Mov. Disord. Clin. Pract. 2018, 5, 14–28. [Google Scholar] [CrossRef]
- Mazuel, L.; Chassain, C.; Jean, B.; Pereira, B.; Cladière, A.; Speziale, C.; Durif, F. Proton MR Spectroscopy for Diagnosis and Evaluation of Treatment Efficacy in Parkinson Disease. Radiology 2016, 278, 505–513. [Google Scholar] [CrossRef]
| Participants Characteristics | Controls N = 35 | PD N = 35 | p-Value | Akinetic-Rigid PD N = 19 | Mixed PD N = 16 | p-Value |
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||
| Age [years] | 55 (50;66) | 59 (54; 66) | 0.14 | 60 (54; 67) | 59 (55; 66) | 0.64 |
| Age at diagnosis [years] | 54 (49; 60) | 52 (47; 60) | 55 (53; 59) | 0.37 | ||
| PD duration [years] | 4.7 (2.5; 7.7) | 6.0 (3.6; 8.8) | 3.5 (1.5; 5.4) | 0.03 | ||
| MDS-UPDRS3 total score; range (0–132) | 1 (0; 2) | 34 (24; 43) | <0.0001 | 34 (24; 43) | 34.5 (25.5; 45.5) | 0.95 |
| MDS-UPDRS3 rigidity subscore; range (0–20) | 0 (0; 0) | 7 (3; 8) | <0.0001 | 7 (5; 8) | 4 (2.5; 8) | 0.10 |
| Tapping hits (left or more affected hand) | 186 (180; 205) | 164 (134; 184) | 0.0001 | 163 (143; 178) | 176 (126; 190) | 0.80 |
| Tremor amplitude (mm) (left or more affected hand) | 0.9 (0.7; 1.1) | 1.1 (0.8; 1.3) | 0.019 | 1.1 (0.8; 1.2) | 1.2 (0.8; 1.9) | 0.26 |
| N (%) | N (%) | N (%) | N % | |||
| Education | ||||||
| Low | 8 (22.9) | 17 (48.6) | 0.08 | 12 (63.2) | 5 (31.3) | 0.06 |
| Medium | 8 (22.9) | 6 (17.1) | 4 (21.1) | 2 (12.5) | ||
| High | 19 (54.3) | 12 (34.3) | 3 (15.8) | 9 (56.3) | ||
| Clinically more affected side | ||||||
| Left | 12 (34.3) | 5 (26.3) | 7 (43.8) | 0.24 | ||
| Right | 14 (40.0) | 7 (36.8) | 7 (43.8 | |||
| No preference | 9 (25.7) | 7 (36.8) | 2 (12.5) |
| Neuroimaging Data | Controls N = 35 | PD N = 35 | p-Value | Akinetic-Rigid PD N = 19 | Mixed PD N = 16 | p-Value |
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||
| R2* (1/s), SN (N = 70) | 45.6 (40.8; 51.1) | 48.0 (39.6; 61.8) | 0.38 | 45.5 (40.4; 56) | 53.4 (36.1; 62.4) | 0.84 |
| R2* (1/s), GP (N = 70) | 43.4 (40.4; 47.7) | 44.1 (37.9; 47.2) | 0.43 | 39.9 (37.9; 46.2) | 46.0 (38.2; 47.8) | 0.41 |
| Striatum | ||||||
| GABA (mM) (N = 68) | 1.9 (1.8; 2.1) | 2.0 (1.8; 2.2) | 0.30 | 2.0 (1.6; 2.2) | 2.1 (1.9; 2.4) | 0.13 |
| Glx (mM) (N = 69) | 10.5 (9.1; 11.3) | 10.5 (8.9; 11.7) | 0.88 | 9.6 (8.8; 11.0) | 11.4 (10.1; 12.3) | 0.04 |
| Myo-inositol (mM) (N = 69) | 4.3 (3.9; 4.7) | 4.4 (3.9; 5.1) | 0.37 | 4.3 (3.9; 4.8) | 4.6 (4.0; 5.2) | 0.30 |
| Total creatine (mM) (N = 69) | 7.5 (7.1; 7.8) | 7.2 (6.5; 7.6) | 0.04 | 6.7 (6.4; 7.4) | 7.4 (6.9; 7.8) | 0.03 |
| Thalamus | ||||||
| GABA (mM) (N = 69) | 1.9 (1.7; 2.4) | 2.1 (1.9; 2.4) | 0.21 | 2.2 (1.9; 2.4) | 2.0 (1.9; 2.1) | 0.06 |
| Glx (mM) (N = 69) | 7.2 (6.6; 8.3) | 7.7 (6.8; 8.5) | 0.15 | 7.5 (6.8; 8.3) | 7.8 (7.2; 8.9) | 0.22 |
| Myo-inositol (mM) (N = 69) | 4.6 (4.2; 4.8) | 4.9 (4.3; 5.4) | 0.19 | 4.7 (3.9; 5.3) | 5.0 (4.5; 5.5) | 0.64 |
| Total creatine (mM) (N = 69) | 6.3 (6.1; 6.8) | 6.3 (6.0; 6.8) | 0.77 | 6.2 (6.0; 6.5) | 6.5 (6.2; 6.8) | 0.06 |
| rs (95% CI) | Tapping Hits Non-dominant or More Affected Hand | Tremor Amplitude (mM) Non-Dominant Hand or More Affected Hand | MDS-UPDRS3 Total Score | MDS-UPDRS3 Rigidity Subscore |
|---|---|---|---|---|
| Age (years) | −0.10 (−0.42, 0.24) | 0.09 (−0.25, 0.41) | 0.34 (0.01, 0.61) | 0.19 (−0.16, 0.49) |
| Age at diagnosis (years) | −0.11 (−0.43, 0.23) | 0.17 (−0.18, 0.47) | 0.26 (−0.08, 0.54) | 0.07 (−0.27, 0.39) |
| R2* (1/s) SN | 0.11 (−0.23, 0.43) | 0.02 (−0.32, 0.35) | 0.39 (0.07, 0.64) | 0.33 (−0.01, 0.59) |
| R2* (1/s) GP | −0.04 (−0.37, 0.29) | 0.00 (−0.34, 0.33) | 0.32 (−0.01, 0.59) | 0.32 (−0.01, 0.59) |
| Striatum | ||||
| GABA | 0.31 (−0.14, 0.65) | 0.13 (−0.32, 0.53) | 0.37 (−0.08, 0.69) | 0.30 (−0.15, 0.65) |
| Glx | −0.30 (−0.66, 0.16) | 0.03 (−0.42, 0.47) | −0.14 (−0.55, 0.32) | −0.23 (−0.61, 0.24) |
| Myo-inositol | 0.49 (0.06, 0.77) | −0.34 (−0.68, 0.12) | 0.30 (−0.16., 0.66) | 0.03 (−0.42, 0.47) |
| Total creatine | 0.06 (−0.39, 0.49) | −0.29 (−0.65, 0.18) | 0.14 (−0.32, 0.55) | 0.01 (−0.44, 0.45) |
| Thalamus | ||||
| GABA | −0.11 (−0.55, 0.38) | −0.20 (−0.61, 0.30) | −0.01 (−0.47, 0.46) | −0.15 (−0.58, 0.34) |
| Glx | 0.16 (−0.30, 0.56) | −0.03 (−0.47, 0.42) | 0.15 (−0.31, 0.56) | 0.11 (−0.35, 0.52) |
| Myo-inositol | −0.10 (−0.52, 0.36) | 0.03 (−0.42, 0.47) | −0.19 (−0.58, 0.28) | −0.30 (−0.65, 0.17) |
| Total creatine | 0.26 (−0.20, 0.63) | −0.01 (−0.45, 0.43) | 0.01 (−0.44, 0.45) | −0.12 (−0.54, 0.34) |
| Tapping Hits | Tremor Amplitude (mm) | |||||
|---|---|---|---|---|---|---|
| Exp(β) | 95% CI | Exp(β) | 95% CI | |||
| Study groups (N = 70) | ||||||
| Intercept | 227.30 | 190.62 | 271.04 | 0.54 | 0.30 | 1.00 |
| Akinetic-rigid PD vs. controls | 0.91 | 0.85 | 0.97 | 1.06 | 0.87 | 1.30 |
| Mixed PD vs. controls | 0.87 | 0.81 | 0.93 | 1.32 | 1.08 | 1.62 |
| High education vs. lower levels | 1.05 | 1.00 | 1.10 | 0.95 | 0.80 | 1.12 |
| Age [per 10 years] | 0.98 | 0.96 | 1.01 | 1.06 | 0.96 | 1.18 |
| Non-dominant hand | 0.90 | 0.86 | 0.95 | 1.18 | 1.12 | 1.25 |
| More affected side | 0.92 | 0.86 | 0.99 | 1.11 | 1.02 | 1.22 |
| Brain iron (N = 35 PD patients) | ||||||
| R2* [log 1/s] | 0.96 | 0.84 | 1.10 | 0.87 | 0.70 | 1.08 |
| Globus pallidus vs. substantia nigra | 0.99 | 0.94 | 1.06 | 0.98 | 0.92 | 1.04 |
| Neurometabolites (N = 35 PD patients) | ||||||
| GABA | 1.01 | 0.85 | 1.22 | 1.03 | 0.84 | 1.25 |
| Striatum vs. thalamus | 0.99 | 0.91 | 1.07 | 0.97 | 0.89 | 1.05 |
| Glutamate and glutamine | 0.89 | 0.75 | 1.07 | 1.22 | 1.02 | 1.47 |
| Striatum vs. thalamus | 1.02 | 0.94 | 1.12 | 0.93 | 0.85 | 1.01 |
| Myo-inositol | 1.27 | 1.06 | 1.53 | 1.09 | 0.87 | 1.36 |
| Striatum vs. thalamus | 1.02 | 0.95 | 1.10 | 0.97 | 0.90 | 1.05 |
| Total creatine | 1.01 | 0.69 | 1.49 | 1.16 | 0.73 | 1.84 |
| Striatum vs. thalamus | 1.00 | 0.92 | 1.08 | 0.96 | 0.88 | 1.04 |
| Β | 95% CI | ||
|---|---|---|---|
| Intercept | −4.10 | −46.71 | 38.51 |
| Akinetic-rigid PD vs. mixed PD | −4.13 | −14.40 | 6.14 |
| High education | −4.71 | −15.53 | 6.11 |
| Age [per 10 years] | 7.22 | 0.38 | 14.06 |
| Brain iron | |||
| R2*, globus pallidus [log 1/s] | 19.72 | −8.70 | 48.15 |
| R2*, substantia nigra [log 1/s] | 10.19 | −7.41 | 27.78 |
| Neurometabolites | |||
| Striatum | |||
| GABA | 7.71 | −21.54 | 36.95 |
| Glutamate and glutamine | −5.48 | −33.40 | 22.45 |
| Myo-inositol | 0.06 | −31.11 | 31.23 |
| Total creatine | 14.59 | −38.66 | 67.84 |
| Thalamus | |||
| GABA | −9.83 | −46.20 | 26.54 |
| Glutamate and glutamine | 1.18 | −28.92 | 31.28 |
| Myo-inositol | −29.00 | −58.21 | 0.21 |
| Total creatine | −5.49 | −75.39 | 64.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesch, B.; Casjens, S.; Woitalla, D.; Dharmadhikari, S.; Edmondson, D.A.; Zella, M.A.S.; Lehnert, M.; Lotz, A.; Herrmann, L.; Muhlack, S.; et al. Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease. Cells 2019, 8, 96. https://doi.org/10.3390/cells8020096
Pesch B, Casjens S, Woitalla D, Dharmadhikari S, Edmondson DA, Zella MAS, Lehnert M, Lotz A, Herrmann L, Muhlack S, et al. Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease. Cells. 2019; 8(2):96. https://doi.org/10.3390/cells8020096
Chicago/Turabian StylePesch, Beate, Swaantje Casjens, Dirk Woitalla, Shalmali Dharmadhikari, David A. Edmondson, Maria Angela Samis Zella, Martin Lehnert, Anne Lotz, Lennard Herrmann, Siegfried Muhlack, and et al. 2019. "Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease" Cells 8, no. 2: 96. https://doi.org/10.3390/cells8020096
APA StylePesch, B., Casjens, S., Woitalla, D., Dharmadhikari, S., Edmondson, D. A., Zella, M. A. S., Lehnert, M., Lotz, A., Herrmann, L., Muhlack, S., Kraus, P., Yeh, C.-L., Glaubitz, B., Schmidt-Wilcke, T., Gold, R., van Thriel, C., Brüning, T., Tönges, L., & Dydak, U. (2019). Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease. Cells, 8(2), 96. https://doi.org/10.3390/cells8020096







