Self-Assembling Scaffolds Supported Long-Term Growth of Human Primed Embryonic Stem Cells and Upregulated Core and Naïve Pluripotent Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintenance of Human ESCs in 2-D culture
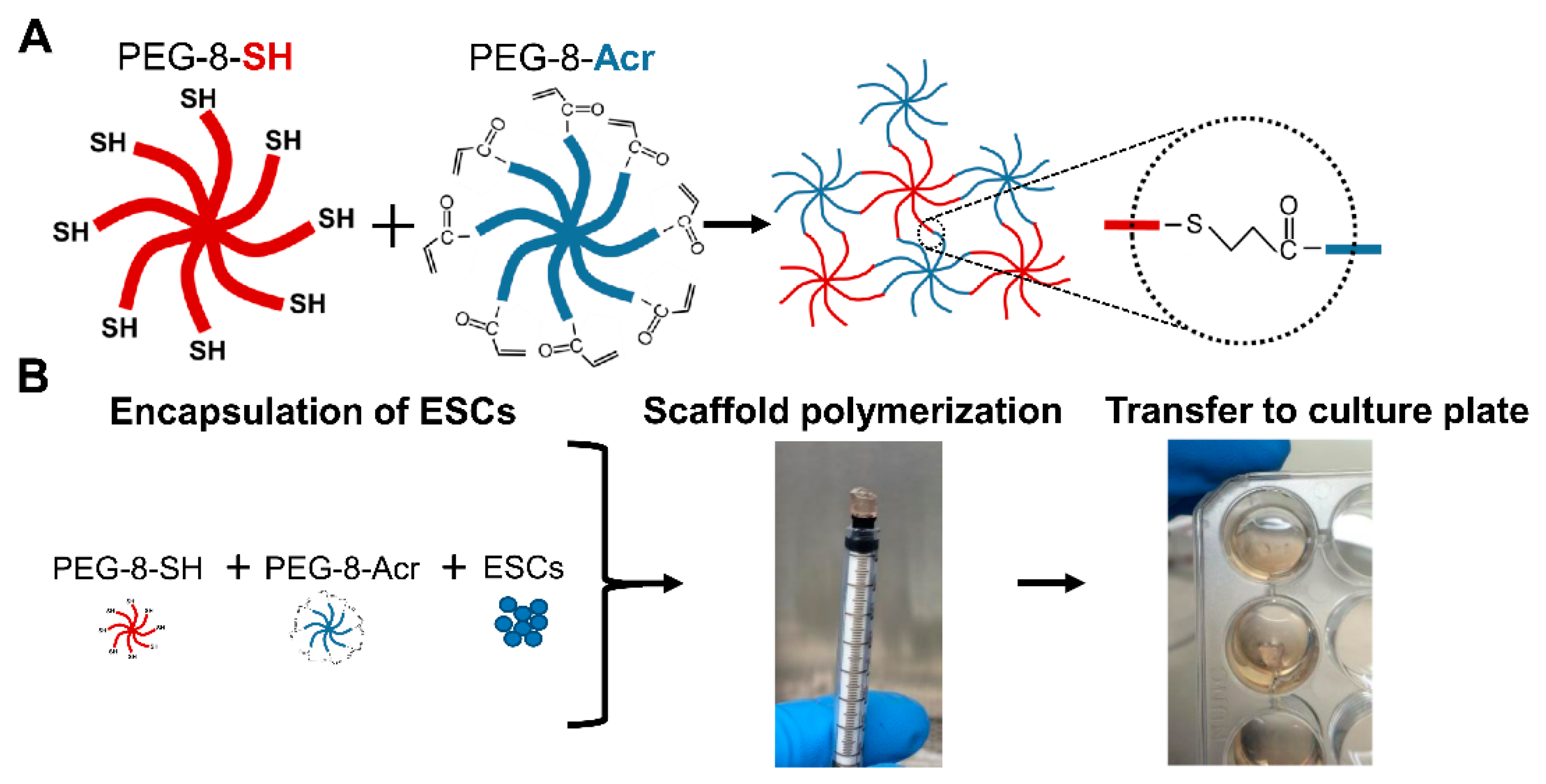
2.2. Selection and Composition of Scaffolds for 3-D Culture of Human ESCs
2.3. Cell Proliferation and Viability Assays
2.4. Differentiation of Human ESCs
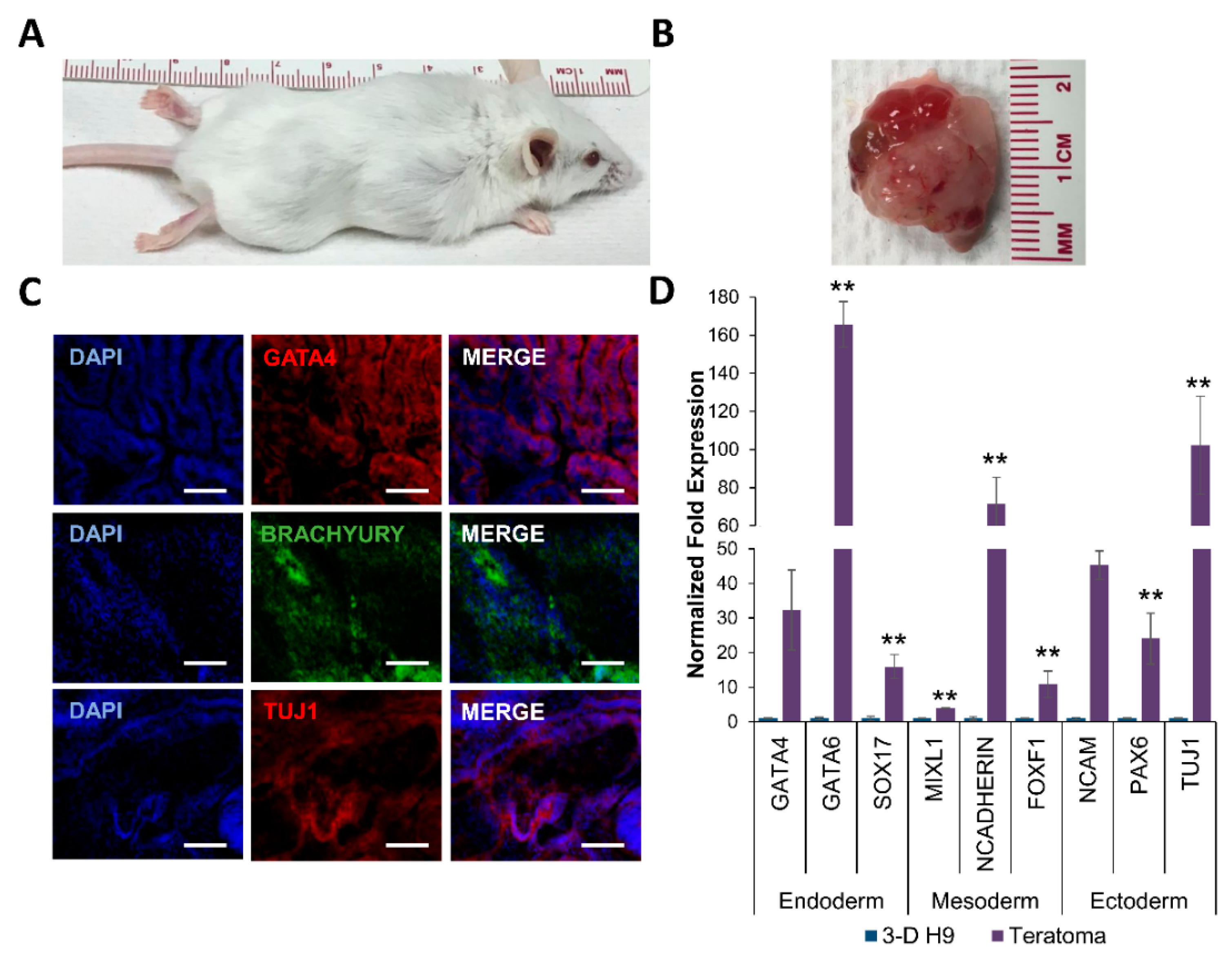
2.5. Teratoma Assay
2.6. Gene Expression Analysis
2.7. Immunocytochemical Analysis
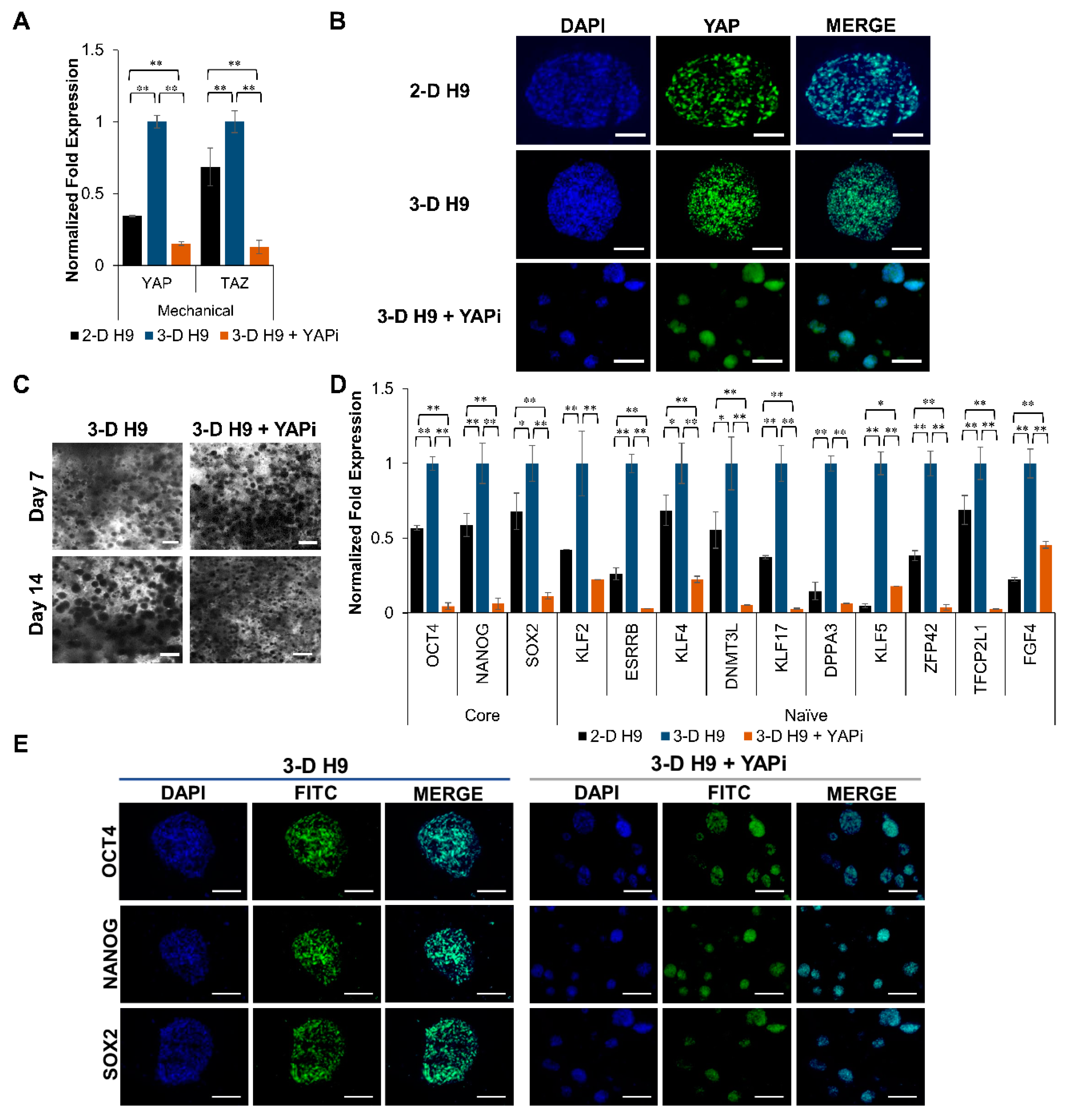
2.8. Effect of YAP Inhibitor on Human ESCs Grown in 3-D Self-Assembling Scaffolds
2.9. Statistical analysis
3. Results
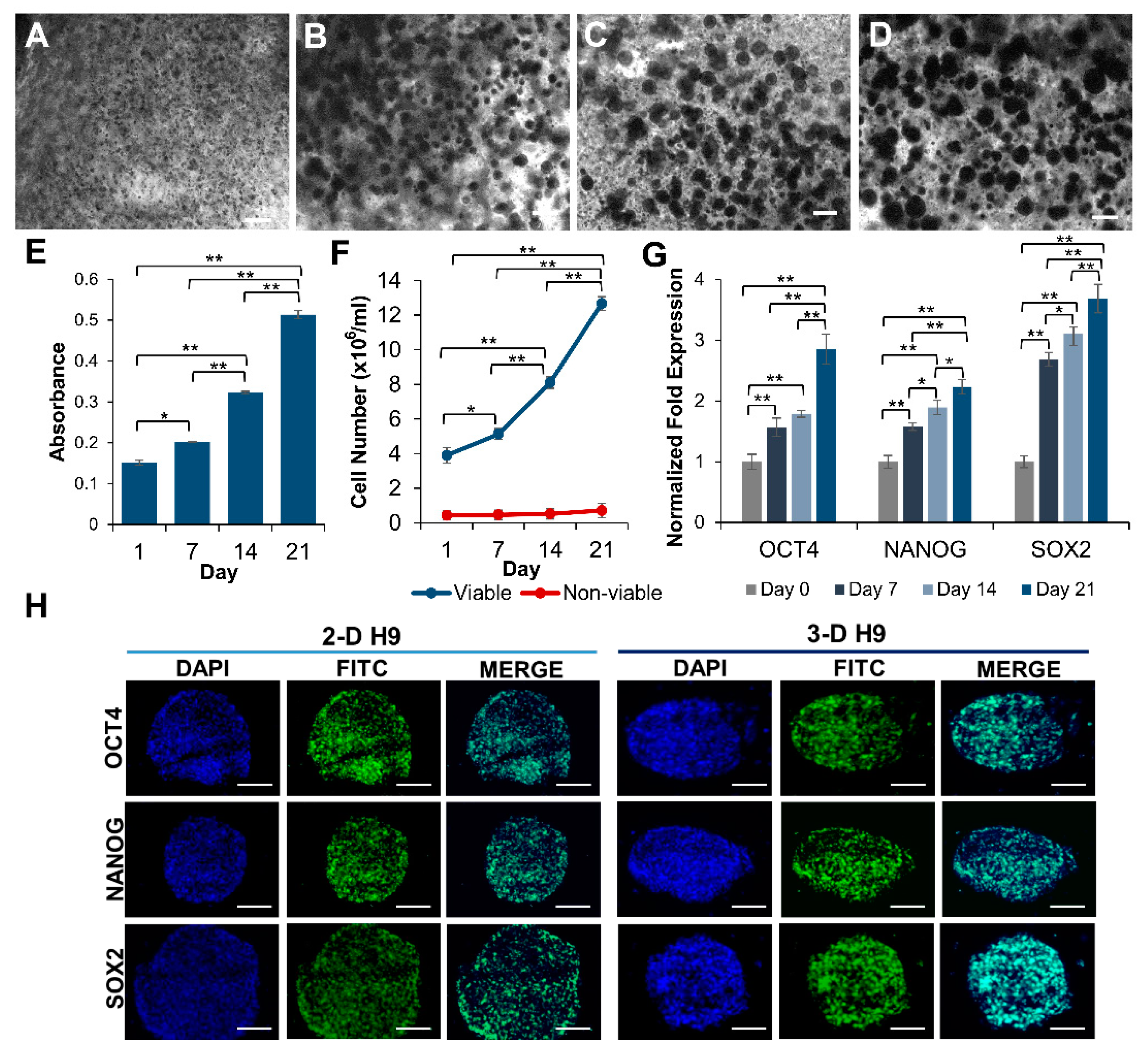
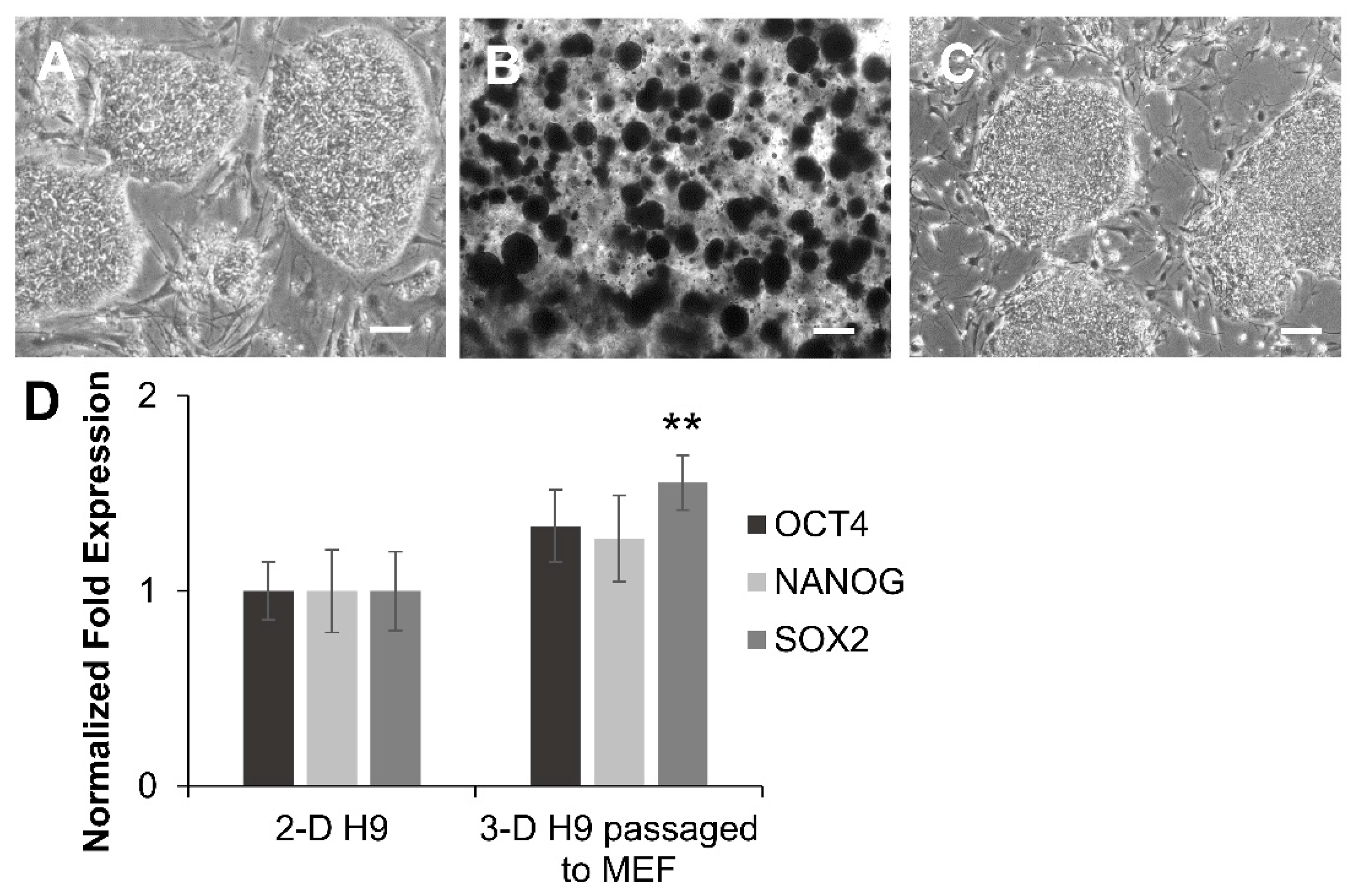
3.1. Growth and Characterization of H9 Cells Grown under 3-D Culture Conditions
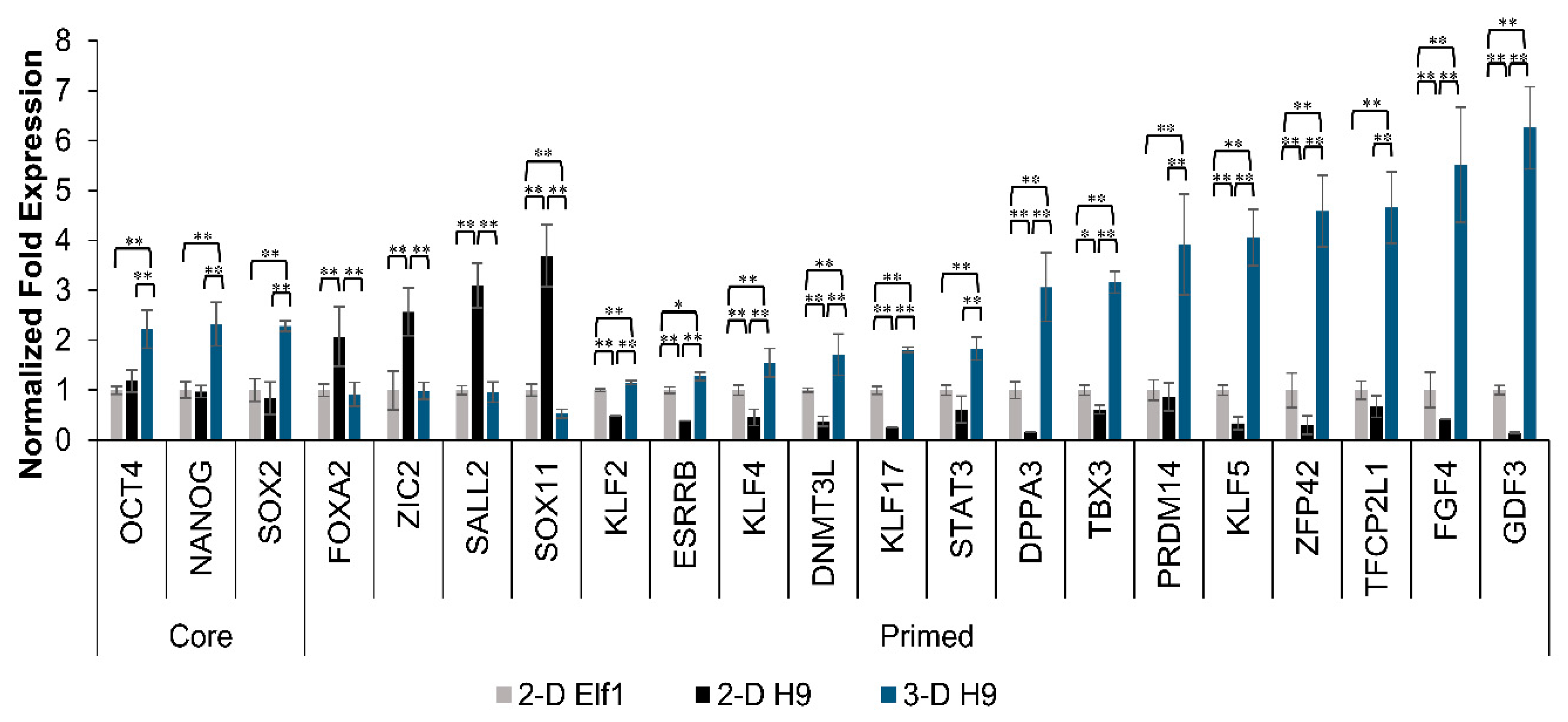
3.2. Maintenance of Pluripotency in H9 Cells Grown under 3-D Culture Conditions
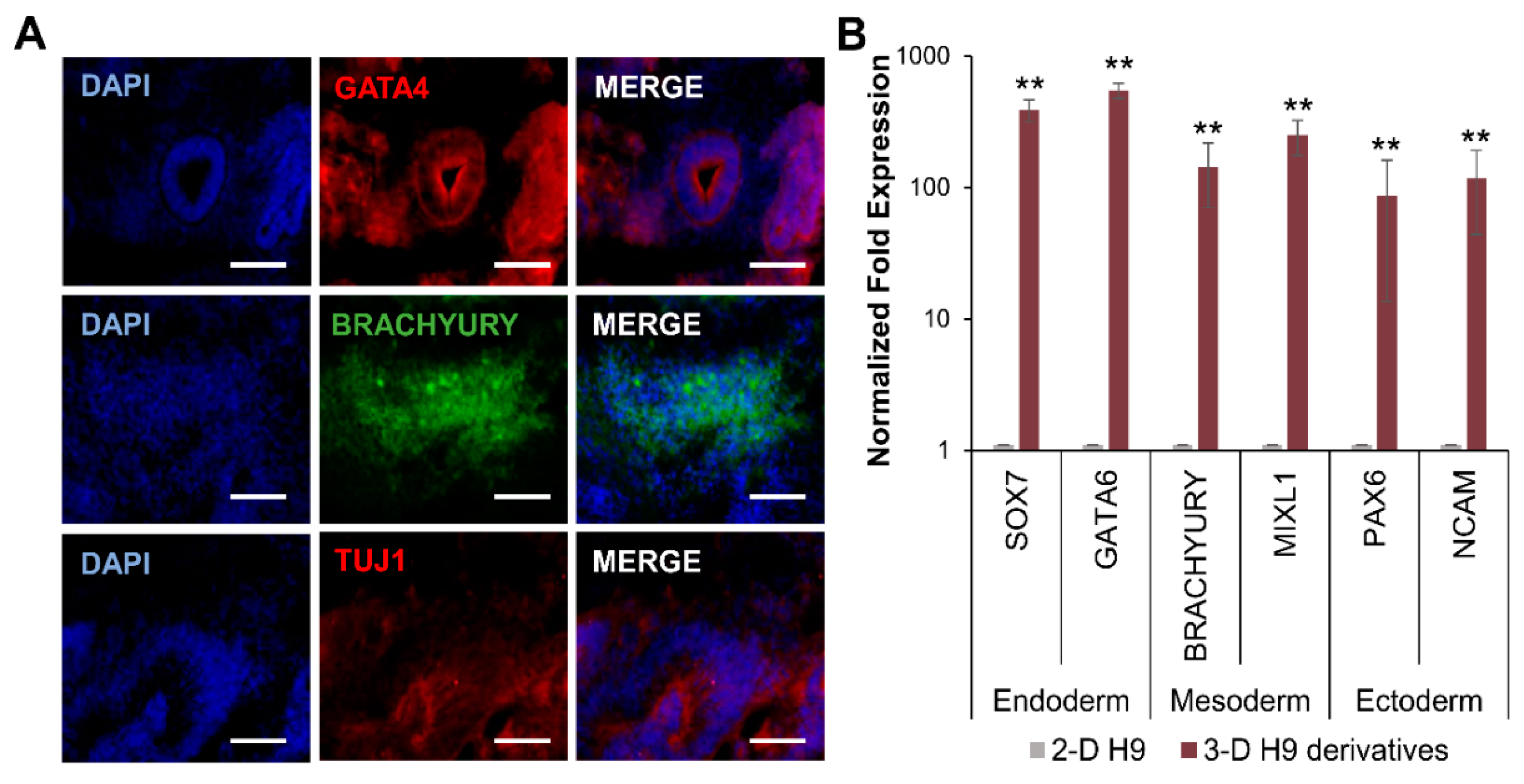
3.3. Differentiation of H9 Cells Grown in 3-D Self-Assembling Scaffolds
3.4. Expression of Naïve Pluripotent Markers in H9 Cells Grown under 3-D Culture Conditions and the Effect of YAP Inhibition
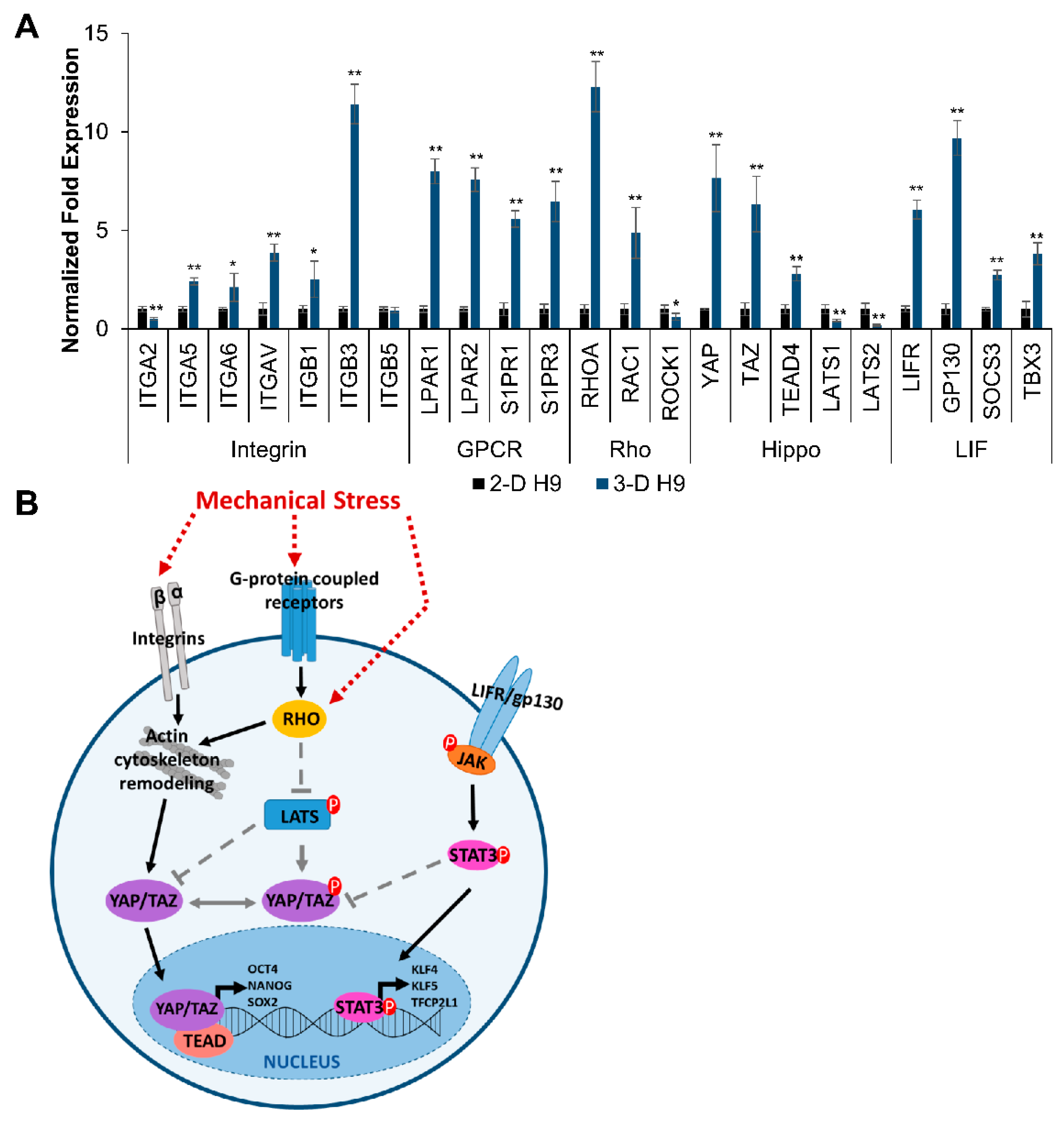
3.5. Mechanism of Regulation of Pluripotent Genes in H9 Cells Grown under 3-D Culture Conditions
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotech. 2000, 18, 399–404. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Nakamura, T.; Okamoto, I.; Sasaki, K.; Yabuta, Y.; Iwatani, C.; Tsuchiya, H.; Seita, Y.; Nakamura, S.; Yamamoto, T.; Saitou, M. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 2016, 537, 57–62. [Google Scholar] [CrossRef]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef]
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. [Google Scholar] [CrossRef]
- Morgani, S.; Nichols, J.; Hadjantonakis, A.-K. The many faces of pluripotency: In vitro adaptations of a continuum of in vivo states. BMC Dev. Biol. 2017, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Ganz, K.; Shi, L.; et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 2014, 15, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yamauchi, T.; Izpisua Belmonte, J.C. An overview of mammalian pluripotency. Development 2016, 143, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, C.; Wang, L.; Liu, Y.; Mu, X.; Ma, Y.; Li, L. Maintenance of human embryonic stem cells on gelatin. Chin. Sci. Bull. 2009, 54, 4214. [Google Scholar] [CrossRef]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.; Domogatskaya, A.; Strom, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Kalaskar, D.M.; Downes, J.E.; Murray, P.; Edgar, D.H.; Williams, R.L. Characterization of the interface between adsorbed fibronectin and human embryonic stem cells. J. R. Soc. Interface 2013, 10, 20130139. [Google Scholar] [CrossRef]
- Braam, S.R.; Zeinstra, L.; Litjens, S.; Ward-van Oostwaard, D.; van den Brink, S.; van Laake, L.; Lebrin, F.; Kats, P.; Hochstenbach, R.; Passier, R.; et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 2008, 26, 2257–2265. [Google Scholar] [CrossRef]
- Richards, M.; Fong, C.Y.; Chan, W.K.; Wong, P.C.; Bongso, A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002, 20, 933–936. [Google Scholar] [CrossRef]
- Jozefczuk, J.; Drews, K.; Adjaye, J. Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J. Vis. Exp. 2012. [Google Scholar] [CrossRef]
- Kent, L. Culture and maintenance of human embryonic stem cells. J. Vis. Exp. 2009. [Google Scholar] [CrossRef]
- Akhmanova, M.; Osidak, E.; Domogatsky, S.; Rodin, S.; Domogatskaya, A. Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int. 2015, 2015, 35. [Google Scholar] [CrossRef]
- Cosson, S.; Lutolf, M.P. Chapter 7—Microfluidic patterning of protein gradients on biomimetic hydrogel substrates. In Methods in Cell Biology; Piel, M., Théry, M., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 91–102. [Google Scholar]
- Infantes, E.C.; Prados, A.B.H.; Contreras, I.D.; Cahuana, G.M.; Hmadcha, A.; Bermudo, F.M.; Soria, B.; Huamán, J.R.T.; Bergua, F.J.B. Nitric oxide and hypoxia response in pluripotent stem cells. Redox Biol. 2015, 5, 417–418. [Google Scholar] [CrossRef][Green Version]
- Musah, S.; Morin, S.A.; Wrighton, P.J.; Zwick, D.B.; Jin, S.; Kiessling, L.L. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano 2012, 6, 10168–10177. [Google Scholar] [CrossRef] [PubMed]
- Peerani, R.; Rao, B.M.; Bauwens, C.; Yin, T.; Wood, G.A.; Nagy, A.; Kumacheva, E.; Zandstra, P.W. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007, 26, 4744–4755. [Google Scholar] [CrossRef] [PubMed]
- Szablowska-Gadomska, I.; Zayat, V.; Buzanska, L. Influence of low oxygen tensions on expression of pluripotency genes in stem cells. Acta Neurobiol. Exp. 2011, 71, 86–93. [Google Scholar]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 2009, 15, 205–219. [Google Scholar] [CrossRef]
- Janmey, P.A.; Miller, R.T. Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011, 124, 9–18. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and rho gtpase signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3d culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Lund, A.W.; Yener, B.; Stegemann, J.P.; Plopper, G.E. The natural and engineered 3d microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng. Part B Rev. 2009, 15, 371–380. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef]
- Saha, K.; Pollock, J.F.; Schaffer, D.V.; Healy, K.E. Designing synthetic materials to control stem cell phenotype. Curr. Opin. Chem. Biol. 2007, 11, 381–387. [Google Scholar] [CrossRef]
- Mammoto, A.; Mammoto, T.; Ingber, D.E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012, 125, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Krupinski, P.; Chickarmane, V.; Peterson, C. Simulating the mammalian blastocyst—Molecular and mechanical interactions pattern the embryo. PLOS Comput. Biol. 2011, 7, e1001128. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Perez-Cruet, M.; Chavez, F.; Chaudhry, G.R. Simplified three-dimensional culture system for long-term expansion of embryonic stem cells. World J. Stem Cells 2015, 7, 1064–1077. [Google Scholar] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Ware, C.B.; Nelson, A.M.; Mecham, B.; Hesson, J.; Zhou, W.; Jonlin, E.C.; Jimenez-Caliani, A.J.; Deng, X.; Cavanaugh, C.; Cook, S.; et al. Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4484–4489. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Kraehenbuehl, T.P.; Langer, R.; Ferreira, L.S. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat. Methods 2011, 8, 731–736. [Google Scholar] [CrossRef]
- Qin, H.; Hejna, M.; Liu, Y.; Percharde, M.; Wossidlo, M.; Blouin, L.; Durruthy-Durruthy, J.; Wong, P.; Qi, Z.; Yu, J.; et al. Yap induces human naive pluripotency. Cell Rep. 2016, 14, 2301–2312. [Google Scholar] [CrossRef]
- Beers, J.; Gulbranson, D.R.; George, N.; Siniscalchi, L.I.; Jones, J.; Thomson, J.A.; Chen, G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 2012, 7, 2029–2040. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human ipsc derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Walsh, P.; Clarke, D.L.; Rowley, J.A.; Fellner, T. Scalable passaging of adherent human pluripotent stem cells. PLoS ONE 2014, 9, e88012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Li, Y.; Poh, Y.C.; Yokohama-Tamaki, T.; Wang, N.; Tanaka, T.S. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE 2010, 5, e15655. [Google Scholar] [CrossRef]
- Chowdhury, F.; Na, S.; Li, D.; Poh, Y.C.; Tanaka, T.S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010, 9, 82–88. [Google Scholar] [CrossRef]
- Higuchi, S.; Watanabe, T.M.; Kawauchi, K.; Ichimura, T.; Fujita, H. Culturing of mouse and human cells on soft substrates promote the expression of stem cell markers. J. Biosci. Bioeng. 2014, 117, 749–755. [Google Scholar] [CrossRef]
- McKee, C.; Hong, Y.; Yao, D.; Chaudhry, G.R. Compression induced chondrogenic differentiation of embryonic stem cells in three-dimensional polydimethylsiloxane scaffolds. Tissue Eng. Part A 2017, 23, 426–435. [Google Scholar] [CrossRef]
- McKee, C.; Beeravolu, N.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Mesenchymal stem cells transplanted with self-assembling scaffolds differentiated to regenerate nucleus pulposus in an ex vivo model of degenerative disc disease. Appl. Mater. Today 2019. [Google Scholar] [CrossRef]
- Keung, A.J.; Asuri, P.; Kumar, S.; Schaffer, D.V. Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells. Integr. Biol. 2012, 4, 1049–1058. [Google Scholar] [CrossRef]
- Sun, Y.; Villa-Diaz, L.G.; Lam, R.H.W.; Chen, W.; Krebsbach, P.H.; Fu, J. Mechanics regulates fate decisions of human embryonic stem cells. PLoS ONE 2012, 7, e37178. [Google Scholar] [CrossRef]
- Jang, M.; Lee, S.T.; Kim, J.W.; Yang, J.H.; Yoon, J.K.; Park, J.C.; Ryoo, H.M.; van der Vlies, A.J.; Ahn, J.Y.; Hubbell, J.A.; et al. A feeder-free, defined three-dimensional polyethylene glycol-based extracellular matrix niche for culture of human embryonic stem cells. Biomaterials 2013, 34, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.G.; Robey, P.G. Human pluripotent stem cell culture: Considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Gerecht, S.; Burdick, J.A.; Ferreira, L.S.; Townsend, S.A.; Langer, R.; Vunjak-Novakovic, G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11298–11303. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Leung, M.; Hopper, R.; Ellenbogen, R.; Zhang, M. Feeder-free self-renewal of human embryonic stem cells in 3d porous natural polymer scaffolds. Biomaterials 2010, 31, 404–412. [Google Scholar] [CrossRef]
- Lei, Y.; Schaffer, D.V. A fully defined and scalable 3d culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, E5039–E5048. [Google Scholar] [CrossRef]
- Wei, J.; Han, J.; Zhao, Y.; Cui, Y.; Wang, B.; Xiao, Z.; Chen, B.; Dai, J. The importance of three-dimensional scaffold structure on stemness maintenance of mouse embryonic stem cells. Biomaterials 2014, 35, 7724–7733. [Google Scholar] [CrossRef]
- Li, X.; Ma, R.; Gu, Q.; Liang, L.; Wang, L.; Zhang, Y.; Wang, X.; Liu, X.; Li, Z.; Fang, J.; et al. A fully defined static suspension culture system for large-scale human embryonic stem cell production. Cell Death Dis. 2018, 9, 892. [Google Scholar] [CrossRef]
- Collier, A.J.; Panula, S.P.; Schell, J.P.; Chovanec, P.; Plaza Reyes, A.; Petropoulos, S.; Corcoran, A.E.; Walker, R.; Douagi, I.; Lanner, F.; et al. Comprehensive cell surface protein profiling identifies specific markers of human naive and primed pluripotent states. Cell Stem Cell 2017, 20, 874–890. [Google Scholar] [CrossRef]
- Rostovskaya, M.; Stirparo, G.G.; Smith, A. Capacitation of human naïve pluripotent stem cells for multi-lineage differentiation. Development 2019, 146, dev172916. [Google Scholar] [CrossRef]
- Guo, G.; von Meyenn, F.; Santos, F.; Chen, Y.; Reik, W.; Bertone, P.; Smith, A.; Nichols, J. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Rep. 2016, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Cheng, A.W.; Saha, K.; Kim, J.; Lengner, C.J.; Soldner, F.; Cassady, J.P.; Muffat, J.; Carey, B.W.; Jaenisch, R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse escs. Proc. Natl. Acad. Sci. USA 2010, 107, 9222–9227. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Sasaki, H. Epiblast formation by tead-yap-dependent expression of pluripotency factors and competitive elimination of unspecified cells. Dev. Cell 2019, 50, 139–154. [Google Scholar] [CrossRef]
- Kilens, S.; Meistermann, D.; Moreno, D.; Chariau, C.; Gaignerie, A.; Reignier, A.; Lelièvre, Y.; Casanova, M.; Vallot, C.; Nedellec, S.; et al. Parallel derivation of isogenic human primed and naive induced pluripotent stem cells. Nat. Commun. 2018, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Guo, G.; Loos, R.; Nichols, J.; Ficz, G.; Krueger, F.; Oxley, D.; Santos, F.; Clarke, J.; Mansfield, W.; et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 2014, 158, 1254–1269. [Google Scholar] [CrossRef]
- Sim, Y.J.; Kim, M.S.; Nayfeh, A.; Yun, Y.J.; Kim, S.J.; Park, K.T.; Kim, C.H.; Kim, K.S. 2i maintains a naive ground state in escs through two distinct epigenetic mechanisms. Stem Cell Rep. 2017, 8, 1312–1328. [Google Scholar] [CrossRef]
- Van der Jeught, M.; Taelman, J.; Duggal, G.; Ghimire, S.; Lierman, S.; Chuva de Sousa Lopes, S.M.; Deforce, D.; Deroo, T.; De Sutter, P.; Heindryckx, B. Application of small molecules favoring naive pluripotency during human embryonic stem cell derivation. Cell. Reprogramming 2015, 17, 170–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, K.; Li, C.; Lai, A.C.K.; Foo, J.J.; Chan, V. Progress in integrative biomaterial systems to approach three-dimensional cell mechanotransduction. Bioengineering 2017, 4, 72. [Google Scholar] [CrossRef]
- Finch-Edmondson, M.; Sudol, M. Framework to function: Mechanosensitive regulators of gene transcription. Cell. Mol. Biol. Lett. 2016, 21, 28. [Google Scholar] [CrossRef]
- Baxter, M.A.; Camarasa, M.V.; Bates, N.; Small, F.; Murray, P.; Edgar, D.; Kimber, S.J. Analysis of the distinct functions of growth factors and tissue culture substrates necessary for the long-term self-renewal of human embryonic stem cell lines. Stem Cell Res. 2009, 3, 28–38. [Google Scholar] [CrossRef]
- Mei, Y.; Saha, K.; Bogatyrev, S.R.; Yang, J.; Hook, A.L.; Kalcioglu, Z.I.; Cho, S.W.; Mitalipova, M.; Pyzocha, N.; Rojas, F.; et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010, 9, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Vitillo, L.; Kimber, S.J. Integrin and fak regulation of human pluripotent stem cells. Curr. Stem Cell Rep. 2017, 3, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Yun, J.I.; Jo, Y.S.; Mochizuki, M.; van der Vlies, A.J.; Kontos, S.; Ihm, J.E.; Lim, J.M.; Hubbell, J.A. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials 2010, 31, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the hippo-yap pathway by g-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef]
- Lam, R.M.; Chesler, A.T. Shear elegance: A novel screen uncovers a mechanosensitive gpcr. J. Gen. Physiol. 2018, 150, 907. [Google Scholar] [CrossRef]
- Varelas, X. The hippo pathway effectors taz and yap in development, homeostasis and disease. Development 2014, 141, 1614–1626. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.L.; et al. Force triggers yap nuclear entry by regulating transport across nuclear pores. Cell 2017, 171, 1397–1410. [Google Scholar] [CrossRef]
- Sasaki, H. Roles and regulations of hippo signaling during preimplantation mouse development. Dev. Growth Differ. 2017, 59, 12–20. [Google Scholar] [CrossRef]
- Ohgushi, M.; Minaguchi, M.; Sasai, Y. Rho-signaling-directed yap/taz activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell 2015, 17, 448–461. [Google Scholar] [CrossRef]
- Hsiao, C.; Lampe, M.; Nillasithanukroh, S.; Han, W.; Lian, X.; Palecek, S.P. Human pluripotent stem cell culture density modulates yap signaling. Biotechnol. J. 2016, 11, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Varelas, X.; Sakuma, R.; Samavarchi-Tehrani, P.; Peerani, R.; Rao, B.M.; Dembowy, J.; Yaffe, M.B.; Zandstra, P.W.; Wrana, J.L. Taz controls smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 2008, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.; Abujarour, R.; et al. The role of yap transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Papaspyropoulos, A.; Bradley, L.; Thapa, A.; Leung, C.Y.; Toskas, K.; Koennig, D.; Pefani, D.E.; Raso, C.; Grou, C.; Hamilton, G.; et al. Rassf1a uncouples wnt from hippo signalling and promotes yap mediated differentiation via p73. Nat. Commun. 2018, 9, 424. [Google Scholar] [CrossRef]
- Young, R.A. Control of the embryonic stem cell state. Cell 2011, 144, 940–954. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Feng, W.; Yu, Y.; Jeong, K.; Guo, W.; Lu, Y.; Mills, G.B. Verteporfin inhibits yap function through up-regulating 14-3-3σ sequestering yap in the cytoplasm. Am. J. Cancer Res. 2015, 6, 27–37. [Google Scholar]
- Onishi, K.; Zandstra, P.W. Lif signaling in stem cells and development. Development 2015, 142, 2230. [Google Scholar] [CrossRef]
- Daheron, L.; Opitz, S.L.; Zaehres, H.; Lensch, M.W.; Andrews, P.W.; Itskovitz-Eldor, J.; Daley, G.Q. Lif/stat3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 2004, 22, 770–778. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | ||
|---|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | Product Length | |
| ACTIN | AATCTGGCACCACACCTTCTAC | ATAGCACAGCCTGGATAGCAAC | 170 |
| BRACHYURY | TGCTTCCCTGAGACCCAGTT | GATCACTTCTTTCCTTTGCATCAAG | 121 |
| DNMT3L | CTGCTCCATCTGCTGCTCC | ATCCACACACTCGAAGCAGT | 85 |
| DPPA3 | AGACCAACAAACAAGGAGCCT | CCCATCCATTAGACACGCAGA | 88 |
| ESRRB | GACATTGCCTCTGGCTACCA | CTCCGTTTGGTGATCTCGCA | 131 |
| FGF4 | CGTGGTGAGCATCTTCGGC | GTAGGACTCGTAGGCGTTGT | 145 |
| FOXA2 | GGGAGCGGTGAAGATGGA | TCATGTTGCTCACGGAGGAGTA | 89 |
| FOXF1 | AAGCCGCCCTATTCCTACATC | GCGCTTGGTGGGTGAACT | 63 |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC | 101 |
| GATA4 | TCCCTCTTCCCTCCTCAAAT | TCAGCGTGTAAAGGCATCTG | 194 |
| GATA6 | CCCACAACACAACCTACAGC | GCGAGACTGACGCCTATGTA | 131 |
| GDF3 | GTCTCCCGAGACTTATGCTACG | AGTAGAGGAGCTTCTGCAGGCA | 136 |
| GP130 | GGAGTGAAGAAGCAAGTGGGA | AGGCAATGTCTTCCACACGA | 128 |
| HMBS | AGGAGTTCAGTGCCATCATCCT | CACAGCATACATGCATTCCTCA | 104 |
| ITGA2 | TTGCGTGTGGACATCAGTCT | GCTGGTATTTGTCGGACATCT | 158 |
| ITGA5 | GCCGATTCACATCGCTCTCAA | GTCTTCTCCACAGTCCAGCAA | 139 |
| ITGA6 | CGAAACCAAGGTTCTGAGCCC | CTTGGATCTCCACTGAGGCAG | 151 |
| ITGAV | AGGAGAAGGTGCCTACGAAGC | GCACAGGAAAGTCTTGCTAAGG | 105 |
| ITGB1 | GGATTCTCCAGAAGGTGGTTT | TGCCACCAAGTTTCCCATCT | 143 |
| ITGB3 | CATGGATTCCAGCAATGTCCTC | TTGAGGCAGGTGGCATTGAAG | 126 |
| ITGB5 | GCCTTTCTGTGAGTGCGACAA | CCGATGTAACCTGCATGGCAC | 111 |
| KLF17 | TCAGGAAGGGACTGGTAGAA | GTACCCGCATATGTCGTCTAAG | 206 |
| KLF2 | CCAAGAGTTCGCATCTGAAGG | CCGTGTGCTTTCGGTAGTG | 132 |
| KLF4 | CGAACCCACACAGGTGAGAA | TACGGTAGTGCCTGGTCAGTTC | 75 |
| KLF5 | ACCCTGGTTGCACAAAAGTT | CAGCCTTCCCAGGTACACTT | 100 |
| LATS1 | CTCTGCACTGGCTTCAGATG | TCCGCTCTAATGGCTTCAGT | 145 |
| LATS2 | ACATTCACTGGTGGGGACTC | GTGGGAGTAGGTGCCAAAAA | 147 |
| LIFR | CACCTTCCAAAATAGCGAGTATGG | ATGGTTCCGACCGAGACGAGTT | 159 |
| MIXL1 | CCGAGTCCAGGATCCAGGTA | CTCTGACGCCGAGACTTGG | 58 |
| NANOG | AAAGAATCTTCACCTATGCC | GAAGGAAGAGGAGAGACAGT | 110 |
| N-CADHERIN | TGTTTGGCCTGGCGTTCTTT | AGGAGACAGAAACGAAGCCA | 156 |
| NCAM | AGGAGACAGAAACGAAGCCA | GGTGTTGGAAATGCTCTGGT | 161 |
| OCT4 | CCCCTGGTGCCGTGAA | GCAAATTGCTCGAGTTCTTTCTG | 97 |
| PAX6 | CTTTGCTTGGGAAATCCGAG | AGCCAGGTTGCGAAGAACTC | 103 |
| PRDM14 | CCTTGTGTGGTATGGAGACTGC | CTTTCACATCTGTAGCCTTCTGC | 126 |
| RAC1 | ATGTCCGTGCAAAGTGGTATC | CTCGGATCGCTTCGTCAAACA | 249 |
| RHOA | CATCCGGAAGAAACTGGT | TCCCACAAAGCCAACTC | 168 |
| ROCK1 | GGTGGTCGGTTGGGGTATTTT | CGCCCTAACCTCACTTCCC | 196 |
| SALL2 | GGCTTGCCTTATGGTATGTCCG | TGGCACTGAGTGCTGTTGTGGA | 115 |
| SOCS3 | ATTCGGGACCAGCCCCC | AAACTTGCTGTGGGTGACCA | 121 |
| SOX11 | CGACGACCTAATGTTCGACC | GACAGGGATAGGTTCCCCG | 105 |
| SOX17 | CGCACGGAATTTGAACAGTA | GGATCAGGGACCTGTCACAC | 182 |
| SOX2 | TTGCTGCCTCTTTAAGACTAGGA | CTGGGGCTCAAACTTCTCTC | 75 |
| SOX7 | ACGCCGAGCTCAGCAAGAT | TCCACGTACGGCCTCTTCTG | 73 |
| STAT3 | CTTTGAGACCGAGGTGTATCAC | GGTCAGCATGTTGTACCACAG | 133 |
| TAZ | GAGGGTGTATGGTGGAGATAAA | CCAACTGTAGCAAACAGGATTAG | 86 |
| TBX3 | GGACACTGGAAATGGCCGAAG | GCTGCTTGTTCACTGGAGGAC | 123 |
| TBX3 | CGGACATACTTGTTCCCCGA | GCAGGGTGAGCTGTTTTCTTTT | 154 |
| TEAD4 | CCAAGCTCTGGATGTTGGAGTTC | GATGTCCACGGCTTCGAGGTA | 161 |
| TFCP2L1 | TTTGTGGGACCCTGCGAAG | TGCTTAAACGTGTCAATCTGGA | 129 |
| TUJ1 | GGCCAAGTTCTGGGAAGTCA | CGAGTCGCCCACGTAGTTG | 70 |
| YAP | GCTGCCACCAAGCTAGATAA | GTGCATGTGTCTCCTTAGATCC | 101 |
| ZFP42 | CGCAATCGCTTGTCCTCAGA | GCTCTCAACGAACGCTTTCC | 130 |
| ZIC2 | CGCTCCGAGAACCTCAAGAT | CCCTCAAACTCACACTGGAA | 71 |
| Antibody | Primary | Secondary |
|---|---|---|
| BRACHYURY | Rabbit Polyclonal | Anti-Rabbit Alexa Fluor 488 |
| GATA4 | Mouse Polyclonal | Anti-Mouse-Cy3 |
| NANOG | Rabbit Polyclonal | Anti-Rabbit Alexa Fluor 488 |
| OCT4 | Rabbit Polyclonal | Anti-RabbitAlexa Fluor 488 |
| SOX2 | Rabbit polyclonal | Anti-Mouse Alexa Fluor 488 |
| TUJ1 | Mouse Polyclonal | Anti-Mouse-Cy3 |
| YAP | Rabbit Polyclonal | Anti-Rabbit Alexa Fluor 488 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKee, C.; Brown, C.; Chaudhry, G.R. Self-Assembling Scaffolds Supported Long-Term Growth of Human Primed Embryonic Stem Cells and Upregulated Core and Naïve Pluripotent Markers. Cells 2019, 8, 1650. https://doi.org/10.3390/cells8121650
McKee C, Brown C, Chaudhry GR. Self-Assembling Scaffolds Supported Long-Term Growth of Human Primed Embryonic Stem Cells and Upregulated Core and Naïve Pluripotent Markers. Cells. 2019; 8(12):1650. https://doi.org/10.3390/cells8121650
Chicago/Turabian StyleMcKee, Christina, Christina Brown, and G. Rasul Chaudhry. 2019. "Self-Assembling Scaffolds Supported Long-Term Growth of Human Primed Embryonic Stem Cells and Upregulated Core and Naïve Pluripotent Markers" Cells 8, no. 12: 1650. https://doi.org/10.3390/cells8121650
APA StyleMcKee, C., Brown, C., & Chaudhry, G. R. (2019). Self-Assembling Scaffolds Supported Long-Term Growth of Human Primed Embryonic Stem Cells and Upregulated Core and Naïve Pluripotent Markers. Cells, 8(12), 1650. https://doi.org/10.3390/cells8121650





