Non-Coding RNAs in IGF-1R Signaling Regulation: The Underlying Pathophysiological Link between Diabetes and Cancer
Abstract
1. Introduction
2. Role of IGF-1R Signaling in the Pathogenesis of DM and Cancer
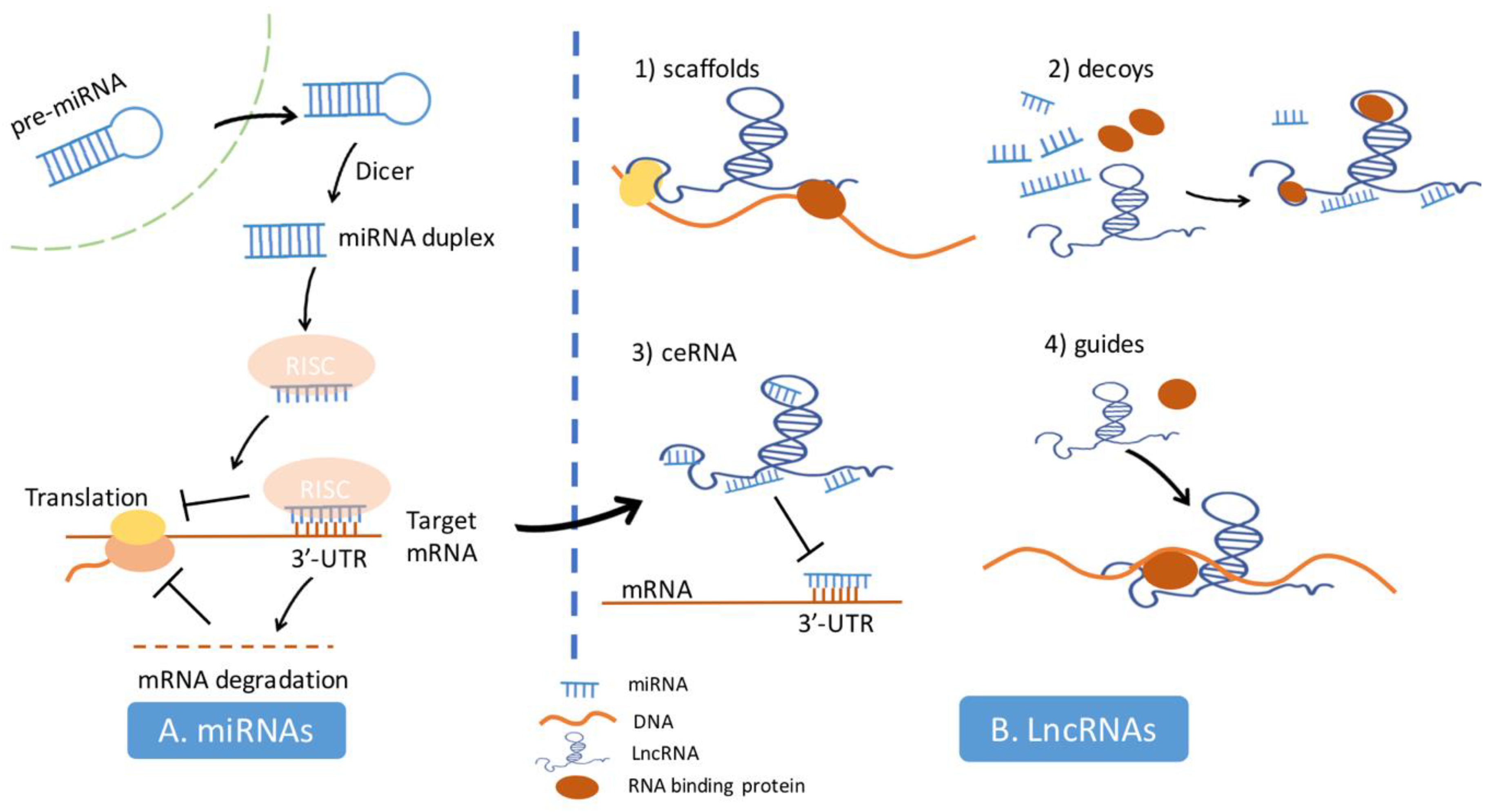
3. The Mechanism of miRNAs and lncRNAs in the Regulation of Cell Biology Processes
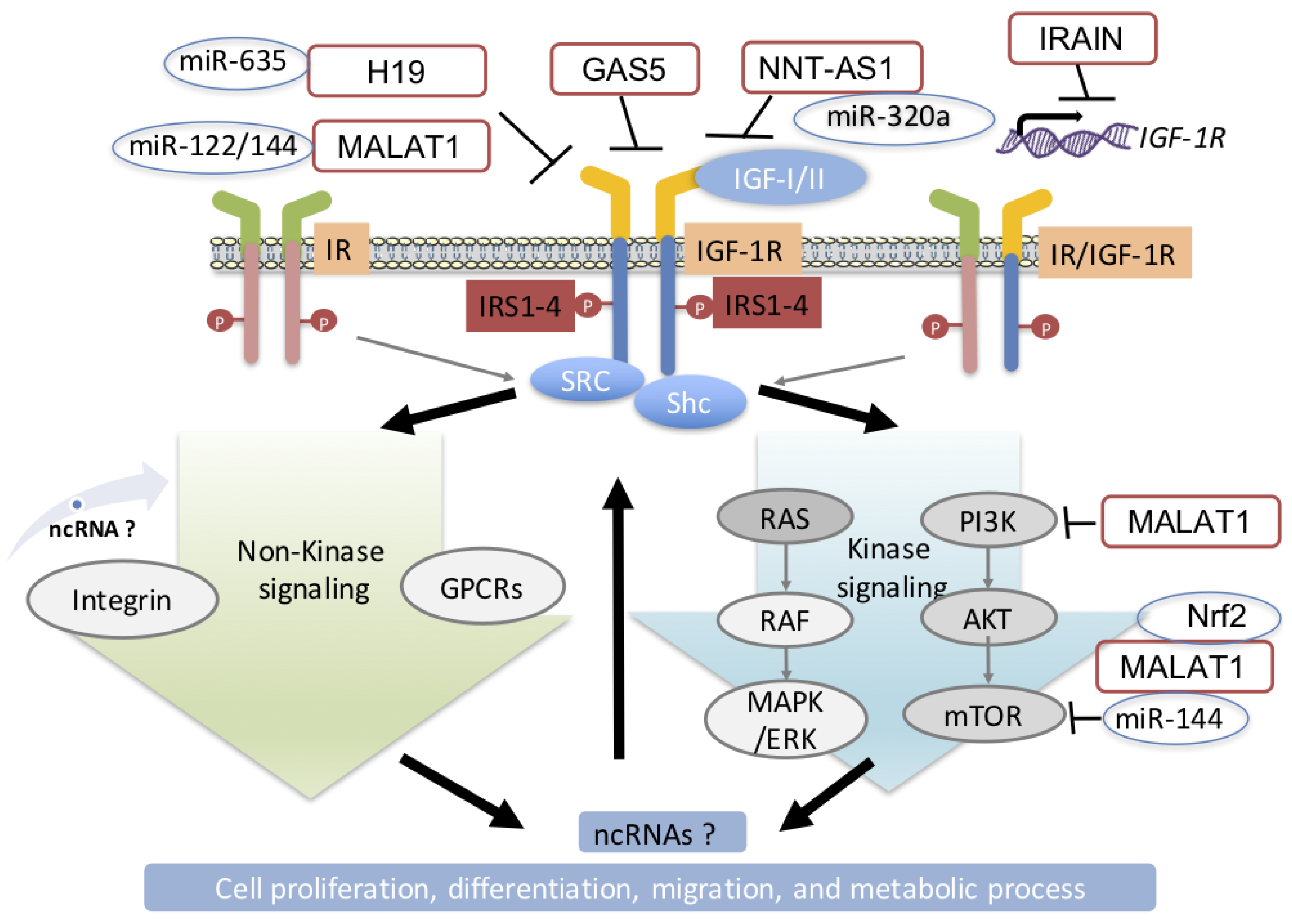
4. Modulation of IGF-1R in Diabetes and Cancer by miRNAs
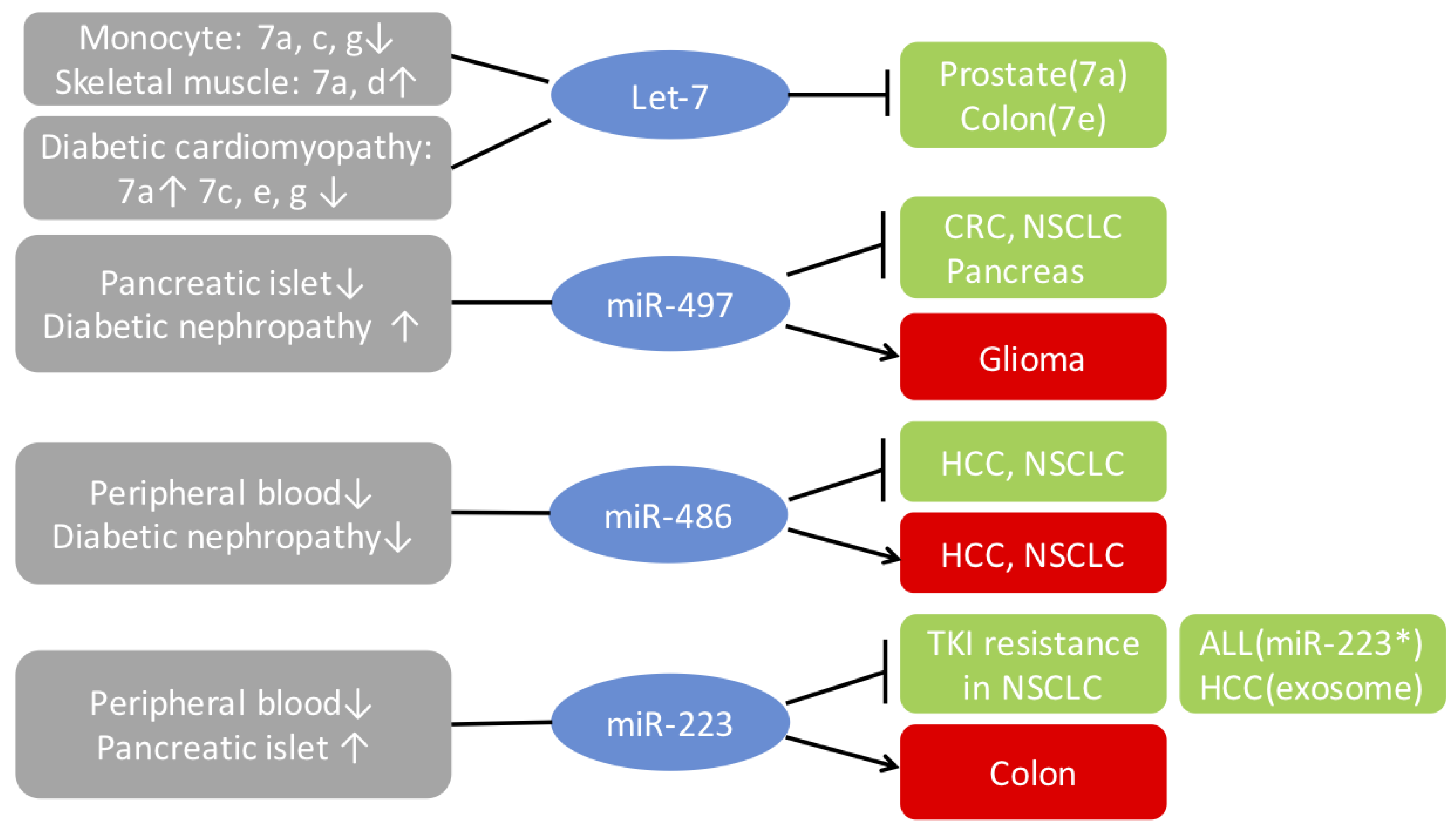
4.1. Let-7 Family
4.2. MiR-497
4.3. MiR-486
4.4. MiR-223
5. Regulation of Insulin/IGF Signaling by lncRNA in DM and Cancer
5.1. MALAT1
5.2. GAS5
5.3. IRAIN
5.4. Other lncRNAs that Regulate the IGF-1R
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ben, Q.; Cai, Q.; Li, Z.; Yuan, Y.; Ning, X.; Deng, S.; Wang, K. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: A case-control study. Eur. J. Cancer. 2011, 47, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Solomon, C.G.; Hu, F.B.; Rosner, B.A.; Hankinson, S.E.; Colditz, G.A.; Manson, J.E.; Nurses’ Health, S. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses’ Health Study. Diabetes Care 2003, 26, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Mantzoros, C.S.; Wolk, A. Diabetes and risk of endometrial cancer: A population-based prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Deka, A.; Jacobs, E.J.; Newton, C.C.; Hildebrand, J.S.; McCullough, M.L.; Limburg, P.J.; Gapstur, S.M. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 2010, 139, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int. J. Cancer 2007, 121, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 2007, 50, 1365–1374. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef]
- Everhart, J.; Wright, D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995, 273, 1605–1609. [Google Scholar] [CrossRef]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- Bansal, D.; Bhansali, A.; Kapil, G.; Undela, K.; Tiwari, P. Type 2 diabetes and risk of prostate cancer: A meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013, 16, 151–158. [Google Scholar] [CrossRef]
- Ding, J.; Li, C.; Tang, J.; Yi, C.; Liu, J.Y.; Qiu, M. Higher Expression of Proteins in IGF/IR Axes in Colorectal Cancer is Associated with Type 2 Diabetes Mellitus. Pathol. Oncol. Res. 2016, 22, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Kohlgruber, A.; Lynch, L. Adipose tissue inflammation in the pathogenesis of type 2 diabetes. Curr. Diab. Rep. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Girnita, L.; Varani, G.; Calin, G.A. Decrypting noncoding RNA interactions, structures, and functional networks. Genome Res. 2019, 29, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Cimmino, A.; Fabbri, M.; Ferracin, M.; Wojcik, S.E.; Shimizu, M.; Taccioli, C.; Zanesi, N.; Garzon, R.; Aqeilan, R.I.; et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 5166–5171. [Google Scholar] [CrossRef]
- Calin, G.A.; Trapasso, F.; Shimizu, M.; Dumitru, C.D.; Yendamuri, S.; Godwin, A.K.; Ferracin, M.; Bernardi, G.; Chatterjee, D.; Baldassarre, G.; et al. Familial cancer associated with a polymorphism in ARLTS1. N. Engl. J. Med. 2005, 352, 1667–1676. [Google Scholar] [CrossRef]
- Fabbri, M.; Garzon, R.; Andreeff, M.; Kantarjian, H.M.; Garcia-Manero, G.; Calin, G.A. MicroRNAs and noncoding RNAs in hematological malignancies: Molecular, clinical and therapeutic implications. Leukemia 2008, 22, 1095–1105. [Google Scholar] [CrossRef]
- Sevignani, C.; Calin, G.A.; Nnadi, S.C.; Shimizu, M.; Davuluri, R.V.; Hyslop, T.; Demant, P.; Croce, C.M.; Siracusa, L.D. MicroRNA genes are frequently located near mouse cancer susceptibility loci. Proc. Natl. Acad. Sci. USA 2007, 104, 8017–8022. [Google Scholar] [CrossRef]
- Shah, M.Y.; Ferrajoli, A.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. microRNA Therapeutics in Cancer—An Emerging Concept. EBioMedicine 2016, 12, 34–42. [Google Scholar] [CrossRef]
- Smolle, M.A.; Calin, H.N.; Pichler, M.; Calin, G.A. Noncoding RNAs and immune checkpoints-clinical implications as cancer therapeutics. FEBS J. 2017, 284, 1952–1966. [Google Scholar] [CrossRef]
- Redis, R.S.; Calin, G.A. SnapShot: Non-coding RNAs and Metabolism. Cell Metab. 2017, 25, 220–220.e1. [Google Scholar] [CrossRef]
- Redis, R.S.; Vela, L.E.; Lu, W.; Ferreira de Oliveira, J.; Ivan, C.; Rodriguez-Aguayo, C.; Adamoski, D.; Pasculli, B.; Taguchi, A.; Chen, Y.; et al. Allele-Specific Reprogramming of Cancer Metabolism by the Long Non-coding RNA CCAT2. Mol. Cell 2016, 61, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, M.M.; Yuen, J.S.; Protheroe, A.S.; Pollak, M.; Macaulay, V.M. The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res. 2008, 14, 6364–6370. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009, 30, 586–623. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef] [PubMed]
- Girnita, A.; Zheng, H.; Gronberg, A.; Girnita, L.; Stahle, M. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene 2012, 31, 352–365. [Google Scholar] [CrossRef]
- Girnita, L.; Worrall, C.; Takahashi, S.; Seregard, S.; Girnita, A. Something old, something new and something borrowed: Emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol. Life Sci. 2014, 71, 2403–2427. [Google Scholar] [CrossRef]
- Yoshihara, H.; Fukushima, T.; Hakuno, F.; Saeki, Y.; Tanaka, K.; Ito, A.; Yoshida, M.; Iemura, S.; Natsume, T.; Asano, T.; et al. Insulin/insulin-like growth factor (IGF) stimulation abrogates an association between a deubiquitinating enzyme USP7 and insulin receptor substrates (IRSs) followed by proteasomal degradation of IRSs. Biochem. Biophys. Res. Commun. 2012, 423, 122–127. [Google Scholar] [CrossRef]
- Ijuin, T.; Takenawa, T. Regulation of insulin signaling and glucose transporter 4 (GLUT4) exocytosis by phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, skeletal muscle, and kidney enriched inositol polyphosphate phosphatase (SKIP). J. Biol. Chem. 2012, 287, 6991–6999. [Google Scholar] [CrossRef]
- Crudden, C.; Ilic, M.; Suleymanova, N.; Worrall, C.; Girnita, A.; Girnita, L. The dichotomy of the Insulin-like growth factor 1 receptor: RTK and GPCR: Friend or foe for cancer treatment? Growth Horm. IGF Res. 2015, 25, 2–12. [Google Scholar] [CrossRef]
- Crudden, C.; Shibano, T.; Song, D.; Suleymanova, N.; Girnita, A.; Girnita, L. Blurring Boundaries: Receptor Tyrosine Kinases as functional G Protein-Coupled Receptors. Int. Rev. Cell Mol. Biol. 2018, 339, 1–40. [Google Scholar] [CrossRef]
- Economou, M.A.; Wu, J.; Vasilcanu, D.; Rosengren, L.; All-Ericsson, C.; van der Ploeg, I.; Menu, E.; Girnita, L.; Axelson, M.; Larsson, O.; et al. Inhibition of VEGF secretion and experimental choroidal neovascularization by picropodophyllin (PPP), an inhibitor of the insulin-like growth factor-1 receptor. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Vasilcanu, R.; Vasilcanu, D.; Sehat, B.; Yin, S.; Girnita, A.; Axelson, M.; Girnita, L. Insulin-like growth factor type-I receptor-dependent phosphorylation of extracellular signal-regulated kinase 1/2 but not Akt (protein kinase B) can be induced by picropodophyllin. Mol. Pharmacol. 2008, 73, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Worrall, C.; Suleymanova, N.; Crudden, C.; Trocoli Drakensjo, I.; Candrea, E.; Nedelcu, D.; Takahashi, S.I.; Girnita, L.; Girnita, A. Unbalancing p53/Mdm2/IGF-1R axis by Mdm2 activation restrains the IGF-1-dependent invasive phenotype of skin melanoma. Oncogene 2017, 36, 3274–3286. [Google Scholar] [CrossRef]
- Girnita, L.; Takahashi, S.I.; Crudden, C.; Fukushima, T.; Worrall, C.; Furuta, H.; Yoshihara, H.; Hakuno, F.; Girnita, A. Chapter Seven—When Phosphorylation Encounters Ubiquitination: A Balanced Perspective on IGF-1R Signaling. Prog. Mol. Biol. Transl. Sci. 2016, 141, 277–311. [Google Scholar] [CrossRef]
- Suleymanova, N.; Crudden, C.; Shibano, T.; Worrall, C.; Oprea, I.; Tica, A.; Calin, G.A.; Girnita, A.; Girnita, L. Functional antagonism of beta-arrestin isoforms balance IGF-1R expression and signalling with distinct cancer-related biological outcomes. Oncogene 2017, 36, 5734–5744. [Google Scholar] [CrossRef]
- Garg, N.; Thakur, S.; McMahan, C.A.; Adamo, M.L. High fat diet induced insulin resistance and glucose intolerance are gender-specific in IGF-1R heterozygous mice. Biochem. Biophys. Res. Commun. 2011, 413, 476–480. [Google Scholar] [CrossRef]
- Xuan, S.; Kitamura, T.; Nakae, J.; Politi, K.; Kido, Y.; Fisher, P.E.; Morroni, M.; Cinti, S.; White, M.F.; Herrera, P.L.; et al. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J. Clin. Investig. 2002, 110, 1011–1019. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, S.; Meng, H. Role of the Insulin-Like Growth Factor Type 1 Receptor in the Pathogenesis of Diabetic Encephalopathy. Int. J. Endocrinol. 2015, 2015, 626019. [Google Scholar] [CrossRef]
- Spampinato, D.; Pandini, G.; Iuppa, A.; Trischitta, V.; Vigneri, R.; Frittitta, L. Insulin/insulin-like growth factor I hybrid receptors overexpression is not an early defect in insulin-resistant subjects. J. Clin. Endocrinol. Metab. 2000, 85, 4219–4223. [Google Scholar] [CrossRef][Green Version]
- Boni-Schnetzler, M.; Schmid, C.; Meier, P.J.; Froesch, E.R. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am. J. Physiol. 1991, 260, E846–E851. [Google Scholar] [CrossRef] [PubMed]
- Holly, J.M.P.; Biernacka, K.; Perks, C.M. The Neglected Insulin: IGF-II, a Metabolic Regulator with Implications for Diabetes, Obesity, and Cancer. Cells 2019, 8, 1207. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, R.; Kanety, H.; Papa, M.Z.; Lunenfeld, B.; Karasik, A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 1993, 268, 26055–26058. [Google Scholar] [PubMed]
- Mitsiades, C.S.; Mitsiades, N.; Koutsilieris, M. The Akt pathway: Molecular targets for anti-cancer drug development. Curr. Cancer Drug Targets 2004, 4, 235–256. [Google Scholar] [CrossRef]
- Li, S.; Pinard, M.; Wang, Y.; Yang, L.; Lin, R.; Hiscott, J.; Su, B.; Brodt, P. Crosstalk between the TNF and IGF pathways enhances NF-kappaB activation and signaling in cancer cells. Growth Horm IGF Res. 2015, 25, 253–261. [Google Scholar] [CrossRef]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; Macdonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004, 432, 226–230. [Google Scholar] [CrossRef]
- Zhu, H.; Leung, S.W. Identification of microRNA biomarkers in type 2 diabetes: A meta-analysis of controlled profiling studies. Diabetologia 2015, 58, 900–911. [Google Scholar] [CrossRef]
- Panda, A.C.; Grammatikakis, I.; Yoon, J.H.; Abdelmohsen, K. Posttranscriptional regulation of insulin family ligands and receptors. Int. J. Mol. Sci. 2013, 14, 19202–19229. [Google Scholar] [CrossRef]
- Zhu, H.; Shyh-Chang, N.; Segre, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef]
- Frost, R.J.; Olson, E.N. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 21075–21080. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Shi, E.; Tan, Z.; Xiong, J.; Yan, L.; Jiang, X. Inhibition of the Let-7 Family MicroRNAs Induces Cardioprotection Against Ischemia-Reperfusion Injury in Diabetic Rats. Ann. Thorac. Surg. 2016, 102, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Chavali, V.; Tyagi, S.C.; Mishra, P.K. Differential expression of dicer, miRNAs, and inflammatory markers in diabetic Ins2+/− Akita hearts. Cell BioChem. Biophys. 2014, 68, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Luscher, T.F.; Cosentino, F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur. Heart J. 2016, 37, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Baldeon, R.L.; Weigelt, K.; de Wit, H.; Ozcan, B.; van Oudenaren, A.; Sempertegui, F.; Sijbrands, E.; Grosse, L.; van Zonneveld, A.J.; Drexhage, H.A.; et al. Type 2 Diabetes Monocyte MicroRNA and mRNA Expression: Dyslipidemia Associates with Increased Differentiation-Related Genes but Not Inflammatory Activation. PLoS ONE 2015, 10, e0129421. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective Effect of let-7 miRNA Family in Regulating Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 2017, 66, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Sjogren, R.J.; Egan, B.; Krook, A. miRNA let-7 expression is regulated by glucose and TNF-alpha by a remote upstream promoter. BioChem. J. 2015, 472, 147–156. [Google Scholar] [CrossRef]
- Chiu, S.C.; Chung, H.Y.; Cho, D.Y.; Chan, T.M.; Liu, M.C.; Huang, H.M.; Li, T.Y.; Lin, J.Y.; Chou, P.C.; Fu, R.H.; et al. Therapeutic potential of microRNA let-7: Tumor suppression or impeding normal stemness. Cell Transpl. 2014, 23, 459–469. [Google Scholar] [CrossRef]
- Shi, Z.M.; Wang, X.F.; Qian, X.; Tao, T.; Wang, L.; Chen, Q.D.; Wang, X.R.; Cao, L.; Wang, Y.Y.; Zhang, J.X.; et al. MiRNA-181b suppresses IGF-1R and functions as a tumor suppressor gene in gliomas. RNA 2013, 19, 552–560. [Google Scholar] [CrossRef]
- Li, Z.; Pan, W.; Shen, Y.; Chen, Z.; Zhang, L.; Zhang, Y.; Luo, Q.; Ying, X. IGF1/IGF1R and microRNA let-7e down-regulate each other and modulate proliferation and migration of colorectal cancer cells. Cell Cycle 2018, 17, 1212–1219. [Google Scholar] [CrossRef]
- Lang, H.; Xiang, Y.; Lin, N.; Ai, Z.; You, Z.; Xiao, J.; Liu, D.; Yang, Y. Identification of a Panel of MiRNAs as Positive Regulators of Insulin Release in Pancreatic Beta-Cells. Cell Physiol. BioChem. 2018, 48, 185–193. [Google Scholar] [CrossRef]
- Zeng, L.Q.; Wei, S.B.; Sun, Y.M.; Qin, W.Y.; Cheng, J.; Mitchelson, K.; Xie, L. Systematic profiling of mRNA and miRNA expression in the pancreatic islets of spontaneously diabetic Goto-Kakizaki rats. Mol. Med. Rep. 2015, 11, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Samandari, N.; Mirza, A.H.; Kaur, S.; Hougaard, P.; Nielsen, L.B.; Fredheim, S.; Mortensen, H.B.; Pociot, F. Influence of Disease Duration on Circulating Levels of miRNAs in Children and Adolescents with New Onset Type 1 Diabetes. Noncoding RNA 2018, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, S.; Xu, R.; Gao, S.; Yin, J. Melatonin Attenuates Endothelial-to-Mesenchymal Transition of Glomerular Endothelial Cells via Regulating miR-497/ROCK in Diabetic Nephropathy. Kidney Blood Press. Res. 2018, 43, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Fang, W.T.; Mao, T.; Gu, Z.T.; Yang, Y.; Chen, W.H. Recurrence pattern of histological node-negative squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with lymphadenectomy. Zhonghua Wai Ke Za Zhi 2013, 51, 908–911. [Google Scholar] [PubMed]
- Xu, Y.; Chen, J.; Gao, C.; Zhu, D.; Xu, X.; Wu, C.; Jiang, J. MicroRNA-497 inhibits tumor growth through targeting insulin receptor substrate 1 in colorectal cancer. Oncol. Lett. 2017, 14, 6379–6386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, J.W.; Wang, T.X.; You, L.; Zheng, L.F.; Shu, H.; Zhang, T.P.; Zhao, Y.P. Insulin-like growth factor 1 receptor (IGF-1R) as a target of MiR-497 and plasma IGF-1R levels associated with TNM stage of pancreatic cancer. PLoS ONE 2014, 9, e92847. [Google Scholar] [CrossRef]
- Luo, M.; Shen, D.; Zhou, X.; Chen, X.; Wang, W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery 2013, 153, 836–847. [Google Scholar] [CrossRef]
- Ma, W.; Kang, Y.; Ning, L.; Tan, J.; Wang, H.; Ying, Y. Identification of microRNAs involved in gefitinib resistance of non-small-cell lung cancer through the insulin-like growth factor receptor 1 signaling pathway. Exp. Ther. Med. 2017, 14, 2853–2862. [Google Scholar] [CrossRef]
- Zhu, D.; Tu, M.; Zeng, B.; Cai, L.; Zheng, W.; Su, Z.; Yu, Z. Up-regulation of miR-497 confers resistance to temozolomide in human glioma cells by targeting mTOR/Bcl-2. Cancer Med. 2017, 6, 452–462. [Google Scholar] [CrossRef]
- Matsha, T.E.; Kengne, A.P.; Hector, S.; Mbu, D.L.; Yako, Y.Y.; Erasmus, R.T. MicroRNA profiling and their pathways in South African individuals with prediabetes and newly diagnosed type 2 diabetes mellitus. Oncotarget 2018, 9, 30485–30498. [Google Scholar] [CrossRef][Green Version]
- Flowers, E.; Gadgil, M.; Aouizerat, B.E.; Kanaya, A.M. Circulating micrornas associated with glycemic impairment and progression in Asian Indians. Biomark. Res. 2015, 3, 22. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Chen, H.; Si, H.; Li, X.; Ding, X.; Sheng, Q.; Chen, P.; Zhang, H. Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes. Acta Diabetol. 2014, 51, 823–831. [Google Scholar] [CrossRef]
- Regmi, A.; Liu, G.; Zhong, X.; Hu, S.; Ma, R.; Gou, L.; Zafar, M.I.; Chen, L. Evaluation of Serum microRNAs in Patients with Diabetic Kidney Disease: A Nested Case-Controlled Study and Bioinformatics Analysis. Med. Sci. Monit. 2019, 25, 1699–1708. [Google Scholar] [CrossRef]
- Jiang, M.; Li, X.; Quan, X.; Yang, X.; Zheng, C.; Hao, X.; Qu, R.; Zhou, B. MiR-486 as an effective biomarker in cancer diagnosis and prognosis: A systematic review and meta-analysis. Oncotarget 2018, 9, 13948–13958. [Google Scholar] [CrossRef]
- Gao, Z.J.; Yuan, W.D.; Yuan, J.Q.; Yuan, K.; Wang, Y. miR-486-5p functions as an oncogene by targeting PTEN in non-small cell lung cancer. Pathol. Res. Pract. 2018, 214, 700–705. [Google Scholar] [CrossRef]
- Wang, J.; Tian, X.; Han, R.; Zhang, X.; Wang, X.; Shen, H.; Xue, L.; Liu, Y.; Yan, X.; Shen, J.; et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene 2014, 33, 1181–1189. [Google Scholar] [CrossRef]
- Sun, H.; Cui, C.; Xiao, F.; Wang, H.; Xu, J.; Shi, X.; Yang, Y.; Zhang, Q.; Zheng, X.; Yang, X.; et al. miR-486 regulates metastasis and chemosensitivity in hepatocellular carcinoma by targeting CLDN10 and CITRON. Hepatol. Res. 2015, 45, 1312–1322. [Google Scholar] [CrossRef]
- Huang, X.P.; Hou, J.; Shen, X.Y.; Huang, C.Y.; Zhang, X.H.; Xie, Y.A.; Luo, X.L. MicroRNA-486-5p, which is downregulated in hepatocellular carcinoma, suppresses tumor growth by targeting PIK3R1. FEBS J. 2015, 282, 579–594. [Google Scholar] [CrossRef]
- Peng, Y.; Dai, Y.; Hitchcock, C.; Yang, X.; Kassis, E.S.; Liu, L.; Luo, Z.; Sun, H.L.; Cui, R.; Wei, H.; et al. Insulin growth factor signaling is regulated by microRNA-486, an underexpressed microRNA in lung cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 15043–15048. [Google Scholar] [CrossRef]
- Youness, R.A.; El-Tayebi, H.M.; Assal, R.A.; Hosny, K.; Esmat, G.; Abdelaziz, A.I. MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol. Lett. 2016, 12, 2567–2573. [Google Scholar] [CrossRef]
- Deiuliis, J.A.; Syed, R.; Duggineni, D.; Rutsky, J.; Rengasamy, P.; Zhang, J.; Huang, K.; Needleman, B.; Mikami, D.; Perry, K.; et al. Visceral Adipose MicroRNA 223 Is Upregulated in Human and Murine Obesity and Modulates the Inflammatory Phenotype of Macrophages. PLoS ONE 2016, 11, e0165962. [Google Scholar] [CrossRef]
- Li, Y.; Deng, S.; Peng, J.; Wang, X.; Essandoh, K.; Mu, X.; Peng, T.; Meng, Z.X.; Fan, G.C. MicroRNA-223 is essential for maintaining functional beta-cell mass during diabetes through inhibiting both FOXO1 and SOX6 pathways. J. Biol. Chem. 2019. [Google Scholar] [CrossRef]
- Lu, H.; Buchan, R.J.; Cook, S.A. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc. Res. 2010, 86, 410–420. [Google Scholar] [CrossRef]
- Liu, D.; Pan, J.; Zhao, D.; Liu, F. MicroRNA-223 inhibits deposition of the extracellular matrix by airway smooth muscle cells through targeting IGF-1R in the PI3K/Akt pathway. Am. J. Transl. Res. 2018, 10, 744–752. [Google Scholar]
- Zhao, F.Y.; Han, J.; Chen, X.W.; Wang, J.; Wang, X.D.; Sun, J.G.; Chen, Z.T. miR-223 enhances the sensitivity of non-small cell lung cancer cells to erlotinib by targeting the insulin-like growth factor-1 receptor. Int. J. Mol. Med. 2016, 38, 183–191. [Google Scholar] [CrossRef]
- Gusscott, S.; Kuchenbauer, F.; Humphries, R.K.; Weng, A.P. Notch-mediated repression of miR-223 contributes to IGF1R regulation in T-ALL. Leuk. Res. 2012, 36, 905–911. [Google Scholar] [CrossRef]
- Josse, C.; Bouznad, N.; Geurts, P.; Irrthum, A.; Huynh-Thu, V.A.; Servais, L.; Hego, A.; Delvenne, P.; Bours, V.; Oury, C. Identification of a microRNA landscape targeting the PI3K/Akt signaling pathway in inflammation-induced colorectal carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G229–G243. [Google Scholar] [CrossRef]
- Aucher, A.; Rudnicka, D.; Davis, D.M. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 2013, 191, 6250–6260. [Google Scholar] [CrossRef]
- Liu, W.; Kang, L.; Han, J.; Wang, Y.; Shen, C.; Yan, Z.; Tai, Y.; Zhao, C. miR-342-3p suppresses hepatocellular carcinoma proliferation through inhibition of IGF-1R-mediated Warburg effect. Onco. Targets Ther. 2018, 11, 1643–1653. [Google Scholar] [CrossRef]
- Png, K.J.; Halberg, N.; Yoshida, M.; Tavazoie, S.F. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 2011, 481, 190–194. [Google Scholar] [CrossRef]
- Li, Y.; Xu, K.; Xu, K.; Chen, S.; Cao, Y.; Zhan, H. Roles of Identified Long Noncoding RNA in Diabetic Nephropathy. J. Diabetes Res. 2019, 2019, 5383010. [Google Scholar] [CrossRef]
- Leti, F.; Morrison, E.; DiStefano, J.K. Long noncoding RNAs in the pathogenesis of diabetic kidney disease: Implications for novel therapeutic strategies. Per. Med. 2017, 14, 271–278. [Google Scholar] [CrossRef]
- Sun, X.; Wong, D. Long non-coding RNA-mediated regulation of glucose homeostasis and diabetes. Am. J. Cardiovasc. Dis. 2016, 6, 17–25. [Google Scholar]
- Wang, Y.; Hu, Y.; Sun, C.; Zhuo, S.; He, Z.; Wang, H.; Yan, M.; Liu, J.; Luan, Y.; Dai, C.; et al. Down-regulation of Risa improves insulin sensitivity by enhancing autophagy. FASEB J. 2016, 30, 3133–3145. [Google Scholar] [CrossRef]
- Ji, P.; Diederichs, S.W.; Boing, S.; Metzger, R.; Schneider, P.M.; Tidow, N.; Brandt, B.; Buerger, H.; Bulk, E.; Thomas, M. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003, 22, 8031. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Zhan, X.; Sun, L.; Gao, J.; Cao, Y.; Qiu, H. Construction of competitive endogenous RNA network reveals regulatory role of long non-coding RNAs in type 2 diabetes mellitus. J. Cell Mol. Med. 2017, 21, 3204–3213. [Google Scholar] [CrossRef]
- Chen, J.; Ke, S.; Zhong, L.; Wu, J.; Tseng, A.; Morpurgo, B.; Golovko, A.; Wang, G.; Cai, J.J.; Ma, X.; et al. Long noncoding RNA MALAT1 regulates generation of reactive oxygen species and the insulin responses in male mice. BioChem. Pharmacol. 2018, 152, 94–103. [Google Scholar] [CrossRef]
- Puthanveetil, P.; Chen, S.; Feng, B.; Gautam, A.; Chakrabarti, S. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell Mol. Med. 2015, 19, 1418–1425. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yao, J.; Li, X.M.; Song, Y.C.; Wang, X.Q.; Li, Y.J.; Yan, B.; Jiang, Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014, 5, e1506. [Google Scholar] [CrossRef]
- Carter, G.; Miladinovic, B.; Patel, A.A.; Deland, L.; Mastorides, S.; Patel, N.A. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015, 4, 102–107. [Google Scholar] [CrossRef]
- Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Hum. Genom. 2018, 12, 41. [Google Scholar] [CrossRef]
- Lin, H.; Xing, W.; Li, Y.; Xie, Y.; Tang, X.; Zhang, Q. Downregulation of serum long noncoding RNA GAS5 may contribute to insulin resistance in PCOS patients. Gynecol. Endocrinol. 2018, 34, 784–788. [Google Scholar] [CrossRef]
- Shi, X.; Sun, M.; Liu, H.; Yao, Y.; Kong, R.; Chen, F.; Song, Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol. Carcinog. 2015, 54, E1–E12. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Pickard, M., Vl; Farzaneh, F.; Williams, G. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009, 28, 195–208. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Meng, X.; Zhou, S.; Xiao, S.; Li, X.; Liu, S.; Yu, P. Long noncoding RNA GAS5 impairs the proliferation and invasion of endometrial carcinoma induced by high glucose via targeting miR-222-3p/p27. Am. J. Transl. Res. 2019, 11, 2413–2421. [Google Scholar]
- Cortot, A.B.; Repellin, C.E.; Takeshi, S.; Marzia, C.; Kreshnik, Z.; Dalia, E.; Christensen, J.G.; Kwok-Kin, W.; Gray, N.S.; Jänne, P.A. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013, 73, 834–843. [Google Scholar] [CrossRef]
- Amandine, H.; Marie, W.; Benoît, B.; Martine, A.; Corine, T.; Nathalie, R.; Sandrine, D.; Florence, D.F.; Denis, M.S.; Jacques, C. Insulin-like growth factor-1 receptor inhibition overcomes gefitinib resistance in mucinous lung adenocarcinoma. J. Pathol. 2011, 225, 83–95. [Google Scholar]
- Dong, S.; Qu, X.; Li, W.; Zhong, X.; Li, P.; Yang, S.; Chen, X.; Shao, M.; Zhang, L. The long non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyrosine kinase inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR via downregulation of the IGF-1R expression. J. Hematol. Oncol. 2015, 8, 43. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Sun, Y.; Yu, D.; Wen, X.; Wang, H.; Cui, J.; Wang, G.; Hoffman, A.R.; Hu, J.F. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014, 42, 9588–9601. [Google Scholar] [CrossRef]
- Kang, L.; Sun, J.; Wen, X.; Cui, J.; Wang, G.; Hoffman, A.R.; Hu, J.F.; Li, W. Aberrant allele-switch imprinting of a novel IGF1R intragenic antisense non-coding RNA in breast cancers. Eur. J. Cancer 2015, 51, 260–270. [Google Scholar] [CrossRef]
- Pian, L.; Wen, X.; Kang, L.; Li, Z.; Nie, Y.; Du, Z.; Yu, D.; Zhou, L.; Jia, L.; Chen, N.; et al. Targeting the IGF1R Pathway in Breast Cancer Using Antisense lncRNA-Mediated Promoter cis Competition. Mol. Ther. Nucleic Acids 2018, 12, 105–117. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Qiu, T.; Wang, Y.; Ricketts, D.M.; Qi, C. Upregulation of long non-coding RNA NNT-AS1 promotes osteosarcoma progression by inhibiting the tumor suppressive miR-320a. Cancer Biol. Ther. 2018. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, F.; Zhou, J.; Yan, L.; Jurczak, M.J.; Lee, H.Y.; Yang, L.; Mueller, M.; Zhou, X.B.; Dandolo, L.; et al. The H19/let-7 double-negative feedback loop contributes to glucose metabolism in muscle cells. Nucleic Acids Res. 2014, 42, 13799–13811. [Google Scholar] [CrossRef]
- Zhang, N.; Geng, T.; Wang, Z.; Zhang, R.; Cao, T.; Camporez, J.P.; Cai, S.Y.; Liu, Y.; Dandolo, L.; Shulman, G.I.; et al. Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Farzi-Molan, A.; Babashah, S.; Bakhshinejad, B.; Atashi, A.; Fakhr Taha, M. Down-regulation of the non-coding RNA H19 and its derived miR-675 is concomitant with up-regulation of insulin-like growth factor receptor type 1 during neural-like differentiation of human bone marrow mesenchymal stem cells. Cell Biol. Int. 2018, 42, 940–948. [Google Scholar] [CrossRef]
- Nigi, L.; Grieco, G.E.; Ventriglia, G.; Brusco, N.; Mancarella, F.; Formichi, C.; Dotta, F.; Sebastiani, G. MicroRNAs as Regulators of Insulin Signaling: Research Updates and Potential Therapeutic Perspectives in Type 2 Diabetes. Int. J. Mol. Sci. 2018, 19, 3705. [Google Scholar] [CrossRef]
- Deiuliis, J.A. MicroRNAs as regulators of metabolic disease: Pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. (Lond.) 2016, 40, 88–101. [Google Scholar] [CrossRef]
- Crudden, C.; Girnita, A.; Girnita, L. Targeting the IGF-1R: The Tale of the Tortoise and the Hare. Front. Endocrinol. (Lausanne) 2015, 6, 64. [Google Scholar] [CrossRef]
- Crudden, C.; Song, D.; Cismas, S.; Trocme, E.; Pasca, S.; Calin, G.A.; Girnita, A.; Girnita, L. Below the Surface: IGF-1R Therapeutic Targeting and Its Endocytic Journey. Cells 2019, 8, 1223. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Li, J.; Chi, D.; Sahnoune, I.; Calin, S.; Girnita, L.; Calin, G.A. Non-Coding RNAs in IGF-1R Signaling Regulation: The Underlying Pathophysiological Link between Diabetes and Cancer. Cells 2019, 8, 1638. https://doi.org/10.3390/cells8121638
Chen B, Li J, Chi D, Sahnoune I, Calin S, Girnita L, Calin GA. Non-Coding RNAs in IGF-1R Signaling Regulation: The Underlying Pathophysiological Link between Diabetes and Cancer. Cells. 2019; 8(12):1638. https://doi.org/10.3390/cells8121638
Chicago/Turabian StyleChen, Baoqing, Junyan Li, Dongmei Chi, Iman Sahnoune, Steliana Calin, Leonard Girnita, and George A. Calin. 2019. "Non-Coding RNAs in IGF-1R Signaling Regulation: The Underlying Pathophysiological Link between Diabetes and Cancer" Cells 8, no. 12: 1638. https://doi.org/10.3390/cells8121638
APA StyleChen, B., Li, J., Chi, D., Sahnoune, I., Calin, S., Girnita, L., & Calin, G. A. (2019). Non-Coding RNAs in IGF-1R Signaling Regulation: The Underlying Pathophysiological Link between Diabetes and Cancer. Cells, 8(12), 1638. https://doi.org/10.3390/cells8121638





