The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology
Abstract
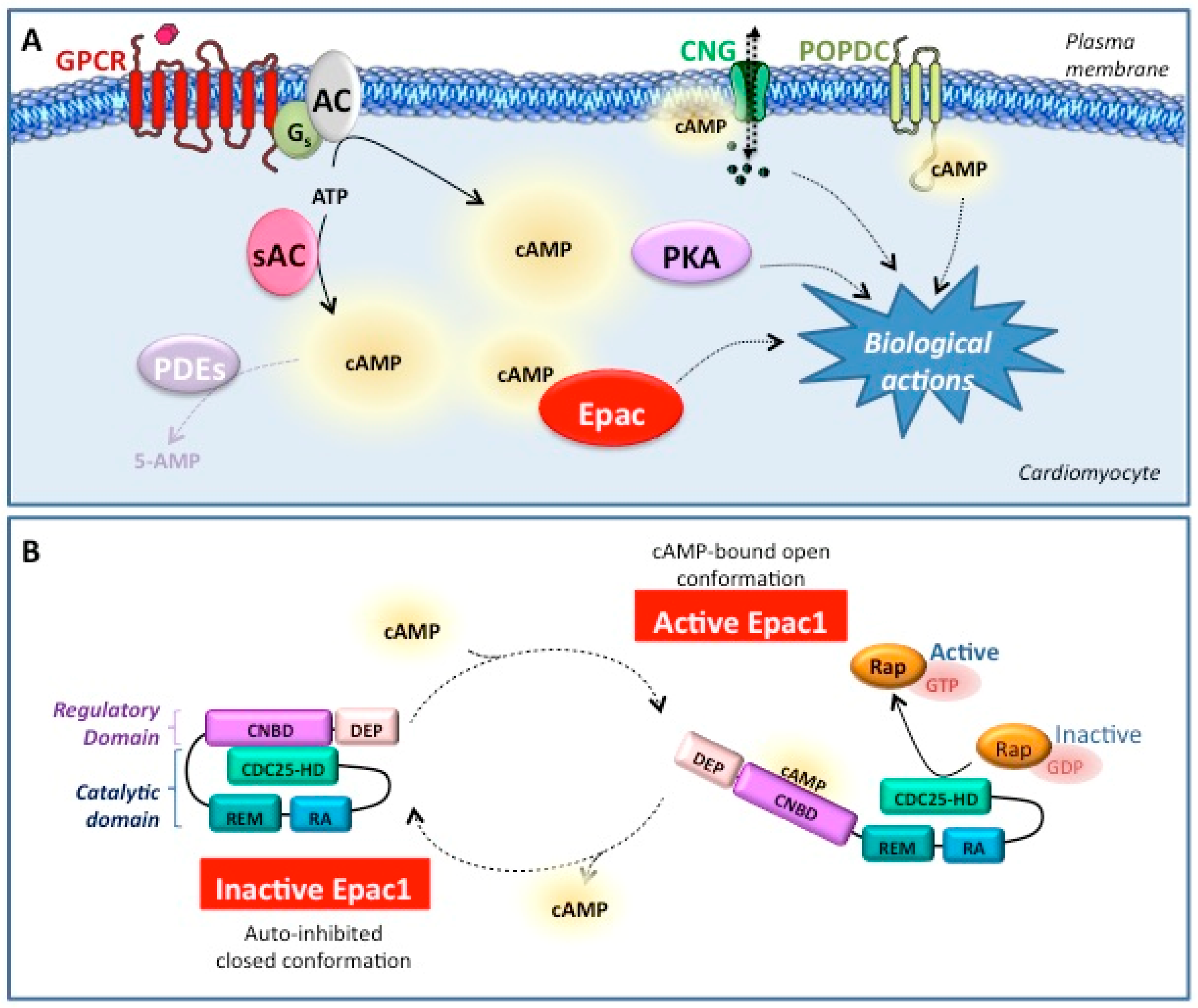
1. Introduction
2. Epac1 Protein
2.1. Epac Genes and Transcripts
2.2. Epac1 Structure
3. Small-Molecule Epac1 Modulators
3.1. Epac1 Agonists
3.1.1. Cyclic Nucleotides
3.1.2. Non-Cyclic Nucleotide Small Molecules
3.2. Epac1 Competitive Inhibitors
3.3. Epac1 Noncompetitive Inhibitors
3.3.1. Compound 5376753
3.3.2. Compound AM-001
3.4. Epac1 Uncompetitive Inhibitors
| Usual Name | IUPAC Chemical Name | Chemical Structure | Targeted Isoform | EC/IC50 (µM) | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| AGONISTS | ||||||
| Tolbu- tamide | N-(butylcarbamoyl)-4- methylbenzenesulfonamide |  | Epac1 | > 2000 | O | [27] |
| I942 | N-[(2,4-dimethylphenyl)sulfonyl]-2-(2- naphthyloxy)acetamide |  | Epac1 | 50 | O | [30] |
| ANTAGONISTS | ||||||
| ESI-08 | 4-cyclohexyl-2-(2,5- dimethylbenzylthio)-6-oxo-1,6- dihydropyrimidine-5-carbonitrile |  | Epac1 & Epac2 | unk | C | [33] |
| HJC0197 | 4-cyclopentyl-2-(2,5- dimethylbenzylsulfanyl)-6-oxo-1,6 dihydropyrimidine-5-carbonitrile |  | Epac1 & Epac2 | unk | C | [33] |
| ESI-09 | 3-(5-tert-butylisoxazol-3-yl)-2-[(3- chlorophenyl)hydrazono]-3- oxopropionitrile |  | Epac1 & Epac2 | 3.2 | C | [34] |
| HJC0726 | 2-(5-(tert-butyl)isoxazol-3-yl)-N-(3,5- dichlorophenyl)-2-oxoacetohydrazonoyl cyanide |  | Epac1 & Epac2 | 2.4 | C | [38] |
| NY0123 | 2-(5-(tert-butyl)isoxazol-3-yl)-2-oxo-N- (3,4,5-trichlorophenyl) acetohydrazonoyl cyanide |  | Epac1 & Epac2 | 2.4 | C | [38] |
| NY0460 | N-(3-trifluoromethyl-4-chlorophenyl)-2- oxo-2-(5-phenylisoxazol-3- yl)acetohydrazonoyl cyanide |  | Epac1 & Epac2 | 2.4 | C | [39] |
| NY0562 | 2-(benzo[d]isoxazol-3-yl)-N-(4-chloro- 3-(trifluoromethyl)phenyl)-2- oxoacetohydrazonoyl cyanide |  | Epac1 & Epac2 | 2.7 | C | [39] |
| ZL0524 | (E)-N-(3,5-dichlorophenyl)-2-oxo-2- (5,6,7,8-tetrahydronaphthalen-2- yl)acetohydrazonoyl cyanide |  | Epac1 & Epac2 | 3.6 | C | [40] |
| 5376753 | 5-{[5-(2,4-dichlorophenyl)-2- furyl]methylene}-2-thioxodihydro- 4,6(1H,5H)-pyrimidinedione |  | Epac1 & Epac2 | 4 | NC | [44] |
| AM-001 | 3-amino-N-(4-fluorophenyl)-4-phenyl-6- (2-thienyl)thieno[2,3-b]pyridine-2- carboxamide |  | Epac1 | 48 | NC | [45] |
| (R)-CE3F4 | (2R)-5,7-dibromo-6-fluoro-2-methyl-1,2, 3,4-tetrahydroquinoline-1-carbaldehyde |  | Epac1 | 4.2 | UC | [21,47] |
3.5. Conclusion on Epac1 Inhibitors
4. Role of Epac Proteins in Cardiac Diseases
4.1. Epac1 and Pathological Cardiac Remodelling Leading to HF
4.2. Epigenetic Regulation of Epac1 During Cardiac Remodelling
4.3. Role of Epac1 in Other Cardiac Disorders
4.3.1. Atrial and Ventricular Arrhythmias
4.3.2. Cardiac Ischemia
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| β-AR | β-adrenergic receptor |
| cAMP | 3′,5′-Cyclic adenosine monophosphate |
| sAC | soluble adenylyl cyclase |
| AKAPs | A-kinase anchoring proteins |
| ATP | adenosine triphosphate |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CDC25-HD | cell division cycle 25 homology domain |
| CNBD | cyclic-nucleotide-binding domain |
| CNG | cyclic nucleotide gated |
| DEP | Dishevelled, Egl-10, Pleckstrin |
| Epac | exchange protein directly activated by cAMP |
| ER | endoplasmic reticulum |
| GEF | guanine-nucleotide-exchange factor |
| GPCRs | G protein-coupled receptors |
| GRK | G-protein receptor kinase |
| GRP75 | chaperone glucose-regulated protein 75 |
| HDAC | histone deacetylase |
| HF | heart failure |
| IDH2 | isocitrate dehydrogenase 2 |
| IK | potassium K+-current |
| IP3 | inositol-1,4,5-trisphosphate |
| IP3R1 | IP3 receptor 1 (IP3R1) |
| I/R | ischemia-reperfusion |
| MEF2 | myocyte enhancer factor 2 |
| MI | myocardial infarction |
| mPTP | mitochondrial permeability transition pore |
| NFAT | nuclear factor of activated T |
| PDE | phosphodiesterases |
| PKA | protein kinase A |
| PKD | protein kinase D |
| PKG | protein kinase G |
| PLC | phospholipase C |
| POPDC | popeye domain-containing proteins |
| RA | Ras-association domain |
| REM | Ras-exchange motif |
| RyR | ryanodine receptors |
| ROS | reactive oxygen species |
| SAR | structure-activity relationship |
| TRPC | transient Receptor Potential Canonical |
| VDAC1 | voltage-dependent anion channel 1 |
References
- Halls, M.L.; Cooper, D.M.F. Adenylyl cyclase signalling complexes—Pharmacological challenges and opportunities. Pharmacol. Ther. 2017, 172, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pozdniakova, S.; Ladilov, Y. Functional Significance of the Adcy10-Dependent Intracellular cAMP Compartments. J. Cardiovasc. Dev. Dis. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Bobin, P.; Belacel-Ouari, M.; Bedioune, I.; Zhang, L.; Leroy, J.; Leblais, V.; Fischmeister, R.; Vandecasteele, G. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch. Cardiovasc. Dis. 2016, 109, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Dekker, F.J.; Maarsingh, H. Exchange protein directly activated by cAMP (epac): A multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 2013, 65, 670–709. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Schindler, R. New kids on the block: The Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscle. Cell. Signal. 2017, 40, 156–165. [Google Scholar] [CrossRef]
- Sartiani, L.; Mannaioni, G.; Masi, A.; Novella Romanelli, M.; Cerbai, E. The hyperpolarization-activated cyclic nucleotide-gated channels: From biophysics to pharmacology of a unique family of ion channels. Pharmacol. Rev. 2017, 69, 354–395. [Google Scholar] [CrossRef]
- Robichaux, W.G.; Cheng, X. Intracellular cAMP sensor EPAC: Physiology, pathophysiology, and therapeutics development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef]
- Ercu, M.; Klussmann, E. Roles of A-kinase anchoring proteins and phosphodiesterases in the cardiovascular system. J. Cardiovasc. Dev. Dis. 2018, 5. [Google Scholar] [CrossRef]
- Lezoualc’h, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- El-Armouche, A.; Eschenhagen, T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail. Rev. 2009, 14, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Levine, T.B.; Olivari, M.T.; Garberg, V.; Lura, D.; Francis, G.S.; Simon, A.B.; Rector, T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 1984, 311, 819–823. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef]
- Banerjee, U.; Cheng, X. Exchange protein directly activated by cAMP encoded by the mammalian rapgef3 gene: Structure, function and therapeutics. Gene 2015, 570, 157–167. [Google Scholar] [CrossRef]
- Niimura, M.; Miki, T.; Shibasaki, T.; Fujimoto, W.; Iwanaga, T.; Seino, S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J. Cell. Physiol. 2009, 219, 652–658. [Google Scholar] [CrossRef]
- Rehmann, H.; Arias-Palomo, E.; Hadders, M.A.; Schwede, F.; Llorca, O.; Bos, J.L. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 2008, 455, 124–127. [Google Scholar] [CrossRef]
- Rehmann, H.; Das, J.; Knipscheer, P.; Wittinghofer, A.; Bos, J.L. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 2006, 439, 625–628. [Google Scholar] [CrossRef]
- Singhmar, P.; Huo, X.; Eijkelkamp, N.; Berciano, S.R.; Baameur, F.; Mei, F.C.; Zhu, Y.; Cheng, X.; Hawke, D.; Mayor, F., Jr.; et al. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc. Natl. Acad. Sci. USA 2016, 113, 3036–3041. [Google Scholar] [CrossRef]
- Enserink, J.M.; Christensen, A.E.; de Rooij, J.; van Triest, M.; Schwede, F.; Genieser, H.G.; Døskeland, S.O.; Blank, J.L.; Bos, J.L. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 2002, 4, 901–906. [Google Scholar] [CrossRef]
- Courilleau, D.; Bouyssou, P.; Fischmeister, R.; Lezoualc’h, F.; Blondeau, J.-P. The (R)-enantiomer of CE3F4 is a preferential inhibitor of human exchange protein directly activated by cyclic AMP isoform 1 (Epac1). Biochem. Biophys. Res. Commun. 2013, 440, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Schwede, F.; Bertinetti, D.; Langerijs, C.N.; Hadders, M.A.; Wienk, H.; Ellenbroek, J.H.; de Koning, E.J.P.; Bos, J.L.; Herberg, F.W.; Genieser, H.G.; et al. Structure-guided design of selective Epac1 and Epac2 agonists. PLoS Biol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Laxman, S.; Riechers, A.; Sadilek, M.; Schwede, F.; Beavo, J.A. Hydrolysis products of cAMP analogs cause transformation of Trypanosoma brucei from slender to stumpy-like forms. Proc. Natl. Acad. Sci. USA 2006, 103, 19194–19199. [Google Scholar] [CrossRef] [PubMed]
- Vliem, M.J.; Ponsioen, B.; Schwede, F.; Pannekoek, W.-J.; Riedl, J.; Kooistra, M.R.H.; Jalink, K.; Genieser, H.-G.; Bos, J.L.; Rehmann, H. 8-pCPT-2′-O-Me-cAMP-AM: An improved Epac-selective cAMP analogue. Chembiochem. Eur. J. Chem. Biol. 2008, 9, 2052–2054. [Google Scholar] [CrossRef] [PubMed]
- Poppe, H.; Rybalkin, S.D.; Rehmann, H.; Hinds, T.R.; Tang, X.-B.; Christensen, A.E.; Schwede, F.; Genieser, H.-G.; Bos, J.L.; Doskeland, S.O.; et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat. Methods 2008, 5, 277–278. [Google Scholar] [CrossRef]
- Holz, G.G.; Chepurny, O.G.; Schwede, F. Epac-selective cAMP analogs: New tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell. Signal. 2008, 20, 10–20. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Katoh, M.; Shibasaki, T.; Minami, K.; Sunaga, Y.; Takahashi, H.; Yokoi, N.; Iwasaki, M.; Miki, T.; Seino, S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 2009, 325, 607–610. [Google Scholar] [CrossRef]
- Tsalkova, T.; Gribenko, A.V.; Cheng, X. Exchange protein directly activated by cyclic AMP isoform 2 is not a direct target of sulfonylurea drugs. Assay Drug Dev. Technol. 2011, 9, 88–91. [Google Scholar] [CrossRef]
- Parnell, E.; Smith, B.O.; Palmer, T.M.; Terrin, A.; Zaccolo, M.; Yarwood, S.J. Regulation of the inflammatory response of vascular endothelial cells by EPAC1. Br. J. Pharmacol. 2012, 166, 434–446. [Google Scholar] [CrossRef]
- Parnell, E.; McElroy, S.P.; Wiejak, J.; Baillie, G.L.; Porter, A.; Adams, D.R.; Rehmann, H.; Smith, B.O.; Yarwood, S.J. Identification of a novel, small molecule partial agonist for the cyclic AMP sensor, EPAC1. Sci. Rep. 2017, 7, 294. [Google Scholar] [CrossRef]
- Wiejak, J.; van Basten, B.; Luchowska-Stańska, U.; Hamilton, G.; Yarwood, S.J. The novel exchange protein activated by cyclic AMP 1 (EPAC1) agonist, I942, regulates inflammatory gene expression in human umbilical vascular endothelial cells (HUVECs). Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Tsalkova, T.; Mei, F.C.; Cheng, X. A fluorescence-based high-throughput assay for the discovery of exchange protein directly activated by cyclic AMP (EPAC) antagonists. PLoS ONE 2012, 7, e30441. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tsalkova, T.; Mei, F.C.; Hu, Y.; Cheng, X.; Zhou, J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg. Med. Chem. Lett. 2012, 22, 4038–4043. [Google Scholar] [CrossRef] [PubMed]
- Almahariq, M.; Tsalkova, T.; Mei, F.C.; Chen, H.; Zhou, J.; Sastry, S.K.; Schwede, F.; Cheng, X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 2013, 83, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, H. Epac-inhibitors: Facts and artefacts. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Boulton, S.; Selvaratnam, R.; Ahmed, R.; Van, K.; Cheng, X.; Melacini, G. Mechanisms of specific versus nonspecific interactions of aggregation-prone inhibitors and attenuators. J. Med. Chem. 2019, 62, 5063–5079. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Boulton, S.; Mei, F.; Ye, N.; Melacini, G.; Zhou, J.; Cheng, X. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: Defining the ESI-09 “therapeutic window”. Sci. Rep. 2015, 5, 9344. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, Y.; Chen, H.; Liu, Z.; Mei, F.C.; Wild, C.; Chen, H.; Cheng, X.; Zhou, J. Structure-activity relationship studies of substituted 2-(isoxazol-3-yl)-2-oxo-N’-phenyl-acetohydrazonoyl cyanide analogues: Identification of potent exchange proteins directly activated by cAMP (EPAC) antagonists. J. Med. Chem. 2015, 58, 6033–6047. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, Y.; Liu, Z.; Mei, F.C.; Chen, H.; Wang, P.; Cheng, X.; Zhou, J. Identification of novel 2-(benzo[d]isoxazol-3-yl)-2-oxo-N-phenylacetohydrazonoyl cyanide analoguesas potent EPAC antagonists. Eur. J. Med. Chem. 2017, 134, 62–71. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Chen, H.; Wang, P.; Mei, F.C.; Ye, N.; Cheng, X.; Zhou, J. Structure-activity relationships of 2-substituted phenyl-N-phenyl-2-oxoacetohydrazonoyl cyanides as novel antagonists of exchange proteins directly activated by cAMP (EPACs). Bioorg. Med. Chem. Lett. 2017, 27, 5163–5166. [Google Scholar] [CrossRef]
- Tsalkova, T.; Mei, F.C.; Li, S.; Chepurny, O.G.; Leech, C.A.; Liu, T.; Holz, G.G.; Woods, V.L.; Cheng, X. Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc. Natl. Acad. Sci. USA 2012, 109, 18613–18618. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tsalkova, T.; Chepurny, O.G.; Mei, F.C.; Holz, G.G.; Cheng, X.; Zhou, J. Identification and characterization of small molecules as potent and specific EPAC2 antagonists. J. Med. Chem. 2013, 56, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Rogers, K.E.; McCammon, J.A.; Insel, P.A. Identification and validation of modulators of exchange protein activated by cAMP (Epac) activity: Structure-function implications for Epac activation and inhibition. J. Biol. Chem. 2014, 289, 8217–8230. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Rogers, K.E.; Aroonsakool, N.; McCammon, J.A.; Insel, P.A. Allosteric inhibition of Epac: Computational modeling and experimental validation to identify allosteric sites and inhibitors. J. Biol. Chem. 2014, 289, 29148–29157. [Google Scholar] [CrossRef] [PubMed]
- Laudette, M.; Coluccia, A.; Sainte-Marie, Y.; Solari, A.; Fazal, L.; Sicard, P.; Silvestri, R.; Mialet-Perez, J.; Pons, S.; Ghaleh, B.; et al. Identification of a pharmacological inhibitor of Epac1 that protects the heart against acute and chronic models of cardiac stress. Cardiovasc. Res. 2019, 115, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zucker, R.S. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J. Neurosci. 2005, 25, 208–214. [Google Scholar] [CrossRef][Green Version]
- Courilleau, D.; Bisserier, M.; Jullian, J.-C.; Lucas, A.; Bouyssou, P.; Fischmeister, R.; Blondeau, J.-P.; Lezoualc’h, F. Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac. J. Biol. Chem. 2012, 287, 44192–44202. [Google Scholar] [CrossRef]
- Boulton, S.; Selvaratnam, R.; Blondeau, J.-P.; Lezoualc’h, F.; Melacini, G. Mechanism of selective enzyme inhibition through uncompetitive regulation of an allosteric agonist. J. Am. Chem. Soc. 2018, 140, 9624–9637. [Google Scholar] [CrossRef]
- Westley, A.M.; Westley, J. Enzyme inhibition in open systems. Superiority of uncompetitive agents. J. Biol. Chem. 1996, 271, 5347–5352. [Google Scholar] [CrossRef]
- Swynghedauw, B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999, 79, 215–262. [Google Scholar] [CrossRef]
- Ulucan, C.; Wang, X.; Baljinnyam, E.; Bai, Y.; Okumura, S.; Sato, M.; Minamisawa, S.; Hirotani, S.; Ishikawa, Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1662–H1672. [Google Scholar] [CrossRef] [PubMed]
- Métrich, M.; Lucas, A.; Gastineau, M.; Samuel, J.-L.; Heymes, C.; Morel, E.; Lezoualc’h, F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ. Res. 2008, 102, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Fazal, L.; Laudette, M.; Paula-Gomes, S.; Pons, S.; Conte, C.; Tortosa, F.; Sicard, P.; Sainte-Marie, Y.; Bisserier, M.; Lairez, O.; et al. Multifunctional mitochondrial Epac1 controls myocardial cell death. Circ. Res. 2017, 120, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Marcantoni, A.; Gastineau, M.; Birkedal, R.; Rochais, F.; Garnier, A.; Lompré, A.-M.; Vandecasteele, G.; Lezoualc’h, F. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ. Res. 2005, 97, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Cheng, H.; Lao, D.H.; Na, L.; van Oort, R.J.; Brown, J.H.; Wehrens, X.H.T.; Chen, J.; Bers, D.M. Epac2 mediates cardiac β1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation 2013, 127, 913–922. [Google Scholar] [CrossRef]
- Laurent, A.C.; Bisserier, M.; Lucas, A.; Tortosa, F.; Roumieux, M.; De Régibus, A.; Swiader, A.; Sainte-Marie, Y.; Heymes, C.; Vindis, C.; et al. Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy. Cardiovasc. Res. 2015, 105, 55–64. [Google Scholar] [CrossRef]
- Bobin, P.; Varin, A.; Lefebvre, F.; Fischmeister, R.; Vandecasteele, G.; Leroy, J. Calmodulin kinase II inhibition limits the pro-arrhythmic Ca2+ waves induced by cAMP-phosphodiesterase inhibitors. Cardiovasc. Res. 2016, 110, 151–161. [Google Scholar] [CrossRef]
- Okumura, S.; Fujita, T.; Cai, W.; Jin, M.; Namekata, I.; Mototani, Y.; Jin, H.; Ohnuki, Y.; Tsuneoka, Y.; Kurotani, R.; et al. Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. J. Clin. Investig. 2014, 124, 2785–2801. [Google Scholar] [CrossRef]
- Cai, W.; Fujita, T.; Hidaka, Y.; Jin, H.; Suita, K.; Prajapati, R.; Liang, C.; Umemura, M.; Yokoyama, U.; Sato, M.; et al. Disruption of Epac1 protects the heart from adenylyl cyclase type 5-mediated cardiac dysfunction. Biochem. Biophys. Res. Commun. 2016, 475, 1–7. [Google Scholar] [CrossRef]
- Fujita, T.; Umemura, M.; Yokoyama, U.; Okumura, S.; Ishikawa, Y. The role of Epac in the heart. Cell. Mol. Life Sci. 2017, 74, 591–606. [Google Scholar] [CrossRef]
- Mangmool, S.; Shukla, A.K.; Rockman, H.A. beta-Arrestin-dependent activation of Ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J. Cell Biol. 2010, 189, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Berthouze-Duquesnes, M.; Lucas, A.; Saulière, A.; Sin, Y.Y.; Laurent, A.-C.; Galés, C.; Baillie, G.; Lezoualc’h, F. Specific interactions between Epac1, β-arrestin2 and PDE4D5 regulate β-adrenergic receptor subtype differential effects on cardiac hypertrophic signaling. Cell. Signal. 2013, 25, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, R.A.; Weeks, K.L. Histone deacetylases in cardiovascular and metabolic diseases. J. Mol. Cell. Cardiol. 2019, 130, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Métrich, M.; Laurent, A.-C.; Breckler, M.; Duquesnes, N.; Hmitou, I.; Courillau, D.; Blondeau, J.-P.; Crozatier, B.; Lezoualc’h, F.; Morel, E. Epac activation induces histone deacetylase nuclear export via a Ras-dependent signalling pathway. Cell. Signal. 2010, 22, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Nash, C.A.; Brown, L.M.; Malik, S.; Cheng, X.; Smrcka, A.V. Compartmentalized cyclic nucleotides have opposing effects on regulation of hypertrophic phospholipase Cε signaling in cardiac myocytes. J. Mol. Cell. Cardiol. 2018, 121, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nash, C.A.; Wei, W.; Irannejad, R.; Smrcka, A.V. Golgi localized β1-adrenergic receptors stimulate Golgi PI4P hydrolysis by PLCε to regulate cardiac hypertrophy. eLife 2019, 8, e48167. [Google Scholar] [CrossRef]
- Pereira, L.; Ruiz-Hurtado, G.; Morel, E.; Laurent, A.-C.; Métrich, M.; Domínguez-Rodríguez, A.; Lauton-Santos, S.; Lucas, A.; Benitah, J.-P.; Bers, D.M.; et al. Epac enhances excitation-transcription coupling in cardiac myocytes. J. Mol. Cell. Cardiol. 2012, 52, 283–291. [Google Scholar] [CrossRef]
- Zhang, L.; Malik, S.; Pang, J.; Wang, H.; Park, K.M.; Yule, D.I.; Blaxall, B.C.; Smrcka, A.V. Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell 2013, 153, 216–227. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, A.; Ruiz-Hurtado, G.; Sabourin, J.; Gómez, A.M.; Alvarez, J.L.; Benitah, J.-P. Proarrhythmic effect of sustained EPAC activation on TRPC3/4 in rat ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 87, 74–78. [Google Scholar] [CrossRef]
- Pfleger, J.; Gresham, K.; Koch, W.J. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat. Rev. Cardiol. 2019, 16, 612–622. [Google Scholar] [CrossRef]
- Pereira, L.; Rehmann, H.; Lao, D.H.; Erickson, J.R.; Bossuyt, J.; Chen, J.; Bers, D.M. Novel Epac fluorescent ligand reveals distinct Epac1 vs. Epac2 distribution and function in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 3991–3996. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Métrich, M.; Fernández-Velasco, M.; Lucas, A.; Leroy, J.; Perrier, R.; Morel, E.; Fischmeister, R.; Richard, S.; Bénitah, J.P.; et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J. Physiol. 2007, 583, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hurtado, G.; Morel, E.; Domínguez-Rodríguez, A.; Llach, A.; Lezoualc’h, F.; Benitah, J.-P.; Gomez, A.M. Epac in cardiac calcium signaling. J. Mol. Cell. Cardiol. 2013, 58, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Aflaki, M.; Qi, X.-Y.; Xiao, L.; Ordog, B.; Tadevosyan, A.; Luo, X.; Maguy, A.; Shi, Y.; Tardif, J.-C.; Nattel, S. Exchange protein directly activated by cAMP mediates slow delayed-rectifier current remodeling by sustained β-adrenergic activation in guinea pig hearts. Circ. Res. 2014, 114, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Hothi, S.S.; Gurung, I.S.; Heathcote, J.C.; Zhang, Y.; Booth, S.W.; Skepper, J.N.; Grace, A.A.; Huang, C.L.-H. Epac activation, altered calcium homeostasis and ventricular arrhythmogenesis in the murine heart. Pflug. Arch. 2008, 457, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hothi, S.S.; Salvage, S.C.; Jeevaratnam, K.; Grace, A.A.; Huang, C.L.-H. Arrhythmic effects of Epac-mediated ryanodine receptor activation in Langendorff-perfused murine hearts are associated with reduced conduction velocity. Clin. Exp. Pharmacol. Physiol. 2017, 44, 686–692. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Zheng, J.-K.; Wang, W.-W.; Kong, F.-Q.; Wu, X.-X.; Jiang, J.-K.; Pan, J.-X. Exchange-protein activated by cAMP (EPAC) regulates L-type calcium channel in atrial fibrillation of heart failure model. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2200–2207. [Google Scholar]
- Prajapati, R.; Fujita, T.; Suita, K.; Nakamura, T.; Cai, W.; Hidaka, Y.; Umemura, M.; Yokoyama, U.; Knollmann, B.C.; Okumura, S.; et al. Usefulness of exchanged protein directly activated by cAMP (Epac)1-inhibiting therapy for prevention of atrial and ventricular arrhythmias in mice. Circ. J. 2019, 83, 295–303. [Google Scholar] [CrossRef]
- Yang, Z.; Kirton, H.M.; Al-Owais, M.; Thireau, J.; Richard, S.; Peers, C.; Steele, D.S. Epac2-Rap1 signaling regulates reactive oxygen species production and susceptibility to cardiac arrhythmias. Antioxid. Redox Signal. 2017, 27, 117–132. [Google Scholar] [CrossRef]
- Qiao, J.; Mei, F.C.; Popov, V.L.; Vergara, L.A.; Cheng, X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J. Biol. Chem. 2002, 277, 26581–26586. [Google Scholar] [CrossRef]
- Ong, S.-B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Khaliulin, I.; Bond, M.; James, A.F.; Dyar, Z.; Amini, R.; Johnson, J.L.; Suleiman, M.-S. Functional and cardioprotective effects of simultaneous and individual activation of protein kinase A and Epac. Br. J. Pharmacol. 2017, 174, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.; Varin, A.; Nicolas, V.; Courilleau, D.; Mateo, P.; Caubere, C.; Rouet, P.; Gomez, A.-M.; Vandecasteele, G.; et al. A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death. Cell Death Dis. 2016, 7, e2198. [Google Scholar] [CrossRef] [PubMed]
- Surinkaew, S.; Aflaki, M.; Takawale, A.; Chen, Y.; Qi, X.-Y.; Gillis, M.-A.; Shi, Y.-F.; Tardif, J.-C.; Chattipakorn, N.; Nattel, S. Exchange protein activated by cyclic-adenosine monophosphate (Epac) regulates atrial fibroblast function and controls cardiac remodelling. Cardiovasc. Res. 2019, 115, 94–106. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouvet, M.; Blondeau, J.-P.; Lezoualc’h, F. The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology. Cells 2019, 8, 1543. https://doi.org/10.3390/cells8121543
Bouvet M, Blondeau J-P, Lezoualc’h F. The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology. Cells. 2019; 8(12):1543. https://doi.org/10.3390/cells8121543
Chicago/Turabian StyleBouvet, Marion, Jean-Paul Blondeau, and Frank Lezoualc’h. 2019. "The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology" Cells 8, no. 12: 1543. https://doi.org/10.3390/cells8121543
APA StyleBouvet, M., Blondeau, J.-P., & Lezoualc’h, F. (2019). The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology. Cells, 8(12), 1543. https://doi.org/10.3390/cells8121543





