Platyconic Acid A, Platycodi Radix-Derived Saponin, Suppresses TGF-β1-Induced Activation of Hepatic Stellate Cells via Blocking SMAD and Activating the PPARγ Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of PA
2.3. Cell Culture
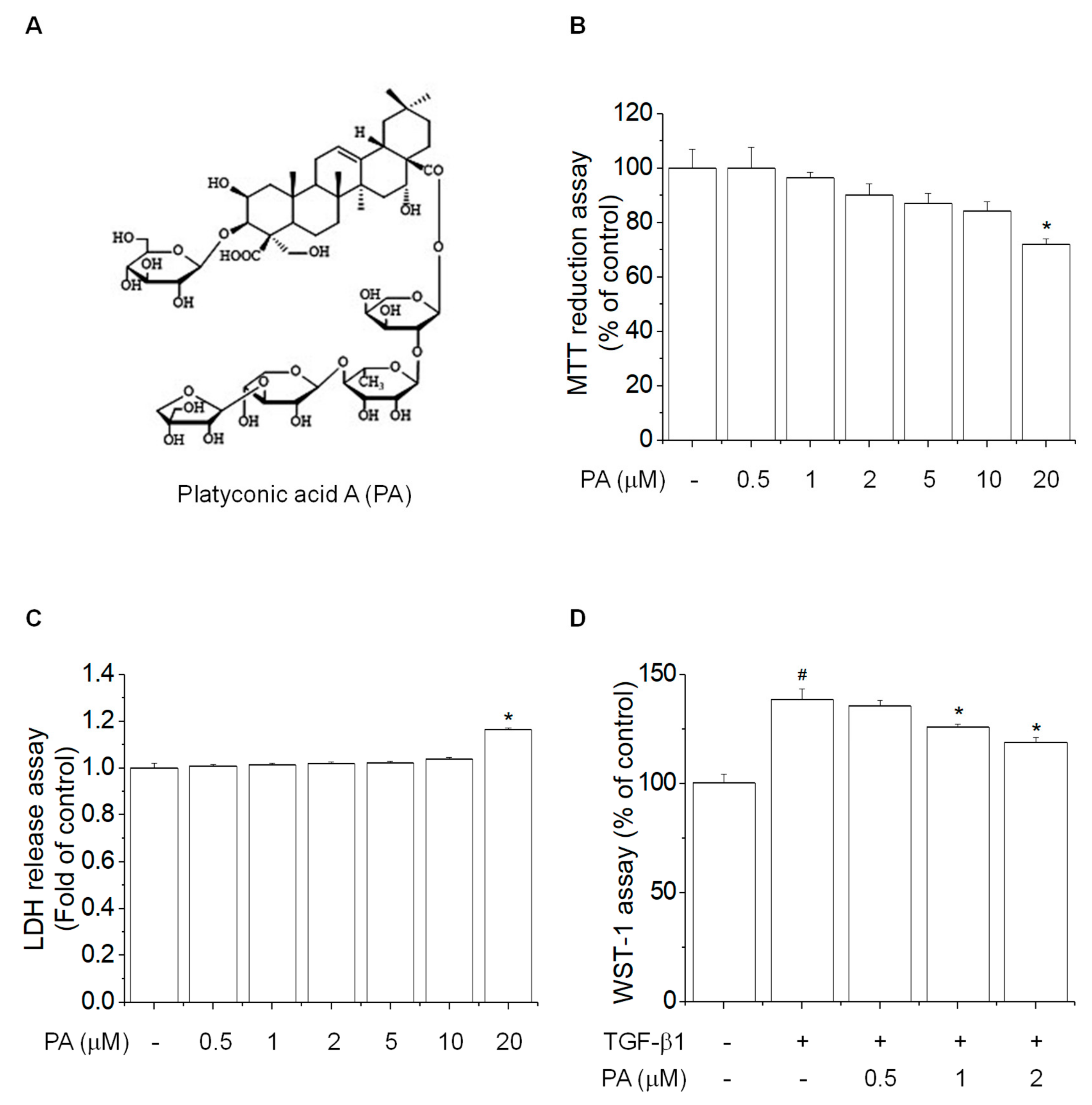
2.4. Cell Viability Assay
2.5. Real Time-Polymerase Chain Reaction
2.6. Luciferase Assay
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. PA Reduces TGF-β1-Induced HSCs Proliferation
3.2. PA Reduces TGF-β1-Induced HSCs Activation
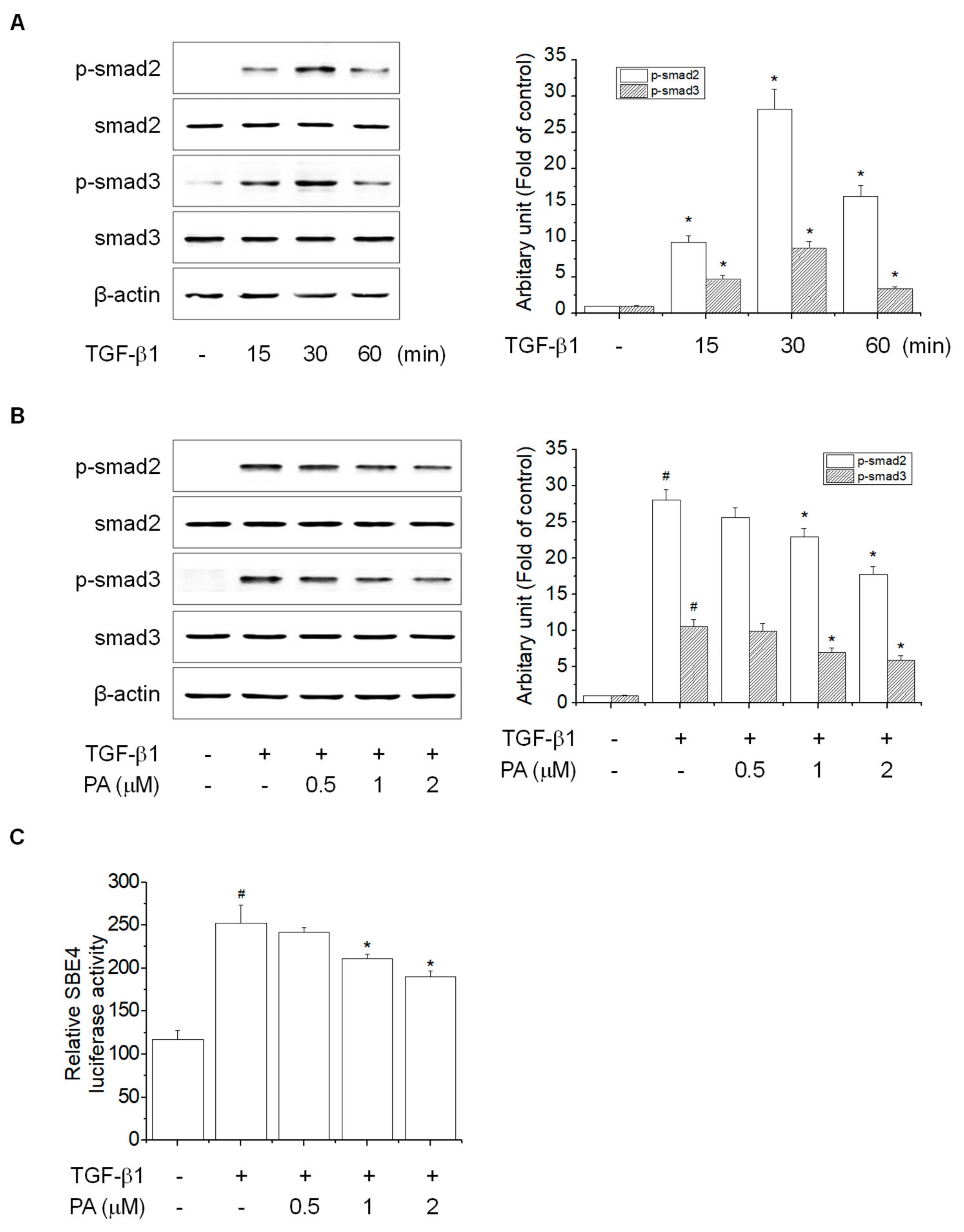
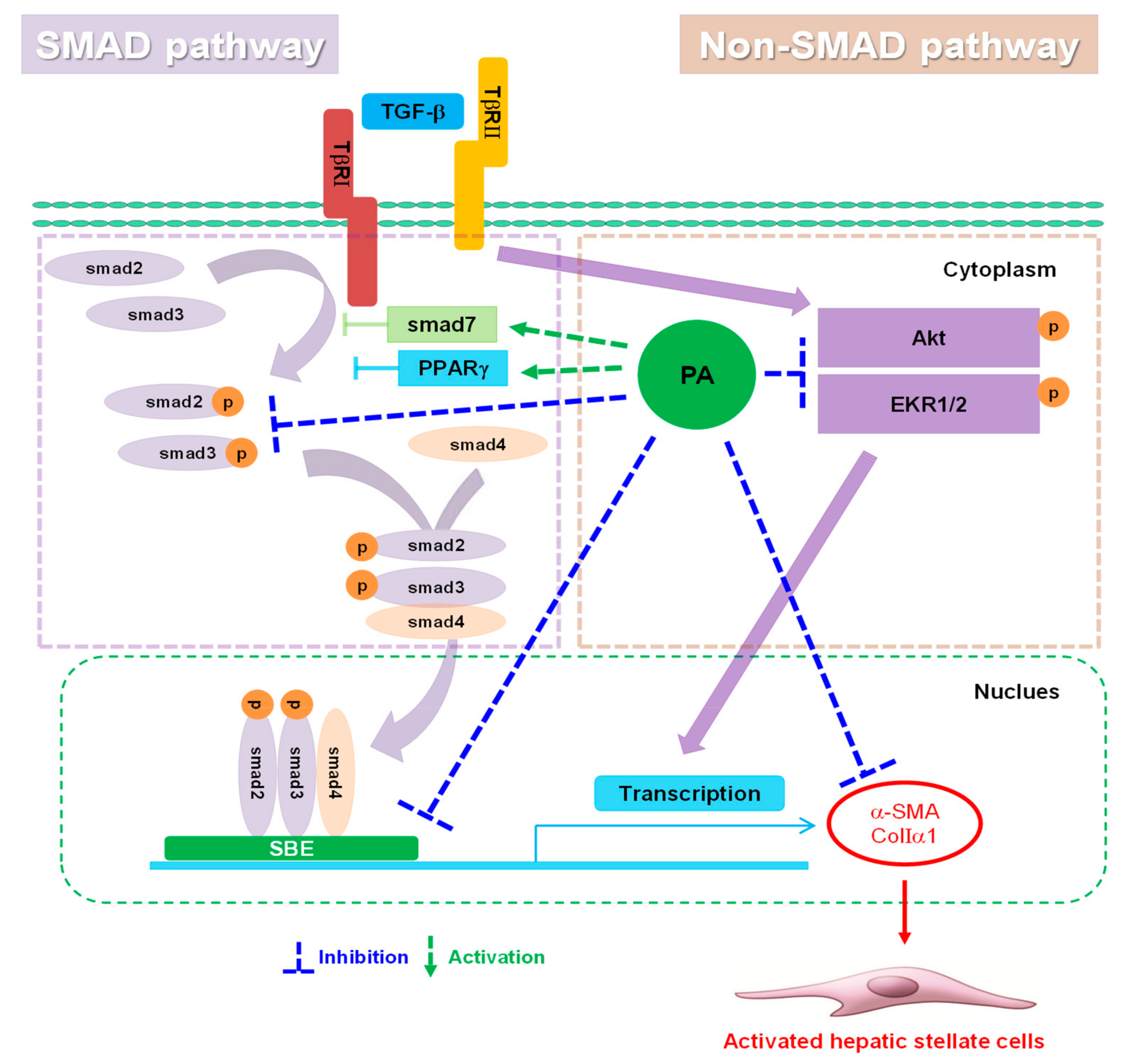
3.3. PA Reduces TGF-β1-Induced HSCs Activation by Blocking a SMAD-Dependent Signal Pathway
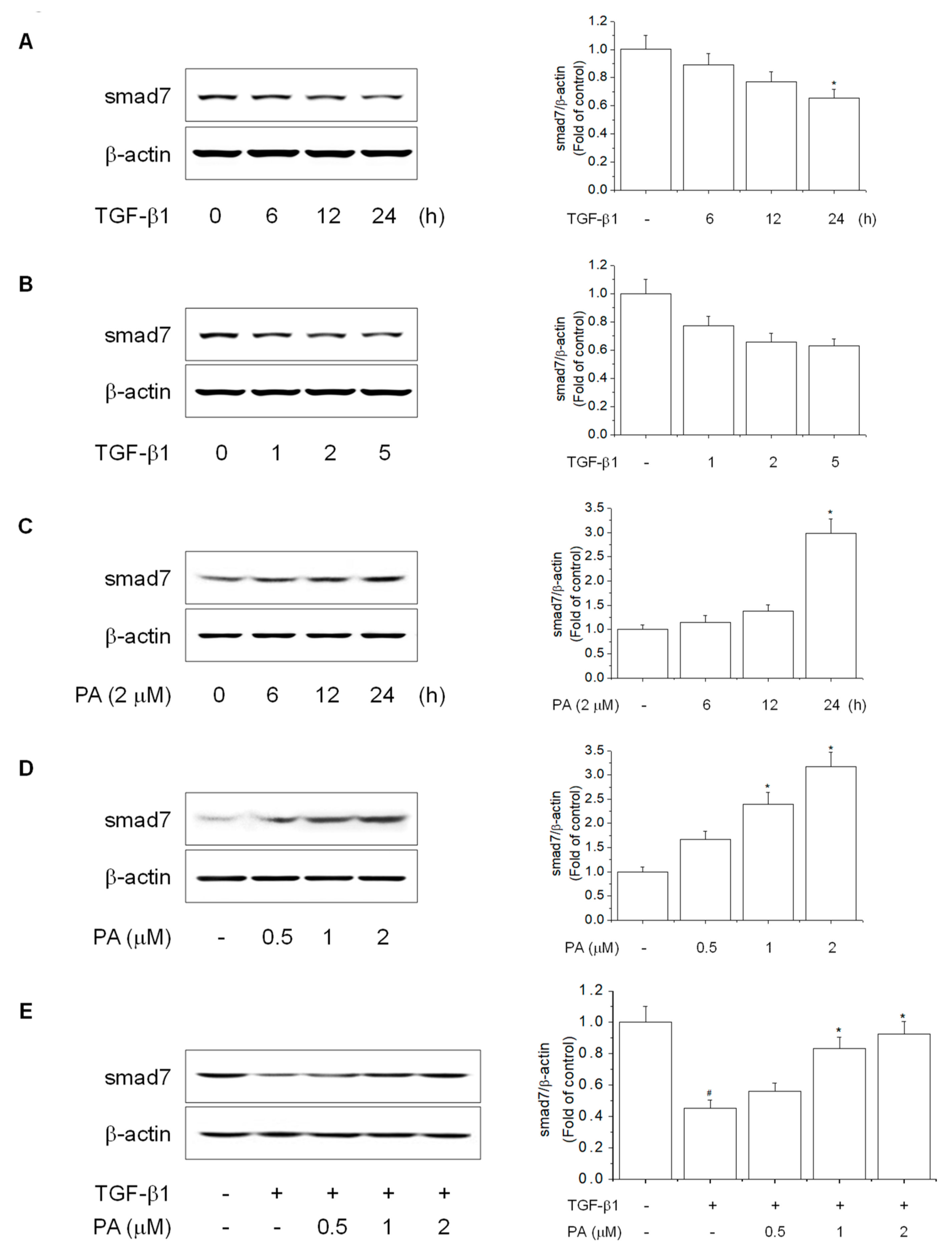
3.4. PA Reduces TGF-β1-Induced HSCs Activation by Upregulation of PPARγ
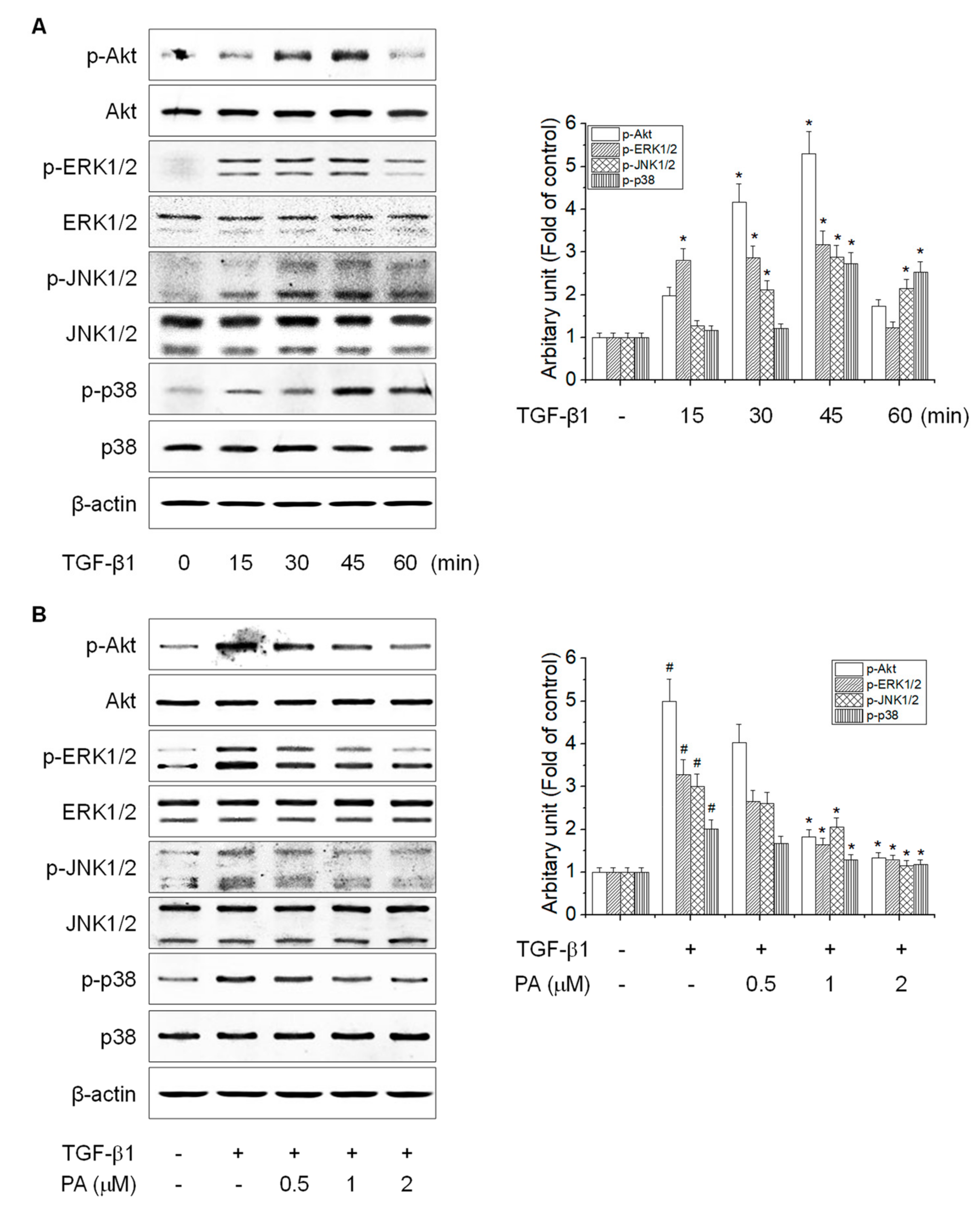
3.5. PA Reduces TGF-β1-Induced HSCs Activation by Blocking the SMAD-Independent Signal Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-SMA | alpha-smooth muscle actin |
| ECM | extracellular matrix |
| PA | platyconic acid A |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| SBE | smad binding elements |
| TGF-β | transforming growth factor-β |
References
- Roy, S.; Trautwein, C.; Luedde, T.; Roderburg, C. A General Overview on Non-coding RNA-Based Diagnostic and Therapeutic Approaches for Liver Diseases. Front. Pharmacol. 2018, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; French, S.W.; Zakhari, S.; Malnick, S.; Seitz, H.K.; Cohen, L.B.; Salaspuro, M.; Voinea-Griffin, A.; Barasch, A.; Kirpich, I.A.; et al. Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage. Exp. Mol. Pathol. 2017, 102, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.C.; Friedman, S.L.; Mann, D.A. Emerging and disease-specific mechanisms of hepatic stellate cell activation. Semin. Liver Dis. 2015, 35, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Investig. 2013, 123, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, S.; Zhang, P. Effect of Exogenous Fetuin-A on TGF-β/Smad Signaling in Hepatic Stellate Cells. Biomed. Res. Int. 2016, 2016, 8462615. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; An, H.J.; Kim, W.H.; Gwon, M.G.; Gu, H.; Park, Y.Y.; Park, K.K. Anti-fibrotic Effects of Synthetic Oligodeoxynucleotide for TGF-β1 and Smad in an Animal Model of Liver Cirrhosis. Mol. Ther. Nucleic Acids 2017, 8, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Namsen, R.; Rojanasthien, N.; Sireeratawong, S.; Rojsanga, P.; Nimlamool, W.; Potikanond, S. Thunbergia laurifolia Exhibits Antifibrotic Effects in Human Hepatic Stellate Cells. Evid. Based Complement. Alternat. Med. 2017, 2017, 3508569. [Google Scholar] [CrossRef]
- Kuo, L.M.; Chen, P.J.; Sung, P.J.; Chang, Y.C.; Ho, C.T.; Wu, Y.H.; Hwang, T.L. The Bioactive Extract of Pinnigorgia sp. Induces Apoptosis of Hepatic Stellate Cells via ROS-ERK/JNK-Caspase-3 Signaling. Mar. Drugs. 2018, 16, 19. [Google Scholar] [CrossRef]
- Li, J.F.; Lu, G.F.; Zou, Y.Y. Demethylbellidifolin inhibits proliferation and activation of hepatic stellate cells. J. Investig. Surg. 2011, 24, 171–177. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Lee, G.H.; Kim, Y.A.; Chung, Y.C.; Jeong, H.G. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 2017, 65, 317–326. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, J.; He, L.N.; Li, B.H.; Ding, Y.N.; Chen, Y.W.; Fan, J.G. Prolyl oligopeptidase attenuates hepatic stellate cell activation through induction of Smad7 and PPAR-γ. Exp. Ther. Med. 2017, 13, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Nuwormegbe, S.A.; Sohn, J.H.; Kim, S.W. A PPAR-Gamma Agonist Rosiglitazone Suppresses Fibrotic Response in Human Pterygium Fibroblasts by Modulating the p38 MAPK Pathway. Invest. Ophthalmol. Vis. Sci. 2017, 58, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Jeong, H.G. Protective effect of Platycodi radix on carbon tetrachloride-induced hepatotoxicity. Food Chem. Toxicol. 2002, 40, 517–525. [Google Scholar] [CrossRef]
- Lee, K.J.; You, H.J.; Park, S.J.; Kim, Y.S.; Chung, Y.C.; Jeong, T.C.; Jeong, H.G. Hepatoprotective effects of Platycodon grandiflorum on acetaminophen-induced liver damage in mice. Cancer Lett. 2001, 174, 73–81. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandiflorus—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Han, E.H.; Park, B.H.; Kim, H.G.; Hwang, Y.P.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Platycodi Radix suppresses development of atopic dermatitis-like skin lesions. Environ. Toxicol. Pharmacol. 2012, 33, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jin, S.W.; Han, E.H.; Park, B.H.; Kim, H.G.; Khanal, T.; Hwang, Y.P.; Do, M.T.; Lee, H.S.; Chung, Y.C.; et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine 2014, 21, 1053–1061. [Google Scholar] [CrossRef]
- Choi, J.H.; Hwang, Y.P.; Lee, H.S.; Jeong, H.G. Inhibitory effect of Platycodi Radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 1272–1279. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Kim, H.G.; Choi, C.Y.; Lee, H.S.; Ryu, S.Y.; Chung, Y.C.; Hwang, Y.J.; Um, Y.J.; Jeong, T.C.; et al. Saponins, especially platyconic acid A, from Platycodon grandiflorum reduce airway inflammation in ovalbumin-induced mice and PMA-exposed A549 cells. J. Agric. Food Chem. 2015, 63, 1468–1476. [Google Scholar] [CrossRef]
- Jeong, H.M.; Han, E.H.; Jin, Y.H.; Hwang, Y.P.; Kim, H.G.; Park, B.H.; Kim, J.Y.; Chung, Y.C.; Lee, K.Y.; Jeong, H.G. Saponins from the roots of Platycodon grandiflorum stimulate osteoblast differentiation via p38 MAPK- and ERK-dependent RUNX2 activation. Food Chem. Toxicol. 2010, 48, 3362–3368. [Google Scholar] [CrossRef]
- Choi, J.H.; Han, Y.; Kim, Y.A.; Jin, S.W.; Lee, G.H.; Jeong, H.M.; Lee, H.S.; Chung, Y.C.; Lee, Y.C.; Kim, E.J.; et al. Platycodin D Inhibits Osteoclastogenesis by Repressing the NFATc1 and MAPK Signaling Pathway. J. Cell. Biochem. 2017, 118, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Hwang, S.J.; Choi, J.H.; Jeong, H.G. Saponins derived from the roots of Platycodon grandiflorum inhibit HT-1080 cell invasion and MMPs activities: Regulation of NF-kappaB activation via ROS signal pathway. Cancer Lett. 2008, 268, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, J.Y.; Jung, K.S.; Choi, C.Y.; Chung, Y.C.; Kim, D.H.; Jeong, H.G. Suppressive effects of Platycodon grandiflorum on the progress of carbon tetrachloride-induced hepatic fibrosis. Arch. Pharm. Res. 2004, 27, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jin, S.W.; Kim, H.G.; Khanal, T.; Hwang, Y.P.; Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Platycodi Radix attenuates dimethylnitrosamine-induced liver fibrosis in rats by inducing Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 2013, 56, 231–239. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Kim, H.G.; Khanal, T.; Song, G.Y.; Nam, M.S.; Lee, H.S.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Saponins, especially platycodin D, from Platycodon grandiflorum modulate hepatic lipogenesis in high-fat diet-fed rats and high glucose-exposed HepG2 cells. Toxicol. Appl. Pharmacol. 2013, 267, 174–183. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Kim, S.J.; Lee, H.S.; Chung, Y.C.; Kim, E.J.; Lee, Y.C.; Jeong, H.G. Saponins from the roots of Platycodon grandiflorum ameliorate high fat diet-induced non-alcoholic steatohepatitis. Biomed. Pharmacother. 2017, 86, 205–212. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yoo, D.S.; Choi, C.W.; Cha, M.R.; Kim, Y.S.; Lee, H.S.; Lee, K.R.; Ryu, S.Y. Platyconic acid A, a genuine triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules 2008, 13, 2871–2879. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Qian, S.; Liu, L. Evodiamine ameliorates liver fibrosis in rats via TGF-β1/Smad signaling pathway. J. Nat. Med. 2018, 72, 145–154. [Google Scholar] [CrossRef]
- Hu, Z.; You, P.; Xiong, S.; Gao, J.; Tang, Y.; Ye, X.; Xia, Y.; Zhang, D.; Liu, Y. Carapax Trionycis extracts inhibit fibrogenesis of activated hepatic stellate cells via TGF-β1/Smad and NFκB signaling. Biomed. Pharmacother. 2017, 95, 11–17. [Google Scholar] [CrossRef]
- Yoshida, K.; Matsuzaki, K. Differential Regulation of TGF-β/Smad Signaling in Hepatic Stellate Cells between Acute and Chronic Liver Injuries. Front. Physiol. 2012, 3, 53. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, Q.; Huang, Q.X.; Liu, J.T.; He, Y.H.; Lu, J.J.; Bai, X.Y. Activation of PPARγ is required for hydroxysafflor yellow A of Carthamus tinctorius to attenuate hepatic fibrosis induced by oxidative stress. Phytomedicine 2013, 20, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, J.; Lu, H.; Zhang, G.; Liu, Y.; Wang, J.; Zhang, Y.; Shang, H.; Ji, H.; Chen, X.; et al. MicroRNA-130a and -130b enhance activation of hepatic stellate cells by suppressing PPARγ expression: A rat fibrosis model study. Biochem. Biophys. Res. Commun. 2015, 465, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wu, Y.L.; Li, X.; Zhang, Y.; Xia, K.L.; Cui, B.W.; Lian, L.H.; Nan, J.X. Oligomeric proanthocyanidin derived from grape seeds inhibited NF-κB signaling in activated HSC: Involvement of JNK/ERK MAPK and PI3K/Akt pathways. Biomed. Pharmacother. 2017, 93, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Foo, N.P.; Lin, S.H.; Lee, Y.H.; Wu, M.J.; Wang, Y.J. α-Lipoic acid inhibits liver fibrosis through the attenuation of ROS-triggered signaling in hepatic stellate cells activated by PDGF and TGF-β. Toxicology 2011, 282, 39–46. [Google Scholar] [CrossRef]
- Kuo, L.M.; Kuo, C.Y.; Lin, C.Y.; Hung, M.F.; Shen, J.J.; Hwang, T.L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 2014, 19, 3327–3344. [Google Scholar] [CrossRef]
- Ni, M.M.; Xu, T.; Wang, Y.R.; He, Y.H.; Zhou, Q.; Huang, C.; Meng, X.M.; Li, J. Inhibition of IRF3 expression reduces TGF-β1-induced proliferation of hepatic stellate cells. J. Physiol. Biochem. 2016, 72, 9–23. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68–69, 435–451. [Google Scholar] [CrossRef]
- Khanal, T.; Choi, J.H.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Protective effects of saponins from the root of Platycodon grandiflorum against fatty liver in chronic ethanol feeding via the activation of AMP-dependent protein kinase. Food Chem. Toxicol. 2009, 47, 2749–2754. [Google Scholar] [CrossRef]
- Greuter, T.; Malhi, H.; Gores, G.J.; Shah, V.H. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: Exploiting similarities and differences in pathogenesis. JCI Insight 2017, 2, 95354. [Google Scholar] [CrossRef]
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 11033–11053. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Murata, M.; Yamaguchi, T.; Matsuzaki, K. TGF-β/Smad signaling during hepatic fibro-carcinogenesis (review). Int. J. Oncol. 2014, 45, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Alsamman, M.; Scholten, D.; Weiskirchen, R. Endoglin in liver fibrogenesis: Bridging basic science and clinical practice. World J. Biol. Chem. 2014, 5, 180–203. [Google Scholar]
- Li, J.; Li, X.; Xu, W.; Wang, S.; Hu, Z.; Zhang, Q.; Deng, X.; Wang, J.; Zhang, J.; Guo, C. Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver Int. 2015, 35, 1222–1233. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Yin, L.; Xu, L.; Qi, Y.; Xu, Y.; Sun, H.; Lin, Y.; Liu, K.; Peng, J. Potent effects of dioscin against liver fibrosis. Sci. Rep. 2015, 5, 9713. [Google Scholar] [CrossRef]


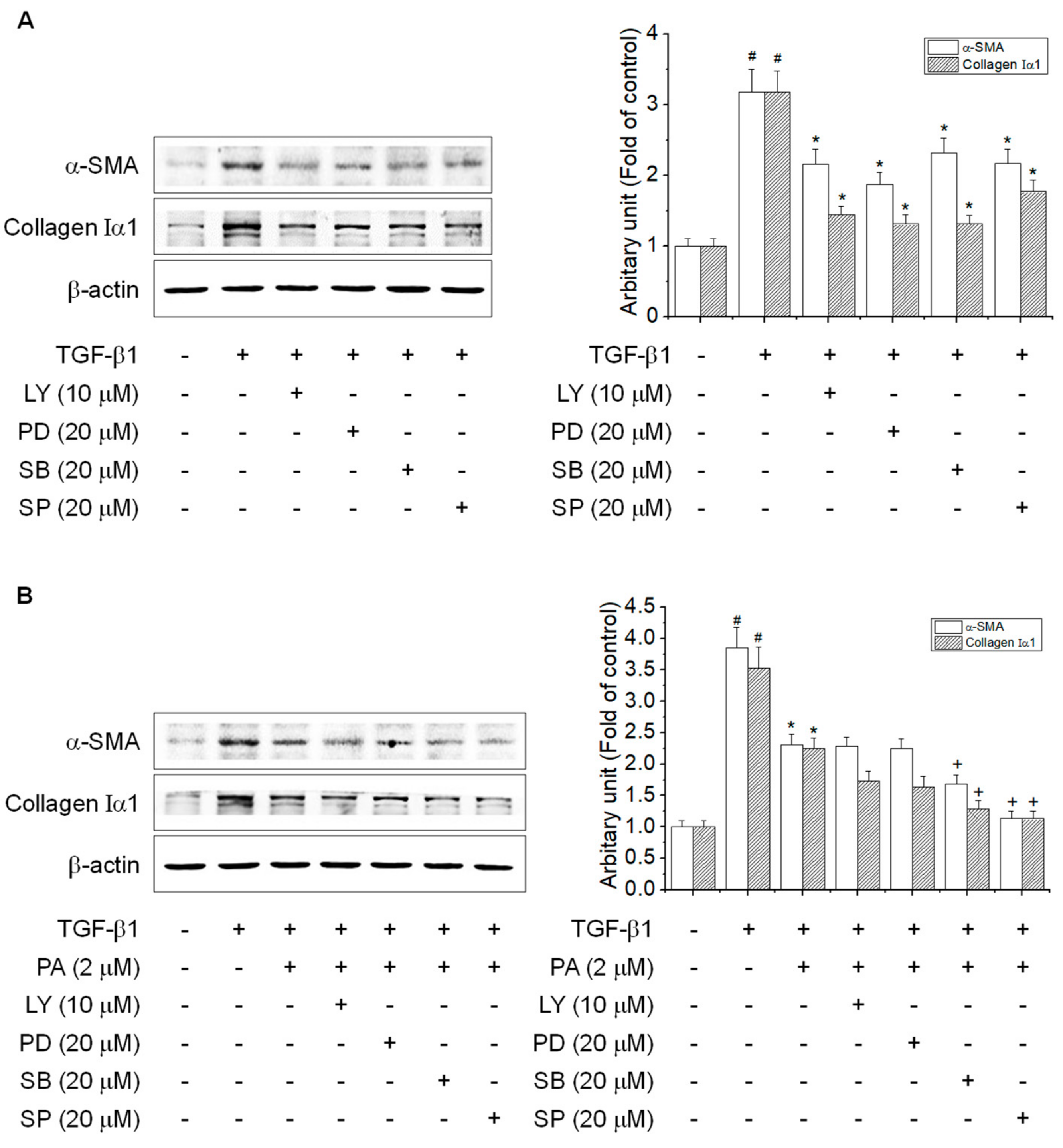
| Gene | Forward | Reverse |
|---|---|---|
| α-SMA | CATCACCAACTGGGACGACA | TCCGTTAGCAAGGTCGGATG |
| ColIa1 | AATCAGCTGGAGTTTCCGTG | TTGGAAACCTTGAGGACCAGG |
| GAPDH | GGCAAGTTCAATGGCACAGT | AAGGTGGAGGAATGGGAGTT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Kim, S.M.; Lee, G.H.; Jin, S.W.; Lee, H.S.; Chung, Y.C.; Jeong, H.G. Platyconic Acid A, Platycodi Radix-Derived Saponin, Suppresses TGF-β1-Induced Activation of Hepatic Stellate Cells via Blocking SMAD and Activating the PPARγ Signaling Pathway. Cells 2019, 8, 1544. https://doi.org/10.3390/cells8121544
Choi JH, Kim SM, Lee GH, Jin SW, Lee HS, Chung YC, Jeong HG. Platyconic Acid A, Platycodi Radix-Derived Saponin, Suppresses TGF-β1-Induced Activation of Hepatic Stellate Cells via Blocking SMAD and Activating the PPARγ Signaling Pathway. Cells. 2019; 8(12):1544. https://doi.org/10.3390/cells8121544
Chicago/Turabian StyleChoi, Jae Ho, Seul Mi Kim, Gi Ho Lee, Sun Woo Jin, Hyun Sun Lee, Young Chul Chung, and Hye Gwang Jeong. 2019. "Platyconic Acid A, Platycodi Radix-Derived Saponin, Suppresses TGF-β1-Induced Activation of Hepatic Stellate Cells via Blocking SMAD and Activating the PPARγ Signaling Pathway" Cells 8, no. 12: 1544. https://doi.org/10.3390/cells8121544
APA StyleChoi, J. H., Kim, S. M., Lee, G. H., Jin, S. W., Lee, H. S., Chung, Y. C., & Jeong, H. G. (2019). Platyconic Acid A, Platycodi Radix-Derived Saponin, Suppresses TGF-β1-Induced Activation of Hepatic Stellate Cells via Blocking SMAD and Activating the PPARγ Signaling Pathway. Cells, 8(12), 1544. https://doi.org/10.3390/cells8121544





