Abstract
Cardiac Progenitor Cells (CPCs) show great potential as a cell resource for restoring cardiac function in patients affected by heart disease or heart failure. CPCs are proliferative and committed to cardiac fate, capable of generating cells of all the cardiac lineages. These cells offer a significant shift in paradigm over the use of human induced pluripotent stem cell (iPSC)-derived cardiomyocytes owing to the latter’s inability to recapitulate mature features of a native myocardium, limiting their translational applications. The iPSCs and direct reprogramming of somatic cells have been attempted to produce CPCs and, in this process, a variety of chemical and/or genetic factors have been evaluated for their ability to generate, expand, and maintain CPCs in vitro. However, the precise stoichiometry and spatiotemporal activity of these factors and the genetic interplay during embryonic CPC development remain challenging to reproduce in culture, in terms of efficiency, numbers, and translational potential. Recent advances in biomaterials to mimic the native cardiac microenvironment have shown promise to influence CPC regenerative functions, while being capable of integrating with host tissue. This review highlights recent developments and limitations in the generation and use of CPCs from stem cells, and the trends that influence the direction of research to promote better application of CPCs.
1. Cardiac Regeneration—A Problem to Solve or A Solution with Promise?
With morbidity rates associated with cardiovascular diseases in the decline in the developed world from improved treatments and pharmacological intervention, scientists and clinicians have been approaching therapies recently for these diseases with vigor. However, there is still no reliable therapy for acute cardiac conditions like myocardial infarction (MI), which account for nearly half of all cardiovascular deaths in the industrialized world [1,2]. Regenerative medicine-based strategies for infarcted myocardium have shown promise in preclinical animal models as well as early clinical trials [3]. Whilst these have demonstrated some physiological improvements in ventricular function, they were associated with very low cell retention after some weeks, suggesting a paracrine effect of transplanted cells rather than functional integration within the damaged tissue [4].
The heart was long viewed as a post-mitotic or terminally differentiated organ with no ability to regenerate or repair, a dogma that has been challenged abundantly in recent years [5,6]. Cardiac regeneration, following injury, is still an unresolved debate over whether it is attributed to dedifferentiation and proliferation of resident cardiomyocytes or from an inherent trigger in differentiation of cardiac stem or progenitor cells in putative cell niches within the heart [7,8,9,10,11]. The turnover of cardiomyocytes in the adult heart is around 1% per year which is insufficient to counter the loss caused by MI that can lead to loss of around 1 billion cardiomyocytes [12]. Therefore, the only long-term solution relies on heart transplantation, but this does not come without its own issues such as insufficient number of donors coupled with the requirement for a life-long immunosuppressive therapy. This catapulted research towards cell-based therapies for cardiac regeneration [13]. Cardiomyocytes are the main cardiac cell type that is lost in cardiovascular disorders, like heart failure, myocardial infarction, and ischemia, and therefore, replacing these cells could potentially restore heart function. However, transplanting cardiomyocytes to repair diseased hearts has shown to yield only transient responses as most cells are eventually lost in the host environment [14,15]. This is because cardiomyocytes have very limited proliferative ability and as a result, they are unable to repopulate and replenish the damaged tissue efficiently [16,17]. Furthermore, other cell types like smooth muscle cells, and endothelial cells can suffer from collateral damage and their functional renewal is vital for effective heart regeneration [18]. This puts emphasis on the role of a precursor cell type capable of extensive expansion and differentiation into the key cell players of cardiac regeneration.
Even though some level of cell turnover has been observed in the adult heart, cells with self-renewal or potency capabilities are generally considered lacking in this tissue [19]. Nevertheless, several studies report the evidence of a progenitor population from resident cardiac stem cells (CSCs) in the heart, called Cardiac Progenitor Cells (CPCs) [20,21,22,23]. In contrast to terminally differentiated cardiomyocytes, CPCs are highly proliferative and can theoretically differentiate into all the necessary cardiac cell types for effective reconstitution of damaged cardiac tissue and promoting its neovascularization [14,18,20,21,24,25,26,27,28,29,30,31]. Therefore, CPCs present a more effective cell source than cardiomyocytes for cell-based regenerative strategies. However, the application of CPCs has not been straight-forward particularly in chronic infarcts, where CPCs are associated with senescence, decreased telomerase activity and increased apoptosis [7]. Cell therapy using CPCs generally involve transplantation of in vitro-expanded CPC populations which in turn yield mild improvements in cardiac function [32]. However, long term prognosis with such therapies are poor owing to reduced cell viability and inefficient engraftment into the host tissue. This is compounded by the somewhat hostile microenvironment created by MI, from scar formation and associated inflammatory or tissue alterations, which compromises the effectiveness of such therapies [33,34,35]. There are also reports that the administration of CPCs predisposed the risk of cardiac arrhythmias and teratoma formation [36]. Therefore, better understanding of the CPC cell behavior in dynamic pathophysiological microenvironments could aid in developing strategies to optimize their contribution to cardiac repair.
Various approaches have been developed to generate CPCs ex vivo, in the hope of obtaining reliable source of cells that can trigger mechanisms of cardiac regeneration. For example, CPCs from the heart tissue (also known as putative CPCs) can be isolated and expanded in vitro [27,37,38,39,40]. However, such cells are hard to access and are present in low numbers in the tissue, making them extremely challenging to harvest and realize their potential [41]. Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), are thought to be a superior alternative cell source since they could potentially provide an unlimited supply of cardiac progenitor cells. However, ESC-based therapy faces several challenges like immunogenicity, high risk of tumor formation and the characteristic ethical concerns, which have prevented their clinical application [42,43]. On the other hand, iPSCs avoid the ethical issues associated with ESCs and allows for the development of patient-specific derived CPCs, which represents an advantage over other cell sources in the creation of immune-compatible cardiac therapies [44,45]. However, with issues surrounding the safety of iPSC-based therapies, in terms of the potential risk of tumor formation associated with such therapies or immune rejection of iPS-derived cells from a common donor, scientists are looking at reprogramming from a different perspective [46,47,48]. Reprogramming patient somatic cells into other cell types, bypassing the step of stem cell generation, can potentially overcome issues with translating iPSC technology. This process is known as direct cellular reprogramming or transdifferentiation, and might represent a more robust approach to rapidly generate sufficient numbers of CPCs from somatic cells for therapy [49].
This review focuses on the ongoing progress and limitations in generating CPCs from iPSCs and through direct reprogramming. It will start by providing a concise introduction about the various cardiac progenitor cells identified in embryonic and adult heart tissues. The review will then move towards discussing reprogramming approaches that were successful in generating CPCs and the functionality of these CPC-derived cells. Strategies to improve efficiencies of current protocols and tissue engineering advances to mimic CPC microenvironment and in vivo applications of CPCs will also be evaluated. Finally, the review will finish with a summary of existing challenges and limitations and future directions for CPC research, hopefully convincing readers it is a promising strategy for cardiac regeneration (Figure 1).
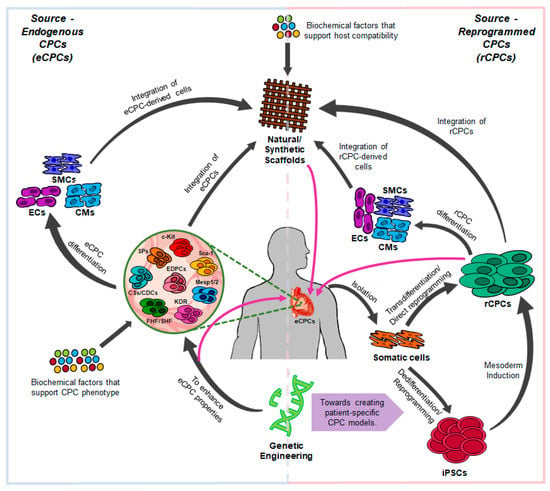
Figure 1.
The interplay between genetics and biomaterials for understanding Cardiac Progenitor Cells (CPCs) biology, function, and its regenerative applications. eCPCs (endogenous CPCs), rCPCs (reprogrammed CPCs), iPSCs (induced Pluripotent Stem Cells), SPs (Side Population-derived CPCs), CSs/CDCs (Cardiospheres/Cardiosphere-Derived Cells), EDPCs (Epicardium-derived CPCs), FHF/SHF (First Heart Field-/Second Heart Field-derived CPCs) CMs (Cardiomyocytes), SMCs (Smooth Muscle Cells), ECs (Endothelial Cells).
2. Cardiac Progenitor Cells (CPCs) In Vivo
Progenitor cells are distinct from embryonic stem cells as they have a predetermined differentiation fate and therefore, their ability to self-renew and differentiate into other cell types is restricted [19]. CPCs generate cells of the three cardiac lineages: cardiomyocytes, smooth muscle cells and endothelial cells. These cells are also responsible for the maintenance of cardiac homeostasis under physiological and pathological conditions [50]. Several studies have identified and isolated multiple CPC populations from distinct stages of cardiac development and heart locations. These cells are collectively characterized according to their cell surface and genetic marker expression profiles. The various CPCs reported to date are described below and their specific features are summarized in Table 1.

Table 1.
Types of CPCs identified in the heart tissue.
2.1. c-KIT-Expressing CPCs
The first-ever detected CPCs were isolated from female rats and were characterized by the expression of the stem cell surface marker c-KIT [28]. These CPCs are present throughout the ventricular and atrial myocardium, particularly in the atria and the ventricular apex [28]. These progenitor cells also express the cardiac transcription factors NKX2.5, GATA4, and MEF2C, and are negative for hematopoietic lineage markers, such as CD45, CD34, CD3, CD14, CD16, CD19, CD20 and CD56 [50,51,52]. They are self-renewing, clonogenic and are able to differentiate into the three cardiac cell types in vitro and in vivo [28,53]. The c-KIT receptor binds to the Stem Cell Factor (SCF) which leads to the activation of the signaling pathways Phosphoinositide 3-kinase/Protein Kinase B (PI3K/AKT) and p38 Mitogen-Activated Protein Kinase (MAPK) [54,55]. These pathways regulate a variety of CPC functions like self-renewal, proliferation, survival, and migration [54,55,56,57]. Even though c-KIT CPCs contribute to the generation of cardiomyocytes at earlier stages of embryonic development and right after birth, this ability is mostly lost in the adult heart and very low percentages of new cardiomyocytes seem to originate from these CPCs [58,59,60]. Therefore, the improvement of cardiac function by c-KIT CPCs might be a result of paracrine factors rather than the production of de novo cardiomyocytes [58,61]. Furthermore, c-KIT expression is considered necessary but not sufficient to define CPCs [62].
2.2. SCA1-Expressing CPCs
Another CPC population present in adult hearts expresses the Stem Cell Antigen 1 (SCA1). The cells were first identified in adult mouse hearts [11] and are predominantly located in the atrium, the intra-atrial septum, the atrium-ventricular boundary and scattered within the epicardial layer [37]. SCA1 is a cell surface protein of the Ly6 gene family and it was initially used to isolate hematopoietic stem cells [63]. Additionally, SCA1 is widely expressed by stem and progenitor cells from a variety of tissues, including the heart, and it has roles in cell survival, proliferation and differentiation [63]. Several studies have shown that SCA1 CPCs are negative for hematopoietic lineage markers and are able to differentiate into the three cardiac lineages [11,64]. These CPCs also have the ability of homing in response to injury and contribute to neovascularization in vivo [11,65,66]. Although this CPC population seems promising for cardiac regeneration, their translational relevance is not without caveats. First, all the SCA1 CPC populations identified to date display different gene expression profiles and distinct differentiation potential [37,66,67,68,69,70,71]. In addition, several studies have shown that the benefits resulted from the transplantation of these CPCs might be predominantly due to paracrine mechanisms as these cells differentiate into cardiomyocytes with very low efficiency [66,68,70,71]. Finally, SCA1 is only present in murine cells and a human ortholog of SCA1 has yet to be identified [63]. Therefore, the nature of the epitope target of SCA1 in humans and the nature of regeneration of the associated CPC population have yet to be determined.
2.3. MESP1/2-Expressing CPCs
During the development of mesoderm, embryonic cells express the transcription factor Mesoderm Posterior Protein 1/2 (MESP1/2), which is essential for proper cell migration [15,72,73,74]. MESP1/2 expression marks the first step in the commitment of the nascent mesoderm into the myocardial lineages, and it describes the first population of multipotent cardiac progenitor cells that produce the various cardiac cell types of the heart [72,75]. Although MESP1/2 CPCs show increased cardiac potential, in comparison to other CPC types, they are not irreversibly committed to the cardiac fate [76]. Consequently, there is a possibility that these cells will differentiate into derivates of the paraxial mesoderm and skeletal muscle [77,78]. Furthermore, MESP1/2 is only transiently expressed during embryonic development, which increases the difficulty of tracking the expansion and differentiation of the CPCs [79,80].
2.4. KDR/FLK1-Expressing CPCs
During cell movement from the primitive streak to the anterior regions of the embryo, the precardiac mesodermal cells start to express a receptor for Vascular Endothelial Growth Factor (VEGF) called KDR/FLK1 [20,81]. These FLK1-expressing progenitor cells have the ability to generate cells from both hematopoietic and cardiac lineages [20,81,82,83]. Selection between these two lineages is determined by the levels of FLK1 activity [81]. For example, high expression of FLK1 promotes differentiation towards hematopoietic lineages, whereas low or absent FLK1 expression stimulates cells to follow the cardiac fate [81,82]. These negative FLK1-expressing cells further generate a second FLK1+ cell population that represents the first multipotent cardiac progenitor cells that are permanently committed to the cardiogenic fate [20,29]. Because KDR/FLK1 displays a broad expression, it is frequently used in combination with other cardiac markers, such as Platelet-Derived Growth Factor-alpha (PDGFRα), C-X-C chemokine Receptor type 4 (CXCR4) and sometimes MESP1/2, to enrich for CPCs [79,80].
2.5. CPCs from the First and Second Heart Fields
The cardiac mesoderm contains two unique progenitor cell pools that give rise to the primary and secondary heart fields [20,22]. The two fields develop sequentially and display distinctive molecular profiles that lead to the formation of different heart components. CPCs of the first heart field (FHF) express the transcription factor NKX2.5, whereas CPCs from the second heart field (SHF) express the transcription factor ISL1 [15,22,40,75,84]. FHF-derived CPCs are more difficult to isolate owing to a lack of unique markers except for NKX2.5 [84]. The hyperpolarisation-activated nucleotide-gated cation channel HCN4 has been suggested as an additional marker for FHF, however, this marker might isolate a more restricted CPC that preferentially generates cells of the conduction system [85,86,87]. Regardless of the markers, FHF-CPCs predominantly differentiate into cardiomyocytes and have some tendency towards smooth muscle lineages [21]. On the other hand, ISL1 CPCs can generate cells of all the three cardiac lineages and they are responsible for producing most of the cardiomyocytes (around 40%) during heart development [22,30,40]. In addition, these CPCs have also been identified in the adult heart, specifically in the outflow tract, atria and right ventricle [30,40].
2.6. Epicardium-Derived CPCs
Several studies have demonstrated that a specific CPC population present in the postnatal and adult heart is derived from the epicardium. They express the transcription factor Wilms tumor 1 (WT1) and are originally derived from CPCs of the second heart field [88]. Additionally, these CPCs emerge from epicardial cells that migrate into the myocardium and undergo epithelial-to-mesenchymal transition (EMT) [88,89]. The epicardial-derived CPCs can differentiate into several different cell types such as coronary smooth muscle cells, cardiomyocytes, endothelial cells, perivascular and cardiac interstitial fibroblasts, albeit with varying efficiencies [51,88,89,90,91,92,93]. Even though WT1 CPCs could potentially be an additional cell source for cardiac regeneration, these cells seem to share some characteristics with c-KIT CPCs: they participate in cardiomyocyte formation during cardiac development but are present at extremely low levels in the adult heart [9,88,90,92]. Stimulatory factors like peptide thymosin beta 4 (Tβ4) can potentially reactivate the developmental program of adult epicardial cells, however, the reactivated cells still exhibit distinct phenotype from their embryonic counterparts, raising doubts about their cardiogenic potential [92].
2.7. Side Population-Derived CPCs
Side populations (SPs) have been detected in various tissues, including the heart, and are enriched for stem and progenitor cell activity [38,94,95,96,97,98]. These cells are generally identified in vitro by their ability to export the DNA Hoechst dye from their nuclei when stained [94,95]. This dye efflux is performed by an ATP (Adenosine Triphosphate)-binding cassette transporter (also known as ABC transporter protein) that is present in their cellular membranes [94,95]. The phenomenon causes the side population cells to contain much lower concentrations of the dye in their nuclei compared to other cell types, allowing for their isolation using cell sorting techniques [95]. The main ABC transporter protein used to identify cardiac SPs is the ABCG2, which was demonstrated to have a role in stem cell proliferation and differentiation and is expressed in SP cells during early development and in the postnatal heart [38,94,95,99]. These cardiac SPs can be found in the perivascular and interstitial areas of the heart, and display self-renewal, homing and multipotency [94,97,100,101,102]. Noseda et al. (2015) demonstrated that cardiac SPs, co-expressing SCA1 and PDGFRα, displayed high clonogenicity and multilineage potential [103]. They particurlaly demonstrated that clones derived from cardiac SPs subjected to long-term propagation (more than 10 months and 300 doublings) still resembled freshly isolated SP cells, showing maintainance of phenotype, self-renewal and tri-lineage capacity and absence of replicative senescence. However, cardiac SPs exhibit a few disadvantages that could potentially prevent their clinical application. For instance, the differentiation potential of human SPs has not been thoroughly investigated [38]. In addition, the multipotency of SPs might be attributed to their heterogeneous nature as they are composed of several subpopulations with distinct differentiation potential (cardiac, hematopoietic and mesenchymal) [38,104]. Therefore, it is still inconclusive on which markers can predict the SP subpopulation with the best cardiac potential.
2.8. Cardiosphere-Derived CPCs
Cardiospheres contain a mixture of stromal, mesenchymal and progenitor cells that are isolated from human heart biopsy cultures [39,52]. They represent a niche-like environment containing a mixture of cells, with cardiac-committed cells in the center and supporting cells, such as mesenchymal and endothelial progenitor cells, in the periphery of the spherical cluster [105,106]. Many cells can be harvested from these cell clusters and they are called cardiosphere-derived cells (CDCs) [52,105]. However, like in the case of c-KIT and epicardial CPCs, the regenerative potential of CDCs is questionable as it has been shown that cardiac repair by these cells mainly results from paracrine mechanisms rather than cell generation [105].
3. Generation of CPCs from Human iPSCs
Native CPCs are present in very low numbers in the heart tissue, and therefore, a larger source of cells is required for efficient cardiac regeneration [41]. The reprogramming of human adult somatic cells into embryonic stem cell-like cells (known as iPSCs) using defined factors opened new possibilities for the generation of patient-specific pluripotent cells. In turn, human iPSCs could potentially offer an unlimited source of differentiated cells and in the process, offer the chance to recreate the development process of CPCs in vitro [121]. This section will provide a detailed description and assessment of current methods used to induce, expand and maintain CPCs derived from iPSCs.
Several techniques have been developed to modulate cardiac differentiation in iPSCs (Table 2). However, the efficiencies for cardiac differentiation can vary considerably between iPSC lines [121,122,123,124]. Regardless of the type of culture employed, the first step in all protocols involves the dedifferentiation of a chosen cell type into a pluripotent state using conventional reprogramming factors, such as OCT4, SOX2, KFL4 and c-MYC [44,45]. Once pluripotency has been achieved, the following step is to induce cardiac differentiation of the iPSCs. Different methods have been employed to accomplish differentiation of iPSCs into cardiomyocytes: embryoid body (EB); monolayer-based cultures supplemented with growth factors, serum or small molecules, matrices, and co-culture with visceral endodermal stromal (END2) layers [15,122,125]. Recent protocols utilise a monolayer culture with a serum-free medium, such as mTeSR1 or E8 medium, which maintains iPSC pluripotency and self-renewal in a feeder-free culture [126,127]. Unfortunately, these studies predominantly focused on the generation of iPSC-cardiomyocytes and not necessarily the homogeneity of CPC population entering the cardiac lineages.

Table 2.
Protocols producing CPCs as target cells or as intermediate cells from iPSCs.
In addition to the nature of the pluripotent culture employed, the type and timing of growth factors and/or small molecules added throughout the protocol affects cardiomyocyte differentiation efficiency. Early differentiation protocols only employed growth factors that modulate key signaling pathways involved in cardiomyogenesis, like Bone Morphogenic Protein (BMP), Activin/Nodal and Fibroblast Growth Factor (FGF) signaling pathways [15,128]. Such factors included Activin A, BMP2/4 and FGF2 which induce cardiac mesoderm formation in iPSCs [15,29,122,128,129]. Lian et al. (2012) demonstrated that iPSC differentiation towards cardiac lineages could be accomplished by exclusively using small molecule modulators of the Wingless (WNT) signaling pathway [130]. Minami et al. (2012) also showed that combining analogous WNT modulators during the early and middle stages of the cardiac differentiation process can further enhance the protocol’s efficiency [131]. Many protocols rely on adding a Glycogen Synthase Kinase (GSK3) inhibitor, such as CHIR99021 (CHIR), to the medium for 24 h to activate the canonical WNT signaling [126,130,132]. Induction of the canonical WNT signaling stimulates the expression of the mesoderm marker Brachyury (T) in undifferenced iPSCs, initiating mesoderm induction [126,132]. Once T+ cells have been established, the WNT signaling is then suppressed to direct the mesodermal cells towards the cardiac fate [126]. Several inhibitory molecules can be used, like XAV939, inhibitor of WNT production (IWP), inhibitor of WNT response (IWR) or an exogenous β-catenin small hairpin RNA (shRNA). After 3/4 days of WNT signaling suppression, iPSC-derived T+ mesodermal cells begin to express cardiac transcription factors, like NKX2.5, ISL1, FLK1, and PDGFRα, which transitions into the CPC population.
More recent studies have been successful in generating CPCs from iPSCs using a single small molecule, potentially reducing costs, time and labor. For instance, the immunosuppressant cyclosporin-A (CSA) was shown to stimulate differentiation of FLK1-positive mesodermal cells into FLK1+/CXCR4+/VE-cadherin− CPCs and cardiomyocytes [133,134]. When CSA was added to the medium, the CPC and cardiomyocyte yield was 10 to 20 times higher compared to untreated cells. Additionally, the generated cardiomyocytes exhibited molecular, structural and functional properties of adult cardiomyocytes. However, additional factors and/or other protocols may be required to produce cells from the other cardiac lineages as FLK1+/CXCR4+/VE-cadherin− CPCs have an exceptionally low endothelial potential and cannot differentiate into smooth muscle cells [110,133]. Furthermore, the study used co-culture with END2 cells to induce cardiac differentiation in iPSCs, which prevents reproducibility of the protocol due to the presence of END2-derived growth factors at unknown concentrations [135]. Another study also demonstrated that treating human iPSCs with the cardiogenic small molecule isoxazole (ISX-9) for 7 days stimulated the expression of CPC markers [136]. These CPCs expressed NKX2.5, GATA4, ISL1, and MEF2C and were able to generate cardiomyocytes, smooth muscle cells and endothelial cells under basal differentiation conditions. Furthermore, ISX-9 seems to modulate key signaling pathways involved in cardiomyogenesis, like VEGF, Activin A and canonical and non-canonical WNT signaling. The study also demonstrated that the small molecule might participate in CPC generation by upregulating activators involved in both canonical and non-canonical WNT pathways in a temporal and sequential manner (WNT3A at day 3 and WNT5A and WNT11 at day 7, respectively).
Therefore, the application of iPSC technology in CPC research has great prospects for improving current cardiac regeneration approaches through the development of novel cell therapies, disease models and drug screens. However, most studies using iPSCs in cardiac regeneration predominantly focus on producing cardiomyocytes and improving their maturation [15,126,130,137,138,139,140,141,142]. Current knowledge about associating this with the generation of iPSC-CPCs, however, remain limited.
4. Direct Reprogramming into CPCs
The discovery of iPSC reprogramming prompted studies to evaluate if it would be possible to reprogram somatic cells directly into other cell types without an iPSC intermediate stage, a process known as transdifferentiation or direct reprogramming. Transdifferentiation has shown to be a much quicker process than dedifferentiation into iPSCs, with the former taking only a few days to achieve, whereas the latter can last up to 3 weeks plus differentiation time to produce the desired cell lineages. With the added advantage of avoiding potential cumulative mutation or epigenetic changes, generally associated during complex iPSC reprogramming processes, direct reprogramming of somatic cells can potentially offer a simpler, faster and safer alternative to generate cells compared to iPSC dedifferentiation [41]. Most transdifferentiation studies in the cardiac field involve the generation of fully differentiated cardiac cells, particularly cardiomyocytes, rather than cardiac progenitor cells [159,160,161,162,163,164,165,166,167,168,169,170,171,172]. Potentially, using transdifferentiation protocols to generate CPCs might be a superior approach for regenerative medicine applications. This section focuses on the current approaches that are associated with producing CPCs from direct reprogramming.
4.1. Partial Somatic Cell Reprogramming into CPCs
Some studies have developed transdifferentiation protocols that involve a transient stage of pluripotency of somatic cells before they continue into CPC fates. The use of reprogramming factors (OCT4, SOX2, KLF4 and C-MYC) seems to be enough to initiate resetting of epigenetic memory of somatic cells towards a stem cell path (partial reprogramming), but the factors alone are insufficient to directly activate cardiac lineage-specific genes for directed differentiation [159]. In order to achieve lineage commitment, signaling molecules involved in cardiogenesis, like BMPs, WNT modulators and FGFs, need to be activated in the cultures [14,159], similar to differentiation protocols for cardiomyocytes from iPSCs. One study demonstrated that secondary mouse embryonic fibroblasts can be converted into CPCs using a technique developed by Wang et al. (2014) called Cell Activation and Signaling-Directed (CASD) lineage conversion [165], which combines reprogramming and cardiac-specific factors to induce cell activation and direct cell fate towards cardiogenesis, respectively [14]. Zhang et al. (2016) transiently exposed the mouse fibroblasts to reprogramming medium containing doxycycline and JAK inhibitor 1 (JI1) for 6 days, and then to transdifferentiation medium with CHIR99021 and JI1 for 2 days to induce cardiac differentiation. Following this, the cells are treated with a mixture of CHIR99021, BMP4, Activin A, and SU5402 (inhibitor of FGF, VEGF, and PDGF signaling) for 3 days. The obtained CPCs from this protocol expressed the proliferative marker Ki-67, the typical cardiac transcription factors GATA4, MEF2C, TBX5 and NKX2.5, and the cell surface molecules FLK1 and PDGFRα and were capable of producing cells from the three cardiac lineages. Efe et al. (2011) also demonstrated that transient expression of pluripotent markers (OCT4, SOX2, KLF4 and C-MYC) followed by exposure to chemically defined media containing BMP4 and the JAK inhibitor JI1 induced cardiac conversion of mouse embryonic and tail-tip fibroblasts [159]. JI1 was added to the reprogramming media for 9 days and from day 9, BMP4 was added and the media was subsequently changed to RPMI supplemented with N2 and B27 lacking vitamin A for 5 additional days. This protocol upregulated the expression of several CPC markers such as NKX2.5, GATA4, and FLK1 by day 9/10.
Wang et al. (2014) were able to significantly reduce the number of reprogramming factors to successfully stimulate cardiac transformation in mouse fibroblasts [165]. This protocol involved a single transcription factor (OCT4) and a cocktail of small molecules: an activin A/TGF-β receptor (ALK4/5/7) inhibitor (SB431542), GSK inhibitor (CHIR), Lysine (K)-Specific Demethylase 1 (LSD1/KDM1) inhibitor (parnate/tranylcypromine) and an adenylyl cyclase activator (forskolin). Mouse fibroblasts were first exposed to the reprogramming media, containing the small molecules, for 15 days. This was followed by media change to RPMI supplemented with N2 and B27 lacking vitamin A and addition of BMP4 during the first 5 days. CPC markers, like FLK1, MESP1, ISL1, GATA4, and Ki-67, can be detected around days 15–20. These cells went on to differentiate into cardiomyocytes, endothelial cells and smooth muscle cells under specific conditions. Another study developed an entirely chemical reprogramming protocol that utilised a larger combination of small molecules compared to Wang et al. (2014): CHIR, the ALK5 inhibitor RepSox, forskolin, the histone deacetylase (HDAC) 1 inhibitor valproic acid (VPA), parnate and the retinoid pathway activator TTNPB [173]. Mouse fibroblasts were exposed to the reprogramming cocktail for 16 days and CPC markers could be detected around day 8-20. The markers identified included SCA1, ABCG2, WT1, FLK1, and MESP1, demonstrating that the protocol can generate CPC populations. Most of the studies described protocols predominantly focused on their ability to generate cardiomyocytes from somatic cells using some iPS factors, and whilst CPCs were observed in some of these studies, their characteristics were not necessarily a focus of their attention and would warrant some investigation in their potency independently.
4.2. Direct Somatic Reprogramming into CPCs
Direct reprogramming of somatic cells involves the transdifferentiation into other cell types without an iPSC intermediate stage. One study showed that CPCs can be directly generated from adult mouse fibroblasts from different tissues (cardiac, lung and tail tip) using a 11- (MESP1, MESP2, GATA4, GATA6, BAF60C, SRF, ISL1, NKX2.5, IRX4, TBX5 and TBX20) or a 5- Factor (MEF2C, TBX5, GATA4 NKX2.5, BAF60C) reprogramming protocol [24]. Both protocols led to the formation of CPCs expressing the genes NKX2.5, MEF2C, MESP1, TBX20, IRX4, and the cell surface protein CXCR4, independently of factor combination and tissue origin of the fibroblasts. The CPCs also showed downregulation of fibroblasts-specific genes, such as FSP1, and could differentiate into the three cardiac lineages. Furthermore, adding a canonical WNT activator, and a JAK/STAT activator during the reprogramming process can increase the protocol efficiency, leading to the production of more CPCs. Even though the 11-factor and 5-factor protocols generated CPCs with comparable characteristics, they differ in the amount of CPC colonies generated, with the former producing more, and in the expression of smooth muscle cell and endothelial cell markers in CPC-differentiated cells, with the 5-factor protocol-based CPCs generating more of these markers than the 11-factor system. Another study showed that human dermal fibroblasts can be directly reprogrammed into CPCs by overexpressing the genes MESP1 and ETS2 [174]. In this specific reprogramming protocol, human dermal fibroblasts are converted into CPCs through a 4-day co-expression of ETS2 and MESP1 using lentiviral vectors, which is then followed by Activin A and BMP2 treatment for another 2 days. Human ETS2 is a transcription factor involved in development, apoptosis and oncogenic transformation and when co-expressed with MESP1, induces the expression of BMP2, initiates the Activin A/Nodal signaling and stimulates the emergence of CD31/PECAM-1 (endothelial cells) and KDR cells (CPCs). ETS2 could potentially be substituted by other ETS transcripts, such as ETS1, FLI1, ETV1, ETV5, ERG and ETV that are also highly abundant in the developing heart, and might function similarly to ETS2 in reprogramming human somatic cells into CPCs.
All these protocols described required the use of viral vectors, usually lentiviruses, to deliver the reprogramming factors into cells. This implied host cell genome changes which could potentially affect its suitability for translational applications. One method that addresses this concern is through the delivery of reprogramming proteins, related to transcription factors, directly into cells. These proteins can modulate the gene expression of cells to convert them into other cell types. For example, using a nonviral-based protein delivery system with the cardiac transcription factors GATA4, HAND2, MEF2C, and TBX5 induces reprogramming of human dermal fibroblasts into CPCs [41]. Additionally, adding growth factors such as BMP4, Activin A and basic Fibroblast Growth Factor (bFGF) can further stimulate and sustain potency towards a CPC state. This combination increased the cellular expression of CPC markers (FLK1 and ISL1) and decreased the expression of fibroblast-specific markers (COL1A2 and FSP1). Furthermore, the protocol demonstrated high efficiency in direct transdifferentiation, converting more than 80% of the human dermal fibroblasts into CPCs.
4.3. Somatic Reprogramming into Cardiospheres
Recent studies have shown that adult skin fibroblasts from mouse and human can be converted into cardiospheres that, in turn, have the potential to generate CPCs [175,176]. For this, the skin cells were first reprogrammed with the Yamanaka factors SOX2, KLF4 and OCT4 overnight, followed by media change to Knockout Serum Replacement-based media for 18 days and finally treatment with the GSK3 inhibitor BIO and Oncostatin for 2 days [175,176]. The resulted cardiospheres resembled endogenous cardiospheres formed from the cellular outgrowth of cardiac explants in vitro [39], but produced a higher number of MESP1, ISL1-, and NKX2.5- expressing cells [175,176]. On passaging, the cardiospheres became enriched with CPCs expressing c-KIT, FLK1 and CXCR4, which were able to differentiate into cardiomyocytes [175]. However, human cardiospheres do not display spontaneous beating and fail to propagate in vitro compared to mouse cardiopsheres, suggesting different signaling pathways being utilized for somatic reprogramming into cardiospheres in both mice and humans [175,176].
4.4. In Vivo Direct Reprogramming
One exciting potential of direct reprogramming is its application in vivo, in which endogenous cardiac cells would be directly converted into CPCs to repair the damaged myocardium. This approach could represent an improvement in promoting cardiac regeneration as it bypasses the several issues associated with cellular transplantation [166,177]. In addition, it avoids the need for cell harvesting, expansion, maintenance, and/or effective delivery systems, which are current challenges faced by cellular in vitro methods. In vivo direct reprogramming takes advantage of the heart native environment that might contain extracellular matrix proteins and growth factors that could make cells more permissive for functional reprogramming and lead to the formation of more mature cardiac cells [160,177,178,179,180]. In a study using an in vivo zebrafish model [181], cardiac ventricular injury induced the expression of Notch and RALDH2 in atrial cardiomyocytes, which caused the cells to lose their sarcomeric organization and re-express CPC transcription factors, such as GATA4, HAND2, NKX2.5, TBX5, TBX20 and MEF2. Once these dedifferentiated atrial cardiomyocytes reached the ventricle, they further expressed ventricle-specific markers, like Iroquois Homeobox Protein Ziro 1 (IRX1A) and ventricular Myosin Heavy Chain (vMHC), and differentiated into ventricular cardiomyocytes. Another study demonstrated that adult murine atrial and ventricular cardiomyocytes can acquire properties of CPCs through spontaneous dedifferentiation in vitro [182]. The dedifferentiated cardiomyocytes gave rise to CPCs that expressed the cardiac markers c-KIT, GATA4, and NKX2.5, self-organised into cardiospheres and were able to differentiate into functional cardiomyocytes and endothelial cells [182]. These results were further investigated by Zhang et al. (2015) in vivo using a MI mouse model [183]. They specifically analysed DNA methylome changes during cardiomyocyte dedifferentiation and observed that cardiomyocyte-specific genes, like Myosin Light Chain Kinase 3 (MYLK3) and Myosin Heavy Chain 6 and 7 (MYH6 and MYH7), became hypermethylated (repressed), whereas cell cycle and proliferation genes, such as Epiregulin (EREG) and SRY-box 4 (SOX4), were hypomethylated (upregulated) in the generated CPCs. This concept could potentially be applied in in vivo CPC reprogramming. However, the molecular mechanisms involved in somatic cell dedifferentiation are not fully elucidated and more information is needed to identify the factors responsible.
Although in vivo reprogramming shows great potential, it has only been employed to derive fully differentiated cardiac cells, specifically cardiomyocytes, and not CPCs as such [160,177,178,179,180,184,185]. Therefore, even though direct reprogramming seems to be a suitable approach to generate CPCs, there are still some issues that influence its application in regenerative therapeutics. These include sub-optimal efficiencies in transdifferentiation protocols for CPC generation and lack of in-depth characteristics of CPC potency, differentiation potential and functionality of their derivatives.
5. In Vitro Culture of CPCs Derived Through Reprogramming Protocols
Establishing reprogramming protocols to generate CPCs from iPSCs and somatic cells is essential to advance CPC research for cardiac regeneration. However, the field also faces issues regarding the isolation, propagation, and expansion of CPCs in vitro. This section focuses on the current methods that have been successful in isolating, expanding and maintaining CPCs in vitro.
5.1. Isolation of CPCs
Isolation of CPCs is usually performed based on their characteristic gene expression patterns and surface markers (see Table 1). For example, ISL1 and NKX2.5 genes are frequently used to identify CPCs [186]. However, these genes are transiently expressed in cells which can lead to the isolation of a heterogeneous cell population containing various CPCs with distinct self-renewal and differentiation potential [186]. When using only cell surface markers, a combination of at least two markers is frequently used as a single surface marker seems insufficient to discriminate a CPC signature. For instance, Nsair et al. (2012) demonstrated that the co-expression of two cell surface markers, FLT1 (VEGFR1) and FLT4 (VEGFR3) specifically identifies ISL1/NKX2.5-expressing CPCs [187]. This combination was also shown to be more effective in identifying homogenous CPC populations (approximately 89% pure) compared to other combinations, such as FLK1 alone or FLK1 with PDGFRα. Furthermore, the isolated CPCs were able to differentiate into the three cardiac lineages and engraft into the host tissue. One study by Nelson et al. (2008) used the cell surface markers CXCR4 and FLK1 to isolate a more restricted CPC from a heterogeneous FLK1 positive population [188]. Zhou et al. (2017) also demonstrated that the marker SIX2 is able to target temporally distinct cell subpopulations from second heart field-associated CPCs [189]. One very recent study (Torán et al., 2019) used proteomic and genomic approaches to comprehensively characterize the proteome of human adult c-KIT CPCs [190]. It was demonstrated that these CPCs highly express 4 surface markers: GPR4 (G protein-coupled receptor 4), CACNG7 (calcium voltage-gated channel auxiliary subunit gamma 7), CDH5 (VE-cadherin) and F11R (F11 receptor) in comparison to mesenchymal stem cells, human dermal fibroblasts and cardiac fibroblasts. More research, however, will be required to further clarify the role of these proteins in CPC functions.
Thus, new markers are continuously being discovered to isolate specific CPC populations. However, they are frequently identified in CPCs derived from neonatal/adult tissue but fewer in ESC-CPCs and iPSC-CPCs [107,133,134,190,191,192]. Further validation of such markers is vital to assign a common signature that accurately identifies these cells.
5.2. Expansion and Maintenance of iPSC-CPCs
The maintenance of β-catenin concentration seems to be an efficient method for CPC expansion in vitro [187,193]. Applying GSK3 inhibitors, like WNT3A, CHIR, or 6-bromoindirubin-3′-oxime/BIO, can promote CPC expansion and suppress myocytic differentiation, leading to the formation of a relatively homogenous CPC colony [193]. Furthermore, the combination of a WNT/β-catenin inhibitor (IQ-1) and a ROCK inhibitor (Thiazovivn) is also able to expand CPCs in a feeder-free medium for a minimum of 4 weeks, while maintaining their multipotent state (more than 90% remained multipotent) [187]. IQ-1 is a selective β-catenin inhibitor that targets the signaling mediated by the protein’s interaction with p300. This suppresses p300 pro-differentiation function and stimulates a pluripotency state. Furthermore, WNT signaling seems to interact with other signaling pathways, such as Notch and FGF signaling, to stimulate the expansion of CPCs [194,195]. For example, activation of the Notch signaling by Notch1 represses expansion, self-renewal and β-catenin activity in CPCs [195]. Activation of both WNT and FGF signaling pathways enhances ISL1 CPCs in a cooperative manner [194]. Therefore, using biomolecules that inhibit and activate the Notch and FGF signaling, respectively, together with WNT activators might facilitate CPC expansion. Notably, inhibition of FGF signaling has also been demonstrated to enhance CPC expansion, but this inhibition is suggested to affect only a subset of CPCs (expressing SCA1) [196] and therefore, warrants further investigation.
Several studies have shown that persistent inhibition of the BMP signaling enhances expansion of CPCs and prevents their differentiation [186,197,198]. For example, the BMP inhibitor Gremlin 2 (GREM2), whose expression initiates in NKX2.5+ CPCs after cardiac mesoderm specification and follows cardiac lineage differentiation, promotes proliferation of CPCs from iPSCs by suppressing the BMP4 receptor activity [197]. This effect was demonstrated to be consistent across distinct iPSCs lines and independent of the differentiation method used. However, GREM2 is also able to induce differentiation of CPCs into the cardiac cell subtypes. Therefore, timing and potency of this BMP antagonist may need careful evaluation to CPCs and avoid spontaneous differentiation. Notably, GREM2 appears to only increase the number of KDRlow and NKX2.5+ CPCs in vitro, and its function seems to be lost in the adult heart. Ao et al. (2012) used a second-generation BMP inhibitor called Dorsomorphin homologue 1 (DMH1) that was able to enrich CPCs, expressing Branchyury, MESP1 and ISL1 markers, from pluripotent cells [198]. Additionally, DMH1 was shown to be a more selective inhibitor of BMP type 1 receptors compared with other BMP inhibitors. This selective inhibition is, therefore, best applied during early stages of cardiac differentiation (pre-mesoderm and cardiac mesoderm stages) in order to increase the number of CPCs.
Another molecule that enhances CPC expansion in vitro is Ascorbic acid (AA) [143]. AA was shown to enhance the expansion of isolated iPSC-derived FLK1+/CXCR4+ CPCs through the MEK-ERK1/2 pathway by promoting collagen synthesis. However, the effects of AA on other CPC types need to be evaluated before AA can be used as a universal factor for efficient CPC expansion. Birket et al. (2015) used a cocktail of molecules modulators of the FGF, VEGF, PDGF, BMP, Nodal, AKT and hedgehog signaling pathways (SU5402, DMH1, SB431542, Insulin-Like Growth Factor 1 (IGF1) and Smoothened Agonist (SAG)) that was capable of expanding CPCs for more than 40 population doublings [186]. However, this study used MYC-transduced iPSC lines and consequently, the method needs further assessment using CPCs derived from non-transgenic iPSCs. Bao and colleagues (2017) developed two protocols, with and without serum, to maintain self-renewal and stimulate expansion of human iPSC-derived epicardial CPCs for long periods of time [152,153]. Both methods involve the addition of a TGF-β inhibitor, such as SB431542 or A83-01, to the medium. The epicardial CPCs can either be cultured in LaSR basal medium, which contains albumin, or in RPMI with ascorbic acid and insulin (RPMI/Vc/Ins), a xeno-free/chemically defined medium. Cells kept in LaSR basal medium can be maintained for up to 2 months, whereas CPCs in RPMI/Vc/Ins can be cultured for approximately 24 days before they start undergoing epithelial-to-mesenchymal transition (EMT) and lose their morphology. The use of a gentler dissociating buffer (Versene) also seemed to improve expansion efficiency of the CPCs from human pluripotent stem cells (iPSCs and ESCs) after 8 passages [152]. One study developed a Good Manufacturing Practice (GMP)-compatible system for the expansion of CPCs, using stirred tank bioreactors and microcarrier technology [199]. Human CPCs from three different donors were inoculated with microcarriers (Cytodex 1 coated with CELLstartTMCTSTM) for up to 7 days. The microcarrier-based stirred cultures lead to a cell suspension increase of 3-fold and greater cell viabilities compared with standard static T-flask monolayers. Furthermore, the CPCs in the culture system expressed the markers CD44, CD105, CD166, KDR, GATA4, and TBX5. This method provides tight control of environmental cues to mimic physiological conditions, which could potentially improve the production of high-quality CPCs for therapeutic applications.
5.3. Expansion and Maintenance of Transdifferentiated CPCs
CPCs derived from direct reprogramming of somatic cells seem to have similar requirements as iPSCs-CPCs for expansion and maintenance. For instance, adding a canonical WNT activator and a JAK/STAT activator to the cultures was shown to maintain the proliferative and multipotent state of the CPCs for several passages (over 20 and 30 passages for a 5- and 11- Factor reprogramming protocol, respectively) without continuous expression of the reprogramming factors [24]. However, CPC maintenance and expansion potential can be negatively affected when utilising somatic cells from tissues other than cardiac tissue, like lung and skin tissues. Furthermore, fibroblast-derived CPCs can be alternatively expanded and maintained using a combination of signaling molecules (BMP4, Activin A, CHIR, and SU5402) that synergistically repress cardiac differentiation and sustain CPC self-renewal [14]. In this case, the CPCs’ undifferentiated morphology, gene expression pattern and cell surface molecule expression remain the same for more than 18 passages regardless of the tissue origin of the donor cells.
Overall, the requirements for in vitro culture of CPCs involved the precise temporal activation and suppression of several signaling pathways. It remains challenging to expand CPCs while maintaining their self-renewal and multipotent differentiation potential as the process is extremely complex, preventing the development of standard conditions yet. This can be more complicated when considering CPCs derived from iPSCs and direct reprogramming and their associated characteristics [186,200,201,202]. Therefore, more comparative studies of current protocols will be imperative to establish standard in vitro culture conditions that are optimal for the isolation, expansion and maintenance of specific CPCs.
6. Strategies to Improve CPC Reprogramming
Strategies for producing CPCs are still developing with time. Whilst the concept of CPC generation through reprogramming or transdifferentiation has taken precedence to produce desired cardiac lineages, the protocols suffer from poor efficiency or lack of mechanistic insight to achieve the target population and desired functional improvement. Strategies to accelerate proliferation and extend replicative lifespan of CPCs are being essentially employed to understand and potentially overcome the inherent limitations of patient CPC populations derived from compromised, aged, or damaged myocardium. With developments in genetic engineering approaches and factors, such as CRISPR gene editing, epigenetic modulators and/or microRNAs, and its significance in cardiac development, there seems scope for applying this in the field of CPC regeneration and address some of the current limitations. This section will describe examples of such strategies in the context of CPCs.
6.1. Genetic Engineering with PIM1
Genetic engineering with PIM1, has been applied in CPCs to enhance their properties, like proliferation, survival and differentiation [203]. Pro-viral insertion site for the moloney murine leukemia virus (PIM1), a proto-oncogene serine/threonine-protein kinase, is highly expressed in bone marrow, tumor cells and fetal heart and is associated with many signaling pathways, mostly related to anti-cell apoptosis and cell cycle regulation [204]. Mohsin and colleagues (2013) genetically modified patient-derived human CPCs (hCPCs) with PIM1 kinase (termed hCPCeP) to increase proliferation, telomere length, survival and decrease expression of cellular senescence markers, rejuvenating the phenotypic and functional properties of hCPCs, in an effort to ameliorate the cumulative effects of age and disease [205]. The PIM1-engineered cells also showed increased commitment to the three cardiac lineages [203]. Interestingly, the effect of PIM1 in hCPCeP normalizes after several rounds of passaging, consistent with the notion that PIM-1 can transiently increase mitosis coupled with telomere stability (increased TERT activity) and without resultant oncogenic transformation through a c-MYC synergy. These properties of hCPCeP can be modulated by targeted localization of PIM1 in mitochondrial or nuclear components, conferring an optimal stem cell trait irrespective of patient-associated cell heterogeneity [206]. Furthermore, intramyocardial injection of hCPCeP into cardiomyopathic challenged-SCID mice demonstrate increased cellular engraftment and differentiation with improved vasculature and reduced infarct size [203]. Similar results were also observed when using murine CPCs [207] but these earlier studies relied largely on viral delivery methods to induce PIM1 overexpression. In an alternative strategy, a non-viral modified plasmid-minicircle (MC) was used as a vehicle to deliver PIM1 into mouse CPCs (mCPCs) in vitro and the myocardium in vivo [208]. Mice with PIM1-MC injection showed increased protection compared to control groups measured by ejection fraction at 3- and 7-days post injury, supporting the potential of a non-cell based therapeutic approach for treatment of ischemic heart disease and MI.
6.2. CRISPR in Context with CPCs
In an effort to identify previously unknown regulators of cardiomyocyte differentiation from human ESCs (hESCs) through quantitative proteomics, Murry lab [209] demonstrated that DAB2 (Disabled 2) plays a functional role in cardiac lineage specification towards cardiomyocytes by being preferentially upregulated in CPCs. CRISPR/Cas9 deletion of Dab2 in zebrafish embryos was used to show increase in WNT/β-catenin signaling and consequent decrease in cardiomyocyte number, suggesting that inhibiting WNT/β-catenin signaling by DAB2 (or analogous inhibitors like the Dickkopf WNT signaling Pathway Inhibitor 1 (DKK1)) can be crucial in maintaining cardiomyocyte numbers from CPCs in the developing heart. Supporting this mechanism, the same lab, using antisense knockdown and CRISPR/Cas9 mutagenesis in hESCs and zebrafish, went on to demonstrate that Alpha Protein Kinase 2 (ALPK2) is temporally expressed during specification of CPCs (but not in endocardial-like endothelial cells), and cardiac commitment through negative regulation of WNT/β-catenin signaling [210]. In a more recent study [211], CRISPR-mediated ablation of Furin gene in mouse CPCs, whose product is a natural target of Nkx2.5 repression during heart development, produces abnormalities in embryo characterized by reduced proliferation of CPCs and their premature differentiation, suggesting Furin mediates some aspects of Nkx2.5 function in heart and is necessary for CPC differentiation. This role of Furin in the maturation of CPCs is, in part, mediated by the modulation of the BMP pathway by Nkx2.5. Therefore, genetic engineering using CRISPR has been pivotal in recent years to identify mechanisms associated with CPCs and continue to show promise with a perpetual trend in CRISPR advances.
6.3. Epigenetic Modulators
Distinct cell types display different epigenetic profiles that leads to differential gene expression. Cellular reprogramming is associated with changes in the epigenetic signature of cells. During these epigenetic transitions, proteins called epigenetic modulators bind to specific regions of the chromatin and regulate the transcription of genes. Therefore, inhibition and/or overexpression of these modulators might affect cardiac reprogramming efficiency [41,212]. For example, knockdown of the polycomb ring finger pro-oncogene Bmi1 in several fibroblast types (murine embryonic, neonatal and adult tip tail and adult cardiac fibroblasts) results in the activation of core cardiac transcription factors, such as GATA4, ISL1 and TBX20, which converts the cells into cardiomyocytes [212]. Additionally, Zhou et al. (2016) demonstrated that silencing of Bmi1 allowed for efficient cardiomyocyte reprogramming using just two factors (MEF2C and TBX5). The induced cardiomyocytes displayed features of advanced maturity, such as contractile activity, sarcomere structures and periodic calcium oscillation. Therefore, it would be useful to investigate the role of Bmi1 in the context of CPC reprogramming, considering the significance of ISL1 upregulation under Bmi1 depletion. Another epigenetic modulator that could potentially be employed in CPC reprogramming is the BAF chromatin remodeling protein BAF60A. BAF60A is thought to have a role in the maintenance of CPC self-renewal thought interaction with TBX1 [213,214]. TBX1 seems to recruit BAF60A onto the promoter region of WNT5A gene, upregulating its expression in CPCs [214]. WNT5A is a non-canonical WNT pathway ligand that is highly expressed in CPCs derived from the SHF, and it cooperates with another non-canonical WNT ligand, called WNT11, to induce development of CPCs from the two heart fields [215]. Accurate identification of the cellular epigenetic barriers could potentially reduce the number of reprogramming factors employed to generate CPCs and ultimately, lead to faster and safer protocols.
6.4. MicroRNAs
MicroRNAs are short non-coding RNA molecules that bind to messenger RNA and repress gene expression. MicroRNAs show a promising alternative to traditional reprogramming protocols as they are easily delivered and display low toxicity in animal models [184]. In addition, several microRNA transcripts can be packed into a single delivery vector, which could potentially increase reprogramming efficiency. However, most studies have mainly examined the use of microRNAs in converting somatic cells directly into cardiomyocytes, not CPCs as such [180,184,216]. Nevertheless, microRNAs have been shown to modulate CPC functions [217,218,219] (see Table 3). Sirish et al. (2012) investigated the miRNA expression changes in CPC development [219]. They identified 8 differentially expressed microRNAs (miR-103, -130a, -17, -130b, -208b, -185, -200b and -486) in mouse neonatal and adult LIN−/c-KIT+ CPCs. The target proteins of microRNAs were predicted to be predominantly involved in cell proliferation, with a few proteins having roles in cell organisation, development, metabolic process, adhesion, homeostasis, activation, communication, and motility. The group also demonstrated that overexpression of the miR-17-92 cluster, which targets cell cycle proteins, in adult CPCs increased their proliferative capacity by 2-fold in vivo. Two studies showed that the microRNAs miR-1, -499 and -204 repress proliferation and stimulate differentiation in committed SCA1+ CPCs [217,218]. Additionally, Xiao et al. (2012) revealed that inhibition of miR-204 suppressed CPC differentiation and promoted proliferation without affecting cell viability [218]. A study in 2016 identified several microRNAs that regulate cardiac fate, like let-7, miR-18, miR-302 and the miR-17-92 cluster, in MESP1+ CPCs [220]. It was also shown that the CPCs were particularly enriched for the miR-322/-503 cluster which targets the CUG-binding protein Elav-like family member 1 (CELF1). Ectopic CELF1 expression promoted neural lineage-specification at the expense of cardiomyocyte differentiation in the CPCs. Therefore, miR-322/-503 may be a key regulator in promoting the cardiac program in early mesodermal cells by cross-suppressing other lineages. Garate et al. (2018) investigated the expression of microRNAs during the differentiation of human pluripotent stem cells (hPSCs) towards mesoderm and cardiac cells [221]. They found several microRNA families (miR-302, C19MC, miR-17/92 and miR-26) that were highly expressed in EpCAM/CD326-negative and NCAM/CD56-positive mesoendodermal progenitor cells (MPCs) [222]. The microRNA families identified were speculated to be associated with the epithelial to mesenchymal transition occurring during the development of mesoderm. However, the specific roles of the microRNAs in CPCs will need to be determined as MPCs are able to generate all the mesoendodermal lineages, including cardiovascular, hematoendothelial and mesenchymal. One very recent study by Cheng et al. (2019) showed that the ischemic heart secretes microRNAs (miR-1a, miR-133a, miR-208a and miR-499) that mobilised LIN−/c-KIT+ bone marrow progenitor cells (BM PCs) into the site of injury, where they proliferated and promoted vascularisation [223]. These results demonstrated the principle of employing microRNAs to target endogenous progenitor cells to enhance ischemic cardiovascular repair. Therefore, as molecular mechanisms regulated by microRNAs during CPC development get explored more, they offer a suitable choice of target for improving CPC generation from iPSCs or for transdifferentiation.

Table 3.
Role of microRNAs in CPC biology.
7. Tissue Engineering with CPCs and CPC-Derived Cardiomyocytes
Several studies have demonstrated that the cells generated from CPCs, particularly cardiomyocytes, display an immature phenotype similar to that of embryonic cardiac cells [3]. However, when the CPCs are transplanted into a host environment, the differentiated cells reach a more advanced maturity, such as greater organisation of sarcomeres and formation of gap junctions (in the case of cardiomyocytes) and development of tubular-like structures (for smooth muscle and endothelial cells) [11,24,27,28]. Furthermore, CPCs seem to have distinct differentiation potential in vitro and in vivo [96,111]. This could mean that the microenvironment of the heart might have a key role in CPC functions. Stem and progenitor cells reside in specific tissue microenvironments, called niches, which provide protection and support to the cells [241]. A way to potentially enhance CPC regenerative potential could be to mimic their microenvironment. Cardiac tissue engineering aims to achieve this goal by combining multiple microenvironment components, such as cells, extracellular matrix (ECM) and biochemical factors like BMP2, VEGF, bFGF, DKK1, and IGF1, to create cardiac tissue constructs. Therefore, determining the ideal matrix for supporting CPCs and their derivatives is paramount. In principle, the scaffold matrix should be biodegradable, immune-privileged, provide electrical and/or mechanical properties for cell coupling and assembly, and support vascularisation [242,243]. Two types of materials are typically employed in the production of scaffolds for tissue engineering: natural matrices and synthetic matrices. This section will describe different types of scaffolds that have been used in combination with CPCs and CPC-derived cardiomyocytes (Table 4).

Table 4.
Cardiac tissue engineering strategies with biomaterials using CPCs.
7.1. Natural Scaffolds
Natural matrices have the advantage of being composed of native ECM cues that modulate cell behavior [243,244]. These scaffolds can comprise pure ECM elements, like hydrogels made from natural materials such as fibrin, alginate, gelatin, and collagen, or acellular tissue which displays the biochemical and biomechanical properties (tensile strength and composition) of the native ECM tissue [245,246]. Three independent studies used a fibrin patch seeded with CPCs (murine and human) to develop a tissue construct, which was then tested in vivo [247,248,249]. Vallée et al. (2012) specifically utilized BMP2-primed murine ESCs seeded onto fibrin matrices as single cells, small cluster and embryoid bodies [249]. These constructs were then engrafted onto myocardial infarcted rat hearts, which led to a reduction in remodeling and deterioration of cardiac functions. Seeded cells were identified by the expression of the cardiac genes MESP1, NKX2.5, MEF2C, TBX6 and GATA4, speculating a CPC-related population. The transplanted cells were also able to colonize the outer connective tissue where they differentiated into cardiomyocytes and promoted neovascularization. The results from Vallée et al. (2012) encouraged two other studies to apply their tissue engineering approach with human CPCs [247,248]. Bellamy et al. (2015) and Menasché et al. (2015) seeded human CPCs, expressing the markers SSEA1 and ISL1, in a fibrinogen patch [247,248]. The two studies differed in the number of CPCs used, Bellamy et al. (2015) used 700,000 cells whereas Menasché et al. (2015) used 4 million cells; and in the in vivo model chosen, myocardial infarction rats and a 68-year-old patient suffering from severe heart failure, respectively. Improvement of contractility and attenuation of ventricular remodeling was observed in both studies. It was also shown that these benefits were likely a result of paracrine factors secreted by the transplanted CPCs rather than de novo generation of tissue. Gaetani and colleagues (2012 and 2015) used 3D printing with SCA1+/CD105+ fetal CPCs, which are referred to as human fetal cardiomyocyte progenitor cells (hCMPCs), and three types of natural scaffolds (pure, RGD-modified alginate and a hyaluronic acid/gelatin-based matrix) [250,251]. The hCMPCs were able to migrate from the scaffolds, colonize the surrounding areas and form tubular-like structures [250,251]. Another study by Christoforou et al. (2013) used murine iPSC-derived CPCs mixed within a fibrin/Matrigel hydrogel that were applied in polydimethylsiloxane (PDMS) molds and cultured for 14 days in vitro [157]. These CPCs expressed NKX2.5, GATA4, c-KIT and either FLK1 or SCA1 and differentiated into mature cardiomyocytes that aligned into unidirectional myofilament and displayed abundant electromechanical connections. This study also concluded that accessibility to oxygen and nutrients within tissue constructs greatly affects integration of the implanted cells.
Native ECM generally comprise of various components such as glycosaminoglycans (GAGs), collagen, fibrinogen, hyaluronic acid and hydroxyapatite (HA) [246]. To mimic this, recent studies have applied natural scaffolds generated from the decellularisation of tissues. This technique removes any cells present in the tissue while preserving its original 3D architecture and ECM. Two studies have combined decellularised scaffolds with iPSC-derived CPCs [252,253]. Lu et al. (2013) used human iPSC-CPCs that were positive (low) for the marker KDR and negative for c-KIT to repopulate a whole decellularised mouse heart [252]. The CPCs differentiated into cardiomyocytes, endothelial cells and smooth muscle cells, and efficiency to a specific lineage could be changed with the addition of growth factors. The recellularised scaffolds displayed vessel-like structures, spontaneous contraction, uniform wave propagation in some regions, and the ECM seemed to stimulate proliferation of CPCs and formation of wider myofilaments of cardiomyocytes. However, drawbacks of this study included the uneven recellularisation of the heart constructs which led to weaker mechanical forces and incomplete synchronization, and inability to generate cells of the conduction system and cardiac fibroblasts. Although natural scaffolds retain the ultrastructure and biological information of the native tissue, there is a risk of immunological reaction, disease transmission (in case of animal-derived materials) and are generally variable in their physical properties [243,245].
7.2. Synthetic Scaffolds
The ideal synthetic scaffold should be biocompatible, degradable, display a surface that allows for cell attachment, migration and differentiation, and a macrostructure that supports cell growth and nutrient and waste exchange [245]. Structure and properties of synthetic scaffolds, like the associated mechanics, chemistry and degradation rate, can be easily customised for the type of cells being used [243,245,246]. Two studies employed self-assembling peptide nanofibres with CPCs and tested the constructs in vivo [254,255]. Both studies used two distinct experimental designs: Padin-Iruegas et al. (2009) seeded adult rat Lin− c-KIT+ CPCs onto nanofibres tethered with IGF1, whereas Tokunaga et al. (2010) used adult mouse SCA1+ CPCs mixed with Puramatrix® (3D Matrix, Ltd.) (no tethered factors). The CPCs in Tokunaga et al. (2010) nanofibres minimally contributed to de novo cardiomyocyte generation and had no differentiation potential towards endothelial lineages [255]. The benefits observed were associated to effects from paracrine signaling. On the other hand, Padin-Iruegas et al. (2009) showed that continued IGF1 release from nanofibres enhanced CPC survival and proliferation, and stimulated differentiation into cardiomyocytes, smooth muscle cells and endothelial cells [254]. Additionally, the regenerated cardiomyocytes were able to couple with resident cardiomyocytes, and the smooth muscle cells and endothelial cells formed vascular structures. These studies demonstrated that functionalising self-assembling peptide nanofibres can potentially support long-term CPC survival, proliferation and differentiation, and lead to a more robust maturity of the CPC-derived cells, especially if applied in the human CPC context. Li et al. (2011) used a solution made of mouse cardiosphere-derived cells and degradable poly(N-isopropylacrylamide) hydrogel and performed in vitro testing of the effects of scaffold stiffness and presence/absence of collagen on the cells’ functions [256]. The hydrogels with medium stiffness and collagen were optimal for cardiosphere-derived cells differentiation into cardiomyocytes, which displayed the highest expression of maturation genes (MYH6 and cTNT). Unfortunately, there were no reports on the effects of the hydrogels on cardiosphere-derived cells differentiation potential towards smooth muscle cells and endothelial cells. Liu et al. (2015) also employed nanofibres with CPCs, but they used poly(l-lactic acid) and mouse ESC-derived CPCs [257]. These CPCs were positive for ISL1 and GATA4 and differentiated into the three cardiac lineages in both in vitro and in vivo conditions. Additionally, differentiation potential towards endothelial lineages was improved in vivo compared to that of in vitro. The scaffolds supported CPC survival, engraftment, proliferation and integration with the host tissue, and stimulated the expression of intercellular coupling proteins (connexin 43) and maturation of cardiomyocytes.
One study used a novel concept called “scaffold-in-scaffold” to promote human CPC growth and differentiation in vitro [258]. The aim of this approach was to create a structure with different physical characteristics to better mimic the ECM microarchitecture. The multitexture 3D scaffold was composed of a polyethylene glycol diacrylate (PEGDa) woodpile and a softer PEGDa hydrogel. Human LIN− SCA1+ CPCs seeded on these scaffolds highly differentiated into cardiomyocytes, which aligned in an orderly manner. However, robust cardiomyocyte maturation, such as sarcomeric organisation and formation of gap junctions, was not achieved. In addition, there were no reports on the differentiation potential towards other cardiac lineages.
Synthetic biomaterials are a great promise to constructing 3D microenvironments with adjustable features. However, they still come with a few limitations, such as poor biocompatibility, incomplete polymer degradation, and some toxicity, that will need to be addressed systematically to achieve better cellular responses.
A significant trend that has been popular with human Pluripotent Stem Cell-Cardiomyocytes (hPSC-CMs), has been the implementation of electrically-compatible scaffolds or biomaterials (in 2D or 3D) compatible with standard electrophysiology measurements to stimulate hPSC-CM electrical behavior and consequently its mature electrophysiological phenotypes (see Table 5). This would be a strategy for exploration with CPCs as we improve our understanding of the CPC niche. Furthermore, while most of the studies described above employed ESC-derived or putative CPCs on scaffolds, studies using patient-specific CPCs from iPSCs or from transdifferentiation in engineered scaffolds to model phenotypes are very rare. Therefore, with potential improvements in cardiac tissue engineering and mechanistic understanding of responses in situ, the CPC niche can be exploited to assess normal and disease-associated cardiac cell behavior to produce better regenerative outcomes (Figure 2).

Table 5.
In vitro cardiac tissue engineering techniques with biomaterials to stimulate and record hPSC-CM electrical activity.
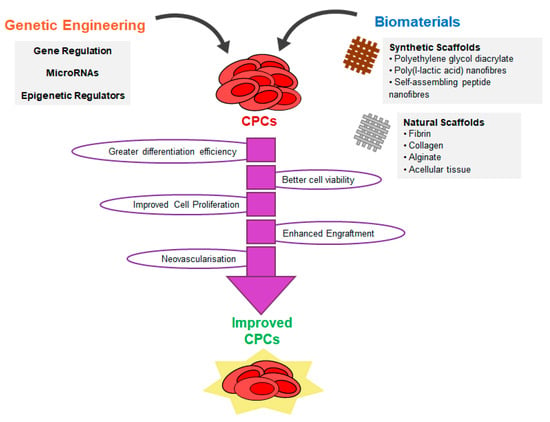
Figure 2.
Promising strategies to improve CPC characteristics and functionality. Strategies for producing CPCs to date through reprogramming or transdifferentiation has been associated with poor efficiency or lack of mechanistic insight to achieve the target population and desired functional improvement. With a range of tools for genetic engineering or gene modulation, and with advances in tissue engineering approaches, new strategies have been applied in this field to accelerate proliferation, enhance differentiation, extend replicative lifespan or improve functionality or engraftment of CPCs (detailed in Section 6 and Section 7).
8. In Vivo Applications of Human CPCs
The end-goal of in vitro and animal in vivo studies in CPC research is to provide enough evidence regarding the efficacy and safety of cell therapies for further application in human trials. This is not without the caveat that, despite promising results from in vitro and animal models, the translation to clinical trials still suffer from serious inefficiencies in desirable outcomes over long term, costing billions of dollars in the process [280]. Even though there is not yet an agreement on the CPC population that displays the best regenerative capacity, a variety of CPCs have been used or are being used in clinical trials, which are summarized in Table 6.

Table 6.
Past and ongoing clinical trials using CPCs.
The first-ever clinical trial using CPCs, called SCIPIO (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy) used human LIN− c-KIT+ CPCs to improve postinfarction left ventricular dysfunction. However, this study has now been retracted due to concerns about the randomisation and lack of integrity of certain data [281,282]. In 2012, the randomised phase I trial CADUCEUS (CArdiosphere-Derived aUtologous stem CELLs to reverse ventricUlar dySfunction) employed cardiosphere-derived cells to reduce scarring after myocardial infarction [283]. These cells were obtained from endomyocardial biopsy specimens and were transplanted into patients 1.5–3 months post-myocardial infarction using intracoronary infusion. The results showed that the cells led to an improvement in viable heart tissue and a reduction of scarring. Differentiation potential of cardiosphere-derived cells towards cardiac lineages remained to be elucidated and thus, it is likely that the benefits observed in the CADUCEUS study were a result of paracrine factors. In the same year, another phase I trial called ALCADIA (AutoLogous human CArdiac-Derived stem cell to treat Ischemic cArdiomyopathy) used autologous human CPCs in combination with a controlled released of bFGF in patients suffering from ischemic cardiomyopathy and heart failure [284,285]. These CPCs expressed the mesenchymal surface markers CD105 and CD90 and were also derived from endomyocardial biopsy specimens. The cells were injected intramyocardially and a biodegradable gelatin hydrogel sheet containing bFGF was then implanted on the epicardium, which covered the injection sites areas. However, as in the case of the CADUCEUS study, the benefits observed, such as attenuation of adverse ventricular remodelling and neovascularisation, were probably due to paracrine mechanisms as there was no compelling evidence that the employed CPCs can differentiate into cardiomyocytes in vivo [284,286]. A more recent trial published in 2018, named ESCORT (Transplantation of Human Embryonic Stem Cell-derived Progenitors in Severe Heart Failure), used hESC-derived CPCs, expressing the markers SSEA1/CD15 and ISL1, embedded in a fibrin gel [287]. The scaffold was then delivered onto the epicardium of the infarct area. The aim of the study was to confirm the safety and feasibility of the therapy rather than evaluating its regenerative effects in the patients. Further investigation will be needed to thoroughly assess the benefits of the fibrin gel patch in severe heart failure.
There are also reports on phase I and II clinical trials assessing the use of autologous cardiosphere-derived cells in paediatric patients suffering from hypoplastic left heart syndrome [288,289]. The phase I TICAP (Transcoronary Infusion of CArdiac Progenitor cells in patients with single ventricle physiology) demonstrated that the approach was safe and feasible for improving cardiac function after 18 months [288]. The safety of the therapy was also analysed at 36 months post-transplantation [290]. There was no tumour formation and the initial observed benefits were enhanced, with attenuation of ventricular stiffness and improvement of ventriculoarterial coupling. The results obtained from TICAP were further confirmed by the phase II PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) [289]. Furthermore, the therapy is currently being tested in a phase III trial (APOLLON) [291] and applied in paediatric patients diagnosed with dilated cardiomyopathy (phase I trial TICAP-DCM: Transcoronary Infusion of CArdiac Progenitor cells in paediatric Dilated CardioMyopathy) [292], for which results are still waiting.
Most trials involving CPCs come with limitations in employing small sample sizes or lack of blinded assessment, which ultimately leads to inconclusive results regarding the therapies’ efficiency in recovering from cardiac disorders. In addition, it is still inconclusive whether the positive results are attributed to intracoronary infusion of CPCs themselves or from paracrine factors as speculated by some trials. It will, therefore, be imperative to perform future clinical trials with a broader assessment of study subjects and an established human reproducible model to better explore the CPCs’ regenerative capacity in human hearts.
9. Current Challenges and Limitations
There is still a lot of debate on the effect that CPCs play a role in cardiac regeneration and repair in the context of diseases like MI, demonstrating increased left ventricular ejection fraction, decreased infarct size, and an increase in hemodynamic function following infusion of autologous CPCs. Even though there is a growing emphasis on the application of CPCs for cardiac regeneration, its impact is still obscure, particularly owing to its heterogeneous nature and mechanistic silencing from deep-rooted complexities associated with the nature of the cardiomyopathic disease. For example, there is still no consensus regarding which CPC population is the ideal cell type for cell-based regenerative therapies and which combination of markers accurately characterise CPCs. Additionally, the characteristic epigenetic, gene, protein and secretome profiles of most CPCs remain unclear [19,41]. This could elucidate how phenotypes and genotypes of CPCs alter throughout their development and their effects on self-renewal and potency potentials. Furthermore, not many studies have investigated and compared the therapeutic efficacy of different CPCs. The ideal CPC type should be able to tolerate autologous transplantation, expand extensively in vitro, differentiate into mature cardiac cell subtypes and integrate with the host cells [299].
Viral transduction remains the main approach applied in most reprogramming processes (both in vitro and in vivo) as it shows the greatest efficiency. However, this is associated with a risk of genome integration and activation of oncogenic genes. In addition, the currently developed protocols require the use of both reprogramming and growth factors which substantially increases their complexity and final cost. It is, therefore, imperative to develop a more effective and simpler gene transfer methods that ensure cell therapies are safe and display a good cost-benefit ratio.
Furthermore, the populations of CPC-derived cells are heterogeneous and frequently represent immature cells, which could potentially lead to arrhythmias, lower long-term stability and poor integration when transplanted [3,300]. The mechanisms involved in cardiac lineage subtype specification will need to be fully investigated and optimised to produce purer and more mature populations of the desired cell types from the CPCs. With the growing pace of CRISPR strategies and its potential to address limitations associated with genetic control and regulation, it will not be surprising that this will be applied to CPCs for this purpose in the very near future.
Epigenetic profiles seem to strongly affect reprogramming efficiencies for both iPSCs and transdifferentiation technologies. For example, using cells from non-cardiac tissue organs or aged tissue negatively affects the cardiogenesis capability of iPSCs [301]. The success of reprogramming a cell fate relies on the ability to overcome the several epigenetic barriers present in somatic cells. The more distinct the donor somatic cells are from the cardiac tissue, the higher the number of epigenetic barriers that need to be overcome and consequently, the harder it is to reprogram the cells. Therefore, understanding the epigenetic regulatory mechanisms involved CPC formation might be vital to improving reprogramming efficiency.
Another limitation in CPC research is that many studies have been performed in rodent models, which display distinct cardiac anatomy and physiology from the human heart. Additionally, current techniques developed using animal cells will need to be further validated for human context. For example, the direct reprogramming protocol involving the three core cardiac genes GATA4, MEF2C and TBX5 (also known as GMT) was demonstrated to induce mouse fibroblasts into cardiac cells, but it was insufficient to convert human fibroblasts [164].
For future preclinical trials, the relationship between the number of CPCs and their effects on cardiac regeneration and the appropriate frequency of administration of each cell therapy needs to be further investigated [299]. In addition, molecules and/or cells are very often directly injected into the heart during open-surgery. This is an invasive approach that could cause additional injury and pain to the patients. Other less invasive methods, such as intracoronary and intravenous injection, have been employed to deliver cells to the heart. However, these techniques rely on correct homing of cells into the damaged tissue, and very often the delivered cells become trapped in other organs [302,303]. Consequently, other delivery systems that are less aggressive and show the best efficacy and safety need to be developed before CPCs can be applied in regenerative medicine strategies.
There are sufficient reports that support the existence of CPCs within specialized niche structures in the myocardium [241]. For therapeutic applications, these CPCs can be isolated and cultured in vitro, prior to transplantation into the affected heart or, the local microenvironment can be modulated to recruit CPCs to the infarct area. Current biomaterial strategies (discussed in Table 4 and Table 5) have exploited both these methods for functional improvements but do not report complete recovery under physiological conditions or pathological insults. This is evident in the lack of clinical trials with CPCs using biomaterials (Table 6). This offers an opportunity to integrate engineering with mechanistic modulation (perhaps through genetic engineering) to contextualize CPC behavior with disease factors.
The difficulties described above rely, to some extent, on the incomplete understanding of the heart development and cardiac regeneration processes. Increasing this knowledge will clarify the precise stoichiometry of the cardiac factors and optimal culture conditions to accurately mimic the development of CPCs in vitro.
10. Final Thoughts—Controversies Surrounding CPCs
It does seem that the debate surrounding CPCs and adult heart repair is taking a full circle—it is there, it is not there, it is there, etc? With the first evidence in rodents supporting the notion of c-KIT+ cells from bone marrow or adult heart to replace damaged myocardial tissue, from Piero Anversa’s lab, and subsequent retractions of 31 papers from his group owing to unreliable data, it has encouraged the field to challenge the theory by more robust techniques in mouse models [58,61,304,305]. Results from such studies showed that cardiomyocyte generation from a c-KIT+ cells was an extremely rare event. Notably, more recently, the data from Li et al. (2018) showed compelling evidence to support endogenous stem cell to myocyte conversion in embryonic but not in adult heart [306].
Ironically, a more recent work in 2019, by Narino et al., has demonstrated that c-KIT expression labels a heterogeneous cardiac cell population, with cells low in c-KIT expression enriched for CSCs while c-KIT high expressers having endothelial/mast cell differentiation potential [307]. This study went on to show that adult c-KIT-labeled CSCs in mouse “can be myogenic” and help to regenerate after injury and to counteract effects of aging on cardiac structure and function, thus boldly suggesting that CSCs as the bonafide endogenous source of cardiomyocytes in healthy/pathological heart. Consequently, they identified c-KIT haploinsufficiency, generated usually in lineage-tracing studies, prevents efficient labeling of true CSCs on one hand while affecting the regenerative potential of these cells on the other, which perhaps could have been the oversight in the rival camp. Nevertheless, irrespective of the c-KIT controversy, there is no denying that animal studies and clinical trials have appreciated the benefit of a range of cell types for CPCs from many different sources through cellular transplantation approaches ([308,309,310] and Table 1, Table 4 and Table 6). Furthermore, there is an emerging theory that injected/infused CPCs can induce a reconditioning of the injured heart through paracrine signalling or that these cells stimulate an acute inflammatory response when these cells die and are cleared, resulting in a secondary acute healing response [311].
Therefore, as implied in Table 1, there is still no consensus on an endogenous CPC type that is critical for myocardial repair and regeneration but there is growing consensus that regeneration associated with these CPCs are not robust enough to repair severe myocardial damage such as in MI (commented in [307]). While this review does not offer to bias the reader for one or the other theory, in light of these recent studies, it offers the field impetus to interrogate other strategies and CPC sources (like from stem cells or transdifferentiation of somatic cells) to provide mechanistic insights into how CPCs can be more functionally significant in the context of cardiac regenerative medicine.
11. Future Directions
Heart failure patients are typically elderly, and suffer from chronic cardiomyopathies and associated complications like diabetes, hypertension, etc. Notably, they possess CPCs with compromised regenerative potential, insufficient to recover lost cardiac function [312,313]. The propensity of CPCs to affect cardiac repair is influenced by several factors, including genetics [314], epigenetic dysregulation [315], environmental stress [315], disease progression and pathogenesis [316,317], heart load [318], medication, and aging [319,320]. Nevertheless, discovery of CPC characteristics has revolutionized the conceptual view of treatment for heart disease, supported by the capacity of CPCs to form functionally integrated cardiomyocytes and vasculature [321]. Therefore, it is rational to enhance potential of CPCs from the adult or reprogrammed cell sources prior to adoptive transfer into a damaged myocardium. Hence, CPC research is gaining momentum to improve its feasibility for cardiac regenerative therapeutics. Advances in this field are progressing towards combining optimised reprogramming approaches from iPSCs and somatic cells with tissue engineering strategies. This will undoubtedly bring advances in genomics, epigenomics, and proteomics of CPCs and their differentiated counterparts, to realise their full potential. Future regenerative approaches might bring together genetic engineering (a very tested strategy in iPSCs), the addition of multiple stimuli (mechanical, electrical and biochemical factors) and tissue engineering approaches to develop a meticulously controlled system that maximises CPC regenerative capacity, and that could potentially be applied in cell therapy, disease modelling, and drug screening (Figure 1).
Funding
This research was funded by the Engineering and Physical Sciences Research Council (EPSRC), UK, grant number EP/L015072/1 and the APC was funded by the same.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Executive summary: Heart disease and stroke statistics—2014 Update: A report from the American Heart Association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V. Global burden of cardiovascular disease. J. Am. Coll. Cardiol. 2014, 64, 520–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Witman, N.; Sahara, M. Cardiac progenitor cells in basic biology and regenerative medicine. Stem Cells Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Van Laake, L.W.; Davidson, S.M.; Engel, F.B.; Hausenloy, D.J.; Lecour, S.; Leor, J.; Perrino, C.; Schulz, R.; Ytrehus, K.; et al. Position paper of the European society of cardiology working group cellular biology of the heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Graham, E.; Bergmann, O. Dating the heart: Exploring cardiomyocyte renewal in humans. Physiology 2017, 32, 33–41. [Google Scholar] [CrossRef]
- Urbanek, K.; Torella, D.; Sheikh, F.; De Angelis, A.; Nurzynska, D.; Silvestri, F.; Beltrami, C.A.; Bussani, R.; Beltrami, A.P.; Quaini, F.; et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc. Natl. Acad. Sci. USA 2005, 102, 8692–8697. [Google Scholar] [CrossRef]
- Bloomekatz, J.; Galvez-Santisteban, M.; Chi, N.C. Myocardial plasticity: Cardiac development, regeneration and disease. Curr. Opin. Genet. Dev. 2016, 40, 120–130. [Google Scholar] [CrossRef]
- van Berlo, J.H.; Molkentin, J.D. An emerging consensus on cardiac regeneration. Nat. Med. 2014, 20, 1386–1393. [Google Scholar] [CrossRef]
- Liang, S.X.; Phillips, W.D. Migration of resident cardiac stem cells in myocardial infarction: Migration of cardiac stem cells. Anat. Rec. 2013, 296, 184–191. [Google Scholar] [CrossRef]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Lemcke, H.; David, R. Stem cell therapy in heart diseases—Cell types, mechanisms and improvement strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, N.; Huang, Y.; Spencer, C.I.; Fu, J.; Yu, C.; Liu, K.; Nie, B.; Xu, T.; Li, K.; et al. Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell 2016, 18, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef]
- Lam, J.T.; Moretti, A.; Laugwitz, K.-L. Multipotent progenitor cells in regenerative cardiovascular medicine. Pediatric Cardiol. 2009, 30, 690–698. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Ebrahimi, B. Cardiac progenitor reprogramming for heart regeneration. Cell Regen. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Birket, M.J.; Mummery, C.L. Pluripotent stem cell derived cardiovascular progenitors—A developmental perspective. Dev. Biol. 2015, 400, 169–179. [Google Scholar] [CrossRef]
- Kattman, S.J.; Huber, T.L.; Keller, G.M. Multipotent Flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell 2006, 11, 723–732. [Google Scholar] [CrossRef]
- Wu, S.M.; Fujiwara, Y.; Cibulsky, S.M.; Clapham, D.E.; Lien, C.; Schultheiss, T.M.; Orkin, S.H. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 2006, 127, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-L.; Liang, X.; Shi, Y.; Chu, P.-H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef]
- Henning, R.J. Stem cells in cardiac repair. Future Cardiol. 2011, 7, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Lalit, P.A.; Salick, M.R.; Nelson, D.O.; Squirrell, J.M.; Shafer, C.M.; Patel, N.G.; Saeed, I.; Schmuck, E.G.; Markandeya, Y.S.; Wong, R.; et al. Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell 2016, 18, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, N.; Miller, R.A.; Hill, C.M.; Jie, C.C.; McCallion, A.S.; Gearhart, J.D. Mouse ES cell–derived cardiac precursor cells are multipotent and facilitate identification of novel cardiac genes. J. Clin. Investig. 2008, 118, 894–903. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kawaguchi, N.; Ellison, G.M.; Matsuoka, R.; Shin’oka, T.; Kurosawa, H. Characterization of long-term cultured c-kit + cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010, 19, 105–116. [Google Scholar] [CrossRef]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; LeCapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Yang, L.; Soonpaa, M.H.; Adler, E.D.; Roepke, T.K.; Kattman, S.J.; Kennedy, M.; Henckaerts, E.; Bonham, K.; Abbott, G.W.; Linden, R.M.; et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008, 453, 524–528. [Google Scholar] [CrossRef]
- Moretti, A.; Caron, L.; Nakano, A.; Lam, J.T.; Bernshausen, A.; Chen, Y.; Qyang, Y.; Bu, L.; Sasaki, M.; Martin-Puig, S.; et al. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006, 127, 1151–1165. [Google Scholar] [CrossRef]
- Tallini, Y.N.; Greene, K.S.; Craven, M.; Spealman, A.; Breitbach, M.; Smith, J.; Fisher, P.J.; Steffey, M.; Hesse, M.; Doran, R.M.; et al. c-Kit expression identifies cardiovascular precursors in the neonatal heart. Proc. Natl. Acad. Sci. USA 2009, 106, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.J.; Schulman, S.P.; Hare, J.M. Stem cell therapy for cardiac repair: Ready for the next step. Circulation 2006, 114, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; Schellings, M.; Pinto, Y.; Heymans, S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: A temporal and spatial window. Cardiovasc. Res. 2006, 69, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Deddens, J.C.; Sadeghi, A.H.; Hjortnaes, J.; van Laake, L.W.; Buijsrogge, M.; Doevendans, P.A.; Khademhosseini, A.; Sluijter, J.P.G. Modeling the human scarred heart in vitro: Toward new tissue engineered models. Adv. Healthc. Mater. 2017, 6, 1600571. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Gonzalez-Quesada, C.; Frangogiannis, N.G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J. Mol. Cell. Cardiol. 2010, 48, 504–511. [Google Scholar] [CrossRef]
- Le, T.Y.L.; Thavapalachandran, S.; Kizana, E.; Chong, J.J. New developments in cardiac regeneration. Heart Lung Circ. 2017, 26, 316–322. [Google Scholar] [CrossRef]
- Van Vliet, P.; Roccio, M.; Smits, A.M.; van Oorschot, A. a. M.; Metz, C.H.G.; van Veen, T. a. B.; Sluijter, J.P.G.; Doevendans, P.A.; Goumans, M.-J. Progenitor cells isolated from the human heart: A potential cell source for regenerative therapy. Neth Heart J. 2008, 16, 163–169. [Google Scholar] [CrossRef]
- Sandstedt, J.; Jonsson, M.; Kajic, K.; Sandstedt, M.; Lindahl, A.; Dellgren, G.; Jeppsson, A.; Asp, J. Left atrium of the human adult heart contains a population of side population cells. Basic Res. Cardiol. 2012, 107, 255. [Google Scholar] [CrossRef]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.G.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef]
- Laugwitz, K.-L.; Moretti, A.; Lam, J.; Gruber, P.; Chen, Y.; Woodard, S.; Lin, L.-Z.; Cai, C.-L.; Lu, M.M.; Reth, M.; et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005, 433, 647–653. [Google Scholar] [CrossRef]
- Li, X.-H.; Li, Q.; Jiang, L.; Deng, C.; Liu, Z.; Fu, Y.; Zhang, M.; Tan, H.; Feng, Y.; Shan, Z.; et al. Generation of functional human cardiac progenitor cells by high-efficiency protein transduction: Protein-generated cardiac progenitor cells. Stem Cells Transl. Med. 2015, 4, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Pessina, A.; Gribaldo, L. The key role of adult stem cells: Therapeutic perspectives. Curr. Med. Res. Opin. 2006, 22, 2287–2300. [Google Scholar] [CrossRef] [PubMed]
- Cedar, S. The function of stem cells and their future roles in healthcare. Br. J. Nurs. 2006, 15, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 2018, 557, 619–620. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Ronen, D.; Benvenisty, N. Genomic stability in reprogramming. Curr. Opin. Genet. Dev. 2012, 22, 444–449. [Google Scholar] [CrossRef]
- Margariti, A.; Kelaini, S.; Cochrane, A. Direct reprogramming of adult cells: Avoiding the pluripotent state. Stem Cells Cloning: Adv. Appl. 2014, 7, 19. [Google Scholar] [CrossRef]
- Xu, J.; Lian, W.; Li, L.; Huang, Z. Generation of induced cardiac progenitor cells via somatic reprogramming. Oncotarget 2017, 8, 29442. [Google Scholar] [CrossRef]
- Sassoli, C. Cardiac progenitor cells as target of cell and growth factor-based therapies for myocardial regeneration. J. Stem Cell Res. Ther. 2013, 9, 004. [Google Scholar] [CrossRef]
- Le, T.; Chong, J. Cardiac progenitor cells for heart repair. Cell Death Discov. 2016, 2, 16052. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.J.; Stirparo, G.G.; Papait, R.; Scarfò, M.; et al. Adult c-kitpos cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 2013, 154, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Edling, C.E.; Hallberg, B. c-Kit—A hematopoietic cell essential receptor tyrosine kinase. Int. J. Biochem. Cell Biol. 2007, 39, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Vajravelu, B.N.; Hong, K.U.; Al-Maqtari, T.; Cao, P.; Keith, M.C.L.; Wysoczynski, M.; Zhao, J.; Moore IV, J.B.; Bolli, R. c-Kit promotes growth and migration of human cardiac progenitor cells via the PI3K-AKT and MEK-ERK pathways. PLoS ONE 2015, 10, e0140798. [Google Scholar] [CrossRef]
- Kuang, D.; Zhao, X.; Xiao, G.; Ni, J.; Feng, Y.; Wu, R.; Wang, G. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res. Cardiol. 2008, 103, 265–273. [Google Scholar] [CrossRef]
- Ayach, B.B.; Yoshimitsu, M.; Dawood, F.; Sun, M.; Arab, S.; Chen, M.; Higuchi, K.; Siatskas, C.; Lee, P.; Lim, H.; et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Natl. Acad. Sci. USA 2006, 103, 2304–2309. [Google Scholar] [CrossRef]
- van Berlo, J.H.; Kanisicak, O.; Maillet, M.; Vagnozzi, R.J.; Karch, J.; Lin, S.-C.J.; Middleton, R.C.; Marbán, E.; Molkentin, J.D. c-Kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014, 509, 337–341. [Google Scholar] [CrossRef]
- Jesty, S.A.; Steffey, M.A.; Lee, F.K.; Breitbach, M.; Hesse, M.; Reining, S.; Lee, J.C.; Doran, R.M.; Nikitin, A.Y.; Fleischmann, B.K.; et al. c-Kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. USA 2012, 109, 13380–13385. [Google Scholar] [CrossRef]
- Zaruba, M.-M.; Soonpaa, M.; Reuter, S.; Field, L.J. Cardiomyogenic potential of c-Kit +–Expressing cells derived from neonatal and adult mouse hearts. Circulation 2010, 121, 1992–2000. [Google Scholar] [CrossRef]
- Sultana, N.; Zhang, L.; Yan, J.; Chen, J.; Cai, W.; Razzaque, S.; Jeong, D.; Sheng, W.; Bu, L.; Xu, M.; et al. Resident c-kit+ cells in the heart are not cardiac stem cells. Nat. Commun. 2015, 6, 8701. [Google Scholar] [CrossRef] [PubMed]
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: C-Kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Stanford, W.L. Concise review: Stem cell antigen-1: Expression, function, and enigma. Stem Cells 2007, 25, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Nagai, T.; Nishigaki, N.; Oyama, T.; Nishi, J.; Wada, H.; Sano, M.; Toko, H.; Akazawa, H.; Sato, T.; et al. Adult cardiac sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 2004, 279, 11384–11391. [Google Scholar] [CrossRef]
- Tateishi, K.; Ashihara, E.; Takehara, N.; Nomura, T.; Honsho, S.; Nakagami, T.; Morikawa, S.; Takahashi, T.; Ueyama, T.; Matsubara, H.; et al. Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J. Cell Sci. 2007, 120, 1791–1800. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Q.; Nakamura, Y.; Lee, J.; Zhang, G.; From, A.H.L.; Zhang, J. The role of the Sca-1 +/CD31 − cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 2006, 24, 1779–1788. [Google Scholar] [CrossRef]
- Van Vliet, P.; Smits, A.M.; De Boer, T.P.; Korfage, T.H.; Metz, C.H.G.; Roccio, M.; Van Der Heyden, M.A.G.; Van Veen, T.A.B.; Sluijter, J.P.G.; Doevendans, P.A.; et al. Foetal and adult cardiomyocyte progenitor cells have different developmental potential. J. Cell. Mol. Med. 2010, 14, 861–870. [Google Scholar] [CrossRef][Green Version]
- Huang, C.; Gu, H.; Yu, Q.; Manukyan, M.C.; Poynter, J.A.; Wang, M. Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS ONE 2011, 6, e29246. [Google Scholar] [CrossRef]
- Takamiya, M.; Haider, K.H.; Ashraf, M. Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration. PLoS ONE 2011, 6, e25265. [Google Scholar] [CrossRef]
- Uchida, S.; De Gaspari, P.; Kostin, S.; Jenniches, K.; Kilic, A.; Izumiya, Y.; Shiojima, I.; grosse Kreymborg, K.; Renz, H.; Walsh, K.; et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Rep. 2013, 1, 397–410. [Google Scholar] [CrossRef]
- Ge, Z.; Lal, S.; Le, T.Y.L.; dos Remedios, C.; Chong, J.J.H. Cardiac stem cells: Translation to human studies. Biophys. Rev. 2015, 7, 127–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- David, R.; Jarsch, V.B.; Schwarz, F.; Nathan, P.; Gegg, M.; Lickert, H.; Franz, W.-M. Induction of MesP1 by Brachyury(T) generates the common multipotent cardiovascular stem cell. Cardiovasc. Res. 2011, 92, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schwartz, R.J. Transient Mesp1 expression: A driver of cardiac cell fate determination. Transcription 2013, 4, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Takagi, A.; Inoue, T.; Saga, Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 2000, 127, 3215–3226. [Google Scholar]
- Habib, M.; Caspi, O.; Gepstein, L. Human embryonic stem cells for cardiomyogenesis. J. Mol. Cell. Cardiol. 2008, 45, 462–474. [Google Scholar] [CrossRef]
- Wu, S.M.; Chien, K.R.; Mummery, C. Origins and fates of cardiovascular progenitor cells. Cell 2008, 132, 537–543. [Google Scholar] [CrossRef]
- Saga, Y.; Miyagawa-Tomita, S.; Takagi, A.; Kitajima, S.; Miyazaki, J.; Inoue, T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development 1999, 126, 3437–3447. [Google Scholar]
- Chan, S.S.-K.; Shi, X.; Toyama, A.; Arpke, R.W.; Dandapat, A.; Iacovino, M.; Kang, J.; Le, G.; Hagen, H.R.; Garry, D.J.; et al. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell 2013, 12, 587–601. [Google Scholar] [CrossRef]
- Bondue, A.; Tännler, S.; Chiapparo, G.; Chabab, S.; Ramialison, M.; Paulissen, C.; Beck, B.; Harvey, R.; Blanpain, C. Defining the earliest step of cardiovascular progenitor specification during embryonic stem cell differentiation. J. Cell Biol. 2011, 192, 751–765. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Diaz, A.D.; Benham, A.; Xu, X.; Wijaya, C.S.; Fa’ak, F.; Luo, W.; Soibam, B.; Azares, A.; et al. Mesp1 marked cardiac progenitor cells repair infarcted mouse hearts. Sci. Rep. 2016, 6, 31457. [Google Scholar] [CrossRef]
- Ema, M. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood 2006, 107, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kouskoff, V.; Lacaud, G.; Schwantz, S.; Fehling, H.J.; Keller, G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 13170–13175. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Jiang, X.; Martin-Puig, S.; Caron, L.; Zhu, S.; Shao, Y.; Roberts, D.J.; Huang, P.L.; Domian, I.J.; Chien, K.R. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 2009, 460, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, G.; Lin, L.; Lowe, J.; Zhang, Q.; Bu, L.; Chen, Y.; Chen, J.; Sun, Y.; Evans, S.M. HCN4 dynamically marks the first heart field and conduction system precursors. Circ. Res. 2013, 113, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Später, D.; Abramczuk, M.K.; Buac, K.; Zangi, L.; Stachel, M.W.; Clarke, J.; Sahara, M.; Ludwig, A.; Chien, K.R. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell Biol. 2013, 15, 1098–1106. [Google Scholar] [CrossRef]
- Garcia-Frigola, C.; Shi, Y.; Evans, S.M. Expression of the hyperpolarization-activated cyclic nucleotide-gated cation channel HCN4 during mouse heart development. Gene Expr. Patterns 2003, 3, 777–783. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef]
- van Tuyn, J.; Atsma, D.E.; Winter, E.M.; van der Velde-van Dijke, I.; Pijnappels, D.A.; Bax, N.A.M.; Knaän-Shanzer, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; van der Laarse, A.; et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 2007, 25, 271–278. [Google Scholar] [CrossRef]
- Cai, C.-L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008, 454, 104–108. [Google Scholar] [CrossRef]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Bollini, S.; Vieira, J.M.N.; Howard, S.; Dubè, K.N.; Balmer, G.M.; Smart, N.; Riley, P.R. Re-activated adult epicardial progenitor cells are a heterogeneous population molecularly distinct from their embryonic counterparts. Stem Cells Dev. 2014, 23, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Dubé, K.N.; Riley, P.R. Epicardial progenitor cells in cardiac regeneration and neovascularisation. Vasc. Pharmacol. 2013, 58, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.M.; Meeson, A.P.; Robertson, S.M.; Hawke, T.J.; Richardson, J.A.; Bates, S.; Goetsch, S.C.; Gallardo, T.D.; Garry, D.J. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev. Biol. 2004, 265, 262–275. [Google Scholar] [CrossRef]
- Pfister, O.; Oikonomopoulos, A.; Sereti, K.-I.; Sohn, R.L.; Cullen, D.; Fine, G.C.; Mouquet, F.; Westerman, K.; Liao, R. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ. Res. 2008, 103, 825–835. [Google Scholar] [CrossRef]
- Liang, S.X.; Tan, T.Y.L.; Gaudry, L.; Chong, B. Differentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic model. Int. J. Cardiol. 2010, 138, 40–49. [Google Scholar] [CrossRef]
- Oyama, T.; Nagai, T.; Wada, H.; Naito, A.T.; Matsuura, K.; Iwanaga, K.; Takahashi, T.; Goto, M.; Mikami, Y.; Yasuda, N.; et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J. Cell Biol. 2007, 176, 329–341. [Google Scholar] [CrossRef]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.-M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef]
- Alfakir, M.; Dawe, N.; Eyre, R.; Tyson-Capper, A.; Britton, K.; Robson, S.C.; Meeson, A.P. The temporal and spatial expression patterns of ABCG2 in the developing human heart. Int. J. Cardiol. 2012, 156, 133–138. [Google Scholar] [CrossRef][Green Version]
- Hierlihy, A.M.; Seale, P.; Lobe, C.G.; Rudnicki, M.A.; Megeney, L.A. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002, 530, 239–243. [Google Scholar] [CrossRef]
- Pfister, O.; Mouquet, F.; Jain, M.; Summer, R.; Helmes, M.; Fine, A.; Colucci, W.S.; Liao, R. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ. Res. 2005, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Choi, S.-C.; Park, C.-Y.; Shim, W.-J.; Lim, D.-S. Cardiac side population cells exhibit endothelial differentiation potential. Exp. Mol. Med. 2007, 39, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Noseda, M.; Harada, M.; McSweeney, S.; Leja, T.; Belian, E.; Stuckey, D.J.; Abreu Paiva, M.S.; Habib, J.; Macaulay, I.; de Smith, A.J.; et al. PDGFRα demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat. Commun 2015, 6, 6930. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, K.; Fukushima, S.; Coppen, S.R.; Felkin, L.E.; Varela-Carver, A.; Barton, P.J.R.; Yacoub, M.H.; Suzuki, K. Heterogeneic nature of adult cardiac side population cells. Biochem. Biophys. Res. Commun. 2008, 371, 615–620. [Google Scholar] [CrossRef]
- Chimenti, I.; Smith, R.R.; Li, T.-S.; Gerstenblith, G.; Messina, E.; Giacomello, A.; Marbán, E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef]
- Li, T.-S.; Cheng, K.; Lee, S.-T.; Matsushita, S.; Davis, D.; Malliaras, K.; Zhang, Y.; Matsushita, N.; Smith, R.R.; Marbán, E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 2010, 28, 2088–2098. [Google Scholar] [CrossRef]
- He, J.-Q.; Vu, D.M.; Hunt, G.; Chugh, A.; Bhatnagar, A.; Bolli, R. Human cardiac stem cells isolated from atrial appendages stably express c-kit. PLoS ONE 2011, 6, e27719. [Google Scholar] [CrossRef]
- Hesse, M.; Fleischmann, B.K.; Kotlikoff, M.I. Concise Review: The role of c-kit expressing cells in heart repair at the neonatal and adult stage: C-kit + cells in heart repair. Stem Cells 2014, 32, 1701–1712. [Google Scholar] [CrossRef]
- Freire, A.G.; Nascimento, D.S.; Forte, G.; Valente, M.; Resende, T.P.; Pagliari, S.; Abreu, C.; Carvalho, I.; Nardo, P.D.; Pinto-do-Ó, P. Stable phenotype and function of immortalized Lin− Sca-1+ cardiac progenitor cells in long-term culture: A step closer to standardization. Stem Cells Dev. 2014, 23, 1012–1026. [Google Scholar] [CrossRef]
- Yamashita, J.K.; Takano, M.; Hiraoka-Kanie, M.; Shimazu, C.; Peishi, Y.; Yanagi, K.; Nakano, A.; Inoue, E.; Kita, F.; Nishikawa, S.-I. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005, 19, 1534–1536. [Google Scholar] [CrossRef]
- Lescroart, F.; Chabab, S.; Lin, X.; Rulands, S.; Paulissen, C.; Rodolosse, A.; Auer, H.; Achouri, Y.; Dubois, C.; Bondue, A.; et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat. Cell Biol. 2014, 16, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, T.I.; Appleby, N.; Tsay, E.; Martinez, J.J.; Bailey, L.; Hasaniya, N.; Kearns-Jonker, M. Human neonatal cardiovascular progenitors: Unlocking the secret to regenerative ability. PLoS ONE 2013, 8, e77464. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.-L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Reinecke, H.; Iwata, M.; Torok-Storb, B.; Stempien-Otero, A.; Murry, C.E. Progenitor cells identified by PDGFR-alpha expression in the developing and diseased human heart. Stem Cells Dev. 2013, 22, 1932–1943. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Chandrakanthan, V.; Xaymardan, M.; Asli, N.S.; Li, J.; Ahmed, I.; Heffernan, C.; Menon, M.K.; Scarlett, C.J.; Rashidianfar, A.; et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 2011, 9, 527–540. [Google Scholar] [CrossRef]
- Wessels, A.; Pérez-Pomares, J.M. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells: Epicardially derived cells as cardiac stem cells. Anat. Rec. Part. A: Discov. Mol. Cell. Evol. Biol. 2004, 276A, 43–57. [Google Scholar] [CrossRef]
- Smits, A.; Riley, P. Epicardium-derived heart repair. J. Dev. Biol. 2014, 2, 84–100. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Emmert, L.S.; Martens, A.; Ismail, I.; Schmidt-Richter, I.; Gawol, A.; Seifert, B.; Haverich, A.; Martin, U.; Gruh, I. Higher frequencies of BCRP+ cardiac resident cells in ischaemic human myocardium. Eur. Heart J. 2013, 34, 2830–2838. [Google Scholar] [CrossRef]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marbán, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef]
- Ye, J.; Boyle, A.; Shih, H.; Sievers, R.E.; Zhang, Y.; Prasad, M.; Su, H.; Zhou, Y.; Grossman, W.; Bernstein, H.S.; et al. Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS ONE 2012, 7, e30329. [Google Scholar] [CrossRef]
- Klein, D. iPSCs-based generation of vascular cells: Reprogramming approaches and applications. Cell. Mol. Life Sci. 2018, 75, 1411–1433. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Thompson, S.; Millrod, M.A.; Weinberg, S.; Yuan, X.; Peters, A.; Mahairaki, V.; Koliatsos, V.E.; Tung, L.; Zambidis, E.T. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS ONE 2011, 6, e18293. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.R.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009, 5, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.L.; Zhang, J.; Ng, E.S.; Elliott, D.A.; Elefanty, A.G.; Kamp, T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ. Res. 2012, 111, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Olmer, R.; Haase, A.; Merkert, S.; Cui, W.; Paleček, J.; Ran, C.; Kirschning, A.; Scheper, T.; Glage, S.; Miller, K.; et al. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010, 5, 51–64. [Google Scholar] [CrossRef]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef]
- Drowley, L.; Koonce, C.; Peel, S.; Jonebring, A.; Plowright, A.T.; Kattman, S.J.; Andersson, H.; Anson, B.; Swanson, B.J.; Wang, Q.-D.; et al. Human induced pluripotent stem cell-derived cardiac progenitor cells in phenotypic screening: A transforming growth factor-β type 1 receptor kinase inhibitor induces efficient cardiac differentiation: iPSC-derived cardiac progenitors for phenotypic screening. Stem Cells Transl. Med. 2016, 5, 164–174. [Google Scholar]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Minami, I.; Yamada, K.; Otsuji, T.G.; Yamamoto, T.; Shen, Y.; Otsuka, S.; Kadota, S.; Morone, N.; Barve, M.; Asai, Y.; et al. A Small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2012, 2, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; Olmer, R.; Kropp, C.; Rückert, M.; Jara-Avaca, M.; Robles-Diaz, D.; Franke, A.; Elliott, D.A.; Wojciechowski, D.; Fischer, M.; et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 2014, 3, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Yan, P.; Otsuji, T.G.; Narazaki, G.; Uosaki, H.; Fukushima, H.; Kuwahara, K.; Harada, M.; Matsuda, H.; Matsuoka, S.; et al. Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PLoS ONE 2011, 6, e16734. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Nagasawa, A.; Uosaki, H.; Sugimoto, A.; Yamamizu, K.; Teranishi, M.; Matsuda, H.; Matsuoka, S.; Ikeda, T.; Komeda, M.; et al. Cyclosporin-A potently induces highly cardiogenic progenitors from embryonic stem cells. Biochem. Biophys. Res. Commun. 2009, 379, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Uosaki, H.; Andersen, P.; Shenje, L.T.; Fernandez, L.; Christiansen, S.L.; Kwon, C. Direct contact with endoderm-like cells efficiently induces cardiac progenitors from mouse and human pluripotent stem cells. PLoS ONE 2012, 7, e46413. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Wang, Y.; Tang, Y.; Ali, A.; Hu, H.; Maienschein-Cline, M.; Ashraf, M. Cardiac progenitors induced from human induced pluripotent stem cells with cardiogenic small molecule effectively regenerate infarcted hearts and attenuate fibrosis. Shock 2018, 50, 627–639. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Gai, H. Generation and characterization of functional cardiomyocytes using induced pluripotent stem cells derived from human fibroblasts. Cell Biol. Int. 2009, 33, 1184–1193. [Google Scholar] [CrossRef]
- Uosaki, H.; Fukushima, H.; Takeuchi, A.; Matsuoka, S.; Nakatsuji, N.; Yamanaka, S.; Yamashita, J.K. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE 2011, 6, e23657. [Google Scholar] [CrossRef]
- Zwi, L.; Caspi, O.; Arbel, G.; Huber, I.; Gepstein, A.; Park, I.-H.; Gepstein, L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 2009, 120, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Denning, C.; Borgdorff, V.; Crutchley, J.; Firth, K.S.A.; George, V.; Kalra, S.; Kondrashov, A.; Hoang, M.D.; Mosqueira, D.; Patel, A.; et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Et. Biophys. Acta Mol. Cell Res. 2016, 1863, 1728–1748. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Liu, Z.; Chen, Z.; Wang, J.; Chen, T.; Zhao, X.; Ma, Y.; Qin, L.; Kang, J.; Wei, B.; et al. Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells. Cell Res. 2012, 22, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Liang, H.; Huang, J.; Wang, J.; Chen, Y.; Chen, Z.; Yang, H.-T. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res. 2013, 23, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Blin, G.; Nury, D.; Stefanovic, S.; Neri, T.; Guillevic, O.; Brinon, B.; Bellamy, V.; Rücker-Martin, C.; Barbry, P.; Bel, A.; et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Investig. 2010, 120, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, C.; Martens, A.; Rojas, S.V.; Schnick, T.; Rathert, C.; Schecker, N.; Menke, S.; Glage, S.; Zweigerdt, R.; Haverich, A.; et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur. Heart J. 2011, 32, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Termglinchan, V.; Shao, N.-Y.; Itzhaki, I.; Liu, C.; Ma, N.; Tian, L.; Wang, V.Y.; Chang, A.C.Y.; Guo, H.; et al. A human iPSC double-reporter system enables purification of cardiac lineage subpopulations with distinct function and drug response profiles. Cell Stem Cell 2019, 24, 802–811. [Google Scholar] [CrossRef]
- Ren, Y.; Lee, M.Y.; Schliffke, S.; Paavola, J.; Amos, P.J.; Ge, X.; Ye, M.; Zhu, S.; Senyei, G.; Lum, L.; et al. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J. Mol. Cell. Cardiol. 2011, 51, 280–287. [Google Scholar] [CrossRef]
- Moretti, A.; Bellin, M.; Jung, C.B.; Thies, T.-M.; Takashima, Y.; Bernshausen, A.; Schiemann, M.; Fischer, S.; Moosmang, S.; Smith, A.G.; et al. Mouse and human induced pluripotent stem cells as a source for multipotent Isl1 + cardiovascular progenitors. FASEB J. 2010, 24, 700–711. [Google Scholar] [CrossRef]
- Lian, X.; Bao, X.; Zilberter, M.; Westman, M.; Fisahn, A.; Hsiao, C.; Hazeltine, L.B.; Dunn, K.K.; Kamp, T.J.; Palecek, S.P. Chemically defined, albumin-free human cardiomyocyte generation. Nat. Methods 2015, 12, 595–596. [Google Scholar] [CrossRef]
- Andersen, P.; Tampakakis, E.; Jimenez, D.V.; Kannan, S.; Miyamoto, M.; Shin, H.K.; Saberi, A.; Murphy, S.; Sulistio, E.; Chelko, S.P.; et al. Precardiac organoids form two heart fields via Bmp/Wnt signaling. Nat. Commun. 2018, 9, 3140. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Lian, X.; Qian, T.; Bhute, V.J.; Han, T.; Palecek, S.P. Directed differentiation and long-term maintenance of epicardial cells derived from human pluripotent stem cells under fully defined conditions. Nat. Protoc. 2017, 12, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Lian, X.; Hacker, T.A.; Schmuck, E.G.; Qian, T.; Bhute, V.J.; Han, T.; Shi, M.; Drowley, L.; Plowright, A.T.; et al. Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat. Biomed. Eng. 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Witty, A.D.; Mihic, A.; Tam, R.Y.; Fisher, S.A.; Mikryukov, A.; Shoichet, M.S.; Li, R.-K.; Kattman, S.J.; Keller, G. Generation of the epicardial lineage from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1026–1035. [Google Scholar] [CrossRef]
- Iyer, D.; Gambardella, L.; Bernard, W.G.; Serrano, F.; Mascetti, V.L.; Pedersen, R.A.; Talasila, A.; Sinha, S. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development 2015, 142, 1528–1541. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, H.; Tian, L.; Huo, W.; Zhai, K.; Wang, P.; Ji, G.; Ma, Y. Efficient differentiation of TBX18+/WT1+ epicardial-like cells from human pluripotent stem cells using small molecular compounds. Stem Cells Dev. 2017, 26, 528–540. [Google Scholar] [CrossRef]
- Christoforou, N.; Liau, B.; Chakraborty, S.; Chellapan, M.; Bursac, N.; Leong, K.W. Induced pluripotent stem cell-derived cardiac progenitors differentiate to cardiomyocytes and form biosynthetic tissues. PLoS ONE 2013, 8, e65963. [Google Scholar] [CrossRef]
- Zhang, J.; Tao, R.; Campbell, K.F.; Carvalho, J.L.; Ruiz, E.C.; Kim, G.C.; Schmuck, E.G.; Raval, A.N.; da Rocha, A.M.; Herron, T.J.; et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 2019, 10, 2238. [Google Scholar] [CrossRef]
- Efe, J.A.; Hilcove, S.; Kim, J.; Zhou, H.; Ouyang, K.; Wang, G.; Chen, J.; Ding, S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 2011, 13, 215–222. [Google Scholar] [CrossRef]
- Qian, L.; Huang, Y.; Spencer, C.I.; Foley, A.; Vedantham, V.; Liu, L.; Conway, S.J.; Fu, J.; Srivastava, D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012, 485, 593–598. [Google Scholar] [CrossRef]
- Ieda, M.; Fu, J.-D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-D.; Stone, N.R.; Liu, L.; Spencer, C.I.; Qian, L.; Hayashi, Y.; Delgado-Olguin, P.; Ding, S.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 2013, 1, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Berry, E.C.; Fu, J.; Ieda, M.; Srivastava, D. Reprogramming of mouse fibroblasts into cardiomyocyte-like cells in vitro. Nat. Protoc. 2013, 8, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Muraoka, N.; Inagawa, K.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Kaneda, R.; Suzuki, T.; Kamiya, K.; et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc. Natl. Acad. Sci. USA 2013, 110, 12667–12672. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, N.; Spencer, C.I.; Nie, B.; Ma, T.; Xu, T.; Zhang, Y.; Wang, X.; Srivastava, D.; Ding, S. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 2014, 6, 951–960. [Google Scholar] [CrossRef]
- Mathison, M.; Gersch, R.P.; Nasser, A.; Lilo, S.; Korman, M.; Fourman, M.; Hackett, N.; Shroyer, K.; Yang, J.; Ma, Y.; et al. In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor. J Am Heart Assoc 2012, 1, e005652. [Google Scholar] [CrossRef]
- Protze, S.; Khattak, S.; Poulet, C.; Lindemann, D.; Tanaka, E.M.; Ravens, U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J. Mol. Cell. Cardiol. 2012, 53, 323–332. [Google Scholar] [CrossRef]
- Addis, R.C.; Ifkovits, J.L.; Pinto, F.; Kellam, L.D.; Esteso, P.; Rentschler, S.; Christoforou, N.; Epstein, J.A.; Gearhart, J.D. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J. Mol. Cell. Cardiol. 2013, 60, 97–106. [Google Scholar] [CrossRef]
- Christoforou, N.; Chellappan, M.; Adler, A.F.; Kirkton, R.D.; Wu, T.; Addis, R.C.; Bursac, N.; Leong, K.W. Transcription factors MYOCD, SRF, Mesp1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS ONE 2013, 8, e63577. [Google Scholar] [CrossRef]
- Hirai, H.; Katoku-Kikyo, N.; Keirstead, S.A.; Kikyo, N. Accelerated direct reprogramming of fibroblasts into cardiomyocyte-like cells with the MyoD transactivation domain. Cardiovasc. Res. 2013, 100, 105–113. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Addis, R.C.; Epstein, J.A.; Gearhart, J.D. Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes. PLoS ONE 2014, 9, e89678. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Z.; Yin, C.; Asfour, H.; Chen, O.; Li, Y.; Bursac, N.; Liu, J.; Qian, L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ. Res. 2015, 116, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Huang, C.; Xu, X.; Gu, H.; Ye, Y.; Jiang, C.; Qiu, Z.; Xie, X. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res. 2015, 25, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Islas, J.F.; Liu, Y.; Weng, K.-C.; Robertson, M.J.; Zhang, S.; Prejusa, A.; Harger, J.; Tikhomirova, D.; Chopra, M.; Iyer, D.; et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc. Natl. Acad. Sci. USA 2012, 109, 13016–13021. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Lee, Y.-K.; Ran, X.; Liao, S.-Y.; Yang, J.; Au, K.-W.; Lai, W.-H.; Esteban, M.A.; Tse, H.-F. Generation of induced cardiospheres via reprogramming of skin fibroblasts for myocardial regeneration: Induced cardiospheres for myocardial regeneration. Stem Cells 2016, 34, 2693–2706. [Google Scholar] [CrossRef]
- Lian, W.; Jia, Y.; Li, L.; Huang, Z.; Xu, J. Generation of induced cardiospheres via reprogramming of mouse skin fibroblasts. Curr. Protoc. Stem Cell Biol. 2018, 46, e59. [Google Scholar] [CrossRef]
- Song, K.; Nam, Y.-J.; Luo, X.; Qi, X.; Tan, W.; Huang, G.N.; Acharya, A.; Smith, C.L.; Tallquist, M.D.; Neilson, E.G.; et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 2012, 485, 599–604. [Google Scholar] [CrossRef]
- Sadahiro, T.; Yamanaka, S.; Ieda, M. Direct cardiac reprogramming: Progress and challenges in basic biology and clinical applications. Circ. Res. 2015, 116, 1378–1391. [Google Scholar] [CrossRef]
- Srivastava, D.; DeWitt, N. In vivo cellular reprogramming: The next generation. Cell 2016, 166, 1386–1396. [Google Scholar] [CrossRef]
- Nam, Y.-J.; Song, K.; Luo, X.; Daniel, E.; Lambeth, K.; West, K.; Hill, J.A.; DiMaio, J.M.; Baker, L.A.; Bassel-Duby, R.; et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl. Acad. Sci. USA 2013, 110, 5588–5593. [Google Scholar] [CrossRef]
- Zhang, R.; Han, P.; Yang, H.; Ouyang, K.; Lee, D.; Lin, Y.-F.; Ocorr, K.; Kang, G.; Chen, J.; Stainier, D.Y.R.; et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 2013, 498, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.-S.; Lee, S.-T.; Wawrowsky, K.A.; Cheng, K.; Galang, G.; Malliaras, K.; Abraham, M.R.; Wang, C.; Marbán, E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS ONE 2010, 5, e12559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, J.F.; Qiu, H.; Robb MacLellan, W.; Marbán, E.; Wang, C. Epigenomic reprogramming of adult cardiomyocyte-derived cardiac progenitor cells. Sci. Rep. 2015, 5, 17686. [Google Scholar] [CrossRef]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-Mediated In Vitro and In Vivo Direct Reprogramming of Cardiac Fibroblasts to Cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, L.; Yin, C.; Liu, J.; Qian, L. In vivo cardiac reprogramming using an optimal single polycistronic construct: Figure 1. Cardiovasc. Res. 2015, 108, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Birket, M.J.; Ribeiro, M.C.; Verkerk, A.O.; Ward, D.; Leitoguinho, A.R.; den Hartogh, S.C.; Orlova, V.V.; Devalla, H.D.; Schwach, V.; Bellin, M.; et al. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 970–979. [Google Scholar] [CrossRef]
- Nsair, A.; Schenke-Layland, K.; Van Handel, B.; Evseenko, D.; Kahn, M.; Zhao, P.; Mendelis, J.; Heydarkhan, S.; Awaji, O.; Vottler, M.; et al. Characterization and therapeutic potential of induced pluripotent stem cell-derived cardiovascular progenitor cells. PLoS ONE 2012, 7, e45603. [Google Scholar] [CrossRef]
- Nelson, T.J.; Faustino, R.S.; Chiriac, A.; Crespo-Diaz, R.; Behfar, A.; Terzic, A. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells 2008, 26, 1464–1473. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Guo, C.; Chang, W.; Zhuang, J.; Zhu, P.; Li, X. Temporally distinct Six2 -positive second heart field progenitors regulate mammalian heart development and disease. Cell Rep. 2017, 18, 1019–1032. [Google Scholar] [CrossRef]
- Torán, J.L.; López, J.A.; Gomes-Alves, P.; Aguilar, S.; Torroja, C.; Trevisan-Herraz, M.; Moscoso, I.; Sebastião, M.J.; Serra, M.; Brito, C.; et al. Definition of a cell surface signature for human cardiac progenitor cells after comprehensive comparative transcriptomic and proteomic characterization. Sci. Rep. 2019, 9, 4647. [Google Scholar] [CrossRef]
- Ardehali, R.; Ali, S.R.; Inlay, M.A.; Abilez, O.J.; Chen, M.Q.; Blauwkamp, T.A.; Yazawa, M.; Gong, Y.; Nusse, R.; Drukker, M.; et al. Prospective isolation of human embryonic stem cell-derived cardiovascular progenitors that integrate into human fetal heart tissue. Proc. Natl. Acad. Sci. USA 2013, 110, 3405–3410. [Google Scholar] [CrossRef] [PubMed]
- Skelton, R.J.P.; Costa, M.; Anderson, D.J.; Bruveris, F.; Finnin, B.W.; Koutsis, K.; Arasaratnam, D.; White, A.J.; Rafii, A.; Ng, E.S.; et al. SIRPA, VCAM1 and CD34 identify discrete lineages during early human cardiovascular development. Stem Cell Res. 2014, 13, 172–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qyang, Y.; Martin-Puig, S.; Chiravuri, M.; Chen, S.; Xu, H.; Bu, L.; Jiang, X.; Lin, L.; Granger, A.; Moretti, A.; et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-catenin pathway. Cell Stem Cell 2007, 1, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.D.; Wang, Z.; Lepore, J.J.; Lu, M.M.; Taketo, M.M.; Epstein, D.J.; Morrisey, E.E. Wnt/β-catenin signaling promotes expansion of Isl-1–positive cardiac progenitor cells through regulation of FGF signaling. J. Clin. Investig. 2007, 117, 1794–1804. [Google Scholar] [CrossRef]
- Kwon, C.; Qian, L.; Cheng, P.; Nigam, V.; Arnold, J.; Srivastava, D. A regulatory pathway involving Notch1/β-catenin/Isl1 determines cardiac progenitor cell fate. Nat. Cell Biol. 2009, 11, 951–957. [Google Scholar] [CrossRef]
- Rosenblatt-Velin, N.; Lepore, M.G.; Cartoni, C.; Beermann, F.; Pedrazzini, T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J. Clin. Investig. 2005, 115, 1724–1733. [Google Scholar] [CrossRef]
- Bylund, J.B.; Trinh, L.T.; Awgulewitsch, C.P.; Paik, D.T.; Jetter, C.; Jha, R.; Zhang, J.; Nolan, K.; Xu, C.; Thompson, T.B.; et al. Coordinated proliferation and differentiation of human-induced pluripotent stem cell-derived cardiac progenitor cells depend on bone morphogenetic protein signaling regulation by GREMLIN 2. Stem Cells Dev. 2017, 26, 678–693. [Google Scholar] [CrossRef]
- Ao, A.; Hao, J.; Hopkins, C.R.; Hong, C.C. DMH1, a novel BMP small molecule inhibitor, increases cardiomyocyte progenitors and promotes cardiac differentiation in mouse embryonic stem cells. PLoS ONE 2012, 7, e41627. [Google Scholar] [CrossRef]
- Gomes-Alves, P.; Serra, M.; Brito, C.; Ricardo, C.P.; Cunha, R.; Sousa, M.F.; Sanchez, B.; Bernad, A.; Carrondo, M.J.T.; Rodriguez-Borlado, L.; et al. In vitro expansion of human cardiac progenitor cells: Exploring ’omics tools for characterization of cell-based allogeneic products. Transl. Res. 2016, 171, 96–110.e3. [Google Scholar] [CrossRef]
- Dyer, L.A.; Makadia, F.A.; Scott, A.; Pegram, K.; Hutson, M.R.; Kirby, M.L. BMP signaling modulates hedgehog-induced secondary heart field proliferation. Dev. Biol. 2010, 348, 167–176. [Google Scholar] [CrossRef]
- Gude, N.; Muraski, J.; Rubio, M.; Kajstura, J.; Schaefer, E.; Anversa, P.; Sussman, M.A. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ. Res. 2006, 99, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-S.; Cheng, K.; Malliaras, K.; Matsushita, N.; Sun, B.; Marbán, L.; Zhang, Y.; Marbán, E. Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovasc. Res. 2011, 89, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Khan, M.; Toko, H.; Bailey, B.; Cottage, C.T.; Wallach, K.; Nag, D.; Lee, A.; Siddiqi, S.; Lan, F.; et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J. Am. Coll. Cardiol. 2012, 60, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, A.; Yuan, L.; Bréant, C.; Alitalo, K.; Koskinen, P.J. Developmental expression of Pim kinases suggests functions also outside of the hematopoietic system. Oncogene 2000, 19, 1215–1224. [Google Scholar] [CrossRef]
- Mohsin, S.; Khan, M.; Nguyen, J.; Alkatib, M.; Siddiqi, S.; Hariharan, N.; Wallach, K.; Monsanto, M.; Gude, N.; Dembitsky, W.; et al. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ. Res. 2013, 113, 1169–1179. [Google Scholar] [CrossRef]
- Samse, K.; Emathinger, J.; Hariharan, N.; Quijada, P.; Ilves, K.; Völkers, M.; Ormachea, L.; De La Torre, A.; Orogo, A.M.; Alvarez, R.; et al. Functional effect of Pim1 depends upon intracellular localization in human cardiac progenitor cells. J. Biol. Chem. 2015, 290, 13935–13947. [Google Scholar] [CrossRef]
- Fischer, K.M.; Cottage, C.T.; Wu, W.; Din, S.; Gude, N.A.; Avitabile, D.; Quijada, P.; Collins, B.L.; Fransioli, J.; Sussman, M.A. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 2009, 120, 2077–2087. [Google Scholar] [CrossRef]
- Liu, N.; Wang, B.J.; Broughton, K.M.; Alvarez, R.; Siddiqi, S.; Loaiza, R.; Nguyen, N.; Quijada, P.; Gude, N.; Sussman, M.A. PIM1-minicircle as a therapeutic treatment for myocardial infarction. PLoS ONE 2017, 12, e0173963. [Google Scholar] [CrossRef]
- Hofsteen, P.; Robitaille, A.M.; Chapman, D.P.; Moon, R.T.; Murry, C.E. Quantitative proteomics identify DAB2 as a cardiac developmental regulator that inhibits WNT/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 1002–1007. [Google Scholar] [CrossRef]
- Hofsteen, P.; Robitaille, A.M.; Strash, N.; Palpant, N.; Moon, R.T.; Pabon, L.; Murry, C.E. ALPK2 promotes cardiogenesis in zebrafish and human pluripotent stem cells. iScience 2018, 2, 88–100. [Google Scholar] [CrossRef]
- Dupays, L.; Towers, N.; Wood, S.; David, A.; Stuckey, D.J.; Mohun, T. Furin, a transcriptional target of NKX2-5, has an essential role in heart development and function. PLoS ONE 2019, 14, e0212992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Vaseghi, H.R.; Liu, Z.; Lu, R.; Alimohamadi, S.; Yin, C.; Fu, J.-D.; Wang, G.G.; Liu, J.; et al. Bmi1 is a key epigenetic barrier to direct cardiac reprogramming. Cell Stem Cell 2016, 18, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Lei, I.; Liu, L.; Sham, M.H.; Wang, Z. SWI/SNF in cardiac progenitor cell differentiation: SWI/SNF in Cardiac Progenitors. J. Cell. Biochem. 2013, 114, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fulcoli, F.G.; Ferrentino, R.; Martucciello, S.; Illingworth, E.A.; Baldini, A. Transcriptional control in cardiac progenitors: Tbx1 interacts with the BAF chromatin remodeling complex and regulates Wnt5a. PLoS Genet. 2012, 8, e1002571. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.D.; Miller, M.F.; Wang, Z.; Moon, R.T.; Morrisey, E.E. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development 2012, 139, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, N.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Isomi, M.; Nakashima, H.; Akiyama, M.; Wada, R.; Inagawa, K.; et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014, 33, 1565–1581. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; van Mil, A.; van Vliet, P.; Metz, C.H.G.; Liu, J.; Doevendans, P.A.; Goumans, M.-J. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 859–868. [Google Scholar] [CrossRef]
- Xiao, J.; Liang, D.; Zhang, H.; Liu, Y.; Zhang, D.; Liu, Y.; Pan, L.; Chen, X.; Doevendans, P.A.; Sun, Y.; et al. MicroRNA-204 is required for differentiation of human-derived cardiomyocyte progenitor cells. J. Mol. Cell. Cardiol. 2012, 53, 751–759. [Google Scholar] [CrossRef]
- Sirish, P.; López, J.E.; Li, N.; Wong, A.; Timofeyev, V.; Young, J.N.; Majdi, M.; Li, R.A.; Chen, H.V.; Chiamvimonvat, N. MicroRNA profiling predicts a variance in the proliferative potential of cardiac progenitor cells derived from neonatal and adult murine hearts. J. Mol. Cell. Cardiol. 2012, 52, 264–272. [Google Scholar] [CrossRef]
- Shen, X.; Soibam, B.; Benham, A.; Xu, X.; Chopra, M.; Peng, X.; Yu, W.; Bao, W.; Liang, R.; Azares, A.; et al. miR-322/-503 cluster is expressed in the earliest cardiac progenitor cells and drives cardiomyocyte specification. Proc. Natl. Acad. Sci. USA 2016, 113, 9551–9556. [Google Scholar] [CrossRef]
- Garate, X.; La Greca, A.; Neiman, G.; Blüguermann, C.; Santín Velazque, N.L.; Moro, L.N.; Luzzani, C.; Scassa, M.E.; Sevlever, G.E.; Romorini, L.; et al. Identification of the miRNAome of early mesoderm progenitor cells and cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. 2018, 8, 8072. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.; Zhu, Y.; Schenke-Layland, K.; Kuo, J.; Latour, B.; Ge, S.; Scholes, J.; Dravid, G.; Li, X.; MacLellan, W.R.; et al. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13742–13747. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yang, J.; Zhao, X.; Zhang, E.; Zeng, Q.; Yu, Y.; Yang, L.; Wu, B.; Yi, G.; Mao, X.; et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat. Commun. 2019, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, X.; Zhang, L. microRNAs and cardiac stem cells in heart development and disease. Drug Discov. Today 2019, 24, 233–240. [Google Scholar] [CrossRef]
- Castellan, R.F.P.; Meloni, M. Mechanisms and therapeutic targets of cardiac regeneration: Closing the age gap. Front. Cardiovasc. Med. 2018, 5, 7. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Cui, J.; Sun, M.; Du, W.; Chen, T.; Ming, X.; Zhang, L.; Tian, J.; Li, J.; et al. MiR218 modulates wnt signaling in mouse cardiac stem cells by promoting proliferation and inhibiting differentiation through a positive feedback loop. Sci. Rep. 2016, 6, 20968. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Chen, F.; Cao, N.; Zhou, Z.-W.; Yang, H.-T. miR-142-3p contributes to early cardiac fate decision of embryonic stem cells. Stem Cells Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Ivey, K.N.; Muth, A.; Arnold, J.; King, F.W.; Yeh, R.-F.; Fish, J.E.; Hsiao, E.C.; Schwartz, R.J.; Conklin, B.R.; Bernstein, H.S.; et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2008, 2, 219–229. [Google Scholar] [CrossRef]
- Purvis, N.; Bahn, A.; Katare, R. The role of microRNAs in cardiac stem cells. Stem Cells Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, Q.; Zhou, X.; Zhang, L.; Bao, L.; Zhen, L.; Zhang, Y.; Fan, H.; Liu, Z.; Yu, Z. Neonatal heart-enriched miR-708 promotes differentiation of cardiac progenitor cells in rats. Int. J. Mol. Sci. 2016, 17, 875. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Q.; He, S.; Yang, M.; Maguire, E.M.; An, W.; Afzal, T.A.; Luong, L.A.; Zhang, L.; Xiao, Q. miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 2018, 137, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wu, Y.; Wang, Y.; Yu, D.; Yang, M.; Yang, F.; Feng, C.; Chen, T. MicroRNA-29a promotes smooth muscle cell differentiation from stem cells by targeting YY1. Stem Cell Res. 2016, 17, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Cassano, M.; Messina, G.; Galli, D.; Galvez, B.G.; Curk, T.; Altomare, C.; Ronzoni, F.; Toelen, J.; Gijsbers, R.; et al. miR669a and miR669q prevent skeletal muscle differentiation in postnatal cardiac progenitors. J. Cell Biol. 2011, 193, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Limana, F.; Esposito, G.; D’Arcangelo, D.; Di Carlo, A.; Romani, S.; Melillo, G.; Mangoni, A.; Bertolami, C.; Pompilio, G.; Germani, A.; et al. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS ONE 2011, 6, e19845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sun, Q.; Zhang, Y.; Teng, F.; Sun, J. Up-regulation of miRNA-21 expression promotes migration and proliferation of Sca-1+ cardiac stem cells in mice. Med. Sci. Monit. 2016, 22, 1724–1732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, S.; Huang, M.; Nguyen, P.K.; Gong, Y.; Li, Z.; Jia, F.; Lan, F.; Liu, J.; Nag, D.; Robbins, R.C.; et al. Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation 2011, 124, S27–S34. [Google Scholar] [CrossRef]
- Liu, J.; van Mil, A.; Vrijsen, K.; Zhao, J.; Gao, L.; Metz, C.H.G.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J. Cell. Mol. Med. 2011, 15, 1474–1482. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.-M.; Xi, Y.; Wu, G.; Shelat, H.; Gao, S.; Cheng, J.; Geng, Y.-J. MicroRNA-1/133 targeted dysfunction of potassium channels KCNE1 and KCNQ1 in human cardiac progenitor cells with simulated hyperglycemia. Int. J. Cardiol. 2013, 167, 1076–1078. [Google Scholar] [CrossRef]
- Mauretti, A.; Spaans, S.; Bax, N.A.M.; Sahlgren, C.; Bouten, C.V.C. Cardiac progenitor cells and the interplay with their microenvironment. Stem Cells Int. 2017, 2017, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, R.; Rizzitelli, G.; Chimenti, I.; Barile, L.; Forte, E.; Ionta, V.; Angelini, F.; Sluijter, J.P.G.; Barbetta, A.; Messina, E.; et al. Cardiospheres and tissue engineering for myocardial regeneration: Potential for clinical application. J. Cell. Mol. Med. 2010, 14, 1071–1077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in cardiac tissue engineering. Tissue Eng. Part. B: Rev. 2010, 16, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Elisseeff, J. Controlled differentiation of stem cells. Adv. Drug Deliv. Rev. 2008, 60, 199–214. [Google Scholar] [CrossRef]
- Mendelson, K.; Schoen, F.J. Heart valve tissue engineering: Concepts, approaches, progress, and challenges. Ann. Biomed. Eng. 2006, 34, 1799–1819. [Google Scholar] [CrossRef]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef]
- Bellamy, V.; Vanneaux, V.; Bel, A.; Nemetalla, H.; Emmanuelle Boitard, S.; Farouz, Y.; Joanne, P.; Perier, M.-C.; Robidel, E.; Mandet, C.; et al. Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J. Heart Lung Transplant. 2015, 34, 1198–1207. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report: Figure 1. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef]
- Vallée, J.-P.; Hauwel, M.; Lepetit-Coiffé, M.; Bei, W.; Montet-Abou, K.; Meda, P.; Gardier, S.; Zammaretti, P.; Kraehenbuehl, T.P.; Herrmann, F.; et al. Embryonic stem cell-based cardiopatches improve cardiac function in infarcted rats. Stem Cells Transl. Med. 2012, 1, 248–260. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.G.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P.G. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef] [PubMed]
- Huby, A.-C.; Beigi, F.; Xiang, Q.; Gobin, A.; Taylor, D. Porcine decellularized heart tissue enhance the expression of contractile proteins in human cardiomyocytes and differentiated cardiac progenitor cells. Circ. Res. 2016, 119, A29. [Google Scholar]
- Padin-Iruegas, M.E.; Misao, Y.; Davis, M.E.; Segers, V.F.M.; Esposito, G.; Tokunou, T.; Urbanek, K.; Hosoda, T.; Rota, M.; Anversa, P.; et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation 2009, 120, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Liu, M.-L.; Nagai, T.; Iwanaga, K.; Matsuura, K.; Takahashi, T.; Kanda, M.; Kondo, N.; Wang, P.; Naito, A.T.; et al. Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction. J. Mol. Cell. Cardiol. 2010, 49, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, X.; Matsushita, S.; Guan, J. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials 2011, 32, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, S.; Zhao, C.; Chen, X.; Lei, I.; Wang, Z.; Ma, P.X. Porous nanofibrous poly(l-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering. Acta Biomater. 2015, 26, 105–114. [Google Scholar] [CrossRef]
- Ciocci, M.; Mochi, F.; Carotenuto, F.; Di Giovanni, E.; Prosposito, P.; Francini, R.; De Matteis, F.; Reshetov, I.; Casalboni, M.; Melino, S.; et al. Scaffold-in-scaffold potential to induce growth and differentiation of cardiac progenitor cells. Stem Cells Dev. 2017, 26, 1438–1447. [Google Scholar] [CrossRef]
- Johnson, T.D.; DeQuach, J.A.; Gaetani, R.; Ungerleider, J.; Elhag, D.; Nigam, V.; Behfar, A.; Christman, K.L. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2014, 2, 735–744. [Google Scholar] [CrossRef]
- van Marion, M.H.; Bax, N.A.M.; van Turnhout, M.C.; Mauretti, A.; van der Schaft, D.W.J.; Goumans, M.J.T.H.; Bouten, C.V.C. Behavior of CMPCs in unidirectional constrained and stress-free 3D hydrogels. J. Mol. Cell. Cardiol. 2015, 87, 79–91. [Google Scholar] [CrossRef][Green Version]
- Gaetani, R.; Yin, C.; Srikumar, N.; Braden, R.; Doevendans, P.A.; Sluijter, J.P.G.; Christman, K.L. Cardiac-derived extracellular matrix enhances cardiogenic properties of human cardiac progenitor cells. Cell Transplant. 2016, 25, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- French, K.M.; Boopathy, A.V.; DeQuach, J.A.; Chingozha, L.; Lu, H.; Christman, K.L.; Davis, M.E. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012, 8, 4357–4364. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.L.J.; Narayanan, K.; Gao, S.; Wan, A.C.A. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials 2011, 32, 7571–7580. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Pahlavan, S.; Ashtiani, M.K.; Ansari, H.; Abbasalizadeh, S.; Sayahpour, F.A.; Varzideh, F.; Kostin, S.; Aghdami, N.; Braun, T.; et al. Human embryonic stem cell-derived cardiovascular progenitor cells efficiently colonize in bFGF-tethered natural matrix to construct contracting humanized rat hearts. Biomaterials 2018, 154, 99–112. [Google Scholar] [CrossRef]
- Sánchez, P.L.; Fernández-Santos, M.E.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G.; et al. Acellular human heart matrix: A critical step toward whole heart grafts. Biomaterials 2015, 61, 279–289. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef]
- Silva, A.C.; Rodrigues, S.C.; Caldeira, J.; Nunes, A.M.; Sampaio-Pinto, V.; Resende, T.P.; Oliveira, M.J.; Barbosa, M.A.; Thorsteinsdóttir, S.; Nascimento, D.S.; et al. Three-dimensional scaffolds of fetal decellularized hearts exhibit enhanced potential to support cardiac cells in comparison to the adult. Biomaterials 2016, 104, 52–64. [Google Scholar] [CrossRef]
- Chamberland, C.; Martinez-Fernandez, A.; Beraldi, R.; Nelson, T.J. Embryonic decellularized cardiac scaffold supports embryonic stem cell differentiation to produce beating cardiac tissue. ISRN Stem Cells 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Rajabi-Zeleti, S.; Jalili-Firoozinezhad, S.; Azarnia, M.; Khayyatan, F.; Vahdat, S.; Nikeghbalian, S.; Khademhosseini, A.; Baharvand, H.; Aghdami, N. The behavior of cardiac progenitor cells on macroporous pericardium-derived scaffolds. Biomaterials 2014, 35, 970–982. [Google Scholar] [CrossRef]
- Chimenti, I.; Rizzitelli, G.; Gaetani, R.; Angelini, F.; Ionta, V.; Forte, E.; Frati, G.; Schussler, O.; Barbetta, A.; Messina, E.; et al. Human cardiosphere-seeded gelatin and collagen scaffolds as cardiogenic engineered bioconstructs. Biomaterials 2011, 32, 9271–9281. [Google Scholar] [CrossRef]
- Takehara, N.; Tsutsumi, Y.; Tateishi, K.; Ogata, T.; Tanaka, H.; Ueyama, T.; Takahashi, T.; Takamatsu, T.; Fukushima, M.; Komeda, M.; et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J. Am. Coll. Cardiol. 2008, 52, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Sun, S.; Zhang, X. Implantation of engineered conduction tissue in the rat heart. Mol. Med. Rep. 2019, 19, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, C.; Nan, H.; Yu, Y.; Xiao, Y.; Poon, E.; Yang, G.; Wang, X.; Wang, C.; Li, L.; et al. Graphene sheet-induced global maturation of cardiomyocytes derived from human induced pluripotent stem cells. ACS Appl. Mater. Interfaces 2017, 9, 25929–25940. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, A.; Cherkas, V.; Liu, C.; Braun, G.B.; Kleschevnikov, A.; Miller, Y.I.; Molokanova, E. Graphene biointerfaces for optical stimulation of cells. Sci. Adv. 2018, 4, eaat0351. [Google Scholar] [CrossRef]
- Feiner, R.; Engel, L.; Fleischer, S.; Malki, M.; Gal, I.; Shapira, A.; Shacham-Diamand, Y.; Dvir, T. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016, 15, 679–685. [Google Scholar] [CrossRef]
- Li, J.; Minami, I.; Shiozaki, M.; Yu, L.; Yajima, S.; Miyagawa, S.; Shiba, Y.; Morone, N.; Fukushima, S.; Yoshioka, M.; et al. Human pluripotent stem cell-derived cardiac tissue-like constructs for repairing the infarcted myocardium. Stem Cell Rep. 2017, 9, 1546–1559. [Google Scholar] [CrossRef]
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef]
- Asahi, Y.; Hamada, T.; Hattori, A.; Matsuura, K.; Odaka, M.; Nomura, F.; Kaneko, T.; Abe, Y.; Takasuna, K.; Sanbuissho, A.; et al. On-chip spatiotemporal electrophysiological analysis of human stem cell derived cardiomyocytes enables quantitative assessment of proarrhythmia in drug development. Sci. Rep. 2018, 8, 14536. [Google Scholar] [CrossRef]
- Qian, F.; Huang, C.; Lin, Y.-D.; Ivanovskaya, A.N.; O’Hara, T.J.; Booth, R.H.; Creek, C.J.; Enright, H.A.; Soscia, D.A.; Belle, A.M.; et al. Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. Lab Chip 2017, 17, 1732–1739. [Google Scholar] [CrossRef]
- Banerjee, M.N.; Bolli, R.; Hare, J.M. Clinical studies of cell therapy in cardiovascular medicine: Recent developments and future directions. Circ. Res. 2018, 123, 266–287. [Google Scholar] [CrossRef]
- The Lancet Editors. Expression of concern: The SCIPIO trial. Lancet 2014, 383, 1279. [Google Scholar] [CrossRef]
- The Lancet Editors. Retraction—Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2019, 393, 1084. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Yacoub, M.H.; Terrovitis, J. CADUCEUS, SCIPIO, ALCADIA: Cell therapy trials using cardiac-derived cells for patients with post myocardial infarction LV dysfunction, still evolving. Glob. Cardiol. Sci. Pract. 2013, 2013, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takehara, N.; Ogata, T.; Nakata, M.; Kami, D.; Nakamura, T.; Matoba, S.; Gojo, S.; Sawada, T.; Yaku, H.; Matsubara, H. The alcadia (autologous human cardiac-derived stem cell to treat ischemic cardiomyopathy) trial. Circulation 2012, 126, 2776–2799. [Google Scholar]
- Malliaras, K.; Zhang, Y.; Seinfeld, J.; Galang, G.; Tseliou, E.; Cheng, K.; Sun, B.; Aminzadeh, M.; Marbán, E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol. Med. 2013, 5, 191–209. [Google Scholar] [CrossRef]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Tarui, S.; Ousaka, D.; Eitoku, T.; Kondo, M.; Okuyama, M.; Kobayashi, J.; Baba, K.; Arai, S.; et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: The TICAP prospective phase 1 controlled trial. Circ. Res. 2015, 116, 653–664. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary cardiac progenitor cells in single ventricle physiology: The PERSEUS (cardiac progenitor cell infusion to treat univentricular heart disease) randomized phase 2 trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef]
- Tarui, S.; Ishigami, S.; Ousaka, D.; Kasahara, S.; Ohtsuki, S.; Sano, S.; Oh, H. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: Three-year follow-up of the transcoronary infusion of cardiac progenitor cells in patients with single-ventricle physiology (TICAP) trial. J. Thorac. Cardiovasc. Surg. 2015, 150, 1198–1208. [Google Scholar] [CrossRef]
- Cardiac Stem/Progenitor Cell Infusion in Univentricular Physiology (APOLLON Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT02781922 (accessed on 9 October 2019).
- Transcoronary Infusion of Cardiac Progenitor Cells in Pediatric Dilated Cardiomyopathy. Available online: https://clinicaltrials.gov/ct2/show/NCT03129568 (accessed on 9 October 2019).
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01458405 (accessed on 9 October 2019).
- Sanz-Ruiz, R.; Casado Plasencia, A.; Borlado, L.R.; Fernández-Santos, M.E.; Al-Daccak, R.; Claus, P.; Palacios, I.; Sádaba, R.; Charron, D.; Bogaert, J.; et al. Rationale and design of a clinical trial to evaluate the safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with acute myocardial infarction and left ventricular dysfunction: The randomized multicenter double-blind controlled CAREMI trial (cardiac stem cells in patients with acute myocardial infarction). Circ. Res. 2017, 121, 71–80. [Google Scholar] [PubMed]
- Dilated CardiomYopathy iNtervention with Allogeneic MyocardIally-Regenerative Cells (DYNAMIC). Available online: https://clinicaltrials.gov/ct2/show/NCT02293603 (accessed on 9 October 2019).
- Bolli, R.; Hare, J.M.; March, K.L.; Pepine, C.J.; Willerson, J.T.; Perin, E.C.; Yang, P.C.; Henry, T.D.; Traverse, J.H.; Mitrani, R.D.; et al. Rationale and design of the CONCERT-HF trial (combination of mesenchymal and c-kit + cardiac stem cells as regenerative therapy for heart failure). Circ. Res. 2018, 122, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Regression of Fibrosis & Reversal of Diastolic Dysfunction in HFPEF Patients Treated with Allogeneic CDCs. Available online: https://clinicaltrials.gov/ct2/show/NCT02941705 (accessed on 9 October 2019).
- Sahara, M.; Santoro, F.; Chien, K.R. Programming and reprogramming a human heart cell. EMBO J. 2015, 34, 710–738. [Google Scholar] [CrossRef]
- Amini, H.; Rezaie, J.; Vosoughi, A.; Rahbarghazi, R.; Nouri, M. Cardiac progenitor cells application in cardiovascular disease. J. Cardiovasc. Thorac. Res. 2017, 9, 127–132. [Google Scholar] [CrossRef]
- Sanchez-Freire, V.; Lee, A.S.; Hu, S.; Abilez, O.J.; Liang, P.; Lan, F.; Huber, B.C.; Ong, S.-G.; Hong, W.X.; Huang, M.; et al. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J. Am. Coll. Cardiol. 2014, 64, 436–448. [Google Scholar] [CrossRef]
- Martens, T.P.; Godier, A.F.G.; Parks, J.J.; Wan, L.Q.; Koeckert, M.S.; Eng, G.M.; Hudson, B.I.; Sherman, W.; Vunjak-Novakovic, G. Percutaneous cell delivery into the heart using hydrogels polymerizing in situ. Cell Transplant. 2009, 18, 297–304. [Google Scholar] [CrossRef]
- Beeres, S.L.M.A.; Atsma, D.E.; van Ramshorst, J.; Schalij, M.J.; Bax, J.J. Cell therapy for ischaemic heart disease. Heart 2008, 94, 1214–1226. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, R.; Huang, X.; Zhang, H.; He, L.; Zhang, L.; Tian, X.; Nie, Y.; Hu, S.; Yan, Y.; et al. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016, 26, 119–130. [Google Scholar] [CrossRef]
- He, L.; Li, Y.; Li, Y.; Pu, W.; Huang, X.; Tian, X.; Wang, Y.; Zhang, H.; Liu, Q.; Zhang, L.; et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat. Med. 2017, 23, 1488–1498. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Huang, X.; Bhaloo, S.I.; Zhao, H.; Zhang, S.; Pu, W.; Tian, X.; Li, Y.; Liu, Q.; et al. Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 2018, 138, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Scalise, M.; Cianflone, E.; Mancuso, T.; Aquila, I.; Agosti, V.; Torella, M.; Paolino, D.; Mollace, V.; Nadal-Ginard, B.; et al. Role of c-Kit in myocardial regeneration and aging. Front. Endocrinol. 2019, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-L.; Molkentin, J.D. The elusive progenitor cell in cardiac regeneration: Slip slidin’ away. Circ. Res. 2017, 120, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte regeneration: A consensus statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.W.; Witten, C.M.; Califf, R.M. Clarifying stem-cell therapy’s benefits and risks. N. Engl. J. Med. 2017, 376, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Maliken, B.D.; Molkentin, J.D. Undeniable evidence that the adult mammalian heart lacks an endogenous regenerative stem cell. Circulation 2018, 138, 806–808. [Google Scholar] [CrossRef]
- Writing Group Members; Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; et al. Heart disease and stroke statistics—2012 Update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar]
- Cesselli, D.; Beltrami, A.P.; D’Aurizio, F.; Marcon, P.; Bergamin, N.; Toffoletto, B.; Pandolfi, M.; Puppato, E.; Marino, L.; Signore, S.; et al. Effects of age and heart failure on human cardiac stem cell function. Am. J. Pathol. 2011, 179, 349–366. [Google Scholar] [CrossRef]
- Yao, Y.-G.; Ellison, F.M.; McCoy, J.P.; Chen, J.; Young, N.S. Age-dependent accumulation of mtDNA mutations in murine hematopoietic stem cells is modulated by the nuclear genetic background. Hum. Mol. Genet. 2007, 16, 286–294. [Google Scholar] [CrossRef][Green Version]
- Mohsin, S.; Siddiqi, S.; Collins, B.; Sussman, M.A. Empowering adult stem cells for myocardial regeneration. Circ. Res. 2011, 109, 1415–1428. [Google Scholar] [CrossRef]
- Frati, C.; Savi, M.; Graiani, G.; Lagrasta, C.; Cavalli, S.; Prezioso, L.; Rossetti, P.; Mangiaracina, C.; Ferraro, F.; Madeddu, D.; et al. Resident cardiac stem cells. Curr. Pharm. Des. 2011, 17, 3252–3257. [Google Scholar] [PubMed]
- Leonardini, A.; Avogaro, A. Abnormalities of the cardiac stem and progenitor cell compartment in experimental and human diabetes. Arch. Physiol. Biochem. 2013, 119, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kurazumi, H.; Kubo, M.; Ohshima, M.; Yamamoto, Y.; Takemoto, Y.; Suzuki, R.; Ikenaga, S.; Mikamo, A.; Udo, K.; Hamano, K.; et al. The effects of mechanical stress on the growth, differentiation, and paracrine factor production of cardiac stem cells. PLoS ONE 2011, 6, e28890. [Google Scholar] [CrossRef] [PubMed]
- Torella, D.; Rota, M.; Nurzynska, D.; Musso, E.; Monsen, A.; Shiraishi, I.; Zias, E.; Walsh, K.; Rosenzweig, A.; Sussman, M.A.; et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ. Res. 2004, 94, 514–524. [Google Scholar] [CrossRef]
- Anversa, P.; Rota, M.; Urbanek, K.; Hosoda, T.; Sonnenblick, E.H.; Leri, A.; Kajstura, J.; Bolli, R. Myocardial aging: A stem cell problem. Basic Res. Cardiol. 2005, 100, 482–493. [Google Scholar] [CrossRef]
- Urbanek, K.; Quaini, F.; Tasca, G.; Torella, D.; Castaldo, C.; Nadal-Ginard, B.; Leri, A.; Kajstura, J.; Quaini, E.; Anversa, P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2003, 100, 10440–10445. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).