Role of Fibronectin in Primary Open Angle Glaucoma
Abstract
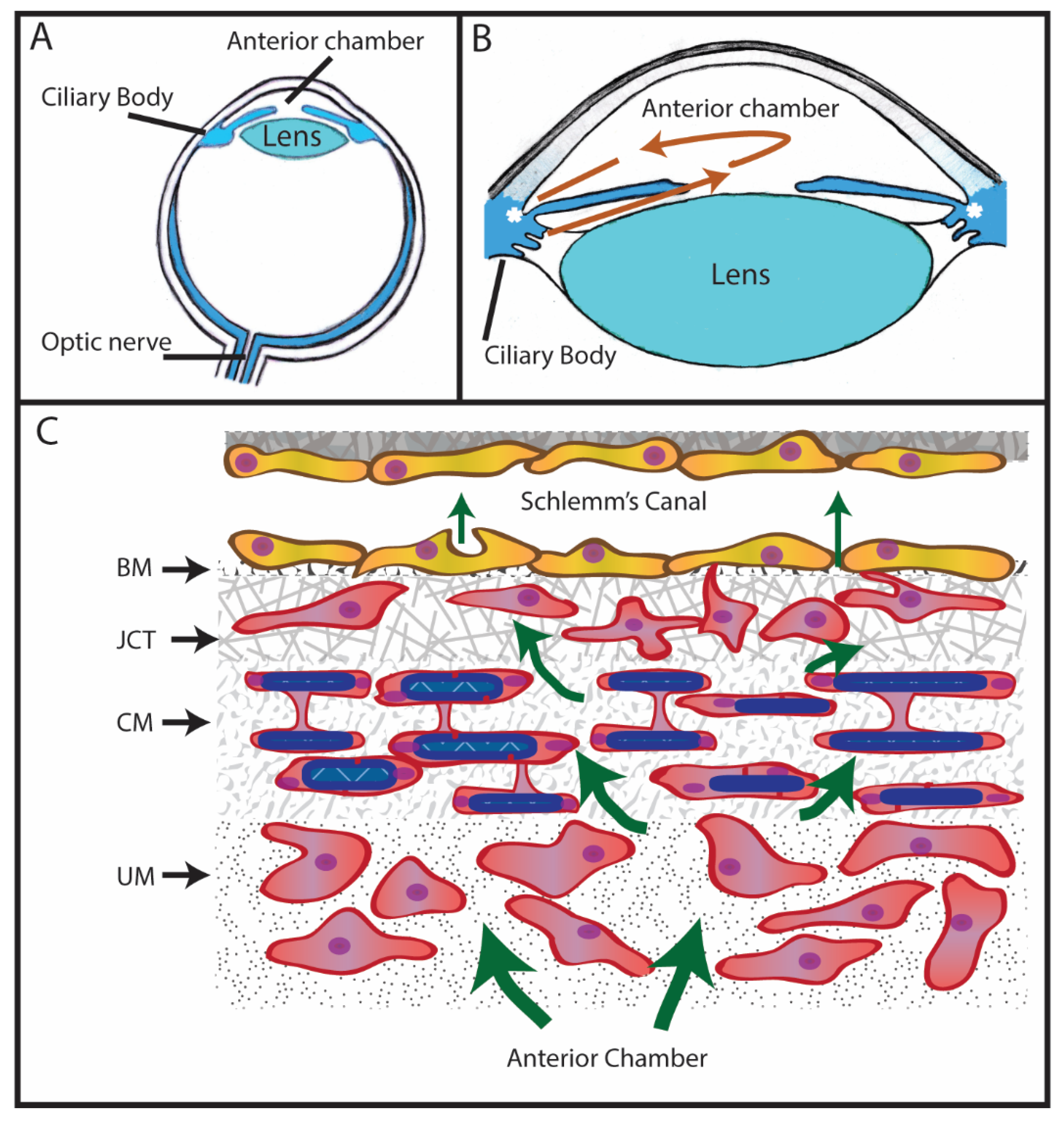
1. Introduction to Glaucoma
2. Expression of Fibronectin in the Trabecular Meshwork
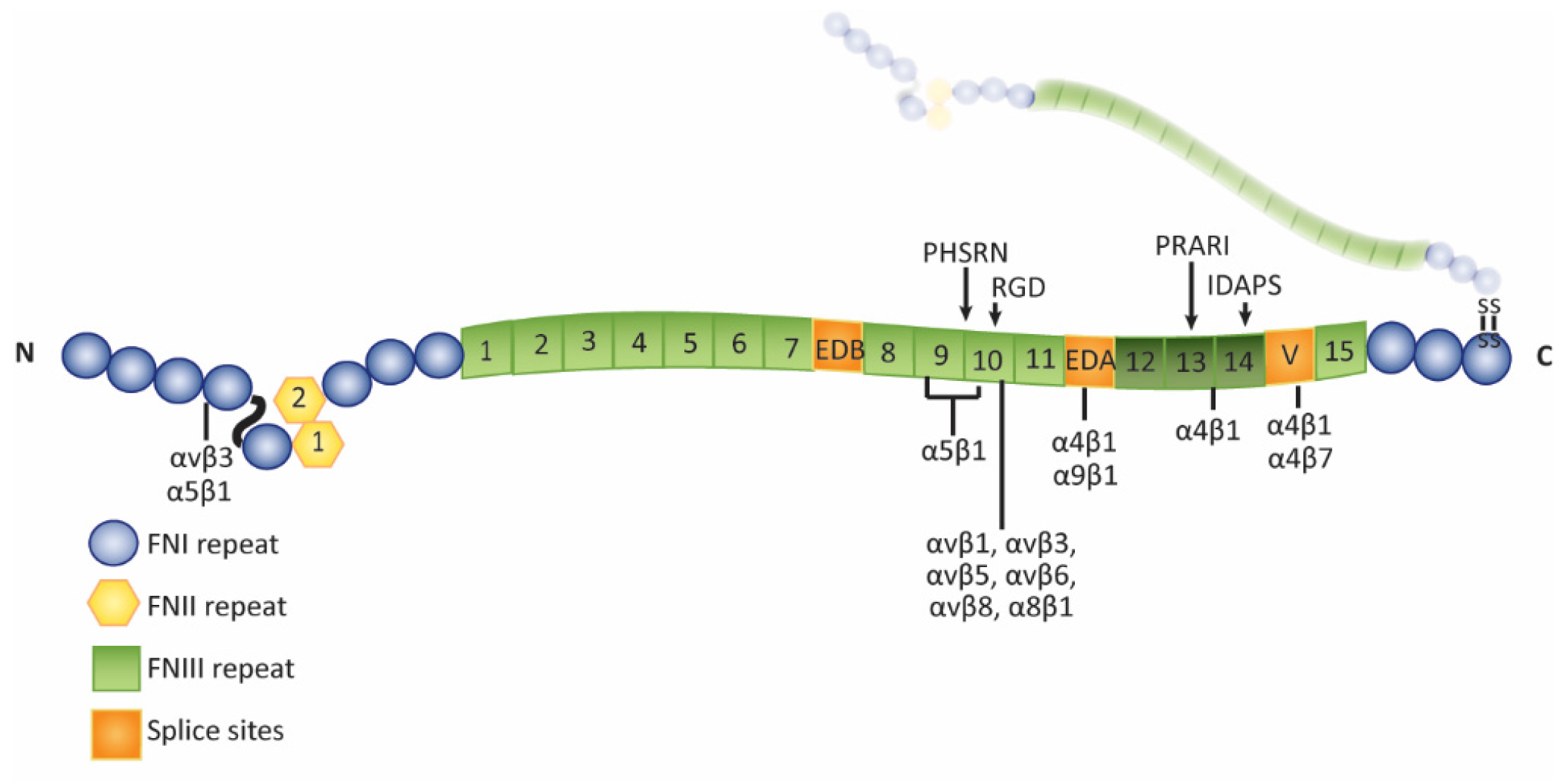
Isoforms of Fibronectin in the Trabecular Meshwork
3. How Fibronectin Affects IOP
3.1. Increased ECM Rigidity
3.2. Fibronectin Regulates Deposition of Collagen IV, Laminin, and Fibrillin
3.3. Bioactivity of HepII Domain Affects IOP
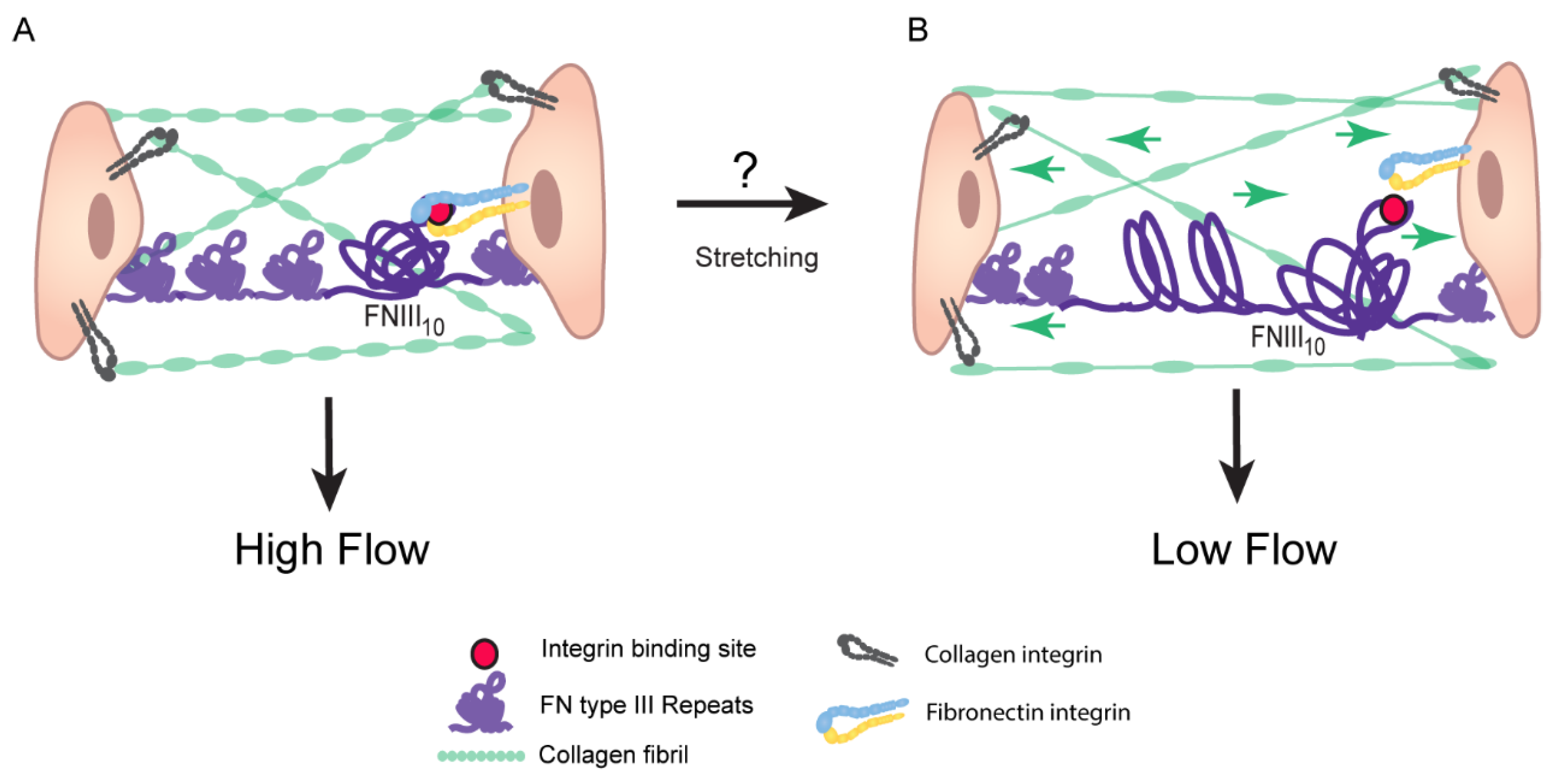
4. Assembly of Fibronectin Fibrils
Fibronectin Fibrils Have Different Conformation and Composition
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Quigley, H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996, 80, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, R.F. Flow of aqueous humor in humans. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3145–3166. [Google Scholar] [PubMed]
- Acott, T.S.; Kelley, M.J. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. What controls aqueous humor outflow resistance? Exp. Eye Res. 2006, 82, 545–557. [Google Scholar] [CrossRef]
- Tamm, E.R.; Braunger, B.M.; Fuchshofer, R. Intraocular pressure and the mechanisms involved in resistance of the aqueous humor flow in the trabecular meshwork outflow pathways. Prog. Mol. Biol. Transl. Sci. 2015, 134, 301–314. [Google Scholar]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef]
- Schroer, A.K.; Merryman, W.D. Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J. Cell Sci. 2015, 128, 1865–1875. [Google Scholar] [CrossRef]
- Hann, C.R.; Springett, M.J.; Wang, X.; Johnson, D.H. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic Res. 2001, 33, 314–324. [Google Scholar] [CrossRef]
- Reid, T.; Kenney, C.; Waring, G.O. Isolation and characterization of fibronectin from bovine aqueous humor. Investig. Ophthalmol. Vis. Sci. 1982, 22, 57–61. [Google Scholar]
- Babizhayev, M.A.; Brodskaya, M.W. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mech. Ageing Dev. 1989, 47, 145–157. [Google Scholar] [CrossRef]
- Floyd, B.B.; Cleveland, P.H.; Worthen, D.M. Fibronectin in human trabecular drainage channels. Investig. Ophthalmol. Vis. Sci. 1985, 26, 797–804. [Google Scholar] [PubMed]
- Tripathi, B.J.; Li, T.; Li, J.; Tran, L.; Tripathi, R.C. Age-related changes in trabecular cells in vitro. Exp. Eye Res. 1997, 64, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, B.H.; Kim, I.S. The measurement of fibronectin concentrations in human aqueous humor. Kor. J. Ophthalmol. 1992, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vesaluoma, M.; Mertaniemi, P.; Mannonen, S.; Lehto, I.; Uusitalo, R.; Sarna, S.; Tarkkanen, A.; Tervo, T. Cellular and plasma fibronectin in the aqueous humour of primary open-angle glaucoma, exfoliative glaucoma and cataract patients. Eye 1998, 12, 886–890. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Li, J.; Chan, W.F.; Tripathi, B.J. Aqueous humor in glaucomatous eyes contains an increased level of TGFβ2. Exp. Eye Res. 1994, 59, 723–727. [Google Scholar] [CrossRef]
- Agarwal, P.; Mohammad Daher, A.; Agarwal, R. Aqueous humor TGF-β2 levels in patients with open-angle glaucoma: A meta-analysis. Mol. Vis. 2015, 21, 612–620. [Google Scholar]
- Prendes, M.A.; Harris, A.; Wirostko, B.M.; Gerber, A.L.; Siesky, B. The role of transforming growth factor β in glaucoma and the therapeutic implications. Br. J. Ophthalmol. 2013, 97, 680–686. [Google Scholar] [CrossRef]
- Min, S.H.; Lee, T.I.; Chung, Y.S.; Kim, H.K. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Kor. J. Ophthlamol. 2006, 20, 162–165. [Google Scholar] [CrossRef]
- Fuchshofer, R. The pathogenic role of transforming growth factor-β2 in glaucomatous damage to the optic nerve head. Exp. Eye Res. 2011, 93, 165–169. [Google Scholar] [CrossRef]
- Medina-Ortiz, W.E.; Belmares, R.; Neubauer, S.; Wordinger, R.J.; Clark, A.F. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-β2. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6779–6788. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; Dimeo, K.; Tong, T.; Peters, D.M. Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp. Eye Res. 2017, 165, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Helbig, H.; Kittredge, K.L.; Coca-Prados, M.; Davis, J.; Palestine, A.G.; Nussenblatt, R.B. Mammalian ciliary-body epithelial cells in culture produced transforming growth factor-beta. Graefes Arch. Clin. Exp. Ophthalmol. 1991, 229, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.B.; Davidson, M.G.; Nasisse, M.P.; Fleisher, L.N.; McGahan, M.C. The lens influences aqueous humor levels of transforming growth factor-beta 2. Graefes Arch. Clin. Exp. Ophthlamol. 1998, 236, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Thomson, C.; de longh, R.U.; Hales, A.M.; Chamberlain, C.G.; McAvoy, J.W. Differential cataractogenic potency of TGF-beta1, -beta2, and -beta3 and their expression in the postnatal rat eye. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1399–1409. [Google Scholar]
- Wallentin, N.; Wickstrom, K.; Lundberg, C. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1410–1418. [Google Scholar]
- Wordinger, R.J.; Clark, A.F.; Agarwal, R.; Lambert, W.; McNatt, L.; Wilson, S.E.; Qu, Z.; Fung, B.K. Cultured human trabecular meshwork cells express functional growth factor receptors. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1575–1589. [Google Scholar]
- Tomarev, S.I.; Wistow, G.; Raymond, V.; Dubois, S.; Malyukova, I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2588–2596. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Yu, A.H.; Welge-Luben, U.; Tamm, E.R. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 715–726. [Google Scholar] [CrossRef]
- Bollinger, K.E.; Crabb, J.S.; Yuan, X.; Putliwala, T.; Clark, A.F.; Crabb, J.W. Quantitative proteomics: TGFβ2 signaling in trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8287–8294. [Google Scholar] [CrossRef]
- Junglas, B.; Yu, A.H.; Welge-Lussen, U.; Tamm, E.R.; Fuchshofer, R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp. Eye Res. 2009, 88, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Junglas, B.; Kuespert, S.; Seleem, A.A.; Struller, T.; Ullmann, S.; Bosl, M.; Bosserhoff, A.; Kostler, J.; Wagner, R.; Tamm, E.R.; et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Path. 2012, 180, 2386–2403. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Vidales, T.; Clark, A.F.; Wordinger, R.J. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp. Eye Res. 2011, 93, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Welge-Lussen, U.; May, C.A.; Lutjen-Drecoll, E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-β1 and TGF-β2. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2229–2238. [Google Scholar]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef]
- Armaly, M.F.; Becker, B. Intraocular pressure response to topical corticosteroids. Fed. Proc. 1965, 24, 1274–1278. [Google Scholar]
- Becker, B.; Ballin, N. Glaucoma and corticosteroid provocative testing. Arch. Ophthalmol. 1965, 74, 621–624. [Google Scholar] [CrossRef]
- Clark, A.F.; Wordinger, R.J. The role of steroids in outflow resistance. Exp. Eye Res. 2009, 88, 752–759. [Google Scholar] [CrossRef]
- Filla, M.S.; Liu, X.; Nguyen, T.D.; Polansky, J.R.; Brandt, C.R.; Kaufman, P.L.; Peters, D.M. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 151–161. [Google Scholar]
- Steely, H.T.; Browder, S.L.; Julian, M.B.; Miggans, S.T.; Wilson, K.L.; Clark, A.F. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2242–2250. [Google Scholar]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef]
- Wordinger, R.J.; Clark, A.F. Effects of glucocorticoids on the trabecular meshwork: Towards a better understanding of glaucoma. Prog. Retin. Eye Res. 1999, 18, 629–667. [Google Scholar] [CrossRef]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, R.B.; Maddineni, P.; Millar, J.C.; Clark, A.F.; Zode, G.S. Increased synthesis and deposition of extracellular matrix proteins leads to endoplasmic reticulum stress in the trabecular meshwork. Sci. Rep. 2017, 7, 14951. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, R.B.; Maddineni, P.; Patel, P.D.; Searby, C.; Sheffield, V.C.; Zode, G.S. Transforming growth factor β2 (TGFβ2) signaling plays a key role in glucocorticoid-induced ocular hypertension. J. Biol. Chem. 2018, 293, 9854–9868. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; Woods, A.; Kaufman, P.L.; Peters, D.M. β1 and β3 integrins cooperate to induce syndecan-4-containing cross-linked actin metworks in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1956–1967. [Google Scholar] [CrossRef]
- Comes, N.; Buie, L.K.; Borras, T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: Implications for glaucoma. Genes Cells 2011, 16, 243–259. [Google Scholar] [CrossRef]
- Kuchtey, J.; Kallberg, M.E.; Gelatt, K.N.; Rinkoshi, T.; Komaromy, A.M.; Kuchtey, R.W. Angiopoietin-like 7 secretion is induced by glaucoma stimuli and its concentration is elevated in glaucomatous aqueous humor. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3438–3448. [Google Scholar] [CrossRef]
- Lo, W.R.; Rowlette, L.L.; Caballero, M.; Yang, P.; Hernandez, M.R.; Borras, T. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 473–485. [Google Scholar] [CrossRef]
- Rozsa, F.W.; Reed, D.M.; Scott, K.M.; Pawar, H.; Moroi, S.E.; Kijek, T.G.; Krafchak, C.M.; Othman, M.I.; Vollrath, D.; Elner, V.M.; et al. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol. Vis. 2006, 12, 125–141. [Google Scholar]
- Zhao, X.; Ramsey, K.E.; Stephan, D.A.; Russell, P. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor beta. Investig. Ophthlamol. Vis. Sci. 2004, 45, 4023–4034. [Google Scholar] [CrossRef] [PubMed]
- Gabelt, B.T.; Kaufman, P.L. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 2005, 24, 612–637. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Roy, S. Effect of high glucose on fibronectin expression and cell proliferation in trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 170–175. [Google Scholar] [PubMed]
- Yue, B.Y.; Higginbotham, E.J.; Chang, I.L. Ascorbic acid modulates the production of fibronectin and laminin by cells from an eye tissue – trabecular meshwork. Exp. Cell Res. 1990, 187, 65–68. [Google Scholar] [CrossRef]
- Zhou, L.; Higginbotham, E.J.; Yue, B.Y. Effects of ascorbic acid on levels of fibronectin, laminin and collagen type I in bovine trabecular meshwork in organ culture. Curr. Eye Res. 1998, 17, 222–227. [Google Scholar] [CrossRef]
- Hysi, P.G.; Khawaja, A.P.; Menni, C.; Tamraz, B.; Wareham, N.; Khaw, K.-T.; Foster, P.J.; Benet, L.Z.; Spector, T.D.; Hammond, C.J. Ascorbic acid metabolites are involved in intraocular pressure control in the general population. Redox. Biol. 2019, 20, 349–353. [Google Scholar] [CrossRef]
- Keller, K.E.; Sun, Y.Y.; Vranka, J.A.; Hayashi, L.; Acott, T.S. Inhibition of hyaluronan synthesis reduces versican and fibronectin levels in trabecular meshwork cells. PLoS ONE 2012, 7, e48523. [Google Scholar] [CrossRef]
- Evanko, S.P.; Potter-Perigo, S.; Petty, L.J.; Workman, G.A.; Wight, T.N. Hyaluronan controls the depostion of fibronectin and collagen and modulates TGF-β1 induction of lung myofibroblasts. Matrix Biol. 2015, 42, 74–92. [Google Scholar] [CrossRef]
- Vogel, S.; Arnoldini, S.; Moller, S.; Schnabelrauch, M.; Hempel, U. Sulfated hyaluronan alters fibronectin matrix assembly and promotes osteogenic differentiation of human bone marrow stromal cells. Sci. Rep. 2016, 6, 36418. [Google Scholar] [CrossRef]
- Knepper, P.A.; Goossens, W.; Hvizd, M.; Palmberg, P.F. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1360–1367. [Google Scholar]
- Knepper, P.A.; Goossens, W.; Palmberg, P.F. Glycosaminoglycan stratification of the juxtacanalicular tissue in normal and primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2414–2425. [Google Scholar] [PubMed]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Ffrench-Constant, C. Alternative splicing of fibronectin – many different proteins but few different functions. Exp. Cell Res. 1995, 221, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Fibronectins; Springer: New York, NY, USA, 1990. [Google Scholar]
- Tripathi, R.C.; Borisuth, N.S.C.; Tripathi, B.J. Growth factors in the aqueous humor and their therapeutic implications in glaucoma and anterior segment disorders of the human eye. Drug Dev. Res. 1991, 22, 1–23. [Google Scholar] [CrossRef]
- Li, J.; Tripathi, B.J.; Tripathi, R.C. Modulation of pre-mRNA splicing and protein production of fibronectin by TGFβ2 in porcine trabecular cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3437–3443. [Google Scholar] [PubMed]
- Keller, K.E.; Kelley, M.J.; Acott, T.S. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Acott, T.S. Pressure-induced expression changes in segmental flow regions of the human trabecular meshwork. Exp. Eye Res. 2017, 158, 67–72. [Google Scholar] [CrossRef]
- Vittal, V.; Rose, A.; Gregory, K.E.; Kelley, M.J.; Acott, T.S. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2857–2868. [Google Scholar] [CrossRef]
- Saito, S.; Yamaji, N.; Yasunaga, K.; Saito, T.; Matsumoto, S.; Katoh, M.; Kobayashi, S.; Masuho, Y. The fibronectin extra domain A activates matrix metalloproteinase gene expression by an interleukin-1-dependent mechanism. J. Biol. Chem. 1999, 274, 30756–30763. [Google Scholar] [CrossRef]
- Acott, T.S.; Wirtz, M.K. Biochemistry of aqueous flow. In The Glaucomas, 2nd ed.; Ritch, R., Shields, M.B., Krupin, T., Eds.; CV Mosby: St. Louis, MO, USA, 1996; Volume 1, pp. 281–305. [Google Scholar]
- Andreasen, P.A.; Egelund, R.; Petersen, H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell. Mol. Life Sci. 2000, 57, 25–40. [Google Scholar] [CrossRef]
- Bradley, J.M.; Kelley, M.J.; Zhu, X.; Anderssohn, A.M.; Alexander, J.P.; Acott, T.S. Effects of mechanical stretching on trabecular matrix metalloproteinases. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1505–1513. [Google Scholar] [PubMed]
- Bradley, J.M.; Vranka, J.; Colvis, C.M.; Conger, D.M.; Alexander, J.P.; Fisk, A.S.; Samples, J.R.; Acott, T.S. Effect of matrix metalloproteinase activity on outflow in perfused human organ culture. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2649–2658. [Google Scholar] [PubMed]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the trabecular meshwork: Promising targets for future glaucoma therapies? Investig. Ophthalmol. Vis. Sci. 2013, 54, 7756–7763. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Bradley, J.M.; Acott, T.S. Differential effects of ADAMTS-1, -4, and -5 in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5769–5777. [Google Scholar] [CrossRef]
- Porter, K.; Lin, Y.; Liton, P.B. Cathepsin B is up-regulated and mediates extracellular matrix degradation in trabecular meshwork cells following phagocytic challenge. PLoS ONE 2013, 8, e68668. [Google Scholar] [CrossRef]
- Johnson, D.H. Histologic findings after argon laser trabeculoplasty in glaucomatous eyes. Exp. Eye Res. 2007, 85, 557–562. [Google Scholar] [CrossRef]
- Parshley, D.E.; Bradley, J.M.; Fisk, A.; Hadaegh, A.; Samples, J.R.; Van Buskirk, E.M.; Acott, T.S. Laser trabeculoplasty induces stromelysin expression by trabecular juxtacanalicular cells. Investig. Ophthalmol. Vis. Sci. 1996, 37, 795–804. [Google Scholar]
- Tian, B.; Geiger, B.; Epstein, D.L.; Kaufman, P.L. Cytoskeletal involvement in the regulation of aqueous humor outflow. Investig. Ophthalmol. Vis. Sci. 2000, 41, 619–623. [Google Scholar]
- Wiederholt, M.; Schafer, R.; Wagner, U.; Lepple-Wienhues, A. Contractile response of the isolated trabecular meshwork and ciliary muscle to cholinergic and adrenergic agents. Ger. J. Ophthalmol. 1996, 5, 146–153. [Google Scholar]
- Nakajima, E.; Nakajima, T.; Minagawa, Y.; Shearer, T.R.; Azuma, M. Contribution of ROCK in contraction of trabecular meshwork: Proposed mechanism for regulating aqueous outflow in monkey and human eyes. J. Pharm. Sci. 2005, 94, 701–708. [Google Scholar] [CrossRef]
- Rao, P.V.; Deng, P.F.; Kumar, J.; Epstein, D.L. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1029–1037. [Google Scholar] [PubMed]
- Pattabiraman, P.P.; Maddala, R.; Rao, P.V. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J. Cell Physiol. 2014, 229, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, P.P.; Rinkoski, T.; Poeschla, E.M.; Proia, A.; Challa, P.; Rao, P.V. RhoA GTPase-induced ocular hypertension in a rodent model is associated with increased fibrogenic activity in the trabecular meshwork. Am. J. Path. 2015, 185, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Doddapattar, P.; Gandhi, C.; Prakash, P.; Dhanesha, N.; Grumbach, I.M.; Dailey, M.E.; Lentz, S.R.; Chauhan, A.K. Fibronectin splicing variants containing extra domain A promote atherosclerosis in mice through toll-like receptor 4. Arterioscler. Throm. Vas. Biol. 2015, 35, 2391–2400. [Google Scholar] [CrossRef]
- Liao, Y.F.; Gotwals, P.J.; Koteliansky, V.E.; Sheppard, D.; Van De Water, L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9 beta 1 and alpha 4 beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 2002, 277, 14467–14474. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Watari, M.; Jerud, E.S.; Young, D.; Ishizaka, S.T.; Rose, J.; Chow, J.C.; Strauss, J.F. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001, 276, 10229–10233. [Google Scholar] [CrossRef]
- Kohan, M.; Muro, A.F.; White, E.S.; Berkman, N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010, 24, 4503–4512. [Google Scholar] [CrossRef]
- Liao, Y.F.; Wieder, K.G.; Classen, J.M.; Van de Water, L. Identification of two amino acids within the EIIIA (ED-A) segment of fibronectin constituting the epitope for two function-blocking monoclonal antibodies. J. Biol. Chem. 1999, 274, 17876–17884. [Google Scholar] [CrossRef]
- Shinde, A.V.; Bystroff, C.; Wang, C.; Vogelezang, M.G.; Vincent, P.A.; Hynes, R.O.; Van de Water, L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J. Biol. Chem. 2008, 283, 2858–2870. [Google Scholar] [CrossRef]
- Gagen, D.; Faralli, J.A.; Filla, M.S.; Peters, D.M. The role of integrins in the trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014, 30, 110–120. [Google Scholar] [CrossRef]
- Schwinn, M.K.; Gonzalez, J.M.; Gabelt, B.T.; Sheibani, N.; Kaufman, P.L.; Peters, D.M. Heparin II domain of fibronectin mediates contractility through an α4β1 co-signaling pathway. Exp. Cell Res. 2010, 316, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Maruyama, I.; Li, Y.; Cheng, E.L.; Yue, B.Y. Expression of integrin receptors in the human trabecular meshwork. Curr. Eye Res. 1999, 19, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Lee, J.; Park, I.; Choi, D.; Lee, S.; Song, S.; Hwang, Y.; Hong, K.Y.; Nakaoka, Y.; Makinen, T.; et al. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J. Clin. Investig. 2014, 124, 3960–3974. [Google Scholar] [CrossRef] [PubMed]
- Grybauskas, A.; Koga, T.; Kuprys, P.V.; Nolan, M.; McCarty, R.; Walker, L.; Green, K.A.; Norkett, W.M.; Yue, B.Y.; Knepper, P.A. ABCB1 transporter and Toll-like receptor 4 in trabecular meshwork cells. Mol. Vis. 2015, 21, 201–212. [Google Scholar]
- Hernandez, H.; Medina-Ortiz, W.E.; Luan, T.; Clark, A.F.; McDowell, C.M. Crosstalk between transforming growth factor beta-2 and toll-like receptor 4 in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1811–1823. [Google Scholar] [CrossRef]
- Ventura, E.; Weller, M.; Macnair, W.; Eschbach, K.; Beisel, C.; Cordazzo, C.; Claassen, M.; Zardi, L.; Burghardt, I. TGF-β induces oncofetal fibronectin that, in turn, modulates TGF-β superfamily signaling in endothelial cells. J. Cell Sci. 2018, 131, jcs209619. [Google Scholar] [CrossRef]
- Mao, Y.; Schwarzbauer, J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005, 24, 389–399. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Tai, V.; Di Paolo, G.; De Camilli, P.; Ginsberg, M.H. Competition for talin results in trans-dominant inhibition of integrin activation. J. Biol. Chem. 2004, 279, 28889–28895. [Google Scholar] [CrossRef]
- Ivaska, J.; Heino, J. Adhesion receptors and cell invasion: Mechanisms of integrin-guided degradation of extracellular matrix. Cell. Mol. Life Sci. 2000, 57, 16–24. [Google Scholar] [CrossRef]
- Lee, J.W.; Juliano, R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol. Cells 2004, 17, 188–202. [Google Scholar]
- Morgan, M.R.; Humphries, M.J.; Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell. Biol. 2007, 8, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.A.; Assoian, R.K. Integrins and cell proliferation: Regulation of cyclin-dependent kinases vis cytoplasmic signaling pathways. J. Cell Sci. 2001, 114, 2553–2560. [Google Scholar] [PubMed]
- Katsumi, A.; Naoe, T.; Matsushita, T.; Kaibuchi, K.; Schwartz, M.A. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J. Biol. Chem. 2005, 250, 19545–19549. [Google Scholar] [CrossRef]
- Borras, T.; Rowlette, L.L.; Tamm, E.R.; Gottanka, J.; Epstein, D.L. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Investig. Ophthalmol. Vis. Sci. 2002, 43, 33–40. [Google Scholar]
- Keller, K.E.; Acott, T.S. The juxtacanalicular region of ocular trabecular meshwork: A tissue with a unique extracellular matrix and specialized function. J. Ocul. Biol. 2013, 1, 3–18. [Google Scholar]
- Keller, K.E.; Aga, M.; Bradley, J.M.; Kelley, M.J.; Acott, T.S. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 2009, 88, 676–682. [Google Scholar] [CrossRef]
- Acott, T.S.; Kelley, M.J.; Keller, K.E.; Vranka, J.A.; Abu-Hassan, D.W.; Li, X.; Aga, M.; Bradley, J.M. Intraocular pressure homeostasis: Maintaining balance in a high-pressure environment. J. Ocul. Pharmacol. Ther. 2014, 30, 94–101. [Google Scholar] [CrossRef]
- Vranka, J.A.; Staverosky, J.A.; Reddy, A.P.; Wilmarth, P.A.; David, L.L.; Acott, T.S.; Russell, P.; Raghunathan, V.K. Biomechanical rigidity and quantitative proteomics analysis of segmental regions of the trabecular meshwork at physiologic and elevated pressure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 246–259. [Google Scholar] [CrossRef]
- Keller, K.E.; Bradley, J.M.; Kelley, M.J.; Acott, T.S. Effects of modifiers of glycosaminoglycan biosynthesis on outflow facility in perfusion culture. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2495–2505. [Google Scholar] [CrossRef]
- Li, A.F.; Tane, N.; Roy, S. Fibronectin overexpression inhibits trabecular meshwork cell monolayer permeability. Mol. Vis. 2004, 10, 750–757. [Google Scholar]
- Wang, C.; Lin, L.; Zhicheng, L. Experimental research on the relationship between the stiffness and the expressions of fibronectin proteins and adaptor proteins of rat trabecular meshwork cells. BMC Ophthalmol. 2017, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, U.; Millar, J.C.; Clark, A.F. Tissue transglutaminase elevates intraocular pressure in mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6197–6211. [Google Scholar] [CrossRef]
- Tovar-Vidales, T.; Roque, R.; Clark, A.F.; Wordinger, R.J. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissue. Investig. Ophthalmol. Vis. Sci. 2008, 49, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Schlunck, G.; Han, H.; Wecker, T.; Kampik, D.; Meyer-ter-Vehn, T.; Grehn, F. Substrate rigidity modulates cell-matrix interactions and protein expression in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, V.K.; Benoit, J.; Kasetti, R.; Zode, G.; Salemi, M.; Phinney, B.S.; Keller, K.E.; Staverosky, J.A.; Murphy, C.J.; Acott, T.; et al. Glaucomatous cell derived matrices differentially modulate non-glaucomatous trabecular meshwork cellular behavior. Acta Biomater. 2018, 71, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chrzanowska-Wodnicka, M.; Brown, J.; Shaub, A.; Belkin, A.M.; Burridge, K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 1998, 141, 539–551. [Google Scholar] [CrossRef]
- Smith, M.L.; Gourdon, D.; Little, W.C.; Kubow, K.E.; Equiluz, A.; Luna-Morris, S.; Vogel, V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS ONE 2007, 5, e268. [Google Scholar] [CrossRef]
- Abu-Lail, N.I.; Ohashi, T.; Clark, R.L.; Erickson, H.P.; Zauscher, S. Understanding the elasticity of fibonectin fibrils: Unfolding strengths of FN-III and GFP domains measured by single molecule force spectroscopy. Matrix Biol. 2006, 25, 175–184. [Google Scholar] [CrossRef]
- Schiller, H.B.; Hermann, M.-R.; Polleux, J.; Vignaud, T.; Zanivan, S.; Friedel, C.C.; Sun, Z.; Raducanu, A.; Gottschalk, K.-E.; Thery, M.; et al. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 2013, 15, 625–636. [Google Scholar] [CrossRef]
- Antia, M.; Baneyx, G.; Kubow, K.E.; Vogel, V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Dis. 2008, 139, 229–249. [Google Scholar] [CrossRef]
- Krammer, A.; Craig, D.; Thomas, W.E.; Schulten, K.; Vogel, V. A structural model for force regulated integrin binding to fibronectin’s RGD-synergy site. Matrix Biol. 2002, 21, 139–147. [Google Scholar] [CrossRef]
- Cao, L.; Nicosia, J.; Larouche, J.; Zhang, Y.; Bachman, H.; Brown, A.C.; Holmgren, L.; Barker, T.H. Detection of an integrin-binding mechanoswitch within fibronectin during tissue formation and fibrosis. ACS Nano 2017, 11, 7110–7117. [Google Scholar] [CrossRef]
- Jones, F.S.; Jones, P.L. The tenascin family of ECM glycoproteins: Structure, function, and regulation during embryonic development and tissue remodeling. Dev. Dyn. 2000, 218, 235–259. [Google Scholar] [CrossRef]
- Jalkanen, S.; Jalkanen, M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J. Cell Biol. 1992, 116, 817–825. [Google Scholar] [CrossRef]
- Wu, Y.J.; La Pierre, D.P.; Wu, J.; Yee, A.J.; Yang, B.B. The interaction of versican with its binding partners. Cell Res. 2005, 15, 483–494. [Google Scholar] [CrossRef]
- Baneyx, G.; Baugh, L.; Vogel, V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc. Natl. Acad. Sci. USA 2002, 99, 5139–5143. [Google Scholar] [CrossRef]
- Pattabiraman, P.P.; Toris, C.B. The exit strategy: Pharmacological modulation of extracellular matrix production and deposition for better aqueous humor drainage. Eur. J. Pharmacol. 2016, 787, 32–42. [Google Scholar] [CrossRef]
- Dallas, S.L.; Sivakumar, P.; Jones, C.J.; Chen, Q.; Peters, D.M.; Mosher, D.F.; Humphries, M.J.; Kielty, C.M. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 2005, 280, 18871–18880. [Google Scholar] [CrossRef]
- Li, S.; Van Den Diepstraten, C.; D’Souza, S.J.; Chan, B.M.C.; Pickering, J.G. Vascular smooth muscle cells orchestrate the assembly of type I collagen via α2β1 integrin, RhoA, and fibronectin polymerization. Am. J. Path. 2003, 163, 1045–1056. [Google Scholar] [CrossRef]
- Sabatier, L.; Chen, D.; Fagotto-Kaufmann, C.; Hubmacher, D.; McKee, M.D.; Annis, D.S.; Mosher, D.F.; Reinhardt, D.P. Fibrillin assembly requires fibronectin. Mol. Biol. Cell 2009, 20, 846–858. [Google Scholar] [CrossRef]
- Sottile, J.; Hocking, D.C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 2002, 13, 3546–3559. [Google Scholar] [CrossRef]
- Velling, T.; Risteli, J.; Wennerberg, K.; Mosher, D.F.; Johansson, S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11 beta 1 and alpha 2 beta 1. J. Biol. Chem. 2002, 277, 37377–37381. [Google Scholar] [CrossRef]
- Murphy, C.G.; Yun, A.J.; Newsome, D.A.; Alvarado, J.A. Localization of extracellular proteins of the human trabecular meshwork by indirect immunofluorescence. Am. J. Ophthalmol. 1987, 104, 33–43. [Google Scholar] [CrossRef]
- Hann, C.R.; Fautsch, M.P. The elastin fiber system between and adjacent to collector channels in the human juxtacanalicular tissue. Investig. Ophthalmol. Vis. Sci. 2011, 52, 45–50. [Google Scholar] [CrossRef]
- Tomasini-Johansson, B.R.; Kaufman, N.R.; Ensenberger, M.G.; Ozeri, V.; Hanski, E.; Mosher, D.F. A 49-residue peptide from adhesin F1 of Streptococcus pyogenes inhibits fibronectin matrix assembly. J. Biol. Chem. 2001, 276, 23430–23439. [Google Scholar] [CrossRef]
- Worthen, D.M.; Cleveland, P.H.; Slight, J.R.; Abare, J. Selective binding affinity of human plasma fibronectin for the collagens I-IV. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1740–1744. [Google Scholar]
- Sylman, J.L.; Artzer, D.T.; Rana, K.; Neeves, K.B. A vascular injury model using focal heat-induced activation of endothelial cells. Integr. Biol. 2015, 7, 801–814. [Google Scholar] [CrossRef]
- Coelho, N.M.; Salmeron-Sanchez, M.; Altankov, G. Fibroblasts remodeling of type IV collagen at a biomaterials interface. Biomater. Sci. 2013, 1, 494–502. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Stahl, R.C.; Carey, D.J. Schwann cells use a novel collagen-dependent mechanism for fibronectin fibril assembly. J. Cell Sci. 1998, 111, 2763–2777. [Google Scholar]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell. Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Dallas, S.L.; Chen, Q.; Sivakumar, P. Dynamics of assembly and reorganization of extracellular matrix proteins. Curr. Top. Dev. Biol. 2006, 75, 1–24. [Google Scholar]
- Acott, T.S.; Westcott, M.; Passo, M.S.; Van Buskirk, E.M. Trabecular meshwork glycosamino glycans in human and cynomolgus monkey eye. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1320–1329. [Google Scholar]
- Filla, M.S.; David, G.; Weinreb, R.N.; Kaufman, P.L.; Peters, D.M. Distribution of syndecans 1–4 within the anterior segment of the human eye: Expression of a variant syndecan-3 and matrix-associated syndecan-2. Exp. Eye Res. 2004, 79, 61–74. [Google Scholar] [CrossRef]
- Sharma, A.; Askari, J.A.; Humphries, M.J.; Jones, E.Y.; Stuart, D.I. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 1999, 18, 1468–1479. [Google Scholar] [CrossRef]
- Santas, A.J.; Bahler, C.; Peterson, J.A.; Filla, M.S.; Kaufman, P.L.; Tamm, E.R.; Johnson, D.H.; Peters, D.M. Effect of heparin II domain of fibronectin on aqueous outflow in cultured anterior segments of human eyes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4796–4804. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Hu, Y.; Gabelt, B.T.; Kaufman, P.L.; Peters, D.M. Identification of the active site in the heparin II domain of fibronectin that increases outflow facility in cultured monkey anterior segments. Investig. Ophthalmol. Vis. Sci. 2009, 50, 235–241. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, J.M.; Faralli, J.A.; Peters, J.M.; Newman, J.R.; Peters, D.M. Effect of heparin II domain of fibronectin on actin cytoskeleton and adherens junctions in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2924–2931. [Google Scholar] [CrossRef]
- Peters, D.M.; Herbert, K.; Biddick, B.; Peterson, J.A. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp. Cell Res. 2005, 303, 218–228. [Google Scholar] [CrossRef]
- Wijelath, E.S.; Rahman, S.; Namekata, M.; Murray, J.; Nishimura, T.; Mostafavi-Pour, Z.; Patel, Y.; Suda, Y.; Humphries, M.J.; Sobel, M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain – enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ. Res. 2006, 99, 853–860. [Google Scholar] [CrossRef]
- Hu, D.N.; Ritch, R.; Liebmann, J.; Liu, Y.; Cheng, B.; Hu, M.S. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J. Glaucoma 2002, 11, 406–410. [Google Scholar] [CrossRef]
- Alexander, J.P.; Samples, J.R.; Acott, T.S. Growth factor and cytokine modulation of trabecular meshwork matrix metalloproteinase and TIMP expression. Curr.Eye Res. 1998, 17, 276–285. [Google Scholar] [CrossRef]
- Gavard, J.; Gutkind, J.S. VEGF controls endothelial-cell permeabiliy by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell. Biol. 2006, 8, 1223–1234. [Google Scholar] [CrossRef]
- Reina-Torres, E.; Wen, J.C.; Liu, K.C.; Li, G.; Sherwood, J.M.; Chang, J.Y.; Challa, P.; Flugel-Koch, C.M.; Stamer, W.D.; Allingham, R.R.; et al. VEGF as a paracrine regulator of conventional outflow facility. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1899–1908. [Google Scholar] [CrossRef]
- Fujimoto, T.; Inoue, T.; Maki, K.; Inoue-Mochita, M.; Tanihara, H. Vascular endothelial growth factor-A increases the aqueous humor outflow facility. PLoS ONE 2016, 11, e0161332. [Google Scholar] [CrossRef]
- McDonald, J.A.; Quade, B.J.; Broekelman, T.J.; LaChance, R.; Forsman, K.; Hasegawa, E.; Akiyama, S.K. Fibronectin’s cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J. Biol. Chem. 1987, 262, 2957–2967. [Google Scholar]
- McKeown-Longo, P.J.; Mosher, D.F. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J. Cell Biol. 1985, 100, 364–374. [Google Scholar] [CrossRef]
- Wu, C.; Bauer, J.S.; Juliano, R.L.; McDonald, J.A. The α5β1 integrin fibronectin receptor, but not the α5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J. Biol. Chem. 1993, 268, 21883–21888. [Google Scholar]
- Filla, M.S.; Faralli, J.A.; Desikan, H.; Peotter, J.L.; Wannow, A.C.; Peters, D.M. Activation of αvβ3 integrin alters fibronectin fibril formation in human trabecular meshwork cells in a ROCK-independent manner. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3897–3913. [Google Scholar] [CrossRef]
- McKeown-Longo, P.J.; Mosher, D.F. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J. Cell Biol. 1983, 97, 466–472. [Google Scholar] [CrossRef]
- Wu, C.; Hughes, P.E.; Ginsberg, M.H.; McDonald, J.A. Identification of a new biological function for the integrin alpha v beta 3: Initiation of fibronectin matrix assembly. Cell Adhes. Commun. 1996, 4, 149–158. [Google Scholar] [CrossRef]
- Gagen, D.; Filla, M.S.; Clark, R.W.; Liton, P.; Peters, D.M. Activated αvβ3 integrin regulates αvβ5 integrin-mediated phagocytosis in trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5000–5011. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Kajiwara, K.; Nada, S.; Okada, M. Src mediates TGF-β-induced intraocular pressure elevation in glaucoma. J. Cell. Physiol. 2018, 34, 1730–1744. [Google Scholar] [CrossRef]
- Filla, M.S.; Schwinn, M.K.; Nosie, A.K.; Clark, R.W.; Peters, D.M. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves β3 integrin signaling. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2952–2959. [Google Scholar] [CrossRef]
- Hill, L.J.; Mead, B.; Blanch, R.J.; Ahmed, Z.; De Cogan, F.; Morgan-Warren, P.J.; Mohamed, S.; Leadbeater, W.; Scott, R.A.; Berry, M.; et al. Decorin reduces intraocular pressure and retinal ganglion cell loss in rodents through fibrolysis of the scarred trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3743–3757. [Google Scholar] [CrossRef]
- Hill, L.J.; Mead, B.; Thomas, C.N.; Foale, S.; Feinstein, E.; Berry, M.; Blanch, R.J.; Ahmed, Z.; Logan, A. TGF-β-induced IOP elevations are mediated by RhoA in the early but not the late fibrotic phase of open angle glaucoma. Mol. Vis. 2018, 24, 712–726. [Google Scholar]
- Zhou, L.; Li, Y.; Yue, B.Y. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int. J. Mol. Med. 1998, 1, 339–346. [Google Scholar] [CrossRef]
- Zhang, Q.; Magnusson, M.K.; Mosher, D.F. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol. Biol. Cell. 1997, 8, 1415–1425. [Google Scholar] [CrossRef]
- Pattabiraman, P.P.; Rao, P.V. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork. Am. J. Physiol. Cell. Physiol. 2010, 298, 749–763. [Google Scholar] [CrossRef]
- Fernandez-Sauze, S.; Grall, D.; Cseh, B.; Van Obberghen-Schilling, E. Regulation of fibronectin matrix assembly and capillary mophogenesis in endothelial cells by Rho family GTPases. Exp. Cell Res. 2009, 15, 2092–2104. [Google Scholar] [CrossRef]
- Baneyx, G.; Baugh, L.; Vogel, V. Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 2001, 98, 14464–14468. [Google Scholar] [CrossRef]
- Chen, Y.; Zardi, L.; Peters, D.M. High-resolution cryo-scanning electron microscopy study of the macromolecular structure of fibronectin fibrils. Scanning 1997, 19, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Bencharit, S.; Cui, C.B.; Siddiqui, A.; Howard-Williams, E.L.; Sondek, J.; Zuobi-Hasona, K.; Aukhil, I. Structural insights into fibronectin type III domain-mediated signaling. J. Mol. Biol. 2007, 367, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chakrabarti, R.; Keats, E.C.; Chen, M.; Chakrabarti, S.; Khan, Z.A. Regulation of vascular endothelial growth factor expression by extra domain B segment of fibronectin in endothelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8333–8343. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.; Klemis, V.; Sens, C.; Lenhard, T.; Jacobi, C.; Samstag, Y.; Wabnitz, G.; Kirschfink, M.; Wallich, R.; Hansch, G.M.; et al. Identification and characterization of a unique role for EDB fibronectin in phagocytosis. J. Mol. Med. 2016, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faralli, J.A.; Filla, M.S.; Peters, D.M. Role of Fibronectin in Primary Open Angle Glaucoma. Cells 2019, 8, 1518. https://doi.org/10.3390/cells8121518
Faralli JA, Filla MS, Peters DM. Role of Fibronectin in Primary Open Angle Glaucoma. Cells. 2019; 8(12):1518. https://doi.org/10.3390/cells8121518
Chicago/Turabian StyleFaralli, Jennifer A., Mark S. Filla, and Donna M. Peters. 2019. "Role of Fibronectin in Primary Open Angle Glaucoma" Cells 8, no. 12: 1518. https://doi.org/10.3390/cells8121518
APA StyleFaralli, J. A., Filla, M. S., & Peters, D. M. (2019). Role of Fibronectin in Primary Open Angle Glaucoma. Cells, 8(12), 1518. https://doi.org/10.3390/cells8121518



