Mechanism-Specific Pharmacodynamics of a Novel Complex-I Inhibitor Quantified by Imaging Reversal of Consumptive Hypoxia with [18F]FAZA PET In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Analysis of Oxygen Consumption Rate
2.2. [18F]FAZA Production and IACS-010759 Formulation
2.3. Animal Models
2.4. [18F]FAZA Imaging and Analysis
2.5. 2,4-Dinitrophenol and Pyruvate to Increase Oxygen Consumption Rate and Retention of [18F]FAZA
2.6. Pimonidazole Immunohistochemistry
2.7. Human PET/CT Scanning
2.8. Statistics
3. Results
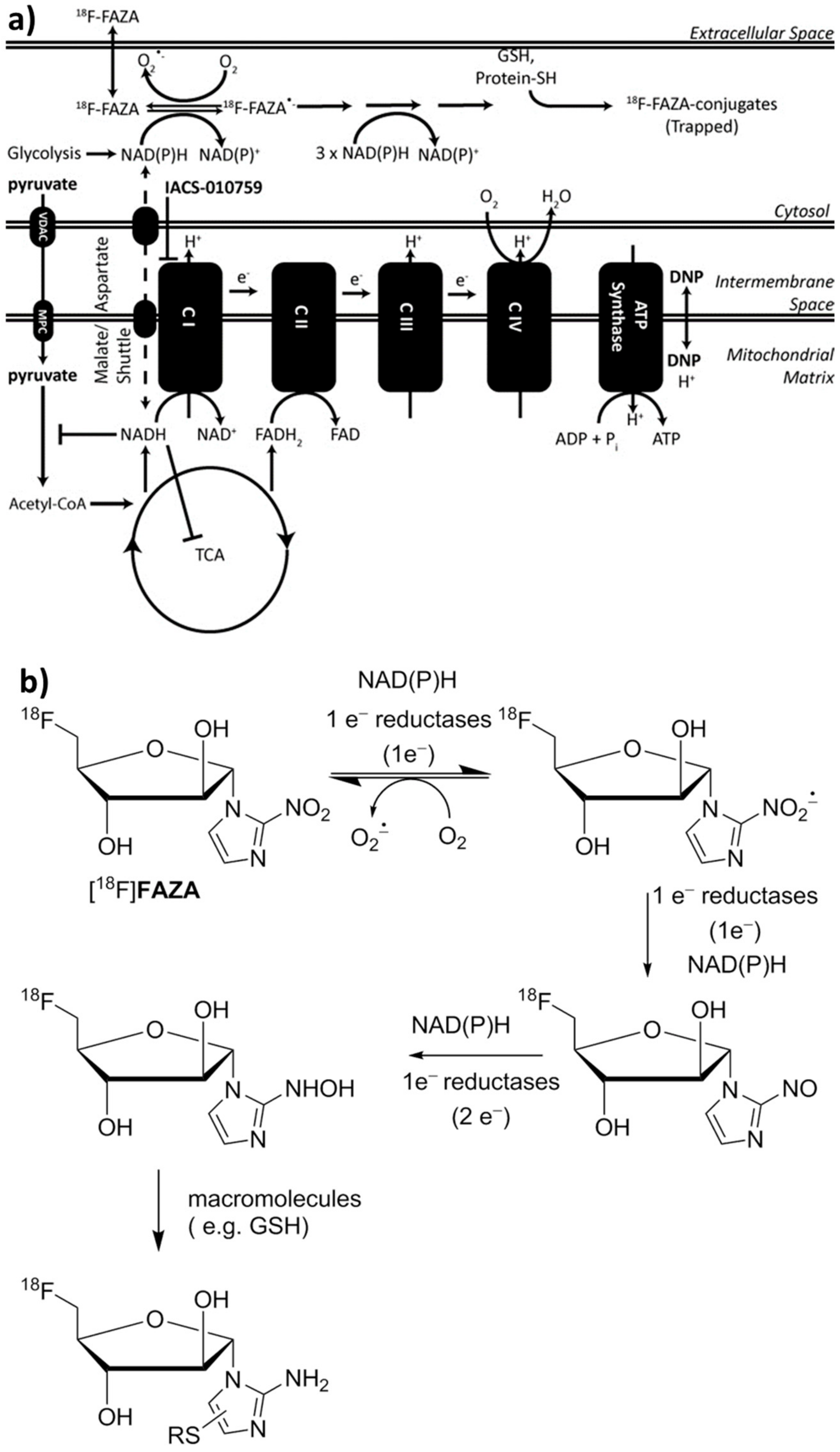
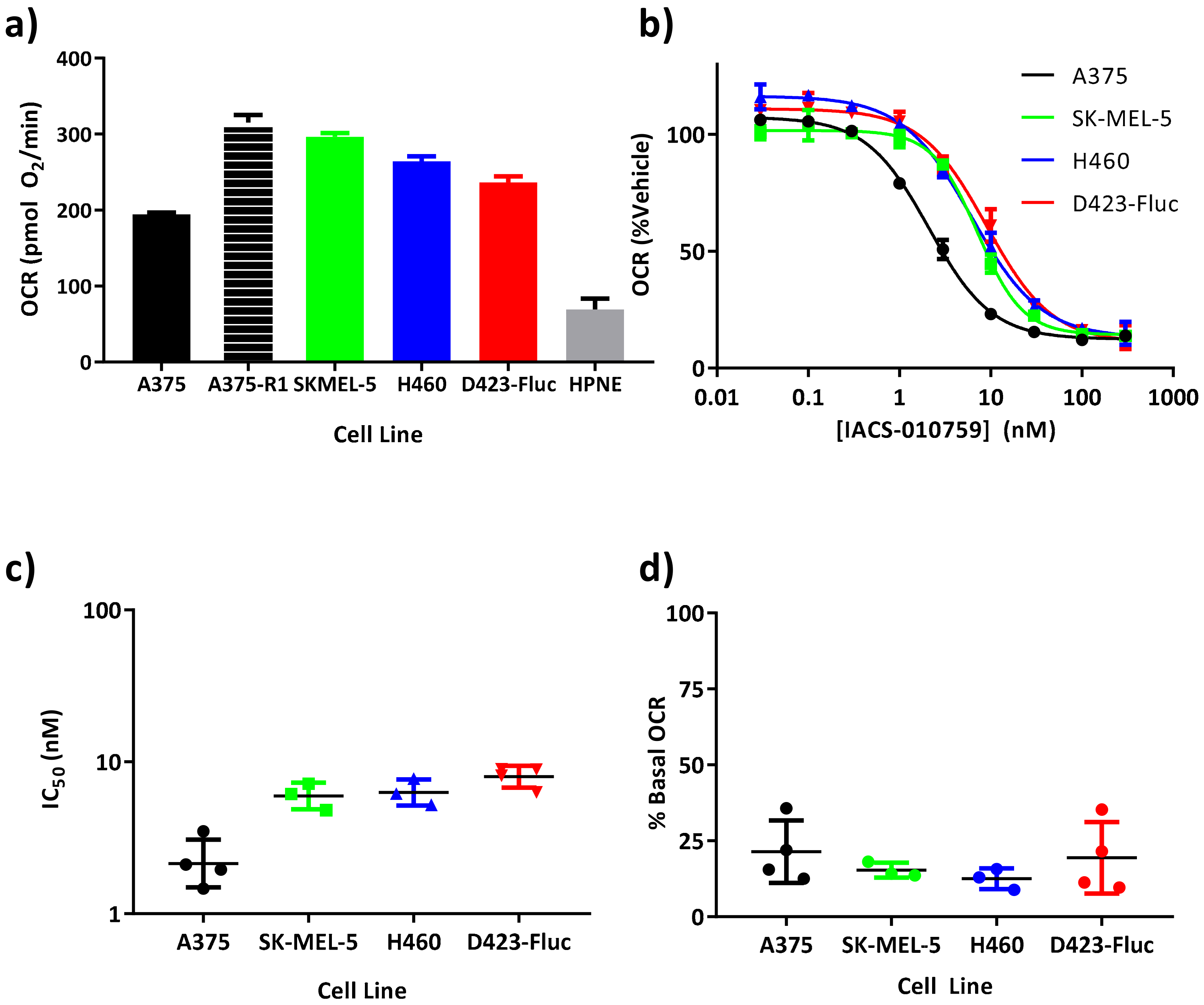
3.1. Inhibition of Oxygen Consumption in Cellulo
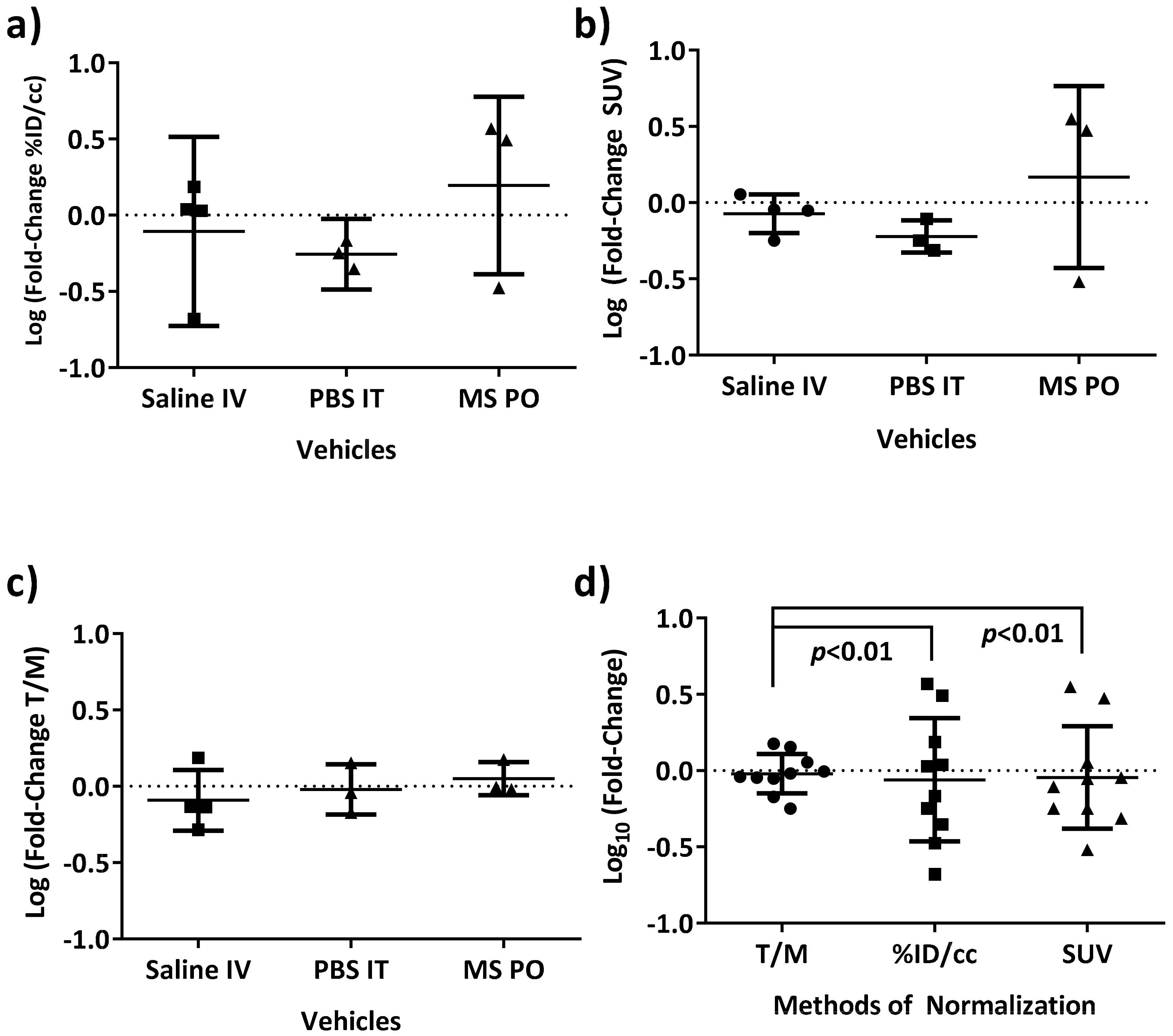
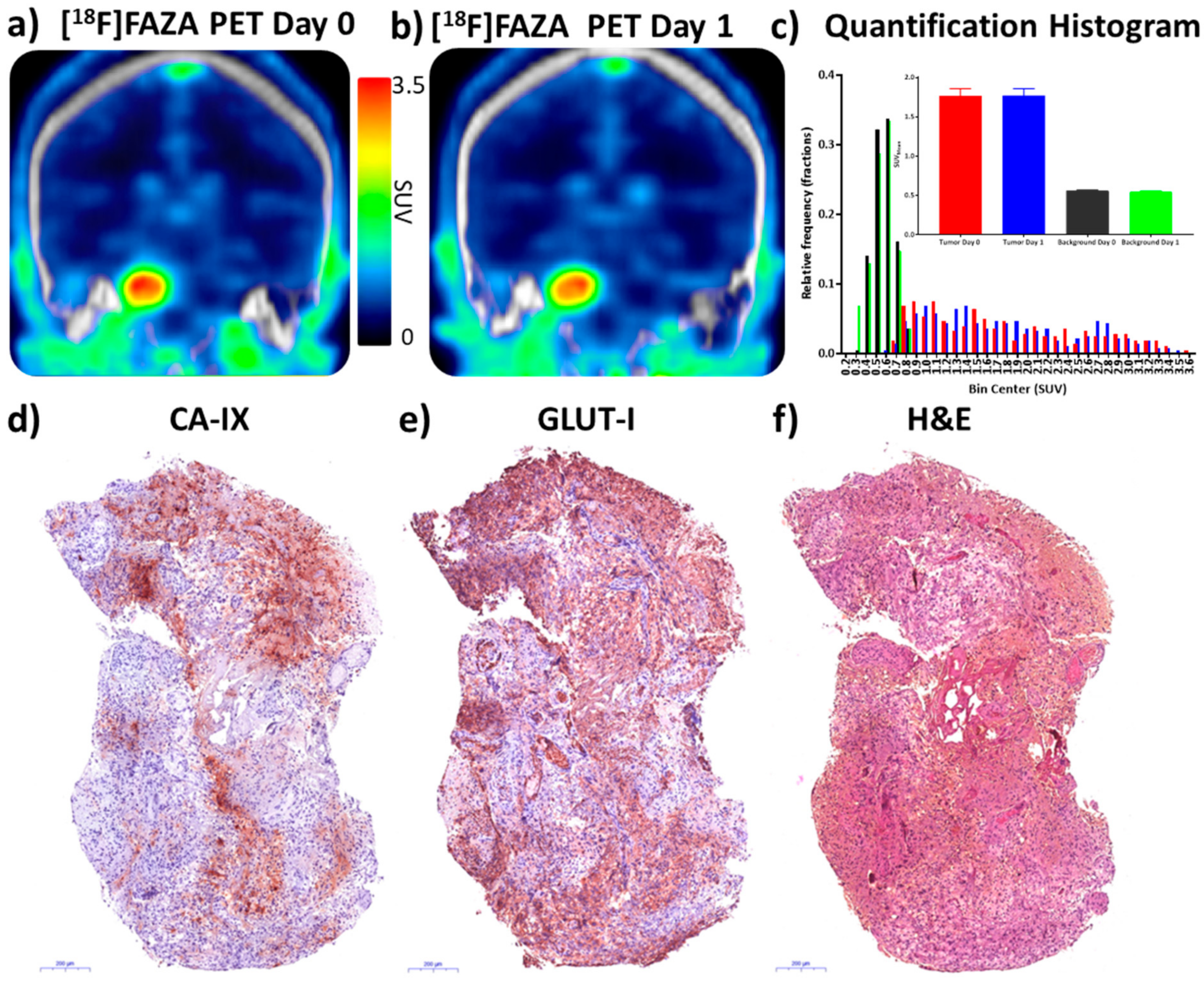
3.2. [18F]FAZA Retention in Tumor-Bearing Mouse Models: Reproducibility and Optimizing Analysis
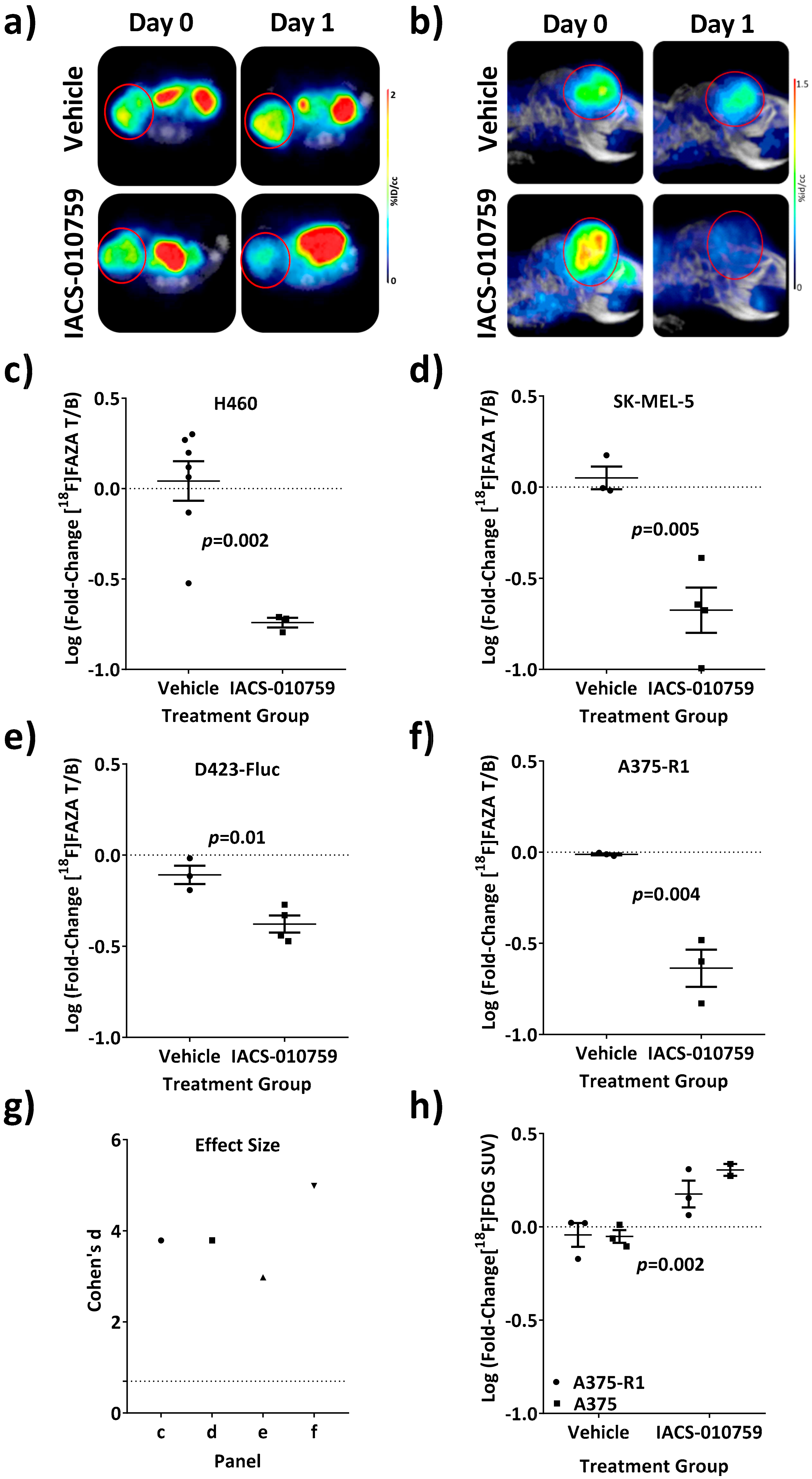
3.3. Reversal of [18F]FAZA Retention In Vivo Across Multiple and Diverse Tumor Models Treated with IACS-010759
3.4. Loss of [18F]FAZA Retention Post-Treatment with IACS-010759 Is Not Related to Changes in the Initial Uptake of [18F]FAZA
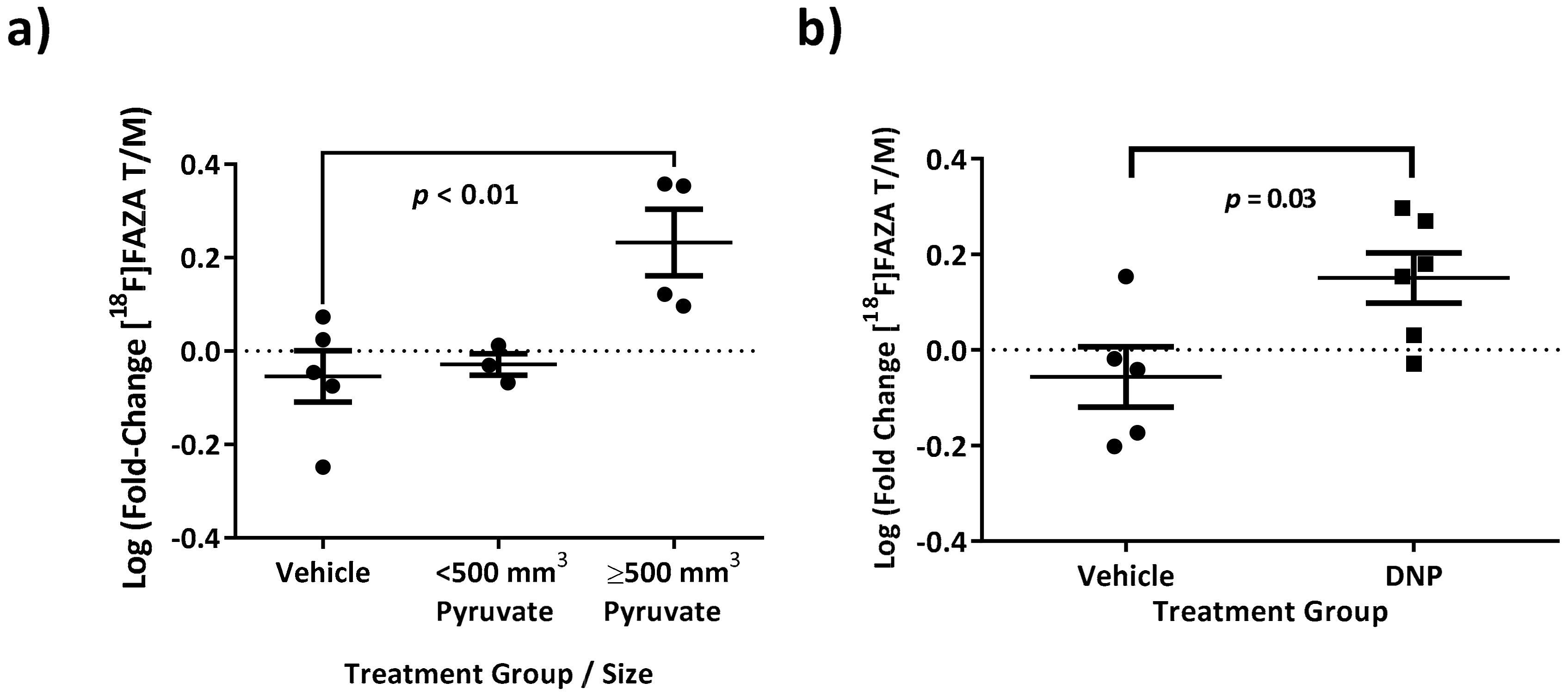
3.5. Increasing [18F]FAZA Retention with Biochemical Drivers of Oxygen Consumption Rate
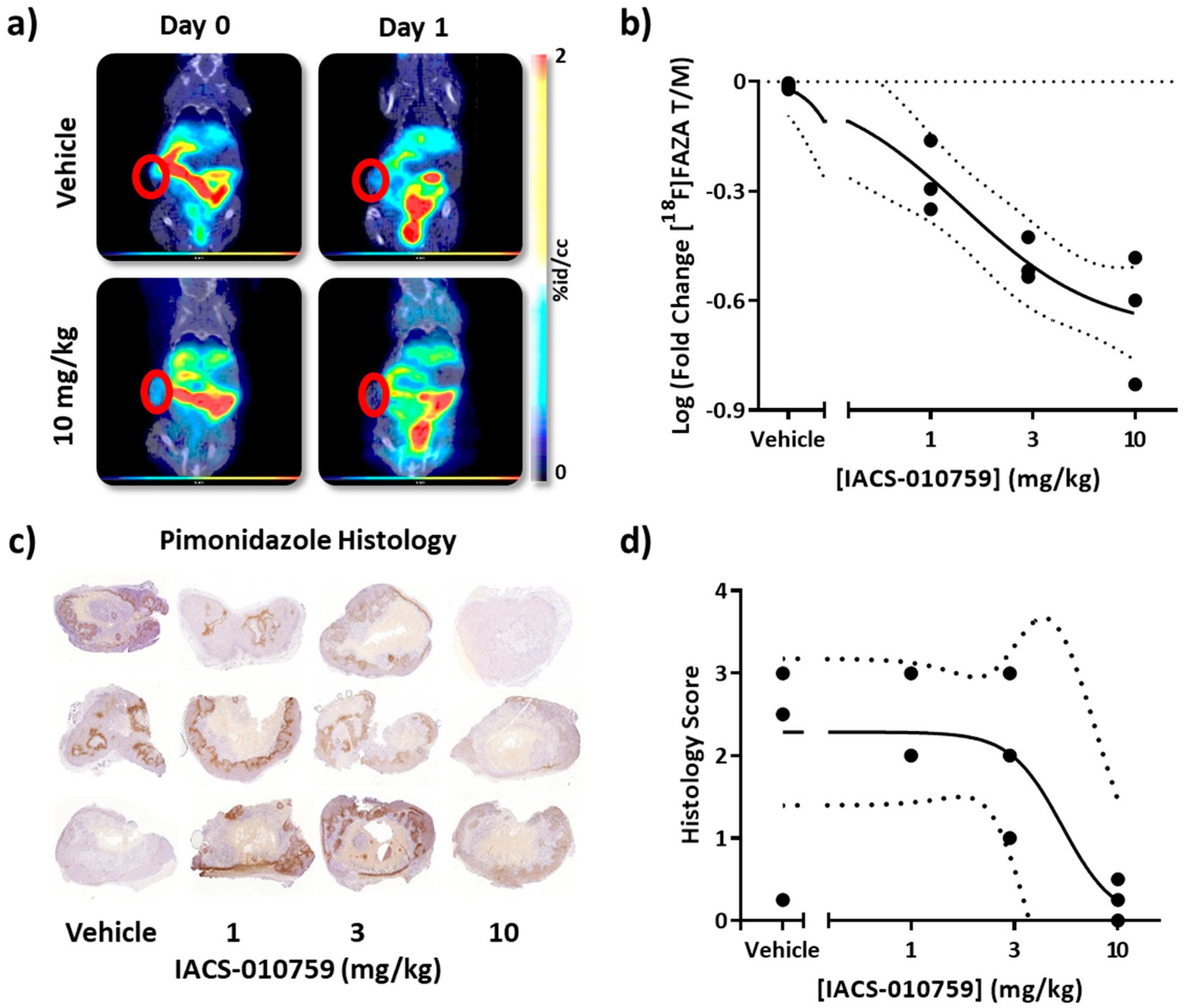
3.6. Dose-Response of IACS-010759-Mediated Reversal of [18F]FAZA Retention in a BRAF Inhibitor-Resistant Melanoma Tumor Model In Vivo
3.7. Precision of [18F]FAZA PET/CT Test-Retest Method in a Patient with Glioblastoma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef]
- West, J.B. Respiratory Physiology: The Essentials, 6th ed.; Williams & Wilkins: Baltimore, MD, USA; London, UK; Los Angeles, CA, USA, 1999. [Google Scholar]
- Cooper, R.A.; Carrington, B.M.; Loncaster, J.A.; Todd, S.M.; Davidson, S.E.; Logue, J.P.; Luthra, A.D.; Jones, A.P.; Stratford, I.; Hunter, R.D.; et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother. Oncol. 2000, 57, 53–59. [Google Scholar] [CrossRef]
- Vaupel, P.; Hockel, M. Blood supply, oxygenation status and metabolic micromilieu of breast cancers: Characterization and therapeutic relevance. Int. J. Oncol. 2000, 17, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Halle, C.; Andersen, E.; Lando, M.; Aarnes, E.K.; Hasvold, G.; Holden, M.; Syljuasen, R.G.; Sundfor, K.; Kristensen, G.B.; Holm, R.; et al. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res. 2012, 72, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Scholz, W. The Contribution of Patho-Anatomical Research to the Problem of Epilepsy. Epilepsia 1959, 1, 36–57. [Google Scholar] [CrossRef]
- Grimes, D.R.; Fletcher, A.G.; Partridge, M. Oxygen consumption dynamics in steady-state tumour models. R. Soc. Open Sci. 2014, 1, 140080. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, C.J.; Dewhirst, M.W. Tumor oxygenation: A matter of supply and demand. Anticancer Res. 1996, 16, 741–749. [Google Scholar]
- Papandreou, I.; Powell, A.; Lim, A.L.; Denko, N. Cellular reaction to hypoxia: Sensing and responding to an adverse environment. Mutat. Res. 2005, 569, 87–100. [Google Scholar] [CrossRef]
- Hockel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Ong, E.T.; Rosner, G.L.; Rehmus, S.W.; Shan, S.; Braun, R.D.; Brizel, D.M.; Secomb, T.W. Arteriolar oxygenation in tumour and subcutaneous arterioles: Effects of inspired air oxygen content. Br. J. Cancer Suppl. 1996, 27, S241–S246. [Google Scholar]
- Dewhirst, M.W.; Kimura, H.; Rehmus, S.W.; Braun, R.D.; Papahadjopoulos, D.; Hong, K.; Secomb, T.W. Microvascular studies on the origins of perfusion-limited hypoxia. Br. J. Cancer Suppl. 1996, 27, S247–S251. [Google Scholar] [PubMed]
- Secomb, T.W.; Hsu, R.; Ong, E.T.; Gross, J.F.; Dewhirst, M.W. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995, 34, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-Inducible Factors: Coupling Glucose Metabolism and Redox Regulation with Induction of the Breast Cancer Stem Cell Phenotype. EMBO J. 2017, 36, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate metabolism in human lung tumors. Cell 2017, 171, 358–371.e359. [Google Scholar] [CrossRef]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic heterogeneity in human lung tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef]
- Sellers, K.; Fox, M.P.; Bousamra, M., 2nd; Slone, S.P.; Higashi, R.M.; Miller, D.M.; Wang, Y.; Yan, J.; Yuneva, M.O.; Deshpande, R.; et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Invest. 2015, 125, 687–698. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Scarbrough, P.M.; Ribeiro, A.; Richardson, R.; Yuan, H.; Sonveaux, P.; Landon, C.D.; Chi, J.T.; Pizzo, S.; Schroeder, T.; et al. Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS ONE 2013, 8, e75154. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.Q.; Sanchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014, 514, 628. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Sriskanthadevan, S.; Jeyaraju, D.V.; Chung, T.E.; Prabha, S.; Xu, W.; Skrtic, M.; Jhas, B.; Hurren, R.; Gronda, M.; Wang, X.; et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood 2015, 125, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Miwa, H.; Shikami, M.; Tsunekawa-Imai, N.; Suganuma, K.; Mizuno, S.; Takahashi, M.; Mizutani, M.; Hanamura, I.; Nitta, M. Importance of glutamine metabolism in leukemia cells by energy production through TCA cycle and by redox homeostasis. Cancer Investig. 2014, 32, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Caro, P.; Kishan, A.U.; Norberg, E.; Stanley, I.A.; Chapuy, B.; Ficarro, S.B.; Polak, K.; Tondera, D.; Gounarides, J.; Yin, H.; et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 2012, 22, 547–560. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Cesi, G.; Walbrecq, G.; Zimmer, A.; Kreis, S.; Haan, C. ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells. Mol. Cancer 2017, 16, 102. [Google Scholar] [CrossRef]
- Gopal, Y.N.; Rizos, H.; Chen, G.; Deng, W.; Frederick, D.T.; Cooper, Z.A.; Scolyer, R.A.; Pupo, G.; Komurov, K.; Sehgal, V.; et al. Inhibition of mTORC1/2 overcomes resistance to MAPK pathway inhibitors mediated by PGC1alpha and oxidative phosphorylation in melanoma. Cancer Res. 2014, 74, 7037–7047. [Google Scholar] [CrossRef]
- Molina, J.R.; Sun, Y.; Protopopova, M.; Gera, S.; Bandi, M.; Bristow, C.; McAfoos, T.; Morlacchi, P.; Ackroyd, J.; Agip, A.-N.A.; et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018, 24, 1036–1046. [Google Scholar] [CrossRef]
- Piert, M.; Machulla, H.J.; Picchio, M.; Reischl, G.; Ziegler, S.; Kumar, P.; Wester, H.J.; Beck, R.; McEwan, A.J.; Wiebe, L.I.; et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J. Nucl. Med. 2005, 46, 106–113. [Google Scholar]
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular imaging of hypoxia. J. Nucl. Med. 2008, 49 (Suppl. 2), 129S–148S. [Google Scholar] [CrossRef]
- Pacheco-Torres, J.; Ballesteros, P.; Lopez-Lurrubia, P.; Cerdan, S. Kinetics and mechanism of bioreduction of nitroimidazoles as hypoxia probes. Proc. Intl. Mag. Reson. Med. 2013, 21, 1886. [Google Scholar]
- Reischl, G.; Ehrlichmann, W.; Bieg, C.; Solbach, C.; Kumar, P.; Wiebe, L.I.; Machulla, H.J. Preparation of the hypoxia imaging PET tracer [18F]FAZA: Reaction parameters and automation. Appl. Radiat. Isot. 2005, 62, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.G.; Zegers, C.M.; Yaromina, A.; Van Elmpt, W.; Dubois, L.; Lambin, P. Current preclinical and clinical applications of hypoxia PET imaging using 2-nitroimidazoles. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 39–57. [Google Scholar] [PubMed]
- Dunn, O.J. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Leonard, P.G.; Satani, N.; Maxwell, D.; Lin, Y.H.; Hammoudi, N.; Peng, Z.; Pisaneschi, F.; Link, T.M.; Lee, G.R.t.; Sun, D.; et al. SF2312 is a natural phosphonate inhibitor of enolase. Nat. Chem. Biol. 2016, 12, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Colla, S.; Aquilanti, E.; Manzo, V.E.; Genovese, G.; Lee, J.; Eisenson, D.; Narurkar, R.; Deng, P.; Nezi, L.; et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature 2012, 488, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Schockel, L.; Glasauer, A.; Basit, F.; Bitschar, K.; Truong, H.; Erdmann, G.; Algire, C.; Hagebarth, A.; Willems, P.H.; Kopitz, C.; et al. Targeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growth. Cancer Metab. 2015, 3, 11. [Google Scholar] [CrossRef]
- Salomon, A.R.; Voehringer, D.W.; Herzenberg, L.A.; Khosla, C. Understanding and exploiting the mechanistic basis for selectivity of polyketide inhibitors of F(0)F(1)-ATPase. Proc. Natl. Acad. Sci. USA 2000, 97, 14766–14771. [Google Scholar] [CrossRef]
- Straud, S.; Zubovych, I.; De Brabander, J.K.; Roth, M.G. Inhibition of iron uptake is responsible for differential sensitivity to V-ATPase inhibitors in several cancer cell lines. PLoS ONE 2010, 5, e11629. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine metabolism in cancer: Understanding the heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Hoareau, R.; Hockley, B.G.; Tluczek, L.J.; Henderson, B.D.; Padgett, H.C.; Scott, P.J. Highlighting the versatility of the Tracerlab synthesis modules. Part 1: Fully automated production of [F]labelled radiopharmaceuticals using a Tracerlab FX(FN). J. Labelled Comp. Radiopharm. 2011, 54, 292–307. [Google Scholar] [CrossRef]
- Yanfei, L.; Han, Y.; Qian, F.; Baofa, Y. Preclinical pharmacokinetics and toxic kinetics study of 2, 4-dinitrophenol (DNP). Pharm. Pharmacol. Int. J. 2016, 4. [Google Scholar] [CrossRef]
- Takakusagi, Y.; Matsumoto, S.; Saito, K.; Matsuo, M.; Kishimoto, S.; Wojtkowiak, J.W.; DeGraff, W.; Kesarwala, A.H.; Choudhuri, R.; Devasahayam, N.; et al. Pyruvate induces transient tumor hypoxia by enhancing mitochondrial oxygen consumption and potentiates the anti-tumor effect of a hypoxia-activated prodrug TH-302. PLoS ONE 2014, 9, e107995. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.J. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002, 352, 3–31. [Google Scholar]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Ponte, K.F.; Berro, D.H.; Collet, S.; Constans, J.M.; Emery, E.; Valable, S.; Guillamo, J.S. In vivo relationship between hypoxia and angiogenesis in human glioblastoma: A multimodal imaging study. J. Nucl. Med. 2017, 58, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Terasaka, S.; Shiga, T.; Hattori, N.; Magota, K.; Kobayashi, H.; Yamaguchi, S.; Houkin, K.; Tanaka, S.; Kuge, Y.; et al. 18F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1383–1392. [Google Scholar] [CrossRef]
- Sancho, P.; Barneda, D.; Heeschen, C. Hallmarks of cancer stem cell metabolism. Br. J. Cancer 2016, 114, 1305–1312. [Google Scholar] [CrossRef]
- Morandi, A.; Indraccolo, S. Linking metabolic reprogramming to therapy resistance in cancer. Biochim. Biophys. Acta 2017, 1868, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Grana, O.; et al. MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Chang, C.P.; Tsao, C.C.; Xu, J. Oligomycin-induced bioenergetic adaptation in cancer cells with heterogeneous bioenergetic organization. J. Biol. Chem. 2010, 285, 12647–12654. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972, 8, 1–34. [Google Scholar]
- Holness, M.J.; Sugden, M.C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans. 2003, 31, 1143–1151. [Google Scholar] [CrossRef]
- Leong, H.S.; Brownsey, R.W.; Kulpa, J.E.; Allard, M.F. Glycolysis and pyruvate oxidation in cardiac hypertrophy—Why so unbalanced? Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2003, 135, 499–513. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1, O(2), and the 3 PHDs: How animal cells signal hypoxia to the nucleus. Cell 2001, 107, 1–3. [Google Scholar] [CrossRef]
- Tran, L.B.; Bol, A.; Labar, D.; Jordan, B.; Magat, J.; Mignion, L.; Gregoire, V.; Gallez, B. Hypoxia imaging with the nitroimidazole 18F-FAZA PET tracer: A comparison with OxyLite, EPR oximetry and 19F-MRI relaxometry. Radiother. Oncol. 2012, 105, 29–35. [Google Scholar] [CrossRef]
- Reischl, G.; Dorow, D.S.; Cullinane, C.; Katsifis, A.; Roselt, P.; Binns, D.; Hicks, R.J. Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA—First small animal PET results. J. Pharmacy Pharm. Sci. 2007, 10, 203–211. [Google Scholar]
- Telford, J.E.; Kilbride, S.M.; Davey, G.P. Complex I is rate-limiting for oxygen consumption in the nerve terminal. J. Biol. Chem. 2009, 284, 9109–9114. [Google Scholar] [CrossRef]
- Zhu, X.H.; Chen, W. In vivo oxygen-17 NMR for imaging brain oxygen metabolism at high field. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 319–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, E.; Liu, H.; Unterschemmann, K.; Ellinghaus, P.; Liu, S.; Gekeler, V.; Cheng, Z.; Berndorff, D.; Gambhir, S.S. 18F-FAZA PET imaging response tracks the reoxygenation of tumors in mice upon treatment with the mitochondrial complex I inhibitor BAY 87-2243. Clin. Cancer. Res. 2015, 21, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, M.; Schmid, M.P.; Potter, R.; Kommata, S.; Georg, D.; Lukic, D.; Dudczak, R.; Kletter, K.; Dimopoulos, J.; Karanikas, G.; et al. Evaluating repetitive 18F-fluoroazomycin-arabinoside (18FAZA) PET in the setting of MRI guided adaptive radiotherapy in cervical cancer. Acta Oncol 2010, 49, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Postema, E.J.; McEwan, A.J.; Riauka, T.A.; Kumar, P.; Richmond, D.A.; Abrams, D.N.; Wiebe, L.I. Initial results of hypoxia imaging using 1-alpha-D: -(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole ( 18F-FAZA). Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Johansen, J.; Kallehauge, J.; Primdahl, H.; Busk, M.; Lassen, P.; Alsner, J.; Sorensen, B.S.; Toustrup, K.; Jakobsen, S.; et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother. Oncol. 2012, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.M.; Beattie, B.J.; Naryanan, M.; Georgi, J.C.; Chen, Q.; Carlin, S.D.; Roble, G.; Zanzonico, P.B.; Gonen, M.; O’Donoghue, J.; et al. Image-guided PO2 probe measurements correlated with parametric images derived from 18F-fluoromisonidazole small-animal PET data in rats. J. Nucl. Med. 2012, 53, 1608–1615. [Google Scholar] [CrossRef]
- Fontanella, A.N.; Schroeder, T.; Hochman, D.W.; Chen, R.E.; Hanna, G.; Haglund, M.M.; Rajaram, N.; Frees, A.E.; Secomb, T.W.; Palmer, G.M.; et al. Quantitative mapping of hemodynamics in the lung, brain, and dorsal window chamber-grown tumors using a novel, automated algorithm. Microcirculation 2013, 20, 724–735. [Google Scholar] [CrossRef]
- Chapman, D.W.; Jans, H.S.; Ma, I.; Mercer, J.R.; Wiebe, L.I.; Wuest, M.; Moore, R.B. Detecting functional changes with [18F]FAZA in a renal cell carcinoma mouse model following sunitinib therapy. EJNMMI Res. 2014, 4, 27. [Google Scholar] [CrossRef][Green Version]
- Cairns, R.A.; Bennewith, K.L.; Graves, E.E.; Giaccia, A.J.; Chang, D.T.; Denko, N.C. Pharmacologically increased tumor hypoxia can be measured by 18F-Fluoroazomycin arabinoside positron emission tomography and enhances tumor response to hypoxic cytotoxin PR-104. Clin. Cancer. Res. 2009, 15, 7170–7174. [Google Scholar] [CrossRef]
- Sarker, D.; Pacey, S.; Workman, P. Use of pharmacokinetic/pharmacodynamic biomarkers to support rational cancer drug development. Biomark Med. 2007, 1, 399–417. [Google Scholar] [CrossRef]
- Gupta, N.; Price, P.M.; Aboagye, E.O. PET for in vivo pharmacokinetic and pharmacodynamic measurements. Eur. J. Cancer 2002, 38, 2094–2107. [Google Scholar] [CrossRef]
- Bertholee, D.; Maring, J.G.; van Kuilenburg, A.B. Genotypes affecting the pharmacokinetics of anticancer drugs. Clin. Pharmacokinet. 2017, 56, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Iakovlev, V.V.; Pintilie, M.; Morrison, A.; Fyles, A.W.; Hill, R.P.; Hedley, D.W. Effect of distributional heterogeneity on the analysis of tumor hypoxia based on carbonic anhydrase IX. Lab. Investig. 2007, 87, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Bettinardi, V.; Fallanca, F.; Incerti, E.; Compierchio, A.; Rossetti, F.; Coliva, A.; Savi, A.; Doglioni, C.; Negri, G.; et al. 18F-FAZA PET/CT in the preoperative evaluation of NSCLC: Comparison with 18F-FDG and immunohistochemistry. Curr. Radiopharm. 2018, 11, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bruine de Bruin, L.; Bollineni, V.R.; Wachters, J.E.; Schuuring, E.; van Hemel, B.M.; van der Wal, J.E.; Slagter-Menkema, L.; de Bock, G.H.; Steenbakkers, R.J.; Langendijk, J.A.; et al. Assessment of hypoxic subvolumes in laryngeal cancer with 18F-fluoroazomycinarabinoside (18F-FAZA)-PET/CT scanning and immunohistochemistry. Radiother. Oncol. 2015, 117, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, T.; Wilkens, J.J. Theoretical analysis of the dose dependence of the oxygen enhancement ratio and its relevance for clinical applications. Radiat. Oncol. 2011, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Brahme, A. Development of radiation therapy optimization. Acta Oncol. 2000, 39, 579–595. [Google Scholar] [CrossRef]
- Lin, A.; Maity, A. Molecular pathways: A novel approach to targeting hypoxia and improving radiotherapy efficacy via reduction in oxygen demand. Clin. Cancer. Res. 2015, 21, 1995–2000. [Google Scholar] [CrossRef]
- Oliva, C.R.; Markert, T.; Ross, L.J.; White, E.L.; Rasmussen, L.; Zhang, W.; Everts, M.; Moellering, D.R.; Bailey, S.M.; Suto, M.J.; et al. Identification of small molecule inhibitors of human cytochrome c oxidase that target chemoresistant glioma cells. J. Biol. Chem. 2016, 291, 24188–24199. [Google Scholar] [CrossRef]
- Oliva, C.R.; Zhang, W.; Langford, C.; Suto, M.J.; Griguer, C.E. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget 2017, 8, 37568–37583. [Google Scholar] [CrossRef]
- Metran-Nascente, C.; Yeung, I.; Vines, D.C.; Metser, U.; Dhani, N.C.; Green, D.; Milosevic, M.; Jaffray, D.; Hedley, D.W. Measurement of tumor hypoxia in patients with advanced pancreatic cancer based on 18F-Fluoroazomyin arabinoside uptake. J. Nucl. Med. 2016, 57, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Servagi-Vernat, S.; Differding, S.; Hanin, F.X.; Labar, D.; Bol, A.; Lee, J.A.; Gregoire, V. A prospective clinical study of [18F]-FAZA PET-CT hypoxia imaging in head and neck squamous cell carcinoma before and during radiation therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Di Perri, D.; Lee, J.A.; Bol, A.; Hanin, F.X.; Janssens, G.; Labar, D.; Robert, A.; Sterpin, E.; Geets, X. Evolution of [18F]fluorodeoxyglucose and [18F]fluoroazomycin arabinoside PET uptake distributions in lung tumours during radiation therapy. Acta Oncol. 2017, 56, 516–524. [Google Scholar] [CrossRef] [PubMed]
Data and materials availability: IACS-010759 and cell lines D423-Fluc and A375-R1 are available upon request with execution of an appropriate MTA. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gammon, S.T.; Pisaneschi, F.; Bandi, M.L.; Smith, M.G.; Sun, Y.; Rao, Y.; Muller, F.; Wong, F.; De Groot, J.; Ackroyd, J.; et al. Mechanism-Specific Pharmacodynamics of a Novel Complex-I Inhibitor Quantified by Imaging Reversal of Consumptive Hypoxia with [18F]FAZA PET In Vivo. Cells 2019, 8, 1487. https://doi.org/10.3390/cells8121487
Gammon ST, Pisaneschi F, Bandi ML, Smith MG, Sun Y, Rao Y, Muller F, Wong F, De Groot J, Ackroyd J, et al. Mechanism-Specific Pharmacodynamics of a Novel Complex-I Inhibitor Quantified by Imaging Reversal of Consumptive Hypoxia with [18F]FAZA PET In Vivo. Cells. 2019; 8(12):1487. https://doi.org/10.3390/cells8121487
Chicago/Turabian StyleGammon, Seth T., Federica Pisaneschi, Madhavi L. Bandi, Melinda G. Smith, Yuting Sun, Yi Rao, Florian Muller, Franklin Wong, John De Groot, Jeffrey Ackroyd, and et al. 2019. "Mechanism-Specific Pharmacodynamics of a Novel Complex-I Inhibitor Quantified by Imaging Reversal of Consumptive Hypoxia with [18F]FAZA PET In Vivo" Cells 8, no. 12: 1487. https://doi.org/10.3390/cells8121487
APA StyleGammon, S. T., Pisaneschi, F., Bandi, M. L., Smith, M. G., Sun, Y., Rao, Y., Muller, F., Wong, F., De Groot, J., Ackroyd, J., Mawlawi, O., Davies, M. A., Vashisht Gopal, Y. N., Di Francesco, M. E., Marszalek, J. R., Dewhirst, M., & Piwnica-Worms, D. (2019). Mechanism-Specific Pharmacodynamics of a Novel Complex-I Inhibitor Quantified by Imaging Reversal of Consumptive Hypoxia with [18F]FAZA PET In Vivo. Cells, 8(12), 1487. https://doi.org/10.3390/cells8121487





