Estrogen Receptors and Melanoma: A Review
Abstract
1. Introduction
2. The Source of Data
- PUBMED
- Ovid MEDLINE
- ISI Web of Science
3. Estrogens and Melanoma: Epidemiological Studies
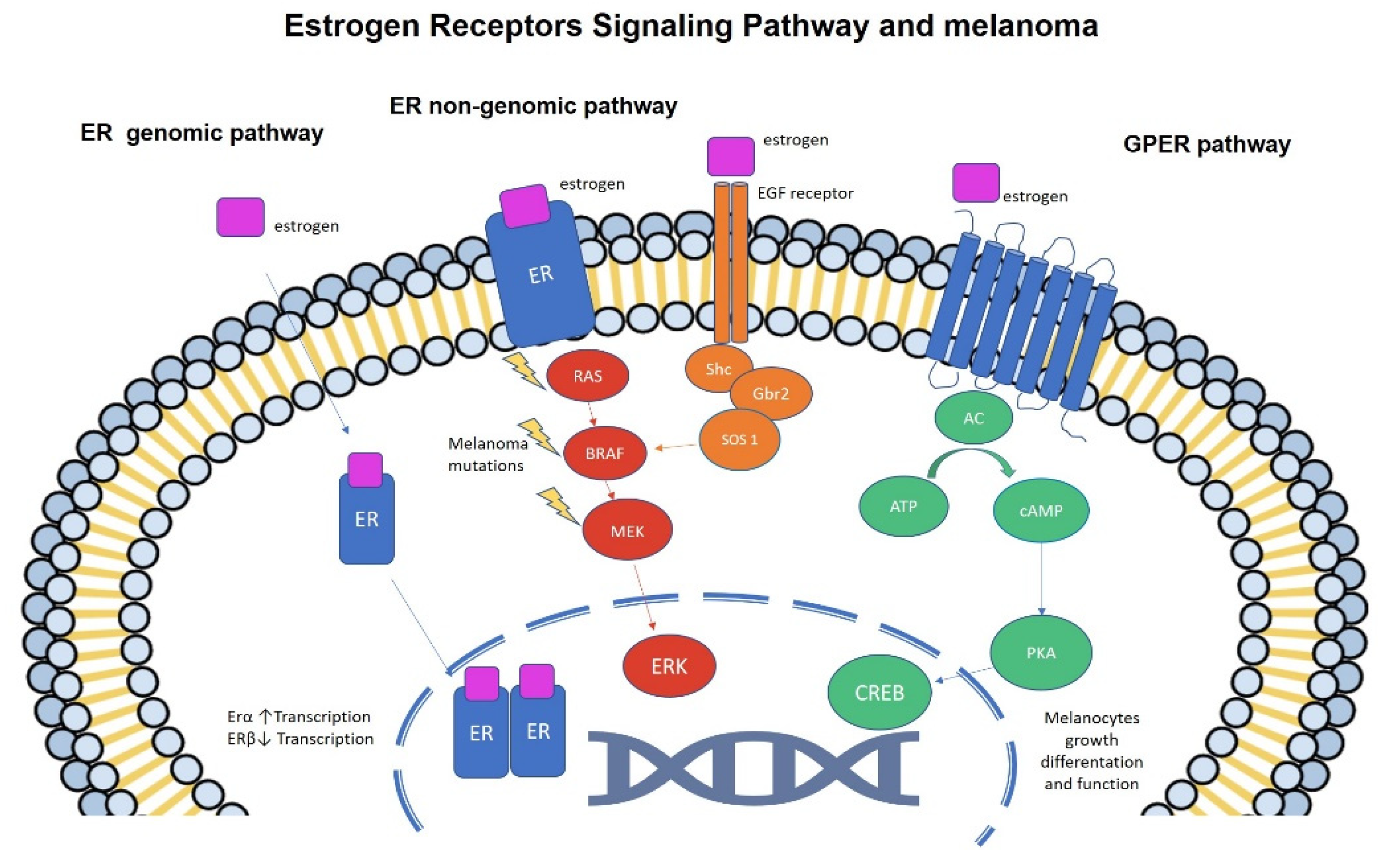
4. Estrogens and Melanoma: Molecular Studies
5. Present and Future Perspectives
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.A.A.; Roesch, A.A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Robsahm, T.E.; Bergva, G.; E Hestvik, U.; Møller, B. Sex differences in rising trends of cutaneous malignant melanoma in Norway, 1954–2008. Melanoma Res. 2013, 23, 70–78. [Google Scholar] [CrossRef]
- Weir, H.K.; Marrett, L.D.; Cokkinides, V.; Barnholtz-Sloan, J.; Patel, P.; Tai, E.; Jemal, A.; Li, J.; Kim, J.; Ekwueme, D.U. Melanoma in adolescents and young adults (ages 15–39 years): United States, 1999–2006. J. Am. Acad. Derm. 2011, 65, S38–S49. [Google Scholar] [CrossRef]
- Reed, K.B.; Brewer, J.D.; Lohse, C.M.; Bringe, K.E.; Pruitt, C.N.; Gibson, L.E. Increasing incidence of melanoma among young adults: An epidemiological study in Olmsted County, Minnesota. Mayo Clin. Proc. 2012, 87, 328–334. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Moser, J.C.; Weaver, A.L.; Markovic, S.N.; Brewer, J.D.; Leontovich, A.A.; Hieken, T.J.; Shuster, L.; Kottschade, L.A.; Olariu, A.; et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017, 6, 2203–2212. [Google Scholar] [CrossRef]
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Lejeune, F.; Kleeberg, U.R.; Coebergh, J.W.; Eggermont, A.M.; de Vries, E. Superior outcome of women with stage I/II cutaneous melanoma: Pooled analysis of four european organisation for research and treatment of cancer phase III trials. J. Clin. Oncol. 2012, 30, 2240–2247. [Google Scholar] [CrossRef]
- Ribero, S.; Longo, C.; Dika, E.; Fortes, C.; Pasquali, S.; Nagore, E.; Glass, D.; Robert, C.; Eggermont, A.M.; Testori, A.; et al. Members of the Melanoma Group of the EORTC. Pregnancy and melanoma: A European-wide survey to assess current management and a critical literature overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 65–69. [Google Scholar] [CrossRef]
- Gandini, S.; Iodice, S.; Koomen, E.; Di Pietro, A.; Sera, F.; Caini, S. Hormonal and reproductive factors in relation to melanoma in women: Current review and meta-analysis. Eur. J. Cancer 2011, 47, 2607–2617. [Google Scholar] [CrossRef]
- Donley, G.M.; Liu, W.T.; Pfeiffer, R.M.; McDonald, E.C.; Peters, K.O.; Tucker, M.A.; Cahoon, E.K. Reproductive factors, exogenous hormone use and incidence of melanoma among women in the United States. Br. J. Cancer 2019, 120, 754–760. [Google Scholar] [CrossRef]
- Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.; Metzler, G.; Moehrle, M.; Breuninger, H.; Garbe, C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer 2008, 112, 1795–1804. [Google Scholar] [CrossRef]
- Joosse, A.; Collette, S.; Suciu, S.; Nijsten, T.; Patel, P.M.; Keilholz, U.; Eggermont, A.M.M.; Coebergh, J.W.W.; De Vries, E. Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: A pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J. Clin. Oncol. 2013, 31, 2337–2346. [Google Scholar] [CrossRef]
- Gamba, C.S.; Clarke, C.A.; Keegan, T.H.; Tao, L.; Swetter, S.M. Melanoma survival disadvantage in young, non-Hispanic white males compared with females. JAMA Derm. 2013, 149, 912–920. [Google Scholar] [CrossRef]
- Roh, M.R.; Eliades, P.; Gupta, S.; Grant-Kels, J.M.; Tsao, H. Cutaneous melanoma in women. Int. J. Womens Dermatol. 2017, 3, S11–S15. [Google Scholar] [CrossRef]
- Courtenay, W.H.; Keeling, R.P. Men, gender, and health: Toward an interdisciplinary approach. J. Am. Coll. Health 2000, 48, 243–246. [Google Scholar] [CrossRef]
- Abel, G.A.; Shelton, J.; Johnson, S.; Elliss-Brookes, L.; Lyratzopoulos, G. Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br. J. Cancer 2015, 112, S129–S136. [Google Scholar] [CrossRef]
- Micheli, A.; Ciampichini, R.; Oberaigner, W.; Ciccolallo, L.; De Vries, E.; Izarzugaza, I.; Zambon, P.; Gatta, G.; De Angelis, R. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur. J. Cancer 2009, 45, 1017–1027. [Google Scholar] [CrossRef]
- Schwartz, M.R.; Luo, L.; Berwick, M. Sex differences in melanoma. Curr. Epidemiol. Rep. 2019, 6, 112–118. [Google Scholar] [CrossRef]
- Fischer, J.; Jung, N.; Robinson, N.; Lehmann, C. Sex differences in immune responses to infectious diseases. Infection 2015, 43, 399–403. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I. Pharmacogenomics, pharmacokinetics and pharmacodynamics: Interaction with biological differences between men and women. Br. J. Pharm. 2014, 171, 580–594. [Google Scholar] [CrossRef]
- Kemeny, M.M.; Busch, E.; Stewart, A.K.; Menck, H.R. Superior survival of young women with malignant melanoma. Am. J. Surg. 1998, 175, 437–444. [Google Scholar] [CrossRef]
- Mervic, L.; Leiter, U.; Meier, F.; Eigentler, T.; Forschner, A.; Metzler, G.; Bartenjev, I.; Büttner, P.; Garbe, C. Sex differences in survival of cutaneous melanoma are age dependent: An analysis of 7338 patients. Melanoma Res. 2011, 21, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Molife, R.; Lorigan, P.; MacNeil, S. Gender and survival in malignant tumours. Cancer Treat. Rev. 2001, 27, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.; Price, A.; Wagner, M.; Williams, V.; Lorigan, P.; Browne, S.; Miller, J.G.; Mac Neil, S. Investigation of female survival benefit in metastatic melanoma. Br. J. Cancer 1999, 80, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Scoggins, C.R.; Ross, M.I.; Reintgen, D.S.; Noyes, R.D.; Goydos, J.S.; Beitsch, P.D.; Urist, M.M.; Ariyan, S.; Sussman, J.J.; Edwards, M.J.; et al. Gender-related differences in outcome for melanoma patients. Ann. Surg. 2006, 243, 693–698. [Google Scholar] [CrossRef]
- Unger, J.M.; Flaherty, L.E.; Liu, P.; Albain, K.S.; Sondak, V.K. Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer 2001, 91, 1148–1155. [Google Scholar] [CrossRef]
- Korn, E.L.; Liu, P.-Y.; Lee, S.J.; Chapman, J.-A.W.; Niedzwiecki, D.; Suman, V.J.; Moon, J.; Sondak, V.K.; Atkins, M.B.; Eisenhauer, E.A.; et al. Meta-analysis of phase ii cooperative group trials in metastatic stage iv melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J. Clin. Oncol. 2008, 26, 527–534. [Google Scholar] [CrossRef]
- Folkerd, E.J.; Dowsett, M. Influence of sex hormones on cancer progression. J. Clin. Oncol. 2010, 28, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Bieber, A.K.; Martires, K.J.; Stein, J.A.; Grant-Kels, J.M.; Driscoll, M.S.; Pomeranz, M.K. Pigmentation and pregnancy: Knowing what is normal. Obstet. Gynecol. 2016, 129, 168–173. [Google Scholar] [CrossRef]
- Morvillo, V.; Lüthy, I.A.; Bravo, A.I.; Capurro, M.I.; Portela, P.; Calandra, R.S.; Mordoh, J. Androgen receptors in human melanoma cell lines IIB-MEL-LES and IIB-MEL-IAN and in human melanoma metastases. Melanoma Res. 2002, 12, 529–538. [Google Scholar] [CrossRef]
- Holly, E.A.; Cress, R.D.; Ahn, D.K. Cutaneous melanoma in women: Ovulatory life, menopause, and use of exogenous estrogens. Cancer Epidemiol. Biomark. Prev. 1994, 3, 661–668. [Google Scholar] [CrossRef]
- Karagas, M.R.; Stukel, T.A.; Dykes, J.; Miglionico, J.; Greene, M.A.; Carey, M.; Armstrong, B.; Elwood, J.M.; Gallagher, R.P.; Green, A.; et al. A pooled analysis of 10 case–control studies of melanoma and oral contraceptive use. Br. J. Cancer 2002, 86, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Holman, C.D.; Armstrong, B.K.; Heenan, P.J. Cutaneous malignant melanoma in women: Exogenous sex hormones and reproductive factors. Br. J. Cancer 1984, 50, 673–680. [Google Scholar] [CrossRef]
- Gallagher, R.P.; Elwood, J.M.; Hill, G.B.; Coldman, A.J.; Threlfall, W.J.; Spinelli, J.J. Reproductive factors, oral contraceptives and risk of malignant melanoma: Western Canada Melanoma Study. Br. J. Cancer 1985, 52, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Bain, C. Hormonal factors and melanoma in women. Med. J. Aust. 1985, 142, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, R.; Franceschi, S.; Rosso, S.; Bidoli, E.; Colonna, S. Cutaneous malignant melanoma in females: The role of hormonal and reproductive factors. Int. J. Epidemiol. 1990, 19, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Beral, V.; Ramcharan, S.; Faris, R. Malignant melanoma and oral contraceptive use among women in California. Br. J. Cancer 1977, 36, 804–809. [Google Scholar] [CrossRef]
- Hannaford, P.; Villard-Mackintosh, L.; Vessey, M.; Kay, C. Oral contraceptives and malignant melanoma. Br. J. Cancer 1991, 63, 430–433. [Google Scholar] [CrossRef][Green Version]
- Adam, S.A.; Sheaves, J.K.; Wright, N.H.; Mosser, G.; Harris, R.W.; Vessey, M.P. A case-control study of the possible association between oral contraceptives and malignant melanoma. Br. J. Cancer 1981, 44, 45–50. [Google Scholar] [CrossRef]
- Palmer, J.R.; Rosenberg, L.; Strom, B.L.; Harlap, S.; Zauber, A.G.; Warshauer, M.E.; Shapiro, S. Oral contraceptive use and risk of cutaneous malignant melanoma. Cancer Causes Control 1992, 3, 547–554. [Google Scholar] [CrossRef]
- Cabanes, P.A.; Desvignes, V.; Chanteau, M.F.; Mlika, N.; Avril, M.F. Oral contraceptive use and risk of cutaneous malignant melanoma in a case-control study of French women. Cancer Causes Control 1992, 3, 199–205. [Google Scholar]
- Osterlind, A.; Tucker, M.A.; Stone, B.J.; Jensen, O.M. The Danish case-control study of cutaneous malignant melanoma. III. Hormonal and reproductive factors in women. Int. J. Cancer 1988, 42, 821–824. [Google Scholar] [PubMed]
- Helmrich, S.P.; Rosenberg, L.; Kaufman, D.W.; Miller, D.R.; Schottenfeld, D.; Stolley, P.D.; Shapiro, S. Lack of an elevated risk of malignant melanoma in relation to oral contraceptive use. J. Natl. Cancer Inst. 1984, 72, 617–620. [Google Scholar] [PubMed]
- Bain, C.; Hennekens, C.H.; Speizer, F.E.; Rosner, B.; Willett, W.; Belanger, C. Oral contraceptive use and malignant melanoma. J. Natl. Cancer Inst. 1982, 68, 537–539. [Google Scholar] [PubMed]
- Holly, E.A.; Cress, R.D.; Ahn, D.K. Cutaneous melanoma in women. III. Reproductive factors and oral contraceptive use. Am. J. Epidemiol. 1995, 141, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, J.; Olsson, H.; Måsbäck, A.; Ingvar, C.; Jonsson, N. Risk of malignant melanoma in relation to drug intake, alcohol, smoking and hormonal factors. Br. J. Cancer 1996, 73, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Beral, V.; Evans, S.; Shaw, H.; Milton, G. Oral contraceptive use and malignant melanoma in Australia. Br. J. Cancer 1984, 50, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Støer, N.C.; Sakshaug, S.; Graff-Iversen, S.; Vangen, S.; Hofvind, S.; Ursin, G.; Weiderpass, E. Menopausal hormone therapy and risk of melanoma: Do estrogens and progestins have a different role? Int. J. Cancer 2017, 141, 1763–1770. [Google Scholar] [CrossRef]
- Joo, B.S.; Park, S.H.; An, B.M.; Kim, K.S.; Moon, S.E.; Moon, H.S. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil. Steril. 2010, 93, 442–446. [Google Scholar] [CrossRef]
- Althuis, M.D.; Scoccia, B.; Lamb, E.J.; Althuis, M.D.; Scoccia, B.; Lamb, E.J.; Moghissi, K.S.; Westhoff, C.L.; Mabie, J.E.; Brinton, L.A. Melanoma, thyroid, cervical, and colon cancer risk after use of fertility drugs. Am. J. Obstet. Gynecol. 2005, 193, 668–674. [Google Scholar] [CrossRef]
- Brinton, L.A.; Moghissi, K.S.; Scoccia, B.; Lamb, E.J.; Trabert, B.; Niwa, S.; Ruggieri, D.; Westhoff, C.L. Effects of fertility drugs on cancers other than breast and gynecologic malignancies. Fertil. Steril. 2015, 104, 980–988. [Google Scholar] [CrossRef]
- Young, P.; Purdie, D.; Jackman, L.; Molloy, D.; Green, A. A study of infertility treatment and melanoma. Melanoma Res. 2001, 11, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Venn, A.; Watson, L.; Lumley, J.; Gilles, G.; King, C.; Healy, D. Breast and ovarian cancer incidence after infertility and in vitro fertilisation. Lancet 1995, 346, 995–1000. [Google Scholar] [CrossRef]
- Silva Idos, S.; Wark, P.A.; McCormack, V.A.; Mayer, D.; Overton, C.; Little, V.; MacLean, A.B. Ovulation stimulation drugs and cancer risks: A long-term follow-up of a British cohort. Br. J. Cancer 2009, 100, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Yli-Kuha, A.-N.; Gissler, M.; Klemetti, R.; Luoto, R.; Hemminki, E. Cancer morbidity in a cohort of 9175 Finnish women treated for infertility. Hum. Reprod. 2012, 27, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Spaan, M.; Schaapveld, M.; Schats, R.; Kortman, M.; Belt-Dusebout, A.V.D.; Mooij, T.; Burger, C.; Van Leeuwen, F.; Lambalk, C.; Laven, J.; et al. Melanoma risk after ovarian stimulation for in vitro fertilization. Hum. Reprod. 2015, 30, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. Adv. Struct. Saf. Stud. 2016, 1366, 1–10. [Google Scholar]
- Barone, I.; Brusco, L.; Fuqua, S.A. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin. Cancer Res. 2010, 16, 2702–2708. [Google Scholar] [CrossRef]
- Lauriola, M.; Enuka, Y.; Zeisel, A.; D’Uva, G.; Roth, L.; Sharon-Sevilla, M.; Lindzen, M.; Sharma, K.; Nevo, N.; Feldman, M.; et al. Diurnal suppression of EGFR signalling by glucocorticoids and implications for tumour progression and treatment. Nat. Commun. 2014, 5, 5073. [Google Scholar] [CrossRef]
- Zhou, J.H.; Kim, K.B.; Myers, J.N.; Fox, P.S.; Ning, J.; Bassett, R.L.; Hasanein, H.; Prieto, V.G. Immunohistochemical expression of hormone receptors in melanoma of pregnant women, nonpregnant women, and men. Am. J. Derm. 2014, 36, 74–79. [Google Scholar] [CrossRef]
- Dika, E.; Fanti, P.A.; Vaccari, S.; Capizzi, E.; DeGiovanni, A.; Gobbi, A.; Piraccini, B.M.; Ribero, S.; Baraldi, C.; Ravaioli, G.M.; et al. Oestrogen and progesterone receptors in melanoma and nevi: An immunohistochemical study. Eur. J. Derm. 2017, 27, 254–259. [Google Scholar] [CrossRef]
- Glatthaar, H.; Katto, J.; Vogt, T.; Mahlknecht, U. Estrogen Receptor Alpha (ESR1) Single-Nucleotide Polymorphisms (SNPs) Affect Malignant Melanoma Susceptibility and Disease Course. Genet. Epigenet. 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Montagnani Marelli, M.; Casati, L.; Fontana, F.; Moretti, R.M.; Limonta, P. Estrogen Receptor β in Melanoma: From Molecular Insights to Potential Clinical Utility. Front. Endocrinol. 2016, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Mavilia, C.; Massi, D.; Sestini, S.; Grazzini, M.; Brandi, M.L.; Lotti, T. The role of estrogens in melanoma and skin cancer. Carcinogenesis 2009, 30, 720. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Mavilia, C.; Massi, D.; Gozzini, A.; Aragona, P.; Tanini, A.; Sestini, S.; Paglierani, M.; Boddi, V.; Brandi, M.L.; et al. Estrogen receptor expression in cutaneous melanoma: A real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch. Derm. 2009, 145, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Al-Azzawi, F.; Ognjanovic, S.; Bao, S.; Yamamoto, S.; Garibay-Tupas, J.; Samal, B.; Bryant-Greenwood, G. Immunolocalisation of oestrogen receptor beta in human tissues. J. Mol. Endocrinol. 2000, 24, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.M.; Davis, R.J.; Shupnik, M.A. ERbeta in breast cancer--onlooker, passive player, or active protector? Steroids 2008, 73, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Ström, A.; Gustafsson, J.A. Estrogen receptor beta in breast cancer—diagnostic implications. Steroids 2009, 74, 635–641. [Google Scholar] [CrossRef]
- Roger, P.; Sahla, M.E.; Mäkelä, S.; Gustafsson, J.A.; Baldet, P.; Rochefort, H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001, 61, 2537–2541. [Google Scholar]
- Hartman, J.; Edvardsson, K.; Lindberg, K.; Zhao, C.; Williams, C.; Ström, A.; Gustafsson, J.A. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009, 69, 6100–6106. [Google Scholar] [CrossRef]
- Drummond, A.E.; Fuller, P.J. The importance of ERbeta signalling in the ovary. J. Endocrinol. 2010, 205, 15–23. [Google Scholar] [CrossRef]
- Thomas, C.G.; Ström, A.; Lindberg, K.; Gustafsson, J.-Å. Estrogen receptor beta decreases survival of p53-defective cancer cells after DNA damage by impairing G2/M checkpoint signaling. Breast Cancer Res. Treat. 2010, 127, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, K.; Ström, A.; Lock, J.G.; Gustafsson, J.A.; Haldosén, L.A.; Helguero, L.A. Expression of estrogen receptor beta increases integrin alpha1 and integrin beta1 levels and enhances adhesion of breast cancer cells. J. Cell. Physiol. 2010, 222, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.N.; Nanney, L.B.; Boyd, A.S.; King, L.E., Jr.; Ellis, D.L. Oestrogen receptor-beta expression in melanocytic lesions. Exp. Derm. 2006, 15, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Wall, B.; Chen, S. G-protein-coupled receptors and melanoma Pigment. Cell Melanoma Res. 2008, 21, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.C.; Santos, A.E.; Custódio, J.B.A. The activation of the G protein-coupled estrogen receptor (GPER) inhibits the proliferation of mouse melanoma K1735-M2 cells. Chem. Biol. Interact. 2017, 277, 176–184. [Google Scholar] [CrossRef]
- Sun, M.; Xie, H.F.; Tang, Y.; Lin, S.Q.; Li, J.M.; Sun, S.N.; Hu, X.L.; Huang, Y.X.; Shi, W.; Jian, D. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J. Steroid Biochem. Mol. Biol. 2017, 165, 236–246. [Google Scholar] [CrossRef]
- Natale, C.A.; Duperret, E.K.; Zhang, J.; Sadeghi, R.; Dahal, A.; O’Brien, K.T.; Cookson, R.; Winkler, J.D.; Ridky, T.W. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 2016, 5, e15104. [Google Scholar] [CrossRef]
- Fábián, M.; Rencz, F.; Krenács, T.; Brodszky, V.; Hársing, J.; Németh, K.; Balogh, P.; Kárpáti, S. Expression of G protein-coupled oestrogen receptor in melanoma and in pregnancy-associated melanoma. J. Eur. Acad. Derm. Venereol. 2017, 31, 1453–1461. [Google Scholar] [CrossRef]
- Lens, M.B.; Reiman, T.; Husain, A.F. Use of tamoxifen in the treatment of malignant melanoma. Cancer 2003, 98, 1355–1361. [Google Scholar] [CrossRef]
- Beguerie, J.R.; Xingzhong, J.; Valdez, R.P. Tamoxifen vs. non-tamoxifen treatment for advanced melanoma: A meta-analysis. Int. J. Derm. 2010, 49, 1194–1202. [Google Scholar] [CrossRef]
- Ricceri, F.; Fasanelli, F.; Giraudo, M.T.; Sieri, S.; Tumino, R.; Mattiello, A.; Vagliano, L.; Masala, G.; Quirós, J.R.; Travier, N.; et al. Risk of second primary malignancies in women with breast cancer: Results from the European prospective investigation into cancer and nutrition (EPIC). Int. J. Cancer. 2015, 137, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Bouchardy, C.; Schaffar, R.; Neyroud-Caspar, I.; Vlastos, G.; Le Gal, F.A.; Rapiti, E.; Benhamou, S. Antiestrogen therapy for breast cancer modifies the risk of subsequent cutaneous melanoma. Cancer Prev. Res. 2012, 5, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lens, M.; Bataille, V. Melanoma in relation to reproductive and hormonal factors in women: Current review on controversial issues. Cancer Causes Control 2008, 19, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Sheikh, S.; Ahmad, A.; Ali, S.M.; Ahmad, M.U.; Ahmad, I. Orally administered endoxifen inhibits tumor growth in melanoma-bearing mice. Cell Mol. Biol. Lett. 2018, 23, 3. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, S.; Mei, S.; Yang, Z.; Xu, L.; Zhou, N.; Yang, Q.; Shen, Q.; Wang, W.; Le, X.; et al. Pharmacological activation of estrogen receptor beta augments innate immunity to suppress cancer metastasis. Proc. Natl. Acad. Sci. USA 2018, 115, E3673–E3681. [Google Scholar] [CrossRef]
- Natale, C.A.; Li, J.; Zhang, J.; Dahal, A.; Dentchev, T.; Stanger, B.Z.; Ridky, T.W. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. eLife 2018, 7, e31770. [Google Scholar] [CrossRef]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microrna expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef]
- Maillot, G.; Lacroix-Triki, M.; Pierredon, S.; Gratadou, L.; Schmidt, S.; Benes, V.; Roche, H.; Dalenc, F.; Auboeuf, D.; Millevoi, S.; et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009, 69, 8332–8340. [Google Scholar] [CrossRef]
- Iorio, M.V.; Ferracin, M.; Liu, C.-G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef]
- Adams, B.D.; Furneaux, H.; White, B.A. The micro-ribonucleic acid (mirna) mir-206 targets the human estrogen receptor-alpha (eralpha) and represses eralpha messenger rna and protein expression in breast cancer cell lines. Mol. Endocrinol. 2007, 21, 1132–1147. [Google Scholar] [CrossRef]
- Pandey, D.P.; Picard, D. Mir-22 inhibits estrogen signaling by directly targeting the estrogen receptor alpha mrna. Mol. Cell Biol. 2009, 29, 3783–3790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, C.; Wang, J.; Xiao, J.; Gatalica, Z.; Recker, R.R.; Xiao, G.G. Let-7 family mirnas regulate estrogen receptor alpha signaling in estrogen receptor positive breast cancer. Breast Cancer Res. Treat. 2011, 127, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Al-Nakhle, H.; Burns, P.A.; Cummings, M.; Hanby, A.M.; Hughes, T.A.; Satheesha, S.; Shaaban, A.M.; Smith, L.; Speirs, V. Estrogen receptor beta 1 expression is regulated by mir-92 in breast cancer. Cancer Res. 2010, 70, 4778–4784. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Zhang, W.; Chen, L.; Hang, J.; Wang, L.; Zhang, R.; Liao, Y.; Chen, J.; Ma, Q.; Sun, Z.; et al. Analysis of the mirna-mrna-lncrna network in human estrogen receptor-positive and estrogen receptor-negative breast cancer based on tcga data. Gene 2018, 658, 28–35. [Google Scholar] [CrossRef]
- Vrtacnik, P.; Ostanek, B.; Mencej-Bedrac, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef]
- Nagpal, N.; Sharma, S.; Maji, S.; Durante, G.; Ferracin, M.; Thakur, J.K.; Kulshreshtha, R. Essential role of med1 in the transcriptional regulation of er-dependent oncogenic mirnas in breast cancer. Sci. Rep. 2018, 8, 11805. [Google Scholar] [CrossRef]
- Bottner, M.; Thelen, P.; Jarry, H. Estrogen receptor beta: Tissue distribution and the still largely enigmatic physiological function. J. Steroid Biochem. Mol. Biol. 2014, 139, 245–251. [Google Scholar] [CrossRef]
- Gao, L.; Wang, G.; Liu, W.N.; Kinser, H.; Franco, H.L.; Mendelson, C.R. Reciprocal feedback between mir-181a and e-2/er alpha in myometrium enhances inflammation leading to labor. J. Clin. Endocrinol. Metab. 2016, 101, 3646–3656. [Google Scholar] [CrossRef]
- Liu, W.H.; Yeh, S.H.; Lu, C.C.; Yu, S.L.; Chen, H.Y.; Lin, C.Y.; Chen, D.S.; Chen, P.J. Microrna-18a prevents estrogen receptor-alpha expression, promoting proliferation of hepatocellular carcinoma cells. Gastroenterology 2009, 136, 683–693. [Google Scholar] [CrossRef]
- Loven, J.; Zinin, N.; Wahlstrom, T.; Muller, I.; Brodin, P.; Fredlund, E.; Ribacke, U.; Pivarcsi, A.; Pahlman, S.; Henriksson, M. Mycn-regulated micrornas repress estrogen receptor-alpha (esr1) expression and neuronal differentiation in human neuroblastoma. Proc. Natl. Acad. Sci. USA 2010, 107, 1553–1558. [Google Scholar] [CrossRef]
- Vidal-Gomez, X.; Perez-Cremades, D.; Mompeon, A.; Dantas, A.P.; Novella, S.; Hermenegildo, C. Microrna as crucial regulators of gene expression in estradiol-treated human endothelial cells. Cell. Physiol. Biochem. 2018, 45, 1878–1892. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Gasparini, P.; Piovan, C.; Ngankeu, A.; Garofalo, M.; Taccioli, C.; Iorio, M.V.; Li, M.; Volinia, S.; Alder, H.; et al. Microrna cluster 221–222 and estrogen receptor alpha interactions in breast cancer. JNCI-J. Natl. Cancer Inst. 2010, 102, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, F.; De Feo, A.; Coscia, C.; Puglisi, R.; Pedini, F.; Pasquini, L.; Bellenghi, M.; Errico, M.C.; Pagani, E.; Care, A. Exosome-mediated transfer of mir-222 is sufficient to increase tumor malignancy in melanoma. J. Transl. Med. 2016, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, F.; Errico, M.C.; Segnalini, P.; Mattia, G.; Care, A. Microrna-221 and -222 pathway controls melanoma progression. Expert Rev. Anticancer 2008, 8, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Godshalk, S.E.; Paranjape, T.; Nallur, S.; Speed, W.; Chan, E.; Molinaro, A.M.; Bacchiocchi, A.; Hoyt, K.; Tworkoski, K.; Stern, D.F.; et al. A variant in a microrna complementary site in the 3′ utr of the kit oncogene increases risk of acral melanoma. Oncogene 2011, 30, 1542–1550. [Google Scholar] [CrossRef]
- Milevskiy, M.J.G.; Gujral, U.; Marques, C.D.; Stone, A.; Northwood, K.; Burke, L.J.; Gee, J.M.W.; Nephew, K.; Clark, S.; Brown, M.A. Microrna-196a is regulated by er and is a prognostic biomarker in er plus breast cancer. Br. J. Cancer 2019, 120, 621–632. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dika, E.; Patrizi, A.; Lambertini, M.; Manuelpillai, N.; Fiorentino, M.; Altimari, A.; Ferracin, M.; Lauriola, M.; Fabbri, E.; Campione, E.; et al. Estrogen Receptors and Melanoma: A Review. Cells 2019, 8, 1463. https://doi.org/10.3390/cells8111463
Dika E, Patrizi A, Lambertini M, Manuelpillai N, Fiorentino M, Altimari A, Ferracin M, Lauriola M, Fabbri E, Campione E, et al. Estrogen Receptors and Melanoma: A Review. Cells. 2019; 8(11):1463. https://doi.org/10.3390/cells8111463
Chicago/Turabian StyleDika, Emi, Annalisa Patrizi, Martina Lambertini, Nicholas Manuelpillai, Michelangelo Fiorentino, Annalisa Altimari, Manuela Ferracin, Mattia Lauriola, Enrica Fabbri, Elena Campione, and et al. 2019. "Estrogen Receptors and Melanoma: A Review" Cells 8, no. 11: 1463. https://doi.org/10.3390/cells8111463
APA StyleDika, E., Patrizi, A., Lambertini, M., Manuelpillai, N., Fiorentino, M., Altimari, A., Ferracin, M., Lauriola, M., Fabbri, E., Campione, E., Veronesi, G., & Scarfì, F. (2019). Estrogen Receptors and Melanoma: A Review. Cells, 8(11), 1463. https://doi.org/10.3390/cells8111463







