Role of ACTN4 in Tumorigenesis, Metastasis, and EMT
Abstract
1. Introduction
2. ACTN4 as a Predictive Marker for Cancer Development
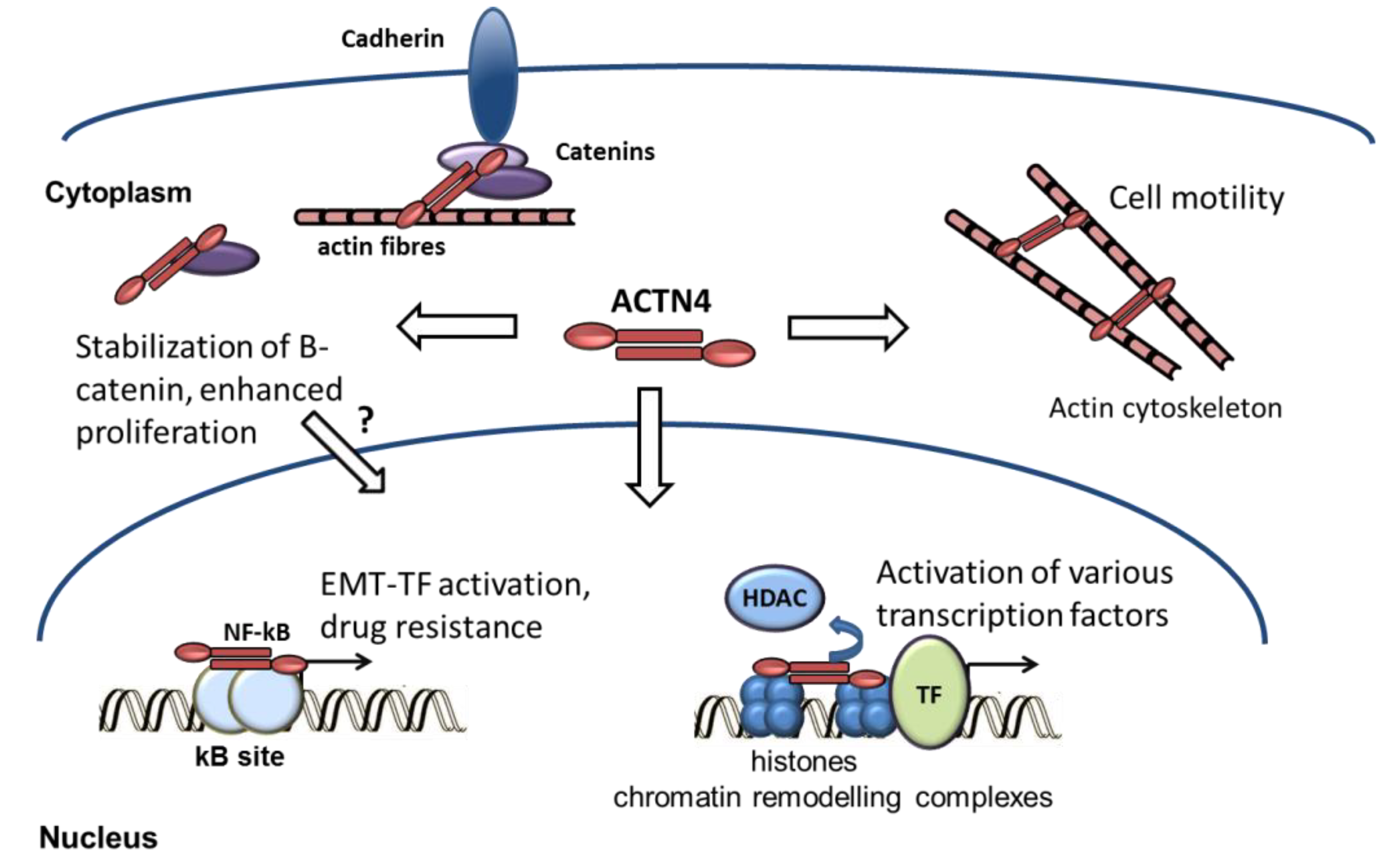
3. ACTN4 and EMT-Related Tumorigenic Activity of Cancer Cells
3.1. EMT Program in Cancer
3.2. Functional Association of ACTN4 with EMT Factors
3.3. ACTN4 and β-Catenin
3.4. ACTN4 and NF-kappaB
3.5. ACTN4 Involvement in MDR
3.6. ACTN4 Effects on Invasion and Migration of Cancer Cells
4. Conclusions and Prospects
Funding
Conflicts of Interest
Abbreviations
| FSGS | familial focal segmental glomerulosclerosis |
| EMT | epithelial-mesenchymal transition |
| EMT-TFs | epithelial-mesenchymal transition transcription factors |
| FISH | fluorescent in situ hybridization |
| IHC | Immunohistochemistry |
| NSCLC | non-small cell lung cancer |
| SCLC | small-cell lung cancer |
| SCC | squamous cell carcinoma |
| CGH | comparative genomic hybridization |
References
- Honda, K.; Yamada, T.; Endo, R.; Ino, Y.; Gotoh, M.; Tsuda, H.; Yamada, Y.; Chiba, H.; Hirohashi, S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J. Cell Biol. 1998, 140, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Adhikari, A.S.; Iyer, S.V.; Hekmatdoost, K.; Welch, D.R.; Iwakuma, T. MTBP suppresses cell migration and filopodia formation by inhibiting ACTN4. Oncogene 2013, 32, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Poch, M.T.; Al-Kassim, L.; Smolinski, S.M.; Hines, R.N. Two distinct classes of CCAAT box elements that bind nuclear factor-Y/alpha-actinin-4: Potential role in human CYP1A1 regulation. Toxicol. Appl. Pharmacol. 2004, 199, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Reineke, E.L.; Lam, M.; Li, X.; Liu, Y.; Gao, C.; Khurana, S.; Kao, H.Y. Alpha-actinin 4 potentiates myocyte enhancer factor-2 transcription activity by antagonizing histone deacetylase 7. J. Biol. Chem. 2006, 281, 35070–35080. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Chakraborty, S.; Cheng, X.; Su, Y.T.; Kao, H.Y. The actin-binding protein, actinin alpha 4 (ACTN4), is a nuclear receptor coactivator that promotes proliferation of MCF-7 breast cancer cells. J. Biol. Chem. 2011, 286, 1850–1859. [Google Scholar] [CrossRef]
- Aksenova, V.; Turoverova, L.; Khotin, M.; Magnusson, K.E.; Tulchinsky, E.; Melino, G.; Pinaev, G.P.; Barlev, N.; Tentler, D. Actin-binding protein alpha-actinin 4 (ACTN4) is a transcriptional co-activator of RelA/p65 sub-unit of NF-κB. Oncotarget 2013, 4, 362–372. [Google Scholar] [CrossRef]
- Zhao, X.; Hsu, K.S.; Lim, J.H.; Bruggeman, L.A.; Kao, H.Y. alpha-Actinin 4 potentiates nuclear factor kappa-light-chain-enhancer of activated B-cell (NF-kappaB) activity in podocytes independent of its cytoplasmic actin binding function. J. Biol. Chem. 2015, 290, 338–349. [Google Scholar] [CrossRef]
- Sharma, S.; Mayank, A.K.; Nailwal, H.; Tripathi, S.; Patel, J.R.; Bowzard, J.B.; Gaur, P.; Donis, R.O.; Katz, J.M.; Cox, N.J.; et al. Influenza A viral nucleoprotein interacts with cytoskeleton scaffolding protein alpha-actinin-4 for viral replication. FEBS J. 2014, 281, 2899–2914. [Google Scholar] [CrossRef]
- Kaplan, J.M.; Kim, S.H.; North, K.N.; Rennke, H.; Correia, L.A.; Tong, H.Q.; Mathis, B.J.; Rodriguez-Perez, J.C.; Allen, P.G.; Beggs, A.H.; et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 2000, 24, 251–256. [Google Scholar] [CrossRef]
- Menez, J.; Le Maux Chansac, B.; Dorothee, G.; Vergnon, I.; Jalil, A.; Carlier, M.F.; Chouaib, S.; Mami-Chouaib, F. Mutant alpha-actinin-4 promotes tumorigenicity and regulates cell motility of a human lung carcinoma. Oncogene 2004, 23, 2630–2639. [Google Scholar] [CrossRef]
- Kikuchi, S.; Honda, K.; Tsuda, H.; Hiraoka, N.; Imoto, I.; Kosuge, T.; Umaki, T.; Onozato, K.; Shitashige, M.; Yamaguchi, U.; et al. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clin. Cancer Res. 2008, 14, 5348–5356. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Honda, K.; Onozato, K.; Takano, M.; Tamai, S.; Imoto, I.; Inazawa, J.; Yamada, T.; Matsubara, O. Actinin-4 gene amplification in ovarian cancer: A candidate oncogene associated with poor patient prognosis and tumor chemoresistance. Mod. Pathol. 2009, 22, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Honda, K. The biological role of actinin-4 (ACTN4) in malignant phenotypes of cancer. Cell Biosci. 2015, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulos, S.N.; Spengler, B.A.; Kisselbach, K.; Evans, A.E.; Biedler, J.L.; Ross, R.A. The human non-muscle alpha-actinin protein encoded by the ACTN4 gene suppresses tumorigenicity of human neuroblastoma cells. Oncogene 2000, 19, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Watabe, Y.; Mori, T.; Yoshimoto, S.; Nomura, T.; Shibahara, T.; Yamada, T.; Honda, K. Copy number increase of ACTN4 is a prognostic indicator in salivary gland carcinoma. Cancer Med. 2014, 3, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kakuya, T.; Mori, T.; Yoshimoto, S.; Watabe, Y.; Miura, N.; Shoji, H.; Onidani, K.; Shibahara, T.; Honda, K. Prognostic significance of gene amplification of ACTN4 in stage I and II oral tongue cancer. Int. J. Oral Maxillofac. Surg. 2017, 46, 968–976. [Google Scholar] [CrossRef]
- Yamada, S.; Yanamoto, S.; Yoshida, H.; Yoshitomi, I.; Kawasaki, G.; Mizuno, A.; Nemoto, T.K. RNAi-mediated down-regulation of alpha-actinin-4 decreases invasion potential in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2010, 39, 61–67. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tsuda, H.; Honda, K.; Takano, M.; Tamai, S.; Imoto, I.; Inazawa, J.; Yamada, T.; Matsubara, O. ACTN4 gene amplification and actinin-4 protein overexpression drive tumor development and histological progression in a high-grade subset of ovarian clear-cell adenocarcinomas. Histopathology 2012, 60, 1073–1083. [Google Scholar] [CrossRef]
- Watanabe, T.; Ueno, H.; Watabe, Y.; Hiraoka, N.; Morizane, C.; Itami, J.; Okusaka, T.; Miura, N.; Kakizaki, T.; Kakuya, T.; et al. ACTN4 copy number increase as a predictive biomarker for chemoradiotherapy of locally advanced pancreatic cancer. Br. J. Cancer 2015, 112, 704–713. [Google Scholar] [CrossRef][Green Version]
- Shoji, H.; Miura, N.; Ueno, H.; Honda, K. Measurement of copy number of ACTN4 to optimize the therapeutic strategy for locally advanced pancreatic cancer. Pancreatology 2018. [Google Scholar] [CrossRef]
- Noro, R.; Honda, K.; Tsuta, K.; Ishii, G.; Maeshima, A.M.; Miura, N.; Furuta, K.; Shibata, T.; Tsuda, H.; Ochiai, A.; et al. Distinct outcome of stage I lung adenocarcinoma with ACTN4 cell motility gene amplification. Ann. Oncol. 2013, 24, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, N.; Shyr, Y.; Yanagisawa, K.; Edgerton, M.; Dang, T.P.; Gonzalez, A.; Nadaf, S.; Larsen, P.; Roberts, J.R.; Nesbitt, J.C.; et al. A training-testing approach to the molecular classification of resected non-small cell lung cancer. Clin. Cancer Res. 2003, 9, 4695–4704. [Google Scholar] [PubMed]
- Miura, N.; Kamita, M.; Kakuya, T.; Fujiwara, Y.; Tsuta, K.; Shiraishi, H.; Takeshita, F.; Ochiya, T.; Shoji, H.; Huang, W.; et al. Efficacy of adjuvant chemotherapy for non-small cell lung cancer assessed by metastatic potential associated with ACTN4. Oncotarget 2016, 7, 33165–33178. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, H.; Fujiwara, Y.; Kakuya, T.; Tsuta, K.; Motoi, N.; Miura, N.; Watabe, Y.; Watanabe, S.I.; Noro, R.; Nagashima, K.; et al. Actinin-4 protein overexpression as a predictive biomarker in adjuvant chemotherapy for resected lung adenocarcinoma. Biomark. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Chang, Y.H.; Wu, C.C.; Tyan, Y.C.; Chang, H.C.; Goan, Y.G.; Lai, W.W.; Cheng, P.N.; Liao, P.C. Alpha-actinin 4 is associated with cancer cell motility and is a potential biomarker in non-small cell lung cancer. J. Thorac. Oncol. 2015, 10, 286–301. [Google Scholar] [CrossRef]
- Gao, Y.; Li, G.; Sun, L.; He, Y.; Li, X.; Sun, Z.; Wang, J.; Jiang, Y.; Shi, J. ACTN4 and the pathways associated with cell motility and adhesion contribute to the process of lung cancer metastasis to the brain. BMC Cancer 2015, 15, 277. [Google Scholar] [CrossRef]
- Noro, R.; Ishigame, T.; Walsh, N.; Shiraishi, K.; Robles, A.I.; Ryan, B.M.; Schetter, A.J.; Bowman, E.D.; Welsh, J.A.; Seike, M.; et al. A Two-Gene Prognostic Classifier for Early-Stage Lung Squamous Cell Carcinoma in Multiple Large-Scale and Geographically Diverse Cohorts. J. Thorac. Oncol. 2017, 12, 65–76. [Google Scholar] [CrossRef]
- Honda, K.; Yamada, T.; Seike, M.; Hayashida, Y.; Idogawa, M.; Kondo, T.; Ino, Y.; Hirohashi, S. Alternative splice variant of actinin-4 in small cell lung cancer. Oncogene 2004, 23, 5257–5262. [Google Scholar] [CrossRef][Green Version]
- Okamoto, N.; Suzuki, H.; Kawahara, K.; Honda, K.; Miura, N.; Hirashima, T.; Tamiya, M.; Morishita, N.; Shiroyama, T.; Tanaka, A.; et al. The alternatively spliced actinin-4 variant as a prognostic marker for metastasis in small-cell lung cancer. Anticancer Res. 2015, 35, 1663–1667. [Google Scholar]
- Miyanaga, A.; Honda, K.; Tsuta, K.; Masuda, M.; Yamaguchi, U.; Fujii, G.; Miyamoto, A.; Shinagawa, S.; Miura, N.; Tsuda, H.; et al. Diagnostic and prognostic significance of the alternatively spliced ACTN4 variant in high-grade neuroendocrine pulmonary tumors. Ann. Oncol. 2013, 24, 84–90. [Google Scholar] [CrossRef]
- Prochazkova, I.; Lenco, J.; Fucikova, A.; Dresler, J.; Capkova, L.; Hrstka, R.; Nenutil, R.; Bouchal, P. Targeted proteomics driven verification of biomarker candidates associated with breast cancer aggressiveness. Biochim. Biophys. Acta 2017, 1865, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Chakraborty, S.; Zhao, X.; Liu, Y.; Guan, D.; Lam, M.; Huang, W.; Yang, S.; Kao, H.Y. Identification of a novel LXXLL motif in alpha-actinin 4-spliced isoform that is critical for its interaction with estrogen receptor alpha and co-activators. J. Biol. Chem. 2012, 287, 35418–35429. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Yan, X.; Chen, Y.; Tao, X.; Wang, J.; Jia, N.; Lyu, T.; Wang, J.; Ding, J.; et al. Estrogen stimulates the invasion of ovarian cancer cells via activation of the PI3K/AKT pathway and regulation of its downstream targets Ecadherin and alphaactinin4. Mol. Med. Rep. 2014, 10, 2433–2440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, C.; Li, J.J.; Deng, T.; Li, B.H.; Geng, P.L.; Zeng, X.T. Actinin-4 as a Diagnostic Biomarker in Serum of Breast Cancer Patients. Med. Sci. Monit. 2019, 25, 3298–3302. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Q.; Tang, H.; Zhang, F.; Zheng, Y.; Wang, S.; Zhang, J.; Wang, Z.; Xie, X. Direct inhibition of ACTN4 by ellagic acid limits breast cancer metastasis via regulation of beta-catenin stabilization in cancer stem cells. J. Exp. Clin. Cancer Res. 2017, 36, 172. [Google Scholar] [CrossRef]
- Nowee, M.E.; Snijders, A.M.; Rockx, D.A.; de Wit, R.M.; Kosma, V.M.; Hamalainen, K.; Schouten, J.P.; Verheijen, R.H.; van Diest, P.J.; Albertson, D.G.; et al. DNA profiling of primary serous ovarian and fallopian tube carcinomas with array comparative genomic hybridization and multiplex ligation-dependent probe amplification. J. Pathol. 2007, 213, 46–55. [Google Scholar] [CrossRef]
- Welsch, T.; Keleg, S.; Bergmann, F.; Bauer, S.; Hinz, U.; Schmidt, J. Actinin-4 expression in primary and metastasized pancreatic ductal adenocarcinoma. Pancreas 2009, 38, 968–976. [Google Scholar] [CrossRef]
- Honda, K.; Yamada, T.; Hayashida, Y.; Idogawa, M.; Sato, S.; Hasegawa, F.; Ino, Y.; Ono, M.; Hirohashi, S. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology 2005, 128, 51–62. [Google Scholar] [CrossRef]
- Fu, L.; Qin, Y.R.; Xie, D.; Chow, H.Y.; Ngai, S.M.; Kwong, D.L.; Li, Y.; Guan, X.Y. Identification of alpha-actinin 4 and 67 kDa laminin receptor as stage-specific markers in esophageal cancer via proteomic approaches. Cancer 2007, 110, 2672–2681. [Google Scholar] [CrossRef]
- Barbolina, M.V.; Adley, B.P.; Kelly, D.L.; Fought, A.J.; Scholtens, D.M.; Shea, L.D.; Stack, M.S. Motility-related actinin alpha-4 is associated with advanced and metastatic ovarian carcinoma. Lab. Investig. 2008, 88, 602–614. [Google Scholar] [CrossRef]
- Koizumi, T.; Nakatsuji, H.; Fukawa, T.; Avirmed, S.; Fukumori, T.; Takahashi, M.; Kanayama, H. The role of actinin-4 in bladder cancer invasion. Urology 2010, 75, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, H.; Ito, K.; Asano, T.; Horiguchi, A.; Hayakawa, M.; Asano, T. Increased expression of alpha-actinin-4 is associated with unfavorable pathological features and invasiveness of bladder cancer. Oncol. Rep. 2013, 30, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Yoshida, A.; Honda, K.; Maeshima, A.M.; Narita, Y.; Yamada, T.; Shibui, S.; Tsuda, H. Immunohistochemical actinin-4 expression in infiltrating gliomas: Association with WHO grade and differentiation. Brain Tumor Pathol. 2014, 31, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yamashita, T.; Yamamoto, S.; Matsunobu, T.; Tsuda, H.; Honda, K.; Yamada, T.; Tamai, S.; Shiotani, A. Histological growth pattern of and alpha-actinin-4 expression in thyroid cancer. Anticancer Res. 2014, 34, 3157–3163. [Google Scholar] [PubMed]
- Shao, H.; Wang, A.; Lauffenburger, D.; Wells, A. Tyro3-mediated phosphorylation of ACTN4 at tyrosines is FAK-dependent and decreases susceptibility to cleavage by m-Calpain. Int. J. Biochem. Cell Biol. 2018, 95, 73–84. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tsuda, H.; Honda, K.; Kita, T.; Takano, M.; Tamai, S.; Inazawa, J.; Yamada, T.; Matsubara, O. Actinin-4 expression in ovarian cancer: A novel prognostic indicator independent of clinical stage and histological type. Mod. Pathol. 2007, 20, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, K.M. alpha-Actinin-4 promotes metastasis in gastric cancer. Lab. Investig. 2017, 97, 1084–1094. [Google Scholar] [CrossRef]
- Quick, Q.; Skalli, O. Alpha-actinin 1 and alpha-actinin 4: Contrasting roles in the survival, motility, and RhoA signaling of astrocytoma cells. Exp. Cell Res. 2010, 316, 1137–1147. [Google Scholar] [CrossRef]
- Hara, T.; Honda, K.; Shitashige, M.; Ono, M.; Matsuyama, H.; Naito, K.; Hirohashi, S.; Yamada, T. Mass spectrometry analysis of the native protein complex containing actinin-4 in prostate cancer cells. Mol. Cell. Proteom. 2007, 6, 479–491. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Tulchinsky, E.; Demidov, O.; Kriajevska, M.; Barlev, N.A.; Imyanitov, E. EMT: A mechanism for escape from EGFR-targeted therapy in lung cancer. Biochim. Biophys. Acta. Rev. Cancer 2019, 1871, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Cheriyamundath, S.; Ben-Ze’ev, A. Cell-cell adhesion: Linking Wnt/beta-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Kourtidis, A.; Lu, R.; Pence, L.J.; Anastasiadis, P.Z. A central role for cadherin signaling in cancer. Exp. Cell Res. 2017, 358, 78–85. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Matsuda, M.; Furuse, M.; Tsukita, S. Regulation of tight junctions during the epithelium-mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003, 116, 1959–1967. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Sato, R.; Semba, T.; Saya, H.; Arima, Y. Concise Review: Stem Cells and Epithelial-Mesenchymal Transition in Cancer: Biological Implications and Therapeutic Targets. Stem Cells 2016, 34, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, Y.; Honda, K.; Idogawa, M.; Ino, Y.; Ono, M.; Tsuchida, A.; Aoki, T.; Hirohashi, S.; Yamada, T. E-cadherin regulates the association between beta-catenin and actinin-4. Cancer Res. 2005, 65, 8836–8845. [Google Scholar] [CrossRef] [PubMed]
- An, H.T.; Yoo, S.; Ko, J. alpha-Actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of Snail expression and beta-catenin stabilization in cervical cancer. Oncogene 2016, 35, 5893–5904. [Google Scholar] [CrossRef]
- Kreimann, E.L.; Morales, F.C.; de Orbeta-Cruz, J.; Takahashi, Y.; Adams, H.; Liu, T.J.; McCrea, P.D.; Georgescu, M.M. Cortical stabilization of beta-catenin contributes to NHERF1/EBP50 tumor suppressor function. Oncogene 2007, 26, 5290–5299. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, J.; Wang, Q.; Song, R.; Liu, H.; Meng, R.; Tao, T.; Si, Y.; Jiang, W.; He, J. NHERF1 regulates actin cytoskeleton organization through modulation of alpha-actinin-4 stability. FASEB J. 2016, 30, 578–589. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Q.; Song, R.; Zhao, C.; Liu, H.; Yang, Y.; Gu, S.; Zhou, D.; He, J. NHERF1 inhibits beta-catenin-mediated proliferation of cervical cancer cells through suppression of alpha-actinin-4 expression. Cell Death Dis. 2018, 9, 668. [Google Scholar] [CrossRef]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grunert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef]
- Tian, B.; Widen, S.G.; Yang, J.; Wood, T.G.; Kudlicki, A.; Zhao, Y.; Brasier, A.R. The NFkappaB subunit RELA is a master transcriptional regulator of the committed epithelial-mesenchymal transition in airway epithelial cells. J. Biol. Chem. 2018, 293, 16528–16545. [Google Scholar] [CrossRef]
- Pires, B.R.; Mencalha, A.L.; Ferreira, G.M.; de Souza, W.F.; Morgado-Diaz, J.A.; Maia, A.M.; Correa, S.; Abdelhay, E.S. NF-kappaB Is Involved in the Regulation of EMT Genes in Breast Cancer Cells. PLoS ONE 2017, 12, e0169622. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Huang, Z.; Yu, F.; Wang, X.; Wang, S.; He, Z.; Lin, J. ACTN4 Promotes the Proliferation, Migration, Metastasis of Osteosarcoma and Enhances its Invasive Ability through the NF-kappaB Pathway. Pathol. Oncol. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yuan, J.; Li, K. EMT transcription factors: Implication in osteosarcoma. Med. Oncol. 2013, 30, 697. [Google Scholar] [CrossRef] [PubMed]
- Babakov, V.N.; Petukhova, O.A.; Turoverova, L.V.; Kropacheva, I.V.; Tentler, D.G.; Bolshakova, A.V.; Podolskaya, E.P.; Magnusson, K.E.; Pinaev, G.P. RelA/NF-kappaB transcription factor associates with alpha-actinin-4. Exp. Cell Res. 2008, 314, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Lomert, E.; Turoverova, L.; Kriger, D.; Aksenov, N.D.; Nikotina, A.D.; Petukhov, A.; Mittenberg, A.G.; Panyushev, N.V.; Khotin, M.; Volkov, K.; et al. Co-expression of RelA/p65 and ACTN4 induces apoptosis in non-small lung carcinoma cells. Cell Cycle 2018, 17, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Tabataba, H.; Liu, X.Y.; Wang, J.Y.; Yan, X.G.; Farrelly, M.; Jiang, C.C.; Guo, S.T.; Liu, T.; Kao, H.Y.; et al. ACTN4 regulates the stability of RIPK1 in melanoma. Oncogene 2018, 37, 4033–4045. [Google Scholar] [CrossRef] [PubMed]
- Kumeta, M.; Yoshimura, S.H.; Harata, M.; Takeyasu, K. Molecular mechanisms underlying nucleocytoplasmic shuttling of actinin-4. J. Cell Sci. 2010, 123, 1020–1030. [Google Scholar] [CrossRef]
- Piskareva, O.; Harvey, H.; Nolan, J.; Conlon, R.; Alcock, L.; Buckley, P.; Dowling, P.; Henry, M.; O’Sullivan, F.; Bray, I.; et al. The development of cisplatin resistance in neuroblastoma is accompanied by epithelial to mesenchymal transition in vitro. Cancer Lett. 2015, 364, 142–155. [Google Scholar] [CrossRef]
- Desai, S.; Barai, A.; Bukhari, A.B.; De, A.; Sen, S. alpha-Actinin-4 confers radioresistance coupled invasiveness in breast cancer cells through AKT pathway. Biochim. Biophys. Acta 2018, 1865, 196–208. [Google Scholar] [CrossRef]
- Ding, Z.; Liang, J.; Lu, Y.; Yu, Q.; Songyang, Z.; Lin, S.Y.; Mills, G.B. A retrovirus-based protein complementation assay screen reveals functional AKT1-binding partners. Proc. Natl. Acad. Sci. USA 2006, 103, 15014–15019. [Google Scholar] [CrossRef]
- Vandermoere, F.; El Yazidi-Belkoura, I.; Demont, Y.; Slomianny, C.; Antol, J.; Lemoine, J.; Hondermarck, H. Proteomics exploration reveals that actin is a signaling target of the kinase Akt. Mol. Cell. Proteom. 2007, 6, 114–124. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D.; Niu, H.; Zhu, G.; Xu, Y.; Ye, D.; Li, J.; Zhang, Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.B.; Fang, H.Y.; Tao, D.Y.; Chen, X.P.; Cao, F.L. COX-2 potentiates cisplatin resistance of non-small cell lung cancer cells by promoting EMT in an AKT signaling pathway-dependent manner. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3838–3846. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Qin, Y.R.; Ming, X.Y.; Zuo, X.B.; Diao, Y.W.; Zhang, L.Y.; Ai, J.; Liu, B.L.; Huang, T.X.; Cao, T.T.; et al. RNA editing of SLC22A3 drives early tumor invasion and metastasis in familial esophageal cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E4631–E4640. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.; Daks, A.; Petrova, V.; Petukhov, A.; Lezina, L.; Shuvalov, O.; Davidovich, P.; Kriger, D.; Lomert, E.; Tentler, D.; et al. Novel isatin-derived molecules activate p53 via interference with Mdm2 to promote apoptosis. Cell Cycle 2018, 17, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Lei, J.X.; LeBlanc, J.; Sodja, C.; Ly, D.; Charlebois, C.; Walker, P.R.; Yamada, T.; Hirohashi, S.; Sikorska, M. Regulation of DNaseY activity by actinin-alpha4 during apoptosis. Cell Death Differ. 2004, 11, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Peng, W.; Ran, Y.; Ge, H.; Zhang, C.; Zou, H.; Ding, Y.; Qi, H. Dysregulated expression of ACTN4 contributes to endothelial cell injury via the activation of the p38-MAPK/p53 apoptosis pathway in preeclampsia. J. Physiol. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Araki, N.; Hatae, T.; Yamada, T.; Hirohashi, S. Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: Analysis by fluorescence ratio imaging. J. Cell Sci. 2000, 113, 3329–3340. [Google Scholar]
- Bolshakova, A.; Petukhova, O.; Turoverova, L.; Tentler, D.; Babakov, V.; Magnusson, K.E.; Pinaev, G. Extra-cellular matrix proteins induce re-distribution of alpha-actinin-1 and alpha-actinin-4 in A431 cells. Cell Biol. Int. 2007, 31, 360–365. [Google Scholar] [CrossRef]
- Fraley, T.S.; Pereira, C.B.; Tran, T.C.; Singleton, C.; Greenwood, J.A. Phosphoinositide binding regulates alpha-actinin dynamics: Mechanism for modulating cytoskeletal remodeling. J. Biol. Chem. 2005, 280, 15479–15482. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Otey, C.; Edlund, M.; Jones, J.C. Interactions of a hemidesmosome component and actinin family members. J. Cell Sci. 2001, 114, 4197–4206. [Google Scholar]
- Celli, L.; Ryckewaert, J.J.; Delachanal, E.; Duperray, A. Evidence of a functional role for interaction between ICAM-1 and nonmuscle alpha-actinins in leukocyte diapedesis. J. Immunol. 2006, 177, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Li, R.; Zhou, R.; Pan, Z.; Xu, L.; Ding, Y.; Zhao, L. LIM kinase 1 interacts with myosin-9 and alpha-actinin-4 and promotes colorectal cancer progression. Br. J. Cancer 2017, 117, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Ito, Y.; Miura, N.; Nagamura, Y.; Nakabo, A.; Fukami, K.; Honda, K.; Sakai, R. Actinin-1 and actinin-4 play essential but distinct roles in invadopodia formation by carcinoma cells. Eur. J. Cell Biol. 2017, 96, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Belot, N.; Rorive, S.; Doyen, I.; Lefranc, F.; Bruyneel, E.; Dedecker, R.; Micik, S.; Brotchi, J.; Decaestecker, C.; Salmon, I.; et al. Molecular characterization of cell substratum attachments in human glial tumors relates to prognostic features. GLIA 2001, 36, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Li, S.; Watkins, S.C.; Wells, A. alpha-Actinin-4 is required for amoeboid-type invasiveness of melanoma cells. J. Biol. Chem. 2014, 289, 32717–32728. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, V.; Khotin, M.G.; Turoverova, L.V.; Iudintseva, N.M.; Magnusson, K.E.; Pinaev, G.P.; Tentler, D.G. Novel splicing isoform of actin-binding protein alpha-actinin 4 in epidermoid carcinoma cells A431. Tsitologiia 2012, 54, 25–32. [Google Scholar]
- Dai, S.; Wang, Z.; Pan, X.; Wang, W.; Chen, X.; Ren, H.; Hao, C.; Han, B.; Chen, N. Functional analysis of promoter mutations in the ACTN4 and SYNPO genes in focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2010, 25, 824–835. [Google Scholar] [CrossRef][Green Version]
- Khotin, M.; Turoverova, L.; Aksenova, V.; Barlev, N.; Borutinskaite, V.V.; Vener, A.; Bajenova, O.; Magnusson, K.E.; Pinaev, G.P.; Tentler, D. Proteomic analysis of ACTN4-interacting proteins reveals it’s a putative involvement in mRNA metabolism. Biochem. Biophys. Res. Commun. 2010, 397, 192–196. [Google Scholar] [CrossRef]
- Trulsson, M.; Yu, H.; Gisselsson, L.; Chao, Y.; Urbano, A.; Aits, S.; Mossberg, A.K.; Svanborg, C. HAMLET binding to alpha-actinin facilitates tumor cell detachment. PLoS ONE 2011, 6, e17179. [Google Scholar] [CrossRef]
- Sen, S.; Dong, M.; Kumar, S. Isoform-specific contributions of alpha-actinin to glioma cell mechanobiology. PLoS ONE 2009, 4, e8427. [Google Scholar] [CrossRef]
- Shao, H.; Wang, J.H.; Pollak, M.R.; Wells, A. alpha-actinin-4 is essential for maintaining the spreading, motility and contractility of fibroblasts. PLoS ONE 2010, 5, e13921. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.S.; Young, P.W. An analysis of splicing, actin-binding properties, heterodimerization and molecular interactions of the non-muscle alpha-actinins. Biochem. J. 2013, 452, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, M.; Kurisu, S.; Yamada, T.; Takenawa, T. alpha-Actinin-4 enhances colorectal cancer cell invasion by suppressing focal adhesion maturation. PLoS ONE 2015, 10, e0120616. [Google Scholar] [CrossRef] [PubMed]
| ACTN4 Status | Cancer Type | Detection Method | Association |
|---|---|---|---|
| Gene amplification | Fallopian tube carcinoma [36] | Comparative genomic hybridization analysis and multiplex ligation-dependent probe amplification | Stages II and III |
| Ovarian cancer [12,18] | FISH, IHC | Chemoresistance and poor prognosis | |
| Locally advanced pancreatic cancer [19] | FISH | Chemoresistance and poor prognosis | |
| Salivary gland carcinoma [15] | FISH | High histological grade and vascular invasions | |
| Tongue cancer [16] | FISH | Poor survival | |
| Lung adenocarcinoma [21] | Tissue microarrays | Advanced stages and poor histological differentiation | |
| Increased expression | Ovarian cancer [46] | IHC and tissue microarrays | Advanced stages and poor survival |
| Invasive pancreatic ductal carcinoma [11] | IHC | Metastases in lymph nodes and distal organs | |
| Oral squamous cell carcinoma [17] | IHC | Invasion areas; no correlation with survival | |
| Non-small cell lung carcinoma (NSCLC) [21,22,25] | cDNA microarrays [22], IHC [21,25] | Poor survival [21,22] Lymph node metastasis [25] | |
| Lung squamous cell carcinoma [27] | Microarray study | High risk of disease recurrence | |
| Brain metastatic tissue [26] | RNA-seq | Distant organ metastases | |
| Colorectal cancer [38] | IHC | Lymph node metastasis | |
| Gastric cancer [47] | RT Profiler PCR Array for Human Cell Motility | Stage IV | |
| Bladder cancer [41,42] | Immunoblotting [41], IHC [41,42] | Invasive tumors, high histological grade | |
| Esophageal cancer [39] | Tissue microarrays and IHC | Advanced stages, lymph node metastasis | |
| Breast cancer [31] | Targeted and discovery proteomic analysis (SRM and iTRAQ) | Positive lymph node status especially in high grade tumors | |
| Thyroid cancer [44] | IHC | Infiltrating tumors | |
| Glioma [43,48] | Immunoblotting [43] IHC [48] | Advanced stages | |
| Reduced expression | Prostate cancer [49] | IHC, immunoblotting | Low and high Gleason grades |
| Specific spliced mRNA variant | Small-cell lung cancer [28,29,30] | cDNA sequencing [28,29,30] IHC [28,29] | Associated with distant metastasis of small-cell lung cancer [28,29] Expressed in 55% of high-grade neurocrine tumors and associated with poorer overall survival [30] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tentler, D.; Lomert, E.; Novitskaya, K.; Barlev, N.A. Role of ACTN4 in Tumorigenesis, Metastasis, and EMT. Cells 2019, 8, 1427. https://doi.org/10.3390/cells8111427
Tentler D, Lomert E, Novitskaya K, Barlev NA. Role of ACTN4 in Tumorigenesis, Metastasis, and EMT. Cells. 2019; 8(11):1427. https://doi.org/10.3390/cells8111427
Chicago/Turabian StyleTentler, Dmitri, Ekaterina Lomert, Ksenia Novitskaya, and Nikolai A. Barlev. 2019. "Role of ACTN4 in Tumorigenesis, Metastasis, and EMT" Cells 8, no. 11: 1427. https://doi.org/10.3390/cells8111427
APA StyleTentler, D., Lomert, E., Novitskaya, K., & Barlev, N. A. (2019). Role of ACTN4 in Tumorigenesis, Metastasis, and EMT. Cells, 8(11), 1427. https://doi.org/10.3390/cells8111427






