Sonodynamic Therapy for Gliomas. Perspectives and Prospects of Selective Sonosensitization of Glioma Cells
Abstract
1. Introduction
2. Ultrasound and Its Medical Applications
2.1. The Thermal Mechanism
2.2. Tension Mechanisms
2.3. Cavitation Mechanisms
3. Sonodynamic Therapy of Cancers: Definitions and Mechanisms
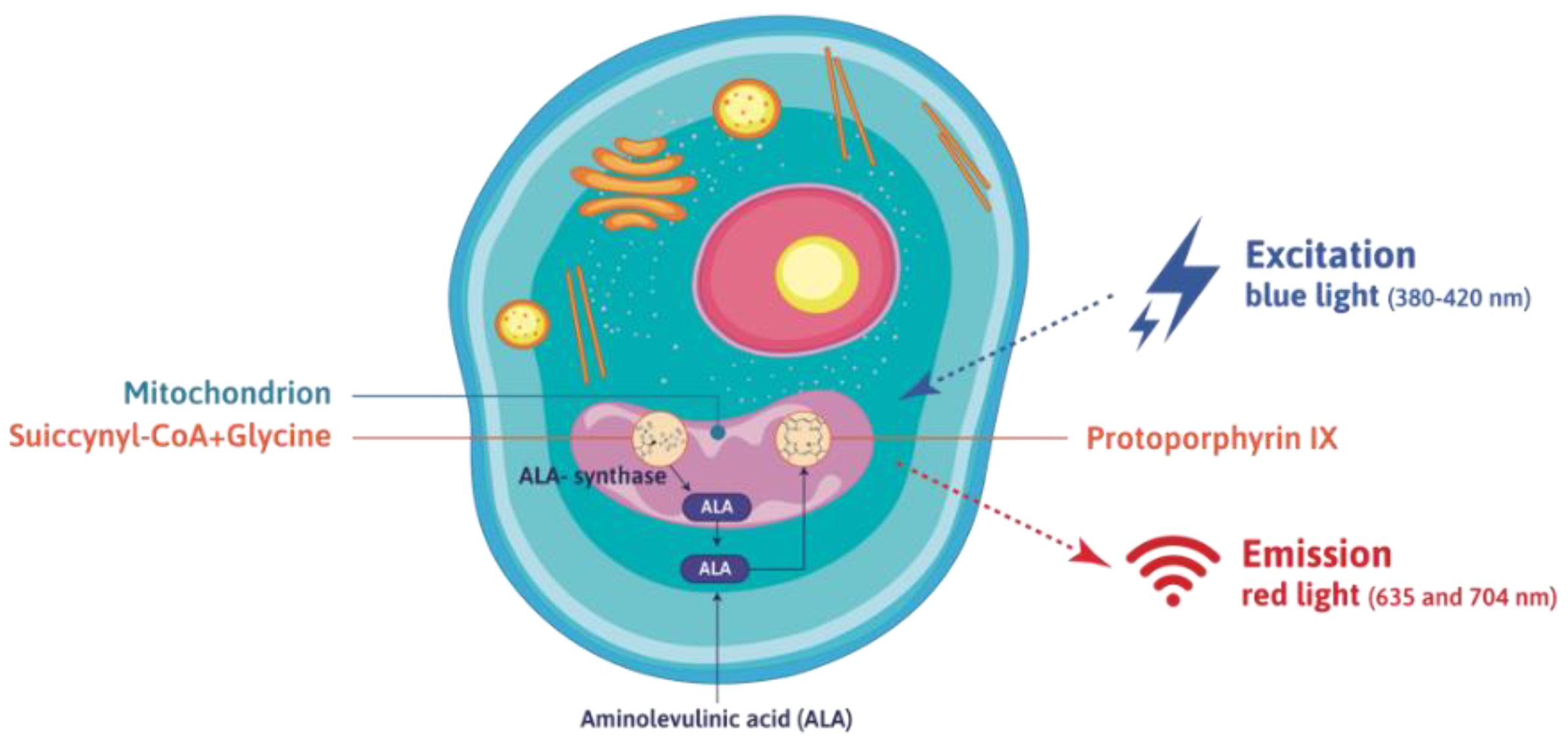
4. 5-Aminolevulinic acid (ALA): A Tumor-Seeking Agent Useful for the Photodynamic Detection of Gliomas
5. ALA and Porphyrins in Pre-Clinical Models of Glioma: Research Review
5.1. In Vitro Studies
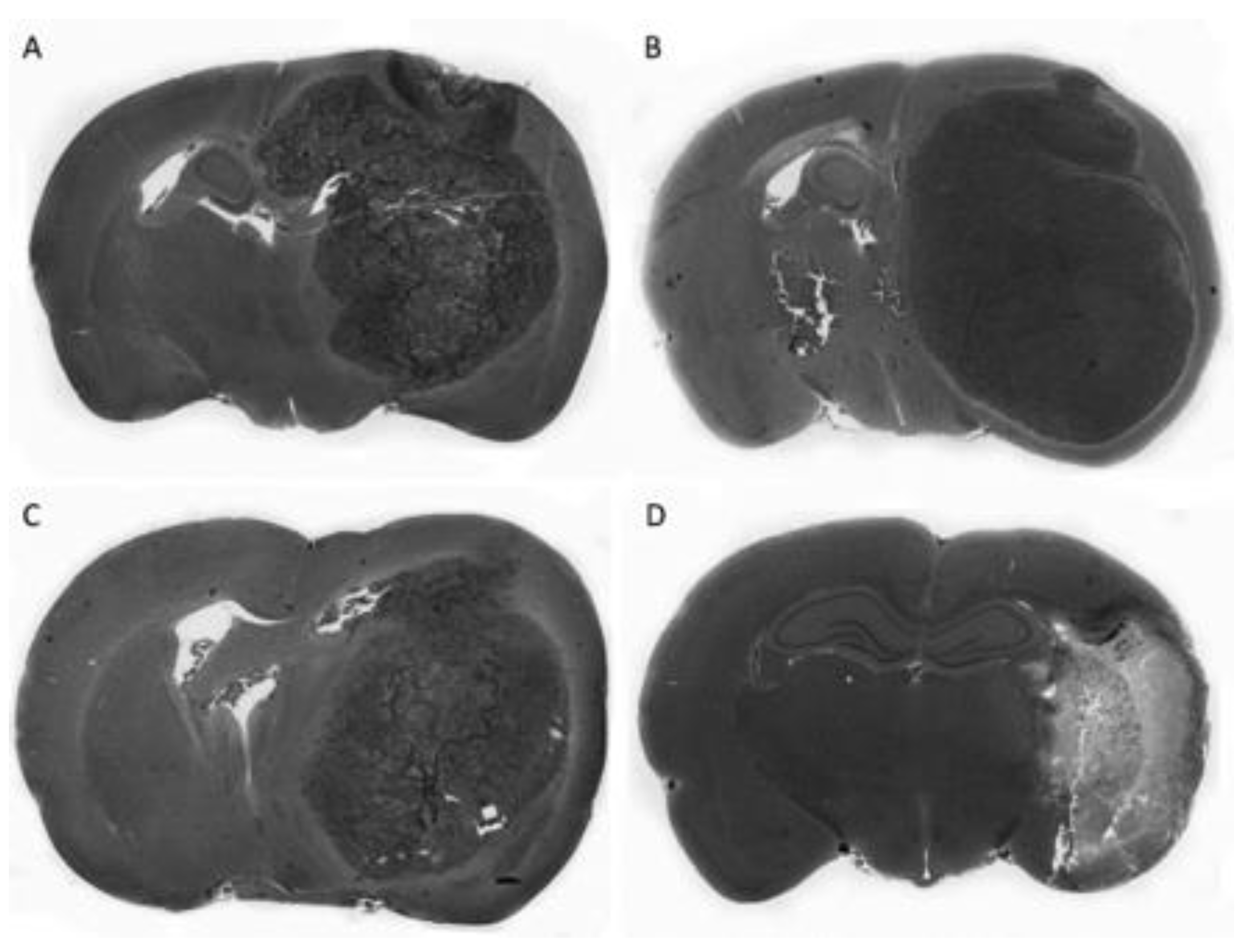
5.2. In Vivo Studies
Author Contributions
Funding
Conflicts of Interest
References
- Eder, K.; Kalman, B. Molecular heterogeneity of glioblastoma and its clinical relevance. Pathol. Oncol. Res. 2014, 20, 777–787. [Google Scholar] [CrossRef]
- Li, K.; Lu, D.; Guo, Y.; Wang, C.; Liu, X.; Liu, Y.; Liu, D. Trends and patterns of incidence of diffuse glioma in adults in the United States, 1973-2014. Cancer Med. 2018, 7, 5281–5290. [Google Scholar] [CrossRef]
- Lukas, R.V.; Mrugala, M.M. Pivotal therapeutic trials for infiltrating gliomas and how they affect clinical practice. Neurooncol. Pract. 2017, 4, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hervey Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neurooncol. 2016, 130, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro. Oncol. 2015, 17, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.; Wickremsekera, A.; Tan, S.T.; Peng, L.; Davis, P.F.; Itinteang, T. Cancer Stem Cell Hierarchy in Glioblastoma Multiforme. Front. Surg. 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.M.; Lee, I.; Kalkanis, S.N. Reoperation for Recurrent Glioblastoma Multiforme. Neurosurg. Clin. N. Am. 2017, 28, 407–428. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Jue, T.R.; McDonald, K.L.; Rovin, R.A. The Survival Effect of Repeat Surgery at Glioblastoma Recurrence and its Trend: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 115, 453–459. [Google Scholar] [CrossRef]
- Cheon, Y.J.; Jung, T.Y.; Jung, S.; Kim, I.Y.; Moon, K.S.; Lim, S.H. Efficacy of Gamma Knife Radiosurgery for Recurrent High-Grade Gliomas with Limited Tumor Volume. J. Korean Neurosurg. Soc. 2018, 61, 516–524. [Google Scholar] [CrossRef]
- Leuthardt, E.C.; Duan, C.; Kim, M.J.; Campian, J.L.; Kim, A.H.; Miller-Thomas, M.M.; Shimony, J.S.; Tran, D.D. Hyperthermic Laser Ablation of Recurrent Glioblastoma Leads to Temporary Disruption of the Peritumoral Blood Brain Barrier. PLoS ONE 2016, 11, e0148613. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Feril, L.B., Jr.; Ikeda-Dantsuji, Y. Sonodynamic therapy. Ultrasonics 2008, 48, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Kim, A.J.; Winkles, J.A.; Eisenberg, H.M.; Woodworth, G.F.; Frenkel, V. Emerging Applications of Therapeutic Ultrasound in Neuro-oncology: Moving Beyond Tumor Ablation. Neurosurgery 2016, 79, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Y.; Wang, P.; Liu, Q.; Zheng, H. Current status and future perspectives of sonodynamic therapy in glioma treatment. Ultrason Sonochem. 2017, 37, 592–599. [Google Scholar] [CrossRef]
- Akimoto, J. Photodynamic Therapy for Malignant Brain Tumors. Neurol. Med. Chir. (Tokyo) 2016, 56, 151–157. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-guided surgery for high-grade gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Miłkowska, K. Ultrasound – mechanisms of action and application in sonodynamic therapy. Postepy Hig. Med. Dosw. 2007, 61, 338–349. [Google Scholar]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. Biol. 2012, 31, 623–634. [Google Scholar] [CrossRef]
- Robertson, V.J.; Ward, A.R. Longwave ultrasound reviewed and reconsidered. Physio-Ther. 1990, 83, 123–130. [Google Scholar] [CrossRef]
- Mahmoud, M.Z.; Alkhorayef, M.; Alzimami, K.S.; Aljuhani, M.S.; Sulieman, A. High-Intensity Focused Ultrasound (HIFU) in uterine fibroid treatment: Review study. Pol. J. Radiol. 2014, 79, 384–390. [Google Scholar] [CrossRef]
- Kujawska, T.; Secomski, W.; Kruglenko, E.; Krawczyk, K.; Nowicki, A. Determination of tissue thermal conductivity by measuring and modeling temperature rise induced in tissue by pulsed focused ultrasound. PLOS One 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- ter Haar, G. Therapeutic ultrasound. Eur. J. Ultrasound 1999, 9, 3–9. [Google Scholar] [CrossRef]
- Barnett, S.B. Biophysical aspects of diagnostic ultrasound. Ultrasound Med. Biol. 2000, 26, S51–S55. [Google Scholar] [CrossRef]
- Kujawska, T.; Secomski, W.; Bilmin, K.; Nowicki, A.; Grieb, P. Impact of thermal effects induced by ultrasound on viability of rat C6 glioma cells. Ultrasonics 2016, 54, 1366–1372. [Google Scholar] [CrossRef]
- Barnett, S.B. Ultrasound. Other non-thermal mechanisms: Acoustic radiation force and streaming. Ultrasound Med. Biol. 1998, 24, S23–S28. [Google Scholar] [CrossRef]
- Duck, F.A. Radiation stress and its bio-effects. European Committee for Medical Ultrasound Safety (ESMUS). Eur. J. Ultrasound 2000, 11, 61–64. [Google Scholar] [CrossRef]
- Miller, M.W.; Mille, D.L.; Brayman, A.A. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med. Biol. 1996, 22, 1131–1154. [Google Scholar] [CrossRef]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy - a review of the synergistic effects of drugs and ultrasound. Ultrasonics Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Fuciarelli, A.F.; Sisk, E.C.; Thomas, R.M.; Miller, D.L. Induction of base damage in DNA solutions by ultrasonic cavitation. Free Radic. Biol. Med. 1995, 18, 231–238. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- McHale, A.P.; Callan, J.F.; Nomikou, N.; Fowley, C.; Callan, B. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. Adv Exp Med Biol. 2016, 880, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Li, S.; Wang, M.; Dai, Z.J. Current Status and Future Perspectives of Sonodynamic Therapy and Sonosensitizers. Asian Pac. J. Cancer Prev. 2015, 16, 4489–4492. [Google Scholar] [CrossRef] [PubMed]
- Mišik, V.; Riesz, P. Free radical intermediates in sonodynamic therapy. Ann. N. Y. Acad Sci 2000, 899, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef]
- Tata, D.B.; Biglow, J.; Tritton, T.R.; Dunn, F. Ultrasound-enhanced hydroxyl radical pro-duction from two clinically employed anticancer drugs, adriamycin and mitomycin C. Ultrason. Sonochem. 1996, 3, 39–45. [Google Scholar] [CrossRef]
- Umemura, S.; Yumita, N.; Nishigaki, R.; Umemura, K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn. J. Cancer Res. 1990, 81, 962–966. [Google Scholar] [CrossRef]
- Grieb, P. 5-Aminolevulinic acid (ALA) and its applications in neurosurgery. Neurol. Neurochir. Pol. 2004, 38, 201–207. [Google Scholar] [CrossRef]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef]
- Mansouri, A.; Mansouri, S.; Hachem, L.D.; Klironomos, G.; Vogelbaum, M.A.; Bernstein, M.; Zadeh, G. The role of 5-aminolevulinic acid in enhancing surgery for high-grade glioma, its current boundaries, and future perspectives: A systematic review. Cancer 2016, 122, 2469–2478. [Google Scholar] [CrossRef]
- Wang, W.; Tabu, K.; Hagiya, Y.; Sugiyama, Y.; Kokubu, Y.; Murota, Y.; Ogura, S.I.; Taga, T. Enhancement of 5-aminolevulinic acid-based fluorescence detection of side population-defined glioma stem cells by iron chelation. Sci. Rep. 2017, 7, 42070. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2009, 23, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Kudo, N.; Yamaguchi, S.; Sumiyoshi, K.; Motegi, H.; Kobayashi, H.; Terasaka, S.; Houkin, K. Porphyrin derivatives-mediated sonodynamic therapy for malignant gliomas in vitro. Ultrasound Med. Biol. 2015, 41, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Song, Y.; Che, Z.; Liu, Q. Calcium overload and in vitro apoptosis of the C6 glioma cells mediated by sonodynamic therapy (hematoporphyrin monomethyl ether and ultrasound). Cell Biochem. Biophys. 2014, 70, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Hu, S.; Wu, C. Apoptotic effect of sonodynamic therapy mediated by hematoporphyrin monomethyl ether on C6 glioma cells in vitro. Acta Neurochir. 2009, 151, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Li, X.Q.; Chen, S.; Cheng, Y.; Deng, J.M.; Wang, Z.G. Glioma stem-like cells are less susceptible than glioma cells to sonodynamic therapy with photofrin. Technol. Cancer Res. Treat. 2012, 11, 615–623. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Wang, K.; Li, X.Q.; Chen, S.; Deng, J.M.; Cheng, Y.; Wang, Z.G. The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with Photofrin in glioma stem-like cells. Ultrasonics 2013, 53, 232–238. [Google Scholar] [CrossRef]
- Ju, D.; Yamaguchi, F.; Zhan, G.; Higuchi, T.; Asakura, T.; Morita, A.; Orimo, H.; Hu, S. Hyperthermotherapy enhances antitumor effect of 5-aminolevulinic acid-mediated sonodynamic therapy with activation of caspase-dependent apoptotic pathway in human glioma. Tumour Biol. 2016, 37, 10415–10426. [Google Scholar] [CrossRef]
- Suehiro, S.; Ohnishi, T.; Yamashita, D.; Kohno, S.; Inoue, A.; Nishikawa, M.; Ohue, S.; Tanaka, J.; Kunieda, T. Enhancement of antitumor activity by using 5-ALA-mediated sonodynamic therapy to induce apoptosis in malignant gliomas: Significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J. Neurosurg. 2018, 129, 1416–1428. [Google Scholar] [CrossRef]
- Bilmin, K.; Kujawska, T.; Secomski, W.; Nowicki, A.; Grieb, P. 5-Aminolevulinic acid-mediated sonosensitization of rat RG2 glioma cells in vitro. Folia Neuropathol. 2016, 54, 234–240. [Google Scholar] [CrossRef]
- Ohmura, T.; Fukushima, T.; Shibaguchi, H.; Yoshizawa, S.; Inoue, T.; Kuroki, M.; Sasaki, K.; Umemura, S. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res. 2011, 31, 2527–2533. [Google Scholar]
- Jeong, E.J.; Seo, S.J.; Ahn, Y.J.; Choi, K.H.; Kim, K.H.; Kim, J.K. Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med. Biol. 2012, 38, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Santos, M.A.; Marcus, S.L.; Hynynen, K. MR-guided Focused Ultrasound Facilitates Sonodynamic Therapy with 5-Aminolevulinic Acid in a Rat Glioma Model. Sci. Rep. 2019, 9, 10465. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilmin, K.; Kujawska, T.; Grieb, P. Sonodynamic Therapy for Gliomas. Perspectives and Prospects of Selective Sonosensitization of Glioma Cells. Cells 2019, 8, 1428. https://doi.org/10.3390/cells8111428
Bilmin K, Kujawska T, Grieb P. Sonodynamic Therapy for Gliomas. Perspectives and Prospects of Selective Sonosensitization of Glioma Cells. Cells. 2019; 8(11):1428. https://doi.org/10.3390/cells8111428
Chicago/Turabian StyleBilmin, Krzysztof, Tamara Kujawska, and Paweł Grieb. 2019. "Sonodynamic Therapy for Gliomas. Perspectives and Prospects of Selective Sonosensitization of Glioma Cells" Cells 8, no. 11: 1428. https://doi.org/10.3390/cells8111428
APA StyleBilmin, K., Kujawska, T., & Grieb, P. (2019). Sonodynamic Therapy for Gliomas. Perspectives and Prospects of Selective Sonosensitization of Glioma Cells. Cells, 8(11), 1428. https://doi.org/10.3390/cells8111428




