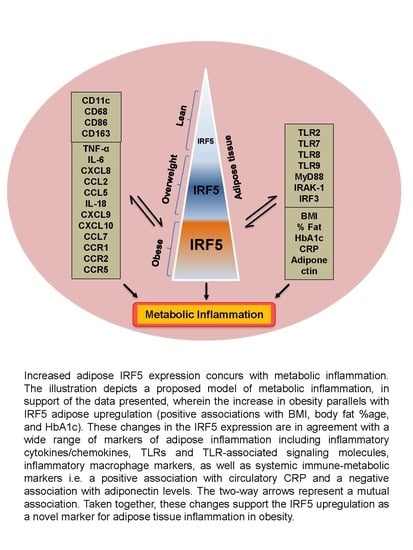
Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric and Physio-Clinical Measurements
2.3. Collection of Subcutaneous Adipose Tissue
2.4. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
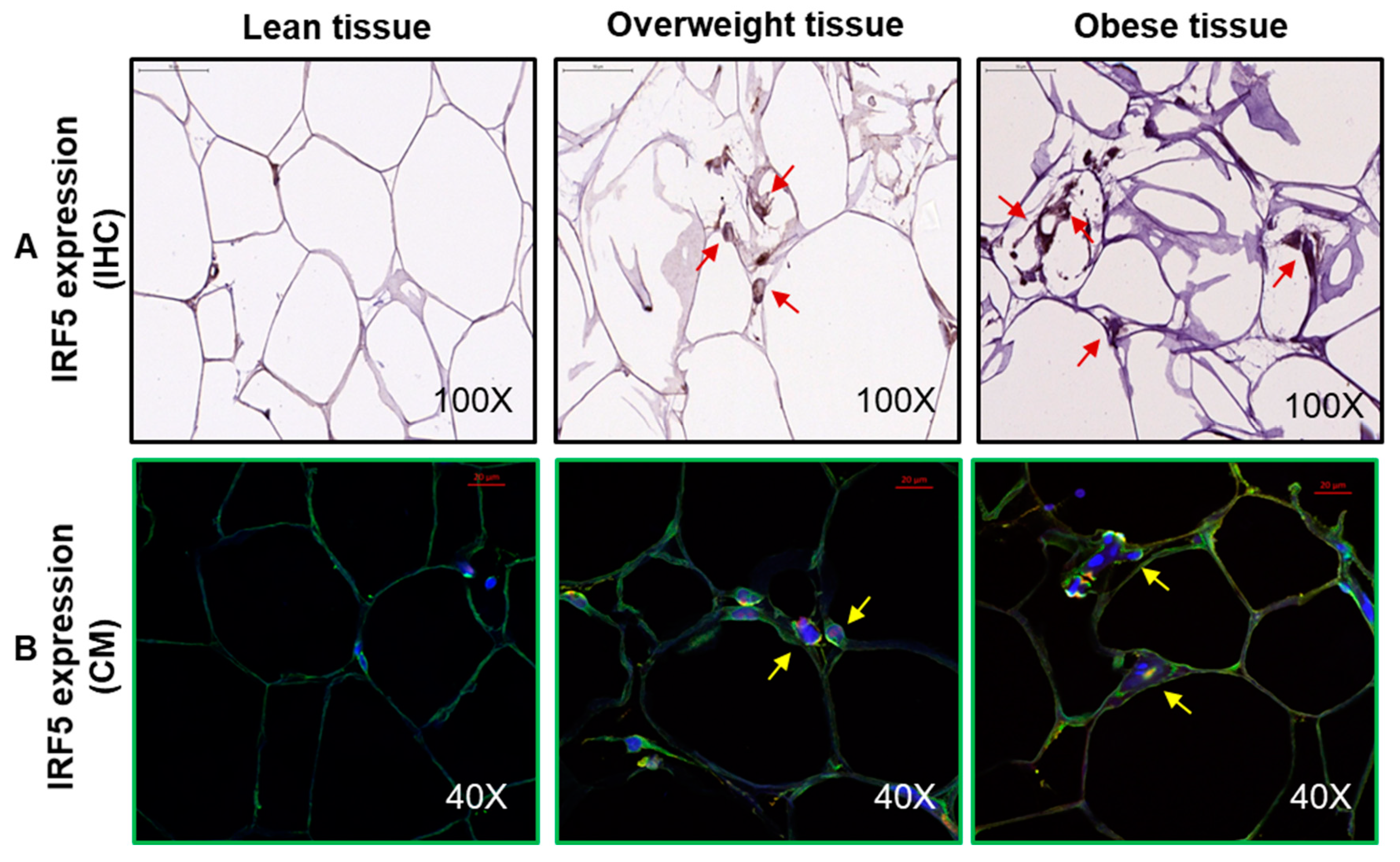
2.5. Immunohistochemistry (IHC)
2.6. Confocal Microscopy
2.7. Statistical Analysis
3. Results
3.1. Increased Adipose IRF-5 Expression in Obesity Correlates with BMI, Body Fat Percentage, Age, and HbA1c
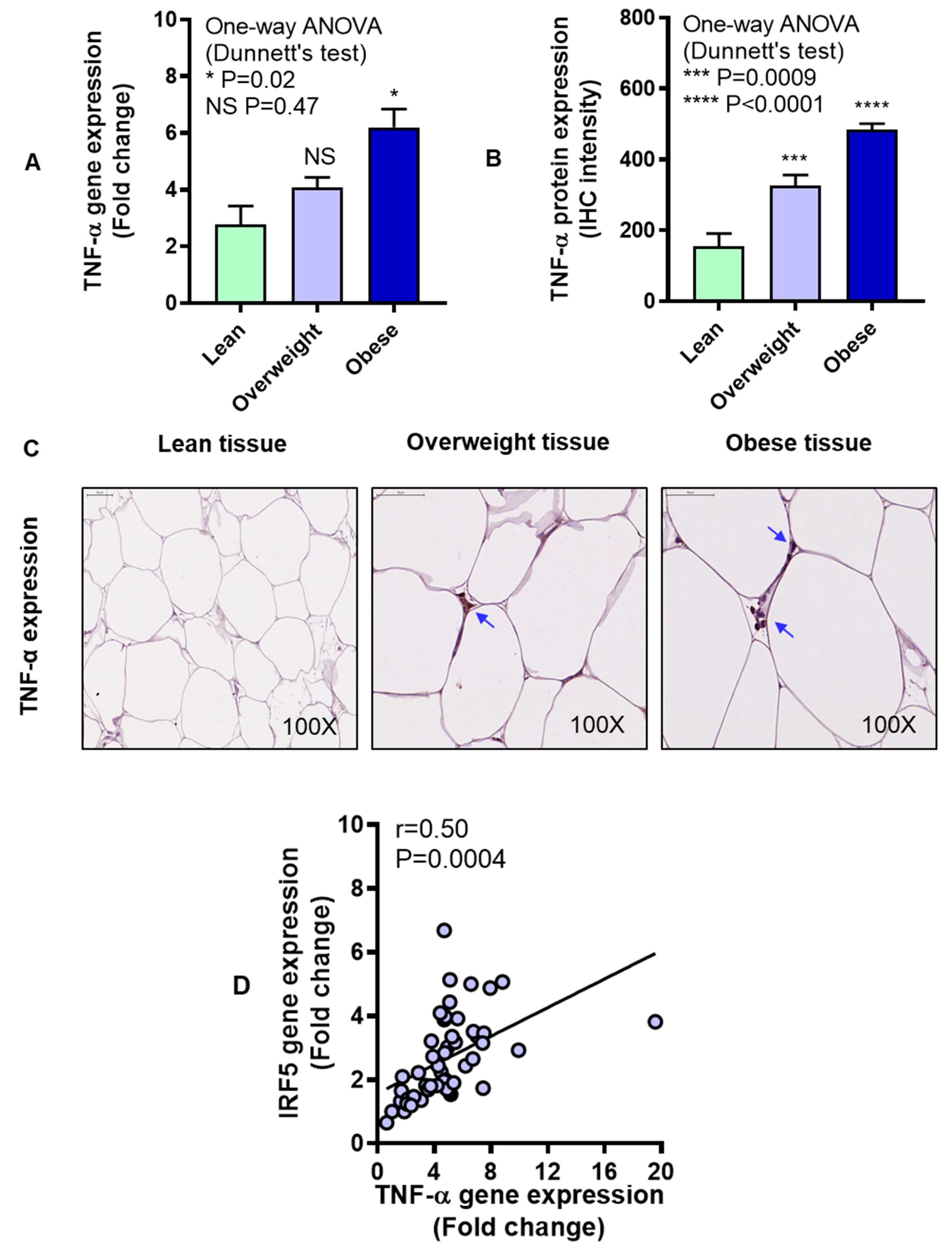
3.2. IRF5 Gene Expression Correlates with that of TNF-α but not IL-6 in Adipose Tissue
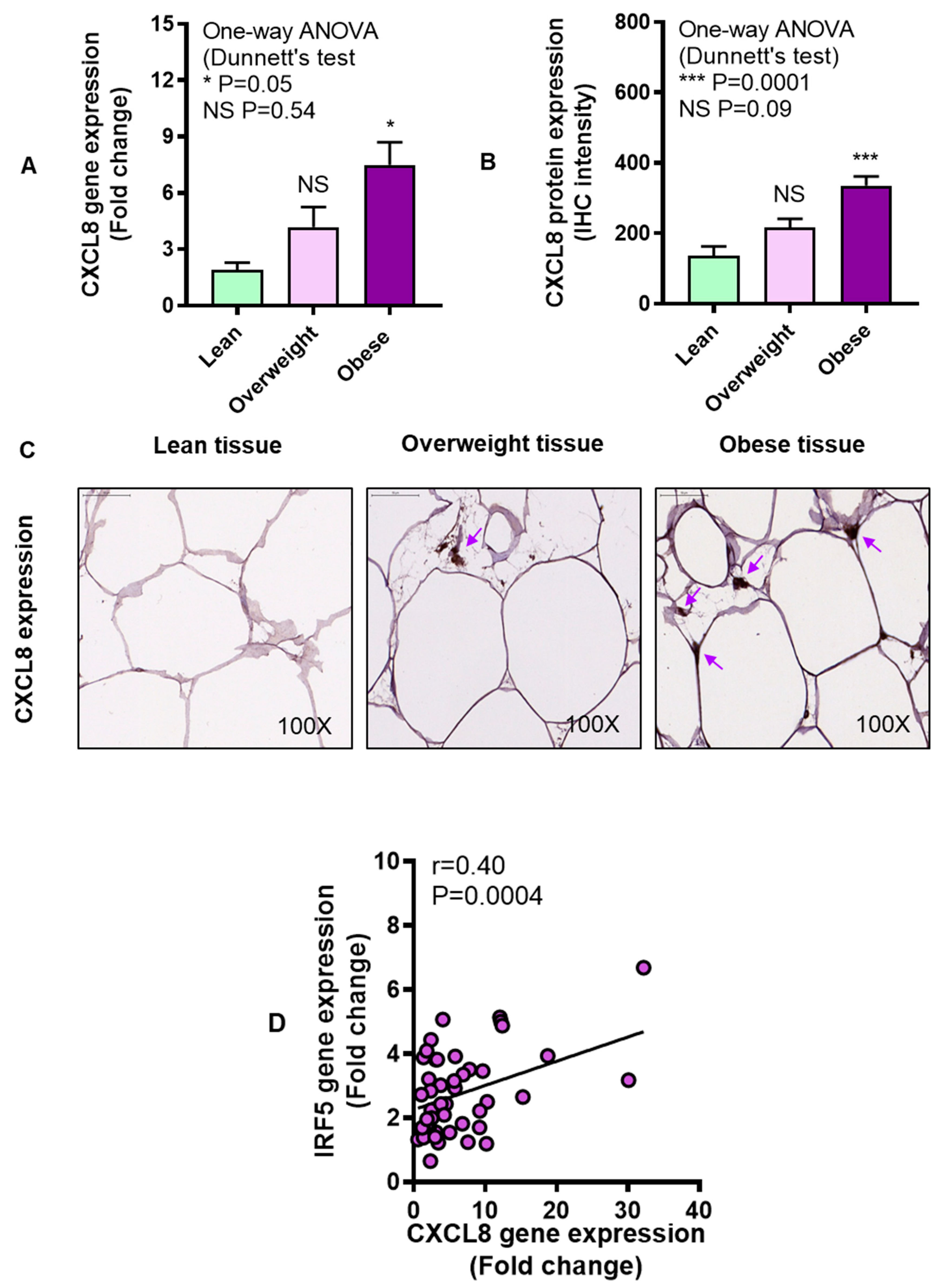
3.3. Adipose CXCL8 Expression is Enhanced in Obese Individuals and Associates with IRF5 Expression
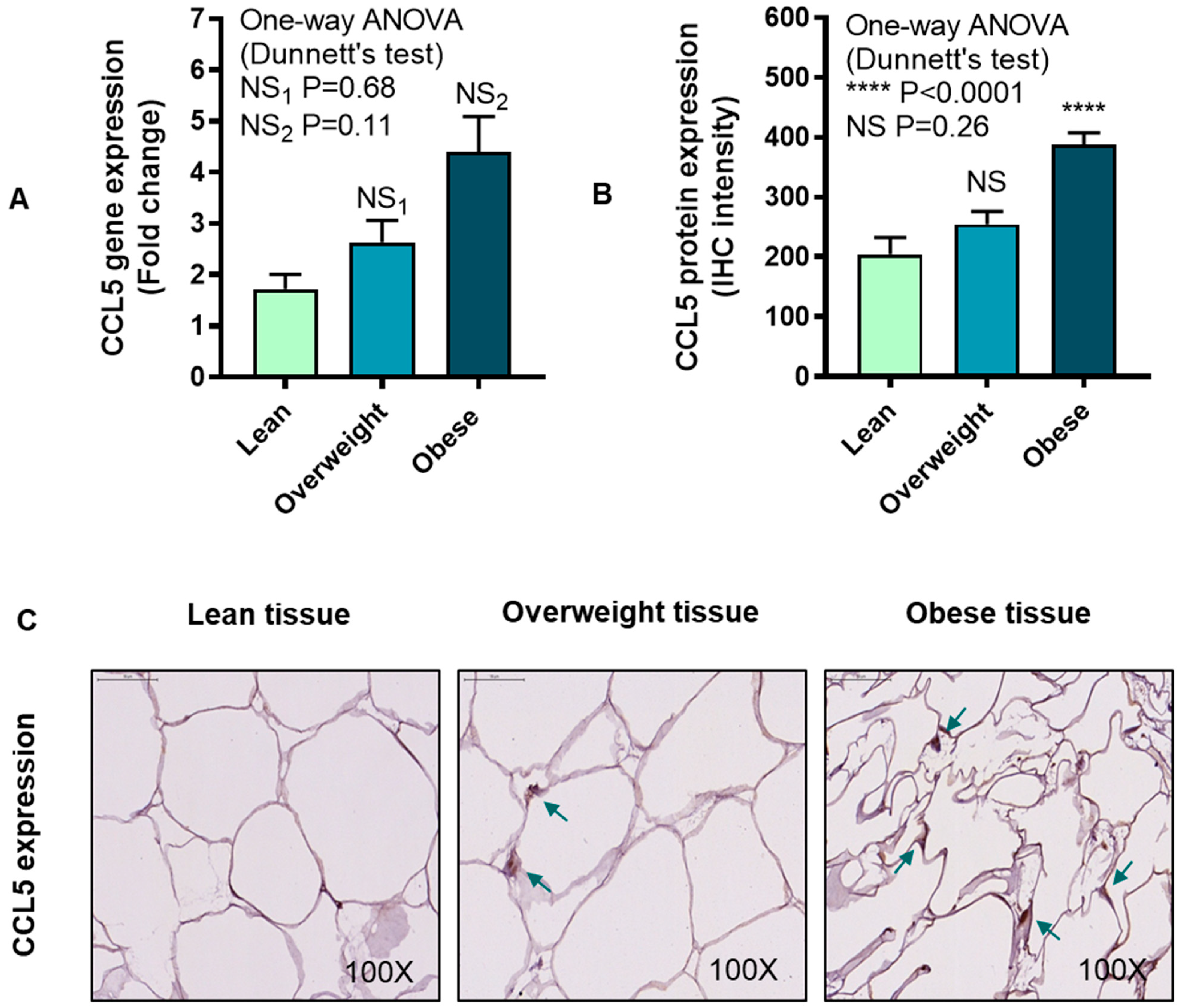
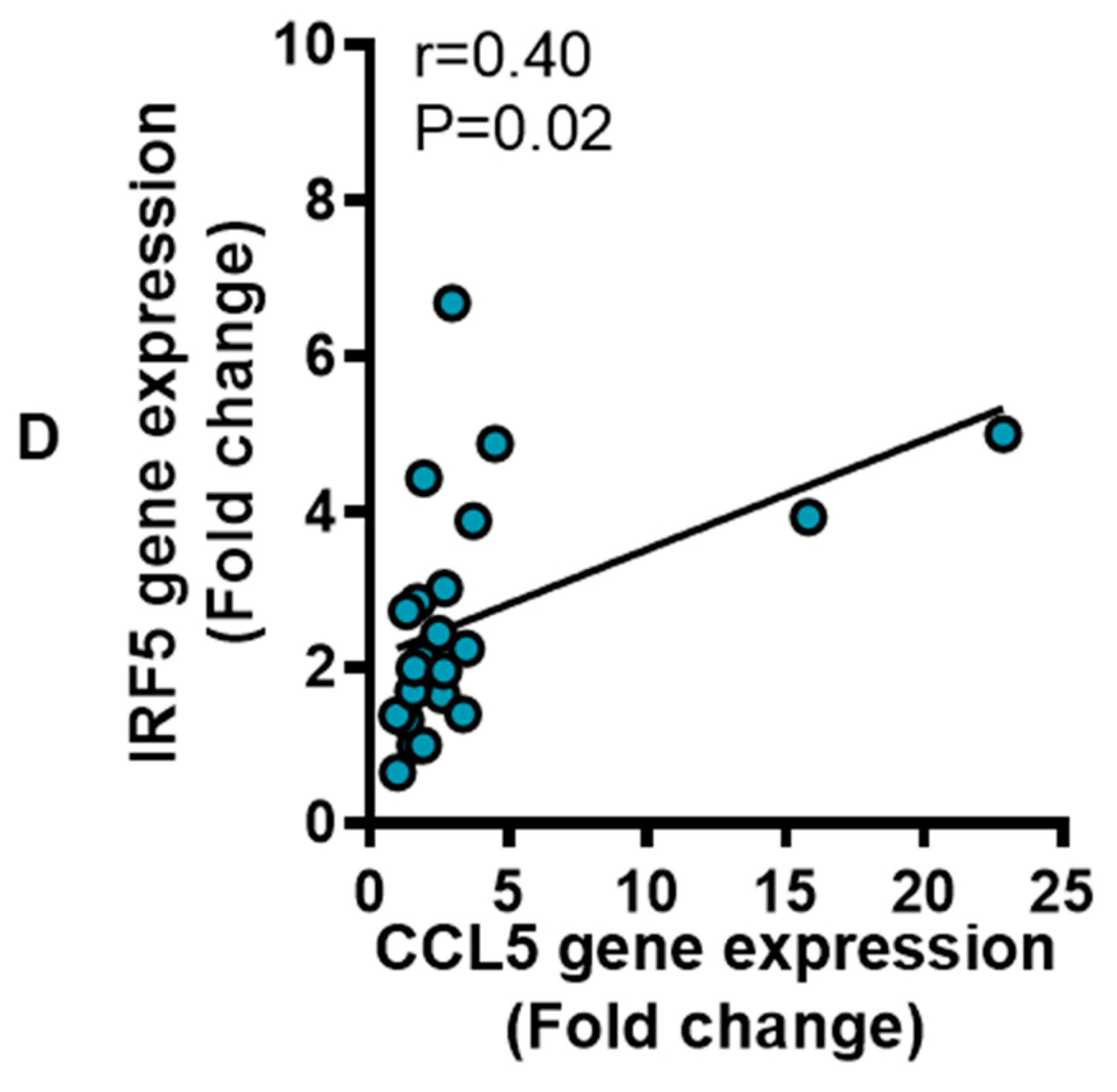
3.4. IRF5 Gene Expression Correlates with that of CCL5 but not CCL2 in Adipose Tissue
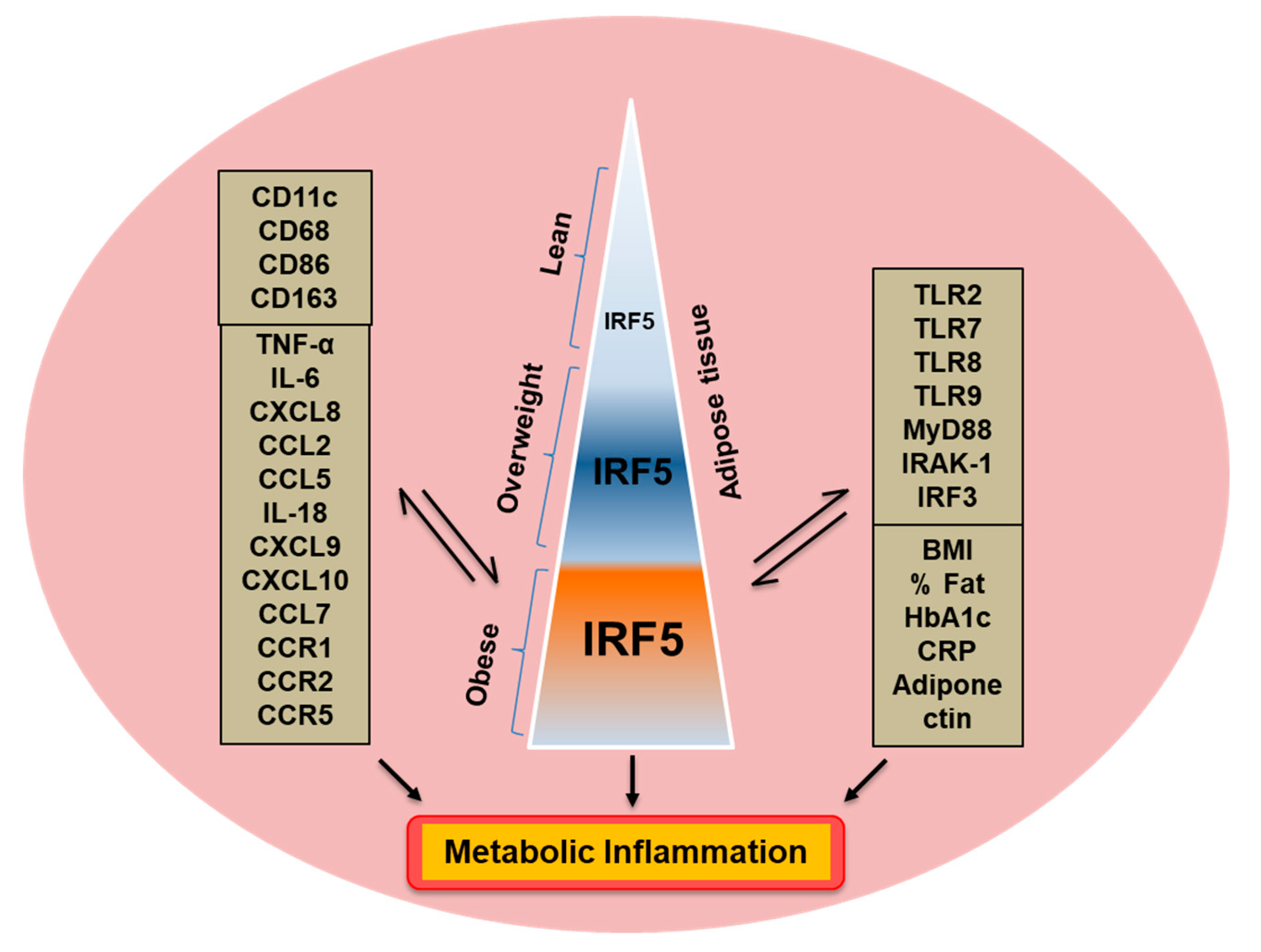
3.5. Relationship of IRF5 Gene Expression with Signature Inflammatory Immune Markers in the Adipose Tissue
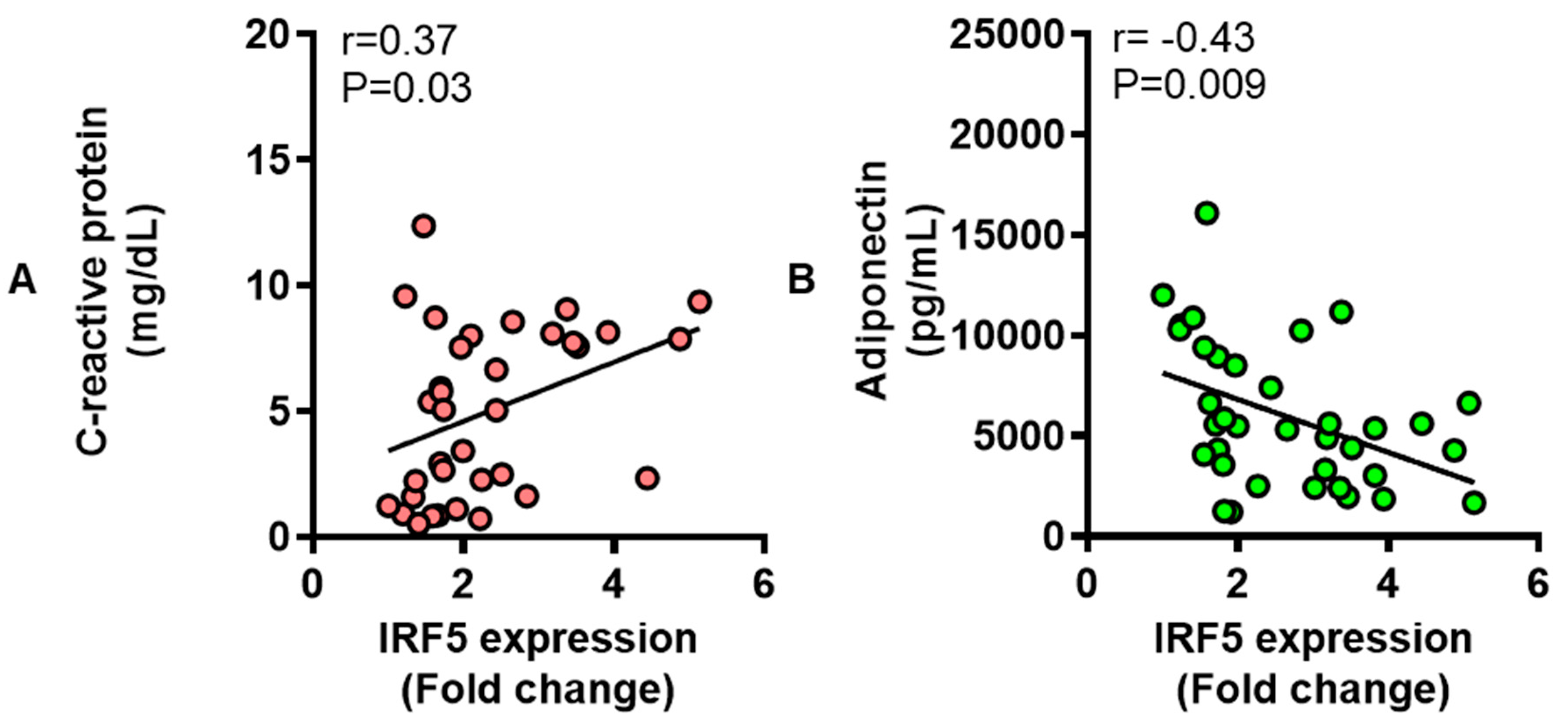
3.6. Adipose IRF5 Gene Expression Correlates with Systemic Immuno-Metabolic Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| T2D | Type-2 diabetes; |
| IRFs | Interferon regulatory factors; |
| BMI | Body mass index; |
| HbA1c | Hemoglobin A1c; |
| CRP | C-reactive protein; |
| TNF-α | Tumor necrosis factor-alpha; |
| IL | Interleukin; |
| CXCL | CXC motif ligand; |
| CCL | CC motif ligand; |
| RT-PCR | Reverse-transcription polymerase chain reaction; |
| MGB | Minor groove binder; |
| FAM | 6-fluorescein amidite; |
| NFQ | Non-fluorescent quencher; |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase; |
| IHC | Immunohistochemistry; |
| DAB | 3,3ʹ-diaminobenzidine; |
| DAPI | 4’,6-diamidino-2-phenylindole; |
| RANTES | Regulated on activation, normal T-cell expressed and secreted; |
| IRAK-1 | Interleukin-1 receptor-associated kinase-1; |
| MyD88 | Myeloid differentiation primary response 88; |
| ATMs | Adipose tissue macrophages. |
References
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. (Lausanne) 2013, 4, 71. [Google Scholar] [CrossRef]
- Moller, D.E. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 2000, 11, 212–217. [Google Scholar] [CrossRef]
- Piya, M.K.; McTernan, P.G.; Kumar, S. Adipokine inflammation and insulin resistance: The role of glucose, lipids and endotoxin. J. Endocrinol. 2013, 216, T1–T15. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Sindhu, S.; Thomas, R.; Shihab, P.; Sriraman, D.; Behbehani, K.; Ahmad, R. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS ONE 2015, 10, e0133494. [Google Scholar] [CrossRef]
- Carey, A.L.; Febbraio, M.A. Interleukin-6 and insulin sensitivity: Friend or foe? Diabetologia 2004, 47, 1135–1142. [Google Scholar] [CrossRef]
- Tack, C.J.; Stienstra, R.; Joosten, L.A.; Netea, M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012, 249, 239–252. [Google Scholar] [CrossRef]
- Ahmad, R.; Thomas, R.; Kochumon, S.; Sindhu, S. Increased adipose tissue expression of IL-18R and its ligand IL-18 associates with inflammation and insulin resistance in obesity. Immun. Inflamm. Dis. 2017, 5, 318–335. [Google Scholar] [CrossRef]
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.C. Interleukin-23: A key cytokine in inflammatory diseases. Ann. Med. 2011, 43, 503–511. [Google Scholar] [CrossRef]
- Yao, L.; Herlea-Pana, O.; Heuser-Baker, J.; Chen, Y.; Barlic-Dicen, J. Roles of the chemokine system in development of obesity, insulin resistance, and cardiovascular disease. J. Immunol. Res. 2014, 2014, 181450. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef]
- Sindhu, S.; Akhter, N.; Arefanian, H.; Al-Roub, A.A.; Ali, S.; Wilson, A.; Al-Hubail, A.; Al-Beloushi, S.; Al-Zanki, S.; Ahmad, R. Increased circulatory levels of fractalkine (CX3CL1) are associated with inflammatory chemokines and cytokines in individuals with type-2 diabetes. J. Diabetes Metab. Disord. 2017, 16, 15. [Google Scholar] [CrossRef]
- Nunemaker, C.S.; Chung, H.G.; Verrilli, G.M.; Corbin, K.L.; Upadhye, A.; Sharma, P.R. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J. Endocrinol. 2014, 222, 267–276. [Google Scholar] [CrossRef]
- Barnes, B.; Lubyova, B.; Pitha, P.M. On the role of IRF in host defense. J. Interferon Cytokine Res. 2002, 22, 59–71. [Google Scholar] [CrossRef]
- Eguchi, J.; Yan, Q.W.; Schones, D.E.; Kamal, M.; Hsu, C.H.; Zhang, M.Q.; Crawford, G.E.; Rosen, E.D. Interferon regulatory factors are transcriptional regulators of adipogenesis. Cell Metab. 2008, 7, 86–94. [Google Scholar] [CrossRef]
- Dalmas, E.; Toubal, A.; Alzaid, F.; Blazek, K.; Eames, H.L.; Lebozec, K.; Pini, M.; Hainault, I.; Montastier, E.; Denis, R.G.; et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat. Med. 2015, 21, 610–618. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Mass, A.; Atizado, V.; Al-Hubail, A.; Al-Ghimlas, F.; Al-Arouj, M.; Bennakhi, A.; Dermime, S.; Behbehani, K. Elevated expression of the toll like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J. Inflamm. 2012, 9, 48. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Dir. Autoimmun. 2010, 11, 145–156. [Google Scholar] [CrossRef]
- Borst, S.E. The role of TNF-alpha in insulin resistance. Endocrine 2004, 23, 177–182. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Roytblat, L.; Rachinsky, M.; Fisher, A.; Greemberg, L.; Shapira, Y.; Douvdevani, A.; Gelman, S. Raised interleukin-6 levels in obese patients. Obes. Res. 2000, 8, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Gallistl, S.; Sudi, K.M.; Aigner, R.; Borkenstein, M. Changes in serum interleukin-6 concentrations in obese children and adolescents during a weight reduction program. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Jardel, C.; Delattre, J.; Hainque, B.; Bruckert, E.; Oberlin, F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation 1999, 99, 2221–2222. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bachmann, R.A.; Chen, J. Interleukin-6 and insulin resistance. Vitam. Horm. 2009, 80, 613–633. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Patsouris, D.; Cao, J.J.; Vial, G.; Bravard, A.; Lefai, E.; Durand, A.; Durand, C.; Chauvin, M.A.; Laugerette, F.; Debard, C.; et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS ONE 2014, 9, e110653. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Al-Roub, A.; Kochumon, S.; Akther, N.; Thomas, R.; Kumari, M.; Koshy, M.S.; Tiss, A.; Hannun, Y.A.; Tuomilehto, J.; et al. The Synergy between Palmitate and TNF-alpha for CCL2 Production Is Dependent on the TRIF/IRF3 Pathway: Implications for Metabolic Inflammation. J. Immunol. 2018, 200, 3599–3611. [Google Scholar] [CrossRef]
- Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Devarajan, S.; Tuomilehto, J.; Ahmad, R. Adipose tissue expression of CCL19 chemokine is positively associated with insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3087. [Google Scholar] [CrossRef]
- Hasan, A.; Akhter, N.; Al-Roub, A.; Thomas, R.; Kochumon, S.; Wilson, A.; Koshy, M.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. TNF-alpha in combination with palmitate enhances IL-8 production via the MyD88- independent TLR4 signaling pathway: Potential relevance to metabolic inflammation. Int. J. Mol. Sci. 2019, 20, 4112. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Kochumon, S.; Shenouda, S.; Wilson, A.; Al-Mulla, F.; Ahmad, R. The cooperative induction of CCL4 in human monocytic cells by TNF-alpha and palmitate requires MyD88 and involves MAPK/NF-kappaB signaling pathways. Int. J. Mol. Sci. 2019, 20, 4658. [Google Scholar] [CrossRef]
- Huber, J.; Kiefer, F.W.; Zeyda, M.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; Zlabinger, G.J.; Stulnig, T.M. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J. Clin. Endocrinol. Metab. 2008, 93, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stepien, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Szelachowska, M.; Kinalska, I. Plasma interleukin 8 concentrations in obese subjects with impaired glucose tolerance. Cardiovasc. Diabetol. 2003, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Weier, M.; Herderschee, J.; Perreau, M.; Calandra, T.; Roger, T.; Giannoni, E. IRF5 is a key regulator of macrophage response to lipopolysaccharide in newborns. Front. Immunol. 2018, 9, 1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, J.; Wang, Y.; Xiao, Y.; Wang, L.; Hua, S. Expression levels of interferon regulatory factor 5 (IRF5) and related inflammatory cytokines associated with severity, prognosis, and causative pathogen in patients with community-acquired pneumonia. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2018, 24, 3620–3630. [Google Scholar] [CrossRef]
- Weiss, M.; Blazek, K.; Byrne, A.J.; Perocheau, D.P.; Udalova, I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediat. Inflamm 2013, 2013, 245804. [Google Scholar] [CrossRef]
- Schoenemeyer, A.; Barnes, B.J.; Mancl, M.E.; Latz, E.; Goutagny, N.; Pitha, P.M.; Fitzgerald, K.A.; Golenbock, D.T. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 2005, 280, 17005–17012. [Google Scholar] [CrossRef]
- Zhao, G.N.; Jiang, D.S.; Li, H. Interferon regulatory factors: At the crossroads of immunity, metabolism, and disease. Biochim. Biophys. Acta 2015, 1852, 365–378. [Google Scholar] [CrossRef]
- Balkhi, M.Y.; Fitzgerald, K.A.; Pitha, P.M. Functional regulation of MyD88-activated interferon regulatory factor 5 by K63-linked polyubiquitination. Mol. Cell Biol. 2008, 28, 7296–7308. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef]
- Kumari, M.; Wang, X.; Lantier, L.; Lyubetskaya, A.; Eguchi, J.; Kang, S.; Tenen, D.; Roh, H.C.; Kong, X.; Kazak, L.; et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J. Clin. Investig. 2016, 126, 2839–2854. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, Y.; Choi, Y.H.; Park, T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J. Nutr. Biochem. 2012, 23, 113–122. [Google Scholar] [CrossRef]
- Ahmad, R.; Kochumon, S.; Thomas, R.; Atizado, V.; Sindhu, S. Increased adipose tissue expression of TLR8 in obese individuals with or without type-2 diabetes: Significance in metabolic inflammation. J. Inflamm. 2016, 13, 38. [Google Scholar] [CrossRef][Green Version]
- Akhter, N.; Madhoun, A.; Arefanian, H.; Wilson, A.; Kochumon, S.; Thomas, R.; Shenouda, S.; Al-Mulla, F.; Ahmad, R.; Sindhu, S. Oxidative Stress Induces Expression of the Toll-Like Receptors (TLRs) 2 and 4 in the Human Peripheral Blood Mononuclear Cells: Implications for Metabolic Inflammation. Cell. Physiol. Biochem. 2019, 53, 1–18. [Google Scholar] [CrossRef]
- Sindhu, S.; Akhter, N.; Kochumon, S.; Thomas, R.; Wilson, A.; Shenouda, S.; Tuomilehto, J.; Ahmad, R. Increased Expression of the Innate Immune Receptor TLR10 in Obesity and Type-2 Diabetes: Association with ROS-Mediated Oxidative Stress. Cell. Physiol. Biochem. 2018, 45, 572–590. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Reviews. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef]
- Kratz, M.; Coats, B.R.; Hisert, K.B.; Hagman, D.; Mutskov, V.; Peris, E.; Schoenfelt, K.Q.; Kuzma, J.N.; Larson, I.; Billing, P.S.; et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014, 20, 614–625. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Devaraj, S.; Singh, U.; Jialal, I. Human C-reactive protein and the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Mohlig, M.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003, 361, 226–228. [Google Scholar] [CrossRef]
- Abraham, P.A.; Attipoe, S.; Kazman, J.B.; Zeno, S.A.; Poth, M.; Deuster, P.A. Role of plasma adiponectin /C-reactive protein ratio in obesity and type 2 diabetes among African Americans. Afr. Health Sci. 2017, 17, 99–107. [Google Scholar] [CrossRef][Green Version]
- Weiss, M.; Byrne, A.J.; Blazek, K.; Saliba, D.G.; Pease, J.E.; Perocheau, D.; Feldmann, M.; Udalova, I.A. IRF5 controls both acute and chronic inflammation. Proc. Natl. Acad. Sci. USA 2015, 112, 11001–11006. [Google Scholar] [CrossRef]
- Al-Shukaili, A.; Al-Ghafri, S.; Al-Marhoobi, S.; Al-Abri, S.; Al-Lawati, J.; Al-Maskari, M. Analysis of inflammatory mediators in type 2 diabetes patients. Int. J. Endocrinol. 2013, 2013, 976810. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Porzia, A.; Mainiero, F.; Costantino, C.; Bertoccini, L.; Ceccarelli, V.; Morini, S.; Baroni, M.G.; Lenzi, A.; et al. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol. 2017, 54, 961–967. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Khoramdelazad, H.; Hassanshahi, G.; Rafatpanah, H.; Hosseini, J.; Mahmoodi, M.; Arababadi, M.K.; Derakhshan, R.; Hasheminasabzavareh, R.; Hosseini-Zijoud, S.M.; et al. Plasma levels of CXCL1 (GRO-alpha) and CXCL10 (IP-10) are elevated in type 2 diabetic patients: Evidence for the involvement of inflammation and angiogenesis/angiostasis in this disease state. Clin. Lab. 2013, 59, 133–137. [Google Scholar] [CrossRef]
- Dworacka, M.; Krzyzagorska, E.; Iskakova, S.; Bekmukhambetov, Y.; Urazayev, O.; Dworacki, G. Increased circulating RANTES in type 2 diabetes. Eur. Cytokine Netw. 2014, 25, 46–51. [Google Scholar] [CrossRef]
- Abdi, R.; Smith, R.N.; Makhlouf, L.; Najafian, N.; Luster, A.D.; Auchincloss, H., Jr.; Sayegh, M.H. The role of CC chemokine receptor 5 (CCR5) in islet allograft rejection. Diabetes 2002, 51, 2489–2495. [Google Scholar] [CrossRef]
- Ahmad, R.; Shihab, P.K.; Thomas, R.; Alghanim, M.; Hasan, A.; Sindhu, S.; Behbehani, K. Increased expression of the interleukin-1 receptor-associated kinase (IRAK)-1 is associated with adipose tissue inflammatory state in obesity. Diabetol. Metab. Syndr. 2015, 7, 71. [Google Scholar] [CrossRef]




| Total Number (N) | 53 (24 Male and 29 Female) |
|---|---|
| Age (Yrs.) | 43.04 ± 1.65 (Range: 24–71) |
| Subgroups (N) | 6 Lean, 18 Overweight and 29 Obese |
| Body mass index (kg/m2) | 31.32 ± 0.72 |
| Body fat (%) | 36.38 ± 0.89 |
| Fasting glucose (mmol/L) | 5.12 ± 0.07 |
| HbA1c (%) | 5.57 ± 0.06 |
| Total cholesterol (mmol/L) | 5.04 ± 0.14 |
| HDL (mmol/L) | 1.30 ± 0.05 |
| LDL (mmol/L) | 3.25 ± 0.13 |
| Triglycerides (mmol/L) | 1.12 ± 0.09 |
| Hypertension (N) | 4 |
| Hyperlipidemia (N) | 3 |
| Therapy | Lipitor, Concor, Aldomet, Eltoxin |
| Cytokines, Chemokines, and Chemokine Receptors | Correlation | Significance |
|---|---|---|
| IL-1β | r = 0.33 | P = 0.04* |
| IL-18 | r = 0.32 | P = 0.04* |
| IL-23A | r = 0.16 | P = 0.25 |
| CXCL9 | r = 0.28 | P = 0.05* |
| CXCL10 | r = 0.34 | P = 0.02* |
| CCL7 | r = 0.30 | P = 0.04* |
| CCL11 | r = 0.22 | P = 0.14 |
| CCL19 | r = 0.20 | P = 0.19 |
| CCR1 | r = 0.30 | P = 0.05* |
| CCR2 | r = 0.53 | P = 0.0002*** |
| CCR5 | r = 0.44 | P = 0.0006*** |
| Receptor | Correlation | Significance |
|---|---|---|
| TLR2 | r = 0.60 | P < 0.0001**** |
| TLR4 | r = 0.001 | P = 0.95 |
| TLR7 | r = 0.62 | P < 0.0001**** |
| TLR8 | r = 0.55 | P < 0.0001**** |
| TLR9 | r = 0.40 | P = 0.005** |
| MyD88 | r = 0.60 | P < 0.0001**** |
| IRAK-1 | r = 0.49 | P = 0.0004*** |
| IRF3 | r = 0.42 | P = 0.005** |
| Markers | Correlation | Significance |
|---|---|---|
| CD11c | r = 0.60 | P < 0.0001**** |
| CD68 | r = 0.63 | P < 0.0001**** |
| CD86 | r = 0.62 | P < 0.0001**** |
| CD163 | r = 0.61 | P < 0.0001**** |
| CD302 | r = 0.11 | P = 0.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindhu, S.; Thomas, R.; Kochumon, S.; Wilson, A.; Abu-Farha, M.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation. Cells 2019, 8, 1418. https://doi.org/10.3390/cells8111418
Sindhu S, Thomas R, Kochumon S, Wilson A, Abu-Farha M, Bennakhi A, Al-Mulla F, Ahmad R. Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation. Cells. 2019; 8(11):1418. https://doi.org/10.3390/cells8111418
Chicago/Turabian StyleSindhu, Sardar, Reeby Thomas, Shihab Kochumon, Ajit Wilson, Mohamed Abu-Farha, Abdullah Bennakhi, Fahd Al-Mulla, and Rasheed Ahmad. 2019. "Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation" Cells 8, no. 11: 1418. https://doi.org/10.3390/cells8111418
APA StyleSindhu, S., Thomas, R., Kochumon, S., Wilson, A., Abu-Farha, M., Bennakhi, A., Al-Mulla, F., & Ahmad, R. (2019). Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation. Cells, 8(11), 1418. https://doi.org/10.3390/cells8111418