Wnt-3a Induces Cytokine Release in Human Mast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Cell Culture
2.3. Treatment with Wnts and Mast Cell Activators
2.4. Measurement of Mediator Release
2.5. Flow Cytometry Analysis and Cell Sorting
2.6. Western Blot Analysis
2.7. Quantitative PCR (qPCR) and RNA Sequencing (RNAseq)
2.8. Statistical Analysis
3. Results
3.1. Human Mast Cells Express FZDs
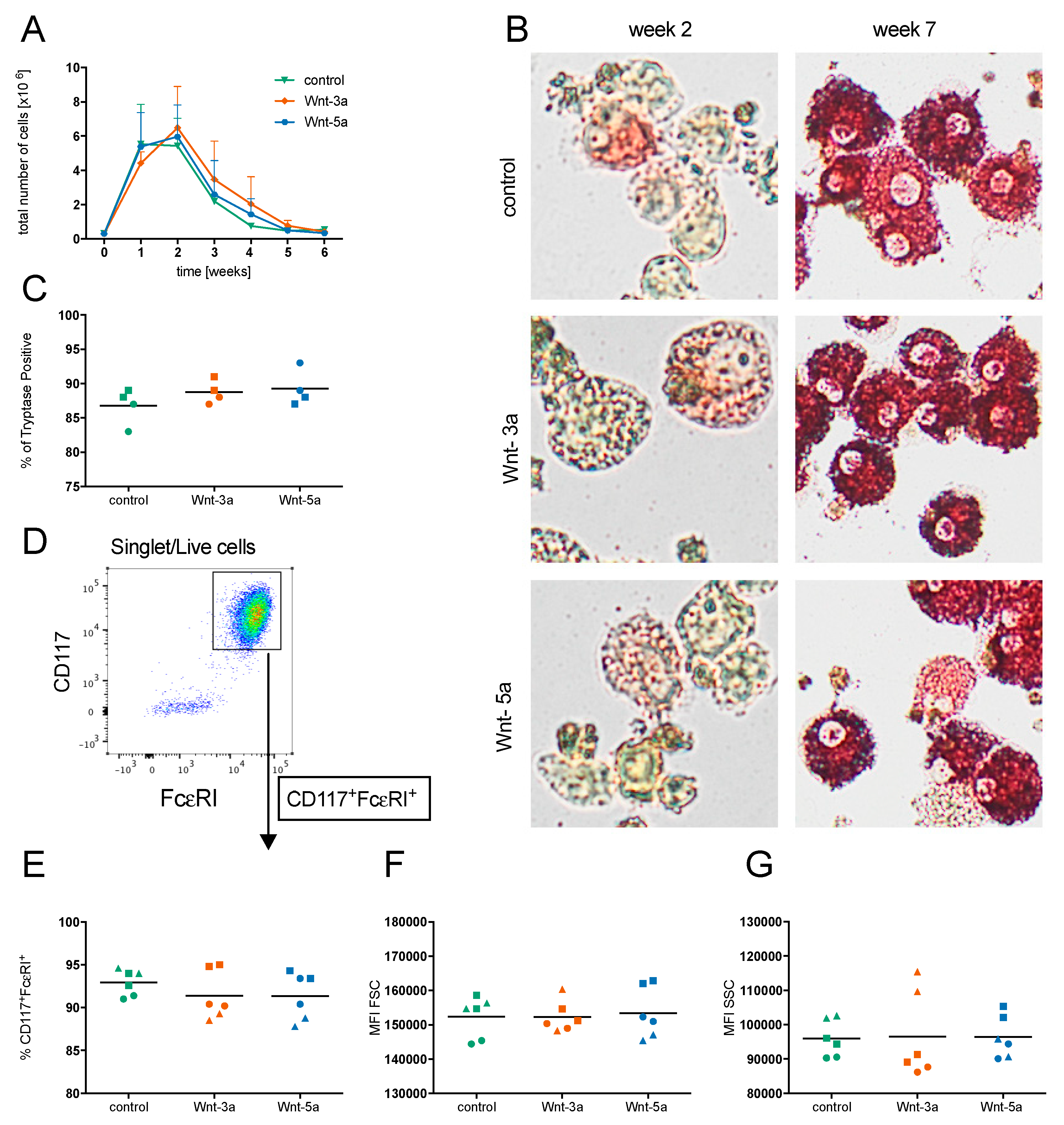
3.2. Wnts Do Not Affect Early Mast Cell Development
3.3. Wnts Do Not Affect Mast Cell Maturation
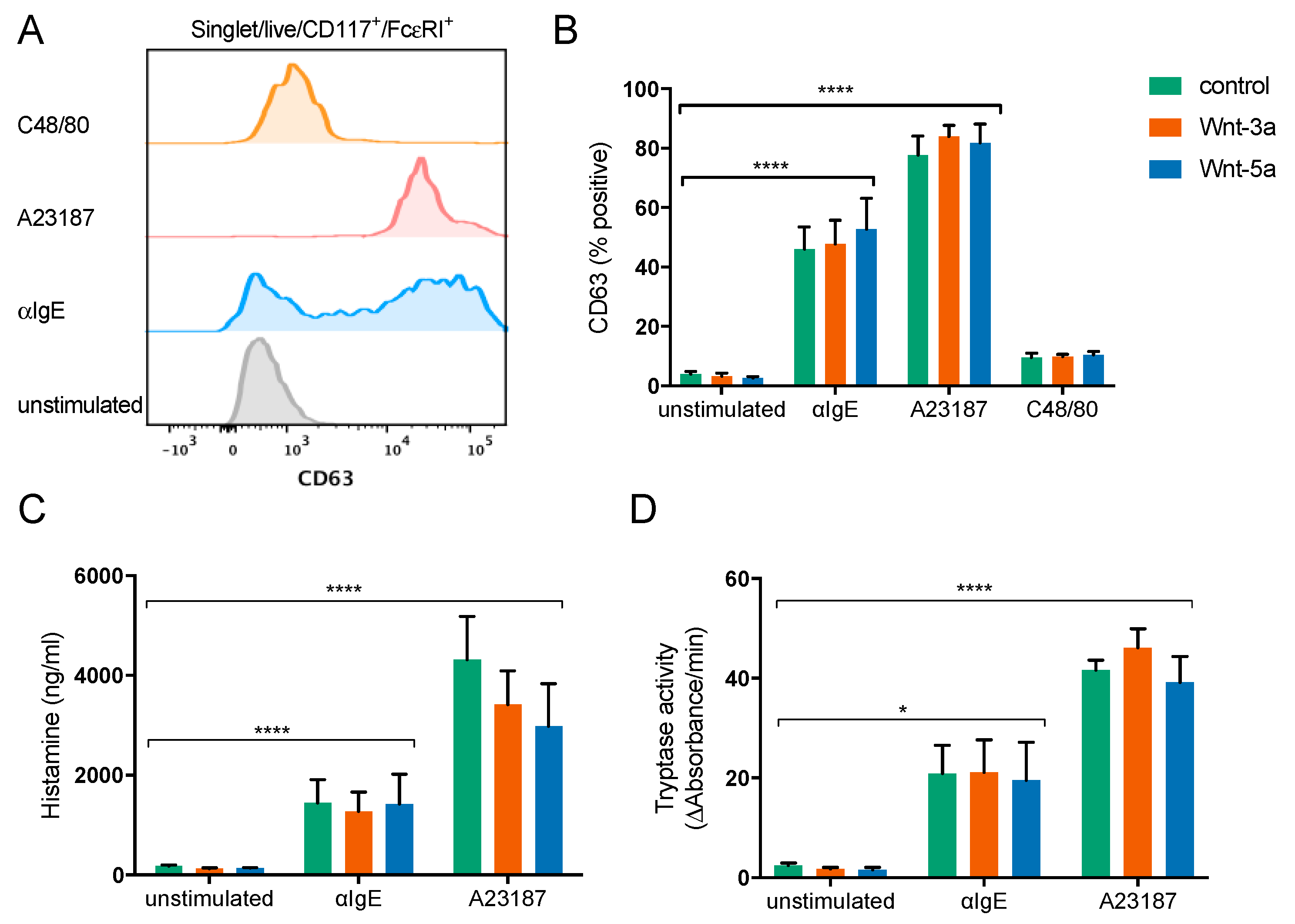
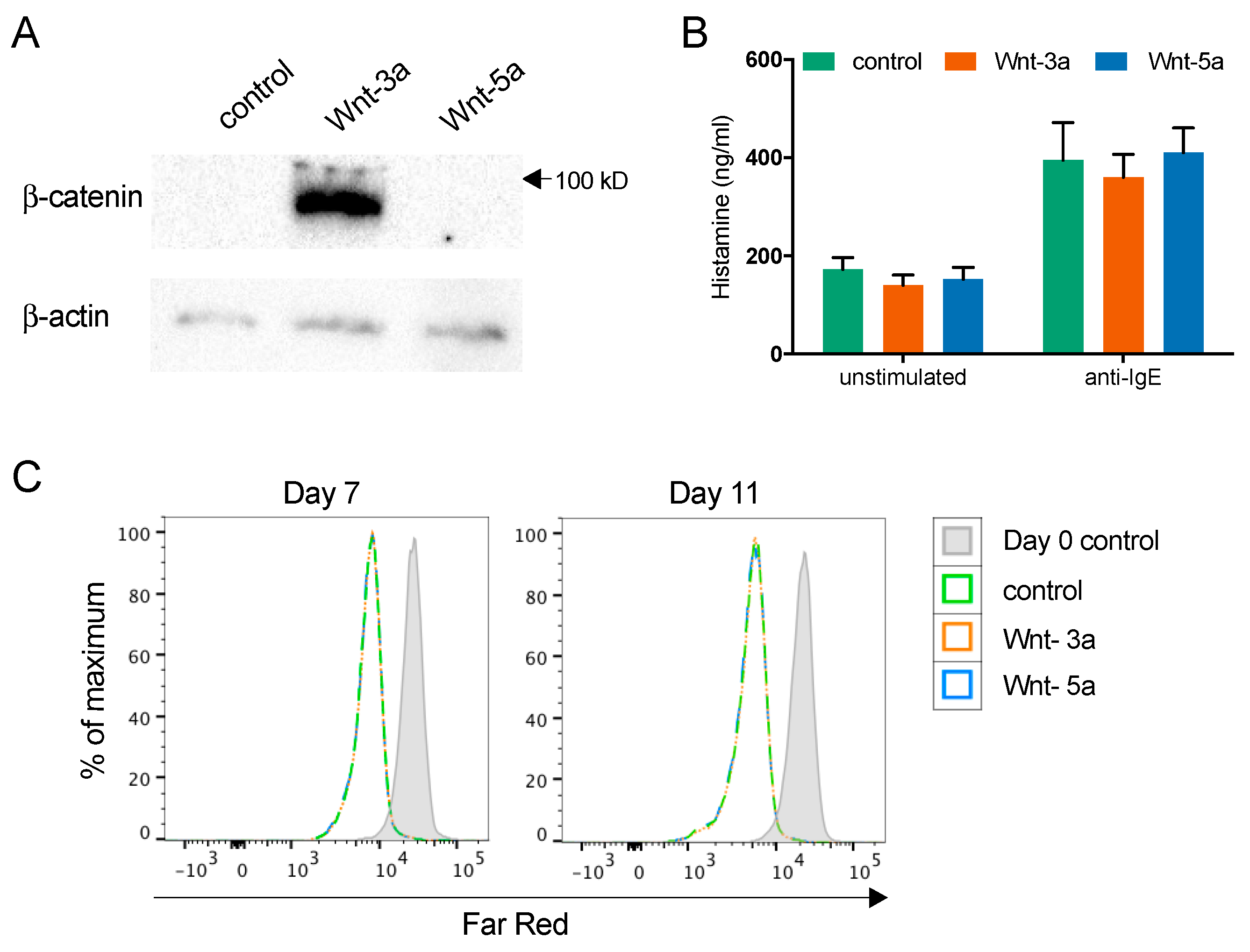
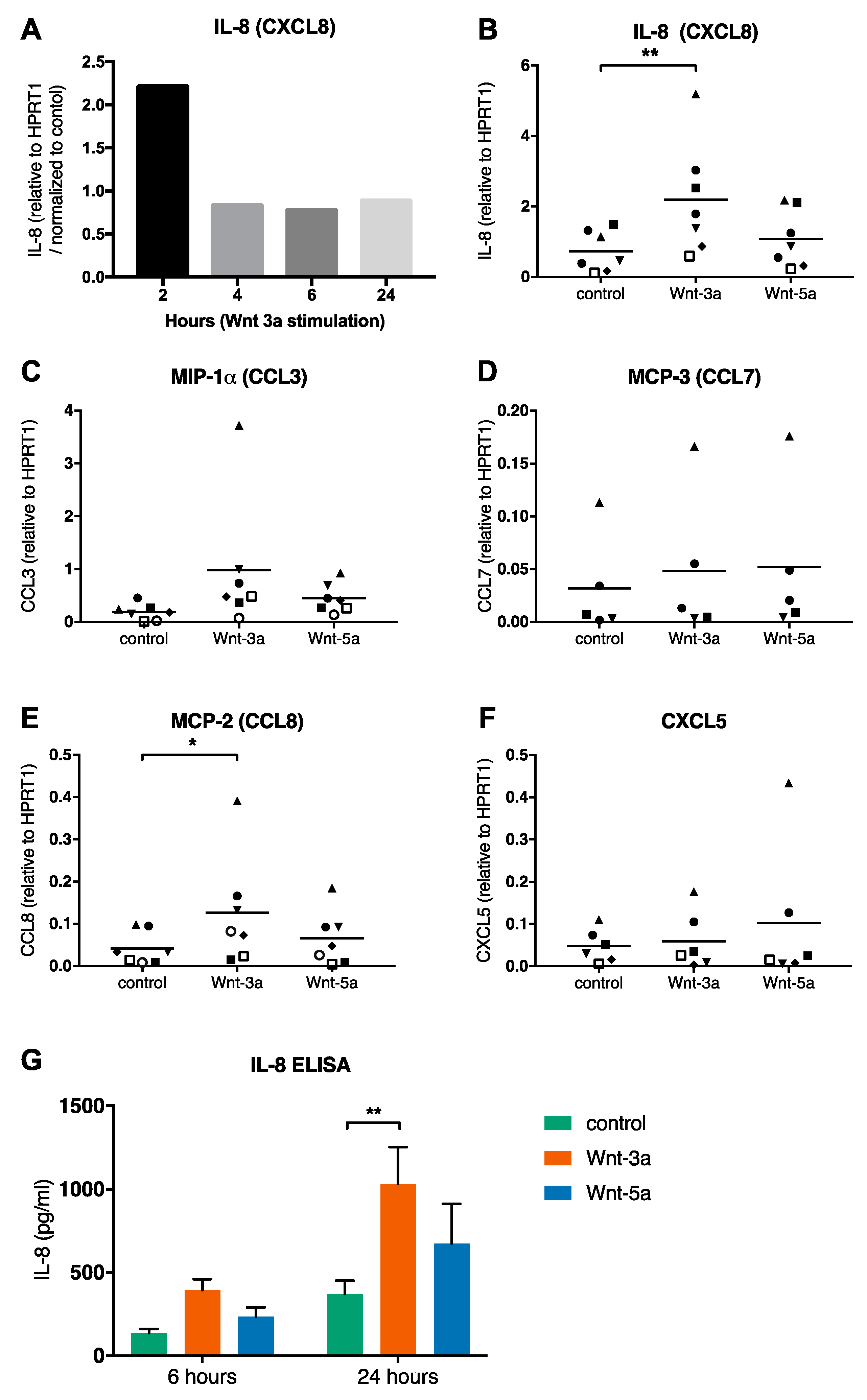
3.4. Wnt-3a Activates Mature Mast Cells and Causes IL-8 Release
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Grootens, J.; Ungerstedt, J.S.; Nilsson, G.; Dahlin, J.S. Deciphering the differentiation trajectory from hematopoietic stem cells to mast cells. Blood Adv. 2018, 2, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Pejler, G.; Ronnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef]
- Schulte, G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol. Rev. 2010, 62, 632–667. [Google Scholar] [CrossRef]
- Schulte, G.; Wright, S.C. Frizzleds as GPCRs—More Conventional Than We Thought! Trends Pharmacol. Sci. 2018, 39, 828–842. [Google Scholar] [CrossRef]
- Reuter, S.; Beckert, H.; Taube, C. Take the Wnt out of the inflammatory sails: Modulatory effects of Wnt in airway diseases. Lab. Investig. 2016, 96, 177–185. [Google Scholar] [CrossRef]
- Sharma, S.; Tantisira, K.; Carey, V.; Murphy, A.J.; Lasky-Su, J.; Celedon, J.C.; Lazarus, R.; Klanderman, B.; Rogers, A.; Soto-Quiros, M.; et al. A role for Wnt signaling genes in the pathogenesis of impaired lung function in asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 328–336. [Google Scholar] [CrossRef]
- Choy, D.F.; Modrek, B.; Abbas, A.R.; Kummerfeld, S.; Clark, H.F.; Wu, L.C.; Fedorowicz, G.; Modrusan, Z.; Fahy, J.V.; Woodruff, P.G.; et al. Gene expression patterns of Th2 inflammation and intercellular communication in asthmatic airways. J. Immunol. 2011, 186, 1861–1869. [Google Scholar] [CrossRef]
- Lento, W.; Congdon, K.; Voermans, C.; Kritzik, M.; Reya, T. Wnt signaling in normal and malignant hematopoiesis. Cold Spring Harb. Perspect Biol. 2013, 5, a008011. [Google Scholar] [CrossRef]
- Feng, Z.; Srivastava, A.S.; Mishra, R.; Carrier, E. A regulatory role of Wnt signaling pathway in the hematopoietic differentiation of murine embryonic stem cells. Biochem. Biophys. Res. Commun. 2004, 324, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, D.J.; Sharma, A.K.; Bruno, E.; Hoffman, R. Role of members of the Wnt gene family in human hematopoiesis. Blood 1998, 92, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Ichii, M.; Frank, M.B.; Iozzo, R.V.; Kincade, P.W. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood 2012, 119, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Nishijima, M.; Tashiro, K.; Kawabata, K. Wnt-beta-Catenin Signaling Promotes the Maturation of Mast Cells. Biomed. Res. Int. 2016, 2016, 2048987. [Google Scholar] [CrossRef]
- Enoksson, M.; Ejendal, K.F.; McAlpine, S.; Nilsson, G.; Lunderius-Andersson, C. Human cord blood-derived mast cells are activated by the Nod1 agonist M-TriDAP to release pro-inflammatory cytokines and chemokines. J. Innate Immun. 2011, 3, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Ronnberg, E.; Dahlin, J.S.; Mazzurana, L.; Safholm, J.; Orre, A.C.; Al-Ameri, M.; Peachell, P.; Adner, M.; Dahlen, S.E.; et al. An Optimized Protocol for the Isolation and Functional Analysis of Human Lung Mast Cells. Front. Immunol. 2018, 9, 2193. [Google Scholar] [CrossRef]
- Harvima, I.T.; Naukkarinen, A.; Harvima, R.J.; Fraki, J.E. Immunoperoxidase and enzyme-histochemical demonstration of human skin tryptase in cutaneous mast cells in normal and mastocytoma skin. Arch. Dermatol. Res. 1988, 280, 363–370. [Google Scholar] [CrossRef]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Ekoff, M.; Grootens, J.; Lof, L.; Amini, R.M.; Hagberg, H.; Ungerstedt, J.S.; Olsson-Stromberg, U.; Nilsson, G. KIT signaling is dispensable for human mast cell progenitor development. Blood 2017, 130, 1785–1794. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Oderup, C.; LaJevic, M.; Butcher, E.C. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J. Immunol. 2013, 190, 6126–6134. [Google Scholar] [CrossRef] [PubMed]
- Halleskog, C.; Dijksterhuis, J.P.; Kilander, M.B.; Becerril-Ortega, J.; Villaescusa, J.C.; Lindgren, E.; Arenas, E.; Schulte, G. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J. Neuroinflamm. 2012, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Halleskog, C.; Mulder, J.; Dahlstrom, J.; Mackie, K.; Hortobagyi, T.; Tanila, H.; Kumar Puli, L.; Farber, K.; Harkany, T.; Schulte, G. WNT signaling in activated microglia is proinflammatory. Glia 2011, 59, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef]
- Dijksterhuis, J.P.; Petersen, J.; Schulte, G. WNT/Frizzled signalling: Receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br. J. Pharmacol. 2014, 171, 1195–1209. [Google Scholar] [CrossRef]
- Willert, K.; Brown, J.D.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R., 3rd; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452. [Google Scholar] [CrossRef]
- Schulte, G.; Bryja, V.; Rawal, N.; Castelo-Branco, G.; Sousa, K.M.; Arenas, E. Purified Wnt-5a increases differentiation of midbrain dopaminergic cells and dishevelled phosphorylation. J. Neurochem. 2005, 92, 1550–1553. [Google Scholar] [CrossRef]
- Halleskog, C.; Schulte, G. Pertussis toxin-sensitive heterotrimeric G(alphai/o) proteins mediate WNT/beta-catenin and WNT/ERK1/2 signaling in mouse primary microglia stimulated with purified WNT-3A. Cell. Signal. 2013, 25, 822–828. [Google Scholar] [CrossRef]
- Skronska-Wasek, W.; Gosens, R.; Konigshoff, M.; Baarsma, H.A. WNT receptor signalling in lung physiology and pathology. Pharmacol. Ther. 2018, 187, 150–166. [Google Scholar] [CrossRef]
- Koopmans, T.; Gosens, R. Revisiting asthma therapeutics: Focus on WNT signal transduction. Drug Discov. Today 2018, 23, 49–62. [Google Scholar] [CrossRef] [PubMed]
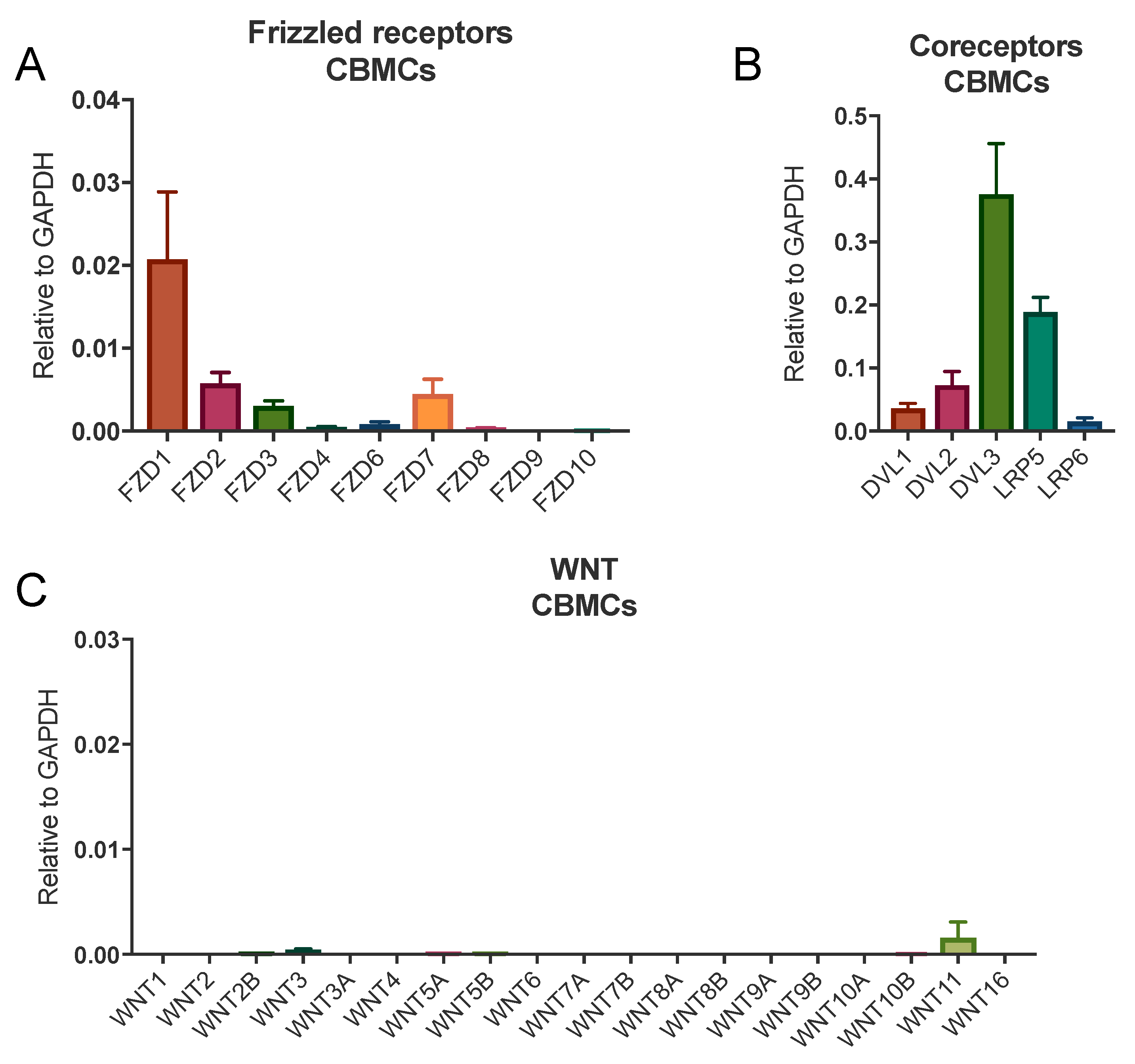

| Gene Name | Donor 1 | Donor 2 | Donor 3 | Average |
|---|---|---|---|---|
| FZD1 | 304 | 176 | 151 | 210 |
| FZD2 | 58 | 8 | 8 | 25 |
| FZD3 | 369 | 137 | 301 | 269 |
| FZD4 | 4 | 6 | 2 | 4 |
| FZD5 | 75 | 96 | 23 | 65 |
| FZD6 | 16 | 8 | 16 | 13 |
| FZD7 | 225 | 59 | 42 | 109 |
| FZD8 | 12 | 2 | 2 | 5 |
| FZD9 | 2 | 0 | 0 | 1 |
| FZD10 | 0 | 0 | 0 | 0 |
| DVL1 | 43 | 33 | 48 | 41 |
| DVL2 | 193 | 156 | 206 | 185 |
| DVL3 | 723 | 331 | 408 | 488 |
| LRP5 | 35 | 16 | 18 | 23 |
| LRP6 | 82 | 104 | 81 | 89 |
| WNT1 | 2 | 0 | 0 | 1 |
| WNT2 | 2 | 2 | 0 | 1 |
| WNT2B | 17 | 23 | 12 | 17 |
| WNT3 | 1 | 2 | 5 | 2 |
| WNT3A | 0 | 0 | 0 | 0 |
| WNT4 | 1 | 5 | 1 | 2 |
| WNT5A | 7 | 2 | 0 | 3 |
| WNT5B | 6 | 2 | 1 | 3 |
| WNT6 | 3 | 0 | 0 | 1 |
| WNT7A | 1 | 0 | 3 | 1 |
| WNT7B | 0 | 0 | 2 | 1 |
| WNT8A | 0 | 0 | 0 | 0 |
| WNT8B | 0 | 0 | 0 | 0 |
| WNT9A | 0 | 0 | 0 | 0 |
| WNT9B | 0 | 0 | 0 | 0 |
| WNT10A | 10 | 0 | 2 | 4 |
| WNT10B | 1 | 5 | 2 | 2 |
| WNT11 | 145 | 131 | 26 | 101 |
| WNT16 | 1 | 0 | 5 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tebroke, J.; Lieverse, J.E.; Säfholm, J.; Schulte, G.; Nilsson, G.; Rönnberg, E. Wnt-3a Induces Cytokine Release in Human Mast Cells. Cells 2019, 8, 1372. https://doi.org/10.3390/cells8111372
Tebroke J, Lieverse JE, Säfholm J, Schulte G, Nilsson G, Rönnberg E. Wnt-3a Induces Cytokine Release in Human Mast Cells. Cells. 2019; 8(11):1372. https://doi.org/10.3390/cells8111372
Chicago/Turabian StyleTebroke, Julia, Joris E. Lieverse, Jesper Säfholm, Gunnar Schulte, Gunnar Nilsson, and Elin Rönnberg. 2019. "Wnt-3a Induces Cytokine Release in Human Mast Cells" Cells 8, no. 11: 1372. https://doi.org/10.3390/cells8111372
APA StyleTebroke, J., Lieverse, J. E., Säfholm, J., Schulte, G., Nilsson, G., & Rönnberg, E. (2019). Wnt-3a Induces Cytokine Release in Human Mast Cells. Cells, 8(11), 1372. https://doi.org/10.3390/cells8111372





