Abstract
Liver fibrosis is a pathophysiologic process involving the accumulation of extracellular matrix proteins as collagen deposition. Advanced liver fibrosis can evolve in cirrhosis, portal hypertension and often requires liver transplantation. At the cellular level, hepatic fibrosis involves the activation of hepatic stellate cells and their transdifferentiation into myofibroblasts. Numerous pro-fibrogenic mediators including the transforming growth factor-β1, the platelet-derived growth factor, endothelin-1, toll-like receptor 4, and reactive oxygen species are key players in this process. Knowledge of the cellular and molecular mechanisms underlying hepatic fibrosis development need to be extended to find novel therapeutic strategies. Antifibrotic therapies aim to inhibit the accumulation of fibrogenic cells and/or prevent the deposition of extracellular matrix proteins. Natural products from terrestrial and marine sources, including sulfur-containing compounds, exhibit promising activities for the treatment of fibrotic pathology. Although many therapeutic interventions are effective in experimental models of liver fibrosis, their efficacy and safety in humans are largely unknown. This review aims to provide a reference collection on experimentally tested natural anti-fibrotic compounds, with particular attention on sulfur-containing molecules. Their chemical structure, sources, mode of action, molecular targets, and pharmacological activity in the treatment of liver disease will be discussed.
1. Introduction
Liver fibrosis is a pathological process that leads to an excessive accumulation of extracellular matrix (ECM) proteins and the loss of the physiological liver tissue architecture [1]. The scar tissue produced in excess and/or not appropriately recovered alters the architecture and limits the proper functioning of the liver (Figure 1). This process is caused by different and repeated insults to the liver like chronic inflammatory lesions, chronic viral hepatitis (hepatitis B or C), bacterial (brucellosis), fungal and parasitic infections (echinococcosis), inborn errors of metabolism, lipid accumulation, iron overload, intense and prolonged use of some drugs (methotrexate, isoniazid, oxyphenisanthin, methyldopa, chlorpromazine, tolbutamide, and amiodarone), and exposure to toxic agents (alcohol) (Figure 2).
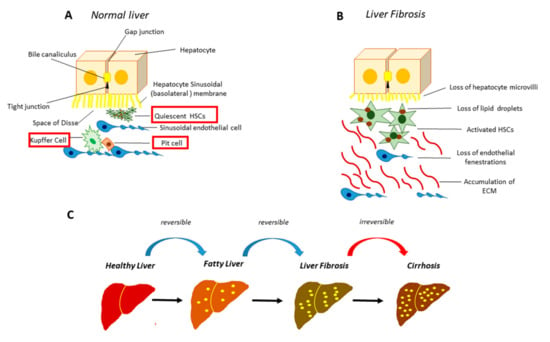
Figure 1.
Normal and fibrotic liver architecture. (A) In the healthy liver the hepatocytes are closely joined by gap junctions. The liver sinusoid, a large fenestrated capillary, is mainly constituted by sinusoidal endothelial cells (SECs), Kupffer cells, specialized hepatic macrophages, and the Pit cells, the natural killer lymphocytes of the liver. The space between the hepatocytes and the sinusoid, called “the space of Disse”, hosts the hepatic stellate cells (HSCs); (B) following HSCs activation, there is a massive accumulation of extracellular matrix (ECM) as well as the loss of microvilli in the hepatocytes and the loss of fenestrae in SECs; (C) stages of hepatic fibrosis.
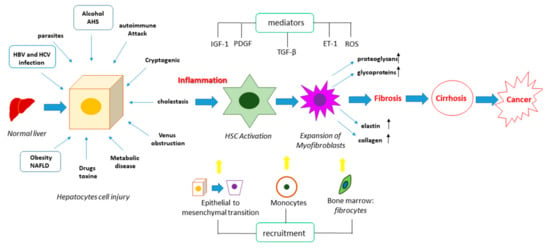
Figure 2.
Pathogenesis of liver fibrosis. Liver fibrosis can be induced by different insults, which can trigger cell death processes in the hepatocytes, leading to the release of pro-fibrogenic mediators such as the transforming growth factor beta (TGF-β), the platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), endothelin 1 (ET-1), and reactive oxygen species (ROS). These can activate HSCs, promoting their differentiation into myofibroblasts, as well as they can stimulate the epithelial to mesenchymal transition and the recruitment of circulating monocytes and fibrocytes from bone marrow. The combination of these events leads to an increased expression and secretion of elastin, collagen, proteoglycans and glycoproteins, causing the accumulation of the ECM and therefore the fibrotic process. This represents a prerequisite for cirrhosis and cancer.
There is also a congenital form of hepatic fibrosis, an autosomal recessive disorder that primarily affects the hepatobiliary and renal system [2]. Liver fibrosis can regress in the initial stages, if the causative agent is removed, on the contrary, it can lead to cirrhosis and liver failure [3,4]. Among the pathological factors, chronic hepatitis C and B infection and alcoholic and non-alcoholic steatohepatitis are the main causes of chronic progressive liver disease, leading in 20–40% of cases to the onset of liver cirrhosis. From the anatomic-pathological point of view, cirrhosis is an advanced stage of fibrosis, characterized by the formation of regenerative nodules of hepatocytes separated by fibrous septa [4]. The cirrhotic process is associated with the necrosis of hepatocytes, the collapse of the reticular support system with consequent deposition of connective tissue, the subversion of the vascular bed, and the nodular regeneration of the remaining parenchyma. This process can take years or decades to develop, therefore a correct staging and an adequate pharmacological therapy of fibrosis are extremely important, not only for the individual prognostic evaluation of the patient, but also for the prevention of disease progression towards cirrhosis and hepatobiliary cancer [5,6].
Therefore, hepatic fibrosis has become a global health problem, associated with a high morbidity and mortality due to the development of several complications, such as portal hypertension and/or consequences such as spontaneous bacterial peritonitis, hepato-renal syndrome, hepato-pulmonary syndrome, cirrhosis and hepatocellular carcinoma [7,8].
To date, liver biopsy is the gold-standard method for the diagnosis of liver fibrosis. The histological examination is useful both to identify the etiology of the liver pathology, to establish the extent of the necrotic-inflammatory lesions and to evaluate the stage of the disease towards cirrhosis. In this scenario, particular attention should be paid to profibrogenic growth factors, cytokines, mediators and cells upstream to the activation of hepatic stellate cells (HSCs) [9]. This review provides the “state of the art” on molecular mechanisms underlying liver fibrosis and the pharmacological potential of natural sulfur-containing compounds as an alternative therapeutic strategy, in order to define new directions in the fight against chronic liver diseases. Since these compounds are powerful antioxidants, due to the unique chemical properties of sulfur atoms, the relationship among reactive oxygen species (ROS)/nitric oxide (NO) production, liver fibrosis and the protective role of sulfur compounds, has been highlighted.
2. Molecular Mechanisms Underpinning Liver Fibrosis
It is well recognized that elevated intracellular concentrations of ROS induce redox imbalance in the liver and are involved in the apoptotic and necrotic process of the hepatocytes. ROS can stimulate the production of pro-fibrogenic mediators by Kupffer cells and the recruitment of circulating inflammatory cells, leading to direct activation of the HSCs [10], involved in the onset of the fibrotic process and its reversion [11,12]. While oxidative stress is a key player in triggering liver damage, finely regulated levels of glutathione (GSH), the most abundant antioxidant thiol in the cell, contribute to maintaining a healthy liver, preserving its functionality [13]. GSH is synthesized inside the cell and partially released in the extracellular space along a gradient concentration. In the extracellular space, GSH is hydrolyzed by γ-glutamyl transpeptidase (GGT), a dimeric enzyme located on the membrane surface [14]. GGT is highly expressed in the liver, where it is responsible for GSH metabolism and detoxification mechanisms [14,15], together with glutathione S-transferases (GSTs), a family of phase II metabolic isozymes, known for their ability to catalyze the conjugation of the reduced form of GSH—via the sulfhydryl group—to electrophilic centers of xenobiotic substrates, making them more water-soluble and more sensitive to detoxification [16].
Oxidative stress in the liver plays a key role in the activation of the pleiotropic cytokine transforming growth factor beta 1 (TGF-β1). TGF-β1 is secreted as a latent complex, and only following cleavage (by proteases), denaturation (by pH modifications), or structure modification (by ROS) of the associated protein latency associated peptide, it can interact with the receptor and activate the TGF-β1 dependent signaling cascades. The TGF-β1 pathway strongly contributes to HSCs activation by modulating the expression and secretion of numerous proteases and their regulators (Figure 3) [17]. TGF-β1 can also self-induce its production thus amplifying its functions [18]. In detail, the TGF-β pathway is activated upon the interaction of type II TGF-β receptor (TGF-β RII) with the ligand. This interaction triggers the phosphorylation of type I TGF-β receptor (TGF-β RI), thus stimulating a signal cascade of phosphorylation events on small mother against decapentaplegic proteins (SMAD) leading to the formation of a complex, which migrates into the nucleus and acts as a transcription factor of several fibrogenic genes. ROS play key roles also in mediating concomitant events, such as inflammation and lipid metabolism dysregulation. These events can be activated by stressful insults including radiations and lead to the rising of the fibrogenic process, mainly through the interplay between the TGF-β pathway and wingless/integrated (Wnt) signaling, which positively regulate each other, while PPAR γ expression decreases and fails to reduce TGF-β for the opposite interplay of the canonical Wnt/β-catenin pathway [19,20]. HSCs proliferation, differentiation and migration can also be regulated by the platelet-derived growth factor (PDGF) signaling [21]. Following the interaction with the eterodimeric receptor, PDGF signaling activates a variety of downstream cascades, including phosphatidylinositol 3-kinase (PI3K)/protein Kinase B (Akt), ras homolog gene family, member A (RhoA)/Rho-associated protein kinase (ROCK), janus kinase 1 (JAK)/signal transducers of activated transcription (STAT), Ras/Raf leading to the activation of several important transcription factors as the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB), STAT1/3 and extracellular signal-regulated kinases 1 and 2 (Erk1/2), which enhance the expression of genes involved in survival, migration and ECM production.
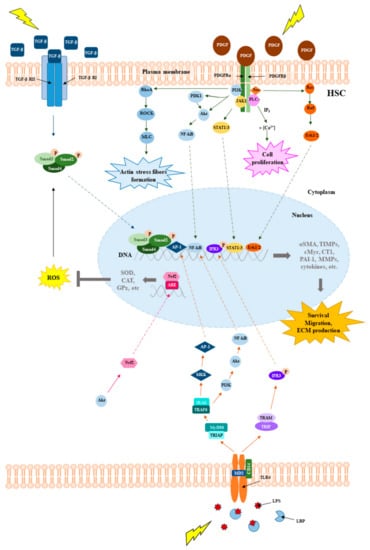
Figure 3.
Schematic representation of the main molecular pathways underlying chronic liver disease. Comparison of the main molecular pathways involved in the pathogenesis of liver disease: TGF-β, PDGF and TLR4 signaling.
The toll like receptor 4 (TLR4) pathway can additionally mediate the inflammatory response, fibrogenesis and survival processes [22] (Figure 3). The impairment of the intestinal barrier, presumably caused by the alteration of the gut microbioma, can allow the microbes to reach the liver through the hepatic portal system, and lipopolysaccharide (LPS) can activate the TLR4 signaling. The activated downstream cascades, myeloid differentiation primary response protein (MyD88)/mitogen-activated protein kinase kinase (MKK)/PI3K and TIR-domain-containing adapter-inducing interferon-β (TRIF)/interferon regulatory factor 3 (IFR3) contribute to the onset of chronic liver disease.
Once HSCs are activated, they show an increase in proliferation, migration, and contact-ability as well as an increase in resistance to apoptosis. At the molecular level, they show a greater expression of smooth muscle actin (α-SMA) and procollagen-I [23], which promote the ability of activated HSCs to deposit collagen and other proteins of matrix in the extracellular space. Furthermore, the activation of HSCs implies an altered regulation of matrix remodeling, involving enzymes such as tissue transglutaminase (tTG), matrix metalloproteinases (MMPs), and their inhibitors (TIMPs) [24]. Among the other factors affecting liver fibrosis is endothelin 1 (ET-1), a peptide widely distributed in the liver, which induces HSC proliferation [25] and the decrease of insulin-like growth factor (IGF), involved in the differentiation, proliferation, and apoptosis of hepatocytes [26]. Consequently, all the cell types, the signaling pathways, and the molecules that play a critical role for the appearance, progression, and reversal of fibrosis can be considered targets for a possible therapy.
3. Natural Sulfur-Containing Compounds for the Treatment of Liver Fibrosis
Despite the increasing understanding of the molecular landscape underlying the pathogenesis of chronic liver diseases, including the inflammation and fibrosis processes, to date there are no effective treatments for hepatic diseases. The removal of the causal agent, such as viruses and parasites but also drugs/toxins, represents the first line of defense against liver related pathologies, but this is often not sufficient to cure advanced stages of fibrosis. The main therapeutic strategies include ROS scavenging, inhibition of hepatic damage, anti-inflammatory action, deactivation and elimination of the cells responsible for the ECM production and degradation, inhibition of cytokine signaling, restoration of the intestinal microbioma, and restoration of the blood flow [27]. In addition to the standardized clinical treatment guidelines from professional associations such as the American Association for the Study of Liver Disease or the European Association for the Study of the Liver, complementary and/or alternative therapies, including the use of natural compounds can offer a good strategy in the cure against chronic liver diseases [27]. In this context, it is notable to identify liver specific bioactive compounds, with negligible immunogenicity and side effects, produced in great amounts at low costs [28,29]. There is a large number of bioactive compounds with documented anti-inflammatory and anti-fibrotic properties, including molecules isolated from the marine environment, although only few marine compounds are currently accepted as drugs in the market [30,31]. Several studies have shown the efficacy of different natural products and phytochemicals present in food and used as food extracts (such as sulforaphane, S-allycysteine, curcumin, proanthocyanidins, garlic extract, ovothiol, coffee, grape skin, or seeds) for preventing or reducing the progression of liver fibrosis with different mechanisms of action and in different animal models. In particular, the use of the numerous antioxidants containing sulfur, able to induce an appreciable/consistent reversion of the fibrotic phenotype in murine models of liver fibrosis, should be highlighted [32,33,34,35,36,37,38].
Sulfur containing low molecular weight compounds are widespread in nature from bacteria to plants, fungi, and animals, playing essential roles in the biology of the cells. Indeed, sulfur atoms can be involved in a great variety of reactions due to their unique chemical properties, as the high number of oxidation states which can form different chemotypes with different functions, including redox activity, metal-binding, and catalysis. In particular, sulfur redox activities are ascribed to its great reactivity against ROS and NO, through the formation of radicals, preventing from oxidative damage, and nitrosothiols, key mediators in NO signaling, having a physiological and therapeutic impact in many tissues, including liver [39,40]. Then, the properties of sulfydryl groups confer major biological activities to the sulfur-containing natural products [41,42]. The relevance of sulfydrilic groups in therapeutic compounds is also highlighted by the finding that a gas as hydrogen sulfide (H2S) regulates fundamental processes in the liver, such as mitochondrial functionality, sensitivity to insulin, and macronutrients metabolism, and is involved in the pathogenesis and treatment of several liver diseases, including hepatic fibrosis, cirrhosis, and cancer [43].
3.1. Glutathione
The most important and widespread low molecular weight sulfur-containing compound in the cells of plants, animals, fungi, bacteria, and archaea is GSH. It is a tripeptide composed of three amino acids: L-cysteine, L-glutamic acid, and glycine, characterized by an unusual gamma peptide linkage between the carboxyl group of the glutamate side chain and the α-amino-group of cysteine (Figure 4A). This unusual gamma amide linkage protects GSH from the hydrolysis by peptidases in the blood [15]. In animal cells, GSH ranges from 0.5 to 10 mM and it is present both in the cytosol and the organelles, both in the reduced (GSH) and oxidized form (GSSG). In the reduced form, the thiol group of cysteinyl residue is able to donate a reducing equivalent to an oxidized acceptor, thus acting as an antioxidant. In healthy cells and tissues, the ratio of GSH versus the disulfide form GSSG is in favor of the reduced form to maintain the reducing intracellular environment and the correct functioning of proteins and enzymes [44]. Therefore, an increased GSSG/GSH ratio is indicative of oxidative stress. GSSG can be converted to the reduced state by glutathione-reductase using NADPH as acceptor of electrons.
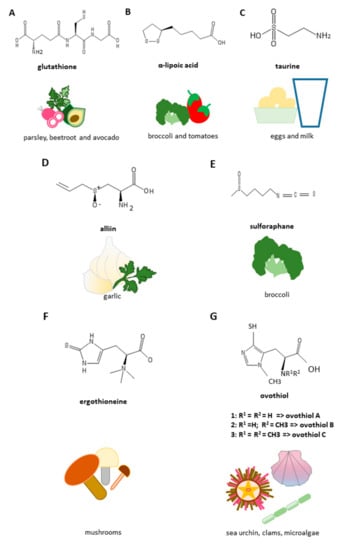
Figure 4.
Structures and richest food sources of anti-fibrotic sulfur-containing compounds. (A) Glutathione; (B) α-lipoic acid; (C) taurine; (D) alliin; (E) sulforaphane; (F) ergothioneine; (G) ovothiol.
Besides neutralizing radicals and peroxides, GSH participates in thiol protection and redox regulation of cellular proteins under oxidative stress by protein S-glutathionylation, a redox-regulated post-translational thiol modification, as well as it represents a natural storage of intracellular NO through the formation of S-nitrosoglutathione [44,45,46]. Although all animal cells produce GSH, its synthesis in the liver has been shown to be essential. Indeed, knockout mice of the enzyme responsible for GSH synthesis die within a month of life due to the absence of hepatic GSH production. Reduced levels of GSH in the cell lead to the appearance of liver diseases. In particular, GSH treatment has shown very promising recovering activities from liver damage induced by oxidative stress in alcohol and non-alcoholic liver diseases [13]. It is not surprising that GSH has been tested as supplement for its detoxification and antioxidant/ROS scavenging activities, being the oxidative stress one of the main pathogenic factors in liver diseases. Parsley, beetroot, spinach, avocados, and asparagus are some of the richest dietary sources of GSH. However, dietary GSH is poorly absorbed by the human body due to the absence of a specific carrier on cell membranes [47]. Although oral administration of GSH improves hepatic metabolism in patients with non-alcoholic liver disease (NAFLD), systemic bioavailability of GSH is low, while the direct administration as intravenous injection in patients suffering from liver disease, results in a significant improvement of liver function even several months after stopping treatment. Since direct supplementation of GSH is not always successful, supply of its sulfur amino acid component, i.e. cysteine, or its derivatives may be more effective at increasing GSH levels. For example, carbon tetrachloride (CCl4)-induced liver fibrosis is attenuated in rats following administration of selenium-glutathione-enriched probiotics by up-regulating the hepatic silent information regulator 1 (SIRT1) and the consequent activation of the signaling cascade, leading to reduction of oxidative stress and endoplasmic reticulum (ER) stress, as well as to the inhibition of mitogen-activated protein kinase (MAPK) signaling [48]. GSH structurally related compounds, as N-acetyl-L-cysteine (NAC), S-Nitroso-N-acetylcysteine (SNAC), S-adenosyl-L-methionine (SAM), and S-allylcysteine (SAC) have been used in clinics to treat liver fibrosis, being precursors for glutathione synthesis and also possessing hepatoprotective roles (reviewed in [30]).
3.2. Lipoic Acid
Lipoic acid (or α-lipoic acid, ALA) is a natural organosulfur compound, introduced through the diet by consuming broccoli, tomatoes, spinach, salads, cabbage, peas, brewer’s yeast, brown rice, and meat (Figure 4B) [49]. Unlike other antioxidants, it can be both fat-soluble and water-soluble, therefore it acts and performs its function on a considerable number of free radicals, both inside and outside the cell. It possesses two sulfur atoms that form a redox-sensitive disulfide bond, and several studies have reported ALA antifibrotic properties. Indeed, the reduced form of dihydrolipoic acid has been shown to modulate the ROS-triggered signaling in previously activated HSCs during thioacetamide-induced hepatic fibrosis [50]. In a rat model of bile duct ligation (BDL)-induced liver fibrosis ALA downregulated the hepatic inhibitor-1 of the plasminogen activator inhibitor-1 (PAI-1) expression by inhibiting the TGF-β signaling mediators, like Smad3, activator protein-1 (AP1), and specificity protein 1 (Sp1) [51]. Yet, in a CCl4-induced liver fibrosis model ALA prevented collagen deposition and oxidative stress, as well as it modulated the expression of the pro-inflammatory cytokine interleukin-6 (IL-6), the inducible nitric oxyde synthase (iNOS), the NF-kB, and the MMP-13 [52]. ALA also provided protection from liver ischemia-reperfusion injury [53] or from tissue damage induced with different treatments, including lipopolysaccharide/D-galactosamine/methotrexate injection [54,55,56,57,58,59], bisphenol-A oral administration [60], a high fat or fructose rich diet [61,62,63] or methionine–choline deficiency [64]. Furthermore, the treatment with ALA of rats with hepatic fibrosis resulted in a reduction in the activity of aspartate transaminase (AST) and alanine transaminase (ALT), improveed hepatic injury by decreasing the deposition of collagen fibers, and restoreed the Akt/mammalian target of rapamycin (mTOR) pathway. All this is possible thanks to its ability to inhibit the TGF-β/Smad3 pathway with consequent suppression of autophagy [65]. In addition, clinical trials consisting in ALA supplementation in patients with NAFLD have reported a modulation of some of the oxidative stress related parameters, including serum malondialdehyde levels and total antioxidant status, as well as a modification of the adipokine profile, known to play a key role in the progression of NAFLD [66,67].
3.3. Taurine
Taurine is an amino sulfonic acid, also referred as an essential amino acid for many species, while for humans is conditional. We basically assume taurine by diet. It is present in eggs, fish, meat, and milk, but not in foods of plant origin, and seafood is considered the richest source (Figure 4C). Taurine-based supplements have also been developed due to several therapeutic applications [68]. In adults, taurine can be synthesized in the liver starting from cysteine and methionine in the presence of sufficient amounts of vitamin B6. Taurine is involved in many cellular biological activities such as bile acid conjugation, cell membrane stabilization, and calcium signaling. In vitro and in vivo studies reported that taurine treatment of HSCs isolated from healthy rats significantly inhibited proliferation, reduced ROS dependent damage, like lipid peroxidation, and provided protection from fibrogenesis, while rats with CCl4-induced liver damage supplemented with taurine were protected from liver histological damage and fibrosis, showing a decrease of oxidative stress markers and fibrogenic factors [69,70]. In addition, taurine protective action seems to be especially displayed in the pericentral region of the liver, where taurine transporter is mostly expressed and this amino acid may be useful against xenobiotics-induced hepatic damages presumably interfering with the NADPH dependent cytochrome P450 2E1 catabolic activity [71]. In vivo studies have reported that taurine administration provides protection against alcohol-induced liver damage in rats by lowering the levels of circulating transaminases and inflammatory cytokines and increasing the hepatic total protein and antioxidant defense, as well as by modulating HSC activation and collagen deposition [72,73]. More recently, the molecular mechanisms of the taurine hepatoprotective activity against alcohol induced damage has been clarified and, in particular, the inhibition of TLR4/MyD88 and NF-κB signaling have been identified as the target pathways [74]. Taurine administration also accelerates alcohol and lipid metabolism in alcoholic fatty liver disease [75]. In addition, taurine reduces ROS damage and increases antioxidant defense in liver previously injured by iron-overloading, resulting in anti-fibrotic and anti-apoptotic properties [76,77]. Moreover, taurine has the ability to attenuate portal hypertension in rats with induced cirrhosis and in cirrhotic patients by acting on vasodilatation through the regulation of NO synthesis [78,79]. Yet, the therapeutic potential of taurine is extended to the diet induced NAFLD showing anti-apoptotic and antioxidant activities in in vitro and ex vivo models and, in addition, a modulation of energetic metabolism in in vivo experiments [80].
3.4. Garlic Derived Sulfur Compounds
Garlic (Allium sativum) is a member of the family Liliaceae, having a huge number of bioactive properties mainly due to its content in organosulfur compounds, which are able to regulate the expression of a wide variety of genes, including the inducible NOS, with a strong impact for human health [81]. The main sulfur compound present in fresh garlic is alliin (Figure 4D) which is metabolized to allicin upon the action of the enzyme allinase when garlic is crushed or cut. Allicin is then metabolized in other compounds, including diallyl sulphide (DAS), di- (DADS) and trisulfide. Intraperitoneal injections of garlic extract in rats with CCl4-induced liver damage elicit an anti-fibrotic effect by modulating HSCs activation, increasing degradation of ECM and leading to liver tissue regeneration and the re-establishment of its function mainly through inhibition of TGF-β1 signaling pathway [33]. SAC, a key component of aged garlic extract, has shown anti-fibrotic properties in liver-damaged rats previously injected with porcine serum, mainly through inhibition of HSCs activation [36]. SAC has been shown to be an endogenous donor of H2S, which plays key roles in the gastrointestinal tract and in the liver. It has been reported that SAC decreases the expression of transaminase levels in rats in which hepatic damage was induced by treatment with CCL4, at the same time leading to a decrease in the levels of gene expression of the main actors of liver damage, such as TGF-β1, tTG, α-SMA, fibronectin, and collagen I [33]. An interesting property of garlic extract is the ability to block the enzymatic activity of tTG; this prevents the covalent stabilization of the fibrils making the matrix more resistant to degradation by specific collagenases [32]. Furthermore, SAC reduced the phosphorylation of SMAD3, signal transducers and transcriptional activators and further inhibited their ability to bind to transcription promoters. Taken together, SAC administration attenuated CCl4-induced liver fibrosis in rats with antioxidant, anti-inflammatory, and antifibrotic effects targeting the STAT3/SMAD3 pathway to inhibit gene transcription [82]. DADS showed protective properties from Trichloromethane-induced liver damage in rats by inhibiting NFkB activation and apoptotic processes and increasing the antioxidant hepatic defenses [83]. Moreover, the treatment with S-allyl-glutathione (SAG) of mice with hepatic fibrosis leads to a dose-dependent decrease in heat shock protein-47 (Hsp47), a specific collagen chaperone and other fibrosis markers. SAG acts also directly on a mannose receptor present on Kupffer cells (hepatic macrophages), thereby indirectly inhibiting the activation of HSCs [84].
The sulfur atoms in these molecules seem to be important for the liver functioning. Indeed, the three major sulfur containing garlic components (diallyl sulfide, disulfide, and trisulfide) have been shown to have a stronger modulatory activity on the hepatic detoxification system along with the increase of the number of sulfur atoms [85]. Many other studies have reported the hepatoprotective properties of garlic and garlic-derived compounds mainly thanks to their ROS scavenging and anti-inflammatory activities [86].
3.5. Sulforaphane
Sulforaphane (SFN) is a dietary isothiocyanate obtained by the enzymatic processing of glucopharanin, a 4-methylsulfinylbutyl glucosinolate, present in cruciferous vegetables, as broccoli and cabbage (Figure 4E). SFN has been shown to protect from BDL-induced liver fibrosis in mice by modulating the nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated inhibition of the TGFβ/Smad signaling pathway, with consequent inhibition of HSCs activation and fibrosis [34]. SFN oral administration had hepatoprotective properties in mice with CCl4-induced liver damage leading to a decrease of ALT serum levels, inhibition of necrotic processes and ROS-induced lipid peroxidation, as well as to an induction of phase 2 detoxification enzymes, including GST [87]. SFN also provides protection from hepatotoxicity induced with a variety of insults, such as cisplatin and LPS, preserving mitochondrial functioning [88] and targeting specific pathways, including MyD88 and anti-TLR-associated activator of interferon (TRIF)-dependent signaling pathways of the TLR system [89]. Feng et al. [90] have investigated the possibility that SFN could inhibit the HSC activation by interfering with microRNAs, which play a key regulative role in the development of liver fibrosis. They demonstrated that SFN is able to downregulate miRNA-423-5p in LX-2 primary human HSC line interfering with their activation. Interestingly, a preclinical phase study reported that CCl4-induced liver fibrosis in rats was prevented by the combination of capsaicin (CPS) and SFN via oral gavage by up-regulating PPARγ and Nrf2 [91]. The doses they used were safe for consumption and could be easily assumed with foods in humans, suggesting the use of functional foods containing these bioactive compounds (chili peppers for CPS and, as mentioned before, cruciferous vegetables for SFN) for prevention of hepatic diseases, although further studies are needed to find the optimal concentrations for use in humans.
3.6. Sulfur-Containing Histidines
Sulfur-containing histidines are amino acid derivatives containing a thiol group on the imidazole ring. They can be distinguished based on the position of such substitution in 2-thiohistidines and 5-thiohistidines. Ergothioneine (ERG), first identified from the ergot fungus Claviceps purpurea, is a trimethyl-L-histidine, characterized by the thiol group on the carbon 2 of the imidazole ring of histidine and three methyl groups on the side chain. The biosynthesis of ERG has been only observed in Actinobacteria (for example, Mycobacterium spp.) and some fungi. In humans it is obtained from dietary sources, mainly mushrooms, and can be accumulated in human tissues such as liver and blood, spleen, kidneys, lungs, heart, intestine, at relatively high concentrations (Figure 4F) [92,93], thanks to its high affinity to the organic cation transporter 1 (OCTN1) [94]. Indeed, the silencing of OCTN1 encoding gene has been shown to prevent ERG absorption in murine tissues [95]. The physiological functions of ERG have not been fully clarified yet. However, several in vitro studies have shown the ability of ERG to eliminate hydroxyl radicals, hypochlorous acid, peroxynitrite [96], singlet oxygen [97], modulates inflammatory responses [98,99,100] and protects against UV and gamma rays [101].
ERG accumulation in tissues suffering a high oxidative stress situation has been proposed to have a protective role. Indeed, induced OCTN1 upregulation and consequent ERG increasing uptake and accumulation in liver of a guinea pig model of NAFLD have been proved to exert hepatoprotective roles both by an antioxidant action, through hsp70 modulation, and by chelating Fe2+ with consequent inhibition of Fenton chemistry [102]. The hsp70 upregulation by ERG has resulted in being protective also for liver injured by ischemia reperfusion leading to a reduction of lipid peroxidation [103]. Moreover, functional studies have demonstrated that OCTN1 expression has a protective role in in vitro and in vivo liver fibrosis models through ERG uptake regulation and consequent gene downregulation of NADPH oxidase 4, a NOX isoform highly expressed in hepatocytes and HSCs, with reduction of oxidative stress and fibrosis [104].
Ovothiols are methyl-5-thiohistidines, having the thiol group in position 5 and the methyl group in position 2 of the imidazole ring of histidine. They can be distinguished in three forms based on the presence/absence of additional methyl groups on the nitrogen group of the side chain: ovothiol A with no additional methyl groups, ovothiol B having one methyl, ovothiol C having two methyl groups [105]. Ovothiols can be synthesized starting from cysteine and histidine in the presence of iron and oxygen thanks to the catalysis of the sulfoxide synthase OvoA and the beta-lyase OvoB [106,107,108]. They are powerful antioxidants, widespread in the marine environment, and most abundant in sea urchin eggs, the richest food source available [109] (Figure 4G). Other marine sources of ovothiols are clams, holoturians, and microalgae [108]. The unique position of the sulfur atom in these molecules confers them a stronger ROS scavenging activity compared to other thiols, like GSH and ERG, protecting also from hydroperoxides and peroxynitrite-induced damage [105]. Ovothiol A has been shown to induce autophagy in a human liver cancer cell line, HepG2 [110]. It also inhibits collagen deposition in a CCl4-induced liver fibrosis murine model, by modulating fibrogenic markers, such as TGF-β, α-SMA, and TIMP-1, as well as by regulating the activity of GGT, a key enzyme involved in the evolution of liver fibrosis [38]. In particular, ovothiol directly inhibits membrane bound GGT activity in human cancer cells, including hepatocarcinoma cells [111]. Consequently, ovothiol exerts its hepatoprotective action by regulating GGT activity and GSH levels.
4. Conclusions and Perspectives
To date there are no drugs for the treatment of liver fibrosis whose causes can be different, mainly chronic alcohol or drug abuse, viral hepatitis B or C, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), a subtype of NAFLD. Therefore, the best way to reduce liver fibrosis consists in eliminating alcohols and drugs, treatment with antiviral drugs for hepatitis B and C, and an appropriate nutrition and lifestyle in the case of NAFLD and/or NASH. Integrating the diet with natural compounds, that have demonstrated antifibrotic properties, and their pharmacological administration, together with antiviral treatment of hepatitis or other insult-specific therapies, can be considered valid therapeutic strategies to regress fibrosis that otherwise could have much more serious consequences. For example, there are several clinical trials for patients with NAFLD that involve the oral administration of sulfur-containing compounds such as GSH, ALA, and taurine [47,66,67,79].
These compounds are pleiotropic molecules acting as ROS and NO scavengers, thiol/disulfide exchange modulators. Thanks to these properties they can regulate several redox-sensitive pathways like TGF-β, PDGF, and TLR pathways, involved in the progression of fibrosis (Figure 5).
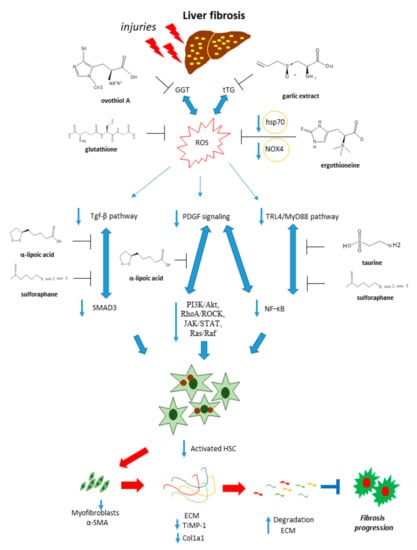
Figure 5.
Antifibrotic properties of sulfur-containing compounds. Sulfur-containing compounds are able to interfere with molecular pathways associated with liver fibrosis, such as TGF-β, PDGF, and TLR4/MyD88 signaling, leading to the inhibition of HSCs activation and the fibrogenesis process.
Some of these natural sulfur compounds can be efficiently acquired by diet through specific membrane transporters as known for ERG and taurine. Others, such as ovothiols of marine origin, have been only recently receiving attention as more potent antioxidants and anti-inflammatory compounds. Several patents have been developed to treat and prevent liver fibrosis through the supplementation of sulfur-containing molecules (Table 1). The most effective compounds seem to be ALA, SAC, and SFN. Ovothiol exerts its anti-fibrotic activity in mice affected by liver fibrosis at doses comparable to ALA, SAC and SFN (Table 1). Future studies need to explore the potential of these small sulfur-containing compounds as dietary supplements and/or new marine drugs for the treatment of liver diseases in humans. Moreover, the discovery of new natural sulfur-containing molecules in the future would help to face the challenge for advanced clinical trials. We expect the discovery of such novel compounds from the ocean, which has recently attracted the interest of the scientific community as a still poorly explored source of food and medicines for mankind.

Table 1.
Therapeutic efficacy of sulfur-containing compounds in liver fibrosis models. The name of compounds, the experimental models, the doses, and the related patents are reported.
Author Contributions
Conceptualization, I.C., A.M., M.B.; methodology, A.M., G.D., M.B.; investigation, I.C., A.M., M.B., G.D.; resources, I.C.; data curation, I.C., A.M., M.B.; project administration, I.C.; writing—original draft preparation, A.M., M.B.; writing—review and editing, I.C., G.D., A.M.; visualization, A.M., M.B.; supervision, I.C.
Funding
This research was funded by Stazione Zoologica Anton Dohrn.
Acknowledgments
Mariarita Brancaccio has been supported by a PhD fellowship funded by the Stazione Zoologica Anton Dohrn (Open University—Stazione Zoologica Anton Dohrn PhD Program). Alfonsina Milito has been supported by a research fellowship funded by the Stazione Zoologica Anton Dohrn.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Akt | Protein Kinase |
| ALA | α-lipoic acid |
| ALT | Alanine transaminase |
| AP-1 | Activator protein-1 |
| AST | Aspartate transaminase |
| BDL | Bile duct ligation |
| cMyc | Cellular myelocytomatosis |
| CPS | Capsaicin |
| DADS | Diallyl disulphide |
| DAS | Diallyl sulphide |
| ECM | Extracellular matrix |
| ERG | Ergothioneine |
| Erk1/2 | Extracellular signal-regulated kinases 1 and 2 |
| ET-1 | Endothelin-1 |
| GGT | γ-glutamyl transpeptidase |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GST | Glutathione S-transferases |
| H2S | Hydrogen sulfide |
| HSC | Hepatic stellate cell |
| Hsp | Heat shock protein |
| IFR3 | Interferon regulatory factor 3 |
| IGF-1 | Insulin-like growth factor 1 |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| LBP | LPS-binding protein |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MKK | Mitogen-activated protein kinase kinase |
| MMPs | Matrix metalloproteinases |
| mTOR | Mammalian target of rapamycin |
| MyD88 | Myeloid differentiation primary response protein |
| NAC | N-acetyl-L-cysteine |
| NAFLD | Non-alcoholic liver disease |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOX | NADPH oxidase |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| OCTN1 | Organic cation transporter 1 |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PDGF | Platelet-derived growth factor |
| PDGFRα/β | Platelet-derived growth factor receptor isoform α/β |
| PDK1 | Phosphoinositide-dependent protein kinase 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PLCγ | Phospholipase C γ |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| Raf | Rapidly accelerated fibrosarcoma |
| RhoA | Ras homolog gene family, member A |
| ROCK | Rho-associated protein kinase |
| ROS | reactive oxygen species |
| SAC | S-allylcysteine |
| SAG | S-allyl-glutathione |
| SAM | S-adenosyl-L-methionine |
| SECs | Sinusoidal endothelial cells |
| SFN | Sulforaphane |
| SIRT1 | Silent information regulator 1 |
| SMAD2/3/4 | Small mother against decapentaplegic protein 2/3/4 |
| SNAC | S-Nitroso-N-acetylcysteine |
| SOD | Superoxide dismutase |
| Sp1 | Specificity protein 1 |
| STAT1/3 | Signal Transducers of Activated Transcription 1/3 |
| TGF-β | Transforming growth factor beta |
| TGF-β RI/II | Transforming growth factor beta receptor type I/II |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TIRAP | TIR-domain containing adapter protein |
| TLR4 | Toll-like receptor 4 |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| TRAM | Translocating chain-associating membrane protein |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| tTG | Tissue transglutaminase |
| Wnt | Wingless/integrated |
| αSMA | α-smooth muscle actin |
References
- Bedossa, P.; Paradis, V. Liver extracellular matrix in health and disease. J. Pathol. 2003, 200, 504–515. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, Q.; Jin, M.; Niu, J.; Gao, Y. Congenital hepatic fibrosis with polycystic kidney disease: Two case reports. Medicine 2019, 98, e15600. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Busletta, C.; Novo, E.; Di Bonzo, L.V.; Cannito, S.; Paternostro, C.; Parola, M. Liver fibrosis: A dynamic and potentially reversible process. Histol. Histopathol. 2010, 25, 1075–1091. [Google Scholar] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, M.; Rombouts, K.; Colagrande, S. Fibrosis in chronic liver diseases: Diagnosis and management. J. Hepatol. 2005, 42, S22–S36. [Google Scholar] [CrossRef]
- Pinter, M.; Trauner, M.; Peck-Radosavljevic, M.; Sieghart, W. Cancer and liver cirrhosis: Implications on prognosis and management. ESMO Open 2016, 1, e000042. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Tacke, F. Liver Fibrosis: From Pathogenesis to Novel Therapies. Dig. Dis. 2016, 34, 410–422. [Google Scholar] [CrossRef]
- Pinzani, M. Pathophysiology of Liver Fibrosis. Dig. Dis. 2015, 33, 492–497. [Google Scholar] [CrossRef]
- Elpek, G.Ö. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J. Gastroenterol. 2014, 20, 7260–7276. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar]
- Safadi, R.; Friedman, S.L. Hepatic fibrosis-role of hepatic stellate cell activation. Med. Gen. Med. 2002, 4, 27. [Google Scholar]
- Zhang, C.-Y.; Yuan, W.-G.; He, P.; Lei, J.-H.; Wang, C.-X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Eggenhoffner, R.; Giacomelli, L. Glutathione in the treatment of liver diseases: Insights from clinical practice. Minerva Gastroenterol. Dietol. 2016, 62, 316–324. [Google Scholar] [PubMed]
- Castellano, I.; Merlino, A. γ-Glutamyltranspeptidases: Sequence, structure, biochemical properties, and biotechnological applications. Cell Mol. Life Sci. 2012, 69, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Merlino, A. Gamma-Glutamyl Transpeptidases: Structure and Function. In Springer Briefs in Biochemistry and Molecular Biology; Springer: Basel, Switzerland, 2013. [Google Scholar]
- Aitken, A.E.; Richardson, T.A.; Morgan, E.T. Regulation of drug-metabolizing enzymes and transporters in inflammation. Ann. Rev. Pharmacol. Toxicol. 2006, 46, 123–149. [Google Scholar] [CrossRef]
- Li, H.-Y.; Ju, D.; Zhang, D.-W.; Li, H.; Kong, L.-M.; Guo, Y.; Li, C.; Wang, X.-L.; Chen, Z.-N.; Bian, H. Activation of TGF-β1-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci. Rep. 2015, 5, 16552. [Google Scholar] [CrossRef]
- Weng, H.-L.; Liu, Y.; Chen, J.-L.; Huang, T.; Xu, L.-J.; Godoy, P.; Hu, J.-H.; Zhou, C.; Stickel, F.; Marx, A.; et al. The etiology of liver damage imparts cytokines transforming growth factor β1 or interleukin-13 as driving forces in fibrogenesis. Hepatology 2009, 50, 230–243. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.N. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget 2017, 8, 90579–90604. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.N. Thermodynamic Aspects and Reprogramming Cellular Energy Metabolism during the Fibrosis Process. Int. J. Mol. Sci. 2017, 18, 2537. [Google Scholar] [CrossRef]
- Ying, H.-Z.; Chen, Q.; Zhang, W.-Y.; Zhang, H.-H.; Ma, Y.; Zhang, S.-Z.; Fang, J.; Yu, C.-H. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review). Mol. Med. Rep. 2017, 16, 7879–7889. [Google Scholar] [CrossRef]
- Guo, J.; Friedman, S.L. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 2010, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Novo, E.; Cannito, S.; Morello, E.; Paternostro, C.; Bocca, C.; Miglietta, A.; Parola, M. Hepatic myofibroblasts and fibrogenic progression of chronic liver diseases. Histol. Histopathol. 2015, 30, 1011–1032. [Google Scholar] [PubMed]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis 2013; A systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-Y.; Wu, J.-Y.; Wu, Y.-B.; Zhong, M.-Z.; Lu, H.-M. Effects of endothelin-1 on hepatic stellate cell proliferation, collagen synthesis and secretion, intracellular free calcium concentration. World J. Gastroenterol. 2004, 10, 2697–2700. [Google Scholar] [CrossRef]
- Adamek, A.; Kasprzak, A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Recent advances in understanding liver fibrosis: Bridging basic science and individualized treatment concepts. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci. Transl. Med. 2013, 5, sr1–sr167. [Google Scholar] [CrossRef]
- Murphy, F.; Arthur, M.; Iredale, J. Developing strategies for liver fibrosis treatment. Expert Opin. Investig. Drug 2002, 11, 1575–1585. [Google Scholar] [CrossRef]
- Weiskirchen, R. Hepatoprotective and Anti-fibrotic Agents: It’s Time to Take the Next Step. Front. Pharmacol. 2016, 6, 303. [Google Scholar] [CrossRef]
- Nair, D.G.; Weiskirchen, R.; Al-Musharafi, S.K. The use of marine-derived bioactive compounds as potential hepatoprotective agents. Acta Pharmacol. Sin. 2015, 36, 158–170. [Google Scholar] [CrossRef]
- D’Argenio, G.; Amoruso, D.C.; Mazzone, G.; Vitaglione, P.; Romano, A.; Ribecco, M.T.; D’Armiento, M.R.; Mezza, E.; Morisco, F.; Fogliano, V.; et al. Garlic extract prevents CCl4-induced liver fibrosis in rats: The role of tissue transglutaminase. Dig. Liver Dis. 2010, 42, 571–577. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Mazzone, G.; Ribecco, M.T.; Lembo, V.; Vitaglione, P.; Guarino, M.; Morisco, F.; Napolitano, M.; Fogliano, V.; Caporaso, N. Garlic extract attenuating rat liver fibrosis by inhibiting TGF-β1. Clin. Nutr. 2013, 32, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.J.; Kim, J.-Y.; Min, A.-K.; Park, K.-G.; Harris, R.A.; Kim, H.-J.; Lee, I.-K. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic. Biol. Med. 2012, 52, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, H.; Takemura, S.; Minamiyama, Y.; Kodai, S.; Tsukioka, T.; Osada-Oka, M.; Kubo, S.; Okada, S.; Suehiro, S. S-allylcysteine is effective as a chemopreventive agent against porcine serum-induced hepatic fibrosis in rats. Osaka City Med. J. 2009, 55, 61–69. [Google Scholar]
- Shin, M.-O.; Moon, J.-O. Effect of dietary supplementation of grape skin and seeds on liver fibrosis induced by dimethylnitrosamine in rats. Nutr. Res. Pract. 2010, 4, 369–374. [Google Scholar] [CrossRef][Green Version]
- Brancaccio, M.; D’Argenio, G.; Lembo, V.; Palumbo, A.; Castellano, I. Antifibrotic Effect of Marine Ovothiol in an In Vivo Model of Liver Fibrosis. Oxid. Med. Cell. Longev. 2018, 2018, 5045734. [Google Scholar] [CrossRef]
- Umbreen, S.; Lubega, J.; Loake, G.J. Sulphur: The heart of nitric oxide-dependent redox signalling. J. Exp. Bot. 2019, 70, 4279–4286. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef]
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic Carbon-Sulfur Bond Formation in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5521–5577. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-D.; Wang, D.-Y.; Li, H.-M.; Guo, J.-C.; Duan, S.-F.; Ji, X.-Y. Hydrogen Sulfide as a Novel Regulatory Factor in Liver Health and Disease. Oxid. Med. Cell. Longev. 2019, 2019, 3831713. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Ruocco, M.R.; Cecere, F.; Di Maro, A.; Chambery, A.; Michniewicz, A.; Parlato, G.; Masullo, M.; De Vendittis, E. Glutathionylation of the iron superoxide dismutase from the psychrophilic eubacterium Pseudoalteromonas haloplanktis. Biochim. Biophys. Acta Proteins Proteomics 2008, 1784, 816–826. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Milczarek, G.; Arasimowicz, M.; Ciszewski, A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 2006, 224, 1363–1372. [Google Scholar] [CrossRef]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Hesketh, J.; Huang, D.; Gan, F.; Hao, S.; Tang, S.; Guo, Y.; Huang, K. Protective effects of selenium-glutathione-enriched probiotics on CCl4-induced liver fibrosis. J. Nutr. Biochem. 2018, 58, 138–149. [Google Scholar] [CrossRef]
- Durrani, A.I.; Schwartz, H.; Nagl, M.; Sontag, G. Determination of free α-lipoic acid in foodstuffs by HPLC coupled with CEAD and ESI-MS. Food Chem. 2010, 120, 1143–1148. [Google Scholar] [CrossRef]
- Foo, N.-P.; Lin, S.-H.; Lee, Y.-H.; Wu, M.-J.; Wang, Y.-J. α-Lipoic acid inhibits liver fibrosis through the attenuation of ROS-triggered signaling in hepatic stellate cells activated by PDGF and TGF-β. Toxicology 2011, 282, 39–46. [Google Scholar] [CrossRef]
- Min, A.-K.; Kim, M.-K.; Seo, H.-Y.; Kim, H.-S.; Jang, B.K.; Hwang, J.S.; Choi, H.-S.; Lee, K.-U.; Park, K.-G.; Lee, I.-K. Alpha-lipoic acid inhibits hepatic PAI-1 expression and fibrosis by inhibiting the TGF-β signaling pathway. Biochem. Biophys. Res. Commun. 2010, 393, 536–541. [Google Scholar] [CrossRef]
- Sadek, K.M.; Saleh, E.A.; Nasr, S.M. Molecular hepatoprotective effects of lipoic acid against carbon tetrachloride-induced liver fibrosis in rats: Hepatoprotection at molecular level. Hum. Exp. Toxicol. 2017, 37, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Alban, F.T.E.; Gyamfi, D.; Van Golen, R.F.; Heger, M. Chapter 10—Reactive Oxygen and Nitrogen Species and Liver Ischemia-Reperfusion Injury: Role of Lipoic Acid. In The Liver; Patel, V.B., Rajendram, R., Preedy, V.R., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 109–119. [Google Scholar]
- Çimen, B.; Çimen, L.; Çetin, I.; Çetin, A. Alpha-lipoic acid alleviates lipopolysaccharide-induced liver damage in rats via antioxidant effect. Dicle Med. J. 2019, 46, 125–132. [Google Scholar] [CrossRef][Green Version]
- El-Feki, M.A.; Amin, H.M.; Abdalla, A.A.; Fesal, M. Immunomodulatory and Anti-oxidant Effects of Alpha-Lipoic Acid and Vitamin E on Lipopolysaccharide-induced Liver Injury in Rats. Middle East J. Appl. Sci. 2016, 6, 460–467. [Google Scholar]
- Gorąca, A.; Huk-Kolega, H.; Kowalczyk, A.; Skibska, B. Anti-oxidative and anti-inflammatory effects of lipoic acid in rat liver. Postep Hig. Med. Dosw. 2015, 69, 270–276. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kaibori, M.; Miki, H.; Nakatake, R.; Tokuhara, K.; Nishizawa, M.; Okumura, T.; Kwon, A.H. Alpha-lipoic acid exerts a liver-protective effect in acute liver injury rats. J. Surg. Res. 2015, 193, 675–683. [Google Scholar] [CrossRef]
- Armagan, I.; Bayram, D.; Candan, I.A.; Yigit, A.; Celik, E.; Armagan, H.H.; Uğuz, A.C. Effects of pentoxifylline and alpha lipoic acid on methotrexate-induced damage in liver and kidney of rats. Environ. Toxicol. Pharmacol. 2015, 39, 1122–1131. [Google Scholar] [CrossRef]
- Çakir, T.; Baştürk, A.; Polat, C.; Aslaner, A.; Durgut, H.; Şehirli, A.Ö.; Gül, M.; Öğünç, A.V.; Gül, S.; Sabuncuoglu, M.Z.; et al. Does alfa lipoic acid prevent liver from methotrexate induced oxidative injury in rats? Acta Cir. Bras. 2015, 30, 247–252. [Google Scholar] [CrossRef]
- Avci, B.; Bahadir, A.; Tuncel, O.K.; Bilgici, B. Influence of α-tocopherol and α-lipoic acid on bisphenol-A-induced oxidative damage in liver and ovarian tissue of rats. Toxicol. Ind. Health 2014, 32, 1381–1390. [Google Scholar] [CrossRef]
- Sena, M.C.; Cipriano, A.M.; Botelho, F.M.; Seiça, M.R. Lipoic Acid Prevents High-Fat Diet-Induced Hepatic Steatosis in Goto Kakizaki Rats by Reducing Oxidative Stress Through Nrf2 Activation. Int. J. Mol. Sci. 2018, 19, 2706. [Google Scholar] [CrossRef]
- Castro, M.C.; Massa, M.L.; Arbeláez, L.G.; Schinella, G.; Gagliardino, J.J.; Francini, F. Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015, 137, 1–6. [Google Scholar] [CrossRef]
- Kaya-Dagistanli, F.; Tanriverdi, G.; Altinok, A.; Ozyazgan, S.; Ozturk, M. The effects of alpha lipoic acid on liver cells damages and apoptosis induced by polyunsaturated fatty acids. Food Chem. Toxicol. 2013, 53, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.N.; Mladenović, D.; Ninković, M.; Ethuričić, I.; Sobajić, S.; Jorgačević, B.; de Luka, S.; Vukicevic, R.J.; Radosavljević, T.S. The effects of α-lipoic acid on liver oxidative stress and free fatty acid composition in methionine-choline deficient diet-induced NAFLD. J. Med. Food 2014, 17, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, J.; Pian, L.; Gui, S.; Lu, B. α-lipoic acid protects against carbon tetrachloride-induced liver cirrhosis through the suppression of the TGF-β/Smad3 pathway and autophagy. Mol. Med. Rep. 2019, 19, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, F.; Hamedi-Shahraki, S.; Hosseinpour-Arjmand, S.; Vaghef-Mehrabany, E.; Ebrahimi-Mameghani, M. Effects of Alpha-Lipoic Acid Supplementation on Oxidative Stress Status in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Iran. Red Crescent Med. J. 2018, 20, e67615. [Google Scholar] [CrossRef]
- Rahmanabadi, A.; Mahboob, S.; Amirkhizi, F.; Hosseinpour-Arjmand, S.; Ebrahimi-Mameghani, M. Oral α-lipoic acid supplementation in patients with non-alcoholic fatty liver disease: Effects on adipokines and liver histology features. Food Funct. 2019, 10, 4941–4952. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Miyazaki, T.; Bouscarel, B.; Ikegami, T.; Honda, A.; Matsuzaki, Y. The Protective Effect of Taurine against Hepatic Damage in a Model of Liver Disease and Hepatic Stellate Cells; Taurine 7; Azuma, J., Schaffer, S.W., Ito, T., Eds.; Springer: New York, NY, USA, 2009; pp. 293–303. [Google Scholar]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; El-Kersh, M.A.; Al-Omair, M.A. Free Radical-Scavenging, Anti-Inflammatory/Anti-Fibrotic and Hepatoprotective Actions of Taurine and Silymarin against CCl4 Induced Rat Liver Damage. PLoS ONE 2015, 10, e0144509. [Google Scholar] [CrossRef]
- Miyazaki, T.; Matsuzaki, Y. Taurine and liver diseases: A focus on the heterogeneous protective properties of taurine. Amino Acids 2014, 46, 101–110. [Google Scholar] [CrossRef]
- Wu, G.; Yang, J.; Sun, C.; Luan, X.; Shi, J.; Hu, J. Effect of Taurine on Alcoholic Liver Disease in Rats; Springer: New York, NY, USA, 2009; pp. 313–322. [Google Scholar]
- Devi, S.L.; Viswanathan, P.; Anuradha, C.V. Regression of liver fibrosis by taurine in rats fed alcohol: Effects on collagen accumulation, selected cytokines and stellate cell activation. Eur. J. Pharmacol. 2010, 647, 161–170. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chiu, C.-C.; Chen, Y.-C.; Chen, M.-L.; Hsu, T.-C.; Tzang, B.-S. Taurine Attenuates Hepatic Inflammation in Chronic Alcohol-Fed Rats Through Inhibition of TLR4/MyD88 Signaling. J. Med. Food 2015, 18, 1291–1298. [Google Scholar] [CrossRef]
- Wu, G.; Tang, R.; Yang, J.; Tao, Y.; Liu, Z.; Feng, Y.; Lin, S.; Yang, Q.; Lv, Q.; Hu, J. Taurine accelerates alcohol and fat metabolism of rats with alcoholic Fatty liver disease. Adv. Exp. Med. Biol. 2015, 803, 793–805. [Google Scholar] [PubMed]
- Zhang, Z.; Liu, D.; Yi, B.; Liao, Z.; Tang, L.; Yin, D.; He, M. Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 2014, 10, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.A.; Gabr, N.S.; Elsaed, W.M. Protective activity of taurine and molecular fibrogenesis in iron overloaded hepatic tissues. Int. J. Pharmacol. 2019, 15, 418–427. [Google Scholar]
- Liang, J.; Deng, X.; Lin, Z.X.; Zhao, L.C.; Zhang, X.L. Attenuation of portal hypertension by natural taurine in rats with liver cirrhosis. World J. Gastroenterol. 2009, 15, 4529–4537. [Google Scholar] [CrossRef]
- Schwarzer, R.; Kivaranovic, D.; Mandorfer, M.; Paternostro, R.; Wolrab, D.; Heinisch, B.; Reiberger, T.; Ferlitsch, M.; Gerner, C.; Trauner, M.; et al. Randomised clinical study: The effects of oral taurine 6g/day vs. placebo on portal hypertension. Aliment. Pharmacol. Ther. 2018, 47, 86–94. [Google Scholar] [CrossRef]
- Gentile, C.L.; Nivala, A.M.; Gonzales, J.C.; Pfaffenbach, K.T.; Wang, D.; Wei, Y.; Jiang, H.; Orlicky, D.J.; Petersen, D.R.; Pagliassotti, M.J.; et al. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am. J. Physiol. Reg. I 2011, 301, R1710–R1722. [Google Scholar] [CrossRef]
- Charron, C.S.; Dawson, H.D.; Novotny, J.A. Garlic Influences Gene Expression In Vivo and In Vitro. J. Nutr. 2016, 146, 444S–449S. [Google Scholar] [CrossRef]
- Gong, Z.; Ye, H.; Huo, Y.; Wang, L.; Huang, Y.; Huang, M.; Yuan, X. S-allyl-cysteine attenuates carbon tetrachloride-induced liver fibrosis in rats by targeting STAT3/SMAD3 pathway. Am. J. Transl. Res. 2018, 10, 1337–1346. [Google Scholar]
- Somade, O.T.; Ugbaja, R.N.; Alli, A.A.; Odubote, O.T.; Yusuf, T.S.; Busari, B.T. Diallyl disulfide, an organo-sulfur compound in garlic and onion attenuates trichloromethane-induced hepatic oxidative stress, activation of NFkB and apoptosis in rats. J. Nutr. Intermed. Metab. 2018, 13, 10–19. [Google Scholar] [CrossRef]
- Takemura, S.; Azuma, H.; Osada-Oka, M.; Kubo, S.; Shibata, T.; Minamiyama, Y. S-allyl-glutathione improves experimental liver fibrosis by regulating Kupffer cell activation in rats. Am. J. Physiol. Gastr. L 2018, 314, G150–G163. [Google Scholar] [CrossRef]
- Wu, C.-C.; Sheen, L.-Y.; Chen, H.-W.; Kuo, W.-W.; Tsai, S.-J.; Lii, C.-K. Differential Effects of Garlic Oil and Its Three Major Organosulfur Components on the Hepatic Detoxification System in Rats. J. Agric. Food Chem. 2002, 50, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, R.; Gao, H.; Xiao, J.; Tipoe, G.L. Chapter 28—Garlic and Liver Diseases. In The Liver; Patel, V.B., Rajendram, R., Preedy, V.R., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 337–347. [Google Scholar]
- Baek, S.-H.; Park, M.; Suh, J.-H.; Choi, H.-S. Protective Effects of an Extract of Young Radish (Raphanus sativus L) Cultivated with Sulfur (Sulfur-Radish Extract) and of Sulforaphane on Carbon Tetrachloride-Induced Hepatotoxicity. Biosci. Biotechnol. Biochem. 2008, 72, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Gaona-Gaona, L.; Molina-Jijón, E.; Tapia, E.; Zazueta, C.; Hernández-Pando, R.; Calderón-Oliver, M.; Zarco-Márquez, G.; Pinzón, E.; Pedraza-Chaverri, J. Protective effect of sulforaphane pretreatment against cisplatin-induced liver and mitochondrial oxidant damage in rats. Toxicology 2011, 286, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, S.; Lee, B.-S.; Jeong, S.Y.; Kim, K.-M.; Ku, S.-K.; Bae, J.-S. Hepatic protective effects of sulforaphane through the modulation of inflammatory pathways. J. Asian Nat. Prod. Res. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.-H.; Li, J.-W.; Sun, H.-T.; He, S.-Q.; Pang, J. Sulforaphane inhibits the activation of hepatic stellate cell by miRNA-423-5p targeting suppressor of fused. Hum. Cell 2019, 32, 403–410. [Google Scholar] [CrossRef]
- Mendivil, E.J.; Sandoval-Rodriguez, A.; Zuñiga-Ramos, L.M.; Santos-Garcia, A.; Armendariz-Borunda, J. Capsaicin and sulforaphane prevent experimental liver fibrosis via upregulation of peroxisome proliferator-activated receptor gamma and nuclear factor (erythroid-derived 2)-like 2. J. Funct. Foods 2019, 52, 382–388. [Google Scholar] [CrossRef]
- Ey, J.; Schömig, E.; Taubert, D. Dietary Sources and Antioxidant Effects of Ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef]
- Tang, R.M.Y.; Cheah, I.K.-M.; Yew, T.S.K.; Halliwell, B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 2018, 8, 1601. [Google Scholar] [CrossRef]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef]
- Kato, Y.; Kubo, Y.; Iwata, D.; Kato, S.; Sudo, T.; Sugiura, T.; Kagaya, T.; Wakayama, T.; Hirayama, A.; Sugimoto, M.; et al. Gene Knockout and Metabolome Analysis of Carnitine/Organic Cation Transporter OCTN1. J. Pharm. Res. 2010, 27, 832–840. [Google Scholar] [CrossRef]
- Franzoni, F.; Colognato, R.; Galetta, F.; Laurenza, I.; Barsotti, M.; Di Stefano, R.; Bocchetti, R.; Regoli, F.; Carpi, A.; Balbarini, A.; et al. An in vitro study on the free radical scavenging capacity of ergothioneine: Comparison with reduced glutathione, uric acid and trolox. Biomed. Pharmacother. 2006, 60, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Rougee, M.; Bensasson, R.V.; Land, E.J.; Pariente, R. Deactivation of singlet molecular oxygen by thiols and related compounds, possible protectors against skin photosensitivity. Photochem. Photobiol. 1988, 47, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Colognato, R.; Laurenza, I.; Fontana, I.; Coppedé, F.; Siciliano, G.; Coecke, S.; Aruoma, O.I.; Benzi, L.; Migliore, L. Modulation of hydrogen peroxide-induced DNA damage, MAPKs activation and cell death in PC12 by ergothioneine. Clin. Nutr. 2006, 25, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Laurenza, I.; Prato, S.D.; Benzi, L.; Colognato, R.; Migliore, L. Modulation of palmitic acid-induced cell death by ergothioneine: Evidence of an anti-inflammatory action. BioFactors 2008, 33, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Gilmour, P.S.; Jimenez, L.A.; Biswas, S.K.; Antonicelli, F.; Aruoma, O.I. Ergothioneine inhibits oxidative stress- and TNF-α-induced NF-κB activation and interleukin-8 release in alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2003, 302, 860–864. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Lo, H.-W.; Korivi, M.; Tsai, Y.-C.; Tang, M.-J.; Yang, H.-L. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes. Free Radic. Biol. Med. 2015, 86, 102–117. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.; Ye, P.; Yew, T.S.Z.; Lim, K.H.C.; Halliwell, B. Liver ergothioneine accumulation in a guinea pig model of non-alcoholic fatty liver disease. A possible mechanism of defence? Free Radic. Res. 2016, 50, 14–25. [Google Scholar] [CrossRef]
- Bedirli, A.; Sakrak, O.; Muhtaroglu, S.; Soyuer, I.; Guler, I.; Riza Erdogan, A.; Sozuer, E.M. Ergothioneine pretreatment protects the liver from ischemia-reperfusion injury caused by increasing hepatic heat shock protein 70. J. Surg. Res. 2004, 122, 96–102. [Google Scholar] [CrossRef]
- Tang, Y.; Masuo, Y.; Sakai, Y.; Wakayama, T.; Sugiura, T.; Harada, R.; Futatsugi, A.; Komura, T.; Nakamichi, N.; Sekiguchi, H.; et al. Localization of Xenobiotic Transporter OCTN1/SLC22A4 in Hepatic Stellate Cells and Its Protective Role in Liver Fibrosis. J. Pharm. Sci. 2016, 105, 1779–1789. [Google Scholar] [CrossRef]
- Palumbo, A.; Castellano, I.; Napolitano, A. Ovothiol: A Potent Natural Antioxidant from Marine Organisms. In Blue Biotechnology; La Barre, S.S., Bates, S., Eds.; Wiley VCH: Weinheim, Germany, 2018; pp. 583–610. [Google Scholar]
- Braunshausen, A.; Seebeck, F.P. Identification and Characterization of the First Ovothiol Biosynthetic Enzyme. J. Am. Chem. Soc. 2011, 133, 1757–1759. [Google Scholar] [CrossRef]
- Naowarojna, N.; Cheng, R.; Chen, L.; Quill, M.; Xu, M.; Zhao, C.; Liu, P. Mini-Review: Ergothioneine and Ovothiol Biosyntheses, an Unprecedented Trans-Sulfur Strategy in Natural Product Biosynthesis. Biochemistry 2018, 57, 3309–3325. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Seebeck, F.P. On ovothiol biosynthesis and biological roles: From life in the ocean to therapeutic potential. Nat. Prod. Rep. 2018, 35, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Migliaccio, O.; D’Aniello, S.; Merlino, A.; Napolitano, A.; Palumbo, A. Shedding light on ovothiol biosynthesis in marine metazoans. Sci. Rep. 2016, 6, 21506. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Russo, M.; Castellano, I.; Napolitano, A.; Palumbo, A. Ovothiol isolated from sea urchin oocytes induces autophagy in the Hep-G2 cell line. Mar. Drugs 2014, 12, 4069–4085. [Google Scholar] [CrossRef]
- Brancaccio, M.; Russo, M.; Masullo, M.; Palumbo, A.; Russo, G.L.; Castellano, I. Sulfur-containing histidine compounds inhibit gamma-glutamyl transpeptidase activity in human cancer cells. J. Biol. Chem. 2019, 294, 14603–14614. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).