MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future?
Abstract
1. Introduction
MicroRNAs and Their Role in Inflammatory Conditions
2. Materials and Methods
3. Results
3.1. MicroRNAs in Inflammatory Heart Diseases
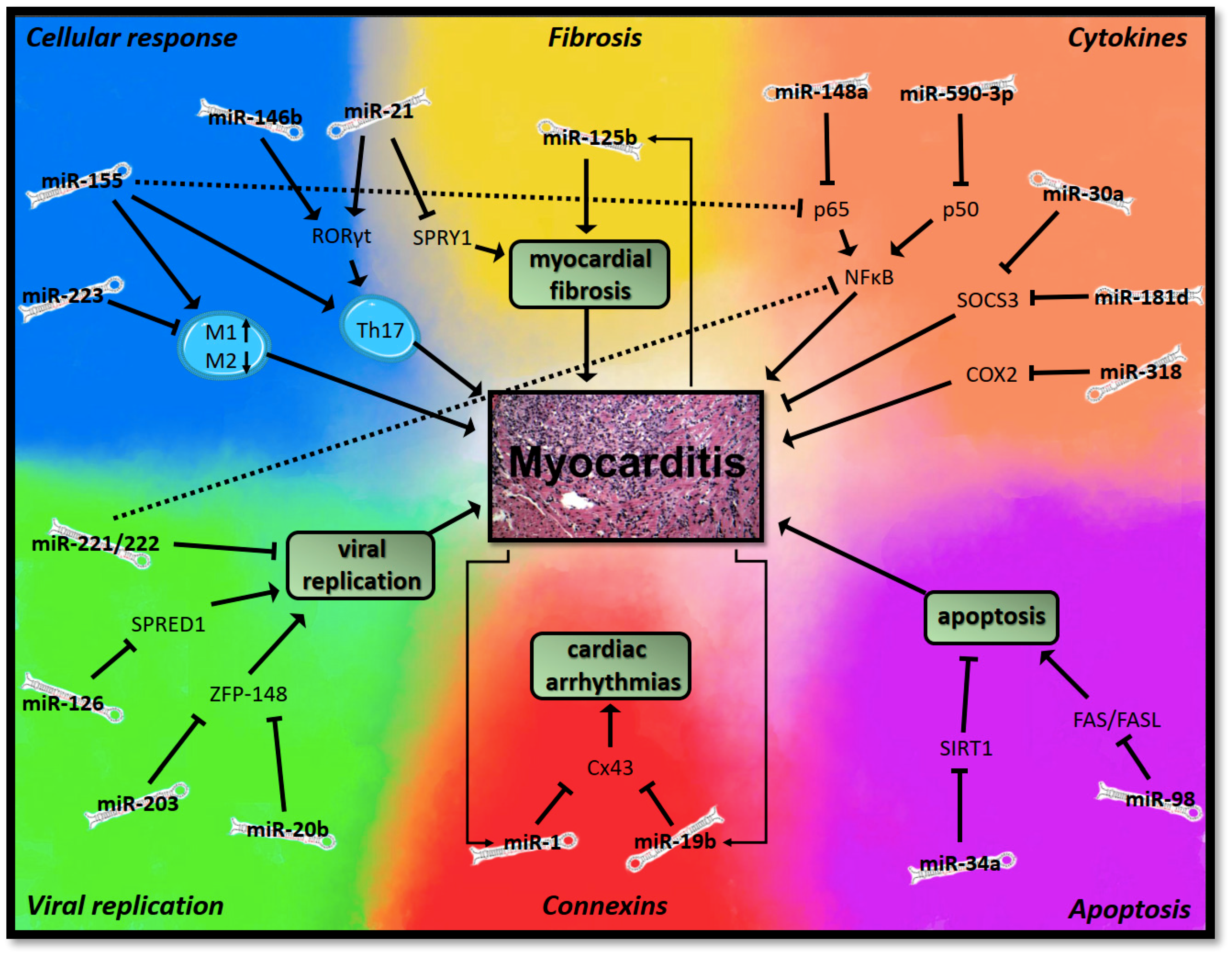
3.1.1. MicroRNAs in Myocarditis
Viral Replication
Cellular Immune Response
Cytokines
Apoptosis
Myocardial Fibrosis
Connexins and Gap Junctions
3.1.2. MicroRNAs in Endocarditis and Pericarditis
3.2. MicroRNAs in Sepsis-Induced Cardiac Dysfunction
4. Discussion
Funding
Conflicts of Interest
Appendix A
Appendix A.1. MicroRNAs in Inflammatory Heart Diseases
Appendix A.1.1. MicroRNAs in Myocarditis
Appendix A.1.2. MicroRNAs in Endocarditis and Pericarditis
Appendix A.2. MicroRNAs in Sepsis-Induced Cardiac Dysfunction
Appendix B

References
- Romaine, S.P.R.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.; Borucka, J.; Petrkova, J.; Petrek, M. Novel insights into miRNA in lung and heart inflammatory diseases. Mediat. Inflamm. 2014, 2014, 259131. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-N.; Li, D.; Xia, J.; Wu, Q.-J.; Wen, R.; Yang, N.; Liu, C.-F. Non-coding RNA: A potential biomarker and therapeutic target for sepsis. Oncotarget 2017, 8, 91765–91778. [Google Scholar] [CrossRef]
- Tian, T.; Wang, J.; Zhou, X. A review: MicroRNA detection methods. Org. Biomol. Chem. 2015, 13, 2226–2238. [Google Scholar] [CrossRef]
- Allegra, A.; Alonci, A.; Campo, S.; Penna, G.; Petrungaro, A.; Gerace, D.; Musolino, C. Circulating microRNAs: New biomarkers in diagnosis, prognosis and treatment of cancer. Int. J. Oncol. 2012, 41, 1897–1912. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Sheedy, F.J.; McCoy, C.E. MicroRNAs: The fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 2011, 11, 163–175. [Google Scholar] [CrossRef]
- Curtale, G.; Renzi, T.A.; Mirolo, M.; Drufuca, L.; Albanese, M.; De Luca, M.; Rossato, M.; Bazzoni, F.; Locati, M. Multi-Step Regulation of the TLR4 Pathway by the miR-125a~99b~let-7e Cluster. Front. Immunol. 2018, 9, 2037. [Google Scholar] [CrossRef]
- Herrero, C.; Hu, X.; Li, W.P.; Samuels, S.; Sharif, M.N.; Kotenko, S.; Ivashkiv, L.B. Reprogramming of IL-10 Activity and Signaling by IFN-γ. J. Immunol. 2003, 171, 5034–5041. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cooper, L.T.; Keren, A.; Sliwa, K.; Matsumori, A.; Mensah, G.A. The global burden of myocarditis: Part 1: A systematic literature review for the global burden of diseases, injuries, and risk factors 2010 study. Glob. Heart 2014, 9, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Tschöepe, C.; Lassner, D.; Schultheiss, H.-P. Myocarditis and inflammatory cardiomyopathy: From diagnosis to treatment. Turk. Kardiyol. Dern. Arsivi Arch. Turk. Soc. Cardiol. 2015, 43, 739–748. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, A.; Patti, G.; Manzoli, A.; Sinagra, G.; Di, L.; Silvestri, F.; Di, S. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: A review. Heart 2001, 85, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Hemida, M.G.; Qiu, Y.; Hanson, P.J.; Zhang, H.M.; Yang, D. MiR-126 promotes coxsackievirus replication by mediating cross-talk of ERK1/2 and Wnt/β-catenin signal pathways. Cell. Mol. Life Sci. 2013, 70, 4631–4644. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Lin, L.; Wu, S.; Guo, Z.; Wang, T.; Qin, Y.; Wang, R.; Zhong, X.; Wu, X.; Wang, Y.; et al. MiR-10a* up-regulates coxsackievirus B3 biosynthesis by targeting the 3D-coding sequence. Nucl. Acids Res. 2013, 41, 3760–3771. [Google Scholar] [CrossRef] [PubMed]
- Desvignes, T.; Lin, L.; Wu, S.; Guo, Z.; Wang, T.; Qin, Y.; Wang, R.; Zhong, X.; Wu, X.; Wang, Y.; et al. miRNA Nomenclature: A View; Incorporating Genetic Origins, Biosynthetic Pathways, and Sequence Variants. Trends Genet. 2015, 31, 613–626. [Google Scholar] [CrossRef]
- Xu, H.-F.; Gao, X.-T.; Lin, J.-Y.; Xu, X.-H.; Hu, J.; Ding, Y.-J.; Zhu, S.-H. MicroRNA-20b suppresses the expression of ZFP-148 in viral myocarditis. Mol. Cell. Biochem. 2017, 429, 199–210. [Google Scholar] [CrossRef]
- Hemida, M.G.; Ye, X.; Zhang, H.M.; Hanson, P.J.; Liu, Z.; McManus, B.M.; Yang, D. MicroRNA-203 enhances coxsackievirus B3 replication through targeting zinc finger protein-148. Cell. Mol. Life Sci. 2013, 70, 277–291. [Google Scholar] [CrossRef]
- Germano, J.F.; Sawaged, S.; Saadaeijahromi, H.; Andres, A.M.; Feuer, R.; Gottlieb, R.A.; Sin, J. Coxsackievirus B infection induces the extracellular release of miR-590-5p, a proviral microRNA. Virology 2019, 529, 169–176. [Google Scholar] [CrossRef]
- Corsten, M.F.; Heggermont, W.; Papageorgiou, A.-P.; Deckx, S.; Tijsma, A.; Verhesen, W.; van Leeuwen, R.; Carai, P.; Thibaut, H.-J.; Custers, K.; et al. The microRNA-221/-222 cluster balances the antiviral and inflammatory response in viral myocarditis. Eur. Heart J. 2015, 36, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Wu, W.; Xue, Y.; Gao, M.; Yan, Y.; Kong, Q.; Pang, Y.; Yang, F. MicroRNA-21 and -146b are involved in the pathogenesis of murine viral myocarditis by regulating TH-17 differentiation. Arch. Virol. 2013, 158, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.M.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Li, X.; Tang, Z.; Wang, X.; Zhong, M.; Suo, Q.; Zhang, Y.; Lv, K. Silencing MicroRNA-155 Attenuates Cardiac Injury and Dysfunction in Viral Myocarditis via Promotion of M2 Phenotype Polarization of Macrophages. Sci. Rep. 2016, 6, 22613. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.F.; Hu, X.; Yan, Y.; Wei, W.; Ma, Y.; Wang, S.; Lu, Z. Wang Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J. Mol. Med. 2016, 94, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Zhang, Z.; Yang, C.; Li, Y. MiR-223/Pknox1 axis protects mice from CVB3-induced viral myocarditis by modulating macrophage polarization. Exp. Cell Res. 2018, 366, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Marques-Rocha, J.L.; Samblas, M.; Milagro, F.I.; Bressan, J.; Martínez, J.A.; Marti, A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015, 29, 3595–3611. [Google Scholar] [CrossRef]
- Gracias, D.T.; Katsikis, P.D. MicroRNAs: Key Components of Immune Regulation. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2011; Volume 780, pp. 15–26. [Google Scholar]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Guo, E.; Qi, Y. Silence of lncRNA CHRF protects H9c2 cells against lipopolysaccharide-induced injury via up-regulating microRNA-221. Exp. Mol. Pathol. 2019, 107, 43–50. [Google Scholar] [CrossRef]
- Bao, J.-L.; Lin, L. MiR-155 and miR-148a reduce cardiac injury by inhibiting NF-κB pathway during acute viral myocarditis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2349–2356. [Google Scholar]
- Deng, Y.-Y.; Zhuo, X.-Z.; Zhao, S.; Yang, G.; Liu, P.-N.; Zhao, Z.; Sun, T.; Liu, J.-H.; Tian, Y.; Zhou, J.; et al. miR-590-3p Is a Novel MicroRNA in Myocarditis by Targeting Nuclear Factor Kappa-B in vivo. Cardiology 2015, 132, 182–188. [Google Scholar]
- Chen, Z.-G.; Liu, H.; Zhang, J.-B.; Zhang, S.-L.; Zhao, L.-H.; Liang, W.-Q. Upregulated microRNA-214 enhances cardiac injury by targeting ITCH during coxsackievirus infection. Mol. Med. Rep. 2015, 12, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sit, A.; Feinberg, M.W. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc. Med. 2014, 24, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Vera, M.P.M.; MICU Registry; Blackwell, T.S.; et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J. Clin. Investig. 2012, 122, 1973–1990. [Google Scholar]
- Pan, A.; Tan, Y.; Wang, Z.; Xu, G.; Xu, H. STAT4 silencing underlies a novel inhibitory role of microRNA-141-3p in inflammation response of mice with experimental autoimmune myocarditis. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H531–H540. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Sun, H.; Yu, Z.; Liu, X.; Luo, X.; Li, C.; Sun, D.; Li, T. MicroRNA-381 protects myocardial cell function in children and mice with viral myocarditis via targeting cyclooxygenase-2 expression. Exp. Ther. Med. 2018, 15, 5510–5516. [Google Scholar] [CrossRef]
- Fan, K.-L.; Li, M.-F.; Cui, F.; Feng, F.; Kong, L.; Zhang, F.-H.; Hao, H.; Yin, M.-X.; Liu, Y. Altered exosomal miR-181d and miR-30a related to the pathogenesis of CVB3 induced myocarditis by targeting SOCS3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2208–2215. [Google Scholar]
- Huang, T.F.; Wu, X.H.; Wang, X.; Lu, I.J. Fas-FasL expression and myocardial cell apoptosis in patients with viral myocarditis. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Jiang, D.; Li, M.; Yu, Y.; Shi, H.; Chen, R. microRNA-34a aggravates coxsackievirus B3-induced apoptosis of cardiomyocytes through the SIRT1-p53 pathway. J. Med. Virol. 2019, 91, 1643–1651. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Zhao, Z.; Jin, Z. Expression of miR-98 in myocarditis and its influence on transcription of the FAS/FASL gene pair. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Xu, H.-F.; Ding, Y.-J.; Zhang, Z.-X.; Wang, Z.-F.; Luo, C.-L.; Li, B.-X.; Shen, Y.-W.; Tao, L.-Y.; Zhao, Z.-Q. MicroRNA-21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Mol. Med. Rep. 2014, 10, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, W.; Lu, S.; Yan, L.; Hu, F.; Wang, Z.; Cheng, B. Androgen receptor regulates cardiac fibrosis in mice with experimental autoimmune myocarditis by increasing microRNA-125b expression. Biochem. Biophys. Res. Commun. 2018, 506, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Dupays, L.; Théveniau-Ruissy, M.; Alcoléa, S.; Jarry-Guichard, T.; Abran, P.; Gros, D. Gap junctional connexins in the developing mouse cardiac conduction system. Novartis Found. Symp. 2003, 250, 80–98; discussion 98–109, 176–279. [Google Scholar] [PubMed]
- Lambiase, P.D.; Tinker, A. Connexins in the heart. Cell Tissue Res. 2015, 360, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Xuan, L.; Liu, Y.; Shao, L.; Ge, H.; Gu, J.; Wei, C.; Zhao, M. Astragalus Root dry extract restores connexin43 expression by targeting miR-1 in viral myocarditis. Phytomedicine 2018, 46, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-F.; Ding, Y.-J.; Shen, Y.-W.; Xue, A.-M.; Xu, H.-M.; Luo, C.-L.; Li, B.-X.; Liu, Y.-L.; Zhao, Z.-Q. MicroRNA- 1 represses Cx43 expression in viral myocarditis. Mol. Cell. Biochem. 2012, 362, 141–148. [Google Scholar] [CrossRef]
- Lin, J.; Xue, A.; Li, L.; Li, B.; Li, Y.; Shen, Y.; Sun, N.; Chen, R.; Xu, H.; Zhao, Z. MicroRNA-19b Downregulates Gap Junction Protein Alpha1 and Synergizes with MicroRNA-1 in Viral Myocarditis. Int. J. Mol. Sci. 2016, 17, 741. [Google Scholar] [CrossRef]
- Ciccacci, C.; Perricone, C.; Politi, C.; Rufini, S.; Ceccarelli, F.; Cipriano, E.; Alessandri, C.; Latini, A.; Valesini, G.; Novelli, G.; et al. A polymorphism upstream MIR1279 gene is associated with pericarditis development in Systemic Lupus Erythematosus and contributes to definition of a genetic risk profile for this complication. Lupus 2017, 26, 841–848. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.-J.; Qian, C.; Dong, Q.; Ding, D.; Wu, Q.-F.; Li, J.; Wang, H.-F.; Li, W.-H.; Xie, Q.; et al. Signal Transducer and Activator of Transcription 3/MicroRNA-21 Feedback Loop Contributes to Atrial Fibrillation by Promoting Atrial Fibrosis in a Rat Sterile Pericarditis Model. Circ. Arrhythm. Electrophysiol. 2016, 9, e003396. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Miranda, M.; Balarini, M.; Caixeta, D.; Bouskela, E. Microcirculatory dysfunction in sepsis: Pathophysiology, clinical monitoring, and potential therapies. Am. J. Physiol. Circ. Physiol. 2016, 311, H24–H35. [Google Scholar] [CrossRef] [PubMed]
- Lipinska-Gediga, M. Sepsis and septic shock—Is a microcirculation a main player? Anestezjol. Intens. Ter. 2016, 48, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, J.; Luyt, C.-E.; Fulla, Y.; Vinsonneau, C.; Cariou, A.; Grabar, S.; Dhainaut, J.-F.; Mira, J.-P.; Chiche, J.-D. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit. Care Med. 2004, 32, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.R. Sepsis-induced myocardial dysfunction. Curr. Opin. Crit. Care 2018, 24, 292–299. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Dalton, A.; Shahul, S. Cardiac dysfunction in critical illness. Curr. Opin. Anaesthesiol. 2018, 31, 158–164. [Google Scholar] [CrossRef]
- Zhang, T.-N.; Yang, N.; Goodwin, J.E.; Mahrer, K.; Li, D.; Xia, J.; Wen, R.; Zhou, H.; Zhang, T.; Song, W.-L.; et al. Characterization of Circular RNA and microRNA Profiles in Septic Myocardial Depression: A Lipopolysaccharide-Induced Rat Septic Shock Model. Inflammation 2019. [Google Scholar] [CrossRef]
- Benz, F.; Roy, S.; Trautwein, C.; Roderburg, C.; Luedde, T. Circulating MicroRNAs as Biomarkers for Sepsis. Int. J. Mol. Sci. 2016, 17, 78. [Google Scholar] [CrossRef]
- Puskarich, M.A.; Nandi, U.; Shapiro, N.I.; Trzeciak, S.; Kline, J.A.; Jones, A.E. Detection of microRNAs in patients with sepsis. J. Acute Dis. 2015, 4, 101–106. [Google Scholar] [CrossRef]
- Yao, L.; Liu, Z.; Zhu, J.; Li, B.; Chai, C.; Tian, Y. Clinical evaluation of circulating microRNA-25 level change in sepsis and its potential relationship with oxidative stress. Int. J. Clin. Exp. Pathol. 2015, 8, 7675–7684. [Google Scholar]
- Xie, J.; Zhang, L.; Fan, X.; Dong, X.; Zhang, Z.; Fan, W. MicroRNA-146a improves sepsis-induced cardiomyopathy by regulating the TLR-4/NF-κB signaling pathway. Exp. Ther. Med. 2019, 18, 779–785. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Feng, J.; Xi, C.; Xu, J.; Sun, L. miR-146a Attenuates Sepsis-Induced Myocardial Dysfunction by Suppressing IRAK1 and TRAF6 via Targeting ErbB4 Expression. Oxid. Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, X.; Zhang, X.; Ha, T.; Ma, H.; Liu, L.; Kalbfleisch, J.H.; Gao, X.; Kao, R.L.; Williams, D.L.; et al. Attenuation of Cardiac Dysfunction in Polymicrobial Sepsis by MicroRNA-146a Is Mediated via Targeting of IRAK1 and TRAF6 Expression. J. Immunol. 2015, 195, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Ji, Y.; Zhang, C.; Guo, X.; Zhang, Y.; Jia, S.; Ma, W.; Fan, Y.; Wang, C. Circulating MiR-146a May be a Potential Biomarker of Coronary Heart Disease in Patients with Subclinical Hypothyroidism. Cell. Physiol. Biochem. 2018, 45, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.I.; Fish, J.E. Micro RNA -146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Yan, X.; Cheng, X.; He, X.; Zheng, W. LncRNA MALAT1 regulates sepsis-induced cardiac inflammation and dysfunction via interaction with miR-125b and p38 MAPK/NFκB. Int. Immunopharmacol. 2018, 55, 69–76. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Ha, T.; Gao, M.; Liu, L.; Wang, R.; Yu, K.; Kalbfleisch, J.H.; Kao, R.L.; Williams, D.L.; et al. MicroRNA-125b Prevents Cardiac Dysfunction in Polymicrobial Sepsis by Targeting TRAF6-Mediated Nuclear Factor κB Activation and p53-Mediated Apoptotic Signaling. J. Infect. Dis. 2016, 214, 1773–1783. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y. MiR-146b protect against sepsis induced mice myocardial injury through inhibition of Notch1. J. Mol. Histol. 2018, 49, 411–417. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Yang, Y.; Wang, Y.; Peng, T.; Chang, J.; Caldwell, C.C.; Zingarelli, B.; Fan, G.-C. Loss of duplexmiR-223 (5p and 3p) aggravates myocardial depression and mortality in polymicrobial sepsis. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 701–711. [Google Scholar] [CrossRef]
- Wang, H.; Bei, Y.; Shen, S.; Huang, P.; Shi, J.; Zhang, J.; Sun, Q.; Chen, Y.; Yang, Y.; Xu, T.; et al. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. J. Mol. Cell. Cardiol. 2016, 94, 43–53. [Google Scholar] [CrossRef]
- Zhang, H.; Caudle, Y.; Shaikh, A.; Yao, B.; Yin, D. Inhibition of microRNA-23b prevents polymicrobial sepsis-induced cardiac dysfunction by modulating TGIF1 and PTEN. Biomed. Pharmacother. 2018, 103, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, Y.; Shaikh, Z.; Li, H.; Zhang, H.; Caudle, Y.; Zheng, S.; Yan, H.; Hu, D.; Stuart, C.; et al. MicroRNA-155 attenuates late sepsis-induced cardiac dysfunction through JNK and β-arrestin 2. Oncotarget 2017, 8, 47317–47329. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bei, Y.; Huang, P.; Zhou, Q.; Shi, J.; Sun, Q.; Zhong, J.; Li, X.; Kong, X.; Xiao, J. Inhibition of miR-155 Protects Against LPS-induced Cardiac Dysfunction and Apoptosis in Mice. Mol. Ther. Nucl. Acids 2016, 5, e374. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirna, M.; Paar, V.; Rezar, R.; Topf, A.; Eber, M.; Hoppe, U.C.; Lichtenauer, M.; Jung, C. MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future? Cells 2019, 8, 1352. https://doi.org/10.3390/cells8111352
Mirna M, Paar V, Rezar R, Topf A, Eber M, Hoppe UC, Lichtenauer M, Jung C. MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future? Cells. 2019; 8(11):1352. https://doi.org/10.3390/cells8111352
Chicago/Turabian StyleMirna, Moritz, Vera Paar, Richard Rezar, Albert Topf, Miriam Eber, Uta C. Hoppe, Michael Lichtenauer, and Christian Jung. 2019. "MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future?" Cells 8, no. 11: 1352. https://doi.org/10.3390/cells8111352
APA StyleMirna, M., Paar, V., Rezar, R., Topf, A., Eber, M., Hoppe, U. C., Lichtenauer, M., & Jung, C. (2019). MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future? Cells, 8(11), 1352. https://doi.org/10.3390/cells8111352






