Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy
Abstract
1. Introduction
2. Androgens and Androgen Receptors (AR) on Bone Health
2.1. The Effects of Androgen and Androgen Receptors on Bone Growth
2.2. The Effects of Androgen and Androgen Receptors on Bone Homeostasis
2.3. Regulation of Bone Turnover in Men
3. The Impacts of Androgen Deficiency/Excess on Bone Remodeling in Human
3.1. Hypogonadism in Men (Androgen Deficiency)
3.2. Isolated Hypogonadotropic Hypogonadism (IHH)
3.3. Klinefelter’s Syndrome (KS)
3.4. Constitutional Delay of Growth and Puberty (CDGP)
3.5. Androgen Deprivation Therapy and Castration
3.6. Aging
3.7. Androgen Insensitivity Syndrome (AIS)
3.8. Hyperandrogenism (Androgen Excessive)
4. Androgens/AR Actions on Skeletons in Animal Studies
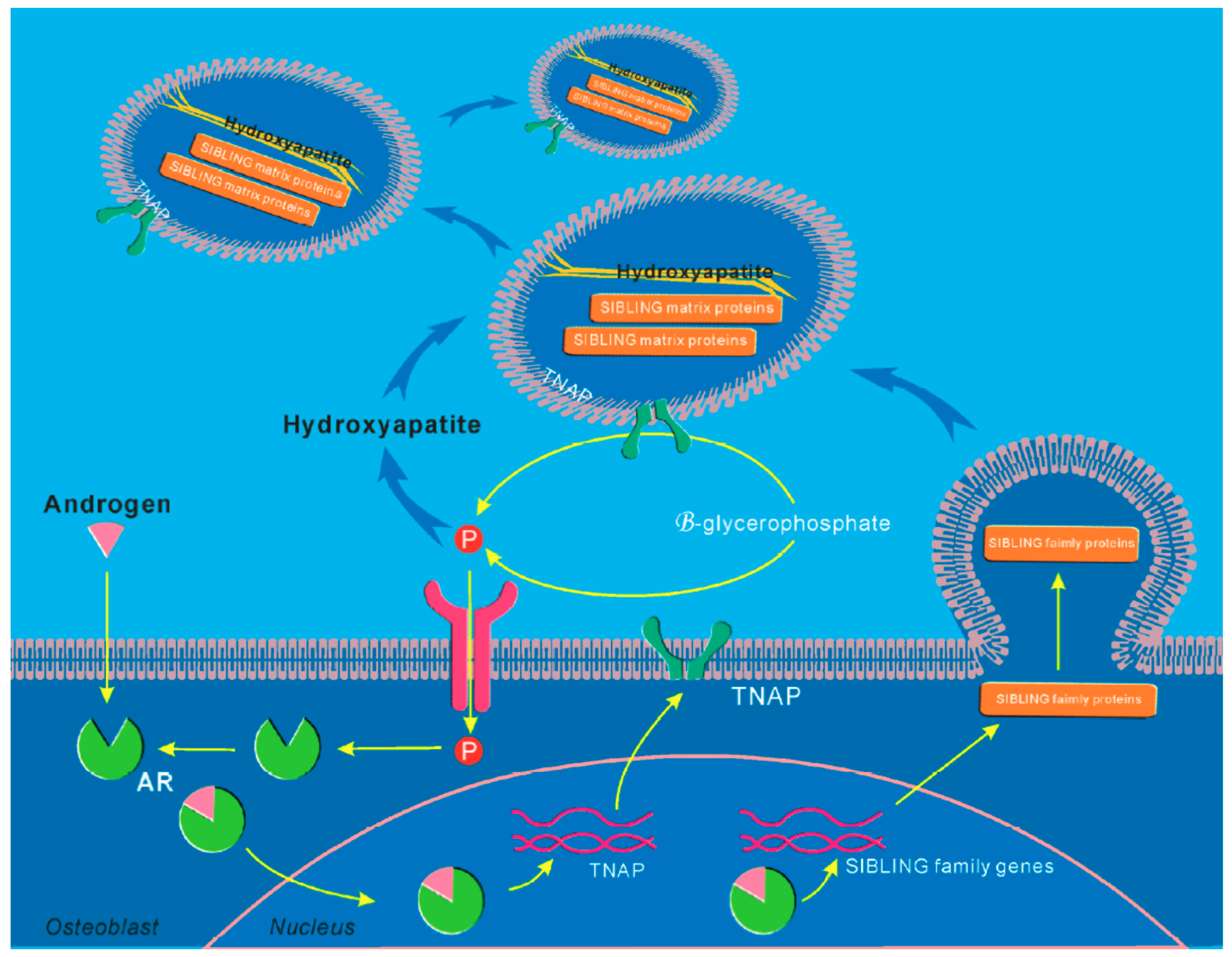
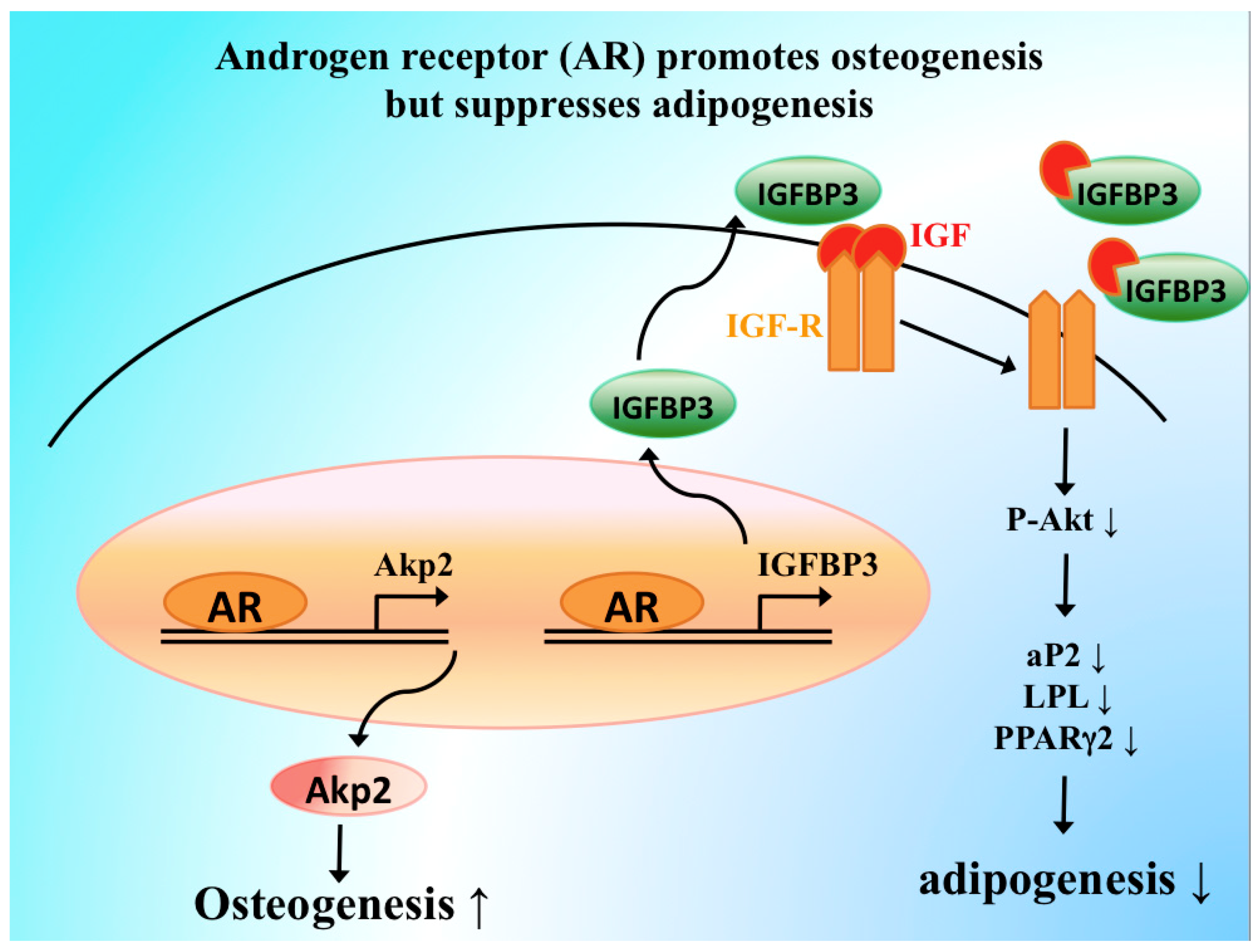
5. Molecular Mechanisms of AR Signaling in Skeletal Stem and Progenitor Cells
6. Effects of Androgen Therapy on Bone
7. Conclusion and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown-Sequard, C.E. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet 1889, 134, 105–107. [Google Scholar] [CrossRef]
- Chang, C.S.; Kokontis, J.; Liao, S.T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 1988, 240, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.N.; Katzenellenbogen, B.S. Androgen-uterine interactions: An assessment of androgen interaction with the testosterone- and estrogen-receptor systems and stimulation of uterine growth and progesterone-receptor synthesis. Mol. Cell. Endocrinol. 1979, 15, 91–108. [Google Scholar] [CrossRef]
- Zava, D.T.; McGuire, W.L. Androgen action through estrogen receptor in a human breast cancer cell line. Endocrinology 1978, 103, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Moverare, S.; Venken, K.; Eriksson, A.L.; Andersson, N.; Skrtic, S.; Wergedal, J.; Mohan, S.; Salmon, P.; Bouillon, R.; Gustafsson, J.A.; et al. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13573–13578. [Google Scholar] [CrossRef] [PubMed]
- Pines, M.; Hurwitz, S. The role of the growth plate in longitudinal bone growth. Poult. Sci. 1991, 70, 1806–1814. [Google Scholar] [CrossRef]
- Shulman, D.I.; Francis, G.L.; Palmert, M.R.; Eugster, E.A.; Lawson Wilkins Pediatric Endocrine Society Drug and Therapeutics Committee. Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development. Pediatrics 2008, 121, e975–e983. [Google Scholar] [CrossRef]
- Cutler, G.B., Jr. The role of estrogen in bone growth and maturation during childhood and adolescence. J. Steroid Biochem. Mol. Biol. 1997, 61, 141–144. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef]
- Morishima, A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar]
- Duan, Y.; Turner, C.H.; Kim, B.T.; Seeman, E. Sexual dimorphism in vertebral fragility is more the result of gender differences in age-related bone gain than bone loss. J. Bone Miner. Res. 2001, 16, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, F.; Venken, K.; Kopchick, J.J.; Torcasio, A.; van Lenthe, G.H.; Boonen, S.; Vanderschueren, D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and igf-1: Evidence from pubertal mouse models. J. Bone Miner. Res. 2010, 25, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.; Moverare-Skrtic, S.; Kopchick, J.J.; Coschigano, K.T.; Ohlsson, C.; Boonen, S.; Bouillon, R.; Vanderschueren, D. Impact of androgens, growth hormone, and igf-i on bone and muscle in male mice during puberty. J. Bone Miner. Res. 2007, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.K.; Vandenput, L.; Moverare Skrtic, S.; Vanderschueren, D.; Boonen, S.; Bouillon, R.; Ohlsson, C. Androgens and the skeleton. Miner. Endocrinol. 2005, 30, 15–25. [Google Scholar]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Van Herck, E.; Nijs, J.; Ederveen, A.G.; De Coster, R.; Bouillon, R. Aromatase inhibition impairs skeletal modeling and decreases bone mineral density in growing male rats. Endocrinology 1997, 138, 2301–2307. [Google Scholar] [CrossRef]
- DiGirolamo, D.J.; Mukherjee, A.; Fulzele, K.; Gan, Y.; Cao, X.; Frank, S.J.; Clemens, T.L. Mode of growth hormone action in osteoblasts. J. Biol. Chem. 2007, 282, 31666–31674. [Google Scholar] [CrossRef]
- Kassem, M.; Blum, W.; Ristelli, J.; Mosekilde, L.; Eriksen, E.F. Growth hormone stimulates proliferation and differentiation of normal human osteoblast-like cells in vitro. Calcif. Tissue Int. 1993, 52, 222–226. [Google Scholar] [CrossRef]
- Li, H.; Bartold, P.M.; Zhang, C.Z.; Clarkson, R.W.; Young, W.G.; Waters, M.J. Growth hormone and insulin-like growth factor i induce bone morphogenetic proteins 2 and 4: A mediator role in bone and tooth formation? Endocrinology 1998, 139, 3855–3862. [Google Scholar]
- Guntur, A.R.; Rosen, C.J. Igf-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013, 2, 437. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rosen, C.J.; Visser, M.; Hannan, M.T.; Harris, T.; Wilson, P.W.; Kiel, D.P. Association between insulin-like growth factor i and bone mineral density in older women and men: The framingham heart study. J. Clin. Endocrinol. Metab. 1998, 83, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Tsai, M.Y.; Xu, Q.; Mu, X.M.; Lardy, H.; Huang, K.E.; Lin, H.; Yeh, S.D.; Altuwaijri, S.; Zhou, X.; et al. Generation and characterization of androgen receptor knockout (arko) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 13498–13503. [Google Scholar] [CrossRef] [PubMed]
- Ucer, S.; Iyer, S.; Bartell, S.M.; Martin-Millan, M.; Han, L.; Kim, H.N.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; Almeida, M.; et al. The effects of androgens on murine cortical bone do not require ar or eralpha signaling in osteoblasts and osteoclasts. J. Bone Miner. Res. 2015, 30, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Sinnesael, M.; Claessens, F.; Laurent, M.; Dubois, V.; Boonen, S.; Deboel, L.; Vanderschueren, D. Androgen receptor (ar) in osteocytes is important for the maintenance of male skeletal integrity: Evidence from targeted ar disruption in mouse osteocytes. J. Bone Miner. Res. 2012, 27, 2535–2543. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. The effects of androgen deficiency on murine bone remodeling and bone mineral density are mediated via cells of the osteoblastic lineage. Endocrinology 1997, 138, 4013–4021. [Google Scholar] [CrossRef]
- Lin, P.W.; Lan, K.C.; Tsai, M.Y.; Shyr, C.R.; Chang, C.; Huang, K.E.; Kang, H.Y. The differential effects of sex hormones on the bone metabolism in mice with androgen receptor deficiency. Adapt. Med. 2018, 10, 143–154. [Google Scholar] [CrossRef]
- Fatayerji, D.; Eastell, R. Age-related changes in bone turnover in men. J. Bone Miner. Res. 1999, 14, 1203–1210. [Google Scholar] [CrossRef]
- Meier, C.; Nguyen, T.V.; Center, J.R.; Seibel, M.J.; Eisman, J.A. Bone resorption and osteoporotic fractures in elderly men: The dubbo osteoporosis epidemiology study. J. Bone Miner. Res. 2005, 20, 579–587. [Google Scholar] [CrossRef]
- Szulc, P.; Montella, A.; Delmas, P.D. High bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: The prospective minos study. Ann. Rheum. Dis. 2008, 67, 1249–1255. [Google Scholar] [CrossRef]
- Bauer, D.C.; Garnero, P.; Harrison, S.L.; Cauley, J.A.; Eastell, R.; Ensrud, K.E.; Orwoll, E.; Osteoporotic Fractures in Men Research Group. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: The mros study. J. Bone Miner. Res. 2009, 24, 2032–2038. [Google Scholar] [CrossRef]
- Sinnesael, M.; Jardi, F.; Deboel, L.; Laurent, M.R.; Dubois, V.; Zajac, J.D.; Davey, R.A.; Carmeliet, G.; Claessens, F.; Vanderschueren, D. The androgen receptor has no direct antiresorptive actions in mouse osteoclasts. Mol. Cell. Endocrinol. 2015, 411, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Kasperk, C.; Helmboldt, A.; Borcsok, I.; Heuthe, S.; Cloos, O.; Niethard, F.; Ziegler, R. Skeletal site-dependent expression of the androgen receptor in human osteoblastic cell populations. Calcif. Tissue Int. 1997, 61, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Jilka, R.L.; Boyce, B.F.; Girasole, G.; Broxmeyer, H.; Dalrymple, S.A.; Murray, R.; Manolagas, S.C. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J. Clin. Investig. 1995, 95, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Kasperk, C.H.; Wakley, G.K.; Hierl, T.; Ziegler, R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J. Bone Miner. Res. 1997, 12, 464–471. [Google Scholar] [CrossRef]
- Kasperk, C.H.; Wergedal, J.E.; Farley, J.R.; Linkhart, T.A.; Turner, R.T.; Baylink, D.J. Androgens directly stimulate proliferation of bone cells in vitro. Endocrinology 1989, 124, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone therapy in men with hypogonadism: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Francis, R.M. The effects of testosterone on osteoporosis in men. Clin. Endocrinol. 1999, 50, 411–414. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Neer, R.M.; Biller, B.M.; Crawford, J.D.; Klibanski, A. Osteopenia in men with a history of delayed puberty. N. Engl. J. Med. 1992, 326, 600–604. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Klibanski, A.; Neer, R.M. A longitudinal evaluation of bone mineral density in adult men with histories of delayed puberty. J. Clin. Endocrinol. Metab. 1996, 81, 1152–1155. [Google Scholar]
- Benito, M.; Gomberg, B.; Wehrli, F.W.; Weening, R.H.; Zemel, B.; Wright, A.C.; Song, H.K.; Cucchiara, A.; Snyder, P.J. Deterioration of trabecular architecture in hypogonadal men. J. Clin. Endocrinol. Metab. 2003, 88, 1497–1502. [Google Scholar] [CrossRef]
- Leifke, E.; Korner, H.C.; Link, T.M.; Behre, H.M.; Peters, P.E.; Nieschlag, E. Effects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal men. Eur. J. Endocrinol. 1998, 138, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mellstrom, D.; Johnell, O.; Ljunggren, O.; Eriksson, A.L.; Lorentzon, M.; Mallmin, H.; Holmberg, A.; Redlund-Johnell, I.; Orwoll, E.; Ohlsson, C. Free testosterone is an independent predictor of bmd and prevalent fractures in elderly men: Mros sweden. J. Bone Miner. Res. 2006, 21, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Melton, L.J., 3rd; O’Fallon, W.M.; Riggs, B.L. Risk factors for spinal osteoporosis in men. Am. J. Med. 1983, 75, 977–983. [Google Scholar] [CrossRef]
- Stanley, H.L.; Schmitt, B.P.; Poses, R.M.; Deiss, W.P. Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men? J. Am. Geriatr. Soc. 1991, 39, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Behre, H.M.; Kliesch, S.; Leifke, E.; Link, T.M.; Nieschlag, E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J. Clin. Endocrinol. Metab. 1997, 82, 2386–2390. [Google Scholar] [CrossRef]
- Lee, M.J.; Ryu, H.K.; An, S.Y.; Jeon, J.Y.; Lee, J.I.; Chung, Y.S. Testosterone replacement and bone mineral density in male pituitary tumor patients. Endocrinol. Metab. 2014, 29, 48–53. [Google Scholar] [CrossRef]
- Basurto, L.; Zarate, A.; Gomez, R.; Vargas, C.; Saucedo, R.; Galvan, R. Effect of testosterone therapy on lumbar spine and hip mineral density in elderly men. Aging Male 2008, 11, 140–145. [Google Scholar] [CrossRef]
- Aversa, A.; Bruzziches, R.; Francomano, D.; Greco, E.A.; Fornari, R.; Di Luigi, L.; Lenzi, A.; Migliaccio, S. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: Results from a 36 months controlled study. Aging Male 2012, 15, 96–102. [Google Scholar] [CrossRef]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Holmes, J.H.; Dlewati, A.; Staley, J.; Santanna, J.; Kapoor, S.C.; et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J. Clin. Endocrinol. Metab. 1999, 84, 1966–1972. [Google Scholar] [CrossRef]
- Haider, A.; Meergans, U.; Traish, A.; Saad, F.; Doros, G.; Lips, P.; Gooren, L. Progressive improvement of t-scores in men with osteoporosis and subnormal serum testosterone levels upon treatment with testosterone over six years. Int. J. Endocrinol. 2014, 2014, 496948. [Google Scholar] [CrossRef]
- Jackson, J.A.; Kleerekoper, M.; Parfitt, A.M.; Rao, D.S.; Villanueva, A.R.; Frame, B. Bone histomorphometry in hypogonadal and eugonadal men with spinal osteoporosis. J. Clin. Endocrinol. Metab. 1987, 65, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Peacock, M.; Aaron, J.E.; Selby, P.L.; Taylor, G.A.; Thompson, J.; Marshall, D.H.; Horsman, A. Osteoporosis in hypogonadal men: Role of decreased plasma 1,25-dihydroxyvitamin d, calcium malabsorption, and low bone formation. Bone 1986, 7, 261–268. [Google Scholar] [CrossRef]
- Baran, D.T.; Bergfeld, M.A.; Teitelbaum, S.L.; Avioli, L.V. Effect of testosterone therapy on bone formation in an osteoporotic hypogonadal male. Calcif. Tissue Res. 1978, 26, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Jones, T.H.; Eastell, R. Treatment of isolated hypogonadotropic hypogonadism effect on bone mineral density and bone turnover. J. Clin. Endocrinol. Metab. 1997, 82, 658–665. [Google Scholar] [CrossRef][Green Version]
- Breuil, V.; Euller-Ziegler, L. Gonadal dysgenesis and bone metabolism. Jt. Bone Spine 2001, 68, 26–33. [Google Scholar] [CrossRef]
- Bojesen, A.; Birkebaek, N.; Kristensen, K.; Heickendorff, L.; Mosekilde, L.; Christiansen, J.S.; Gravholt, C.H. Bone mineral density in klinefelter syndrome is reduced and primarily determined by muscle strength and resorptive markers, but not directly by testosterone. Osteoporos. Int. 2011, 22, 1441–1450. [Google Scholar] [CrossRef]
- Rohani, F.; Alai, M.R.; Moradi, S.; Amirkashani, D. Evaluation of near final height in boys with constitutional delay in growth and puberty. Endocr. Connect. 2018, 7, 456–459. [Google Scholar] [CrossRef]
- Krupa, B.; Miazgowski, T. Bone mineral density and markers of bone turnover in boys with constitutional delay of growth and puberty. J. Clin. Endocrinol. Metab. 2005, 90, 2828–2830. [Google Scholar] [CrossRef]
- Bertelloni, S.; Baroncelli, G.I.; Ferdeghini, M.; Perri, G.; Saggese, G. Normal volumetric bone mineral density and bone turnover in young men with histories of constitutional delay of puberty. J. Clin. Endocrinol. Metab. 1998, 83, 4280–4283. [Google Scholar] [CrossRef][Green Version]
- Ojeda, S.; Lloret, M.; Naranjo, A.; Deniz, F.; Chesa, N.; Dominguez, C.; Lara, P.C. Androgen deprivation in prostate cancer and the long-term risk of fracture. Actas Urol. Esp. 2017, 41, 491–496. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, G.; Chan, P.H.; Hai, J.; Yeung, C.Y.; Fong, C.H.; Woo, Y.C.; Ho, K.L.; Yiu, M.K.; Leung, F.; et al. Androgen deprivation therapy and fracture risk in chinese patients with prostate carcinoma. PLoS ONE 2017, 12, e0171495. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Daneshmand, S. Androgen deprivation therapy: Evidence-based management of side effects. BJU Int. 2013, 111, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Warming, L.; Hassager, C.; Christiansen, C. Changes in bone mineral density with age in men and women: A longitudinal study. Osteoporos. Int. 2002, 13, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hughes, I.A.; Davies, J.D.; Bunch, T.I.; Pasterski, V.; Mastroyannopoulou, K.; MacDougall, J. Androgen insensitivity syndrome. Lancet 2012, 380, 1419–1428. [Google Scholar] [CrossRef]
- Danilovic, D.L.; Correa, P.H.; Costa, E.M.; Melo, K.F.; Mendonca, B.B.; Arnhold, I.J. Height and bone mineral density in androgen insensitivity syndrome with mutations in the androgen receptor gene. Osteoporos. Int. 2007, 18, 369–374. [Google Scholar] [CrossRef]
- Frank, G.R. Role of estrogen and androgen in pubertal skeletal physiology. Med. Pediatr. Oncol. 2003, 41, 217–221. [Google Scholar] [CrossRef]
- Ganie, M.A.; Chakraborty, S.; Sehgal, A.; Sreejith, M.; Kandasamy, D.; Jana, M.; Rashid, A. Bone mineral density is unaltered in women with polycystic ovary syndrome. Horm. Metab. Res. 2018, 50, 754–760. [Google Scholar] [CrossRef]
- Katulski, K.; Slawek, S.; Czyzyk, A.; Podfigurna-Stopa, A.; Paczkowska, K.; Ignaszak, N.; Podkowa, N.; Meczekalski, B. Bone mineral density in women with polycystic ovary syndrome. J. Endocrinol. Investig. 2014, 37, 1219–1224. [Google Scholar] [CrossRef]
- Yang, H.Y.; Lee, H.S.; Huang, W.T.; Chen, M.J.; Chen, S.C.; Hsu, Y.H. Increased risk of fractures in patients with polycystic ovary syndrome: A nationwide population-based retrospective cohort study. J. Bone Miner. Metab. 2018, 36, 741–748. [Google Scholar] [CrossRef]
- Rubin, K.H.; Glintborg, D.; Nybo, M.; Andersen, M.; Abrahamsen, B. Fracture risk is decreased in women with polycystic ovary syndrome: A register-based and population-based cohort study. J. Bone Miner. Res. 2016, 31, 709–717. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Klibanski, A.; Neer, R.M.; Greenspan, S.L.; Rosenthal, D.I.; Crowley, W.F., Jr. Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann. Intern. Med. 1987, 106, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Klibanski, A.; Neer, R.M.; Doppelt, S.H.; Rosenthal, D.I.; Segre, G.V.; Crowley, W.F., Jr. Increases in bone density during treatment of men with idiopathic hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 1989, 69, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, S.L.; Neer, R.M.; Ridgway, E.C.; Klibanski, A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann. Intern. Med. 1986, 104, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, S.L.; Oppenheim, D.S.; Klibanski, A. Importance of gonadal steroids to bone mass in men with hyperprolactinemic hypogonadism. Ann. Intern. Med. 1989, 110, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Klinefelter, H.F., Jr.; Reifenstein, E.C., Jr.; Albright, F., Jr. Syndrome characterized by gynecomastia, aspermatogenesis without a-leydigism, and increased excretion of follicle-stimulating hormone. J. Clin. Endocrinol. Metab. 1942, 2, 615. [Google Scholar] [CrossRef]
- Stagi, S.; Di Tommaso, M.; Manoni, C.; Scalini, P.; Chiarelli, F.; Verrotti, A.; Lapi, E.; Giglio, S.; Dosa, L.; De Martino, M. Bone mineral status in children and adolescents with klinefelter syndrome. Int. J. Endocrinol. 2016, 2016, 3032759. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Birkebaek, N.; Gravholt, C.H. Increased mortality in klinefelter syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 3830–3834. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Birkebaek, N.H.; Gravholt, C.H. Morbidity in klinefelter syndrome: A danish register study based on hospital discharge diagnoses. J. Clin. Endocrinol. Metab. 2006, 91, 1254–1260. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Higgins, C.D.; Schoemaker, M.J.; Wright, A.F.; Jacobs, P.A.; United Kingdom Clinical Cytogenetics Group. Mortality in patients with klinefelter syndrome in britain: A cohort study. J. Clin. Endocrinol. Metab. 2005, 90, 6516–6522. [Google Scholar] [CrossRef]
- Kubler, A.; Schulz, G.; Cordes, U.; Beyer, J.; Krause, U. The influence of testosterone substitution on bone mineral density in patients with klinefelter’s syndrome. Exp. Clin. Endocrinol. 1992, 100, 129–132. [Google Scholar] [CrossRef]
- Tahani, N.; Nieddu, L.; Prossomariti, G.; Spaziani, M.; Granato, S.; Carlomagno, F.; Anzuini, A.; Lenzi, A.; Radicioni, A.F.; Romagnoli, E. Long-term effect of testosterone replacement therapy on bone in hypogonadal men with klinefelter syndrome. Endocrine 2018, 61, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.H.; Pun, K.K.; Wang, C. Loss of bone mass in patients with klinefelter’s syndrome despite sufficient testosterone replacement. Osteoporos. Int. 1993, 3, 3–7. [Google Scholar] [CrossRef]
- Van den Bergh, J.P.; Hermus, A.R.; Spruyt, A.I.; Sweep, C.G.; Corstens, F.H.; Smals, A.G. Bone mineral density and quantitative ultrasound parameters in patients with klinefelter’s syndrome after long-term testosterone substitution. Osteoporos. Int. 2001, 12, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Schipilliti, M.; Vinanzi, C.; Garolla, A.; Di Mambro, A.; Selice, R.; Lenzi, A.; Foresta, C. Bone mass in subjects with klinefelter syndrome: Role of testosterone levels and androgen receptor gene cag polymorphism. J. Clin. Endocrinol. Metab. 2011, 96, E739–E745. [Google Scholar] [CrossRef]
- Luisetto, G.; Mastrogiacomo, I.; Bonanni, G.; Pozzan, G.; Botteon, S.; Tizian, L.; Galuppo, P. Bone mass and mineral metabolism in klinefelter’s syndrome. Osteoporos. Int. 1995, 5, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Wishart, J.M.; O’Loughlin, P.D.; Morris, H.A.; Need, A.G.; Nordin, B.E. Osteoporosis and klinefelter’s syndrome. Clin. Endocrinol. 1992, 36, 113–118. [Google Scholar] [CrossRef]
- Zerbini, C.A.F.; Clark, P.; Mendez-Sanchez, L.; Pereira, R.M.R.; Messina, O.D.; Una, C.R.; Adachi, J.D.; Lems, W.F.; Cooper, C.; Lane, N.E.; et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos. Int. 2017, 28, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Bertelloni, S.; Baroncelli, G.I.; Battini, R.; Perri, G.; Saggese, G. Short-term effect of testosterone treatment on reduced bone density in boys with constitutional delay of puberty. J. Bone Miner. Res. 1995, 10, 1488–1495. [Google Scholar] [CrossRef]
- Mauras, N.; Haymond, M.W.; Darmaun, D.; Vieira, N.E.; Abrams, S.A.; Yergey, A.L. Calcium and protein kinetics in prepubertal boys. Positive effects of testosterone. J. Clin. Investig. 1994, 93, 1014–1019. [Google Scholar] [CrossRef]
- Allen, D.B.; Cuttler, L. Clinical practice. Short stature in childhood--challenges and choices. N. Engl. J. Med. 2013, 368, 1220–1228. [Google Scholar] [CrossRef]
- Joss, E.E.; Schmidt, H.A.; Zuppinger, K.A. Oxandrolone in constitutionally delayed growth, a longitudinal study up to final height. J. Clin. Endocrinol. Metab. 1989, 69, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; McCauley, E.; Brown, D.R.; Dudley, R. Oxandrolone therapy in constitutionally delayed growth and puberty. Bio-technology general corporation cooperative study group. Pediatrics 1995, 96, 1095–1100. [Google Scholar] [PubMed]
- Smith, M.R. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology 2002, 60, 79–85. [Google Scholar] [CrossRef]
- Poulsen, M.H.; Frost, M.; Abrahamsen, B.; Gerke, O.; Walter, S.; Lund, L. Osteoporosis and prostate cancer; a 24-month prospective observational study during androgen deprivation therapy. Scand. J. Urol. 2019, 53, 34–39. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.Y.; Kim, K.J.; Hong, N.; Kim, J.W.; Hah, Y.S.; Koo, K.C.; Kim, J.H.; Cho, K.S. Effect of androgen-deprivation therapy on bone mineral density in patients with prostate cancer: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 113. [Google Scholar] [CrossRef]
- Smith, M.R.; McGovern, F.J.; Fallon, M.A.; Schoenfeld, D.; Kantoff, P.W.; Finkelstein, J.S. Low bone mineral density in hormone-naive men with prostate carcinoma. Cancer 2001, 91, 2238–2245. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Goodwin, J.S. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 2005, 352, 154–164. [Google Scholar] [CrossRef]
- Smith, M.R.; Lee, W.C.; Brandman, J.; Wang, Q.; Botteman, M.; Pashos, C.L. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J. Clin. Oncol. 2005, 23, 7897–7903. [Google Scholar] [CrossRef]
- Smith, M.R.; Boyce, S.P.; Moyneur, E.; Duh, M.S.; Raut, M.K.; Brandman, J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J. Urol. 2006, 175, 136–139. [Google Scholar] [CrossRef]
- Stoch, S.A.; Parker, R.A.; Chen, L.; Bubley, G.; Ko, Y.J.; Vincelette, A.; Greenspan, S.L. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J. Clin. Endocrinol. Metab. 2001, 86, 2787–2791. [Google Scholar] [CrossRef]
- Berruti, A.; Dogliotti, L.; Terrone, C.; Cerutti, S.; Isaia, G.; Tarabuzzi, R.; Reimondo, G.; Mari, M.; Ardissone, P.; De Luca, S.; et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J. Urol. 2002, 167, 2361–2367. [Google Scholar] [CrossRef]
- Smith, M.R.; Finkelstein, J.S.; McGovern, F.J.; Zietman, A.L.; Fallon, M.A.; Schoenfeld, D.A.; Kantoff, P.W. Changes in body composition during androgen deprivation therapy for prostate cancer. J. Clin. Endocrinol. Metab. 2002, 87, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Zebaze, R.M.; Ghasem-Zadeh, A.; Bohte, A.; Iuliano-Burns, S.; Mirams, M.; Price, R.I.; Mackie, E.J.; Seeman, E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: A cross-sectional study. Lancet 2010, 375, 1729–1736. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Kopperdahl, D.L.; Kiel, D.P.; Keaveny, T.M.; Bouxsein, M.L. Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by qct-based finite element analysis. J. Bone Miner. Res. 2011, 26, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Diamond, T.; Campbell, J.; Bryant, C.; Lynch, W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: Longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer 1998, 83, 1561–1566. [Google Scholar] [CrossRef]
- Smith, M.R.; McGovern, F.J.; Zietman, A.L.; Fallon, M.A.; Hayden, D.L.; Schoenfeld, D.A.; Kantoff, P.W.; Finkelstein, J.S. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2001, 345, 948–955. [Google Scholar] [CrossRef]
- Smith, M.R.; Eastham, J.; Gleason, D.M.; Shasha, D.; Tchekmedyian, S.; Zinner, N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J. Urol. 2003, 169, 2008–2012. [Google Scholar] [CrossRef]
- Dunn, J.F.; Nisula, B.C.; Rodbard, D. Transport of steroid hormones: Binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J. Clin. Endocrinol. Metab. 1981, 53, 58–68. [Google Scholar] [CrossRef]
- Roy, T.A.; Blackman, M.R.; Harman, S.M.; Tobin, J.D.; Schrager, M.; Metter, E.J. Interrelationships of serum testosterone and free testosterone index with ffm and strength in aging men. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E284–E294. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J., 3rd; Atkinson, E.J.; O’Fallon, W.M.; Klee, G.G.; Riggs, B.L. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J. Clin. Endocrinol. Metab. 1998, 83, 2266–2274. [Google Scholar]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Pencina, M.; Jasuja, G.K.; Travison, T.G.; Coviello, A.; Orwoll, E.; Wang, P.Y.; Nielson, C.; Wu, F.; Tajar, A.; et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the framingham heart study and applied to three geographically distinct cohorts. J. Clin. Endocrinol. Metab. 2011, 96, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.; Blank, J.B.; Barrett-Connor, E.; Cauley, J.; Cummings, S.; Ensrud, K.; Lewis, C.; Cawthon, P.M.; Marcus, R.; Marshall, L.M.; et al. Design and baseline characteristics of the osteoporotic fractures in men (mros) study—A large observational study of the determinants of fracture in older men. Contemp. Clin. Trials 2005, 26, 569–585. [Google Scholar] [CrossRef]
- Travison, T.G.; Vesper, H.W.; Orwoll, E.; Wu, F.; Kaufman, J.M.; Wang, Y.; Lapauw, B.; Fiers, T.; Matsumoto, A.M.; Bhasin, S. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the united states and europe. J. Clin. Endocrinol. Metab. 2017, 102, 1161–1173. [Google Scholar] [CrossRef]
- Mellstrom, D.; Vandenput, L.; Mallmin, H.; Holmberg, A.H.; Lorentzon, M.; Oden, A.; Johansson, H.; Orwoll, E.S.; Labrie, F.; Karlsson, M.K.; et al. Older men with low serum estradiol and high serum shbg have an increased risk of fractures. J. Bone Miner. Res. 2008, 23, 1552–1560. [Google Scholar] [CrossRef]
- Meier, C.; Nguyen, T.V.; Handelsman, D.J.; Schindler, C.; Kushnir, M.M.; Rockwood, A.L.; Meikle, A.W.; Center, J.R.; Eisman, J.A.; Seibel, M.J. Endogenous sex hormones and incident fracture risk in older men: The dubbo osteoporosis epidemiology study. Arch. Intern. Med. 2008, 168, 47–54. [Google Scholar] [CrossRef]
- Boonen, S.; Vanderschueren, D.; Cheng, X.G.; Verbeke, G.; Dequeker, J.; Geusens, P.; Broos, P.; Bouillon, R. Age-related (type ii) femoral neck osteoporosis in men: Biochemical evidence for both hypovitaminosis d- and androgen deficiency-induced bone resorption. J. Bone Miner. Res. 1997, 12, 2119–2126. [Google Scholar] [CrossRef]
- Ucer, S.; Iyer, S.; Kim, H.N.; Han, L.; Rutlen, C.; Allison, K.; Thostenson, J.D.; De Cabo, R.; Jilka, R.L.; O’Brien, C.; et al. The effects of aging and sex steroid deficiency on the murine skeleton are independent and mechanistically distinct. J. Bone Miner. Res. 2017, 32, 560–574. [Google Scholar] [CrossRef]
- Labrie, F.; Belanger, A.; Cusan, L.; Gomez, J.L.; Candas, B. Marked decline in serum concentrations of adrenal c19 sex steroid precursors and conjugated androgen metabolites during aging. J. Clin. Endocrinol. Metab. 1997, 82, 2396–2402. [Google Scholar] [CrossRef]
- Gurnell, E.M.; Chatterjee, V.K. Dehydroepiandrosterone replacement therapy. Eur. J. Endocrinol. 2001, 145, 103–106. [Google Scholar] [CrossRef][Green Version]
- Greendale, G.A.; Edelstein, S.; Barrett-Connor, E. Endogenous sex steroids and bone mineral density in older women and men: The rancho bernardo study. J. Bone Miner. Res. 1997, 12, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Kritz-Silverstein, D.; Edelstein, S.L. A prospective study of dehydroepiandrosterone sulfate (dheas) and bone mineral density in older men and women. Am. J. Epidemiol. 1993, 137, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, N.; Motos, M.A. Androgen insensitivity syndrome. Gynecol. Endocrinol. 2013, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, B.; Trifiro, M.A. Androgen insensitivity syndrome. In Genereviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Bertelloni, S.; Baroncelli, G.I.; Mora, S. Bone health in disorders of sex differentiation. Sex. Dev. 2010, 4, 270–284. [Google Scholar] [CrossRef]
- Bertelloni, S.; Meriggiola, M.C.; Dati, E.; Balsamo, A.; Baroncelli, G.I. Bone mineral density in women living with complete androgen insensitivity syndrome and intact testes or removed gonads. Sex. Dev. 2017, 11, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Goswami, D.; Trikudanathan, S.; Creighton, S.M.; Conway, G.S. Comparison of bone mineral density and body proportions between women with complete androgen insensitivity syndrome and women with gonadal dysgenesis. Eur. J. Endocrinol. 2008, 159, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Chin, V.L.; Sheffer-Babila, S.; Lee, T.A.; Tanaka, K.; Zhou, P. A case of complete androgen insensitivity syndrome with a novel androgen receptor mutation. J. Pediatr. Endocrinol. Metab. 2012, 25, 1145–1151. [Google Scholar] [CrossRef]
- King, T.F.J.; Wat, W.Z.M.; Creighton, S.M.; Conway, G.S. Bone mineral density in complete androgen insensitivity syndrome and the timing of gonadectomy. Clin. Endocrinol. 2017, 87, 136–140. [Google Scholar] [CrossRef]
- Callewaert, F.; Boonen, S.; Vanderschueren, D. Sex steroids and the male skeleton: A tale of two hormones. Trends Endocrinol. Metab. 2010, 21, 89–95. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Vandenput, L.; Boonen, S.; Lindberg, M.K.; Bouillon, R.; Ohlsson, C. Androgens and bone. Endocr. Rev. 2004, 25, 389–425. [Google Scholar] [CrossRef] [PubMed]
- Taes, Y.; Lapauw, B.; Vandewalle, S.; Zmierczak, H.; Goemaere, S.; Vanderschueren, D.; Kaufman, J.M.; T’Sjoen, G. Estrogen-specific action on bone geometry and volumetric bone density: Longitudinal observations in an adult with complete androgen insensitivity. Bone 2009, 45, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Leary, D.; Schneider, D.L.; Shane, E.; Favus, M.; Quigley, C.A. The contribution of testosterone to skeletal development and maintenance: Lessons from the androgen insensitivity syndrome. J. Clin. Endocrinol. Metab. 2000, 85, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Warne, G.L.; Grover, S.; Zajac, J.D. Hormonal therapies for individuals with intersex conditions: Protocol for use. Treat. Endocrinol. 2005, 4, 19–29. [Google Scholar] [CrossRef]
- Zborowski, J.V.; Cauley, J.A.; Talbott, E.O.; Guzick, D.S.; Winters, S.J. Clinical review 116: Bone mineral density, androgens, and the polycystic ovary: The complex and controversial issue of androgenic influence in female bone. J. Clin. Endocrinol. Metab. 2000, 85, 3496–3506. [Google Scholar] [CrossRef]
- Lingaiah, S.; Morin-Papunen, L.; Piltonen, T.; Puurunen, J.; Sundstrom-Poromaa, I.; Stener-Victorin, E.; Bloigu, R.; Risteli, J.; Tapanainen, J.S. Bone markers in polycystic ovary syndrome: A multicentre study. Clin. Endocrinol. 2017, 87, 673–679. [Google Scholar] [CrossRef]
- Karadag, C.; Yoldemir, T.; Gogas Yavuz, D. Determinants of low bone mineral density in premenopausal polycystic ovary syndrome patients. Gynecol. Endocrinol. 2017, 33, 234–237. [Google Scholar] [CrossRef]
- Noyan, V.; Yucel, A.; Sagsoz, N. The association of bone mineral density with insulin resistance in patients with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 200–205. [Google Scholar] [CrossRef]
- Krishnan, A.; Muthusami, S. Hormonal alterations in pcos and its influence on bone metabolism. J. Endocrinol. 2017, 232, R99–R113. [Google Scholar] [CrossRef]
- Sawalha, A.H.; Kovats, S. Dehydroepiandrosterone in systemic lupus erythematosus. Curr. Rheumatol. Rep. 2008, 10, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.E.; Rodin, A.; Murby, B.; Chapman, M.G.; Fogelman, I. Bone mass in hirsute women with androgen excess. Clin. Endocrinol. 1989, 30, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, S.; Al-Ali, N.; Qurttom, M. Augmentation of bone mineral density in hirsute women. J. Clin. Endocrinol. Metab. 1997, 82, 2821–2825. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, J.M.; Premaor, M.O.; Comim, F.V. Negative impact of polycystic ovary syndrome on bone health: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 633–645. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Lumpkin, C.K., Jr.; Bunn, R.C.; Kemp, S.F.; Fowlkes, J.L. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E735–E745. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef]
- Nestler, J.E.; Powers, L.P.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar] [CrossRef]
- Simberg, N.; Tiitinen, A.; Silfvast, A.; Viinikka, L.; Ylikorkala, O. High bone density in hyperandrogenic women: Effect of gonadotropin-releasing hormone agonist alone or in conjunction with estrogen-progestin replacement. J. Clin. Endocrinol. Metab. 1996, 81, 646–651. [Google Scholar]
- Moghetti, P.; Castello, R.; Zamberlan, N.; Rossini, M.; Gatti, D.; Negri, C.; Tosi, F.; Muggeo, M.; Adami, S. Spironolactone, but not flutamide, administration prevents bone loss in hyperandrogenic women treated with gonadotropin-releasing hormone agonist. J. Clin. Endocrinol. Metab. 1999, 84, 1250–1254. [Google Scholar]
- Prezelj, J.; Kocijancic, A. Antiandrogen treatment with spironolactone and linestrenol decreases bone mineral density in eumenorrhoeic women with androgen excess. Horm. Metab. Res. 1994, 26, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Bertelloni, S.; Baroncelli, G.I.; Sorrentino, M.C.; Costa, S.; Battini, R.; Saggese, G. Androgen-receptor blockade does not impair bone mineral density in adolescent females. Calcif. Tissue Int. 1997, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lingaiah, S.; Morin-Papunen, L.; Risteli, J.; Tapanainen, J.S. Metformin decreases bone turnover markers in polycystic ovary syndrome: A post hoc study. Fertil. Steril. 2019, 112, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in male physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef]
- Vandenput, L.; Ederveen, A.G.; Erben, R.G.; Stahr, K.; Swinnen, J.V.; Van Herck, E.; Verstuyf, A.; Boonen, S.; Bouillon, R.; Vanderschueren, D. Testosterone prevents orchidectomy-induced bone loss in estrogen receptor-alpha knockout mice. Biochem. Biophys. Res. Commun. 2001, 285, 70–76. [Google Scholar] [CrossRef]
- Broulik, P.D. Tamoxifen prevents bone loss in castrated male mice. Horm. Metab. Res. 2000, 32, 181–184. [Google Scholar] [CrossRef]
- Daci, E.; Verstuyf, A.; Moermans, K.; Bouillon, R.; Carmeliet, G. Mice lacking the plasminogen activator inhibitor 1 are protected from trabecular bone loss induced by estrogen deficiency. J. Bone Miner. Res. 2000, 15, 1510–1516. [Google Scholar] [CrossRef]
- Bain, S.D.; Bailey, M.C.; Celino, D.L.; Lantry, M.M.; Edwards, M.W. High-dose estrogen inhibits bone resorption and stimulates bone formation in the ovariectomized mouse. J. Bone Miner. Res. 1993, 8, 435–442. [Google Scholar] [CrossRef]
- Onoe, Y.; Miyaura, C.; Ito, M.; Ohta, H.; Nozawa, S.; Suda, T. Comparative effects of estrogen and raloxifene on b lymphopoiesis and bone loss induced by sex steroid deficiency in mice. J. Bone Miner. Res. 2000, 15, 541–549. [Google Scholar] [CrossRef]
- Poli, V.; Balena, R.; Fattori, E.; Markatos, A.; Yamamoto, M.; Tanaka, H.; Ciliberto, G.; Rodan, G.A.; Costantini, F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994, 13, 1189–1196. [Google Scholar] [CrossRef]
- Lindberg, M.K.; Moverare, S.; Skrtic, S.; Alatalo, S.; Halleen, J.; Mohan, S.; Gustafsson, J.A.; Ohlsson, C. Two different pathways for the maintenance of trabecular bone in adult male mice. J. Bone Miner. Res. 2002, 17, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, L.; Boonen, S.; Van Herck, E.; Swinnen, J.V.; Bouillon, R.; Vanderschueren, D. Evidence from the aged orchidectomized male rat model that 17beta-estradiol is a more effective bone-sparing and anabolic agent than 5alpha-dihydrotestosterone. J. Bone Miner. Res. 2002, 17, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Chen, J.R.; Bellido, T.; Han, L.; Ali, A.A.; O’Brien, C.A.; Plotkin, L.; Fu, Q.; Mancino, A.T.; Wen, Y.; et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 2002, 298, 843–846. [Google Scholar] [PubMed]
- Ishimi, Y.; Miyaura, C.; Ohmura, M.; Onoe, Y.; Sato, T.; Uchiyama, Y.; Ito, M.; Wang, X.; Suda, T.; Ikegami, S. Selective effects of genistein, a soybean isoflavone, on b-lymphopoiesis and bone loss caused by estrogen deficiency. Endocrinology 1999, 140, 1893–1900. [Google Scholar] [CrossRef]
- Ishimi, Y.; Yoshida, M.; Wakimoto, S.; Wu, J.; Chiba, H.; Wang, X.; Takeda, K.; Miyaura, C. Genistein, a soybean isoflavone, affects bone marrow lymphopoiesis and prevents bone loss in castrated male mice. Bone 2002, 31, 180–185. [Google Scholar] [CrossRef]
- Vandenput, L. The Role of Aromatization of Androgens in Oestrogens and of the Oestrogen Receptors in the Development and Maintance of the Male Skeleton; Leuven University Press: Leuven, Belgium, 2002. [Google Scholar]
- Cheng, B.H.; Chu, T.M.; Chang, C.; Kang, H.Y.; Huang, K.E. Testosterone delivered with a scaffold is as effective as bone morphologic protein-2 in promoting the repair of critical-size segmental defect of femoral bone in mice. PLoS ONE 2013, 8, e70234. [Google Scholar] [CrossRef]
- Vidal, O.; Lindberg, M.K.; Hollberg, K.; Baylink, D.J.; Andersson, G.; Lubahn, D.B.; Mohan, S.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc. Natl. Acad. Sci. USA 2000, 97, 5474–5479. [Google Scholar] [CrossRef]
- Ke, H.Z.; Brown, T.A.; Qi, H.; Crawford, D.T.; Simmons, H.A.; Petersen, D.N.; Allen, M.R.; McNeish, J.D.; Thompson, D.D. The role of estrogen receptor-beta, in the early age-related bone gain and later age-related bone loss in female mice. J. Musculoskelet. Neuronal Interact. 2002, 2, 479–488. [Google Scholar]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef]
- Wiren, K.M.; Semirale, A.A.; Hashimoto, J.G.; Zhang, X.W. Signaling pathways implicated in androgen regulation of endocortical bone. Bone 2010, 46, 710–723. [Google Scholar] [CrossRef]
- Wiren, K.M.; Hashimoto, J.G.; Semirale, A.A.; Zhang, X.W. Bone vs. Fat: Embryonic origin of progenitors determines response to androgen in adipocytes and osteoblasts. Bone 2011, 49, 662–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Semirale, A.A.; Zhang, X.W.; Wiren, K.M. Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J. Cell. Biochem. 2011, 112, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Wiren, K.M.; Zhang, X.W.; Toombs, A.R.; Kasparcova, V.; Gentile, M.A.; Harada, S.; Jepsen, K.J. Targeted overexpression of androgen receptor in osteoblasts: Unexpected complex bone phenotype in growing animals. Endocrinology 2004, 145, 3507–3522. [Google Scholar] [CrossRef] [PubMed]
- Wiren, K.M.; Zhang, X.W.; Olson, D.A.; Turner, R.T.; Iwaniec, U.T. Androgen prevents hypogonadal bone loss via inhibition of resorption mediated by mature osteoblasts/osteocytes. Bone 2012, 51, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Notini, A.J.; McManus, J.F.; Moore, A.; Bouxsein, M.; Jimenez, M.; Chiu, W.S.; Glatt, V.; Kream, B.E.; Handelsman, D.J.; Morris, H.A.; et al. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J. Bone Miner. Res. 2007, 22, 347–356. [Google Scholar] [CrossRef]
- Kawano, H.; Sato, T.; Yamada, T.; Matsumoto, T.; Sekine, K.; Watanabe, T.; Nakamura, T.; Fukuda, T.; Yoshimura, K.; Yoshizawa, T.; et al. Suppressive function of androgen receptor in bone resorption. Proc. Natl. Acad. Sci. USA 2003, 100, 9416–9421. [Google Scholar] [CrossRef]
- Khosla, S.; Monroe, D.G. Regulation of bone metabolism by sex steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef]
- Chiang, C.; Chiu, M.; Moore, A.J.; Anderson, P.H.; Ghasem-Zadeh, A.; McManus, J.F.; Ma, C.; Seeman, E.; Clemens, T.L.; Morris, H.A.; et al. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J. Bone Miner. Res. 2009, 24, 621–631. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Niyibizi, C. Progenitors systemically transplanted into neonatal mice localize to areas of active bone formation in vivo: Implications of cell therapy for skeletal diseases. Stem Cells 2006, 24, 1869–1878. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Prockop, D.J.; Fitzpatrick, L.A.; Koo, W.W.; Gordon, P.L.; Neel, M.; Sussman, M.; Orchard, P.; Marx, J.C.; Pyeritz, R.E.; et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999, 5, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Colvard, D.S.; Eriksen, E.F.; Keeting, P.E.; Wilson, E.M.; Lubahn, D.B.; French, F.S.; Riggs, B.L.; Spelsberg, T.C. Identification of androgen receptors in normal human osteoblast-like cells. Proc. Natl. Acad. Sci. USA 1989, 86, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Nordstrand, A.; Bovinder Ylitalo, E.; Thysell, E.; Jernberg, E.; Crnalic, S.; Widmark, A.; Bergh, A.; Lerner, U.H.; Wikstrom, P. Bone cell activity in clinical prostate cancer bone metastasis and its inverse relation to tumor cell androgen receptor activity. Int. J. Mol. Sci. 2018, 19, E1223. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Stribrska, L.; Ramsey, E.E.; Keenan, E.J. Androgen receptors in osteoblast-like cell lines. Calcif. Tissue Int. 1991, 49, 183–187. [Google Scholar] [CrossRef]
- Wiren, K.; Keenan, E.; Zhang, X.; Ramsey, B.; Orwoll, E. Homologous androgen receptor up-regulation in osteoblastic cells may be associated with enhanced functional androgen responsiveness. Endocrinology 1999, 140, 3114–3124. [Google Scholar] [CrossRef]
- Czerwiec, F.S.; Liaw, J.J.; Liu, S.B.; Perez-Stable, C.; Grumbles, R.; Howard, G.A.; Roos, B.A.; Burnstein, K.L. Absence of androgen-mediated transcriptional effects in osteoblastic cells despite presence of androgen receptors. Bone 1997, 21, 49–56. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kakushi, H.; Tohkin, M. Androgens directly stimulate mineralization and increase androgen receptors in human osteoblast-like osteosarcoma cells. Biochem. Biophys. Res. Commun. 1994, 204, 905–911. [Google Scholar] [CrossRef]
- Nakano, Y.; Morimoto, I.; Ishida, O.; Fujihira, T.; Mizokami, A.; Tanimoto, A.; Yanagihara, N.; Izumi, F.; Eto, S. The receptor, metabolism and effects of androgen in osteoblastic mc3t3-e1 cells. Bone Miner. 1994, 26, 245–259. [Google Scholar] [CrossRef]
- Liesegang, P.; Romalo, G.; Sudmann, M.; Wolf, L.; Schweikert, H.U. Human osteoblast-like cells contain specific, saturable, high-affinity glucocorticoid, androgen, estrogen, and 1 alpha,25-dihydroxycholecalciferol receptors. J. Androl. 1994, 15, 194–199. [Google Scholar]
- Masuyama, A.; Ouchi, Y.; Sato, F.; Hosoi, T.; Nakamura, T.; Orimo, H. Characteristics of steroid hormone receptors in cultured mc3t3-e1 osteoblastic cells and effect of steroid hormones on cell proliferation. Calcif. Tissue Int. 1992, 51, 376–381. [Google Scholar] [CrossRef]
- Benz, D.J.; Haussler, M.R.; Thomas, M.A.; Speelman, B.; Komm, B.S. High-affinity androgen binding and androgenic regulation of alpha 1(i)-procollagen and transforming growth factor-beta steady state messenger ribonucleic acid levels in human osteoblast-like osteosarcoma cells. Endocrinology 1991, 128, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Bland, R. Steroid hormone receptor expression and action in bone. Clin. Sci. 2000, 98, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y. The Role of Androgens Acting via the Androgen Receptor in Osteoblasts to Regulate Bone Cell Metabolism. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, December 2017. [Google Scholar]
- Zhuang, Y.H.; Blauer, M.; Pekki, A.; Tuohimaa, P. Subcellular location of androgen receptor in rat prostate, seminal vesicle and human osteosarcoma mg-63 cells. J. Steroid Biochem. Mol. Biol. 1992, 41, 693–696. [Google Scholar] [CrossRef]
- Wiren, K.M.; Zhang, X.; Chang, C.; Keenan, E.; Orwoll, E.S. Transcriptional up-regulation of the human androgen receptor by androgen in bone cells. Endocrinology 1997, 138, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Hicok, K.C.; Schroeder, M.J.; Harris, S.A.; Robinson, J.A.; Khosla, S. Development and characterization of a conditionally immortalized human osteoblastic cell line stably transfected with the human androgen receptor gene. J. Cell. Biochem. 1997, 66, 542–551. [Google Scholar] [CrossRef]
- Wiren, K.M.; Chapman Evans, A.; Zhang, X.W. Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J. Endocrinol. 2002, 175, 683–694. [Google Scholar] [CrossRef]
- Gruber, R.; Czerwenka, K.; Wolf, F.; Ho, G.M.; Willheim, M.; Peterlik, M. Expression of the vitamin d receptor, of estrogen and thyroid hormone receptor alpha- and beta-isoforms, and of the androgen receptor in cultures of native mouse bone marrow and of stromal/osteoblastic cells. Bone 1999, 24, 465–473. [Google Scholar] [CrossRef]
- Mantalaris, A.; Panoskaltsis, N.; Sakai, Y.; Bourne, P.; Chang, C.; Messing, E.M.; Wu, J.H. Localization of androgen receptor expression in human bone marrow. J. Pathol. 2001, 193, 361–366. [Google Scholar] [CrossRef]
- Huang, C.K.; Luo, J.; Lee, S.O.; Chang, C. Concise review: Androgen receptor differential roles in stem/progenitor cells including prostate, embryonic, stromal, and hematopoietic lineages. Stem Cells 2014, 32, 2299–2308. [Google Scholar] [CrossRef]
- Braidman, I.P.; Hainey, L.; Batra, G.; Selby, P.L.; Saunders, P.T.; Hoyland, J.A. Localization of estrogen receptor beta protein expression in adult human bone. J. Bone Miner. Res. 2001, 16, 214–220. [Google Scholar] [CrossRef]
- Abu, E.O.; Horner, A.; Kusec, V.; Triffitt, J.T.; Compston, J.E. The localization of androgen receptors in human bone. J. Clin. Endocrinol. Metab. 1997, 82, 3493–3497. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Shyr, C.R.; Kang, H.Y.; Chang, Y.C.; Weng, P.L.; Wang, S.Y.; Huang, K.E.; Chang, C. The reduced trabecular bone mass of adult arko male mice results from the decreased osteogenic differentiation of bone marrow stroma cells. Biochem. Biophys. Res. Commun. 2011, 411, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Lai, K.P.; Luo, J.; Tsai, M.Y.; Kang, H.Y.; Chen, Y.; Lee, S.O.; Chang, C. Loss of androgen receptor promotes adipogenesis but suppresses osteogenesis in bone marrow stromal cells. Stem Cell Res. 2013, 11, 938–950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, H.Y.; Shyr, C.R.; Huang, C.K.; Tsai, M.Y.; Orimo, H.; Lin, P.C.; Chang, C.; Huang, K.E. Altered tnsalp expression and phosphate regulation contribute to reduced mineralization in mice lacking androgen receptor. Mol. Cell. Biol. 2008, 28, 7354–7367. [Google Scholar] [CrossRef]
- Yatsu, T.; Kusakabe, T.; Kato, K.; Inouye, Y.; Nemoto, K.; Kanno, Y. Selective androgen receptor modulator, yk11, up-regulates osteoblastic proliferation and differentiation in mc3t3-e1 cells. Biol. Pharm. Bull. 2018, 41, 394–398. [Google Scholar] [CrossRef]
- Chang, C.; Yeh, S.; Lee, S.O.; Chang, T.M. Androgen receptor (ar) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: Lessons learned from mice lacking ar in specific cells. Nucl. Recept. Signal. 2013, 11, e001. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsuuw, Y.D.; Huang, F.J.; Shyr, C.R.; Chang, S.Y.; Huang, C.K.; Kang, H.Y.; Huang, K.E. Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. Fertil. Steril. 2006, 85, 1195–1203. [Google Scholar] [CrossRef]
- Davies, A.H.; Zoubeidi, A. The androgen receptor bridges stem cell-associated signaling nodes in prostate stem cells. Stem Cells Int. 2016, 2016, 4829602. [Google Scholar] [CrossRef]
- Huang, C.K.; Lee, S.O.; Lai, K.P.; Ma, W.L.; Lin, T.H.; Tsai, M.Y.; Luo, J.; Chang, C. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology 2013, 57, 1550–1563. [Google Scholar] [CrossRef]
- Huang, C.K.; Tsai, M.Y.; Luo, J.; Kang, H.Y.; Lee, S.O.; Chang, C. Suppression of androgen receptor enhances the self-renewal of mesenchymal stem cells through elevated expression of egfr. Biochim. Biophys. Acta 2013, 1833, 1222–1234. [Google Scholar] [CrossRef]
- Elraiyah, T.; Sonbol, M.B.; Wang, Z.; Khairalseed, T.; Asi, N.; Undavalli, C.; Nabhan, M.; Firwana, B.; Altayar, O.; Prokop, L.; et al. Clinical review: The benefits and harms of systemic testosterone therapy in postmenopausal women with normal adrenal function: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3543–3550. [Google Scholar] [CrossRef]
- Dexheimer, V.; Mueller, S.; Braatz, F.; Richter, W. Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PLoS ONE 2011, 6, e22980. [Google Scholar] [CrossRef] [PubMed]
- Fossett, E.; Khan, W.S. Optimising human mesenchymal stem cell numbers for clinical application: A literature review. Stem Cells Int. 2012, 2012, 465259. [Google Scholar] [CrossRef] [PubMed]
- Fuente-Martin, E.; Argente-Arizon, P.; Ros, P.; Argente, J.; Chowen, J.A. Sex differences in adipose tissue: It is not only a question of quantity and distribution. Adipocyte 2013, 2, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.J.; Janorkar, A.V.; Barnes, A.; Maranon, R.O. A new approach to study the sex differences in adipose tissue. J. Biomed. Sci. 2018, 25, 89. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Petnga, W.; Macaulay, V.M.; Weyer-Czernilofsky, U.; Bogenrieder, T. Insulin-like growth factor (igf) pathway targeting in cancer: Role of the igf axis and opportunities for future combination studies. Target. Oncol. 2017, 12, 571–597. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, J.M. Sex-specific regulation of immune responses by ppars. Exp. Mol. Med. 2017, 49, e364. [Google Scholar] [CrossRef]
- Davis, S.R.; McCloud, P.; Strauss, B.J.; Burger, H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas 2008, 61, 17–26. [Google Scholar] [CrossRef]
- Savvas, M.; Studd, J.W.; Fogelman, I.; Dooley, M.; Montgomery, J.; Murby, B. Skeletal effects of oral oestrogen compared with subcutaneous oestrogen and testosterone in postmenopausal women. BMJ 1988, 297, 331–333. [Google Scholar] [CrossRef]
- Raisz, L.G.; Wiita, B.; Artis, A.; Bowen, A.; Schwartz, S.; Trahiotis, M.; Shoukri, K.; Smith, J. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J. Clin. Endocrinol. Metab. 1996, 81, 37–43. [Google Scholar]
- Watts, N.B.; Notelovitz, M.; Timmons, M.C.; Addison, W.A.; Wiita, B.; Downey, L.J. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstet. Gynecol. 1995, 85, 529–537. [Google Scholar] [CrossRef]
- Savvas, M.; Studd, J.W.; Norman, S.; Leather, A.T.; Garnett, T.J.; Fogelman, I. Increase in bone mass after one year of percutaneous oestradiol and testosterone implants in post-menopausal women who have previously received long-term oral oestrogens. Br. J. Obstet. Gynaecol. 1992, 99, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Tracz, M.J.; Sideras, K.; Bolona, E.R.; Haddad, R.M.; Kennedy, C.C.; Uraga, M.V.; Caples, S.M.; Erwin, P.J.; Montori, V.M. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J. Clin. Endocrinol. Metab. 2006, 91, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Permpongkosol, S.; Khupulsup, K.; Leelaphiwat, S.; Pavavattananusorn, S.; Thongpradit, S.; Petchthong, T. Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J. Sex. Med. 2016, 13, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef]
- Narayanan, R.; Coss, C.C.; Dalton, J.T. Development of selective androgen receptor modulators (sarms). Mol. Cell. Endocrinol. 2018, 465, 134–142. [Google Scholar] [CrossRef]
- Hanada, K.; Furuya, K.; Yamamoto, N.; Nejishima, H.; Ichikawa, K.; Nakamura, T.; Miyakawa, M.; Amano, S.; Sumita, Y.; Oguro, N. Bone anabolic effects of s-40503, a novel nonsteroidal selective androgen receptor modulator (sarm), in rat models of osteoporosis. Biol. Pharm. Bull. 2003, 26, 1563–1569. [Google Scholar] [CrossRef]
- Kearbey, J.D.; Gao, W.; Narayanan, R.; Fisher, S.J.; Wu, D.; Miller, D.D.; Dalton, J.T. Selective androgen receptor modulator (sarm) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm. Res. 2007, 24, 328–335. [Google Scholar] [CrossRef]
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.A.; Steiner, M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef]
- Crawford, J.; Prado, C.M.; Johnston, M.A.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (power trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef]
| Clinical Manifestation | Impacts on Bone in Adults | Reference | |
|---|---|---|---|
| Androgen Deficiency | |||
| Isolated hypogonadotropic hypogonadism (IHH) | Delayed puberty in late teens or early twenties. |
| [54] |
| Klinefelter’s syndrome (KS) | tall stature, small testes, aspermatogenesis, gynecomastia, diminished body hair |
| [55,56] |
| Constitutional delay of growth and puberty (CDGP) | short stature, delay bone age, and puberty |
| [57,58,59] |
| Androgen deprivation therapy | Flushing, decrease libido, anemia, insulin resistance. |
| [60,61,62] |
| Aging | Degeneration of systemic change, sleep disturbance, decrease libido. |
| [63] |
| Androgen insensitivity syndrome (AIS) | 46,XY karyotype , with under masculinized external genitalia depends on residual AR function. Gynecomastia at puberty and infertility in adulthood |
| [64,65,66] |
| Androgen excess | |||
| Polycystic ovary syndrome (PCOS) | Hirsutism, acne, alopecia, seborrhea. Subfertility, menstrual dysfunction. Endometrial hyperplasia |
| [67,68,69,70] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-F.; Lin, P.-W.; Tsai, Y.-R.; Yang, Y.-C.; Kang, H.-Y. Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy. Cells 2019, 8, 1318. https://doi.org/10.3390/cells8111318
Chen J-F, Lin P-W, Tsai Y-R, Yang Y-C, Kang H-Y. Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy. Cells. 2019; 8(11):1318. https://doi.org/10.3390/cells8111318
Chicago/Turabian StyleChen, Jia-Feng, Pei-Wen Lin, Yi-Ru Tsai, Yi-Chien Yang, and Hong-Yo Kang. 2019. "Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy" Cells 8, no. 11: 1318. https://doi.org/10.3390/cells8111318
APA StyleChen, J.-F., Lin, P.-W., Tsai, Y.-R., Yang, Y.-C., & Kang, H.-Y. (2019). Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy. Cells, 8(11), 1318. https://doi.org/10.3390/cells8111318






