Reduced DAXX Expression Is Associated with Reduced CD24 Expression in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Colorectal Tissue Preparation
2.2. Cancer Tissue Extraction for Western Blotting
2.3. Cell Culture
2.4. Transient Transfection and PCR
2.5. Western Blotting for Cell Line
2.6. Immunofluorescence Staining
2.7. Cell Migration and Invasion Assays
2.8. Statistical Analysis
3. Results
3.1. Correlation of DAXX Expression with Clinicopathological Parameters
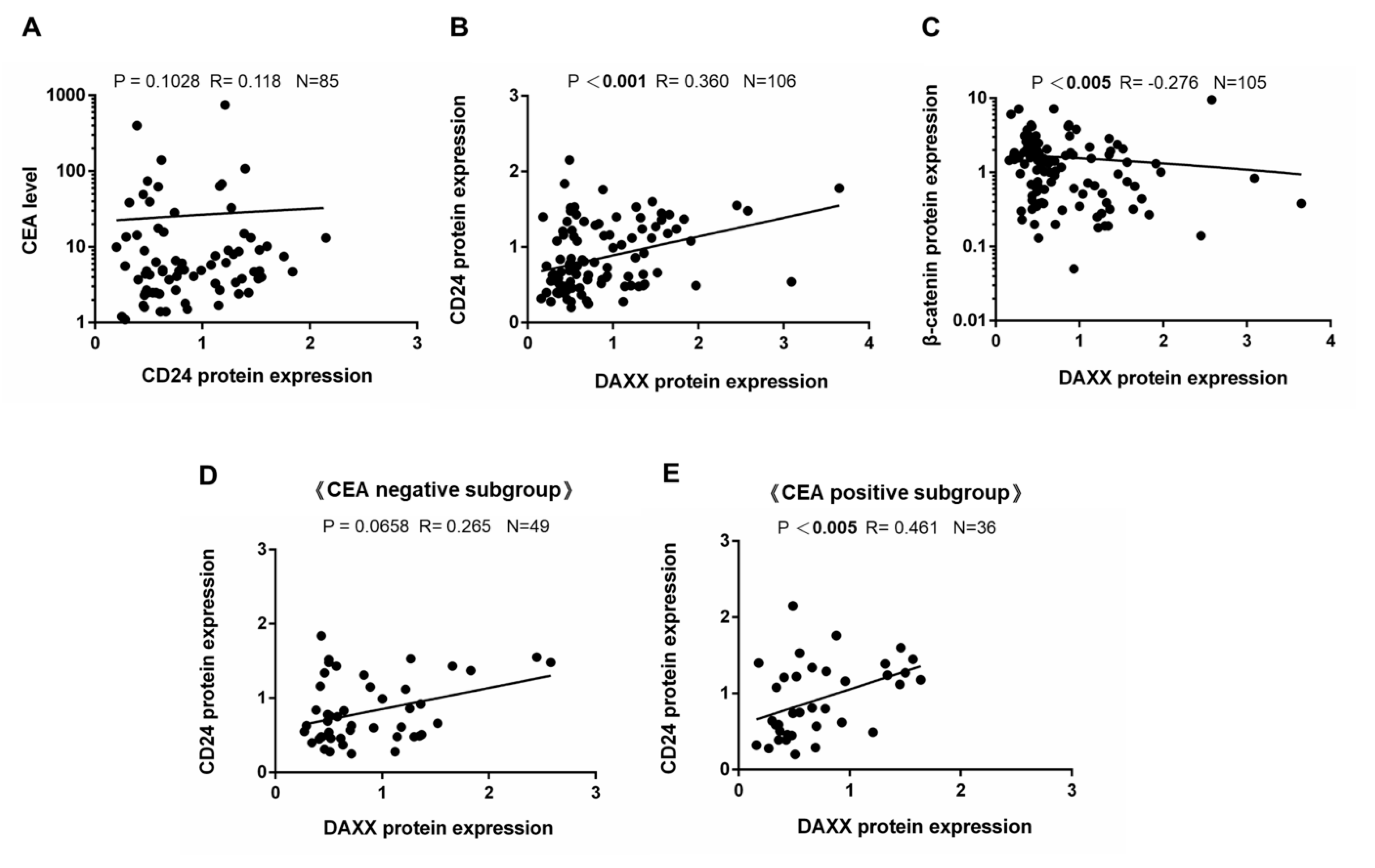
3.2. Correlation of DAXX Expression with CD24 Expression
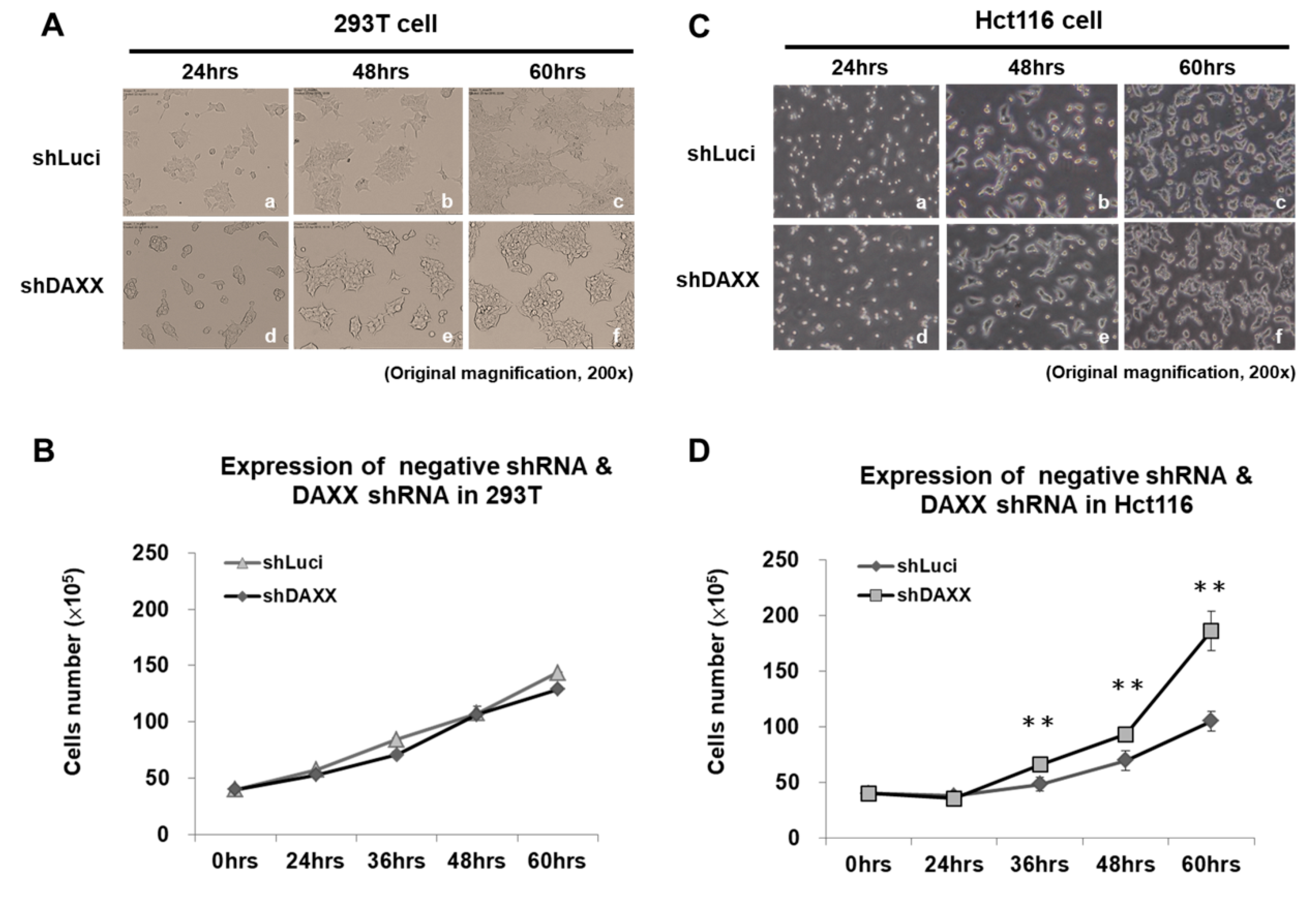
3.3. Correlation of DAXX with CRC Cell Proliferation
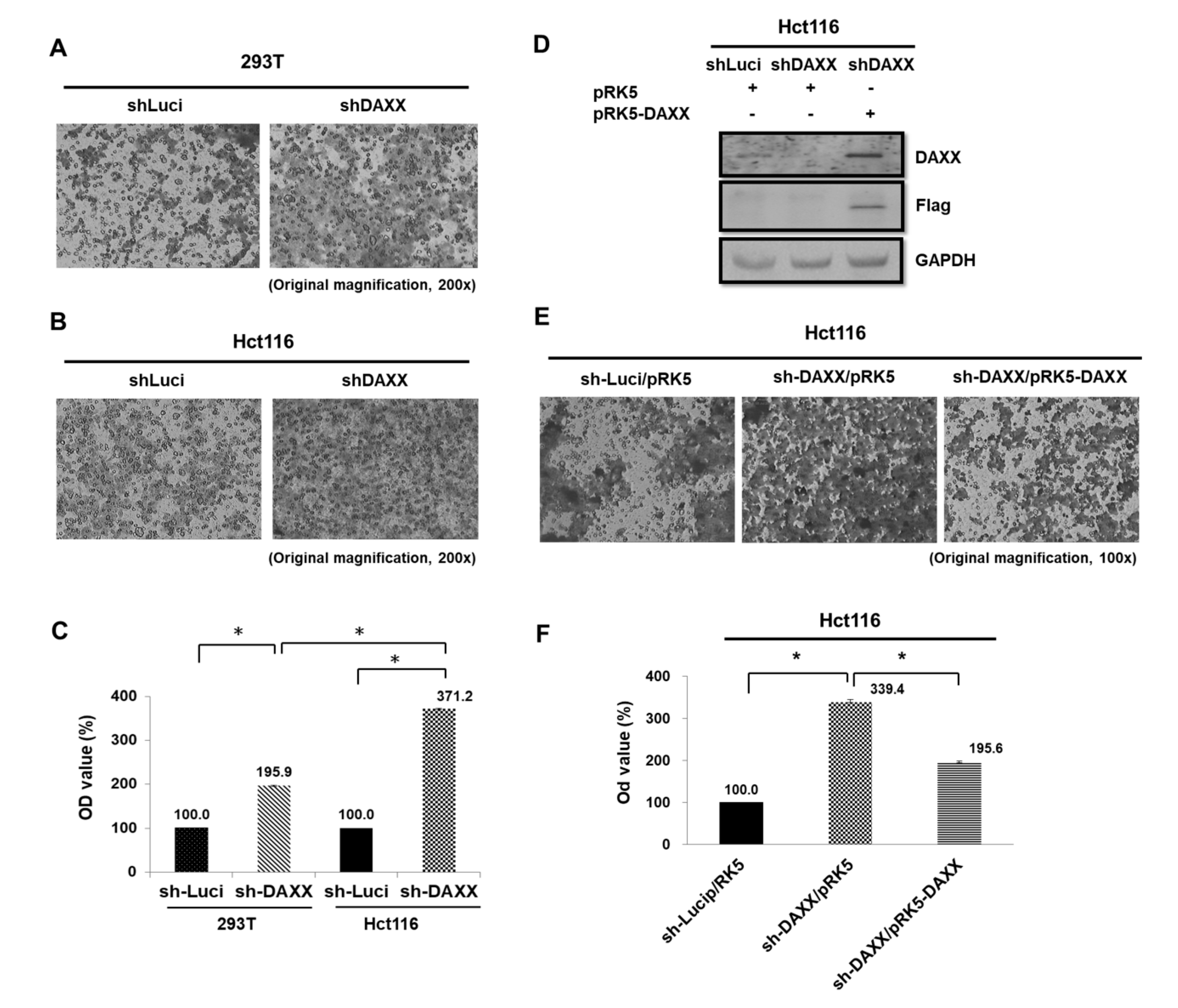
3.4. Correlation of DAXX with CRC Cell Migration
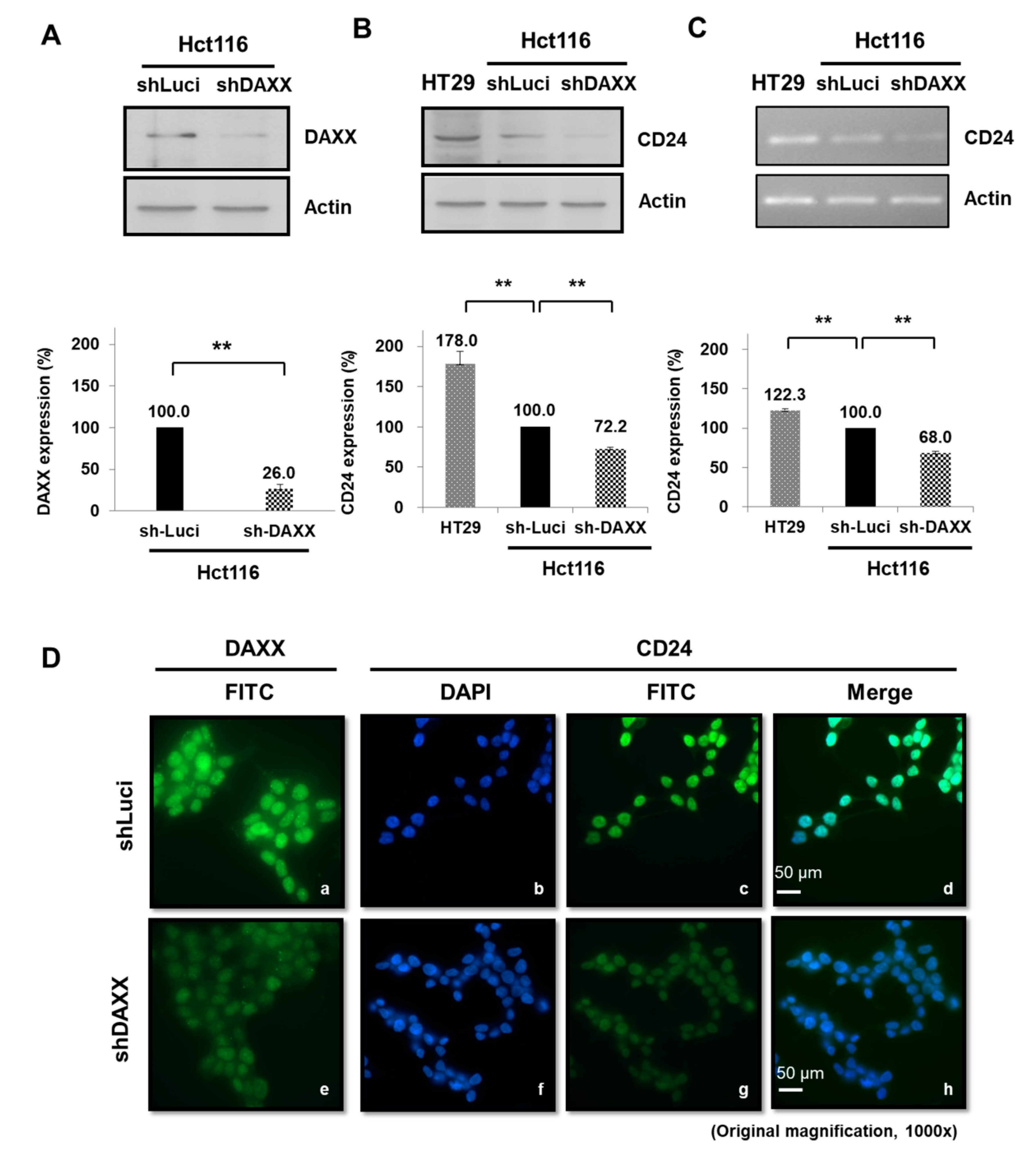
3.5. Regulation of CD24 Expression by DAXX in CRC Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic potential of targeting the wnt/beta-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2016, 36, 1461. [Google Scholar] [CrossRef] [PubMed]
- Porru, M.; Pompili, L.; Caruso, C.; Biroccio, A.; Leonetti, C. Targeting kras in metastatic colorectal cancer: Current strategies and emerging opportunities. J. Exp. Clin. Cancer Res. 2018, 37, 57. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kinugasa, T.; Ohshima, K.; Yuge, K.; Ohchi, T.; Fujino, S.; Shiraiwa, S.; Katagiri, M.; Akagi, Y. Analysis of wnt and beta-catenin expression in advanced colorectal cancer. Anticancer Res. 2015, 35, 4403–4410. [Google Scholar]
- Lee, K.; Piazza, A.G. The interaction between the wnt/β-catenin signaling cascade and pkg activation in cancer. J. Biomed. Res. 2017, 31, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Novellasdemunt, L.; Antas, P.; Li, V.S.W. Targeting wnt signaling in colorectal cancer. A review in the theme: Cell signaling: Proteins, pathways and mechanisms. Am. J. Physiol. Cell Physiol. 2015, 309, C511–C521. [Google Scholar] [CrossRef]
- Korinek, V.; Barker, N.; Moerer, P.; van Donselaar, E.; Huls, G.; Peters, P.J.; Clevers, H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking tcf-4. Nat. Genet. 1998, 19, 379–383. [Google Scholar] [CrossRef]
- Puto, L.A.; Benner, C.; Hunter, T. The daxx co-repressor is directly recruited to active regulatory elements genome-wide to regulate autophagy programs in a model of human prostate cancer. Oncoscience 2015, 2, 362–372. [Google Scholar] [CrossRef][Green Version]
- Merkl, P.E.; Orzalli, M.H.; Knipe, D.M. Mechanisms of host ifi16, pml, and daxx protein restriction of herpes simplex virus 1 replication. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Marinoni, I.; Wiederkeher, A.; Wiedmer, T.; Pantasis, S.; Di Domenico, A.; Frank, R.; Vassella, E.; Schmitt, A.; Perren, A. Hypo-methylation mediates chromosomal instability in pancreatic net. Endocr. Relat. Cancer 2017, 24, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.Y.; Kim, J.I.; Park, E.S.; Mun, J.M.; Park, S.D. The clinical implications of death domain-associated protein (daxx) expression. Korean J. Thorac. Cardiovasc. Surg. 2018, 51, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Khosravi-Far, R.; Chang, H.Y.; Baltimore, D. Daxx, a novel fas-binding protein that activates jnk and apoptosis. Cell 1997, 89, 1067–1076. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Driscoll, D.A.; Lopez-Guisa, J.M.; Strauss, J.F., 3rd. Cloning and expression of primate daxx cdnas and mapping of the human gene to chromosome 6p21.3 in the mhc region. DNA Cell Biol. 1997, 16, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.L.; Cheng, Y.W.; Li, C.H.; Lin, Y.S.; Hsu, H.C.; Kang, J.J. Physiological and functional interactions between tcf4 and daxx in colon cancer cells. J. Biol. Chem. 2006, 281, 15405–15411. [Google Scholar] [CrossRef] [PubMed]
- Saeland, E.; Belo, A.I.; Mongera, S.; van Die, I.; Meijer, G.A.; van Kooyk, Y. Differential glycosylation of muc1 and ceacam5 between normal mucosa and tumour tissue of colon cancer patients. Int. J. Cancer 2012, 131, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahid, A.; Huang, E.H.; Lu, H.; Flanagan, J.; Mallick, A.I.; Gariepy, J. A focused immune response targeting the homotypic binding domain of the carcinoembryonic antigen blocks the establishment of tumor foci in vivo. Int. J. Cancer 2012, 131, 2839–2851. [Google Scholar] [CrossRef]
- Abdul-Wahid, A.; Huang, E.H.; Cydzik, M.; Bolewska-Pedyczak, E.; Gariepy, J. The carcinoembryonic antigen igv-like n domain plays a critical role in the implantation of metastatic tumor cells. Mol. Oncol. 2014, 8, 337–350. [Google Scholar] [CrossRef]
- Abdul-Wahid, A.; Cydzik, M.; Fischer, N.W.; Prodeus, A.; Shively, J.E.; Martel, A.; Alminawi, S.; Ghorab, Z.; Berinstein, N.L.; Gariepy, J. Serum-derived carcinoembryonic antigen (cea) activates fibroblasts to induce a local re-modeling of the extracellular matrix that favors the engraftment of cea-expressing tumor cells. Int. J. Cancer 2018, 143, 1963–1977. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.W. The roles of carcinoembryonic antigen in liver metastasis and therapeutic approaches. Gastroenterol. Res. Pract. 2017, 2017, 7521987. [Google Scholar] [CrossRef]
- Thomas, D.S.; Fourkala, E.O.; Apostolidou, S.; Gunu, R.; Ryan, A.; Jacobs, I.; Menon, U.; Alderton, W.; Gentry-Maharaj, A.; Timms, J.F. Evaluation of serum cea, cyfra21-1 and ca125 for the early detection of colorectal cancer using longitudinal preclinical samples. Br. J. Cancer 2015, 113, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Tiernan, J.P.; Perry, S.L.; Verghese, E.T.; West, N.P.; Yeluri, S.; Jayne, D.G.; Hughes, T.A. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 2013, 108, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Hernandez, J.C.; Dean, A.M.; Rao, P.H.; Darlington, G.J. Cd24-positive cells from normal adult mouse liver are hepatocyte progenitor cells. Stem Cells Dev. 2011, 20, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.F.; Majdic, O.; Gadd, S.; Knapp, W. Signal transduction in lymphocytic and myeloid cells via cd24, a new member of phosphoinositol-anchored membrane molecules. J. Immunol. 1990, 144, 638–641. [Google Scholar]
- Aigner, S.; Sthoeger, Z.M.; Fogel, M.; Weber, E.; Zarn, J.; Ruppert, M.; Zeller, Y.; Vestweber, D.; Stahel, R.; Sammar, M.; et al. Cd24, a mucin-type glycoprotein, is a ligand for p-selectin on human tumor cells. Blood 1997, 89, 3385–3395. [Google Scholar] [PubMed]
- Aigner, S.; Ramos, C.L.; Hafezi-Moghadam, A.; Lawrence, M.B.; Friederichs, J.; Altevogt, P.; Ley, K. Cd24 mediates rolling of breast carcinoma cells on p-selectin. FASEB J. 1998, 12, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Gao, G.; Wang, L.; Wang, T.; Yu, J.; Zhao, Z. Cd24 expression as a marker for predicting clinical outcome in human gliomas. J. Biomed. Biotechnol. 2012, 2012, 517172. [Google Scholar] [CrossRef]
- Yang, X.R.; Xu, Y.; Yu, B.; Zhou, J.; Li, J.C.; Qiu, S.J.; Shi, Y.H.; Wang, X.Y.; Dai, Z.; Shi, G.M.; et al. Cd24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin. Cancer Res. 2009, 15, 5518–5527. [Google Scholar] [CrossRef]
- Sano, A.; Kato, H.; Sakurai, S.; Sakai, M.; Tanaka, N.; Inose, T.; Saito, K.; Sohda, M.; Nakajima, M.; Nakajima, T.; et al. Cd24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2009, 16, 506–514. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, C.R.; Su, W.Y.; Chen, Y.X.; Xu, J.; Fang, J.Y. Cd24 overexpression related to lymph node invasion and poor prognosis of colorectal cancer. Clin. Lab. 2018, 64, 497–505. [Google Scholar] [CrossRef]
- Hosonaga, M.; Arima, Y.; Sugihara, E.; Kohno, N.; Saya, H. Expression of cd24 is associated with her2 expression and supports her2-akt signaling in her2-positive breast cancer cells. Cancer Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Jang, K.; Lee, K.M.; Kim, M.; Kim, J.; Yi, J.Y.; Noh, D.Y.; Shin, I. Cd24 enhances DNA damage-induced apoptosis by modulating nf-kappab signaling in cd44-expressing breast cancer cells. Carcinogenesis 2011, 32, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.P.; Lievre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef] [PubMed]
- Pallegar, N.K.; Ayre, D.C.; Christian, S.L. Repression of cd24 surface protein expression by oncogenic ras is relieved by inhibition of raf but not mek or pi3k. Front. Cell Dev. Biol. 2015, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Shulewitz, M.; Soloviev, I.; Wu, T.; Koeppen, H.; Polakis, P.; Sakanaka, C. Repressor roles for tcf-4 and sfrp1 in wnt signaling in breast cancer. Oncogene 2006, 25, 4361–4369. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Jackson, D.; Seth, R.; Robins, A.; Lobo, D.N.; Tomlinson, I.P.; Ilyas, M. Cd24 is upregulated in inflammatory bowel disease and stimulates cell motility and colony formation. Inflamm. Bowel Dis. 2010, 16, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wang, L.K.; Wang, S.P.; Chang, Y.L.; Wu, Y.Y.; Chen, H.Y.; Hsiao, T.H.; Lai, W.Y.; Lu, H.H.; Chang, Y.H.; et al. Daxx inhibits hypoxia-induced lung cancer cell metastasis by suppressing the hif-1alpha/hdac1/slug axis. Nat. Commun. 2016, 7, 13867. [Google Scholar] [CrossRef]
- Weichert, W.; Denkert, C.; Burkhardt, M.; Gansukh, T.; Bellach, J.; Altevogt, P.; Dietel, M.; Kristiansen, G. Cytoplasmic cd24 expression in colorectal cancer independently correlates with shortened patient survival. Clin. Cancer Res. 2005, 11, 6574–6581. [Google Scholar] [CrossRef]
- Wan, X.; Cheng, C.; Shao, Q.; Lin, Z.; Lu, S.; Chen, Y. Cd24 promotes hcc progression via triggering notch-related emt and modulation of tumor microenvironment. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, J.L.; Kong, X.; Sun, T.T.; Chen, H.Y.; Hong, J.; Fang, J.Y. Cd24 mediates gastric carcinogenesis and promotes gastric cancer progression via stat3 activation. Apoptosis Int. J. Program. Cell Death 2014, 19, 643–656. [Google Scholar] [CrossRef]
- Shapira, S.; Shapira, A.; Starr, A.; Kazanov, D.; Kraus, S.; Benhar, I.; Arber, N. An immunoconjugate of anti-cd24 and pseudomonas exotoxin selectively kills human colorectal tumors in mice. Gastroenterology 2011, 140, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, R.; Ye, P.; Wong, C.; Chen, G.Y.; Zhou, P.; Sakabe, K.; Zheng, X.; Wu, W.; Zhang, P.; et al. Intracellular cd24 disrupts the arf-npm interaction and enables mutational and viral oncogene-mediated p53 inactivation. Nat. Commun. 2015, 6, 5909. [Google Scholar] [CrossRef] [PubMed]
- Duex, J.E.; Owens, C.; Chauca-Diaz, A.; Dancik, G.M.; Vanderlinden, L.A.; Ghosh, D.; Leivo, M.Z.; Hansel, D.E.; Theodorescu, D. Nuclear cd24 drives tumor growth and is predictive of poor patient prognosis. Cancer Res. 2017, 77, 4858–4867. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.W.; Zhou, J.J.; Liu, X.M.; Xu, Y.; Guo, L.J.; Yu, C.; Shi, Q.H.; Fan, H.Y. Death domain-associated protein daxx promotes ovarian cancer development and chemoresistance. J. Biol. Chem. 2013, 288, 13620–13630. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, J.S.; Bader, D.; Kuo, F.; Kozak, C.; Leder, P. Loss of daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 1999, 13, 1918–1923. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, L.; Sun, Y.; Jiang, Z.; Domsic, J.; An, C.; Xing, B.; Tian, J.; Liu, X.; Metz, D.C.; et al. Menin and daxx interact to suppress neuroendocrine tumors through epigenetic control of the membrane metallo-endopeptidase. Cancer Res. 2017, 77, 401–411. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. Daxx/atrx, men1, and mtor pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef]
- Singhi, A.D.; Liu, T.C.; Roncaioli, J.L.; Cao, D.; Zeh, H.J.; Zureikat, A.H.; Tsung, A.; Marsh, J.W.; Lee, K.K.; Hogg, M.E.; et al. Alternative lengthening of telomeres and loss of daxx/atrx expression predicts metastatic disease and poor survival in patients with pancreatic neuroendocrine tumors. Clin. Cancer Res. 2017, 23, 600–609. [Google Scholar] [CrossRef]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.J.; Perren, A. Loss of daxx and atrx are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014, 146, 453–460.e5. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Q.; Li, X.; Zhao, D.; Liu, Y.; Zhao, K.; Liu, Y.; Wang, C.; Jiang, M.; Li, N.; et al. Death domain-associated protein 6 (daxx) selectively represses il-6 transcription through histone deacetylase 1 (hdac1)-mediated histone deacetylation in macrophages. J. Biol. Chem. 2014, 289, 9372–9379. [Google Scholar] [CrossRef]
- Zhong, S.; Salomoni, P.; Ronchetti, S.; Guo, A.; Ruggero, D.; Pandolfi, P.P. Promyelocytic leukemia protein (pml) and daxx participate in a novel nuclear pathway for apoptosis. J. Exp. Med. 2000, 191, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Egan, D.A.; Evans, R.A.; Reed, J.C. Human daxx regulates fas-induced apoptosis from nuclear pml oncogenic domains (pods). EMBO J. 1999, 18, 6037–6049. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pei, H.; Watson, D.K.; Papas, T.S. Eap1/daxx interacts with ets1 and represses transcriptional activation of ets1 target genes. Oncogene 2000, 19, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ohiro, Y.; Usheva, A.; Kobayashi, S.; Duffy, S.L.; Nantz, R.; Gius, D.; Horikoshi, N. Inhibition of stress-inducible kinase pathways by tumorigenic mutant p53. Mol. Cell. Biol. 2003, 23, 322–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Charette, S.J.; Lavoie, J.N.; Lambert, H.; Landry, J. Inhibition of daxx-mediated apoptosis by heat shock protein 27. Mol. Cell. Biol. 2000, 20, 7602–7612. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Sindre, H.; Stamminger, T. Functional interaction between the pp71 protein of human cytomegalovirus and the pml-interacting protein human daxx. J. Virol. 2002, 76, 5769–5783. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Xiao, J.; He, X.; Ke, J.; Zou, Y.; Chen, Y.; Wu, X.; Li, X.; Wang, L.; Wang, J.; et al. Accessing new prognostic significance of preoperative carcinoembryonic antigen in colorectal cancer receiving tumor resection: More than positive and negative. Cancer Biomark. Sect. A Dis. Mark. 2017, 19, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.; Freedman, S.O. Specific carcinoembryonic antigens of the human digestive system. J. Exp. Med. 1965, 122, 467–481. [Google Scholar] [CrossRef]
- Pakdel, A.; Malekzadeh, M.; Naghibalhossaini, F. The association between preoperative serum cea concentrations and synchronous liver metastasis in colorectal cancer patients. Cancer Biomark. Sect. A Dis. Markers 2016, 16, 245–252. [Google Scholar] [CrossRef]
- Bajenova, O.; Chaika, N.; Tolkunova, E.; Davydov-Sinitsyn, A.; Gapon, S.; Thomas, P.; O’Brien, S. Carcinoembryonic antigen promotes colorectal cancer progression by targeting adherens junction complexes. Exp. Cell Res. 2014, 324, 115–123. [Google Scholar] [CrossRef]
- Deng, W.; Gu, L.; Li, X.; Zheng, J.; Zhang, Y.; Duan, B.; Cui, J.; Dong, J.; Du, J. Cd24 associates with egfr and supports egf/egfr signaling via rhoa in gastric cancer cells. J. Transl. Med. 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Ge, C.; Zhao, F.; Chen, T.; Tian, H.; Li, J.; Li, H. Cd24 isoform a promotes cell proliferation, migration and invasion and is downregulated by egr1 in hepatocellular carcinoma. Oncotargets Ther. 2019, 12, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Bretz, N.P.; Salnikov, A.V.; Perne, C.; Keller, S.; Wang, X.; Mierke, C.T.; Fogel, M.; Erbe-Hofmann, N.; Schlange, T.; Moldenhauer, G.; et al. Cd24 controls src/stat3 activity in human tumors. Cell. Mol. Life Sci. 2012, 69, 3863–3879. [Google Scholar] [CrossRef]
- Kristiansen, G.; Sammar, M.; Altevogt, P. Tumour biological aspects of cd24, a mucin-like adhesion molecule. J. Mol. Histol. 2004, 35, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.; Wang, X. Combination of targeting cd24 and inhibiting autophagy suppresses the proliferation and enhances the apoptosis of colorectal cancer cells. Mol. Med. Rep. 2019, 20, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Li, L.; Li, Y.L.; Liu, A.Y.; Yu, M.J.; Wan, Y.P. Distribution and location of daxx in cervical epithelial cells with high risk human papillomavirus positive. Diagn. Pathol. 2014, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Oh, S.H. The role of cd24 in various human epithelial neoplasias. Pathol. Res. Pract. 2005, 201, 479–486. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Attar, A.; Kim, J.; Watson, N.F.; Scholefield, J.H.; Durrant, L.G.; Ilyas, M. Cd24 shows early upregulation and nuclear expression but is not a prognostic marker in colorectal cancer. J. Clin. Pathol. 2009, 62, 1117–1122. [Google Scholar] [CrossRef]
| DAXX Protein Expression | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | DAXX Low | DAXX High | p Vale | |||||
| n | % | n | % | n | % | |||
| Parameter | 106 | 100.0% | 53 | 50.0% | 53 | 50.0% | ||
| Sex | Male | 61 | 57.5% | 35 | 33.0% | 26 | 24.5% | 0.0770 |
| Female | 45 | 42.5% | 18 | 17.0% | 27 | 25.5% | ||
| Differentiation | Well | 4 | 3.8% | 4 | 3.8% | 0 | 0.0% | 0.1274 |
| Moderate | 16 | 15.1% | 7 | 6.6% | 9 | 8.5% | ||
| Poor | 77 | 72.6% | 40 | 37.7% | 37 | 34.9% | ||
| Unknown | 9 | 8.5% | 2 | 1.9% | 7 | 6.6% | ||
| Depth of invasion | T1 | 4 | 3.8% | 3 | 2.8% | 1 | 1.0% | 0.5139 |
| T2 | 1 | 0.9% | 1 | 0.9% | 0 | 0.0% | ||
| T3 | 88 | 83.0% | 44 | 41.5% | 44 | 41.5% | ||
| T4 | 3 | 2.9% | 1 | 1.0% | 2 | 1.9% | ||
| Unknown | 10 | 9.4% | 4 | 3.8% | 6 | 5.6% | ||
| Regional lymph node | N0 | 55 | 51.9% | 29 | 27.4% | 26 | 24.5% | 0.7900 |
| N1 | 42 | 39.6% | 21 | 19.8% | 21 | 19.8% | ||
| Unknown | 9 | 8.5% | 3 | 2.8% | 6 | 5.7% | ||
| Distant metastasis | M0 | 54 | 51.0% | 27 | 25.5% | 27 | 25.5% | 0.7411 |
| M1 | 45 | 42.4% | 24 | 22.6% | 21 | 19.8% | ||
| Unknown | 7 | 6.6% | 2 | 1.9% | 5 | 4.7% | ||
| Lymphatic invasion | Negative | 35 | 33.0% | 19 | 17.9% | 16 | 15.1% | 0.5135 |
| Positive | 53 | 50.0% | 25 | 23.6% | 28 | 26.4% | ||
| Unknown | 18 | 17.0% | 9 | 8.5% | 9 | 8.5% | ||
| Venous invasion | Negative | 71 | 67.0% | 38 | 35.8% | 33 | 31.2% | 0.5653 |
| Positive | 18 | 17.0% | 11 | 10.4% | 7 | 6.6% | ||
| Unknown | 17 | 16.0% | 4 | 3.8% | 13 | 12.2% | ||
| Serum carcinoembryonic antigen (CEA) level | Negative | 49 | 46.2% | 7 | 6.6% | 42 | 39.6.% | <0.001 *** |
| Positive | 36 | 34.0% | 25 | 23.6% | 11 | 10.4% | ||
| Unknown | 21 | 19.8% | 21 | 19.8% | 0 | 0.0% | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Lee, T.-H.; Tzeng, S.-L. Reduced DAXX Expression Is Associated with Reduced CD24 Expression in Colorectal Cancer. Cells 2019, 8, 1242. https://doi.org/10.3390/cells8101242
Chen Y-C, Lee T-H, Tzeng S-L. Reduced DAXX Expression Is Associated with Reduced CD24 Expression in Colorectal Cancer. Cells. 2019; 8(10):1242. https://doi.org/10.3390/cells8101242
Chicago/Turabian StyleChen, Ya-Chun, Tsung-Hsien Lee, and Shu-Ling Tzeng. 2019. "Reduced DAXX Expression Is Associated with Reduced CD24 Expression in Colorectal Cancer" Cells 8, no. 10: 1242. https://doi.org/10.3390/cells8101242
APA StyleChen, Y.-C., Lee, T.-H., & Tzeng, S.-L. (2019). Reduced DAXX Expression Is Associated with Reduced CD24 Expression in Colorectal Cancer. Cells, 8(10), 1242. https://doi.org/10.3390/cells8101242






