Increased Regeneration Following Stress-Induced Lung Injury in Bleomycin-Treated Chimeric Mice with CD44 Knockout Mesenchymal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. MLE Cell-line and Culture
2.2. Mice and Chimerism
2.3. Assessment of Regenerative Capacity
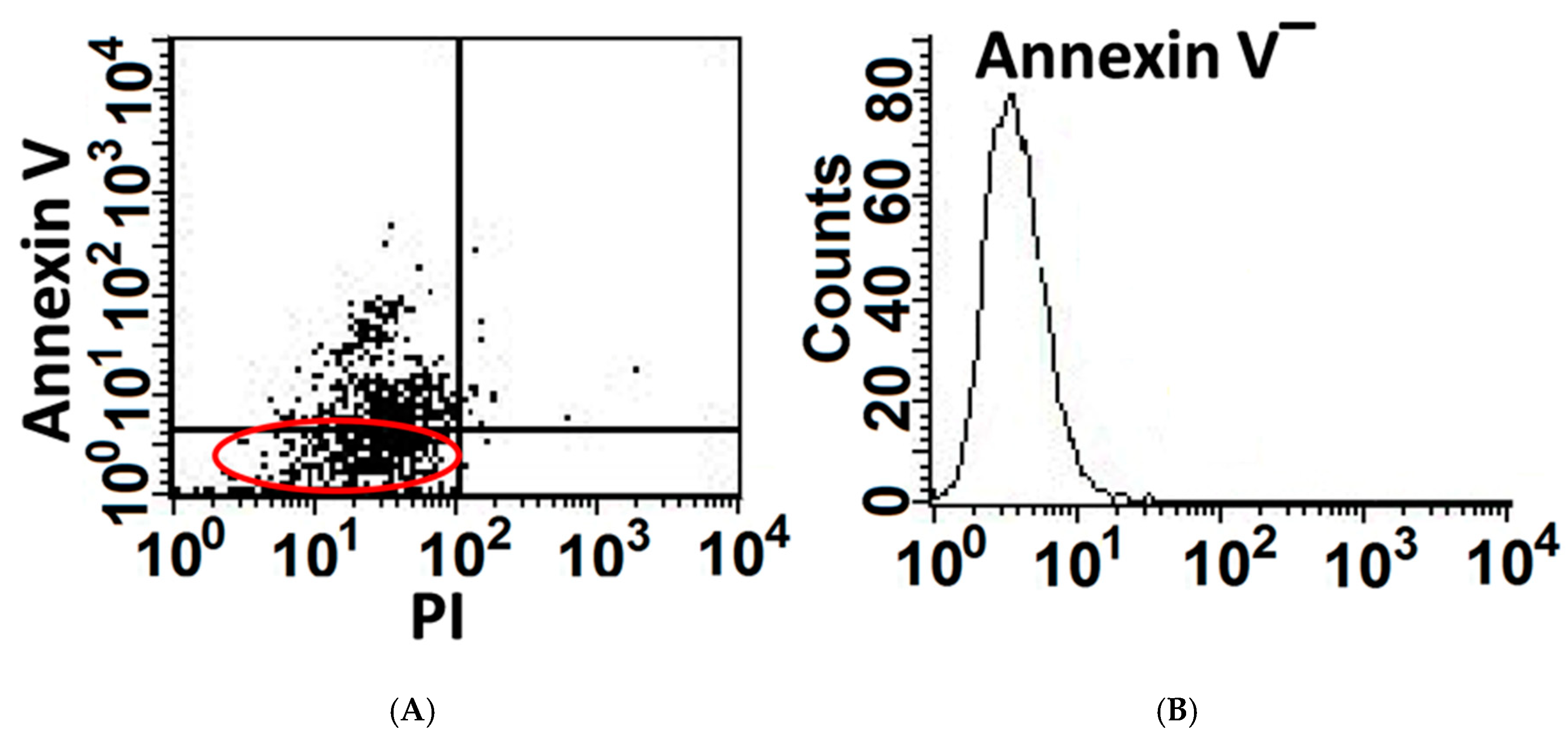
2.4. Annexin-V & BrdU Uptake in Flow Cytometry
2.5. EMT and ATM in IHC
2.6. Lung Mesenchymal Cell Isolation and Culture
2.7. Motility
2.8. Immunofluorescence (IF)
2.9. Immunoblot (IB)
2.10. Phosphorylated Proteins
2.11. Survival Genes
2.12. Data Analysis and Representation
3. Results
3.1. A Portion of AECs, Mouse Lung Epithelial (MLE) Cells, Exposed to Bleomycin Resist Apoptosis and Upregulate Cell-Survival Genes
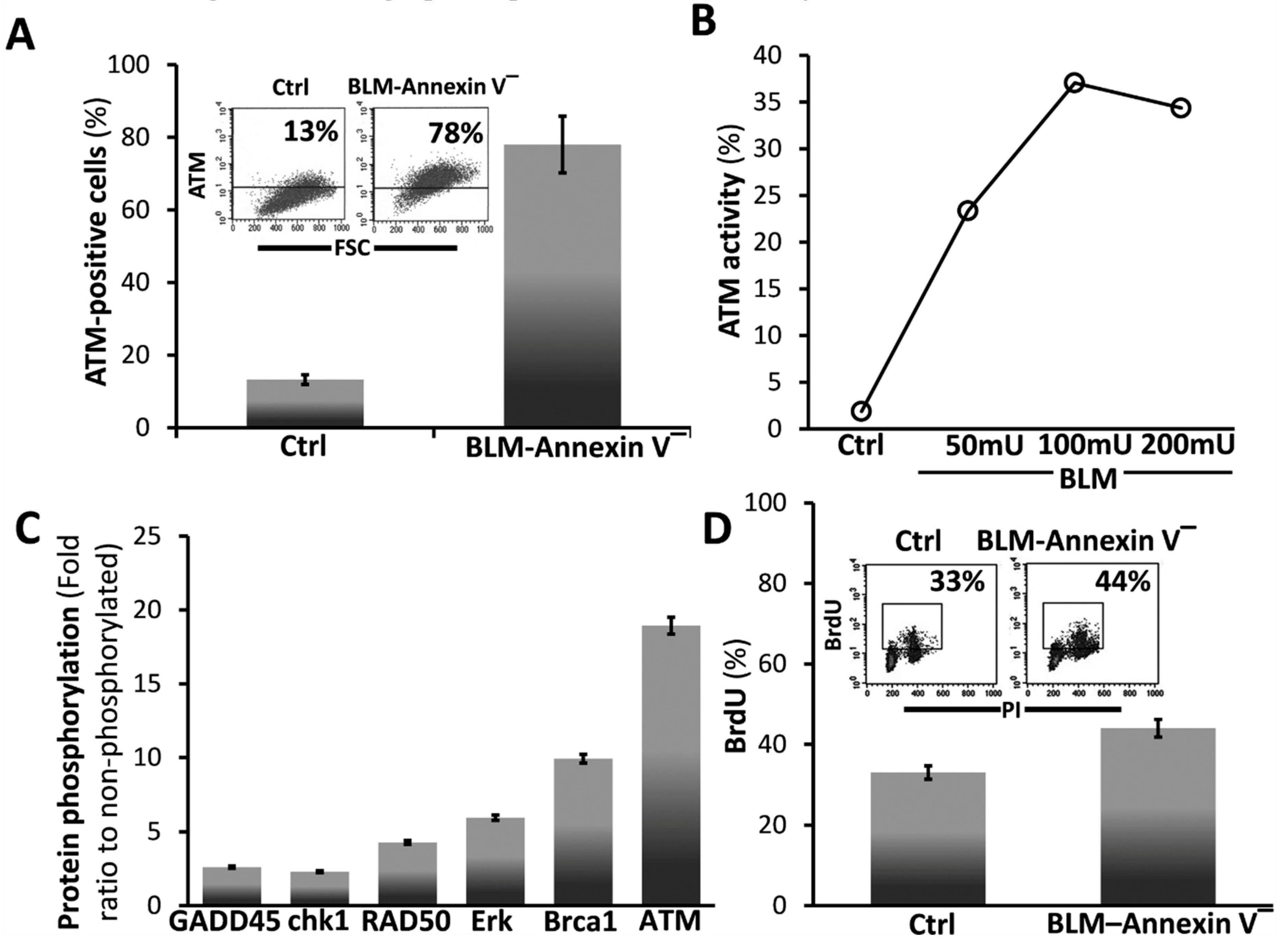
3.2. BLM-Annexin V(−) AECs Upregulate Ataxia Telangiectasia Mutated (ATM) Protein Expression, Activation and Phosphorylation with other Linked Cell-Survival Proteins, and Proliferate
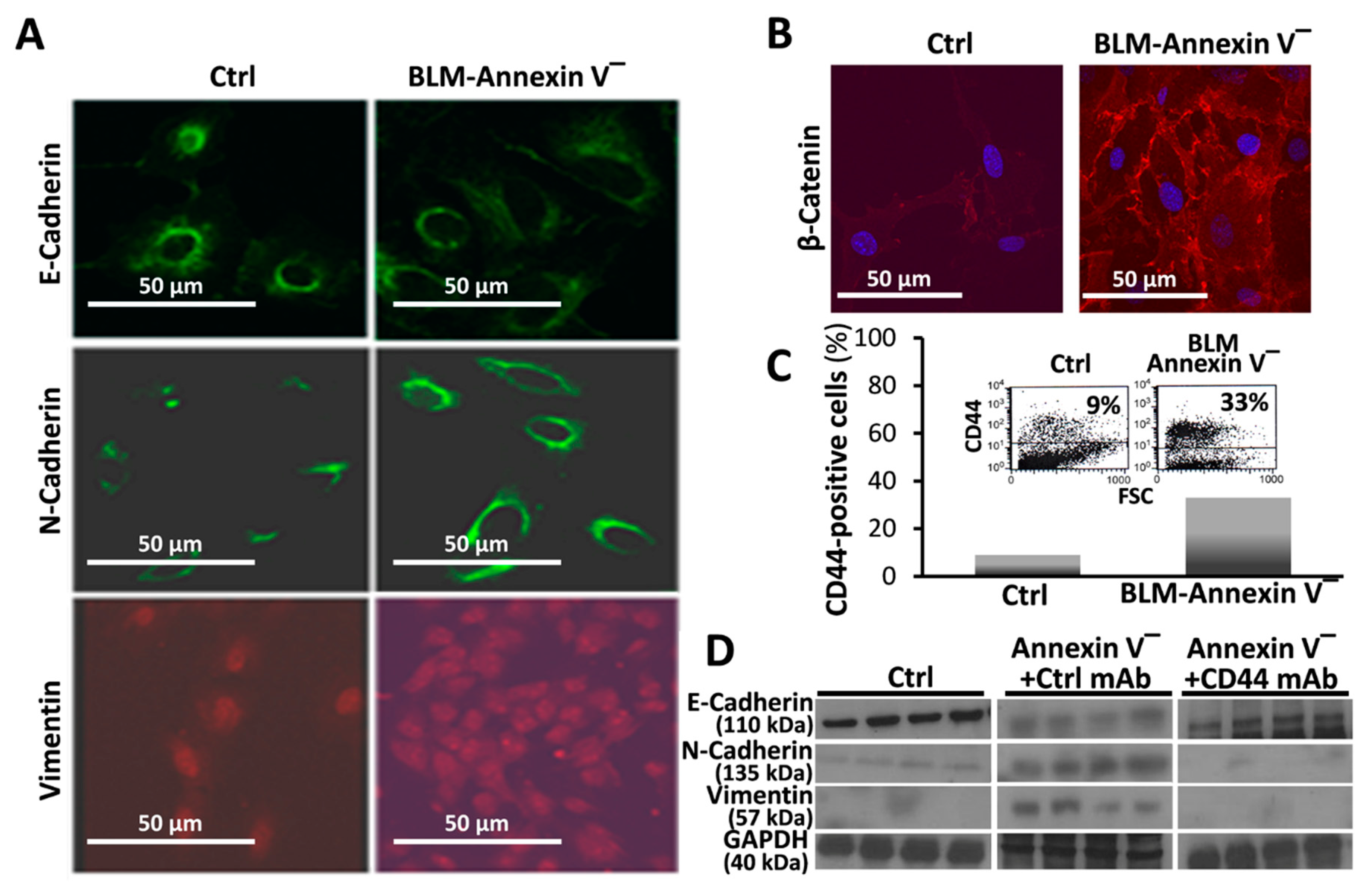
3.3. BLM-Annexin V(−) AECs Perform β-Catenin Nucleus Translocation and a CD44-Mediated Downregulation of E-Cadherin Expression with N-Cadherin and Vimentin Overexpression
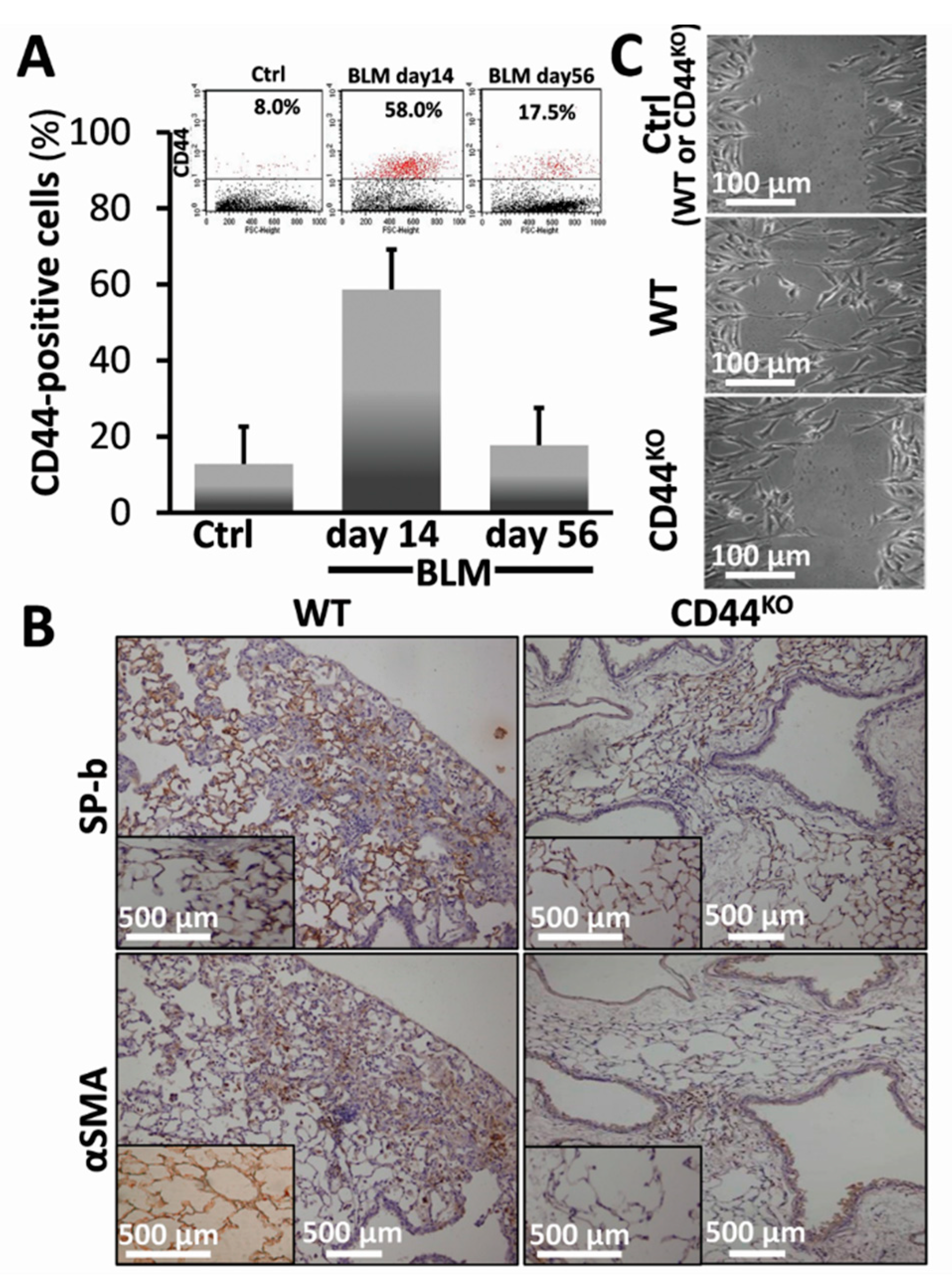
3.4. Bleomycin-Injured Chimeric-CD44KO Mice Decrease EMT Markers in Their Lungs, and Isolated α-SMA+ Cells Decrease their Motility Phenotype In Vitro
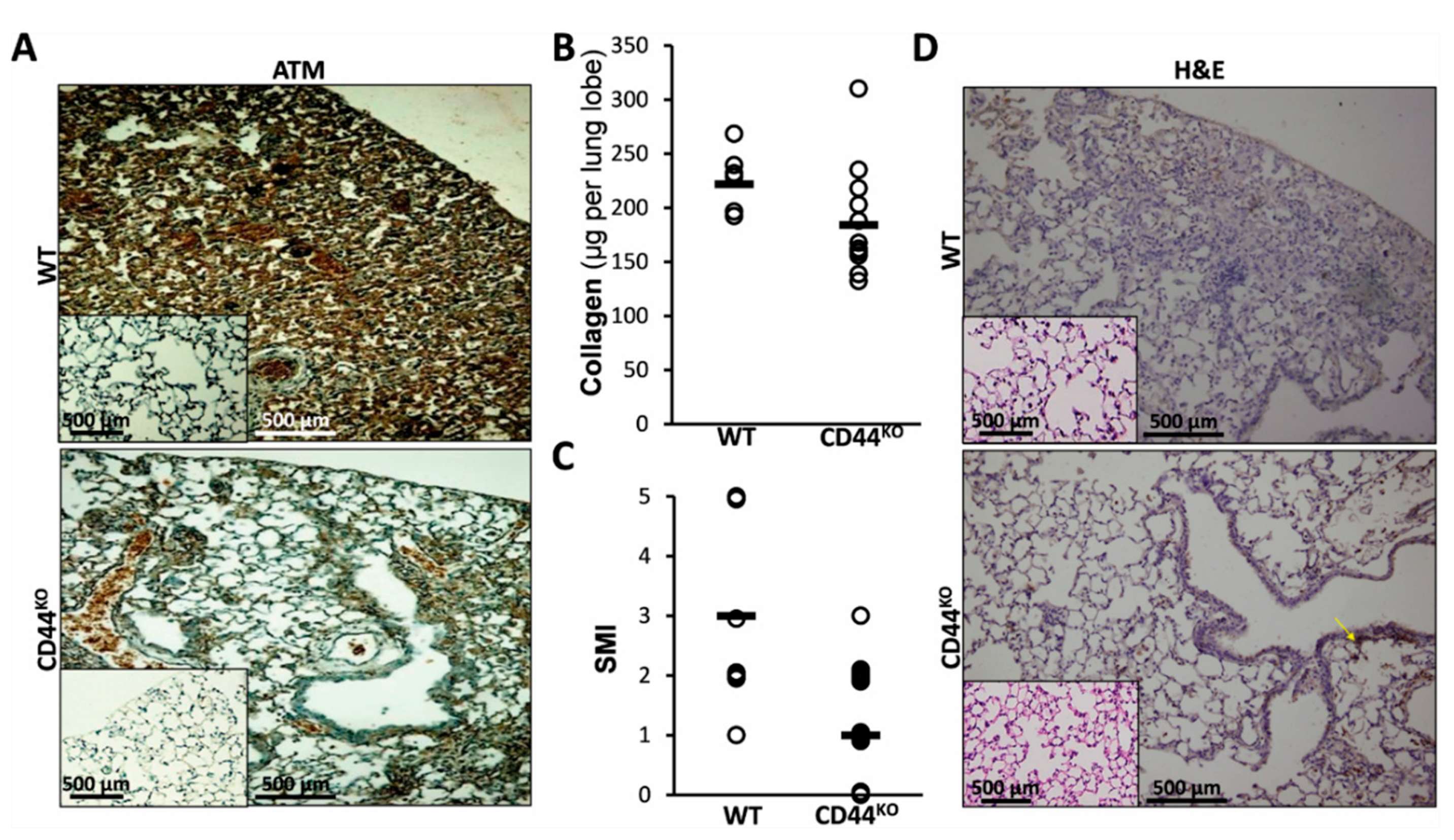
3.5. Normal Lung Regeneration Following Bleomycin-Injury in Chimeric-CD44KO Mice, Compared to Congenic-Controls
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wallach-Dayan, S.B.; Izbicki, G.; Cohen, P.Y.; Gerstl-Golan, R.; Fine, A.; Breuer, R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L790–L796. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.J.; Lehmann, K.; Warne, P.H.; Hill, C.S.; Downward, J. Epithelial to Mesenchymal Transition in Madin-Darby Canine Kidney Cells Is Accompanied by Down-regulation of Smad3 Expression, Leading to Resistance to Transforming Growth Factor-β-induced Growth Arrest. J. Biol. Chem. 2003, 278, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, M.; Mimori, K.; Yokobori, T.; Ishi, H.; Beppu, T.; Nakamori, S.; Baba, H.; Mori, M. Epithelial–mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010, 101, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Lynch, J.P.; Martinez, F.J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Yeh, Y.-H.; Lee, J.-L.; Hsu, Y.-J.; Kuo, C.-T.; Chen, W.-J. Transforming growth factor-β-mediated CD44/STAT3 signaling contributes to the development of atrial fibrosis and fibrillation. Basic Res. Cardiol. 2017, 112, 58. [Google Scholar] [CrossRef]
- Rampanelli, E.; Rouschop, K.M.A.; Claessen, N.; Teske, G.J.D.; Pals, S.T.; Leemans, J.C.; Florquin, S. Opposite role of CD44-standard and CD44-variant-3 in tubular injury and development of renal fibrosis during chronic obstructive nephropathy. Kidney Int. 2014, 86, 558–569. [Google Scholar] [CrossRef]
- Sakuma, Y. Epithelial-to-mesenchymal transition and its role in EGFR-mutant lung adenocarcinoma and idiopathic pulmonary fibrosis. Pathol. Int. 2017, 67, 379–388. [Google Scholar] [CrossRef]
- Tashiro, J.; Rubio, G.A.; Limper, A.H.; Williams, K.; Elliot, S.J.; Ninou, I.; Aidinis, V.; Tzouvelekis, A.; Glassberg, M.K. Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis. Front. Med. (Lausanne) 2017, 4, 118. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Fattman, C.L.; Tan, R.J.; Oury, T.D. Oxidative Stress in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2005, 172, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Bernstein, H.; Payne, C.M.; Garewal, H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: Fail-safe protection against carcinogenesis. Mutat. Res. 2002, 511, 145–178. [Google Scholar] [CrossRef]
- Valerie, K.; Povirk, L.F. Regulation and mechanisms of mammalian double-strand break repair. Oncogene 2003, 22, 5792–5812. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Yacoub, A.; Contessa, J.; Caron, R.; Amorino, G.; Valerie, K.; Hagan, M.P.; Grant, S.; Schmidt-Ullrich, R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 2003, 159, 283–300. [Google Scholar] [CrossRef]
- Tang, S.; Hou, Y.; Zhang, H.; Tu, G.; Yang, L.; Sun, Y.; Lang, L.; Tang, X.; Du, Y.; Zhou, M.; et al. Oxidized ATM promotes abnormal proliferation of breast CAFs through maintaining intracellular redox homeostasis and activating the PI3K-AKT, MEK-ERK, and Wnt-β-catenin signaling pathways. Cell Cycle 2015, 14, 1908–1924. [Google Scholar] [CrossRef] [PubMed]
- Stagni, V.; Manni, I.; Oropallo, V.; Mottolese, M.; Di Benedetto, A.; Piaggio, G.; Falcioni, R.; Giaccari, D.; Di Carlo, S.; Sperati, F.; et al. ATM kinase sustains HER2 tumorigenicity in breast cancer. Nat. Commun. 2015, 6, 6886. [Google Scholar] [CrossRef] [PubMed]
- Schmits, R.; Filmus, J.; Gerwin, N.; Senaldi, G.; Kiefer, F.; Kundig, T.; Wakeham, A.; Shahinian, A.; Catzavelos, C.; Rak, J.; et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 1997, 90, 2217–2233. [Google Scholar] [PubMed]
- Chen, D.; McKallip, R.J.; Zeytun, A.; Do, Y.; Lombard, C.; Robertson, J.L.; Mak, T.W.; Nagarkatti, P.S.; Nagarkatti, M. CD44-deficient mice exhibit enhanced hepatitis after concanavalin A injection: Evidence for involvement of CD44 in activation-induced cell death. J. Immunol. 2001, 166, 5889–5897. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Wallach-Dayan, S.B.; Amir, G.; Breuer, R. Epithelial Cell Apoptosis by Fas Ligand–Positive Myofibroblasts in Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2007, 36, 270–275. [Google Scholar] [CrossRef]
- Egger, C.; Cannet, C.; Gérard, C.; Jarman, E.; Jarai, G.; Feige, A.; Suply, T.; Micard, A.; Dunbar, A.; Tigani, B.; et al. Administration of bleomycin via the oropharyngeal aspiration route leads to sustained lung fibrosis in mice and rats as quantified by UTE-MRI and histology. PLoS ONE 2013, 8, e63432. [Google Scholar] [CrossRef]
- Wallach-Dayan, S.B.; Elkayam, L.; Golan-Gerstl, R.; Konikov, J.; Zisman, P.; Dayan, M.R.; Arish, N.; Breuer, R. Cutting edge: FasL(+) immune cells promote resolution of fibrosis. J. Autoimmun. 2015, 59, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wallach-Dayan, S.B.; Golan-Gerstl, R.; Breuer, R. Evasion of myofibroblasts from immune surveillance: A mechanism for tissue fibrosis. Proc. Natl. Acad. Sci. USA 2007, 104, 20460–20465. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.Y.; Breuer, R.; Wallach-Dayan, S.B. A Profibrotic Phenotype in Naïve and in Fibrotic Lung Myofibroblasts Is Governed by Modulations in Thy-1 Expression and Activation. Mediators Inflamm. 2018, 2018, 4638437. [Google Scholar] [CrossRef] [PubMed]
- Golan-Gerstl, R.; Wallach-Dayan, S.B.; Zisman, P.; Cardoso, W.V.; Goldstein, R.H.; Breuer, R. Cellular FLICE-like inhibitory protein deviates myofibroblast fas-induced apoptosis toward proliferation during lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 47, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.Y.; Breuer, R.; Wallach-Dayan, S.B. Thy1 up-regulates FasL expression in lung myofibroblasts via Src family kinases. Am. J. Respir. Cell Mol. Biol. 2009, 40, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Liu, Z.; Peng, L.; Tang, X.; Meng, F.; Ao, Y.; Zhou, M.; Wang, M.; Cao, X.; Qin, B.; et al. Boosting ATM activity alleviates aging and extends lifespan in a mouse model of progeria. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial−Mesenchymal Transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina (Kaunas) 2019, 55, 83. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Abdullah, A.; Wendt, M.K.; Calve, S. Hyaluronic acid, CD44 and RHAMM regulate myoblast behavior during embryogenesis. Matrix Biol. 2019, 78–79, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.P.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, J.; Liu, L.; Liu, S.; Zhu, R. Hypoxia-Induced Epithelial-Mesenchymal Transition Is Involved in Bleomycin-Induced Lung Fibrosis. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, D.; Liang, J.; Meltzer, E.B.; Gray, A.; Miura, R.; Wogensen, L.; Yamaguchi, Y.; Noble, P.W. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J. Exp. Med. 2011, 208, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Bierhaus, A.; Whyte, A.; Binns, R.M.; Schuh, D.; Müller, M. Expression of CD44 isoforms during bleomycin-or radiation-induced pulmonary fibrosis in rats and mini-pigs. Histochem. Cell Biol. 1996, 105, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Duecker, R.; Baer, P.; Eickmeier, O.; Strecker, M.; Kurz, J.; Schaible, A.; Henrich, D.; Zielen, S.; Schubert, R. Oxidative stress-driven pulmonary inflammation and fibrosis in a mouse model of human ataxia-telangiectasia. Redox Biol. 2018, 14, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Daugherity, E.K.; Balmus, G.; Al Saei, A.; Moore, E.S.; Abi Abdallah, D.; Rogers, A.B.; Weiss, R.S.; Maurer, K.J. The DNA damage checkpoint protein ATM promotes hepatocellular apoptosis and fibrosis in a mouse model of non-alcoholic fatty liver disease. Cell Cycle 2012, 11, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.R.; Zha, Q.; Daniel, L.L.; Singh, M.; Singh, K. Lack of ATM induces structural and functional changes in the heart: Role in β-adrenergic receptor-stimulated apoptosis. Exp. Physiol. 2012, 97, 506–515. [Google Scholar] [CrossRef]
- Russell, R.; Perkhofer, L.; Liebau, S.; Lin, Q.; Lechel, A.; Feld, F.M.; Hessmann, E.; Gaedcke, J.; Güthle, M.; Zenke, M.; et al. Loss of ATM accelerates pancreatic cancer formation and epithelial–mesenchymal transition. Nat. Commun. 2015, 6, 7677. [Google Scholar] [CrossRef]
- Andreassen, C.N.; Overgaard, J.; Alsner, J.; Overgaard, M.; Herskind, C.; Cesaretti, J.A.; Atencio, D.P.; Green, S.; Formenti, S.C.; Stock, R.G.; et al. ATM sequence variants and risk of radiation-induced subcutaneous fibrosis after postmastectomy radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 776–783. [Google Scholar] [CrossRef]
- Chen, W.-T.; Ebelt, N.D.; Stracker, T.H.; Xhemalce, B.; Van Den Berg, C.L.; Miller, K.M. ATM regulation of IL-8 links oxidative stress to cancer cell migration and invasion. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
| Survival Gene | Ctrl | BLM-Annexin V‾ |
|---|---|---|
| P53 and ATM | ||
| P21 | 0 | 2 |
| Chk1 | 0 | 3 |
| Atm | 0 | 6.9 |
| Rad 53 (chk 2) | 0.01 | 2.53 |
| Hus | −1 | 3.16 |
| Gadd 45 | 0 | 12.6 |
| Rpa | 4.5 | 13 |
| Mdm 2 | 8 | 25 |
| TNF NF-KB Activation | ||
| TRAF 4 | 0.2 | 8.86 |
| TRAF 3 | 1.8 | 15.19 |
| TRAF 1 | −1.5 | 1.9 |
| I-TRAF | 0.23 | 5.06 |
| Regulation of Apoptosis | ||
| NAIP 1 | −1 | 2.53 |
| Survivin | 11 | 29 |
| Bik | B3 | 1.9 |
| Bim | −2 | 3.8 |
| Apaf-1 | −0.4 | 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petukhov, D.; Richter-Dayan, M.; Fridlender, Z.; Breuer, R.; Wallach-Dayan, S.B. Increased Regeneration Following Stress-Induced Lung Injury in Bleomycin-Treated Chimeric Mice with CD44 Knockout Mesenchymal Cells. Cells 2019, 8, 1211. https://doi.org/10.3390/cells8101211
Petukhov D, Richter-Dayan M, Fridlender Z, Breuer R, Wallach-Dayan SB. Increased Regeneration Following Stress-Induced Lung Injury in Bleomycin-Treated Chimeric Mice with CD44 Knockout Mesenchymal Cells. Cells. 2019; 8(10):1211. https://doi.org/10.3390/cells8101211
Chicago/Turabian StylePetukhov, Dmytro, Mark Richter-Dayan, Zvi Fridlender, Raphael Breuer, and Shulamit B. Wallach-Dayan. 2019. "Increased Regeneration Following Stress-Induced Lung Injury in Bleomycin-Treated Chimeric Mice with CD44 Knockout Mesenchymal Cells" Cells 8, no. 10: 1211. https://doi.org/10.3390/cells8101211
APA StylePetukhov, D., Richter-Dayan, M., Fridlender, Z., Breuer, R., & Wallach-Dayan, S. B. (2019). Increased Regeneration Following Stress-Induced Lung Injury in Bleomycin-Treated Chimeric Mice with CD44 Knockout Mesenchymal Cells. Cells, 8(10), 1211. https://doi.org/10.3390/cells8101211






