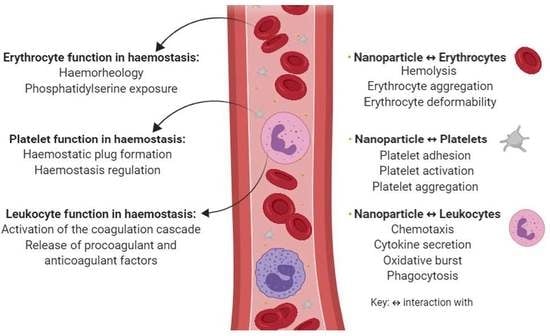
The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis
Abstract
1. Introduction
2. Erythrocyte Function in Hemostasis and the Mechanisms Involved in Nanoparticle Hemocompatibility
2.1. The Influence of Hematocrit on Blood Viscosity and Hemostasis in Connection with Nanoparticle–Erythrocyte Interactions
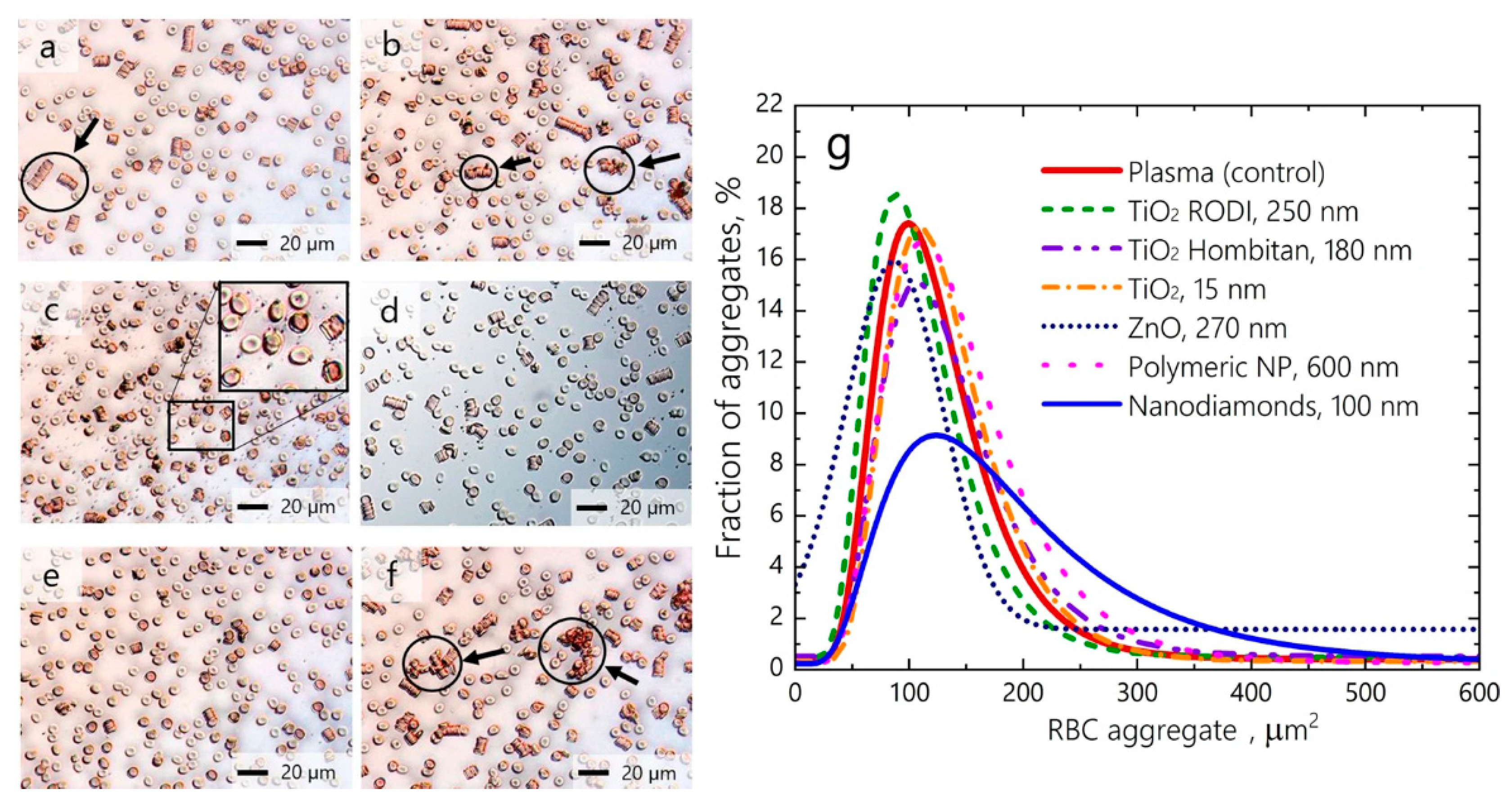
2.2. The Contribution of Erythrocyte Aggregation to Blood Viscosity and Hemostasis in Connection with Nanoparticle–Erythrocyte Interactions
2.3. Erythrocyte Deformability as A Mediator of Blood Viscosity and Hemostasis in Connection with Nanoparticle–Erythrocyte Interactions
2.4. The Influence of Phosphatidylcholine Exposure on Erythrocyte Membranes in Connection with Nanoparticle–Erythrocyte Interactions
3. Platelet Function in Hemostasis and the Mechanisms Involved in Nanoparticle Hemocompatibility
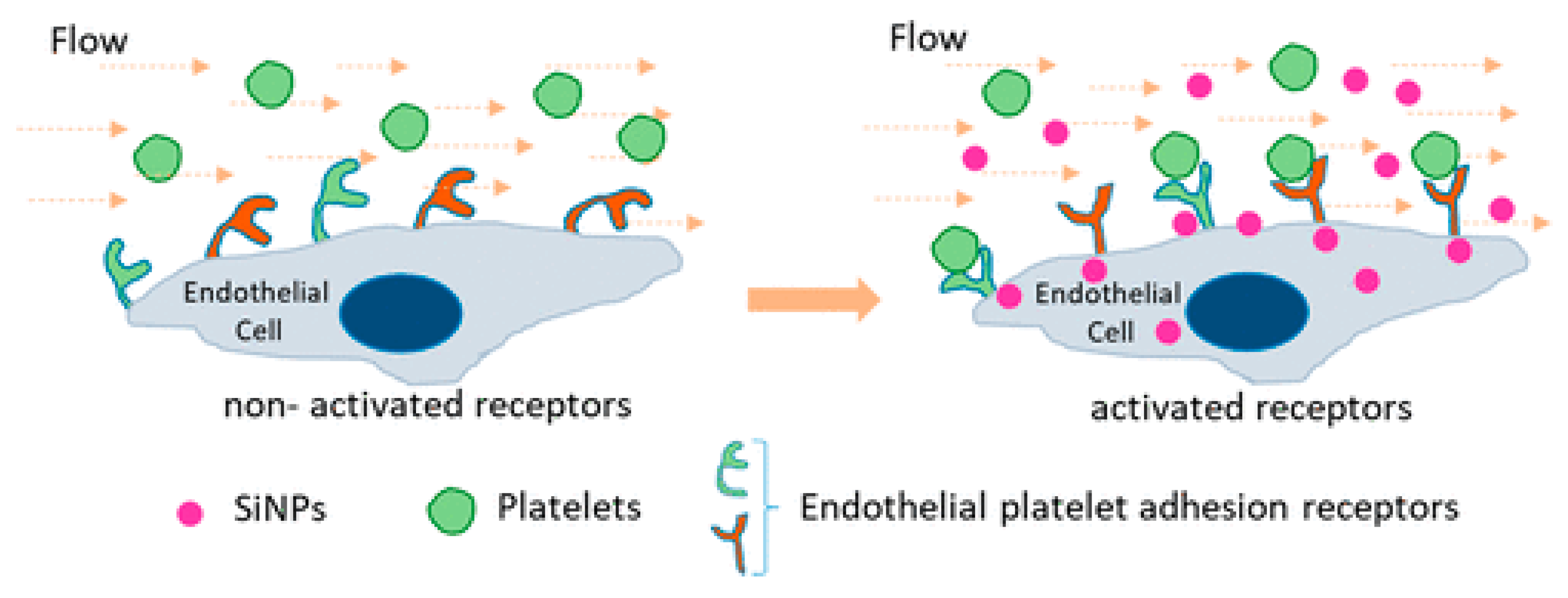
3.1. Platelet Adhesion in Hemostasis and The Effect of Nanoparticle Interactions
3.2. Platelet Activation in Hemostasis and The Effect of Nanoparticle Interactions
3.3. Platelet Aggregation in Hemostasis and the Effect of Nanoparticle Interactions
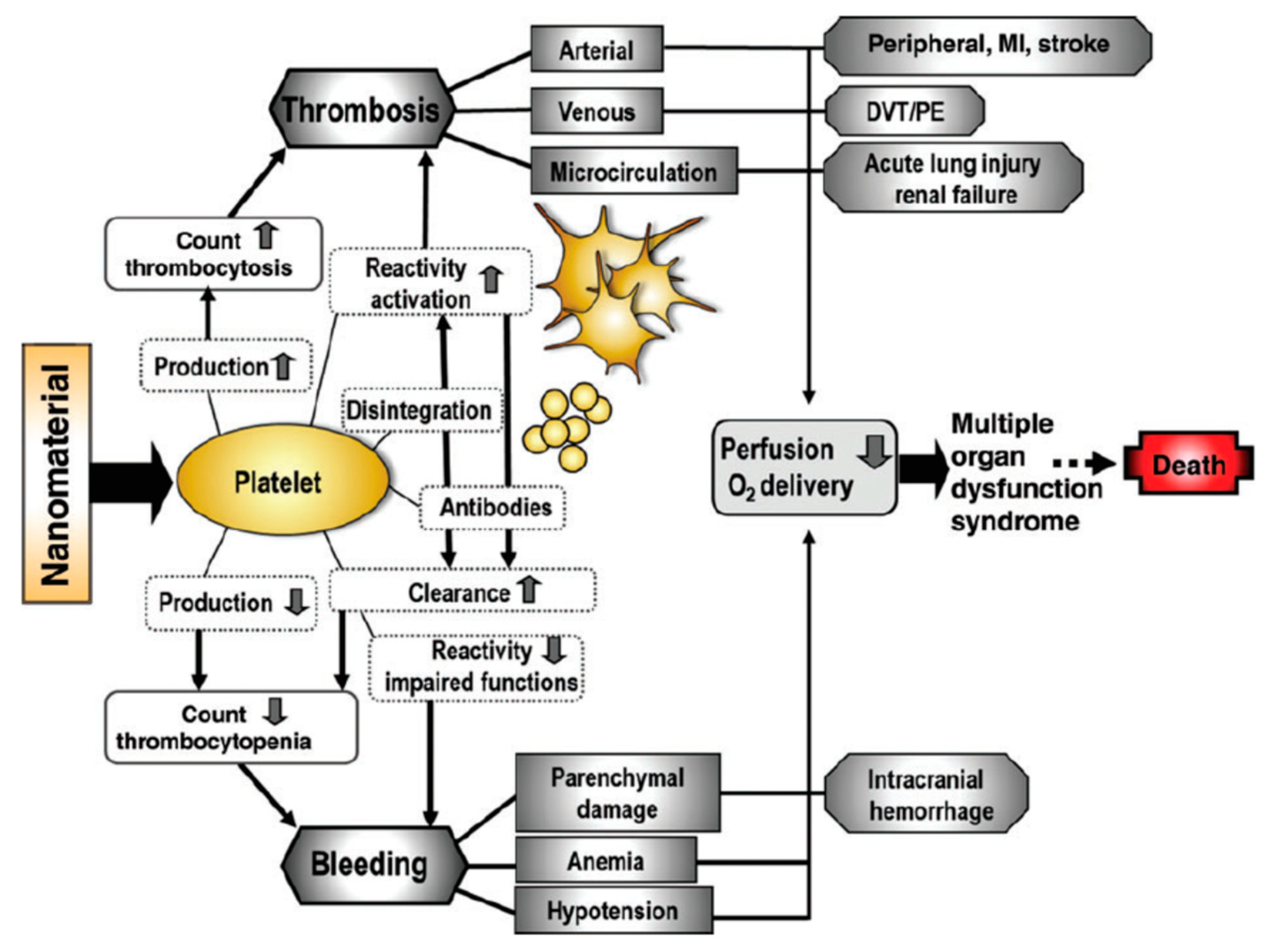
3.4. In Vivo Platelet Toxicities Induced by Nanoparticles
4. Leukocyte Function in Hemostasis and the Mechanisms Involved in Nanoparticle Hemocompatibility
4.1. The Effect of Nanoparticle–Leukocyte Interactions on Chemotaxis
4.2. The Effect of Nanoparticle–Leukocyte Interactions on Cytokine Secretion
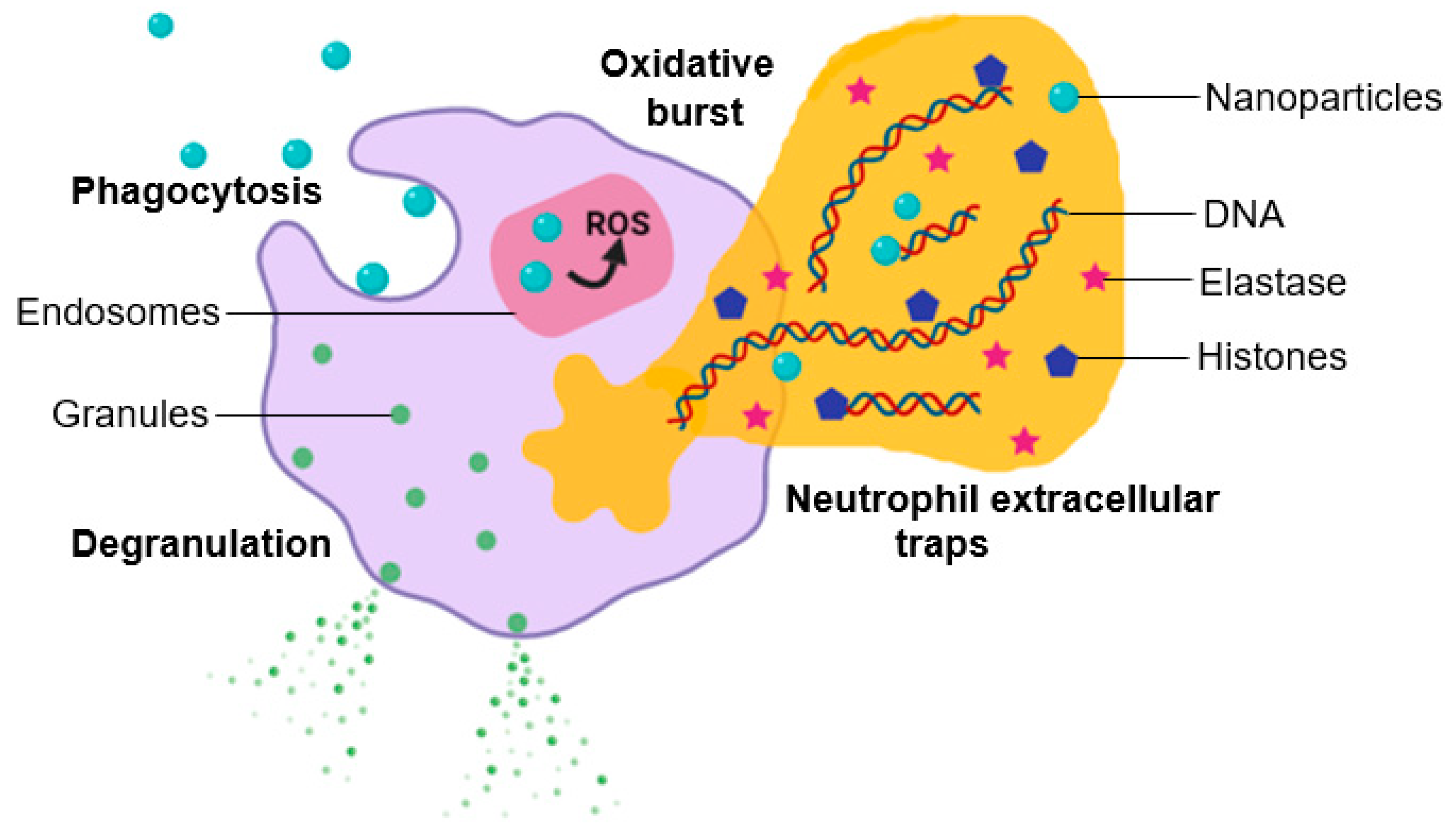
4.3. The Effect of Nanoparticle–Leukocyte Interactions on Oxidative Burst
4.4. The Effect of Nanoparticle–Leukocyte Interactions on Phagocytosis
4.5. Cytotoxicity as a Result of Nanoparticle–Leukocyte Interactions
5. The Influence of Surface Chemistry on Nanoparticle Hemocompatibility
5.1. Surface Charge as A Determining Factor of Nanoparticle Hemocompatability
5.2. Geometry as a Determining Factor of Nanoparticle Hemocompatability
5.3. Porosity as a Determining Factor of Nanoparticle Hemocompatability
5.4. Surface Modification as A Determining Factor of Nanoparticle Hemocompatability
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate; |
| ATP | adenosine triphosphate; |
| EGF | epidermal growth factor; |
| GDP | guanosine diphosphate; |
| GTP | guanosine triphosphate; |
| MMP | matrix metalloproteinase; |
| NO | nitric oxide; |
| PAF | platelet activating factor; |
| PAI-1 | plasminogen activator inhibitor-1; |
| PDGF | platelet derived growth factor; |
| TFPI | tissue factor pathway inhibitor; |
| TGF-β | transforming growth factor β; |
| VEGF | vascular endothelial growth factor; |
| VWF | von Willebrand factor. |
References
- Matus, M.F.; Vilos, C.; Cisterna, B.A.; Fuentes, E.; Palomo, I. Nanotechnology and primary hemostasis: Differential effects of nanoparticles on platelet responses. Vasc. Pharmacol. 2018, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brash, J.L. Blood Compatibility of Nanomaterials. Drug Delivery Nanosystems for Biomedical Applications; Elsevier Inc.: Hamilton, ON, Canada, 2018; pp. 13–31. Available online: https://doi.org/10.1016/B978-0-323-50922-0.00002-X (accessed on 24 June 2019).
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein Corona of Nanoparticles: Distinct Proteins Regulate the Cellular Uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lv, L.; Bai, Y.; Yang, H.; Zhou, H.; Li, C.; Yang, L. Nanotechnology-enabled materials for hemostatic and anti-infection treatments in orthopedic surgery. Int. J. Nanomed. 2018, 13, 8325–8338. [Google Scholar] [CrossRef] [PubMed]
- Zia, F.; Kendall, M.; Watson, S.P.; Mendes, P.M. Platelet aggregation induced by polystyrene and platinum nanoparticles is dependent on surface area. Rsc Adv. 2018, 8, 37789–37794. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Santoso, M.R.; Rezaee, F.; Aghaverdi, H.; Mahmoudi, M.; Perry, G. Advances in Alzheimer’s Diagnosis and Therapy: The Implications of Nanotechnology. Trends Biotechnol. 2017, 35, 937–953. [Google Scholar] [CrossRef]
- Sarmah, D.; Saraf, J.; Kaur, H.; Pravalika, K.; Tekade, R.K.; Borah, A.; Kalia, K.; Dave, K.R.; Bhattacharya, P. Stroke Management: An Emerging Role of Nanotechnology. Micromachines 2017, 8, 262. [Google Scholar] [CrossRef]
- Szebeni, J.; Haima, P. Hemocompatibility of medical devices, blood products, nanomedicines and biologicals. In TECOmedical Clinical & Technical Review; TECOmedical: Sissach, Switzerland, 2013; 40p. [Google Scholar]
- Scanlon, V.C.; Sanders, T. Essentials of Anatomy and Physiology; F.A. Davis: Philadelphia, PA, USA, 2018; pp. 256–314. [Google Scholar]
- Sorlie, P.D.; García-Palmieri, M.R.; Costas, R.; Havlik, R.J. Hematocrit and risk of coronary heart disease: The Puerto Rico Heart Health Program. Am. Hear. J. 1981, 101, 456–461. [Google Scholar] [CrossRef]
- Iwata, H.; Kaibara, M. Activation of factor IX by erythrocyte membranes causes intrinsic coagulation. Blood Coagul. Fibrinolysis 2002, 13, 489–496. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood 2017, 130, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Red blood cells: The forgotten player in hemostasis and thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Du, V.; Huskens, D.; Maas, C.; Al Dieri, R.; De Groot, P.; De Laat, B. New Insights into the Role of Erythrocytes in Thrombus Formation. Semin. Thromb. Hemost. 2013, 40, 72–80. [Google Scholar] [PubMed]
- Mehri, R.; Mavriplis, C.; Fenech, M. Red blood cell aggregates and their effect on non-Newtonian blood viscosity at low hematocrit in a two-fluid low shear rate microfluidic system. PLoS ONE 2018, 13, e0199911. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Intaglietta, M.; Tartakovsky, D.M. Non-Newtonian flow of blood in arterioles: Consequences for wall shear stress measurements. Microcirculation 2014, 21, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Walton, B.L.; Lehmann, M.; Skorczewski, T.; Holle, L.A.; Beckman, J.D.; Cribb, J.A.; Mooberry, M.J.; Wufsus, A.R.; Cooley, B.C.; Homeister, J.W.; et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood 2017, 129, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.F.; Tomaiuolo, M.; Welsh, J.; Poventud-Fuentes, I.; Zhu, L.; Diamond, S.L.; Stalker, T.J. Hemostatic Thrombus Formation in Flowing Blood; Elsevier: Suzhou, China, 2019; pp. 371–391. [Google Scholar] [CrossRef]
- Cooley, M.; Sarode, A.; Hoore, M.; Fedosov, D.A.; Mitragotri, S.; Gupta, A.S. Influence of particle size and shape on their margination and wall-adhesion: Implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 2018, 10, 15350–15364. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Hsu, P.-S.; Tsai, C.-S.; Pan, P.-C.; Chen, Y.-L. Significantly increased low shear rate viscosity, blood elastic modulus, and RBC aggregation in adults following cardiac surgery. Sci. Rep. 2018, 8, 7173. [Google Scholar] [CrossRef]
- L’Acqua, C.; Hod, E. New perspectives on the thrombotic complications of haemolysis. Br. J. Haematol. 2015, 168, 175–185. [Google Scholar] [CrossRef]
- Davenport, R.D. Pathophysiology of hemolytic transfusion reactions. Semin. Hematol. 2005, 42, 165–168. [Google Scholar] [CrossRef]
- Krajewski, S.; Prucek, R.; Panacek, A.; Avci-Adali, M.; Nolte, A.; Straub, A.; Zboril, R.; Wendel, H.P.; Kvitek, L. Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood. Acta Biomater. 2013, 9, 7460–7468. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T. Hemolysis as Expression of Nanoparticles-Induced Cytotoxicity in Red Blood Cells. Biotechnol Mol. Biol. Nanomedicine 2013, 1, 7–12. [Google Scholar]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of Silver Nanoparticles to Red Blood Cells: Size Dependent Adsorption, Uptake, and Hemolytic Activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Tripathy, S.; Adhikary, J.; Chattopadhyay, S.; Mandal, D.; Dash, S.K.; Das, S.; Dey, A.; Dey, S.K.; Das, D.; et al. Surface modification minimizes the toxicity of silver nanoparticles: An in vitro and in vivo study. Jbic J. Boil. Inorg. Chem. 2017, 22, 893–918. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of Silica Nanoparticle Design on Cellular Toxicity and Hemolytic Activity. Acs Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnology 2014, 12, 5. [Google Scholar] [CrossRef]
- Barshtein, G.; Arbell, D.; Yedgar, S. Hemodynamic Functionality of Transfused Red Blood Cells in the Microcirculation of Blood Recipients. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. Mutual interaction of red blood cells influenced by nanoparticles. Sci. Rep. 2019, 9, 5147. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Richard, C.; Scherman, D.; Liu, Y. Hemocompatibility investigation and improvement of near-infrared persistent luminescent nanoparticle ZnGa2O4:Cr3+ by surface PEGylation. J. Mater. Chem. B 2019, 7, 3796–3803. [Google Scholar] [CrossRef]
- Neu, B.; Sowemimo-Coker, S.O.; Meiselman, H.J. Cell-Cell Affinity of Senescent Human Erythrocytes. Biophys. J. 2003, 85, 75–84. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, S. Toxic effects of silver nanoparticles and nanowires on erythrocyte rheology. Food Chem. Toxicol. 2014, 67, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, X.; Dai, H.; Li, S. Nanosize and Surface Charge Effects of Hydroxyapatite Nanoparticles on Red Blood Cell Suspensions. Acs Appl. Mater. Interfaces 2012, 4, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Yedgar, S.; Koshkaryev, A.; Barshtein, G. The red blood cell in vascular occlusion. Pathophysiol. Haemost. Thromb. 2002, 32, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Geekiyanage, N.M.; Balanant, M.A.; Sauret, E.; Saha, S.; Flower, R.; Lim, C.T.; Gu, Y. A coarse-grained red blood cell membrane model to study stomatocyte-discocyteechinocyte morphologies. PLoS ONE 2019, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Shin, S. Advances in the measurement of red blood cell deformability: A brief review. J. Cell. Biotechnol. 2015, 1, 63–79. Available online: http://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JCB-15007 (accessed on 19 June 2019). [CrossRef]
- Kwaan, H.C.; Samama, M. Clinical Thrombosis; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Lin, Y.-C.; Tsai, L.-W.; Perevedentseva, E.; Chang, H.-H.; Sun, D.-S.; Lugovtsov, A.E.; Priezzhev, A.; Mona, J.; Cheng, C.-L. The influence of nanodiamond on the oxygenation states and micro rheological properties of human red blood cells in vitro. J. Biomed. Opt. 2012, 17, 101512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of Mesoporous Silica Nanoparticles with Human Red Blood Cell Membranes: Size and Surface Effects. Acs Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef]
- Kim, J.; Nafiujjaman, M.; Nurunnabi, M.; Lee, Y.-K.; Park, H.-K. Hemorheological characteristics of red blood cells exposed to surface functionalized graphene quantum dots. Food Chem. Toxicol. 2016, 97, 346–353. [Google Scholar] [CrossRef]
- He, Z.; Liu, J.; Du, L. The unexpected effect of PEGylated gold nanoparticles on the primary function of erythrocytes. Nanoscale 2014, 6, 9017–9024. [Google Scholar] [CrossRef]
- Guo, L.; Tong, D.; Yu, M.; Zhang, Y.; Li, T.; Wang, C.; Zhou, P.; Jin, J.; Li, B.; Liu, Y.; et al. Phosphatidylserine-exposing cells contribute to the hypercoagulable state in patients with multiple myeloma. Int. J. Oncol. 2018, 52, 1981–1990. [Google Scholar] [CrossRef]
- Ran, Q.; Xiang, Y.; Liu, Y.; Xiang, L.; Li, F.; Deng, X.; Xiao, Y.; Chen, L.; Chen, L.; Li, Z. Eryptosis Indices as a Novel Predictive Parameter for Biocompatibility of Fe3O4 Magnetic Nanoparticles on Erythrocytes. Sci. Rep. 2015, 5, 16209. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.C.; Myerson, J.W.; Brenner, J.S.; Patel, P.N.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells. Sci. Rep. 2018, 8, 1615. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J. Platelets and Primary Haemostasis. Thromb. Res. 2012, 129, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Feys, H.B.; De Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.E.; Santagostino, E. Platelets: Much more than bricks in a breached wall. Br. J. Haematol. 2017, 178, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Stefanini, L. Platelets at the vascular interface. Res. Pract. Thromb. Haemost. 2018, 2, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Green, L. Physiology of haemostasis. Anaesth. Intensiv. Care Med. 2019, 20, 152–158. [Google Scholar] [CrossRef]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles aggregate human platelets: Potential implications for vascular homeostasis. Int. J. Nanomed. 2012, 7, 631–639. [Google Scholar]
- Saikia, J.; Mohammadpour, R.; Yazdimamaghani, M.; Northrup, H.; Hlady, V.; Ghandehari, H. Silica Nanoparticle–Endothelial Interaction: Uptake and Effect on Platelet Adhesion under Flow Conditions. ACS Appl. Bio Mater. 2018, 1, 1620–1627. [Google Scholar] [CrossRef]
- Feng, L.; Yang, X.; Liang, S.; Xu, Q.; Miller, M.R.; Duan, J.; Sun, Z. Silica nanoparticles trigger the vascular endothelial dysfunction and prethrombotic state via miR-451 directly regulating the IL6R signaling pathway. Part. Fibre Toxicol. 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrews, M.; Yang, Y.; Lang, S.W.; Jin, J.; Cameron-Vendrig, A.; Zhu, G.; Reheman, A.; Ni, H. Platelets in Thrombosis and Hemostasis: Old Topic with New Mechanisms. Cardiovasc. Hematol. Disord. Targets 2013, 12, 126–132. [Google Scholar] [CrossRef]
- Austin, S.K. Haemostasis. Medicine (United Kingdom) 2017, 45, 204–208. [Google Scholar] [CrossRef]
- Radomska, A.; Leszczyszyn, J.; Radomski, M.W. The Nanopharmacology and Nanotoxicology of Nanomaterials: New Opportunities and Challenges. Adv. Clin. Exp. Med. 2016, 25, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Majoral, J.-P. Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers†. Molecules 2018, 23, 622. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Thiagarajan, G.; Herd, H.; Price, R.; Bauer, H.; Hubbard, D.; Burckle, A.; Sadekar, S.; Yu, T.; Anwar, A.; et al. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology 2012, 6, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Campbell, R.A.; Franks, Z.; Gibson, C.C.; Thiagarajan, G.; Vieira-De-Abreu, A.; Sukavaneshvar, S.; Mohammad, S.F.; Li, D.Y.; Ghandehari, H.; et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharm. 2012, 9, 1599–1611. [Google Scholar] [CrossRef]
- Simak, J.; De Paoli, S. The effects of nanomaterials on blood coagulation in hemostasis and thrombosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2017, 9, e1448. [Google Scholar] [CrossRef]
- Šimák, J. The effects of engineered nanomaterials on platelets. In Handbook of Immunological Properties of Engineered Nanomaterials; Dobrovolskaia, M.A., McNeil, S.E., Eds.; World Scientific: Singapore, 2016; pp. 193–259. [Google Scholar]
- Shah, N.B.; Bischof, J.C. Blood protein and blood cell interactions with gold nanoparticles: The need for in vivo studies. BioNanoMaterials 2013, 14, 65–79. [Google Scholar] [CrossRef]
- Gorbet, M.; Sperling, C.; Maitz, M.F.; Siedlecki, C.A.; Werner, C.; Sefton, M.V.; Siedleck, C.A. The blood compatibility challenge. Part 3: Material associated activation of blood cascades and cells. Acta Biomater. 2019, 94, 25–32. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1742706119304313 (accessed on 1 July 2019). [CrossRef] [PubMed]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2019, 128, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., III; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Baharom, F.; Rankin, G.; Blomberg, A.; Smed-Sorensen, A. Human Lung Mononuclear Phagocytes in Health and Disease. Front. Immunol. 2017, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Putzu, L.; Caocci, G.; Di Ruberto, C. Leucocyte classification for leukaemia detection using image processing techniques. Artif. Intell. Med. 2014, 62, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.A.; Rauova, L.; Poncz, M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb. Res. 2010, 125, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Lip, G.Y.H. The role of monocytes in thrombotic disorders: Insights from tissue factor, monocyte-platelet aggregates and novel mechanisms. Thromb. Haemost. 2009, 102, 916–924. [Google Scholar]
- Laridan, E.; Martinod, K.; De Meyer, S.F. Neutrophil extracellular traps in arterial and venous thrombosis. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: Kortrijk, Belgium, 2019; Volume 45, No. 1; pp. 86–93. [Google Scholar]
- Sotiri, I.; Robichaud, M.; Lee, D.; Braune, S.; Gorbet, M.; Ratner, B.D.; Brash, J.L.; Latour, R.A.; Reviakine, I. BloodSurf 2017: News from the blood-biomaterial frontier. Acta Biomater. 2019, 87, 55–60. [Google Scholar] [CrossRef]
- Sperling, C.; Maitz, M.F.; Werner, C. Test methods for hemocompatibility of biomaterials. In Hemocompatibility of Biomaterials for Clinical Applications: Blood-Biomaterials Interactions; Elsevier Ltd.: Dresden, Germany, 2017; pp. 77–104. [Google Scholar]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Bartneck, M.; Keul, H.A.; Singh, S.; Czaja, K.; Bornemann, J.; Bockstaller, M.R.; Moeller, M.; Zwadlo-Klarwasser, G.; Groll, J. Rapid Uptake of Gold Nanorods by Primary Human Blood Phagocytes and Immunomodulatory Effects of Surface Chemistry. Acs Nano 2010, 4, 3073–3086. [Google Scholar] [CrossRef]
- Huang, R.B.; Mocherla, S.; Heslinga, M.J.; Charoenphol, P.; Eniola-Adefeso, O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery (review). Mol. Membr. Boil. 2010, 27, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Tian, J.; Zhao, Y.; Chen, C.; Zhou, R.; Chai, Z. Towards understanding of nanoparticle–protein corona. Arch. Toxicol. 2015, 89, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Simberg, D. Interactions of nanoparticles with plasma proteins: Implication on clearance and toxicity of drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.N.; Rezende Dos Reis, S.R.; Pires, L.P.; Helal-Neto, E.; Sancenón, F.; Barja-Fidalgo, T.C.; de Mattos, R.M.; Nasciutti, L.E.; Martinez-Manez, R.; Santos-Oliveira, R. Avoiding the mononuclear phagocyte system using human albumin for mesoporous silica nanoparticle system. Microporous Mesoporous Mater. 2017, 251, 181–189. [Google Scholar] [CrossRef]
- Vu, V.P.; Gifford, G.B.; Chen, F.; Benasutti, H.; Wang, G.; Groman, E.V.; Scheinman, R.; Saba, L.; Moghimi, S.M.; Simberg, D. Immunoglobulin deposition on biomolecule corona determines complement opsonisation efficiency of preclinical and clinical nanoparticles. Nat. Nanotechnol. 2019, 14, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, E.-J.; Webster, T.J.; Kim, S.-H.; Khang, D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2014, 10, 97–113. [Google Scholar]
- Lin, M.-H.; Lin, C.-F.; Yang, S.-C.; Hung, C.-F.; Fang, J.-Y. The Interplay between Nanoparticles and Neutrophils. J. Biomed. Nanotechnol. 2018, 14, 66–85. [Google Scholar] [CrossRef]
- Batt, J.; School of Dentistry, University of Alberta, Edmonton, T6G 1C9, Canada; Milward, M.; Chapple, I.; Grant, M.; Roberts, H.; Addison, O. TiO2 nanoparticles can selectively bind CXCL8 impacting on neutrophil chemotaxis. Eur. Cells Mater. 2018, 35, 13–24. [Google Scholar] [CrossRef]
- Kojouri, G.A.; Sadeghian, S.; Mohebbi, A.; Dezfouli, M.R.M. The effects of oral consumption of selenium nanoparticles on chemotactic and respiratory burst activities of neutrophils in comparison with sodium selenite in sheep. Biol. Trace Elem. Res. 2012, 146, 160–166. [Google Scholar] [CrossRef]
- Durocher, I.; Noël, C.; Lavastre, V.; Girard, D. Evaluation of the in vitro and in vivo proinflammatory activities of gold (+) and gold (−) nanoparticles. Inflamm. Res. 2017, 66, 981–992. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Urner, M.; Hasler, M.; Roth-Z’Graggen, B.; Aemisegger, C.; Baulig, W.; Athanassiou, E.K.; Regenass, S.; Stark, W.J.; Beck-Schimmer, B. Iron core/shell nanoparticles as magnetic drug carriers: Possible interactions with the vascular compartment. Nanomedicine 2011, 6, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon.”. Eur. J. Clin. Investig. 2018, 48, e12952. [Google Scholar] [CrossRef] [PubMed]
- Rungelrath, V.; Kobayashi, S.D.; DeLeo, F.R. Neutrophils in innate immunity and systems biology-level approaches: An update. Wiley Interdiscip Rev Syst Biol Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nolte, A.; Hossfeld, S.; Post, M.; Niederlaender, J.; Walker, T.; Schlensak, C.; Wendel, H.P. Endotoxins affect diverse biological activity of chitosans in matters of hemocompatibility and cytocompatibility. J. Mater. Sci. Mater. Electron. 2014, 25, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- De Lima, J.M.; Sarmento, R.R.; de Souza, J.R.; Brayner, F.A.; Feitosa, A.P.S.; Padilha, R.; Alves, L.C.; Porto, I.J.; Batista, R.F.B.D.; de Oliveira, J.E.; et al. Evaluation of Hemagglutination Activity of Chitosan Nanoparticles Using Human Erythrocytes. Biomed. Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Sadreddini, S.; Rostamzadeh, D.; Ahmadi, M.; Jadidi-Niaragh, F.; Yousefi, M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. 2016, 80, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Mohapatra, S.; Kong, X.; Li, X. Genetic Vaccines and Therapy. Genet Vaccines Ther. 2008, 9, 1–9. [Google Scholar]
- Mudgal, J.; Mudgal, P.P.; Kinra, M.; Raval, R. Immunomodulatory role of chitosan-based nanoparticles and oligosaccharides in cyclophosphamide-treated mice. Scand. J. Immunol. 2019, 89, e12749. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Aljuffali, I.A.; Lin, C.-F.; Chang, Y.-T.; Fang, J.-Y. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: Lipid nanoparticles versus polymeric nanoparticles. Int. J. Nanomed. 2015, 10, 371–385. [Google Scholar]
- Chua, F. Neutrophil Elastase: Mediator of Extracellular Matrix Destruction and Accumulation. Proc. Am. Thorac. Soc. 2006, 3, 424–427. Available online: http://pats.atsjournals.org/cgi/doi/10.1513/pats.200603-078AW (accessed on 7 July 2019). [CrossRef]
- Sandri, S.; Hebeda, C.B.; Loiola, R.A.; Calgaroto, S.; Paese, K. Direct effects of poly(ε-caprolactone) lipid-core nanocapsules on human immune cells. Nanomedicine 2019, 14, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Adjei, I.M.; Plumton, G.; Sharma, B. Oxidative Stress and Biomaterials: The Inflammatory Link. In Oxidative Stress and Biomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 89–115. [Google Scholar] [CrossRef]
- Pleskova, S.; Kriukov, R.; Gorshkova, E.; Boryakov, A. Characteristics of quantum dots phagocytosis by neutrophil granulocytes. Heliyon 2019, 5, e01439. [Google Scholar] [CrossRef] [PubMed]
- Skivka, L.M.; Prylutska, S.V.; Rudyk, M.P.; Khranovska, N.M.; Opeida, I.V.; Hurmach, V.V.; Prylutskyy, Y.I.; Sukhodub, L.F.; Ritter, U. C60 fullerene and its nanocomplexes with anticancer drugs modulate circulating phagocyte functions and dramatically increase ROS generation in transformed monocytes. Cancer Nanotechnol. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Pujari-Palmer, S.; Chen, S.; Rubino, S.; Weng, H.; Xia, W.; Engqvist, H.; Tang, L.; Ott, M.K. In vivo and in vitro evaluation of hydroxyapatite nanoparticle morphology on the acute inflammatory response. Biomaterials 2016, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xie, F.; Fan, X.; Li, B.; Mei, L.; Zou, H.; Li, W.; Yin, Y.; Liu, Y.; Zhang, H.; et al. Cationic liposomes induce cell necrosis through lysosomal dysfunction and late-stage autophagic flux inhibition. Nanomedicine 2016, 11, 3117–3137. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.B.; Northeved, H.; Ek, P.K.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Boil. Med. 2015, 11, 467–477. [Google Scholar] [CrossRef]
- Bae, C.-S.; Ahn, T. Diacylglycerol in Cationic Nanoparticles Stimulates Oxidative Stress-Mediated Death of Cancer Cells. Lipids 2018, 53, 1059–1067. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Aljuffali, I.A.; Hung, C.-F.; Chen, C.-H.; Fang, J.-Y. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs). Chem. Interact. 2015, 235, 106–114. [Google Scholar] [CrossRef]
- Lotosh, N.Y.; Aliaseva, S.O.; Malashenkova, I.K.; Sorokoumova, G.M.; Vasilov, R.G.; Selischeva, A.A. Cationic Liposomes Cause ROS Generation and Release of Neutrophil Extracellular Traps. Biochem. (Mosc.) Suppl. Ser. A: Membr. Cell Boil. 2019, 13, 40–49. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26348965%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4978509 (accessed on 14 July 2019). [CrossRef]
- Yong, S.B.; Song, Y.; Kim, H.J.; Ain, Q.U.; Kim, Y.H. Mononuclear phagocytes as a target, not a barrier, for drug delivery. J. Control. Release. 2017, 259, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Spitler, R.; Javdani, N.; Ho, J.Q. How do Nanoparticles (NPs) Pass Barriers? In Drug Delivery Systems; World Scientific: Singapore, 2017; pp. 89–111. [Google Scholar]
- Li, W.T.; Chang, H.W.; Yang, W.C.; Lo, C.; Wang, L.Y.; Pang, V.F.; Chen, M.; Jeng, C. Immunotoxicity of Silver Nanoparticles (AgNPs) on the Leukocytes of Common Bottlenose Dolphins (Tursiops truncatus). Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Babin, K.; Goncalves, D.M.; Girard, D. Nanoparticles enhance the ability of human neutrophils to exert phagocytosis by a Syk-dependent mechanism. Biochim. Biophys. Acta – Gen. Subj. 2015, 1850, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Johnson, D.N. Role for nanomaterial-autophagy interaction in neurodegenerative disease. Autophagy 2008, 4, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Colognato, R.; Bonelli, A.; Ponti, J.; Farina, M.; Bergamaschi, E.; Sabbioni, E.; Migliore, L. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis 2008, 23, 377–382. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, F.; Yang, H.; Li, Y. Effects of cobalt nanoparticles on human T cells in vitro. Biol. Trace Elem. Res. 2012, 146, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Crist, R. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2014, 9, 20–35. [Google Scholar]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 1–17. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Zink, J.I.; Nel, A.E. Cationic Polystyrene Nanosphere. ACS Nano 2008, 2, 85–96. [Google Scholar] [CrossRef]
- Thomas, T.P.; Majoros, I.; Kotlyar, A.; Mullen, D.; Banaszak Holl, M.M.; Baker, J.R. Cationic poly(amidoamine) dendrimer induces lysosomal apoptotic pathway at therapeutically relevant concentrations. Biomacromolecules 2009, 10, 3207–3214. [Google Scholar] [CrossRef]
- Futerman, A.H.; Van Meer, G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 2004, 5, 554–565. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation Mechanisms and Signaling Pathways of Autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, L.; Feng, C.; Wen, L.P. Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem. Biophys. Res. Commun. 2005, 337, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Lyles, D.N.; Peifley, K.; Lockett, S.; Neun, B.W.; Hansen, M.; Clogston, J.; Stern, S.T.; McNeil, S.E. Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010, 248, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wu, D.; Shen, X.; Liu, P.X.; Yang, N.; Zhao, B.; Zhang, H.; Sun, Y.; Zhang, L.; Fan, F. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int. J. Nanomedicine 2011, 6, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Noël, C.; Simard, J.C.; Girard, D. Gold nanoparticles induce apoptosis, endoplasmic reticulum stress events and cleavage of cytoskeletal proteins in human neutrophils. Toxicol. Vitr. 2016, 31, 12–22. [Google Scholar] [CrossRef]
- Best, J.P.; Yan, Y.; Caruso, F. The role of particle geometry and mechanics in the biological domain. Adv. Healthc. Mater. 2012, 1, 35–47. [Google Scholar] [CrossRef]
- Decuzzi, P.; Ferrari, M. The receptor-mediated endocytosis of nonspherical particles. Biophys. J. 2008, 94, 3790–3797. [Google Scholar] [CrossRef][Green Version]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Khaliullin, T.O.; Shurin, M.R.; Kisin, E.R.; Yanamala, N.; Fadeel, B.; Chang, J.; Shvedova, A.A. Fibrous nanocellulose, crystalline nanocellulose, carbon nanotubes, and crocidolite asbestos elicit disparate immune responses upon pharyngeal aspiration in mice. J. Immunotoxicol. 2018, 15, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.A.; Hanarp, P.; Sutherland, D.S.; Gold, J.; Mustin, C.; Rouxhet, P.G.; Dufrene, Y.F. Protein adsorption on model surfaces with controlled nanotopography and chemistry. Langmuir 2002, 18, 819–828. [Google Scholar] [CrossRef]
- Ferraz, N.; Hong, J.; Karlsson Ott, M. Procoagulant behavior and platelet microparticle generation on nanoporous alumina. J. Biomater. Appl. 2010, 24, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Greish, K.; McGill, L.D.; Ray, A.; Ghandehari, H. Influence of geometry, porosity, and surface characteristics of silica nanoparticles on acute toxicity: Their vasculature effect and tolerance threshold. ACS Nano 2012, 6, 2289–2301. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Ban, D.K.; Paul, S. Protein corona over silver nanoparticles triggers conformational change of proteins and drop in bactericidal potential of nanoparticles: Polyethylene glycol capping as preventive strategy. Colloids Surfaces B Biointerfaces. 2016, 146, 577–584. [Google Scholar] [CrossRef]
- Hakim, T.S.; Macek, A.S. Role of erythrocyte deformability in the acute hypoxic pressor response in the pulmonary vasculature. Respir Physiol. 1988, 72, 95–107. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kagan, V.E.; Fadeel, B. Close Encounters of the Small Kind: Adverse Effects of Man-Made Materials Interfacing with the Nano-Cosmos of Biological Systems. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.F.; Intaglietta, M. Annual review of biomedical engineering. Blood Substit. 2014, 16, 77–101. [Google Scholar]
- Nel, A.E. Implementation of alternative test strategies for the safety assessment of engineered nanomaterials. J. Intern. Med. 2013, 274, 561–577. [Google Scholar] [CrossRef] [PubMed]

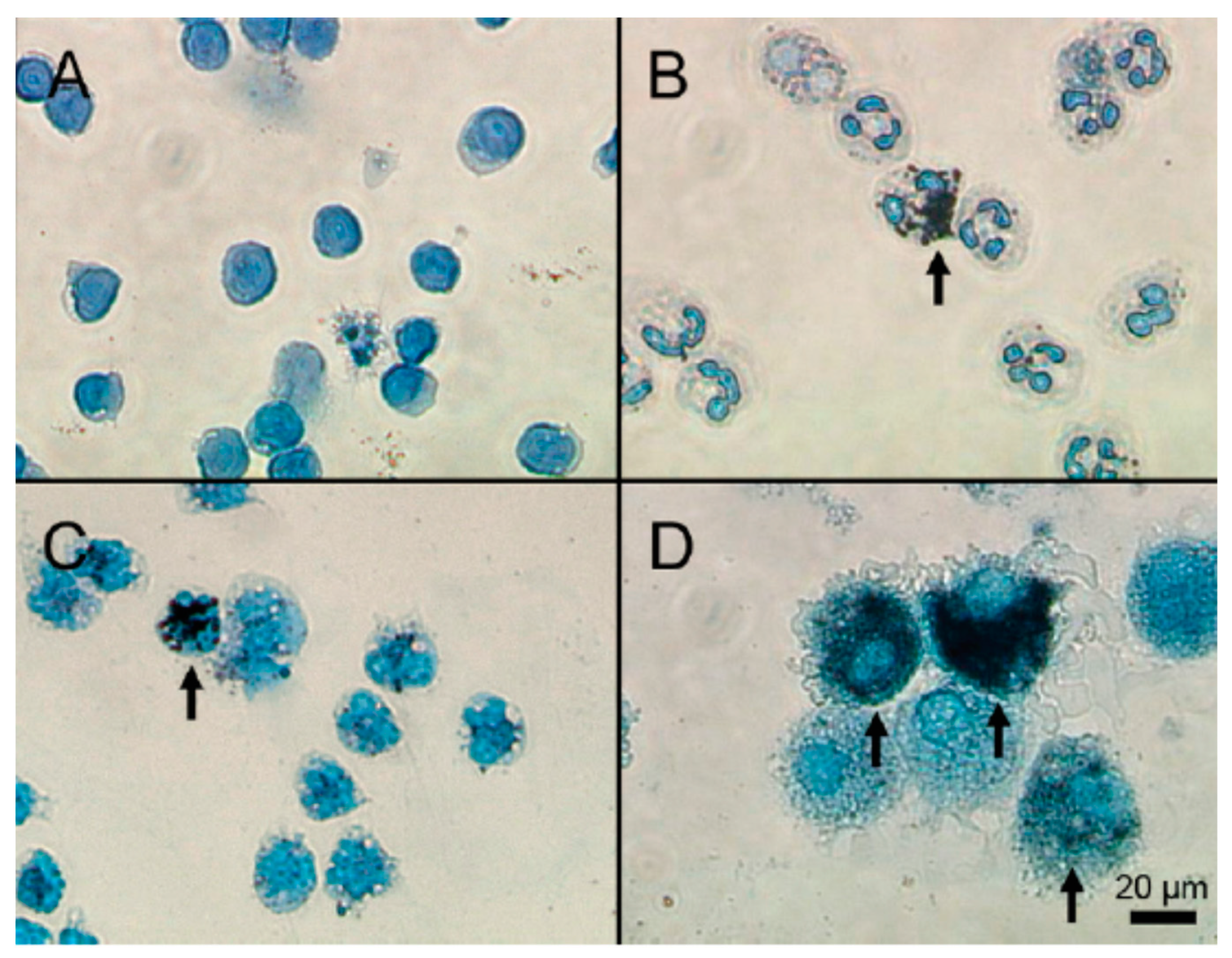

| Granules | Content Class | Factors Released |
|---|---|---|
| Alpha granules | Adhesive glycoproteins | VWF, thrombospondin, P-selectin, fibrinogen, fibronectin, vitronectin |
| Coagulation factors | Plasminogen, kininogens, protein S, factor V, factor XI, factor XIII | |
| Growth factors | IGF, EGF, PDGF, TGF-β | |
| Angiogenic factors | PF4 inhibitor, VEGF | |
| Protease inhibitors | C1-inhibitor, PAI-1, TFPI, α2-antiplasmin, α2-antitripsin, α2-macroglobulin | |
| Immunoglobulins–chemokines | IL8, IL1β, CD40, CXCL4 (platelet basic protein/NAP-2), CXCL (PF4), CXCL1, CXCL5, CCL5 (RANTES), CCL (MIP-1α) | |
| Proteases | MMP2, MMP9 | |
| Dense granules | Amines | Serotonin, histamine |
| Bivalent cations | Ca2+, Mg2+ | |
| Polyphosphates | ADP, ATP, GDP, GTP | |
| Lysosome granules | Enzymes | Acid proteases, glycohydrolases |
| Other soluble mediators | NO, thromboxane A2, defensins, PAF | |
| Leukocyte | Sub-Type | Function | Reference |
|---|---|---|---|
| 1. Granulocytes/polymorphic nuclear cells (PMNs) | Neutrophils | Scavenger of invading pathogens | [69] |
| Basophils | Release of inflammatory and anticoagulant mediators | ||
| Eosinophils | Production of angiogenic and profibrogenic factors | ||
| 2. Monocytes/mononuclear cells | Monocytes/macrophage | Phagocytosis | [70] |
| 3. Lymphocytes | T cells | Orchestration of adaptive immunity | [71] |
| B cells | Humoral immunity through antibody secretion | ||
| Natural killer cells | Removal of tumor and virus-infected cells |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. https://doi.org/10.3390/cells8101209
de la Harpe KM, Kondiah PPD, Choonara YE, Marimuthu T, du Toit LC, Pillay V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells. 2019; 8(10):1209. https://doi.org/10.3390/cells8101209
Chicago/Turabian Stylede la Harpe, Kara M., Pierre P.D. Kondiah, Yahya E. Choonara, Thashree Marimuthu, Lisa C. du Toit, and Viness Pillay. 2019. "The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis" Cells 8, no. 10: 1209. https://doi.org/10.3390/cells8101209
APA Stylede la Harpe, K. M., Kondiah, P. P. D., Choonara, Y. E., Marimuthu, T., du Toit, L. C., & Pillay, V. (2019). The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells, 8(10), 1209. https://doi.org/10.3390/cells8101209