Integrin Activation: Implications for Axon Regeneration
Abstract
:1. Introduction
2. The Challenging Task of Axon Regeneration
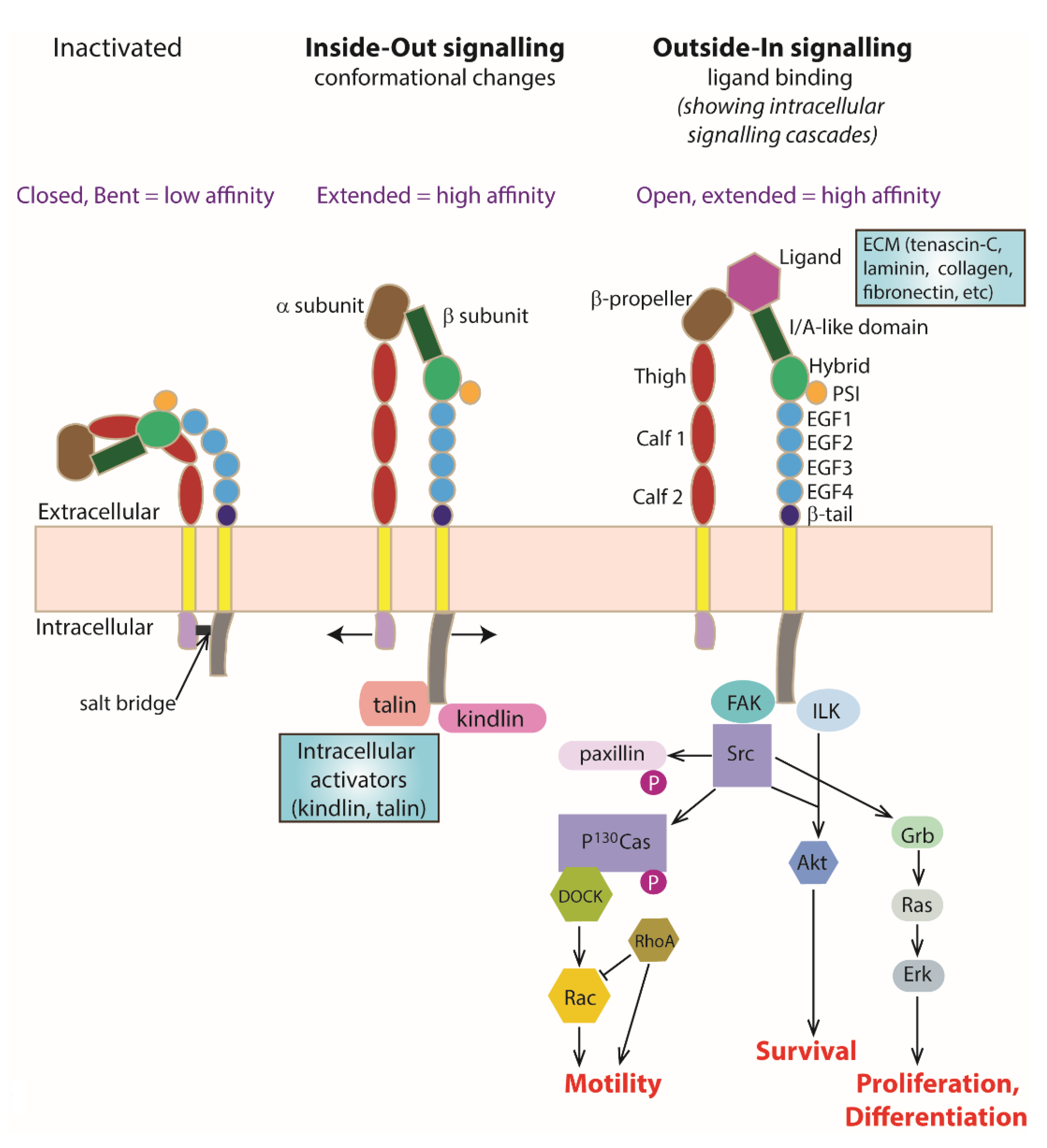
3. Integrin Heterodimers as a Transmembrane Signalling Molecule
4. ‘Inside–Out’ Signalling
4.1. Kindlin
4.2. Talin
4.3. Intracellular Interactions with Kindlin and Talin
- (1)
- The sequential binding model: Kindlin binds to the membrane-distal NxxY motif to induce a slight change in the conformation of the β subunit cytoplasmic tail and this facilitates the binding of talin to the membrane-proximal NPxY motif.
- (2)
- The Cis co-operation model: Simultaneous binding of kindlin and talin to the same β subunit integrin cytoplasmic tail via their respective binding sites.
- (3)
- The Trans co-operation model: Kindlin and talin each bind to different β subunit cytoplasmic tails and then interact with each other to form integrin clustering at focal adhesions.
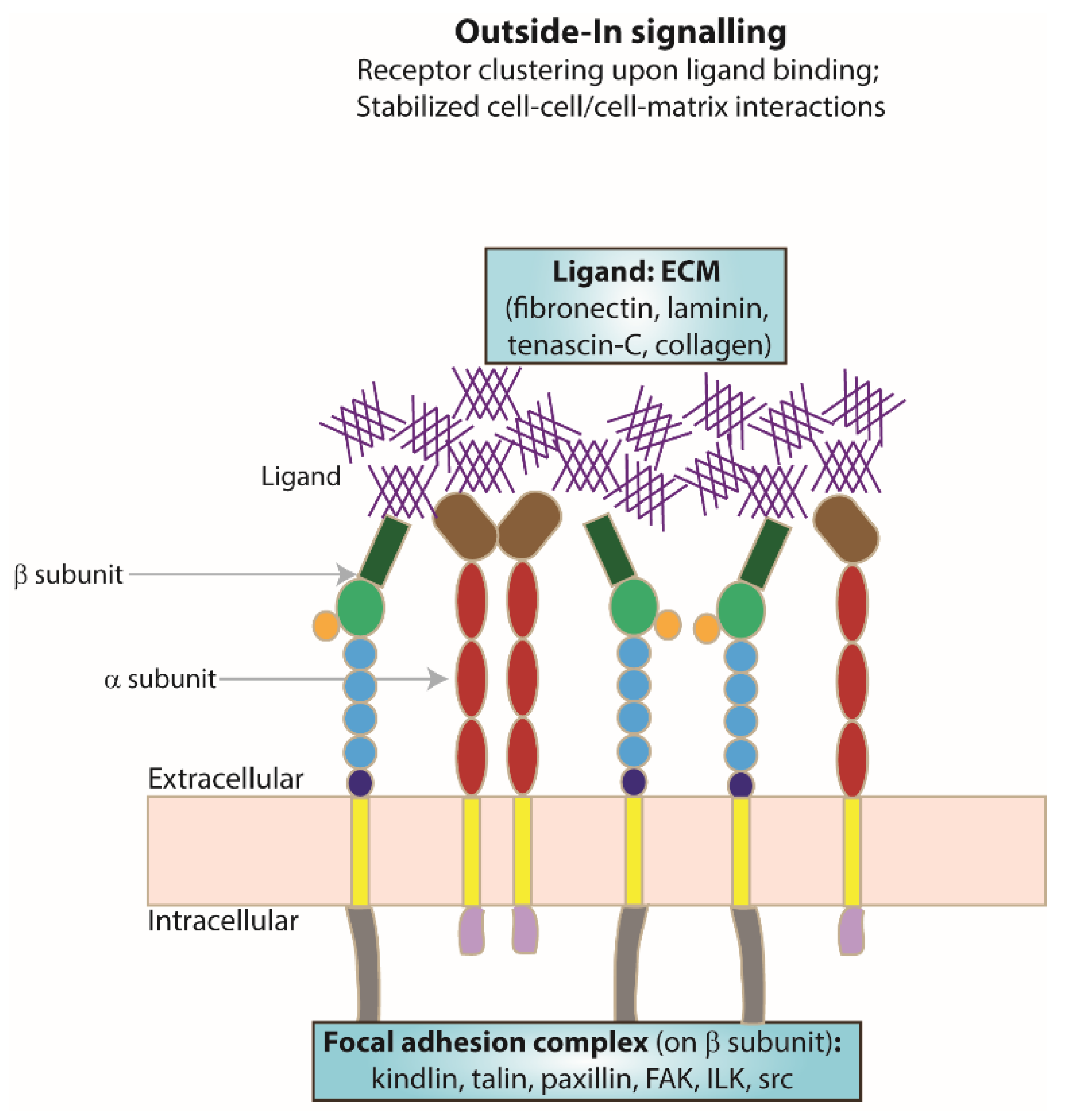
5. ‘Outside–In’ Signalling
Extracellular Ligand Binding
6. Other Factors That Can (Artificially) Modulate Integrin Function
6.1. Divalent Cations
6.2. Integrin-Activating Antibodies
7. Our Findings on Integrin Activation and Axon Regeneration
8. The Outlook for Nervous System Repair
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anton, E.S.; Kreidberg, J.A.; Rakic, P. Distinct functions of α3 and α(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron 1999, 22, 277–289. [Google Scholar] [CrossRef]
- Nikonenko, I.; Toni, N.; Moosmayer, M.; Shigeri, Y.; Muller, D.; Sargent Jones, L. Integrins are involved in synaptogenesis, cell spreading, and adhesion in the postnatal brain. Brain Res. Dev. Brain Res. 2003, 140, 185–194. [Google Scholar] [CrossRef]
- Milner, R.; Huang, X.; Wu, J.; Nishimura, S.; Pytela, R.; Sheppard, D.; ffrench-Constant, C. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J. Cell Sci. 1999, 112 Pt 23, 4271–4279. [Google Scholar] [PubMed]
- Feltri, M.L.; Graus Porta, D.; Previtali, S.C.; Nodari, A.; Migliavacca, B.; Cassetti, A.; Littlewood-Evans, A.; Reichardt, L.F.; Messing, A.; Quattrini, A.; et al. Conditional disruption of β 1 integrin in schwann cells impedes interactions with axons. J. Cell Biol. 2002, 156, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; de Repentigny, Y.; Saulnier, R.; Rippstein, P.; Macklin, W.B.; Kothary, R. Dominant-negative β1 integrin mice have region-specific myelin defects accompanied by alterations in mapk activity. Glia 2006, 53, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.R.; Czvitkovich, S.; Dassie, E.; Vogelaar, C.F.; Faissner, A.; Blits, B.; Gage, F.H.; ffrench-Constant, C.; Fawcett, J.W. α9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Andrews, M.R.; Kwok, J.C.; Heintz, T.G.; Gumy, L.F.; Fassler, R.; Fawcett, J.W. Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 7325–7335. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.; Andrews, M.R.; Chew, D.J.; Moloney, E.B.; Verhaagen, J.; Fassler, R.; Fawcett, J.W. Expression of an activated integrin promotes long-distance sensory axon regeneration in the spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 7283–7297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Q.; Walzer, M.; Wu, Y.H.; Zhou, J.; Dedhar, S.; Snider, W.D. Neurotrophins support regenerative axon assembly over cspgs by an ecm-integrin-independent mechanism. J. Cell Sci. 2006, 119, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Strittmatter, S.M. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Kwok, J.C.; Patani, R.; Ffrench-Constant, C.; Chandran, S.; Fawcett, J.W. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 6289–6295. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.R.; Stelzner, D.J. Plasticity of the corticospinal tract following midthoracic spinal injury in the postnatal rat. J. Comp. Neurol. 1983, 221, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Bregman, B.S. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 1987, 431, 265–279. [Google Scholar] [CrossRef]
- Bates, C.A.; Stelzner, D.J. Extension and regeneration of corticospinal axons after early spinal injury and the maintenance of corticospinal topography. Exp. Neurol. 1993, 123, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Pinkstaff, J.K.; Detterich, J.; Lynch, G.; Gall, C. Integrin subunit gene expression is regionally differentiated in adult brain. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 1541–1556. [Google Scholar]
- Nieuwenhuis, B.; Haenzi, B.; Andrews, M.R.; Verhaagen, J.; Fawcett, J.W. Integrins promote axonal regeneration after injury of the nervous system. Biol. Rev. Camb. Philos. Soc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, A.; Sonnenberg, A. Function and interactions of integrins. Cell Tissue Res. 2001, 305, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Vogelezang, M.G.; Liu, Z.; Relvas, J.B.; Raivich, G.; Scherer, S.S.; ffrench-Constant, C. Α4 integrin is expressed during peripheral nerve regeneration and enhances neurite outgrowth. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 6732–6744. [Google Scholar]
- Wallquist, W.; Zelano, J.; Plantman, S.; Kaufman, S.J.; Cullheim, S.; Hammarberg, H. Dorsal root ganglion neurons up-regulate the expression of laminin-associated integrins after peripheral but not central axotomy. J. Comp. Neurol. 2004, 480, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, P.A.; Mayer, U.; Panjwani, A.; Pountney, D.; Pizzey, J.; Tonge, D.A. Involvement of α7β1 integrin in the conditioning-lesion effect on sensory axon regeneration. Mol. Cell. Neurosci. 2003, 22, 383–395. [Google Scholar] [CrossRef]
- Gardiner, N.J.; Moffatt, S.; Fernyhough, P.; Humphries, M.J.; Streuli, C.H.; Tomlinson, D.R. Preconditioning injury-induced neurite outgrowth of adult rat sensory neurons on fibronectin is mediated by mobilisation of axonal α5 integrin. Mol. Cell. Neurosci. 2007, 35, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Condic, M.L.; Letourneau, P.C. Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature 1997, 389, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Condic, M.L.; Snow, D.M.; Letourneau, P.C. Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 10036–10043. [Google Scholar]
- Condic, M.L. Adult neuronal regeneration induced by transgenic integrin expression. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 4782–4788. [Google Scholar]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.P.; Stehle, T.; Diefenbach, B.; Zhang, R.; Dunker, R.; Scott, D.L.; Joachimiak, A.; Goodman, S.L.; Arnaout, M.A. Crystal structure of the extracellular segment of integrin αvβ3. Science (NY) 2001, 294, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Bankston, L.A.; Arnaout, M.A.; Liddington, R.C. Two conformations of the integrin a-domain (i-domain): A pathway for activation? Structure (Lond. Engl. 1993) 1995, 3, 1333–1340. [Google Scholar] [CrossRef]
- Smith, C.; Estavillo, D.; Emsley, J.; Bankston, L.A.; Liddington, R.C.; Cruz, M.A. Mapping the collagen-binding site in the i domain of the glycoprotein ia/iia (integrin α(2)β(1)). J. Biol. Chem. 2000, 275, 4205–4209. [Google Scholar] [CrossRef] [PubMed]
- Pesho, M.M.; Bledzka, K.; Michalec, L.; Cierniewski, C.S.; Plow, E.F. The specificity and function of the metal-binding sites in the integrin β3 A-domain. J. Biol. Chem. 2006, 281, 23034–23041. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Askari, J.A.; Humphries, M.J.; Bulleid, N.J. Divalent cations regulate the folding and activation status of integrins during their intracellular trafficking. J. Cell Sci. 2011, 124, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.A.; Brunie, L.; Bacher, A.S.; Kessler, H.; Gottschalk, K.E.; Reuning, U. Cytoplasmic salt bridge formation in integrin αvβ3 stabilizes its inactive state affecting integrin-mediated cell biological effects. Cell. Signall. 2014, 26, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, O.; Haas, T.; Plow, E.F.; Qin, J. A structural basis for integrin activation by the cytoplasmic tail of the α IIb-subunit. Proc. Nat. Acad. Sci. USA 2000, 97, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A.; Yan, B.; de Pereda, J.M.; Alvarez, B.G.; Fujioka, Y.; Liddington, R.C.; Ginsberg, M.H. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 2002, 277, 21749–21758. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Bouaouina, M.; Calderwood, D.A. Kindlin-1 and -2 directly bind the c-terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 2009, 284, 11485–11497. [Google Scholar] [CrossRef] [PubMed]
- Ussar, S.; Wang, H.V.; Linder, S.; Fassler, R.; Moser, M. The kindlins: Subcellular localization and expression during murine development. Exp. Cell Res. 2006, 312, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Larjava, H.; Plow, E.F.; Wu, C. Kindlins: Essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008, 9, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, T.M.; Mullen, G.P.; Gilbert, M.M.; Williams, B.D.; Moerman, D.G. The UNC-112 gene in caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J. Cell Biol. 2000, 150, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Senetar, M.A.; Moncman, C.L.; McCann, R.O. Talin2 is induced during striated muscle differentiation and is targeted to stable adhesion complexes in mature muscle. Cell Motil. Cytoskelet. 2007, 64, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Shattil, S.J.; Eto, K.; Tai, V.; Liddington, R.C.; de Pereda, J.M.; Ginsberg, M.H.; Calderwood, D.A. Talin binding to integrin β tails: A final common step in integrin activation. Science (NY) 2003, 302, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A. Talin controls integrin activation. Biochem. Soc. Trans. 2004, 32, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Critchley, D.R. Focal adhesions—The cytoskeletal connection. Curr. Opin. Cell Biol. 2000, 12, 133–139. [Google Scholar] [CrossRef]
- Bledzka, K.; Liu, J.; Xu, Z.; Perera, H.D.; Yadav, S.P.; Bialkowska, K.; Qin, J.; Ma, Y.Q.; Plow, E.F. Spatial coordination of kindlin-2 with talin head domain in interaction with integrin β cytoplasmic tails. J. Biol. Chem. 2012, 287, 24585–24594. [Google Scholar] [CrossRef] [PubMed]
- Margadant, C.; Kreft, M.; de Groot, D.J.; Norman, J.C.; Sonnenberg, A. Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking. Curr. Biol. CB 2012, 22, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Moes, M.; Rodius, S.; Coleman, S.J.; Monkley, S.J.; Goormaghtigh, E.; Tremuth, L.; Kox, C.; van der Holst, P.P.; Critchley, D.R.; Kieffer, N. The integrin binding site 2 (IBS2) in the talin rod domain is essential for linking integrin β subunits to the cytoskeleton. J. Biol. Chem. 2007, 282, 17280–17288. [Google Scholar] [CrossRef] [PubMed]
- Rodius, S.; Chaloin, O.; Moes, M.; Schaffner-Reckinger, E.; Landrieu, I.; Lippens, G.; Lin, M.; Zhang, J.; Kieffer, N. The talin rod IBS2 α-helix interacts with the β3 integrin cytoplasmic tail membrane-proximal helix by establishing charge complementary salt bridges. J. Biol. Chem. 2008, 283, 24212–24223. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Kwok, J.C.; Heller, J.P.; Zhao, R.; Eva, R.; Fawcett, J.W. Full length talin stimulates integrin activation and axon regeneration. Mol. Cell. Neurosci. 2015, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Petrich, B.G. Kindlin: Helper, co-activator, or booster of talin in integrin activation? Curr. Opin. Hematol. 2011, 18, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Legate, K.R.; Zent, R.; Fassler, R. The tail of integrins, talin, and kindlins. Science (NY) 2009, 324, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D.; Otey, C.A.; Hildebrand, J.D.; Parsons, J.T. Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J. Cell Biol. 1995, 130, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Knight, J.D.; Piszczek, G.; Kothary, R.; Qin, J. Biochemical, proteomic, structural, and thermodynamic characterizations of integrin-linked kinase (ilk): Cross-validation of the pseudokinase. J. Biol. Chem. 2011, 286, 21886–21895. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Ale, A.; Santos, D.; Barwig, C.; Freier, T.; Navarro, X.; Udina, E. Substratum preferences of motor and sensory neurons in postnatal and adult rats. Eur. J. Neurosci. 2016, 43, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Springer, T.A. Metal ion and ligand binding of integrin α5β1. Proc. Nat. Acad. Sci. USA 2014, 111, 17863–17868. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science (NY) 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Gailit, J.; Ruoslahti, E. Regulation of the fibronectin receptor affinity by divalent cations. J. Biol. Chem. 1988, 263, 12927–12932. [Google Scholar] [PubMed]
- Lemons, M.L.; Condic, M.L. Combined integrin activation and intracellular cAMP cause Rho GTPase dependent growth cone collapse on laminin-1. Exp. Neurol. 2006, 202, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Byron, A.; Humphries, J.D.; Askari, J.A.; Craig, S.E.; Mould, A.P.; Humphries, M.J. Anti-integrin monoclonal antibodies. J. Cell Sci. 2009, 122, 4009–4011. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.A. The role of natalizumab in the treatment of multiple sclerosis: Benefits and risks. Ther. Adv. Neurol. Disord. 2017, 10, 327–336. [Google Scholar] [CrossRef] [PubMed]
- McLean, L.P.; Cross, R.K. Integrin antagonists as potential therapeutic options for the treatment of crohn’s disease. Expert Opin. Investig. Drugs 2016, 25, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.J.; Cohen, J.A.; Fox, E.J.; Giovannoni, G.; Hartung, H.P.; Havrdova, E.; Schippling, S.; Selmaj, K.W.; Traboulsee, A.; Compston, D.A.S.; et al. Alemtuzumab care-ms II 5-year follow-up: Efficacy and safety findings. Neurology 2017, 89, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Zorner, B.; Schwab, M.E. Anti-nogo on the go: From animal models to a clinical trial. Ann. N. Y. Acad. Sci. 2010, 1198 (Suppl. 1), E22–E34. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.R.; Soleman, S.; Cheah, M.; Tumbarello, D.A.; Mason, M.R.; Moloney, E.; Verhaagen, J.; Bensadoun, J.C.; Schneider, B.; Aebischer, P.; et al. Axonal localization of integrins in the cns is neuronal type and age dependent. eNeuro 2016, 3, 0029-16. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.H.; Andrews, M.R. Restoring axonal localization and transport of transmembrane receptors to promote repair within the injured cns: A critical step in cns regeneration. Neural Regen. Res. 2017, 12, 27–30. [Google Scholar] [PubMed]
| Publication | Andrews et al., 2009 | Tan et al., 2012 | Cheah et al., 2016 |
| Molecule | α9 integrin | Kindlin-1 | α9 integrin + kindlin-1 |
| In vitro model | Adult rat dissociated dorsal root ganglia (DRG) neurons plated on laminin (control) or tenascin-C (TN-C). | Adult rat dissociated DRG neurons plated on laminin (control) or aggrecan. | Adult rat dissociated DRG neurons plated on laminin (control), aggrecan, TN-C, or aggrecan + TN-C. |
| In vitro results | Neurite outgrowth when grown on TN-C rescued by expression of α9 integrin to levels similar to growth on laminin. Growth was significantly higher than wildtype neurons grown on TN-C. | Neurite outgrowth when grown on aggrecan rescued by expression of kindlin-1 to levels similar to growth on laminin. Growth was significantly higher than wildtype neurons grown on aggrecan. | Neurite outgrowth of DRG neurons when grown on aggrecan + TN-C rescued by combined expression of α9 integrin and kindlin-1 to levels similar to growth on laminin. Growth was significantly higher than neurons expressing α9 integrin or kindlin-1 alone grown on aggrecan + TN-C. |
| In vivo model | Unilateral cervical dorsal root crush injury (C5–C8) *, examined 6 weeks post-injury. | Unilateral cervical dorsal root crush injury (C5–C8), examined 6 weeks post-injury. | Unilateral cervical dorsal root crush injury (C5–C8); examined 12 weeks post-injury **. |
| Virus transduction | AAV2-α9 integrin injected into C6, C7 DRGs. AAV2-fGFP as control. | AAV2-kindlin1-mCherry injected into C6, C7 DRGs. AAV2-mCherry and AAV2-fGFP as controls. | AAV5-kindlin1-GFP and AAV-α9integrin-V5 injected into C6, C7 DRGs. AAV5-fGFP as control. |
| Anatomical results | 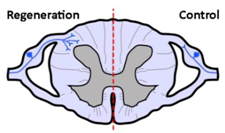 | 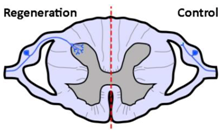 | 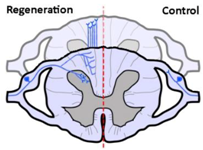 |
| α9 integrin-expressing axons grew into the TN-C-rich DREZ. Control axons did not grow into the CNS at all. | Kindlin1-expressing axons grew beyond the DREZ and into the dorsal horn. Control axons did not grow into the CNS at all. | Axons co-expressing α9 integrin and kindlin-1 grew beyond the TN-C-and-CSPG-rich DREZ and into the dorsal horn topographically, and also within the spinal cord (cuneate fasciculus) to the medulla for a distance of up to 25 mm. Control axons did not grow into the CNS at all. | |
| Behavioural tests | Behavioural recovery to pre-operative levels in α9 integrin group in thermal pain sensory test. | Behavioural recovery to pre-operative levels in kindlin-1 group occurred in both the mechanical pressure and thermal pain sensory tests. | Significant behavioural recovery to near pre-operative levels in combined treatment group in mechanical pressure and thermal pain sensory tests, and ladder-walking (limb proprioception) test. |
| Electro-physiology | N/A | N/A | Significant functional reconnection shown between injured dorsal roots and associated dorsal horn in combined treatment group. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheah, M.; Andrews, M.R. Integrin Activation: Implications for Axon Regeneration. Cells 2018, 7, 20. https://doi.org/10.3390/cells7030020
Cheah M, Andrews MR. Integrin Activation: Implications for Axon Regeneration. Cells. 2018; 7(3):20. https://doi.org/10.3390/cells7030020
Chicago/Turabian StyleCheah, Menghon, and Melissa R. Andrews. 2018. "Integrin Activation: Implications for Axon Regeneration" Cells 7, no. 3: 20. https://doi.org/10.3390/cells7030020
APA StyleCheah, M., & Andrews, M. R. (2018). Integrin Activation: Implications for Axon Regeneration. Cells, 7(3), 20. https://doi.org/10.3390/cells7030020






