Fine Regulation of Neutrophil Oxidative Status and Apoptosis by Ceruloplasmin and Its Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzyme Isolation
2.2. Ethics Statement
2.3. Human Neutrophil Isolation
2.4. Reactive Oxygen Species (ROS) Analysis
2.4.1. Assessment of Extracellular Superoxide Radicals by Cytochrome C Reduction
2.4.2. Assessment of Intracellular ·O2− Formation Using Dihydroethidium
2.4.3. Assessment of Intracellular ROS Formation Using 2′,7′-Dichlorofluorescein-Diacetate (DCFH-DA)
2.5. Apoptosis Detection by Flow Cytometry
2.5.1. Double Alexa Fluor-Conjugated Annexin-V and Propidium Iodide Labeling
2.5.2. Assessment of DNA Fragmentation by PI Staining of Hypo-Diploid Nuclei
2.5.3. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL)
2.6. Statistics
3. Results
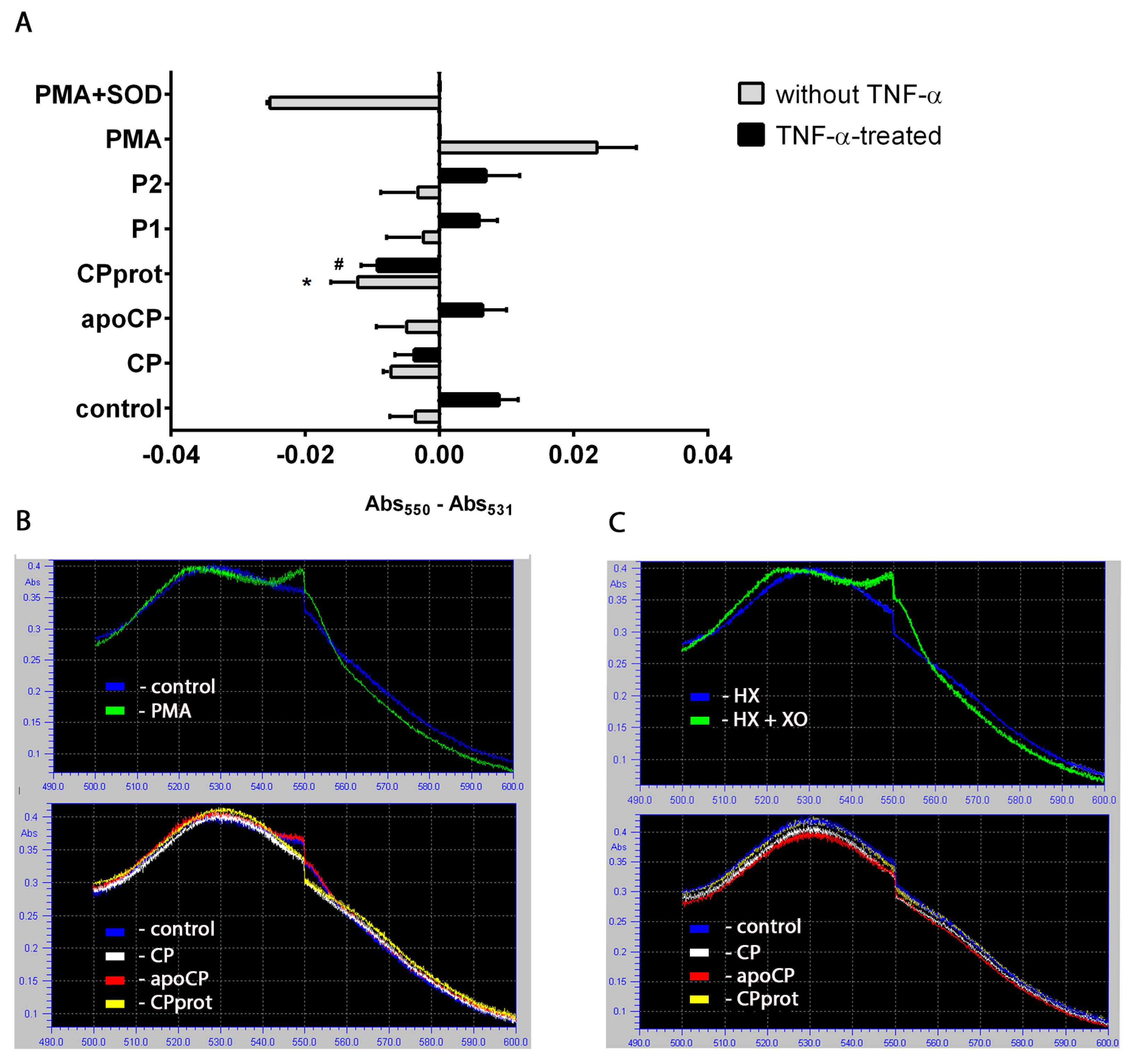
3.1. Intact and Partially-Proteolyzed Ceruloplasmin Have a Dual Effect on the Oxidant Status of Resting and TNF-α-Stimulated Neutrophils
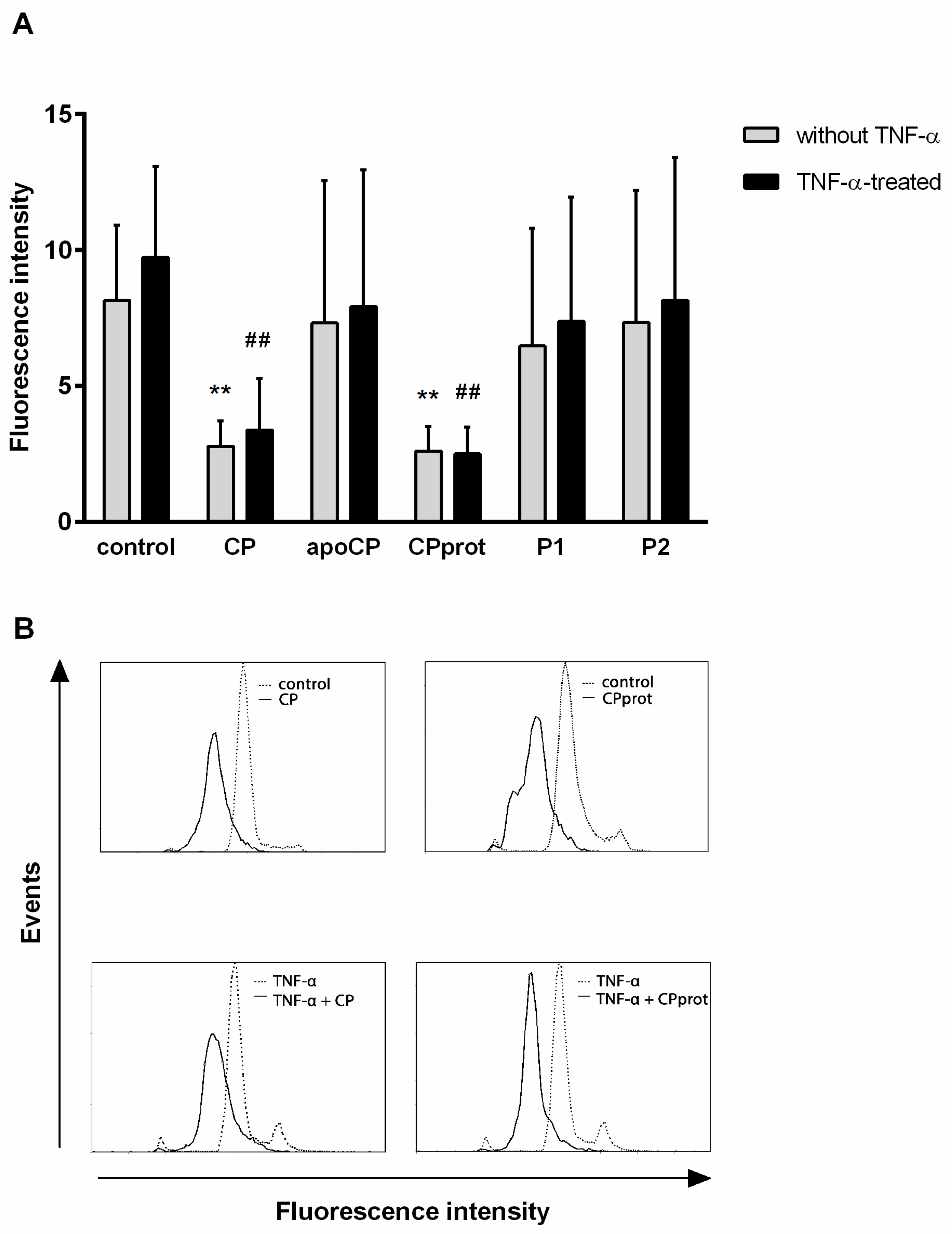
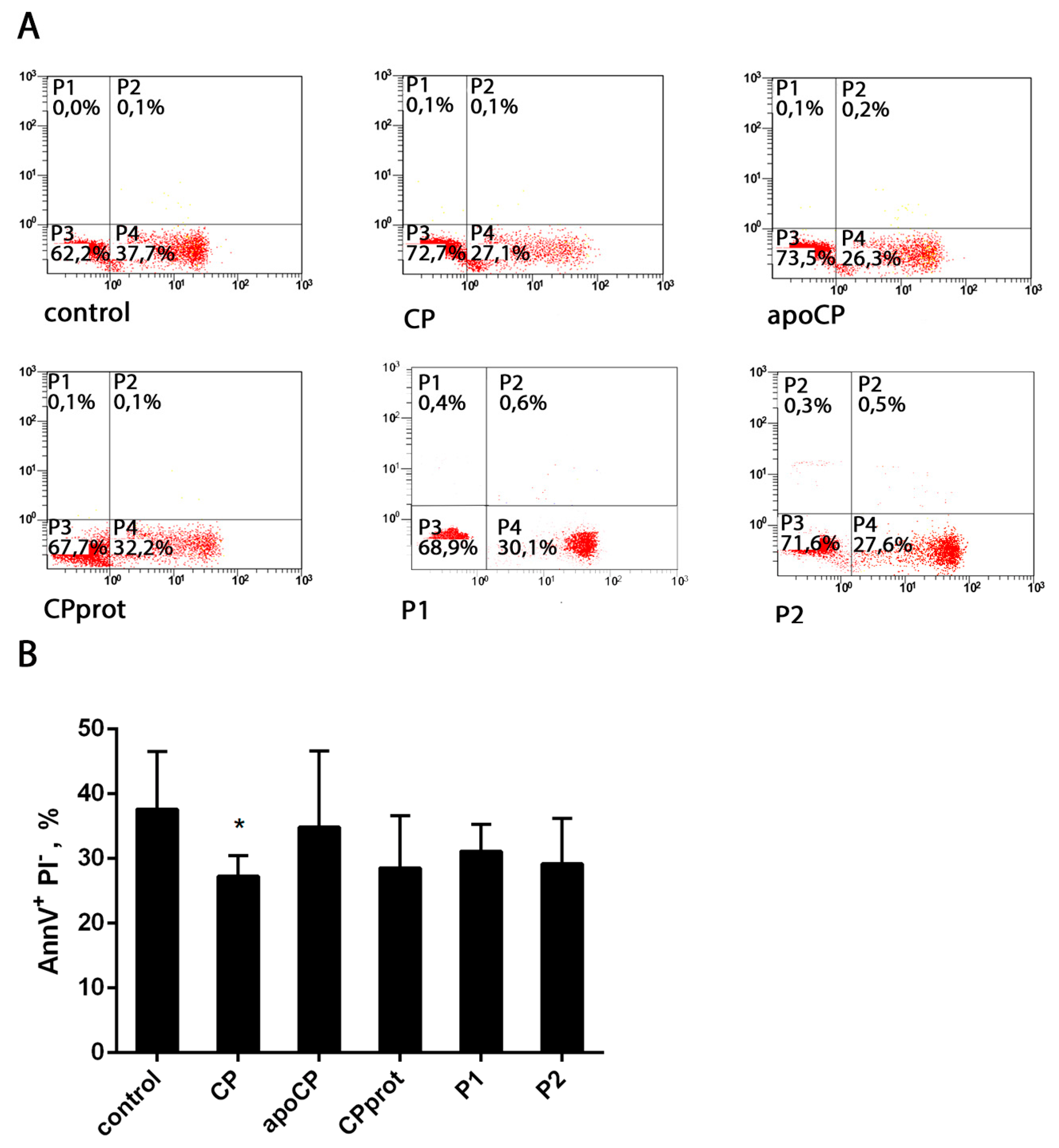
3.2. CP and Its Derivatives Inhibit Spontaneous PMNL Apoptosis
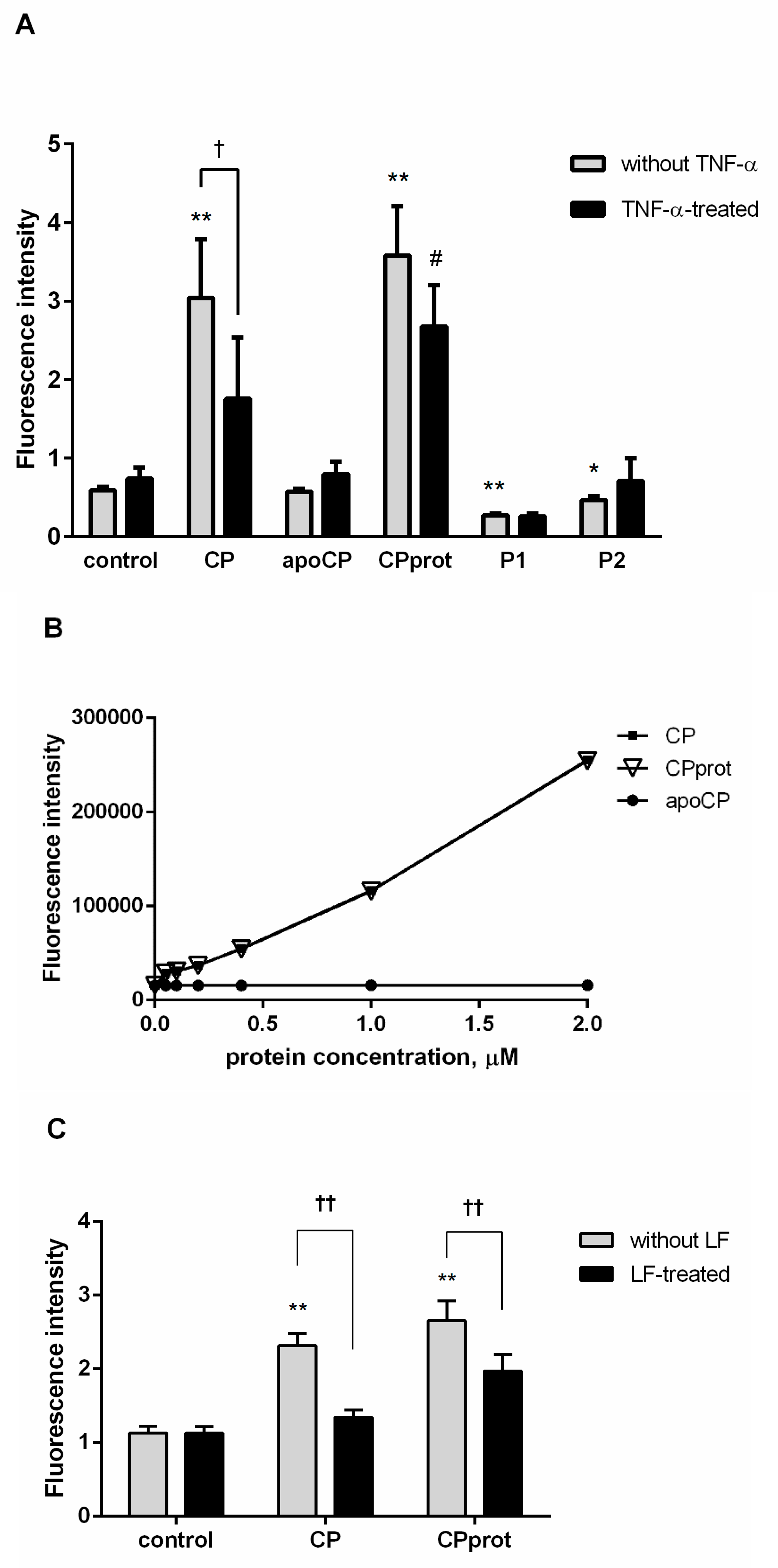
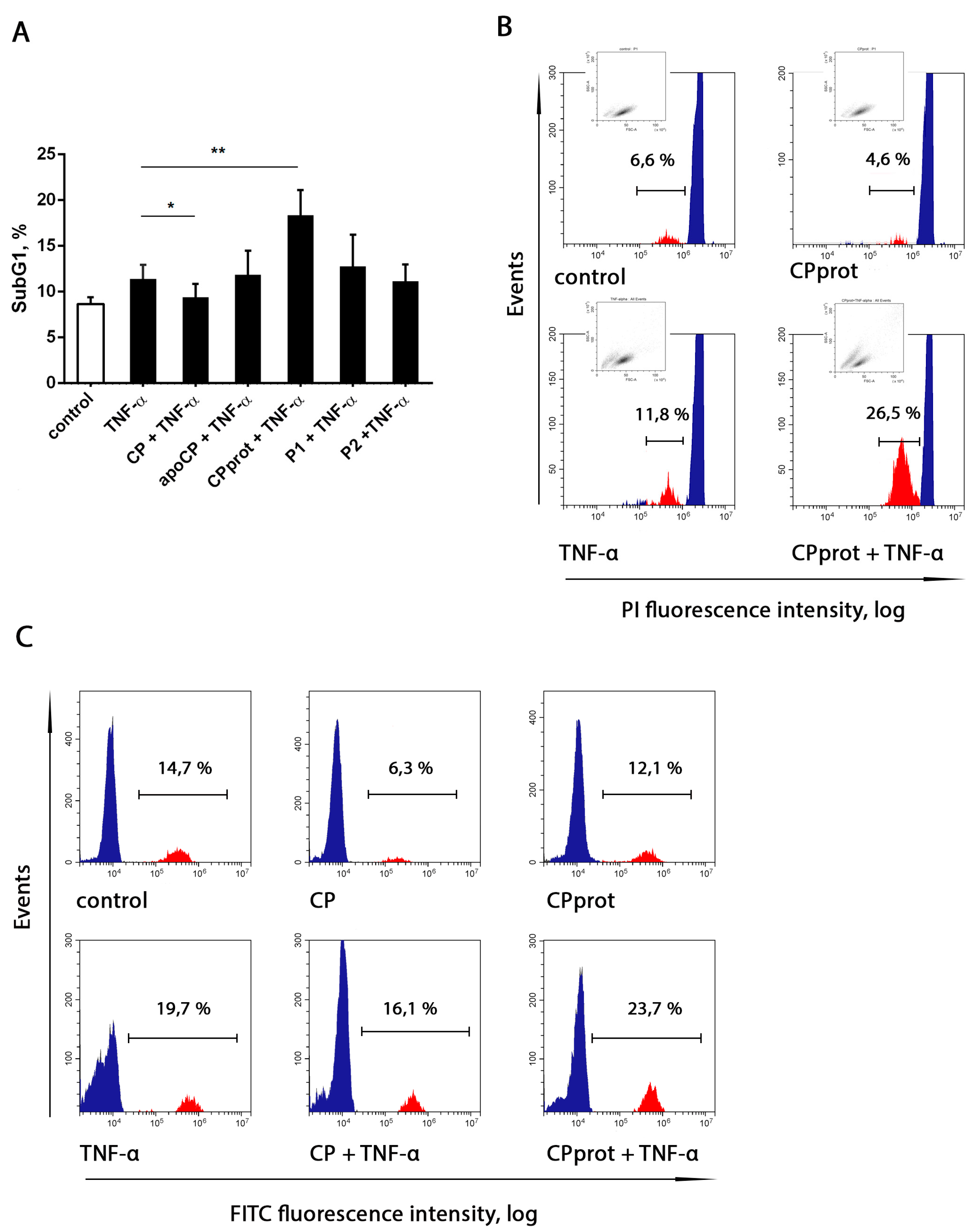
3.3. Proteolyzed Ceruloplasmin Enhances the Pro-Apoptotic Effect of TNF-α, Whereas the Intact Protein Retains Pro-Survival Properties
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holmberg, C.G.; Laurell, C.B. Investigations in Serum Copper. II. Isolation of the Copper Containing Protein, and a Description of some of its Properties. Acta Chem. Scand. 1948, 2, 550–556. [Google Scholar] [CrossRef]
- Linder, M.C. Ceruloplasmin and other copper binding components of blood plasma and their functions: An update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Osaki, S.; Johnson, D.A.; Frieden, E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J. Biol. Chem. 1966, 241, 2746–2751. [Google Scholar] [PubMed]
- Pierre, J.L.; Fontecave, M. Iron and activated oxygen species in biology: The basic chemistry. Biometals 1999, 12, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeeva, E.Y.; Semenova, E.V.; Sokolov, A.V.; Aplin, K.D.; Timofeeva, K.E.; Vasilyev, V.B.; Filatov, M.V. Ceruloplasmin decreases respiratory burst reaction during pregnancy. Free Radic Res. 2016, 50, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Golenkina, E.A.; Kostevich, V.A.; Vasilyev, V.B.; Sud’ina, G.F. Interaction of ceruloplasmin and 5-lipoxygenase. Biochemistry 2010, 75, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Pulina, M.O.; Ageeva, K.V.; Runova, O.L.; Zakharova, E.T.; Vasil’ev, V.B. Identification of leukocyte cationic proteins that interact with ceruloplasmin. Biochemistry 2007, 72, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Pulina, M.O.; Ageeva, K.V.; Ayrapetov, M.I.; Berlov, M.N.; Volgin, G.N.; Markov, A.G.; Yablonsky, P.K.; Kolodkin, N.I.; Zakharova, E.T.; et al. Interaction of ceruloplasmin, lactoferrin, and myeloperoxidase. Biochemistry 2007, 72, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Zakharova, E.T.; Kostevich, V.A.; Samygina, V.R.; Vasilyev, V.B. Lactoferrin, myeloperoxidase, and ceruloplasmin: Complementary gearwheels cranking physiological and pathological processes. Biometals 2014, 27, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.A.; García-Bailo, B.; Borchers, C.H.; Badawi, A.; El-Sohemy, A. Association between the plasma proteome and serum ascorbic acid concentrations in humans. J. Nutr. Biochem. 2013, 24, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Samygina, V.R.; Sokolov, A.V.; Bourenkov, G.; Petoukhov, M.V.; Pulina, M.O.; Zakharova, E.T.; Vasilyev, V.B.; Bartunik, H.; Svergun, D.I. Ceruloplasmin: Macromolecular assemblies with iron-containing acute phase proteins. PLoS ONE 2013, 8, e67145. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Acquasaliente, L.; Kostevich, V.A.; Frasson, R.; Zakharova, E.T.; Pontarollo, G.; Vasilyev, V.B.; De Filippis, V. Thrombin inhibits the anti-myeloperoxidase and ferroxidase functions of ceruloplasmin: Relevance in rheumatoid arthritis. Free Radic. Biol. Med. 2015, 86, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Kostevich, V.A.; Romanico, D.N.; Zakharova, E.T.; Vasilyev, V.B. Two-stage method for purification of ceruloplasmin based on its interaction with neomycin. Biochemistry 2012, 77, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Fling, S.P.; Gregerson, D.S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986, 155, 83–88. [Google Scholar] [CrossRef]
- Sud’ina, G.F.; Brock, T.G.; Pushkareva, M.A.; Galkina, S.I.; Turutin, D.V.; Peters-Golden, M.; Ullrich, V. Sulphatides trigger polymorphonuclear granulocyte spreading on collagen-coated surfaces and inhibit subsequent activation of 5-lipoxygenase. Biochem. J. 2001, 359, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hoffstein, S.T.; Gennaro, D.E.; Manzi, R.M. Neutrophils may directly synthesize both H2O2 and O2− since surface stimuli induce their release in stimulus-specific ratios. Inflammation 1985, 9, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, E.K.; Fridovich, I. The accumulation of superoxide radical during the aerobic action of xanthine oxidase. A requiem for H2O4. Biochim. Biophys. Acta 1976, 430, 182–188. [Google Scholar] [CrossRef]
- Galkina, S.I.; Dormeneva, E.V.; Bachschmid, M.; Pushkareva, M.A.; Sud’ina, G.F.; Ullrich, V. Endothelium-leukocyte interactions under the influence of the superoxide-nitrogen monoxide system. Med. Sci. Monit. 2004, 10, BR307–BR316. [Google Scholar] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Rest, R.F. Measurement of human neutrophil respiratory burst activity during phagocytosis of bacteria. Methods Enzymol. 1994, 236, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta 2014, 1840, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., 2nd; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rothe, G.; Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J. Leukoc. Biol. 1990, 47, 440–448. [Google Scholar] [PubMed]
- Rao, K.M.; Padmanabhan, J.; Kilby, D.L.; Cohen, H.J.; Currie, M.S.; Weinberg, J.B. Flow cytometric analysis of nitric oxide production in human neutrophils using dichlorofluorescein diacetate in the presence of a calmodulin inhibitor. J. Leukoc. Biol. 1992, 51, 496–500. [Google Scholar] [PubMed]
- Richter, J.; Ng-Sikorski, J.; Olsson, I.; Andersson, T. Tumor necrosis factor-induced degranulation in adherent human neutrophils is dependent on CD11b/CD18-integrin-triggered oscillations of cytosolic free Ca2+. Proc. Natl. Acad. Sci. USA 1990, 87, 9472–9476. [Google Scholar] [CrossRef] [PubMed]
- Scheel-Toellner, D.; Wang, K.; Craddock, R.; Webb, P.R.; McGettrick, H.M.; Assi, L.K.; Parkes, N.; Clough, L.E.; Gulbins, E.; Salmon, M.; et al. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood 2004, 104, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.; Moots, R.J.; Edwards, S.W. The dual effects of TNFalpha on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood 2008, 111, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.M.; Kaplan, H.B.; Edelson, H.S.; Weissmann, G. Ceruloplasmin. A scavenger of superoxide anion radicals. J. Biol. Chem. 1979, 254, 4040–4045. [Google Scholar] [PubMed]
- Mukhopadhyay, C.K.; Fox, P.L. Ceruloplasmin copper induces oxidant damage by a redox process utilizing cell-derived superoxide as reductant. Biochemistry 1998, 37, 14222–14229. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Maher, J.; Masters, J.; Angelini, G.D.; Jeremy, J.Y. Does oxidative stress change ceruloplasmin from a protective to a vasculopathic factor? Atherosclerosis 2006, 187, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kwon, H.Y.; Kwon, O.B.; Eum, W.S.; Kang, J.H. Fragmentation of human ceruloplasmin induced by hydrogen peroxide. Biochimie 2000, 82, 175–180. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L.; Pattison, D.I.; Rees, M.D. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1199–1234. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002, 181–182, 223–227. [Google Scholar] [CrossRef]
- Freitas, M.; Ribeiro, D.; Tomé, S.M.; Silva, A.M.; Fernandes, E. Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur. J. Med. Chem. 2014, 86, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.M.; Grima, M.A.; Rayner, B.S.; Hadfield, K.A.; Davies, M.J.; Hawkins, C.L. Comparative reactivity of the myeloperoxidase-derived oxidants hypochlorous acid and hypothiocyanous acid with human coronary artery endothelial cells. Free Radic. Biol. Med. 2013, 65, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; József, L.; Pan, W.; Filep, J.G. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ. Res. 2008, 103, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Mollnau, H.; Eiserich, J.P.; Freeman, B.A.; Daiber, A.; Gehling, U.M.; Brümmer, J.; Rudolph, V.; Münzel, T.; Heitzer, T.; et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. USA 2005, 102, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Grigorieva, D.V.; Gorudko, I.V.; Sokolov, A.V.; Kostevich, V.A.; Vasilyev, V.B.; Cherenkevich, S.N.; Panasenko, O.M. Myeloperoxidase Stimulates Neutrophil Degranulation. Bull. Exp. Biol. Med. 2016, 161, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Srimal, S.; Farber, C.; Sanchez, E.; Kabbash, L.; Asch, A.; Gailit, J.; Wright, S.D. Cytokine-induced respiratory burst of human neutrophils: Dependence on extracellular matrix proteins and CD11/CD18 integrins. J. Cell Biol. 1989, 109, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Decleva, E.; Dri, P.; Menegazzi, R.; Busetto, S.; Cramer, R. Evidence that TNF-induced respiratory burst of adherent PMN is mediated by integrin alpha(L)beta(2). J. Leukoc. Biol. 2002, 72, 718–726. [Google Scholar] [PubMed]
- Chiewchengchol, D.; Wright, H.L.; Thomas, H.B.; Lam, C.W.; Roberts, K.J.; Hirankarn, N.; Beresford, M.W.; Moots, R.J.; Edwards, S.W. Differential changes in gene expression in human neutrophils following TNF-α stimulation: Up-regulation of anti-apoptotic proteins and down-regulation of proteins involved in death receptor signaling. Immun. Inflamm. Dis. 2015, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Salamone, G.; Trevani, A.; Martínez, D.; Vermeulen, M.; Gamberale, R.; Fernández-Calotti, P.; Raiden, S.; Giordano, M.; Geffner, J. Analysis of the mechanisms involved in the stimulation of neutrophil apoptosis by tumour necrosis factor-alpha. Immunology 2004, 113, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Test, S.T. Effect of tumor necrosis factor on the generation of chlorinated oxidants by adherent human neutrophils. J. Leukoc. Biol. 1991, 50, 131–139. [Google Scholar] [PubMed]
- Chatham, W.W.; Turkiewicz, A.; Blackburn, W.D., Jr. Determinants of neutrophil HOCl generation: Ligand-dependent responses and the role of surface adhesion. J. Leukoc. Biol. 1994, 56, 654–660. [Google Scholar] [PubMed]
- Hanlon, W.A.; Stolk, J.; Davies, P.; Humes, J.L.; Mumford, R.; Bonney, R.J. rTNF alpha facilitates human polymorphonuclear leukocyte adherence to fibrinogen matrices with mobilization of specific and tertiary but not azurophilic granule markers. J. Leukoc. Biol. 1991, 50, 43–48. [Google Scholar] [PubMed]
- Segelmark, M.; Persson, B.; Hellmark, T.; Wieslander, J. Binding and inhibition of myeloperoxidase (MPO): A major function of ceruloplasmin? Clin. Exp. Immunol. 1997, 108, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Kurz, T.; Brunk, U.T.; Nilsson, S.E.; Frennesson, C.I. What does the commonly used DCF test for oxidative stress really show? Biochem. J. 2010, 428, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Ageeva, K.V.; Pulina, M.O.; Cherkalina, O.S.; Samygina, V.R.; Vlasova, I.I.; Panasenko, O.M.; Zakharova, E.T.; Vasilyev, V.B. Ceruloplasmin and myeloperoxidase in complex affect the enzymatic properties of each other. Free Radic. Res. 2008, 42, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Ageeva, K.V.; Kostevich, V.A.; Berlov, M.N.; Runova, O.L.; Zakharova, E.T.; Vasilyev, V.B. Study of interaction of ceruloplasmin with serprocidins. Biochemistry 2010, 75, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Loison, F.; Zhu, H.; Karatepe, K.; Kasorn, A.; Liu, P.; Ye, K.; Zhou, J.; Cao, S.; Gong, H.; Jenne, D.E.; et al. Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J. Clin. Investig. 2014, 124, 4445–4458. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Solovyov, K.V.; Kostevich, V.A.; Chekanov, A.V.; Pulina, M.O.; Zakharova, E.T.; Shavlovski, M.M.; Panasenko, O.M.; Vasilyev, V.B. Protection of ceruloplasmin by lactoferrin against hydroxyl radicals is pH dependent. Biochem. Cell Biol. 2012, 90, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L. Evidence for proteolytic fragments in commercial samples of human ceruloplasmin. FEBS Lett. 1971, 18, 321–325. [Google Scholar] [CrossRef]
- Kingston, I.B.; Kingston, B.L.; Putnam, F.W. Chemical evidence that proteolytic cleavage causes the heterogeneity present in human ceruloplasmin preparations. Proc. Natl. Acad. Sci. USA 1977, 74, 5377–5381. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Park, S.Y.; Kim, K.C.; Yum, J.J. Thiol-linked peroxidase activity of human ceruloplasmin. FEBS Lett. 1998, 431, 473–475. [Google Scholar] [CrossRef]
- Francis, N.; Wong, S.H.; Hampson, P.; Wang, K.; Young, S.P.; Deigner, H.P.; Salmon, M.; Scheel-Toellner, D.; Lord, J.M. Lactoferrin inhibits neutrophil apoptosis via blockade of proximal apoptotic signaling events. Biochim. Biophys. Acta 2011, 1813, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Ageeva, K.V.; Pulina, M.O.; Zakharova, E.T.; Vasilyev, V.B. Effect of lactoferrin on oxidative features of ceruloplasmin. Biometals 2009, 22, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, O.M.; Gorudko, I.V.; Sokolov, A.V. Hypochlorous acid as a precursor of free radicals in living systems. Biochemistry (Mosc.) 2013, 78, 1466–1489. [Google Scholar] [CrossRef] [PubMed]
- Golenkina, E.A.; Livenskyi, A.D.; Viryasova, G.M.; Romanova, Y.M.; Sud'ina, G.F.; Sokolov, A.V. Ceruloplasmin-derived peptide is the strongest regulator of oxidative stress and leukotriene synthesis in neutrophils. Biochem. Cell Biol. 2017, 95, 445–449. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of holo-ceruloplasmin, its copper-free (apo-) and partially proteolyzed forms, and synthetic free peptides RPYLKVFNPR (883–892) and RRPYLKVFNPRR (882–893) are available from the authors. |

| Compound | A610/A280 | M (Percentage), kDa |
|---|---|---|
| Intact CP | >0.050 | 132 (94%), 116 (2%), 19 (2%) |
| CPprot | >0.049 | 116 (18%), 72 (16%), 67 (16%), 52 (16%), 19 (34%) |
| Apo-CP | <0.0001 | 132 (94%), 116 (2%), 19 (2%) |
| Abbreviation | Amino Acid Sequence | Molecular Mass (Da) |
|---|---|---|
| P1 | RPYLKVFNPR (883–892) | 1290 |
| P2 | RRPYLKVFNPRR (882–893) | 1600 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golenkina, E.A.; Viryasova, G.M.; Galkina, S.I.; Gaponova, T.V.; Sud’ina, G.F.; Sokolov, A.V. Fine Regulation of Neutrophil Oxidative Status and Apoptosis by Ceruloplasmin and Its Derivatives. Cells 2018, 7, 8. https://doi.org/10.3390/cells7010008
Golenkina EA, Viryasova GM, Galkina SI, Gaponova TV, Sud’ina GF, Sokolov AV. Fine Regulation of Neutrophil Oxidative Status and Apoptosis by Ceruloplasmin and Its Derivatives. Cells. 2018; 7(1):8. https://doi.org/10.3390/cells7010008
Chicago/Turabian StyleGolenkina, Ekaterina A., Galina M. Viryasova, Svetlana I. Galkina, Tatjana V. Gaponova, Galina F. Sud’ina, and Alexey V. Sokolov. 2018. "Fine Regulation of Neutrophil Oxidative Status and Apoptosis by Ceruloplasmin and Its Derivatives" Cells 7, no. 1: 8. https://doi.org/10.3390/cells7010008
APA StyleGolenkina, E. A., Viryasova, G. M., Galkina, S. I., Gaponova, T. V., Sud’ina, G. F., & Sokolov, A. V. (2018). Fine Regulation of Neutrophil Oxidative Status and Apoptosis by Ceruloplasmin and Its Derivatives. Cells, 7(1), 8. https://doi.org/10.3390/cells7010008







