(TCRαβ+) Double-Negative T Cells in Type 1 Diabetes Mellitus
Highlights
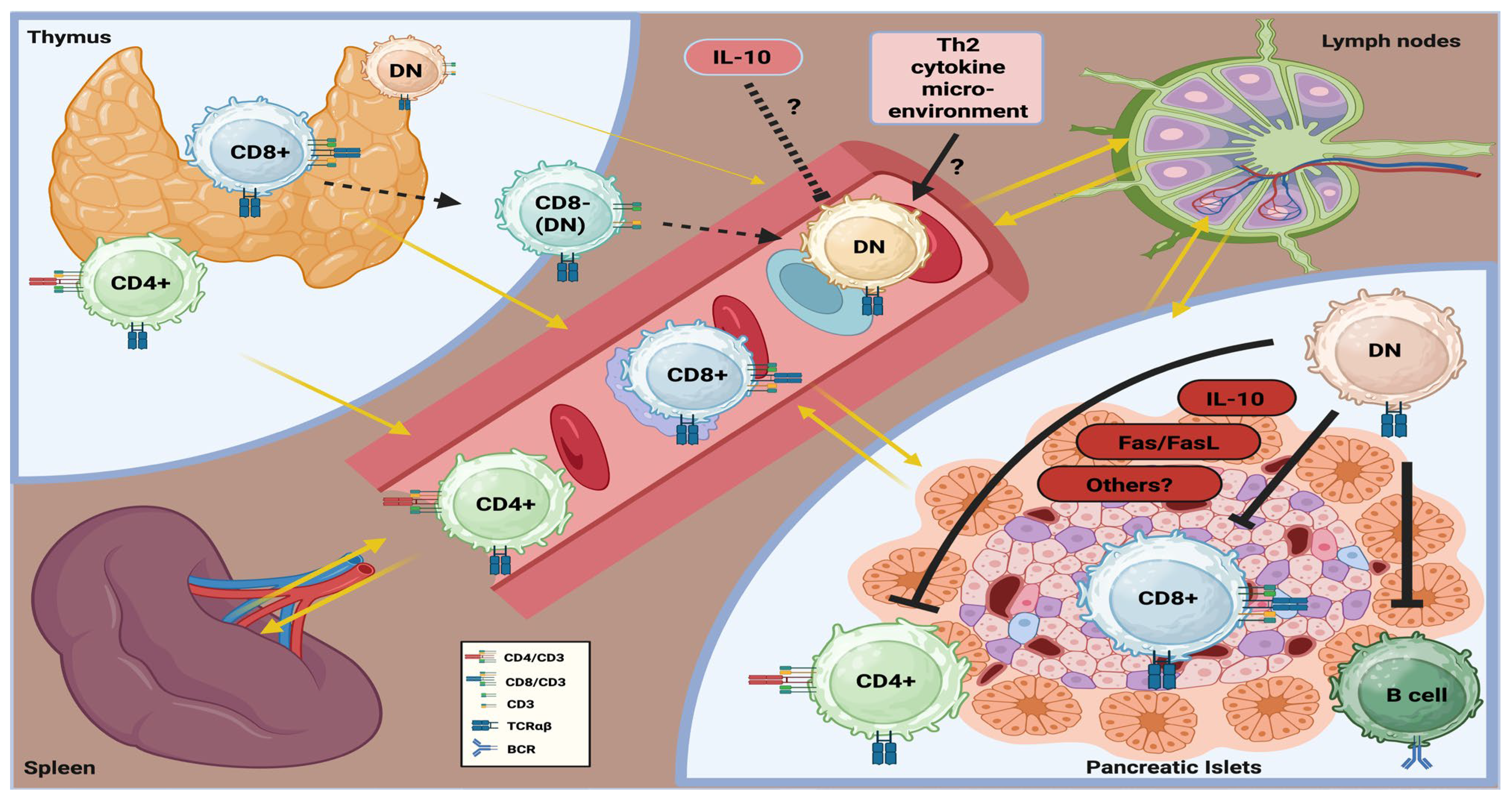
- In murine autoimmune diabetes, TCRαβ+DNT cells appear to exert a predominantly protective role against immune-mediated β-cell injury.
- Very few studies have examined TCRαβ+DNT cells in patients with Type 1 Diabetes Mellitus (T1DM).
- TCRαβ+DNT cells might represent an additional therapeutic target in T1DM and other autoimmune conditions.
- Specific clinical and translational research is needed to better elucidate the role of TCRαβ+DNT cells in T1DM.
Abstract
1. Introduction
2. (TCRαβ+) Double-Negative T Cells
3. (TCRαβ+) DNT Cells in Autoimmune Diabetes (Insights from Experimental Models)
4. (TCRαβ+) DNT Cells in Diabetes Mellitus Type 1: Knowledge Gaps and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Ebrahimpour, Y.; Khatami, S.; Saffar, M.; Fereidouni, A.; Biniaz, Z.; Erfanian, N.; Fereidouni, M. A comprehensive review of novel advances in type 1 diabetes mellitus. J. Diabetes 2025, 17, e70120. [Google Scholar] [CrossRef]
- Vanderniet, J.A.; Jenkins, A.J.; Donaghue, K.C. Epidemiology of type 1 diabetes. Curr. Cardiol. Rep. 2022, 24, 1455–1465. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.-M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.E.; Almdal, T.P.; Carstensen, B. Time trends in mortality rates in type 1 diabetes from 2002 to 2011. Diabetologia 2013, 56, 2401–2404. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Hede, S.M.; Patterson, C.C.; Wild, S.H.; Imperatore, G.; Roglic, G.; Beran, D. Type 1 diabetes in 2017: Global estimates of incident and prevalent cases in children and adults. Diabetologia 2021, 64, 2741–2750. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Lucier, J.; Mathias, P.M. Type 1 Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507713/ (accessed on 15 November 2025).
- Aamodt, K.I.; Powers, A.C. The pathophysiology, presentation and classification of type 1 diabetes. Diabetes Obes. Metab. 2025, 27, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ilonen, J.; Hammais, A.; Laine, A.-P.; Lempainen, J.; Vaarala, O.; Veijola, R.; Simell, O.; Knip, M. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 2013, 62, 3636–3640. [Google Scholar] [CrossRef]
- Willcox, A.; Richardson, S.J.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2008, 155, 173–181. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.; Fu, A.; Kaddis, J.S.; Wasserfall, C.; Schatz, D.A.; Pugliese, A.; Atkinson, M.A. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016, 65, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.C.; Delong, T.; Perdigoto, A.L.; Biru, N.; Brusko, T.M.; Walker, L.S.K. The immunology of type 1 diabetes. Nat. Rev. Immunol. 2024, 24, 435–451. [Google Scholar] [CrossRef]
- Coppieters, K.T.; Dotta, F.; Amirian, N.; Campbell, P.D.; Kay, T.W.; Atkinson, M.A.; Roep, B.O.; von Herrath, M.G. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent-onset and long-term type 1 diabetes patients. J. Exp. Med. 2012, 209, 51–60. [Google Scholar] [CrossRef]
- James, E.A.; Joglekar, A.V.; Linnemann, A.K.; Russ, H.A.; Kent, S.C. The beta cell–immune cell interface in type 1 diabetes (T1D). Mol. Metab. 2023, 78, 101809. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xi, S.; He, G.; Li, Z.; Gang, X.; Sun, C.; Guo, W.; Wang, G. Two to Tango: Dialogue between adaptive and innate immunity in type 1 diabetes. J. Diabetes Res. 2020, 2020, 4106518. [Google Scholar] [CrossRef] [PubMed]
- Leete, P.; Morgan, N.G. Footprints of immune cells in the pancreas in type 1 diabetes; to “B” or not to “B”: Is that still the question? Front. Endocrinol. 2021, 12, 617437. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Bundy, B.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; Moran, A.; Raskin, P.; et al. B-lymphocyte depletion with rituximab and β-cell function: Two-year results. Diabetes Care 2014, 37, 453–459. [Google Scholar] [CrossRef]
- Vecchio, F.; Buono, N.L.; Stabilini, A.; Nigi, L.; Dufort, M.J.; Geyer, S.; Rancoita, P.M.; Cugnata, F.; Mandelli, A.; Valle, A.; et al. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight 2018, 3, e122146. [Google Scholar] [CrossRef]
- Nigi, L.; Pedace, E.; Dotta, F.; Sebastiani, G. Neutrophils in type 1 diabetes: Untangling the intricate web of pathways and hypotheses. Biomolecules 2025, 15, 505. [Google Scholar] [CrossRef]
- Petrelli, A.; Popp, S.K.; Fukuda, R.; Parish, C.R.; Bosi, E.; Simeonovic, C.J. The contribution of neutrophils and NETs to the development of type 1 diabetes. Front. Immunol. 2022, 13, 930553. [Google Scholar] [CrossRef] [PubMed]
- Sabetkam, S.; Kalarestaghi, H.; Mazloumi, Z.; Dizaji Asl, K.; Norouzi, N.; Rafat, A. The dysfunction of natural killer cells is essential for the development of type 1 diabetes. Pathol. Res. Pract. 2023, 247, 154556. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.; Fraker, C.A. Natural killer cells as key mediators in type 1 diabetes immunopathology. Front. Immunol. 2021, 12, 722979. [Google Scholar] [CrossRef]
- Li, H.; Tsokos, G.C. Double-negative T cells in autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 163–172. [Google Scholar] [CrossRef]
- Velikkakam, T.; Gollob, K.J.; Dutra, W.O. Double-negative T cells: Setting the stage for disease control or progression. Immunology 2022, 165, 371–385. [Google Scholar] [CrossRef]
- Dossybayeva, K.; Zhubanova, G.; Mussayeva, A.; Mukusheva, Z.; Dildabayeva, A.; Nauryzbayeva, G.; Akhmaltdinova, L.; Orumbayeva, U.; Tanko, M.; Poddighe, D. Nonspecific increase of αβTCR+ double-negative T cells in pediatric rheumatic diseases. World J. Pediatr. 2024, 20, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zheng, Y.; Sheng, J.; Han, Y.; Yang, Y.; Pan, H.; Yao, J. CD3+CD4−CD8− (double-negative) T cells in inflammation, immune disorders and cancer. Front. Immunol. 2022, 13, 816005. [Google Scholar] [CrossRef]
- Li, X.; Guo, D.; Zou, I.X.; Zhao, L.; Yang, N.; Liu, Y. CD3+CD4−CD8− T cells: A new potential therapeutic target in treating autoimmune diseases. Front. Immunol. 2025, 16, 1683418. [Google Scholar] [CrossRef]
- Poddighe, D.; Maulenkul, T.; Zhubanova, G.; Akhmaldtinova, L.; Dossybayeva, K. Natural Killer T (NKT) cells in autoimmune hepatitis: Current evidence from basic and clinical research. Cells 2023, 12, 2854. [Google Scholar] [CrossRef]
- Pellicci, D.G.; Koay, H.-F.; Berzins, S.P. Thymic development of unconventional T cells: How NKT cells, MAIT cells and γδ T cells emerge. Nat. Rev. Immunol. 2020, 20, 756–770. [Google Scholar] [CrossRef]
- Kukreja, A.; Maclaren, N.K. NKT cells and type 1 diabetes and the “hygiene hypothesis” to explain the rising incidence rates. Diabetes Technol. Ther. 2002, 4, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Tard, C.; Rouxel, O.; Lehuen, A. Regulatory role of natural killer T cells in diabetes. Biomed. J. 2015, 38, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, T.L.; Ling, E.; Haase, C.; Bresson, D.; Urso, B.; von Herrath, M.G. NKG2D blockade facilitates diabetes prevention by antigen-specific Tregs in a virus-induced model of diabetes. J. Autoimmun. 2013, 40, 66–73. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, N.; Flores-Mendoza, G.; Apostolidis, S.A.; Rosetti, F.; Tsokos, G.C.; Crispín, J.C. TCRαβ CD4−CD8− double-negative T cells arise from CD8+ T cells. J. Leukoc. Biol. 2020, 108, 851–857. [Google Scholar] [CrossRef]
- Crispín, J.C.; Tsokos, G.C. Human TCR-alpha beta+ CD4− CD8− T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J. Immunol. 2009, 183, 4675–4681. [Google Scholar] [CrossRef]
- Bristeau-Leprince, A.; Mateo, V.; Lim, A.; Magerus-Chatinet, A.; Solary, E.; Fischer, A.; Rieux-Laucat, F.; Gougeon, M.L. Human TCR alpha/beta+ CD4−CD8− double-negative T cells in patients with autoimmune lymphoproliferative syndrome express restricted Vbeta TCR diversity and are clonally related to CD8+ T cells. J. Immunol. 2008, 181, 440–448. [Google Scholar] [CrossRef]
- Lambert, M.P. Presentation and diagnosis of autoimmune lymphoproliferative syndrome (ALPS). Expert Rev. Clin. Immunol. 2021, 17, 1163–1173. [Google Scholar] [CrossRef]
- Magerus, A.; Rensing-Ehl, A.; Rao, V.K.; Teachey, D.T.; Rieux-Laucat, F.; Ehl, S. Autoimmune lymphoproliferative immunodeficiencies (ALPIDs): A proposed approach to redefining ALPS and other lymphoproliferative immune disorders. J. Allergy Clin. Immunol. 2024, 153, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Sang, A.; Yin, Y.; Zheng, Y.-Y.; Morel, L. Murine models of systemic lupus erythematosus. J. Biomed. Biotechnol. 2011, 2011, 271694. [Google Scholar] [CrossRef]
- Brandt, D.; Hedrich, C.M. TCRαβ+ CD3+ CD4−CD8− double-negative T cells in autoimmunity. Autoimmun. Rev. 2018, 17, 422–430. [Google Scholar] [CrossRef]
- Poddighe, D.; Dossybayeva, K.; Kozhakhmetov, S.; Rozenson, R.; Assylbekova, M. Double-negative T (DNT) cells in patients with systemic lupus erythematosus. Biomedicines 2024, 12, 166. [Google Scholar] [CrossRef]
- Poddighe, D.; Maulenkul, T.; Dossybayeva, K.; Zhubanova, G.; Mukusheva, Z.; Akhmaltdinova, L. Double-negative T cells in pediatric rheumatic diseases. Clin. Exp. Pediatr. 2024, 67, 632–640. [Google Scholar] [CrossRef]
- Chandy, K.G.; Cahalan, M.D.; Grissmer, S. Autoimmune diseases linked to abnormal K+ channel expression in double-negative CD4−CD8− T cells. Eur. J. Immunol. 1990, 20, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Chandy, K.G.; Norton, R.S. Peptide blockers of Kv1.3 channels in T cells as therapeutics for autoimmune disease. Curr. Opin. Chem. Biol. 2017, 38, 97–107. [Google Scholar] [CrossRef]
- Ohya, S.; Kito, H. Ca2+-activated K+ channel KCa3.1 as a therapeutic target for immune disorders. Biol. Pharm. Bull. 2018, 41, 1158–1163. [Google Scholar] [CrossRef]
- Grissmer, S.; Cahalan, M.D.; Chandy, K.G. Abundant expression of type I K+ channels: A marker for lymphoproliferative diseases? J. Immunol. 1988, 141, 1137–1142. [Google Scholar] [CrossRef]
- Grissmer, S.; Hanson, D.C.; Natoli, E.J.; Cahalan, M.D.; Chandy, K.G. CD4−CD8− T cells from mice with collagen arthritis display aberrant expression of type I K+ channels. J. Immunol. 1990, 145, 2105–2109. [Google Scholar] [CrossRef]
- Formby, B.; Hosszufalusi, N.; Chan, E.; Miller, N.; Teruya, M.; Takei, S.; Charles, M.A. Quantitative and functional analyses of spleen and in situ islet immune cells before and after diabetes onset in NOD mice. Autoimmunity 1992, 12, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hosszufalusi, N.; Chan, E.; Granger, G.; Charles, M.A. Quantitative analyses comparing all major spleen cell phenotypes in BB and normal rats: Autoimmune imbalance and double-negative T cells. J. Autoimmun. 1992, 5, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Zipris, D.; Crow, A.R.; Delovitch, T.L. Altered thymic and peripheral T-lymphocyte repertoire preceding onset of diabetes in NOD mice. Diabetes 1991, 40, 429–435. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Constantinou, D.; Mandel, T.E.; Georgiou, H.M. Lymphocyte subsets in thymus and peripheral lymphoid tissues of aging and diabetic NOD mice. Autoimmunity 1994, 17, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Kinder, S.J.; Silvera, P.; Baxter, A.G. Flow cytometric study of T cell development in NOD mice reveals a deficiency in αβTCR+CD4−CD8− thymocytes. J. Autoimmun. 1997, 10, 279–285. [Google Scholar] [CrossRef]
- Goldrath, A.W.; Barber, L.; Chen, K.E.; Alters, S.E. Differences in adhesion markers, activation markers, and TCR in islet infiltrating vs. peripheral lymphocytes in the NOD mouse. J. Autoimmun. 1995, 8, 209–220. [Google Scholar] [CrossRef]
- Ford, M.S.; Chen, W.; Wong, S.; Li, C.; Vanama, R.; Elford, A.R.; Asa, S.L.; Ohashi, P.S.; Zhang, L. Peptide-activated double-negative T cells can prevent autoimmune type 1 diabetes development. Eur. J. Immunol. 2007, 37, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Mohamood, A.S.; Guler, M.L.; Xiao, Z.; Zheng, D.; Hess, A.; Wang, Y.; Yagita, H.; Schneck, J.P.; Hamad, A.R. Protection from autoimmune diabetes and T-cell lymphoproliferation induced by FasL mutation are differentially regulated and can be uncoupled pharmacologically. Am. J. Pathol. 2007, 171, 97–106. [Google Scholar] [CrossRef]
- DeFranco, S.; Bonissoni, S.; Cerutti, F.; Bona, G.; Bottarel, F.; Cadario, F.; Brusco, A.; Loffredo, G.; Rabbone, I.; Corrias, A.; et al. Defective function of Fas in patients with type 1 diabetes associated with autoimmune diseases. Diabetes 2001, 50, 483–488. [Google Scholar] [CrossRef]
- Itoh, N.; Imagawa, A.; Hanafusa, T.; Waguri, M.; Yamamoto, K.; Iwahashi, H.; Mariwaki, M.; Nakajima, H.; Miyagawa, J.; Namba, M.; et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 1997, 186, 613–618. [Google Scholar] [CrossRef]
- Chervonsky, A.V.; Wang, Y.; Wong, F.S.; Visintin, I.; Fravell, R.A.; Janeway, C.A.; Matis, L.A. The role of Fas in autoimmune diabetes. Cell 1997, 89, 17–24. [Google Scholar] [CrossRef]
- Duncan, B.; Nazarov-Stoica, C.; Surls, J.; Kehl, M.; Bona, C.; Casares, S.; Brumeanu, T.D. Double-negative (CD3+4−8−) TCRαβ splenic cells from young NOD mice provide long-lasting protection against type 1 diabetes. PLoS ONE 2010, 5, e11427. [Google Scholar] [CrossRef] [PubMed]
- Dugas, V.; Beauchamp, C.; Chabot-Roy, G.; Hillhouse, E.E.; Lesage, S. Implication of the CD47 pathway in autoimmune diabetes. J. Autoimmun. 2010, 35, 23–32. [Google Scholar] [CrossRef]
- Hillhouse, E.E.; Beauchamp, C.; Chabot-Roy, G.; Dugas, V.; Lesage, S. Interleukin-10 limits the expansion of immunoregulatory CD4−CD8− T cells in autoimmune-prone NOD mice. Immunol. Cell Biol. 2010, 88, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cong, M.; Sun, G.; Wang, P.; Tian, Y.; Shi, W.; Li, X.; You, H.; Zhang, D. Combination of double negative T cells and anti-thymocyte serum reverses type 1 diabetes in NOD mice. J. Transl. Med. 2016, 14, 57. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Ng, T.W.; Wang, Y.; Liu, Q.; Gorantla, V.; Lakkis, F.; Zheng, X.X. Adoptive cell therapy using antigen-specific CD4−CD8− T regulatory cells to prevent autoimmune diabetes and promote islet allograft survival in NOD mice. Diabetologia 2011, 54, 2082–2092. [Google Scholar] [CrossRef]
- Collin, R.; Dugas, V.; Pelletier, A.N.; Chabot-Roy, G.; Lesage, S. The mouse idd2 locus is linked to the proportion of immunoregulatory double-negative T cells, a trait associated with autoimmune diabetes resistance. J. Immunol. 2014, 193, 3503–3512. [Google Scholar] [CrossRef]
- Dugas, V.; Liston, A.; Hillhouse, E.E.; Collin, R.; Chabot-Roy, G.; Pelletier, A.N.; Beauchamp, C.; Hardy, K.; Lesage, S. Idd13 is involved in determining immunoregulatory DN T-cell number in NOD mice. Genes Immun. 2014, 15, 82–87. [Google Scholar] [CrossRef]
- Collin, R.; Doyon, K.; Mullins-Dansereau, V.; Karam, M.; Chabot-Roy, G.; Hillhouse, E.E.; Orthwein, A.; Lesage, S. Genetic interaction between two insulin-dependent diabetes susceptibility loci, Idd2 and Idd13, in determining immunoregulatory DN T cell proportion. Immunogenetics 2018, 70, 495–509. [Google Scholar] [CrossRef]
- Collin, R.; Dugas, V.; Pelletier, A.N.; Chabot-Roy, G.; Lesage, S. Evidence of genetic epistasis in autoimmune diabetes susceptibility revealed by mouse congenic sublines. Immunogenetics 2021, 73, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Zimmerman, S.; Lindahl, A.; Weidanz, J.; Ordovas-Montanes, J.; Kostic, A.; Luber, J.; Robben, M. Single-cell RNA-seq reveals disease-specific CD8+ T cell clonal expansion and high frequency of transcriptionally distinct double-negative T cells in diabetic NOD mice. PLoS ONE 2025, 20, e0317987. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, H.; Pihl, M.; Wahlberg, J.; Ludvigsson, J.; Casas, R. Intralymphatic GAD-alum injection modulates B cell response and induces follicular helper T cells and PD-1+ CD8+ T cells in patients with recent-onset type 1 diabetes. Front. Immunol. 2022, 12, 797172. [Google Scholar] [CrossRef]
- Ludvigsson, J.; Faresjö, M.; Hjorth, M.; Axelsson, S.; Chéramy, M.; Pihl, M.; Vaarala, O.; Forsander, G.; Ivarsson, S.; Johansson, C.; et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N. Engl. J. Med. 2008, 359, 1909–1920. [Google Scholar] [CrossRef]
- Wherrett, D.K.; Bundy, B.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B.; Monzavi, R.; et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: A randomized double-masked controlled trial. Lancet 2011, 378, 319–327. [Google Scholar] [CrossRef]
- Ludvigsson, J.; Krisky, D.; Casas, R.; Battelino, T.; Castaño, L.; Greening, J.; Kordonouri, O.; Osváth-Csincsik, L.; Pozzilli, P.; Robert, J.-J.; et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 2012, 366, 433–442. [Google Scholar] [CrossRef]
- Beam, C.A.; MacCallum, C.; Herold, K.C.; Wherrett, D.K.; Palmer, J.; Ludvigsson, J.; the Type 1 Diabetes TrialNet Study Group. GAD vaccine reduces insulin loss in recently diagnosed type 1 diabetes: Findings from a Bayesian meta-analysis. Diabetologia 2017, 60, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Dietrich, F.; Barcenilla, H.; Tavira, B.; Wahlberg, J.; Achenbach, P.; Ludvigsson, J. Glutamic acid decarboxylase injection into lymph nodes: Beta cell function and immune responses in recent-onset type 1 diabetes patients. Front. Immunol. 2020, 11, 564921. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, F.; Barcenilla, H.; Tavira, B.; Wahlberg, J.; Achenbach, P.; Ludvigsson, J.; Casas, R. Immune response differs between intralymphatic or subcutaneous administration of GAD-alum in individuals with recent onset type 1 diabetes. Diabetes Metab. Res. Rev. 2022, 38, e3500. [Google Scholar] [CrossRef]
- Fajardo-Despaigne, J.E.; Lombard-Vadnais, F.; Pelletier, A.N.; Olazabal, A.; Boutin, L.; Pasquin, S.; Janelle, V.; Legault, L.; Delisle, J.S.; Hillhouse, E.E.; et al. Characterization and effective expansion of CD4−CD8− TCRαβ⁺ T cells from individuals living with type 1 diabetes. Mol. Ther. Methods Clin. Dev. 2024, 33, 101400. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, N.; Rosetti, F.; Crispin, J.C. CD8 is down(regulated) for tolerance. Trends Immunol. 2024, 45, 442–453. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, N.; Apostolidis, S.A.; Penaloza-MacMaster, P.; Martin Villa, J.M.; Barouch, D.H.; Tsokos, G.C.; Crispin, J.C. Programmed cell death 1 and Helios distinguish TCRαβ⁺ double-negative (CD4−CD8−) T cells that derive from self-reactive CD8 T cells. J. Immunol. 2015, 194, 4207–4214. [Google Scholar] [CrossRef]
- Badr, M.E.; Zhang, Z.; Tai, X.; Singer, A. CD8 T cell tolerance results from eviction of immature autoreactive cells from the thymus. Science 2023, 382, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, X.; Deng, J.; Wu, J.; Bai, S.; Yu, R. How do immune cells shape type 1 diabetes? Insights from Mendelian randomization. Front. Endocrinol. 2024, 15, 1402956. [Google Scholar] [CrossRef]
- Fischer, K.; Voelkl, S.; Heymann, J.; Przybylski, G.K.; Mondal, K.; Laumer, M.; Kunz-Schughart, L.; Schmidt, C.A.; Andreesen, R.; Mackensen, A. Isolation and characterization of human antigen-specific TCRαβ⁺ CD4−CD8− double-negative regulatory T cells. Blood 2005, 105, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, S.; Gary, R.; Mackensen, A. Characterization of the immunoregulatory function of human TCRαβ⁺ CD4−CD8− double-negative T cells. Eur. J. Immunol. 2011, 41, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, L.; Young, K.J.; Zhang, L. Suppression of alloimmune responses in vitro and in vivo by CD3⁺CD8−CD4−αβ⁺ regulatory T cells. Transplant. Proc. 2001, 33, 84–85. [Google Scholar] [CrossRef]
- Hillhouse, E.E.; Thiant, S.; Moutuou, M.M.; Lombard-Vadnais, F.; Parat, R.; Delisle, J.-S.; Ahmad, I.; Roy, D.-C.; Guimond, M.; Roy, J.; et al. Double-negative T cells levels correlate with chronic graft-versus-host disease severity. Biol. Blood Marrow Transplant. 2019, 25, 19–25. [Google Scholar] [CrossRef]
- Achita, P.; Dervovic, D.; Ly, D.; Lee, J.B.; Haug, T.; Joe, B.; Hirano, N.; Zhang, L. Infusion of ex vivo expanded human TCRαβ⁺ double-negative regulatory T cells delays onset of xenogeneic graft-versus-host disease. Clin. Exp. Immunol. 2018, 193, 386–399. [Google Scholar] [CrossRef]
- Gao, J.F.; McIntyre, M.S.F.; Juvet, S.C.; Diao, J.; Li, X.; Vanama, R.B.; Mak, T.W.; Cattral, M.S.; Zhang, L. Regulation of antigen-expressing dendritic cells by double-negative regulatory T cells. Eur. J. Immunol. 2011, 41, 2699–2708. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Ma, Y.; Wang, H.; Arp, J.; Jiang, J.; Huang, X.; He, K.M.; Garcia, B.; Madrenas, J.; Zhong, R. Double-negative T cells activated by xenoantigen lyse autologous B and T cells using a perforin/granzyme-dependent, Fas–FasL–independent pathway. J. Immunol. 2006, 177, 6920–6929. [Google Scholar] [CrossRef]
- Haug, T.; Aigner, M.; Peuser, M.M.; Strobl, C.D.; Hildner, K.; Mougiakakos, D.; Bruns, H.; Mackensen, A.; Völkl, S. Human double-negative regulatory T cells induce a metabolic and functional switch in effector T cells by suppressing mTOR activity. Front. Immunol. 2019, 10, 883. [Google Scholar] [CrossRef]
- Sun, G.; Chen, X.; Pan, T.; Song, K.; Xie, H.; Tu, M.; Wan, X.; Yao, W.; Cheng, Y.; Zhou, Z.; et al. Prophylactic infusion of allogeneic double-negative T cells as immune modulators to prevent relapse in high-risk AML patients post-allo-HSCT: A phase I trial. Exp. Hematol. Oncol. 2025, 14, 90. [Google Scholar] [CrossRef]
- Tin, E.; Lee, J.; Zhang, L. Allogeneic double-negative T-cell therapy for acute myeloid leukemia. Curr. Opin. Pharmacol. 2025, 83, 102537. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, H.; Chen, B.; Na, Y.; Khatri, I.; Soares, F.; He, H.H.; Law, A.D.; Pan, T.; Gerbitz, A.; et al. Allogeneic DNT cell therapy synergizes with T cells to promote anti-leukemic activities while suppressing GVHD. J. Exp. Clin. Cancer Res. 2025, 44, 28. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.K.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4⁺ CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.D.; Birch, J.; Accogli, T.; Criado, I.; Khabirova, E.; Parks, C.; Wood, Y.; Young, M.D.; Porter, T.; Richardson, R.; et al. Transcriptional signatures associated with persisting CD19 CAR-T cells in children with leukemia. Nat. Med. 2023, 29, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, B.-Y.; Yu, S.-H.; Chen, S.-J.; Yang, S.-S.; Liu, R.; Chen, L.-J.; Hou, J.; Chen, Z.; Zhao, W.-H.; et al. Long-term remission and survival in patients with relapsed or refractory multiple myeloma after treatment with LCAR-B38M CAR T cells: 5-year follow-up of the LEGEND-2 trial. J. Hematol. Oncol. 2024, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Mackensen, A.; Mougiakakos, D. CAR T-cell therapy in autoimmune diseases. Lancet 2023, 402, 2034–2044. [Google Scholar] [CrossRef]
- Dao, L.T.M.; Vu, T.T.; Nguyen, Q.T.; Hoang, V.T.; Nguyen, T.L. Current cell therapies for systemic lupus erythematosus. Stem Cells Transl. Med. 2024, 13, 859–872. [Google Scholar] [CrossRef]
- Scherlinger, M.; Nocturne, G.; Radic, M.; Launay, D.; Richez, C.; Bousso, P.; Forcade, E.; Meyer, A.; Jorgensen, C.; Bigenwald, C.; et al. Club for Innovative Immunotherapies in Immune-mediated Inflammatory Diseases (C3I). CAR T-cell therapy in autoimmune diseases: Where are we and where are we going? Lancet Rheumatol. 2025, 7, e434–e447. [Google Scholar] [CrossRef]
- Poddighe, D.; Thi Van Nguyen, A.; Phung, L.T.; Le, C.Q.; Hermiston, M.L. Innovative therapies for childhood-onset systemic lupus erythematosus. World J. Pediatr. 2025, 21, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Jouret, M.; Viel, S.; Fournier, B.; Benezech, S.; Avouac, J.; Scherlinger, M.; Belot, A.; C3I Consortium. CAR-T cell therapy for juvenile-onset autoimmune diseases: A promising future? Arthritis Res. Ther. 2025, 27, 102. [Google Scholar] [CrossRef]
- Mohammadi, V.; Maleki, A.J.; Nazari, M.; Siahmansouri, A.; Moradi, A.; Elahi, R.; Esmaeilzadeh, A. Chimeric antigen receptor (CAR)-based cell therapy for type 1 diabetes mellitus (T1DM): Current progress and future approaches. Stem Cell Rev. Rep. 2024, 20, 585–600. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, J. Regulatory T cell-based therapy in type 1 diabetes: Latest breakthroughs and evidence. Int. Immunopharmacol. 2024, 140, 112724. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year, Country [Ref.] | Biological Samples | Main Autoimmune Diabetes Model | DNT Cell Markers | Study Aim | Main Analytical Methods and Equipment | Additional Findings on DNT Cells | Main Conclusion |
|---|---|---|---|---|---|---|---|
| Chandy et al., 1990, USA [44] | Spleen | NOD | CD4− CD8− Thy1.2+ | To assess the voltage-gated K+ channel expression in T cells and, in detail, DNT cells | FACS [n/a] Patch-clamp whole-cell recordings Fluorescence microscopy | -- | “…augmented type l K+ channel expression appears to be a valuable marker for DN T cells associated with murine lupus, autoimmune mellitus and experimental autoimmune encephalomyelitis” |
| Zipris et al., 1991, Canada [51] | Lymph nodes Thymus | NOD | CD4− CD8− | To assess the pattern of thymic T-lymphocyte development and the expression of the peripheral T-lymphocyte repertoire before and at the onset of diabetes | FACS [EPICS V flow cytometer (Coulter)] | “Prediabetic NOD mice displayed an increase in CD4−CD8− T thymocytes, and a reciprocal decrease inCD4+CD8+ T thymocytes. The CPM-accelerated diabetes was characterized by an increase of DN thymocytes in the days following the injection.PS. No information described about peripheral DNT cells.” | “… depletion of CD4+ regulatory T lymphocytes and/or the rerouting of CD4+V8.1+ effector T lymphocytes from the peripheral LN to the pancreas during progression to disease onset mediate the pathogenesis of diabetes.” |
| Formby et al., 1992, USA [49] | Spleen Pancreas (islets) | NOD | CD4− CD8− Thy1.2+ | To perform a quantitative analysis and functional assessment of the inflammatory cells | FACS [n/a (Becton Dickinson)] | -- | “In summary our results show that isolated prediabetic in situ islet immune cells are mostly CD4 positive and double negative T cells.” |
| Hosszufalusi et al., 1992, USA [50] | Spleen | [rats] DP | CD4− CD8− αβTCR+ | To perform a systematic FACS analysis of all major spleen cell populations | FACS [FACScan (Becton Dickinson)] | -- | “… autoimmune diabetes may reflect an immune balance abnormality with a relative shift of cell subset dominance toward double-negative or activated T cells, NK cells and macrophages.” |
| Zhang et al., 1994, China [52] | Spleen Lymph nodes [Thymus] | NOD | CD4− CD8− Thy1.2+ | To detect any immunological alterations that can precede the onset of autoimmune diabetes | FACS [FACScan (Becton Dickinson)] | “The most striking observation was the increase in the proportion of CD4 and CD8 double-negative thymocytes in the long-term diabetics despite the fact that their absolute number was fairly.” PS. No information on DNT cells in peripheral tissues.” | “Our studies indicate a gradual absolute increase, or at least, a sustained percentage increase of CD8+ T cells in both the spleen and pancreatic LN of female NOD mice compared to CD4+T cells which gradually decrease.” |
| Goldrath et al., 1995, USA [54] | Blood Lymph nodes Pancreas (islets) | NOD | CD4− CD8− [αβTCR] [γδTCR] | To characterize the immunophenotype of infiltrating lymphocytes isolated from the islets and compare these phenotypes to those of peripheral lymphocytes | FACS [FACScan (Becton Dickinson)] | “In this study we also find a significant increase in the number of CD3+CD4−CD8− double-negative cells in the infiltrating as compared with the peripheral T cell population (11–17% vs. less than 5% in the periphery).” | “Significantly increased levels of CD4+CD8+ double-positive and CD4−CD8− double negative T cell populations were observed in the infiltrating lymphocytes as compared with peripheral lymphocytes. In addition, within both CD4 and CD8 subpopulations isolated from islet infiltrates, CD11b+ and CD49e+ (adhesion markers) cells were increased with respect to the same subset of cells isolated from the periphery. In contrast, the level of cells that expressed L-selectin was significantly higher in the periphery for both CD4+ and CD8+ cells than for infiltrating cells.” |
| Ford et al., 2007, Canada [55] | Spleen Lymph nodes | P14 RIP-gp P14/RIP-gp | CD4− CD8− Thy1.2+ Vα2+ NK1.1– | To assess the capacity of DNT cells to recognize peptides expressed on self-MHC, to suppress autoreactive CD8+ T cells | MS magnetic column (Miltenyi Biotech) | -- | “In summary, the data presented here demonstrate that DN T cells are activated by peptides presented in the context of self MHC in an antigen-specific fashion and that they can suppress and kill peptide-activated syngeneic CD8+ T cells.” |
| Mohamood et al., 2007, USA [56] | Bone marrow Gut epithelia “Immune-privileged sites” | NOD | CD4− CD8− αβTCR+ | To investigate the role of FasL and DNT cells in autoimmune diabetes | FACS [n/a] | “In addition, we demonstrate genetically, in bone marrow chimeras and haploinsufficient NOD-gld/mice, and pharmacologically, using FasL-neutralizing antibody, that the protective effect of FasL inactivation can be achieved without causing DN T-cell lymphoproliferation” | “…we show that FasL expressed on hematopoiet and nonhematopoietic compartments plays nonredundant roles in the pathogenesis of autoimmune diabetes. Mutation of FasL in either compartment interferes with the autoimmune process and prevents onset of diabetes. Moreover, FasL expressed in the hematopoietic compartment is the dominant regulator of T-cell homeostasis. |
| Duncan et al., 2010, USA [60] | Spleen | NOD | CD4− CD8− αβTCR+ | To analyze the function and phenotype of DNT splenic cells | FACS [FACSAria cell sorter (BD Biosciences)] RT-PCR (IL-10 analysis) | “Their [DNT cells’] suppressive (antidiabetogenic) effect relied mainly on the ability to differentiate into IL-10-secreting TR-1 cells in a Th2-like extra-thymic environment.” | “…this study delineates a new cell population of regulatory cells (DNCD3 [Double negative CD3+4−8− TCRαβ splenic cells]) in young NOD mice with potential anti-diabetogenic effect. The phenotype of DNCD3 splenic cells is CD3+ (CD4−CD8−)CD28+CD69+CD25lowFoxp3-iCTA-4− TCRαβ+) (anti-diabetogenic phenotype) with a predominant Vβ13 gene usage.” |
| Dugas et al., 2010 Canada [61] | Spleen Lymph nodes | NOD | CD4− CD8− B220− CD5low CD1d- tetramer- βTCR+ | To assess the contribution of the CD47 pathway in autoimmune diabetes. | FACS [FACSVantage (BD Biosciences)] | “Decreased proportion of CD4− CD8− T cells in CD47-deficient mice” | “In summary, our observations have permitted the association of a defect in the CD47 pathway with autoimmune diabetes progression and identify at least part of the mechanism by which the disruption of the CD47 pathway accelerates disease onset; through the regulation of DN T cell number.” |
| Hillhouse et al., 2010, Canada [62] | Spleen | NOD | CD4− CD8− | To assess whether the activity of DNT cells is impaired in autoimmune diabetes | FACS [FACSCalibur, FACS LSR (BD Biosciences)] FlowJo | -- | “On the basis of these results, we conclude that, on a per cell basis, the DN T-cell cytotoxic function is not impeded in the autoimmune-prone NOD genetic background” |
| Zhang et al., 2011, USA [64] | Spleen Lymph nodes | NOD | CD4− CD8− | To study whether DNT cells generated from NOD mice retain the antigen-specific regulatory capacity and prevent autoimmune diabetes in vivo | FACS [FACSAria (BD Biosciences)] | -- | “In short, this study demonstrates that beta cell antigen-specific DNT cells can be induced from prediabetic NOD CD4+T cells in vitro, efficiently prevent the onset and progress of autoimmune diabetes in vivo, and also act in conjunction with rapamycin to promote islet allograft survival in NOD mouse models. “ |
| Dugas et al., 2014 Canada [66] | Spleen Lymph nodes | NOD | CD4− CD8− | To assess the impact of major insulin-dependent diabetes (Idd) loci on the number of DNT cells | FACS [FACSCalibur (BD Biosciences)] | “Together, our results show that the regulation of DN T-cell number in NOD mice is at least partially conferred by alleles at the Idd13 locus” | “In conclusion, at least five traits are linked to Idd13, namely, the degree of insulitis, thymic selection as well as the number of merocytic dendritic cells (mcDCs), NKT and DN T cells. […] it is likely that at least two genes within the Idd13 locus contribute to diabetes susceptibility.” |
| Collin et al., 2014 Canada [65] | Spleen Lymph nodes | NOD | CD4− CD8− | To define the genetic basis underlying differences in the proportion of DNT cells between diabetes-prone and diabetes-resistant mice | FACS [FACSCalibur (BD Biosciences)] | Lower DNT cell proportion is associated not only with higher diabetes incidence but also with increased serum IgG autoantibody levels, suggesting DN T cells may influence humoral immune regulation. | “In summary, studying the genetic underpinnings of immunoregulatory DN T cells has revealed that, as for susceptibility to autoimmune diabetes, it is a complex trait. Interestingly, at least two Idd susceptibility loci are linked to the proportion of DN T cells, emphasizing their relevance in contributing to autoimmune diabetes resistance” |
| Liu et al., 2016, China [63] | Spleen Lymph nodes | NOD | CD4− CD8− αβTCR+ | To investigate the regulation of different subsets of T cells in vivo and in vitro and assess the potential therapeutic implications | FACS [FACSAria (BD Biosciences)] | -- | “…ex vivo CD4+ T cell converted DNT cells leads to a long term reversal of new-onset diabetes in NOD mice. […] Combined ATS and DN T cell treatment resulted in significant reversion of new-onset autoimmune diabetes in NOD mice” |
| Collin et al., 2018 Canada [67] | Spleen Lymph nodes [Thymus] | NOD | CD4− CD8− | To explore the genetic factors that can influence the proportion of the DNT cells, with specific regard to Idd2 and Idd13 loci | FACS [FACSCanto, LSRII, Fortessa x-20 FACSCalibur (BD Bioscencies)] | -- | “…we find that genetic interactions between Idd2 and Idd13 loci modulate cell cycle progression, which contributes, at least in part, to defining the proportion of DN T cells in secondary lymphoid organs.” |
| Collin et al., 2021, Canada [68] | Spleen Lymph nodes | NOD | CD4− CD8− | To explore the impact of a chromosome 12 locus in autoimmune diabetes, with specific regard to DN T cells | FACS [n/a] | -- | “…this study identified further complex genetic interactions in defining the proportion of DN T cells, along with evidence of genetic epistasis within a locus on chromosome 12 influencing autoimmune susceptibility.” |
| Islam et al., 2025, USA [69] | Blood Pancreas (islets) | NOD | CD8− CD4− NK1.1− [αβTCR] [γδTCR] | To identify any T-cell clonal expansion and specific transcriptomic signatures, if any, associated with diabetic progression | FACS [Attune NxT (Invitrogen)] Analysis of single-cell gene expression | -- | “…diabetic mice were found to have shockingly high levels of circulating and invading DN T cells and increased exhaustion of potentially immunosuppressive T cell subsets. DN T cell subsets increased during diabetogenesis suggesting that they proliferate more, die less, or are trafficked out of the pancreas more in concert with islet destruction.” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Poddighe, D.; Mussayeva, A.; Dossybayeva, K.; Zhubanova, G.; Galiyeva, D.; Le, K.L.; Tanko, M.N. (TCRαβ+) Double-Negative T Cells in Type 1 Diabetes Mellitus. Cells 2026, 15, 58. https://doi.org/10.3390/cells15010058
Poddighe D, Mussayeva A, Dossybayeva K, Zhubanova G, Galiyeva D, Le KL, Tanko MN. (TCRαβ+) Double-Negative T Cells in Type 1 Diabetes Mellitus. Cells. 2026; 15(1):58. https://doi.org/10.3390/cells15010058
Chicago/Turabian StylePoddighe, Dimitri, Assel Mussayeva, Kuanysh Dossybayeva, Gulsamal Zhubanova, Dinara Galiyeva, Khac Linh Le, and Matthew Naanlep Tanko. 2026. "(TCRαβ+) Double-Negative T Cells in Type 1 Diabetes Mellitus" Cells 15, no. 1: 58. https://doi.org/10.3390/cells15010058
APA StylePoddighe, D., Mussayeva, A., Dossybayeva, K., Zhubanova, G., Galiyeva, D., Le, K. L., & Tanko, M. N. (2026). (TCRαβ+) Double-Negative T Cells in Type 1 Diabetes Mellitus. Cells, 15(1), 58. https://doi.org/10.3390/cells15010058






