Toward Efficient Beige Adipogenesis: Protocol Optimization Using Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
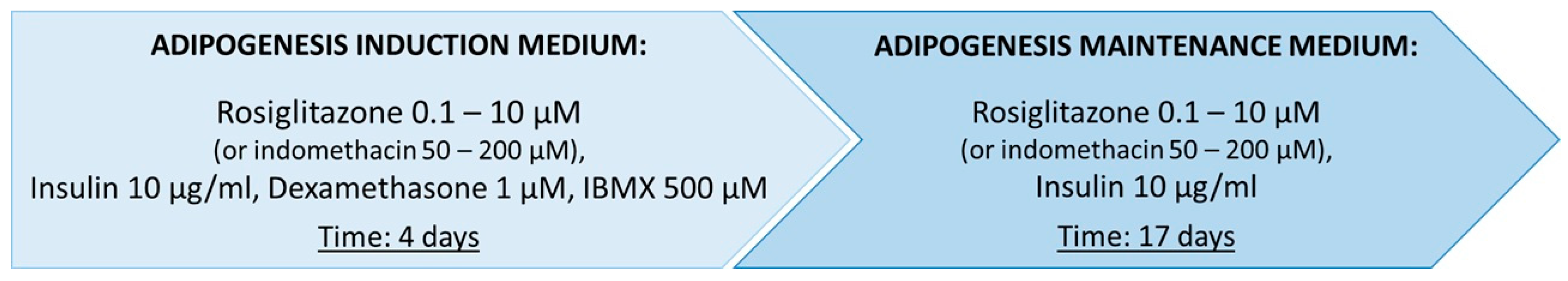
2.1.1. Differentiation of Commercial ADSCs Towards Beige Adipocytes
2.1.2. Isolation and Differentiation Culture of Primary ADSCs
2.1.3. Oil Red O Staining of Lipid Droplets
2.2. Image-Based Quantification of Differentiated Cells
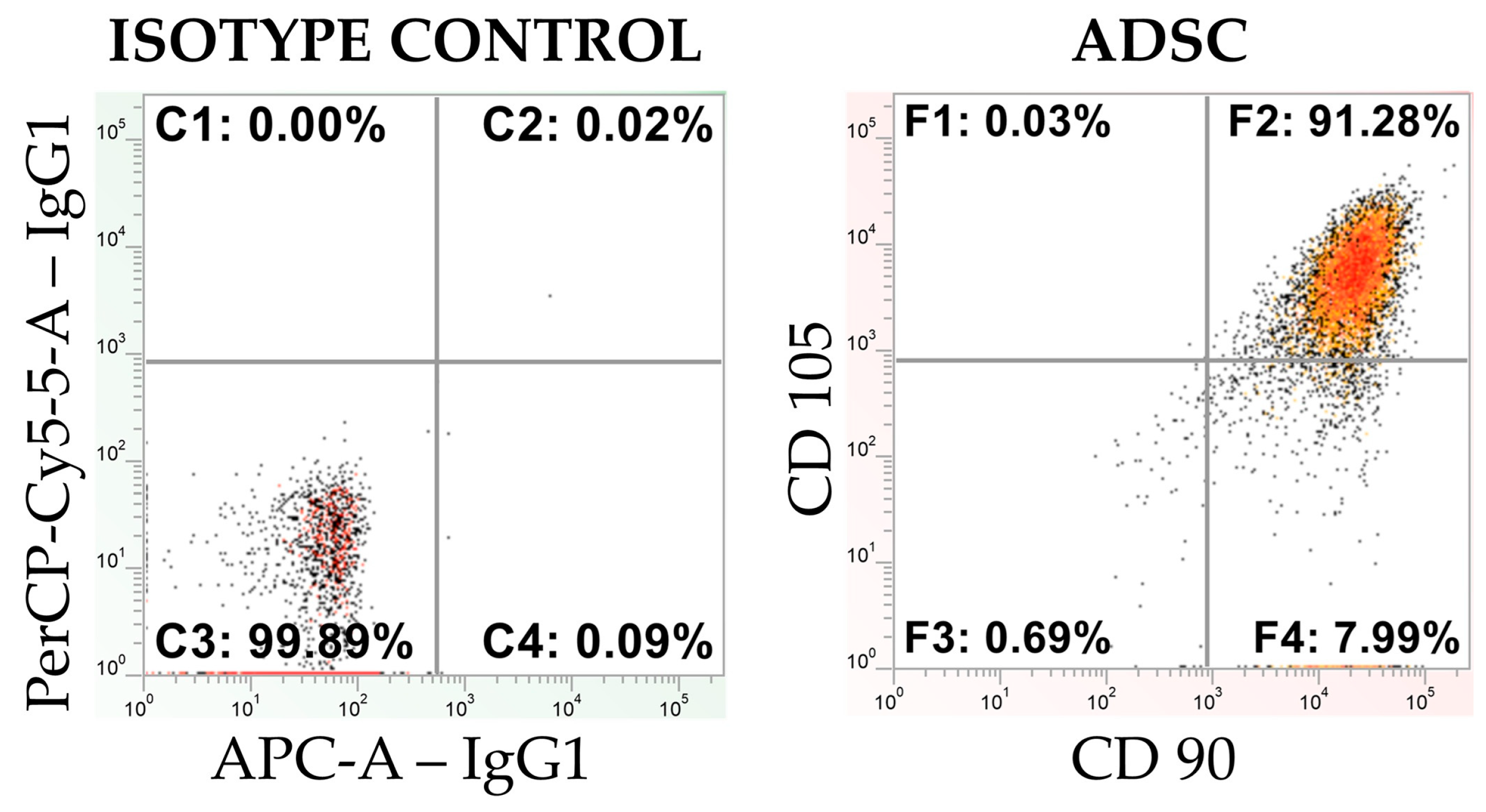
2.3. Flow Cytometry Analysis
2.4. Analysis of UCP1 Gene Expression by RT-qPCR
2.5. Semi-Quantitative Assessment of UCP1 Protein by Western Blot
2.6. Statistical Analysis
3. Results
3.1. Flow Cytometry Analysis of the Commercial and Primary ADSCs
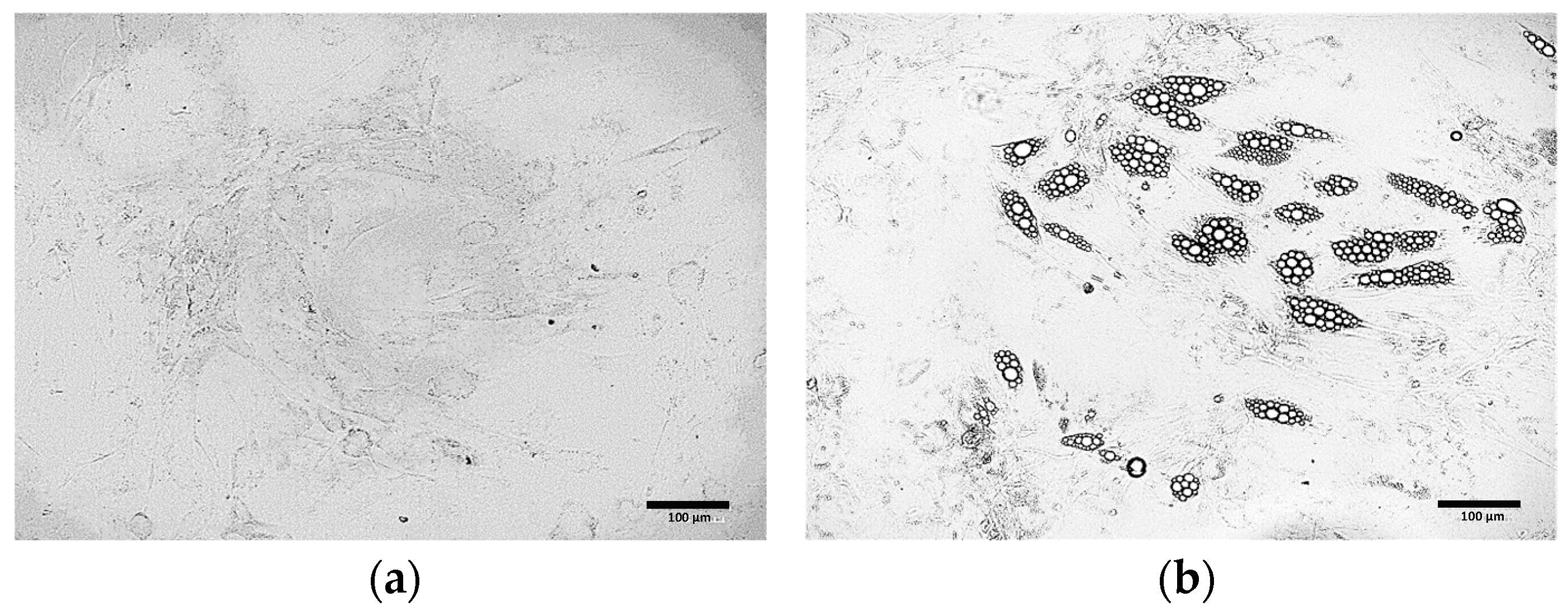
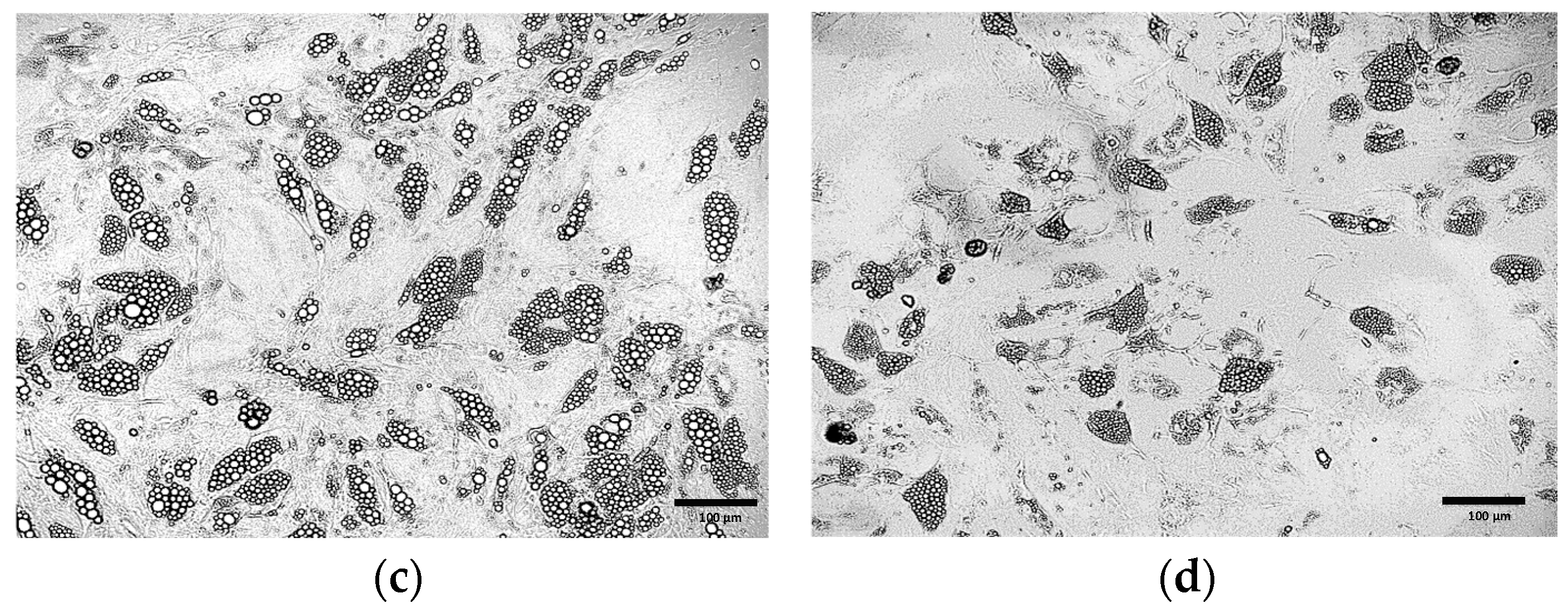
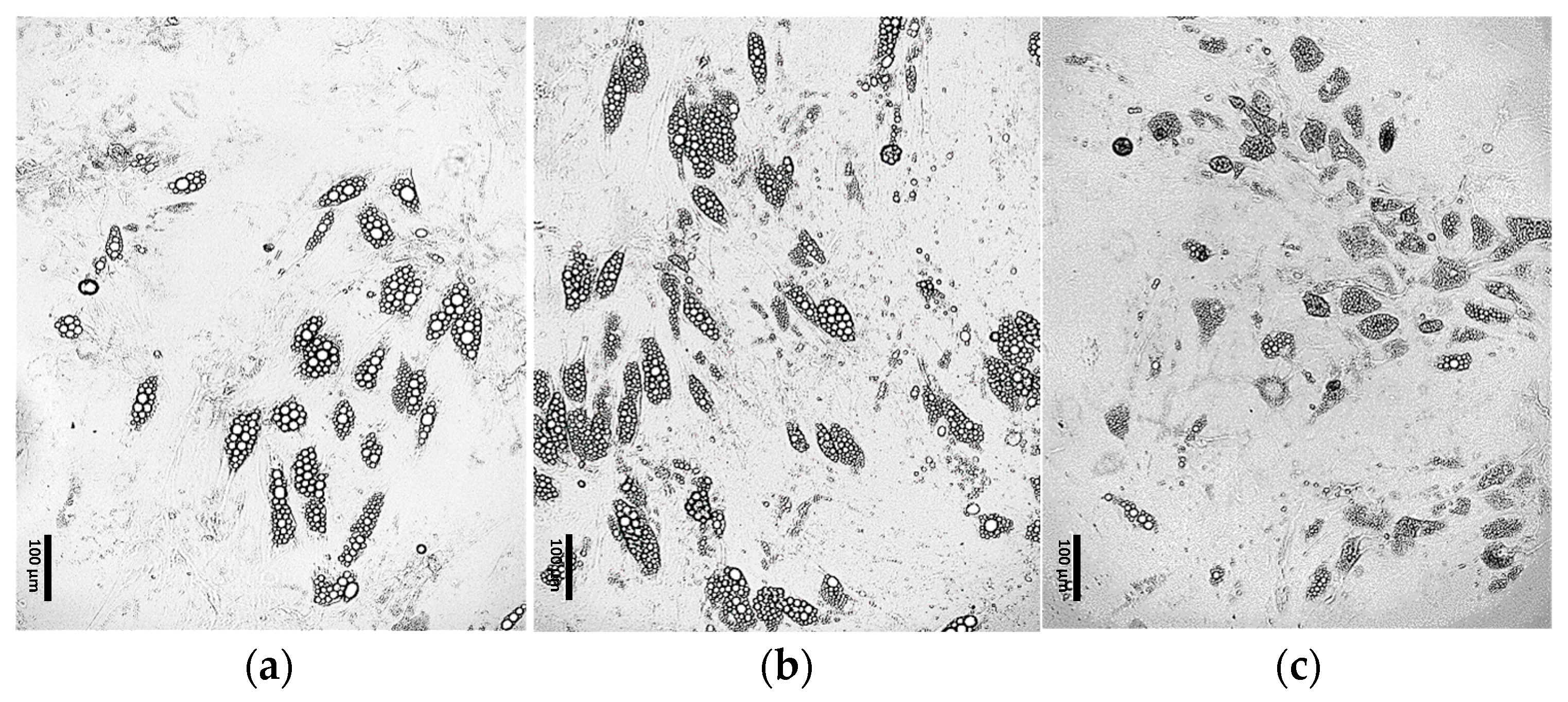
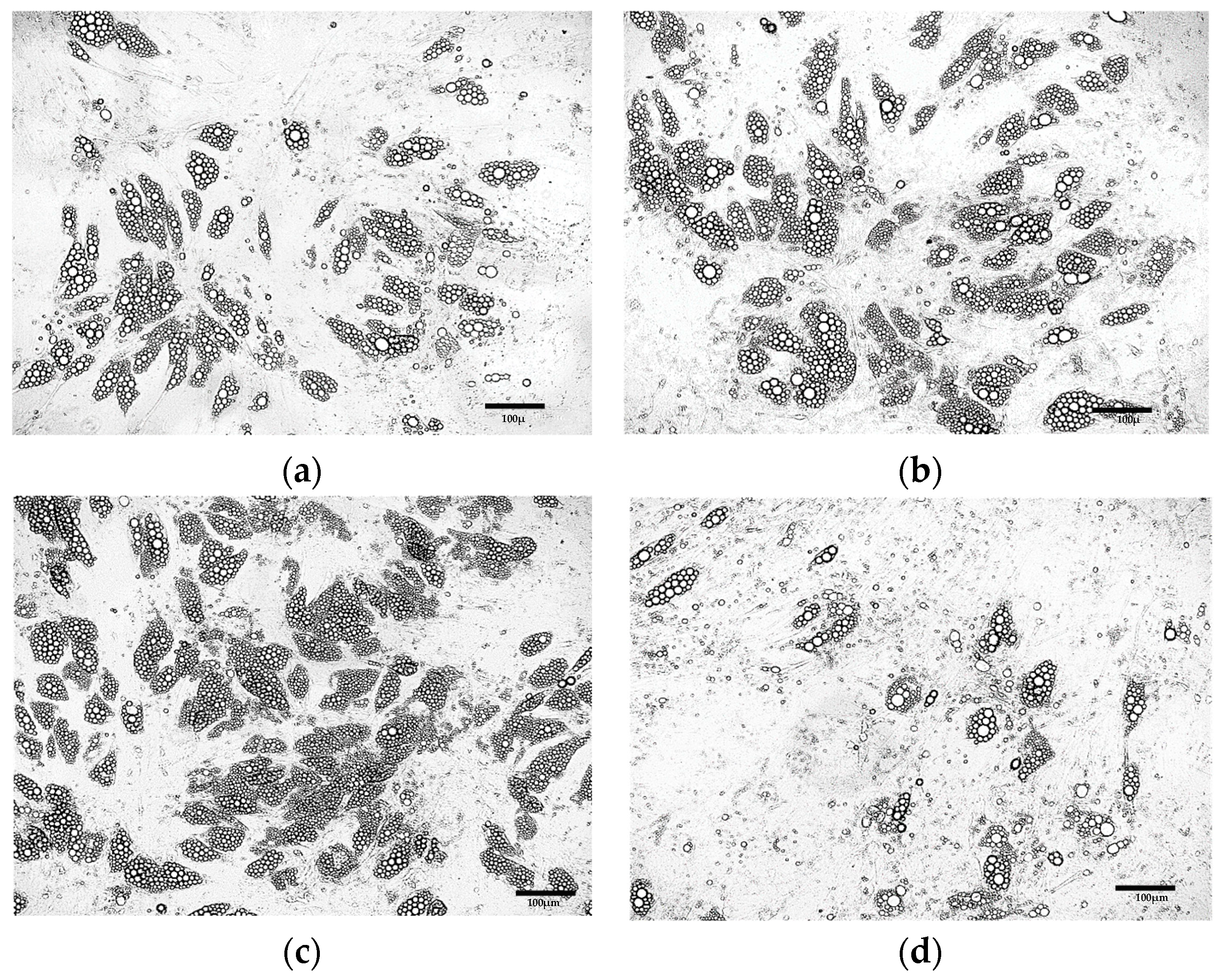
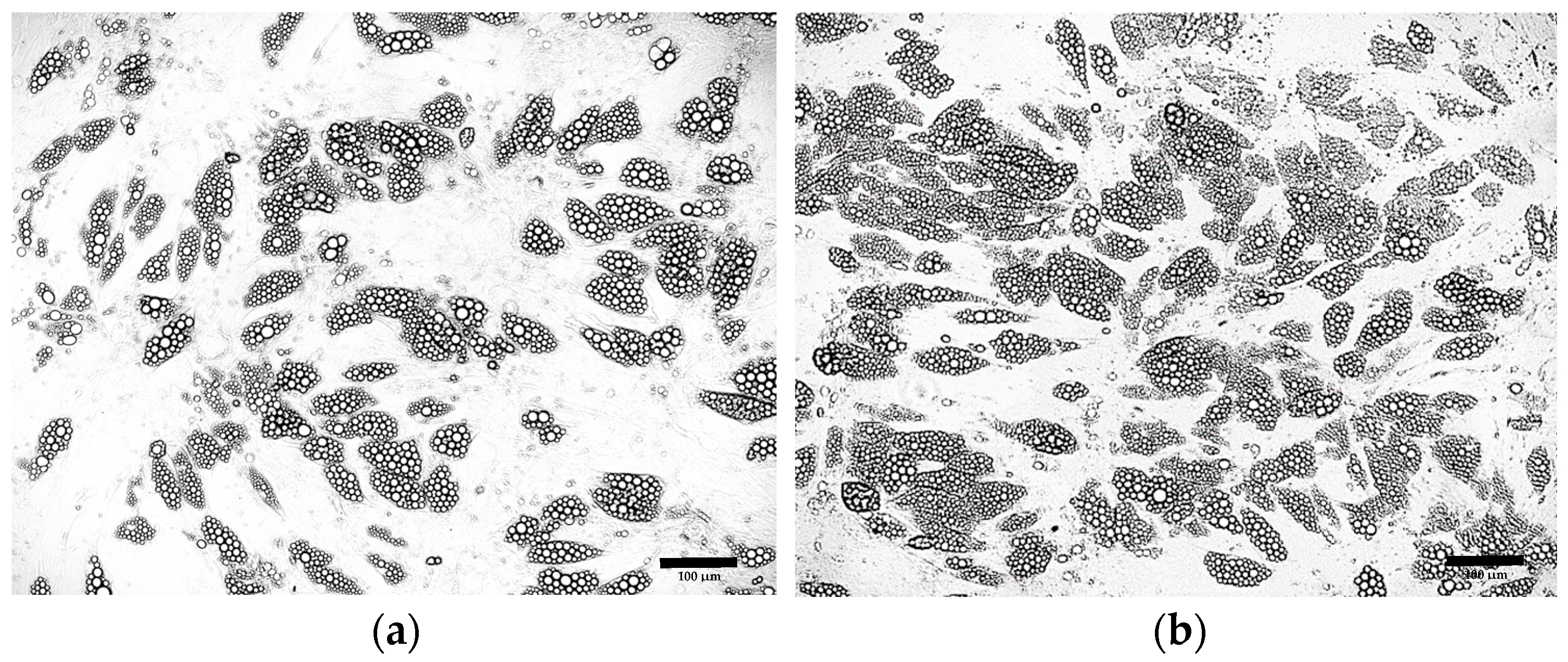
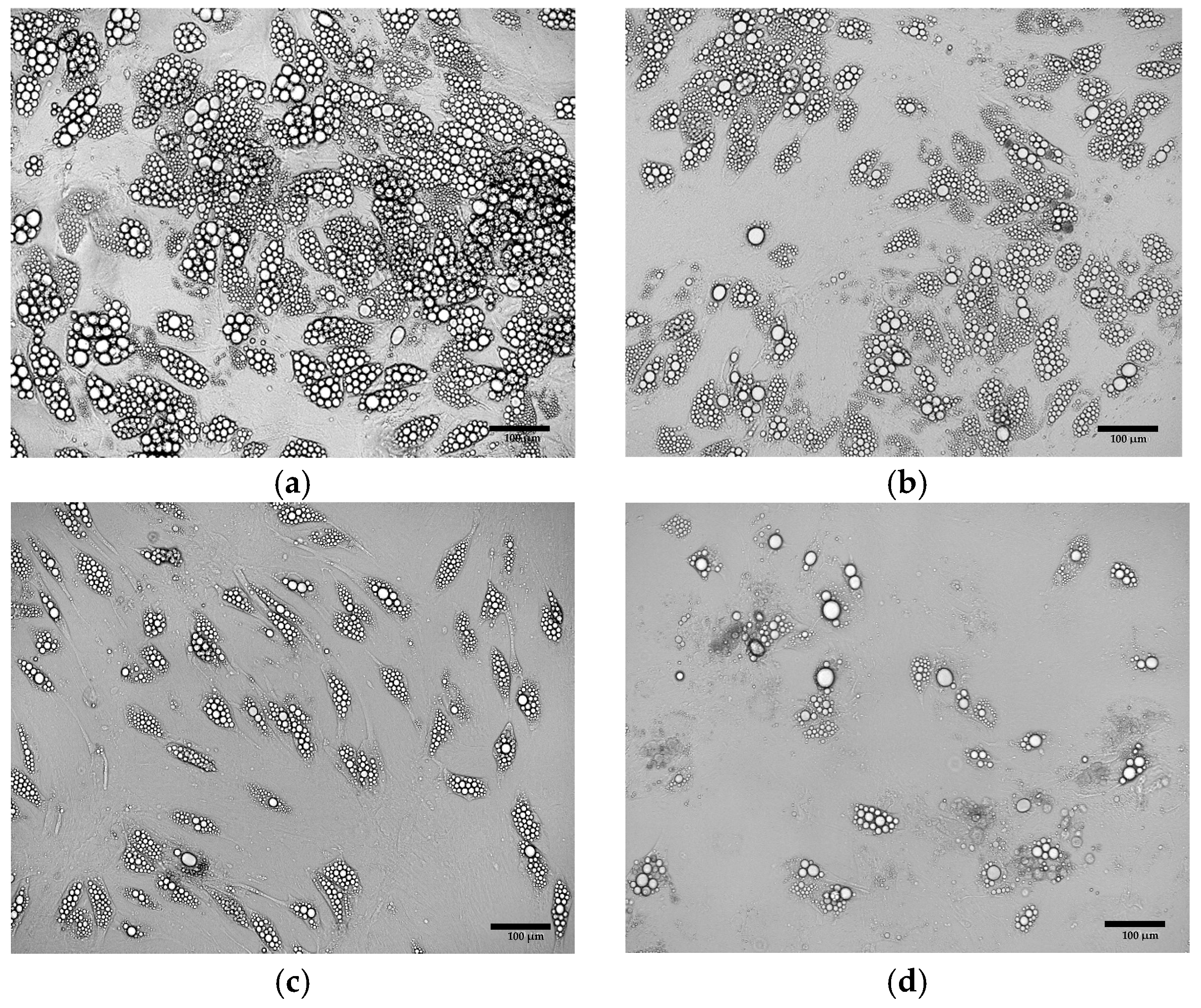
3.2. Comparative Morphological Assessment of ADSCs Differentiated into Beige Adipocytes Using Different Protocols
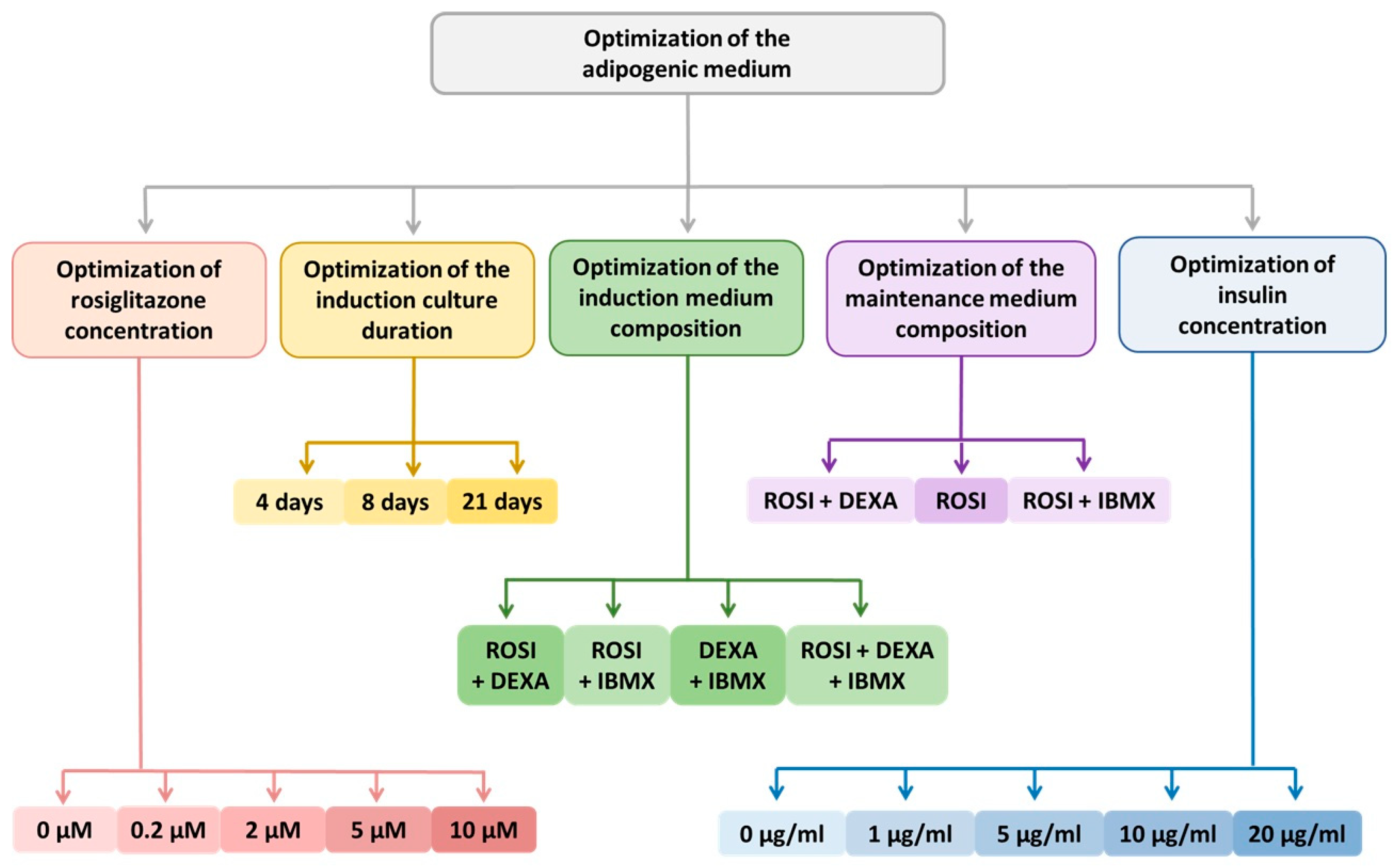
3.2.1. Optimization of the Differentiation Culture Protocol of ADSCs Towards Beige Adipocytes with Rosiglitazone
Optimization of the Induction Medium Composition
Optimization of the Induction Culture Duration
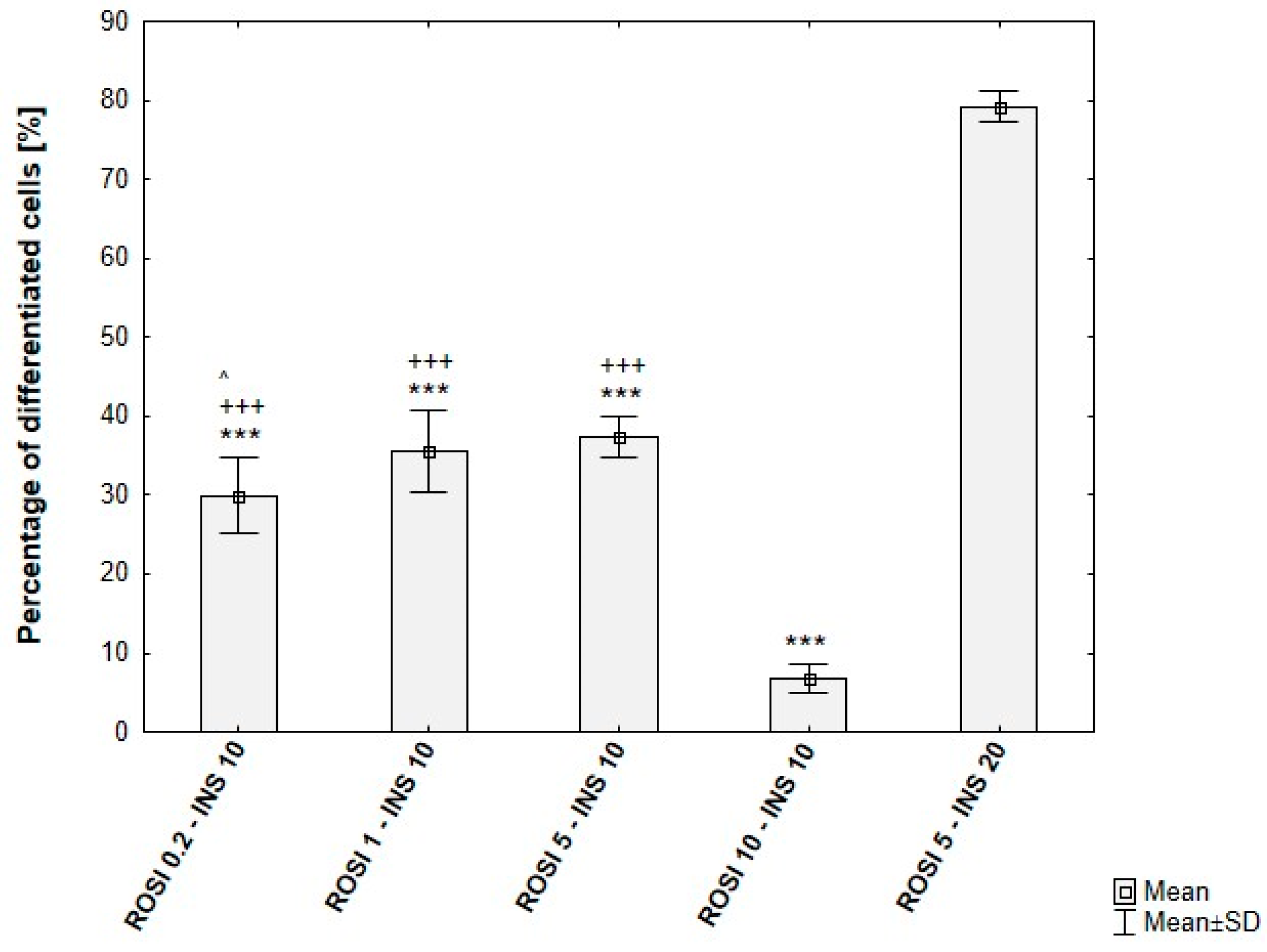
Optimization of Insulin Concentration
Optimization of the Maintenance Medium Composition
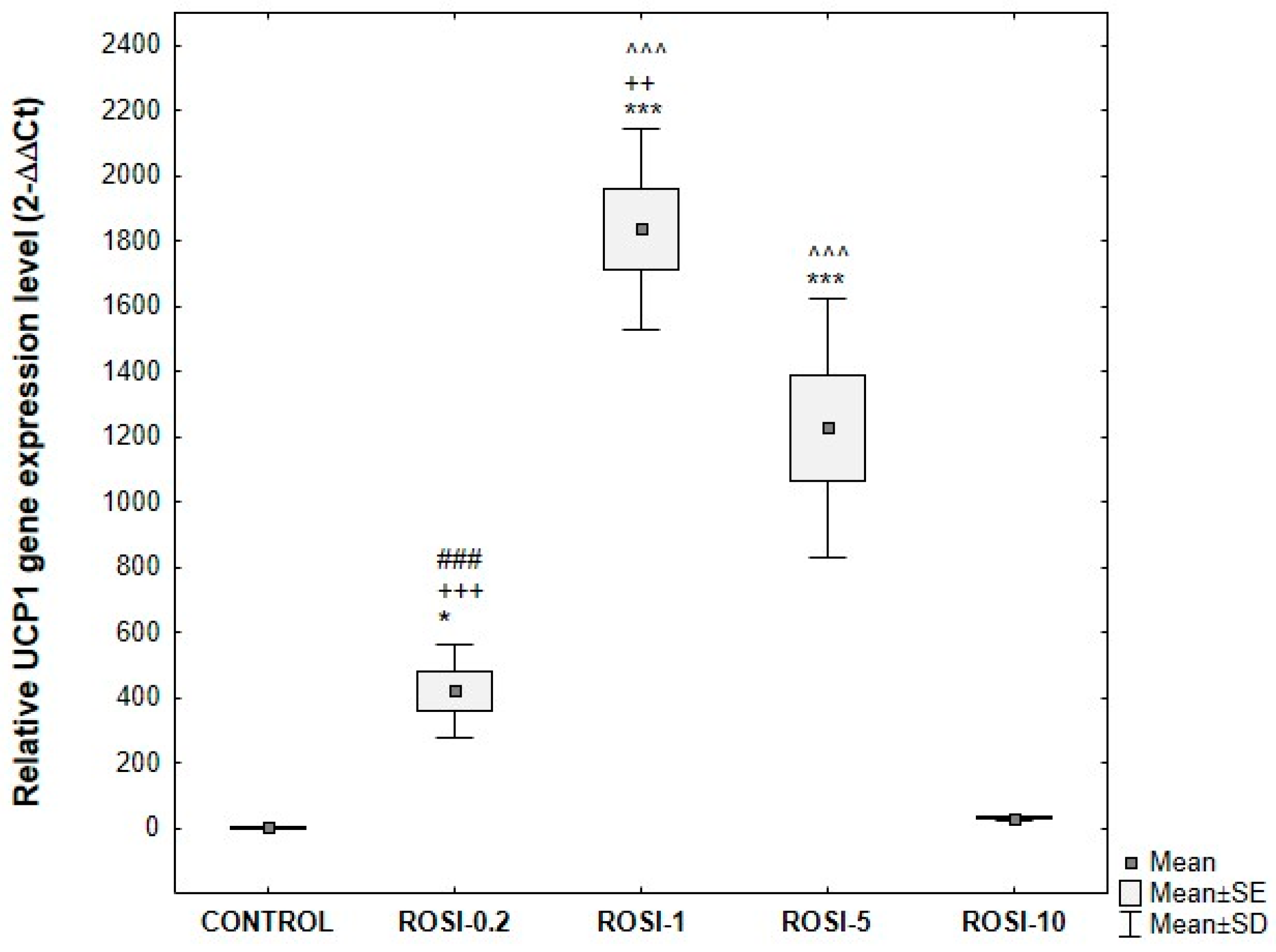
Optimization of Rosiglitazone Concentration
Further Optimization of the Differentiation Protocol Using 5 µM Rosiglitazone
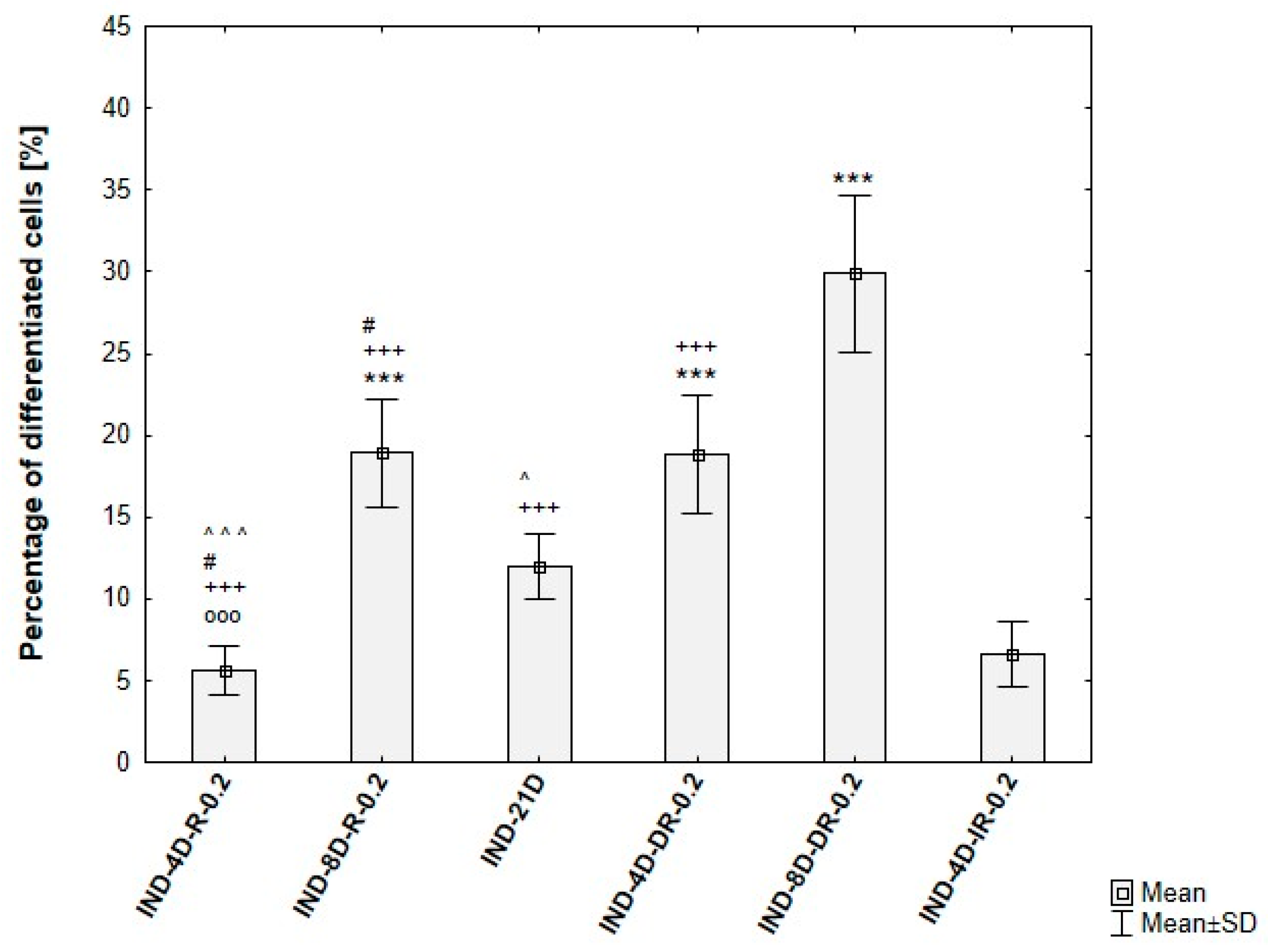
3.2.2. Optimization of the Differentiation Culture Protocol of ADSCs Towards Beige Adipocytes with Indomethacin
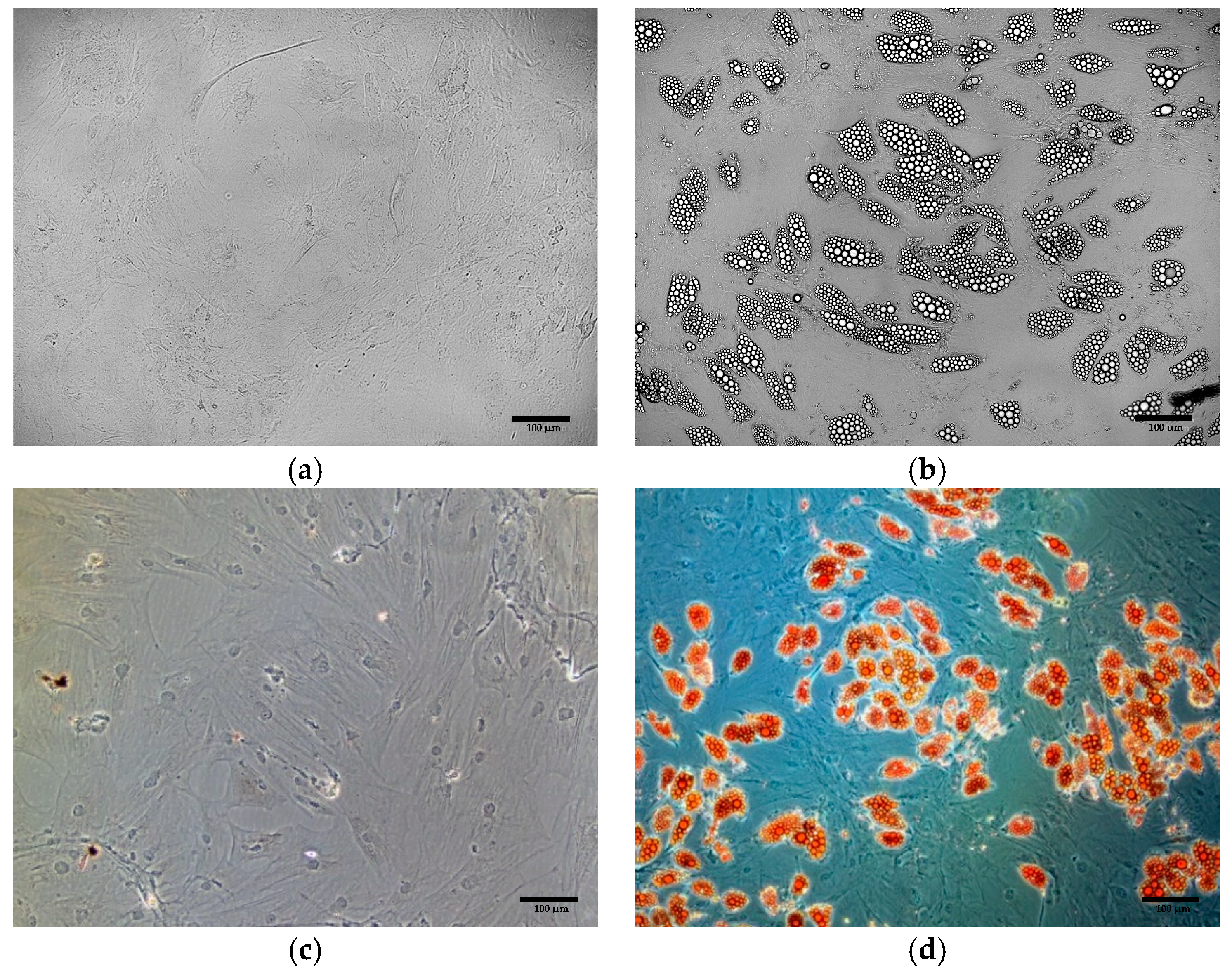
3.3. Oil Red O Staining of Cells Obtained from Differentiation of ADSCs
3.4. Quantitative Verification of Differentiation Efficiency
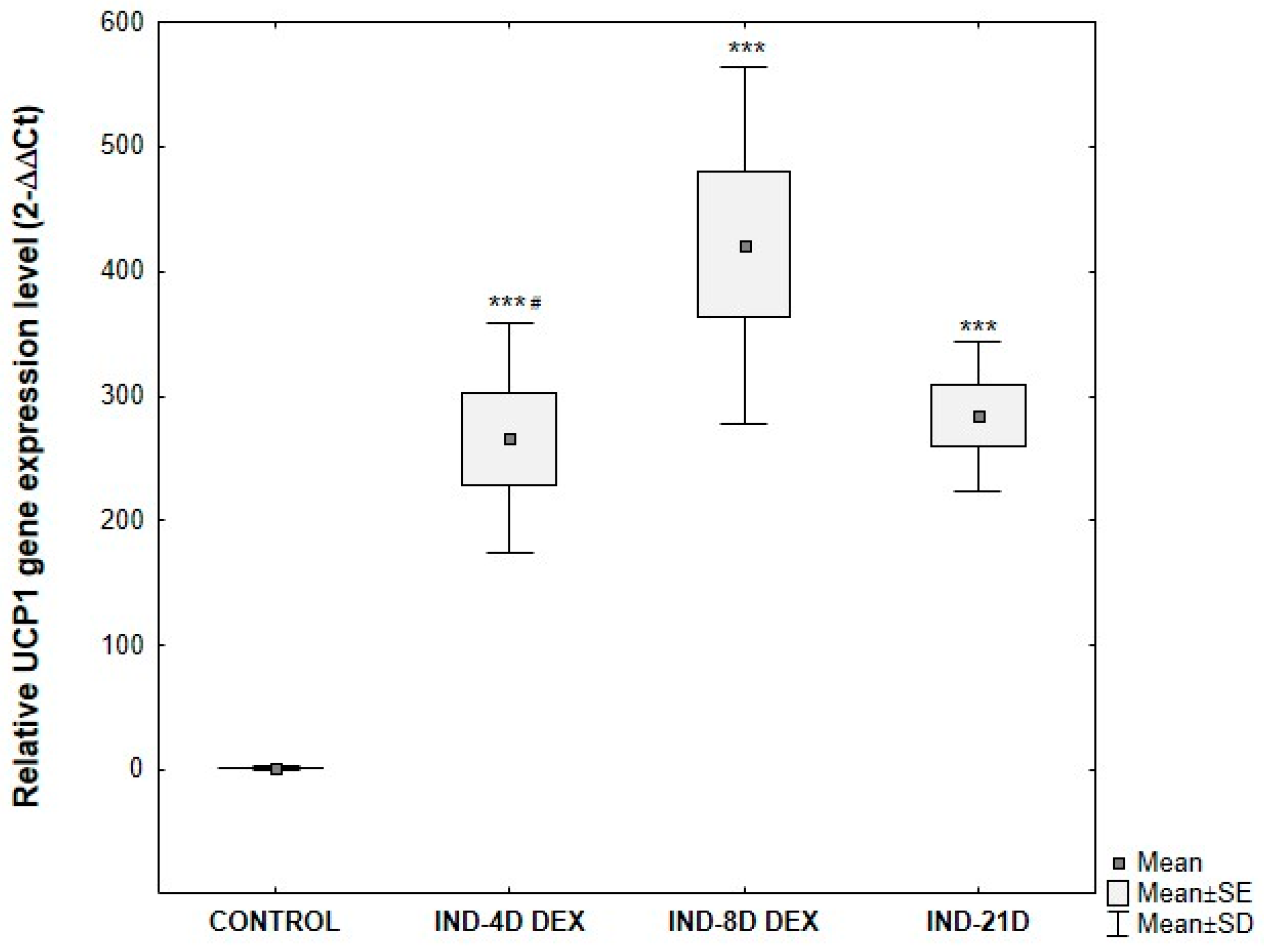
3.5. Analysis of UCP1 Gene Expression in ADSCs Undergoing Adipogenic Differentiation
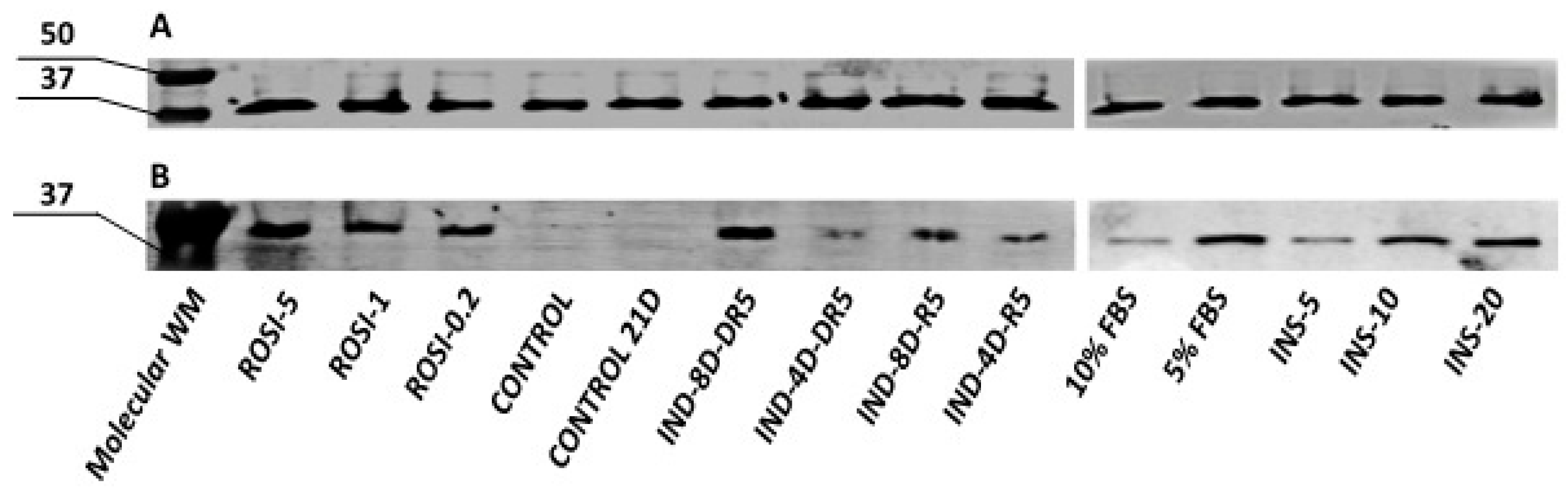
3.6. Semi-Quantitative Analysis of UCP1 Protein by Western Blot in the ADSCs Undergoing Adipogenic Differentiation
3.7. Validation of the Optimized Protocol’s Differentiation Efficiency in Primary ADSCs
3.7.1. Morphological Assessment of Primary ADSCs Differentiated into Beige Adipocytes
3.7.2. Quantitative Verification of Differentiation Efficiency of Primary ADSCs
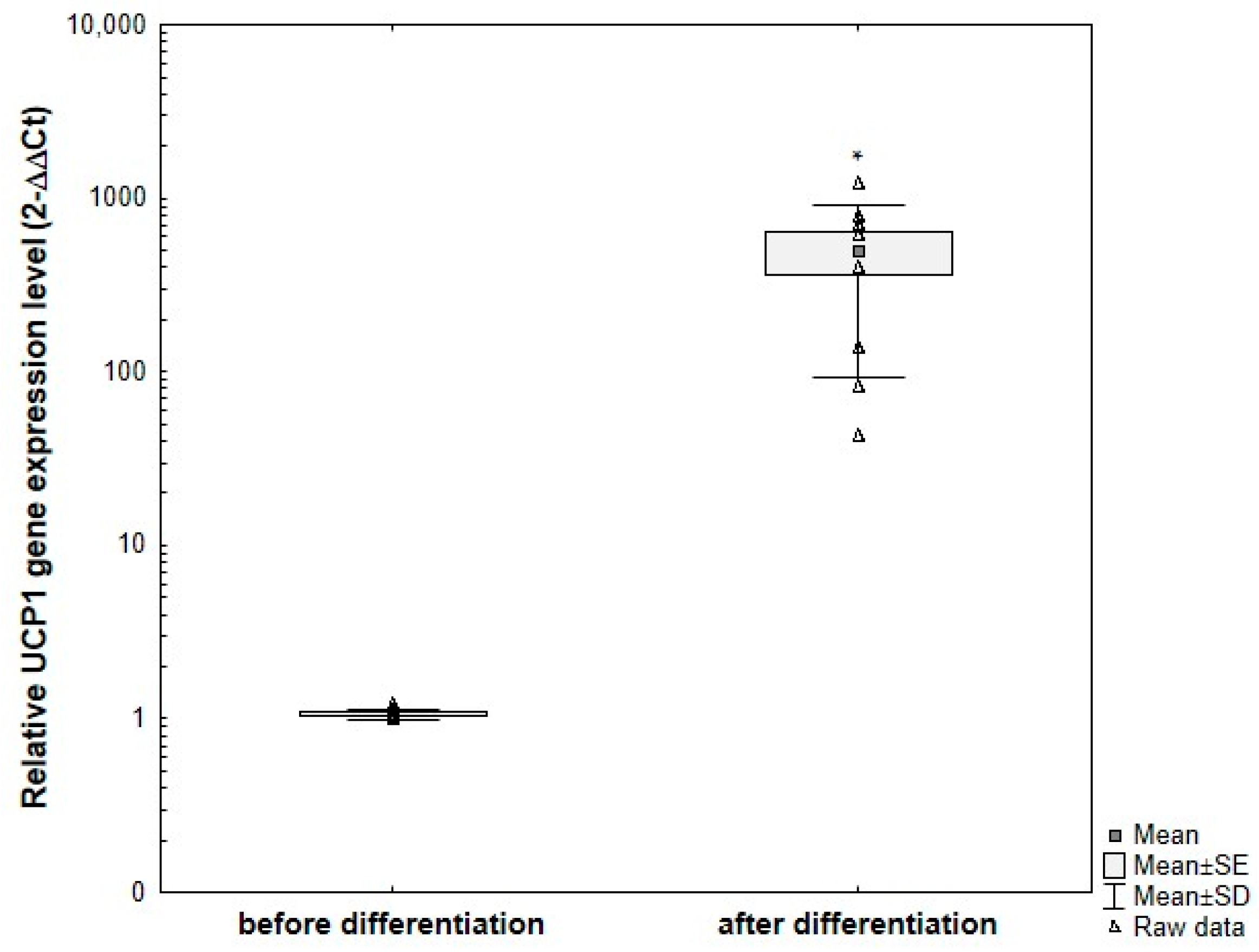
3.7.3. Analysis of UCP1 Gene Expression in Primary ADSCs Undergoing Adipogenic Differentiation
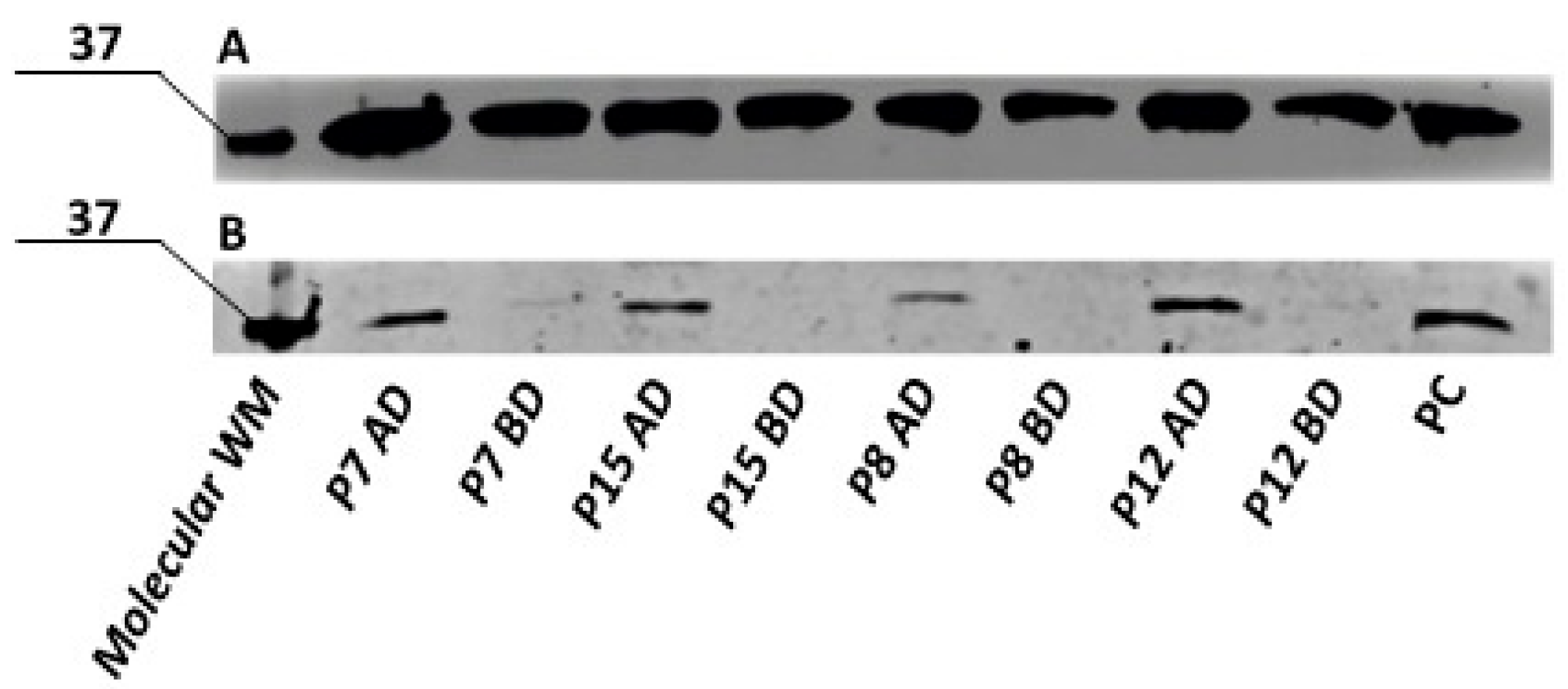
3.7.4. Semi-Quantitative Analysis of UCP1 Protein by Western Blot in the Primary ADSCs Undergoing Adipogenic Differentiation
3.8. Summary
- Rosiglitazone is a more effective inducer of beige adipocyte differentiation from ADSCs compared to indomethacin;
- The optimal differentiation conditions include 5 μM rosiglitazone and 20 μg/mL insulin in the culture medium;
- Extending the induction phase in induction medium to 8 days enhances the efficiency of ADSC differentiation into adipocytes; however, further extension of this period may lead to cytotoxic effects;
- Enrichment of the adipogenic medium with 1 μM dexamethasone throughout the entire differentiation period enhances adipogenic differentiation;
- ADSCs do not undergo spontaneous differentiation into beige adipocytes during long-term (three-week) post-confluent culture.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSCs | Adipose-Derived Stem Cells |
| BAT | Brown Adipose Tissue |
| BM-MSCs | Bone Marrow Mesenchymal Stem Cells |
| DEXA | Dexamethasone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| FBS | Fetal Bovine Serum |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| IBMX | 3-Isobutyl-1-Methylxanthine |
| Il | Interleukin |
| IND | Induction |
| INDO | Indomethacin |
| INS | Insulin |
| MSCs | Mesenchymal Stem Cells |
| PCOS | Polycystic Ovary Syndrome |
| ROSI | Rosiglitazone |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| TNF-A | Tumor Necrosis Factor-Alpha |
| UCP1 | Uncoupling Protein 1 |
| WAT | White Adipose Tissue |
References
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Greenfield, J.R.; Ho, K.K.Y.; Fulham, M.J. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E601–E606. [Google Scholar] [CrossRef]
- Ouellet, V.; Routhier-Labadie, A.; Bellemare, W.; Lakhal-Chaieb, L.; Turcotte, E.; Carpentier, A.C.; Richard, D. Outdoor Temperature, Age, Sex, Body Mass Index, and Diabetic Status Determine the Prevalence, Mass, and Glucose-Uptake Activity of 18F-FDG-Detected BAT in Humans. J. Clin. Endocrinol. Metab. 2011, 96, 192–199. [Google Scholar] [CrossRef]
- Gunawardana, S.C.; Piston, D.W. Reversal of Type 1 Diabetes in Mice by Brown Adipose Tissue Transplant. Diabetes 2012, 61, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Virtanen, K.A.; Richard, D.; Haman, F.; Turcotte, É.E. Brown Adipose Tissue Energy Metabolism in Humans. Front. Endocrinol. 2018, 9, 447. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, T.; Zhao, H.; Huang, Y.; Ye, R.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Zhou, H.; et al. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 2708–2713. [Google Scholar] [CrossRef]
- Magro, B.S.; Dias, D.P.M. Brown and beige adipose tissue: New therapeutic targets for metabolic disorders. Health Sci. Rev. 2024, 10, 100148. [Google Scholar] [CrossRef]
- Singh, A.M.; Zhang, L.; Avery, J.; Yin, A.; Du, Y.; Wang, H.; Li, Z.; Fu, H.; Yin, H.; Dalton, S. Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nat. Commun. 2020, 11, 2758. [Google Scholar] [CrossRef]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic Peroxisome Proliferator-activated Receptor γ (PPARγ) Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-containing Adipocytes Molecularly Distinct from Classic Brown Adipocytes. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, I.; Kim, M. Adipogenic Differentiation of Human Adipose Tissue–Derived Stem Cells Obtained from Cryopreserved Adipose Aspirates. Dermatol. Surg. 2010, 36, 1078–1083. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Péault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytom. A 2010, 77A, 22–30. [Google Scholar] [CrossRef]
- Park, J.-R.; Jung, J.-W.; Seo, M.-S.; Kang, S.-K.; Lee, Y.-S.; Kang, K.-S. DNER modulates adipogenesis of human adipose tissue-derived mesenchymal stem cells via regulation of cell proliferation. Cell Prolif. 2010, 43, 19–28. [Google Scholar] [CrossRef]
- Ghosh, S.; Dean, A.; Walter, M.; Bao, Y.; Hu, Y.; Ruan, J.; Li, R. Cell density-dependent transcriptional activation of endocrine-related genes in human adipose tissue-derived stem cells. Exp. Cell Res. 2010, 316, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, I.; Volti, G.L.; Galvano, F.; Tettamanti, G.; Pluchinotta, F.R.; Bergante, S.; Vanella, L. Diabetic human adipose tissue-derived mesenchymal stem cells fail to differentiate in functional adipocytes. Exp. Biol. Med. 2017, 242, 1079–1085. [Google Scholar] [CrossRef]
- Lauvrud, A.T.; Kelk, P.; Wiberg, M.; Kingham, P.J. Characterization of human adipose tissue-derived stem cells with enhanced angiogenic and adipogenic properties: Angiogenesis and adipogenesis of CD146+ stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 2490–2502. [Google Scholar] [CrossRef]
- Yu, G.; Wu, X.; Dietrich, M.A.; Polk, P.; Scott, L.K.; Ptitsyn, A.A.; Gimble, J.M. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy 2010, 12, 538–546. [Google Scholar] [CrossRef]
- Pisani, D.F.; Djedaini, M.; Beranger, G.E.; Elabd, C.; Scheideler, M.; Ailhaud, G.; Amri, E.-Z. Differentiation of Human Adipose-Derived Stem Cells into “Brite” (Brown-in-White) Adipocytes. Front. Endocrinol. 2011, 29, 87. [Google Scholar] [CrossRef]
- Elabd, C.; Chiellini, C.; Carmona, M.; Galitzky, J.; Cochet, O.; Petersen, R.; Pénicaud, L.; Kristiansen, K.; Bouloumié, A.; Casteilla, L.; et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells 2009, 27, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Mohsen-Kanson, T.; Hafner, A.-L.; Wdziekonski, B.; Takashima, Y.; Villageois, P.; Carrière, A.; Svensson, M.; Bagnis, C.; Chignon-Sicard, B.; Svensson, P.-A.; et al. Differentiation of Human Induced Pluripotent Stem Cells into Brown and White Adipocytes: Role of Pax3. Stem Cells 2014, 32, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwak, H.J.; Cha, J.Y.; Jeong, Y.S.; Rhee, S.D.; Cheon, H.G. The Role of Prolyl Hydroxylase Domain Protein (PHD) during Rosiglitazone-induced Adipocyte Differentiation. J. Biol. Chem. 2014, 289, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Diekman, B.O.; Jain, D.; Guilak, F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: The effects of free fatty acids. Int. J. Obes. 2013, 37, 1079–1087. [Google Scholar] [CrossRef]
- Carey, A.L.; Vorlander, C.; Reddy-Luthmoodoo, M.; Natoli, A.K.; Formosa, M.F.; Bertovic, D.A.; Anderson, M.J.; Duffy, S.J.; Kingwell, B.A. Reduced UCP-1 Content in In Vitro Differentiated Beige/Brite Adipocytes Derived from Preadipocytes of Human Subcutaneous White Adipose Tissues in Obesity. PLoS ONE 2014, 9, e91997. [Google Scholar] [CrossRef]
- Liisberg Aune, U.; Ruiz, L.; Kajimura, S. Isolation and Differentiation of Stromal Vascular Cells to Beige/Brite Cells. J. Vis. Exp. 2013, 28, 50191. [Google Scholar] [CrossRef]
- Yang, D.; Li, N.; Zhang, G. Spontaneous adipogenic differentiation potential of adipose-derived stem cells decreased with increasing cell passages. Mol. Med. Rep. 2018, 17, 6109–6115. [Google Scholar] [CrossRef]
- Wystrychowski, G.; Simka-Lampa, K.; Witkowska, A.; Sobecko, E.; Skubis-Sikora, A.; Sikora, B.; Wojtyna, E.; Golda, A.; Gwizdek, K.; Wróbel, M.; et al. Selected microRNA Expression and Protein Regulator Secretion by Adipose Tissue-Derived Mesenchymal Stem Cells and Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 6644. [Google Scholar] [CrossRef]
- Dudakovic, A.; Camilleri, E.; Riester, S.M.; Lewallen, E.A.; Kvasha, S.; Chen, X.; Radel, D.J.; Anderson, J.M.; Nair, A.A.; Evans, J.M.; et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J. Cell. Biochem. 2014, 115, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, J.; Metcalfe, A.D.; Martin, Y.H. The effect of medium selection on adipose-derived stem cell expansion and differentiation: Implications for application in regenerative medicine. Cytotechnology 2016, 68, 957–967. [Google Scholar] [CrossRef]
- Contador, D.; Ezquer, F.; Espinosa, M.; Arango-Rodriguez, M.; Puebla, C.; Sobrevia, L.; Conget, P. Featured Article: Dexamethasone and rosiglitazone are sufficient and necessary for producing functional adipocytes from mesenchymal stem cells. Exp. Biol. Med. 2015, 240, 1235–1246. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.H.; Heck, B.E.; Shaffer, M.; Yoo, K.H.; Hur, J. PPAR-δ agonist affects adipo-chondrogenic differentiation of human mesenchymal stem cells through the expression of PPAR-γ. Regen. Ther. 2020, 15, 103–111. [Google Scholar] [CrossRef]
- Nuttall, M.E.; Patton, A.J.; Olivera, D.L.; Nadeau, D.P.; Gowen, M. Human Trabecular Bone Cells Are Able to Express Both Osteoblastic and Adipocytic Phenotype: Implications for Osteopenic Disorders. J. Bone Miner. Res. 1998, 13, 371–382. [Google Scholar] [CrossRef]
- Pantoja, C.; Huff, J.T.; Yamamoto, K.R. Glucocorticoid Signaling Defines a Novel Commitment State during Adipogenesis In Vitro. Mol. Biol. Cell 2008, 19, 4032–4041. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Wu, Y.; Fried, S.K. A Modified Protocol to Maximize Differentiation of Human Preadipocytes and Improve Metabolic Phenotypes. Obesity 2012, 20, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Oshina, H.; Sotome, S.; Yoshii, T.; Torigoe, I.; Sugata, Y.; Maehara, H.; Marukawa, E.; Omura, K.; Shinomiya, K. Effects of continuous dexamethasone treatment on differentiation capabilities of bone marrow-derived mesenchymal cells. Bone 2007, 41, 575–583. [Google Scholar] [CrossRef]
- Perl, K.; Ushakov, K.; Pozniak, Y.; Yizhar-Barnea, O.; Bhonker, Y.; Shivatzki, S.; Geiger, T.; Avraham, K.B.; Shamir, R. Reduced changes in protein compared to mRNA levels across non-proliferating tissues. BMC Genom. 2017, 18, 305. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Whyte, N.; Niyibizi, C. Differentiating multipotent mesenchymal stromal cells generate factors that exert paracrine activities on exogenous MSCs: Implications for paracrine activities in bone regeneration. Biochem. Biophys. Res. Commun. 2012, 426, 475–479. [Google Scholar] [CrossRef]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef]
- Maqsood, M.; Kang, M.; Wu, X.; Chen, J.; Teng, L.; Qiu, L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020, 256, 118002. [Google Scholar] [CrossRef] [PubMed]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2020, 41, 53–65. [Google Scholar] [CrossRef] [PubMed]

| Age | BMI | Glu | Ins | HbA1c | HOMA-IR | Cr | TC | HDL | LDL | TG | CRP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 43 | 27.3 | 82.7 | 7.1 | 5.1 | 1.4 | 0.8 | 201.2 | 65.7 | 115.6 | 98.5 | 2.34 |
| SD | 12 | 4.4 | 7.6 | 4.2 | 0.2 | 0.8 | 0.1 | 39.6 | 13.5 | 29.0 | 70.0 | 3.11 |
| Min | 28 | 21.5 | 65 | 2.7 | 4.8 | 0.5 | 0.7 | 166.0 | 48.0 | 69.0 | 49.0 | 0.02 |
| Max | 63 | 36.0 | 88.3 | 15.4 | 5.4 | 3.0 | 1.0 | 267.7 | 87.0 | 150.9 | 252.5 | 8.1 |
| Induction Medium | Maintenance Medium | |||
|---|---|---|---|---|
| Composition | Duration | Composition | Duration | |
| Baseline Protocol | 0.2–10 μM rosiglitazone 1 μM dexamethasone 500 μM IBMX 10 μg/mL insulin | 4 days | 0.2–10 μM rosiglitazone 10 μg/mL insulin | 12–17 days |
| Optimized Protocol | 5 μM rosiglitazone 1 μM dexamethasone 500 μM IBMX 20 μg/mL insulin | 8 days | 5 μM rosiglitazone 1 μM dexamethasone 20 μg/mL insulin | 13 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Simka-Lampa, K.; Kosowska, A.; Garczorz, W.; Kimsa-Furdzik, M.; Wystrychowski, G.; Kruszniewska-Rajs, C.; Muc-Wierzgoń, M.; Francuz, T. Toward Efficient Beige Adipogenesis: Protocol Optimization Using Adipose-Derived Stem Cells. Cells 2026, 15, 54. https://doi.org/10.3390/cells15010054
Simka-Lampa K, Kosowska A, Garczorz W, Kimsa-Furdzik M, Wystrychowski G, Kruszniewska-Rajs C, Muc-Wierzgoń M, Francuz T. Toward Efficient Beige Adipogenesis: Protocol Optimization Using Adipose-Derived Stem Cells. Cells. 2026; 15(1):54. https://doi.org/10.3390/cells15010054
Chicago/Turabian StyleSimka-Lampa, Klaudia, Agnieszka Kosowska, Wojciech Garczorz, Małgorzata Kimsa-Furdzik, Grzegorz Wystrychowski, Celina Kruszniewska-Rajs, Małgorzata Muc-Wierzgoń, and Tomasz Francuz. 2026. "Toward Efficient Beige Adipogenesis: Protocol Optimization Using Adipose-Derived Stem Cells" Cells 15, no. 1: 54. https://doi.org/10.3390/cells15010054
APA StyleSimka-Lampa, K., Kosowska, A., Garczorz, W., Kimsa-Furdzik, M., Wystrychowski, G., Kruszniewska-Rajs, C., Muc-Wierzgoń, M., & Francuz, T. (2026). Toward Efficient Beige Adipogenesis: Protocol Optimization Using Adipose-Derived Stem Cells. Cells, 15(1), 54. https://doi.org/10.3390/cells15010054








