Hypoxia Affects Stem Cell Fate in Patient-Derived Ileum Enteroids in a HIF-1α-Dependent Manner
Highlights
- Low levels of oxygen (i.e., hypoxia) decrease enteroid growth and stem cell proliferation.
- HIF-1α stabilization under normoxic conditions recapitulates the hypoxia-induced loss of stemness.
- Hypoxia, as physiologically present in the intestinal epithelium, regulates intestinal stem cell fate through HIF-1α stabilization.
- Hypoxia-induced HIF-1α stabilization impairs stem cell self-renewal capacity, likely through inhibition of mitochondrial oxidative phosphorylation, which is crucial for intestinal stem cell maintenance.
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Ileum-Derived Enteroid Culture Conditions and Biopsy Collection
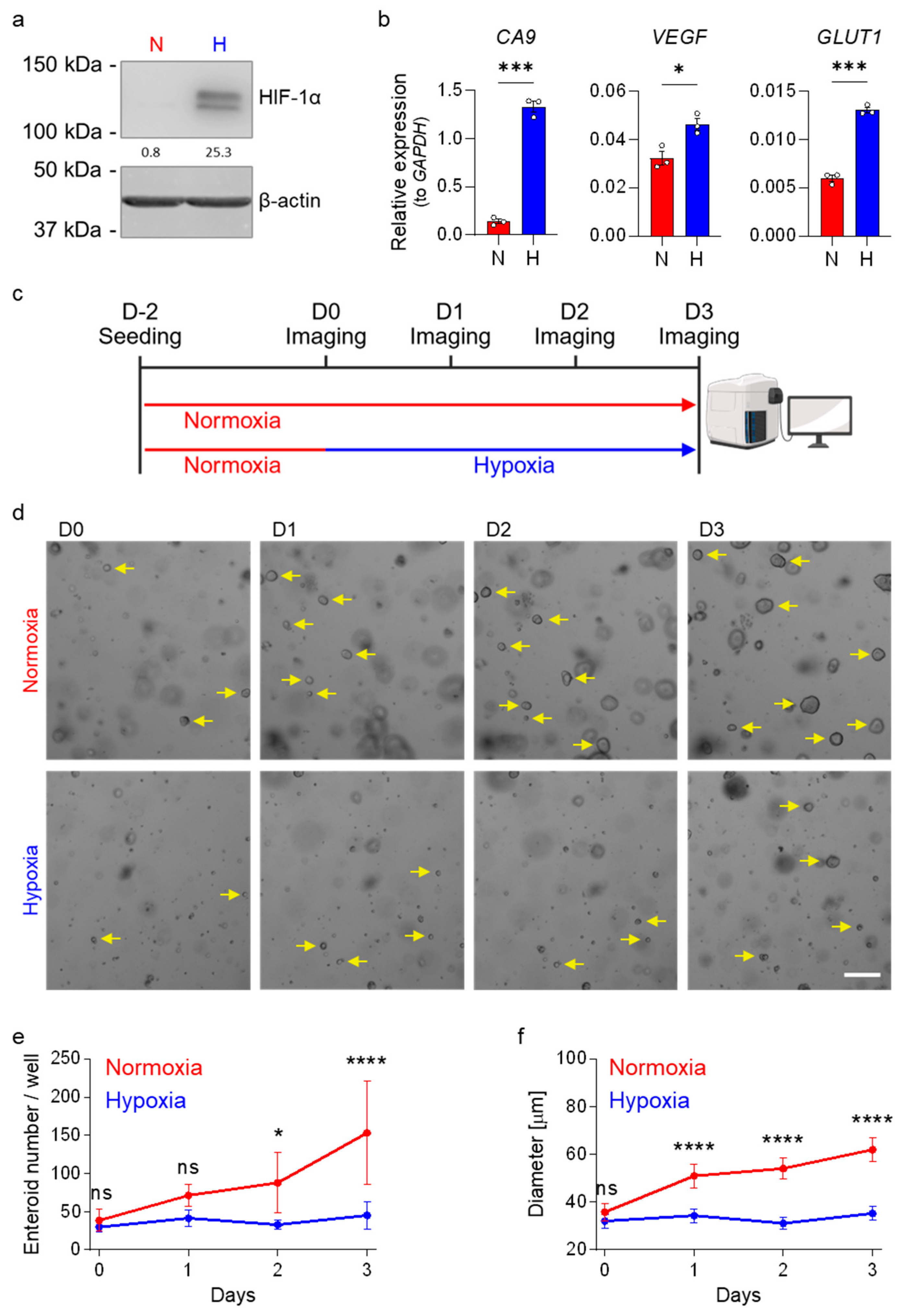
2.2. Assessment of Enteroid Growth in Normoxia or Hypoxia
2.3. Inhibitor Treatments
2.4. Microscopy
2.5. Protein Quantification
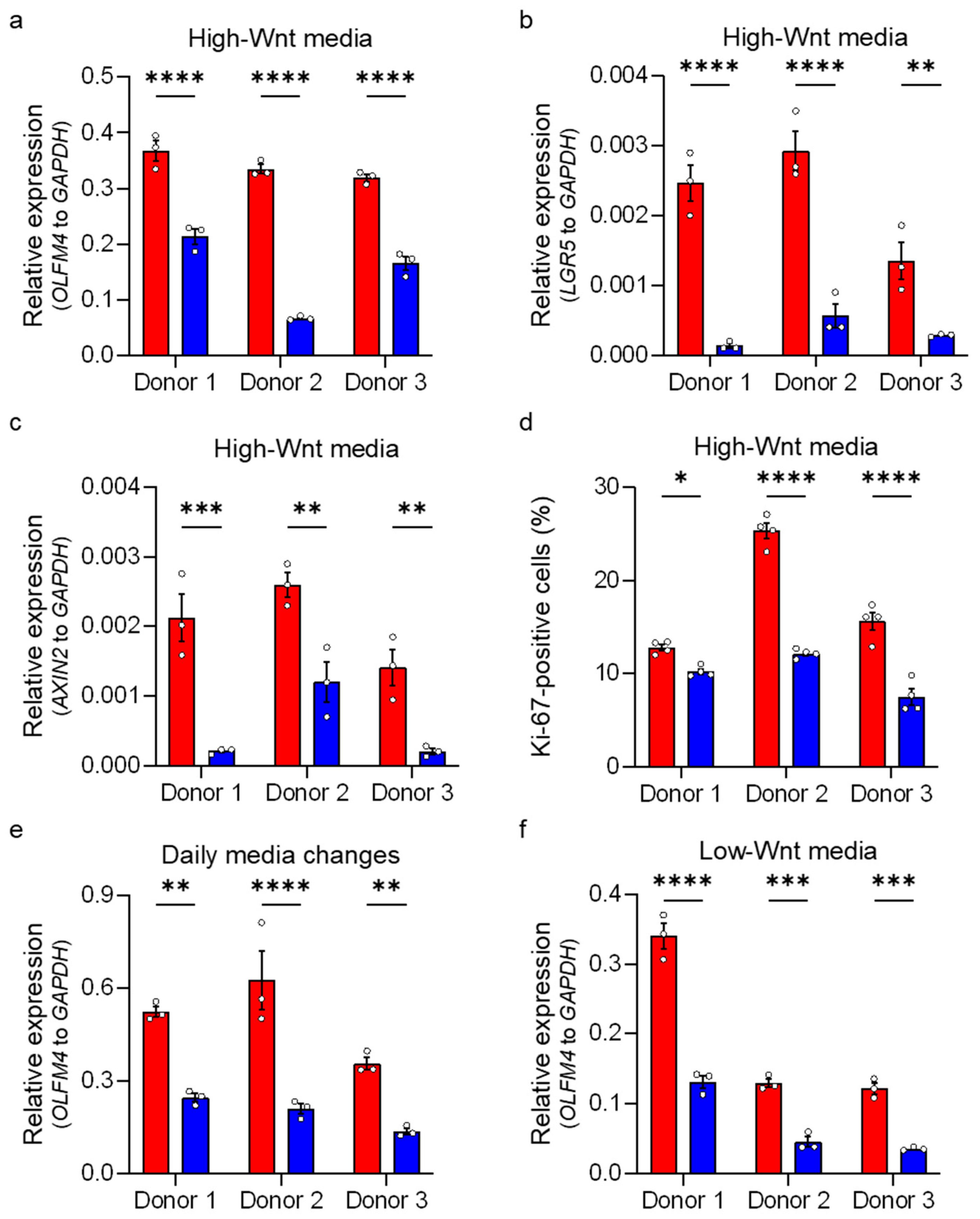
2.6. mRNA Quantification
2.7. Flow Cytometry
2.8. Cytotoxicity Assay
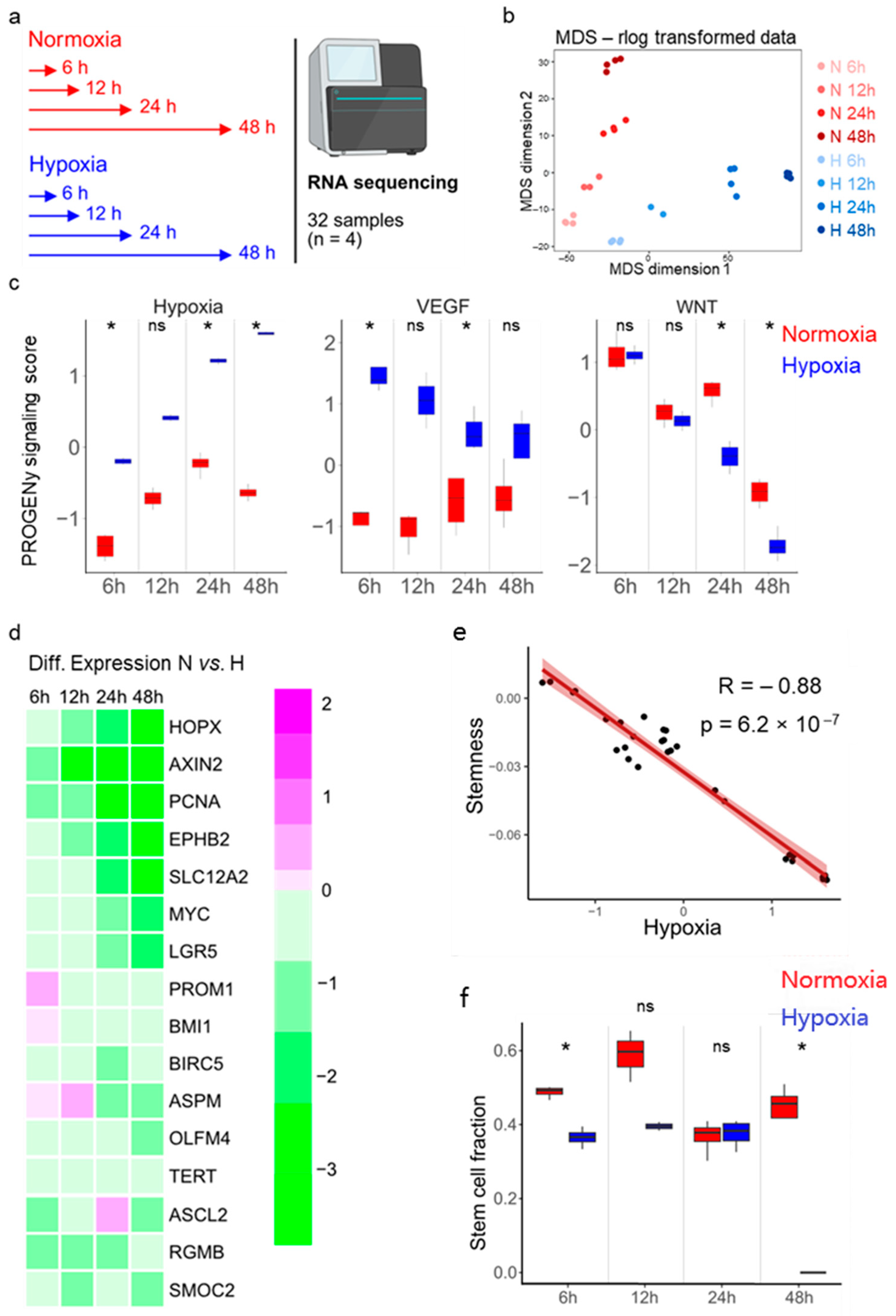
2.9. Bulk RNA Sequencing
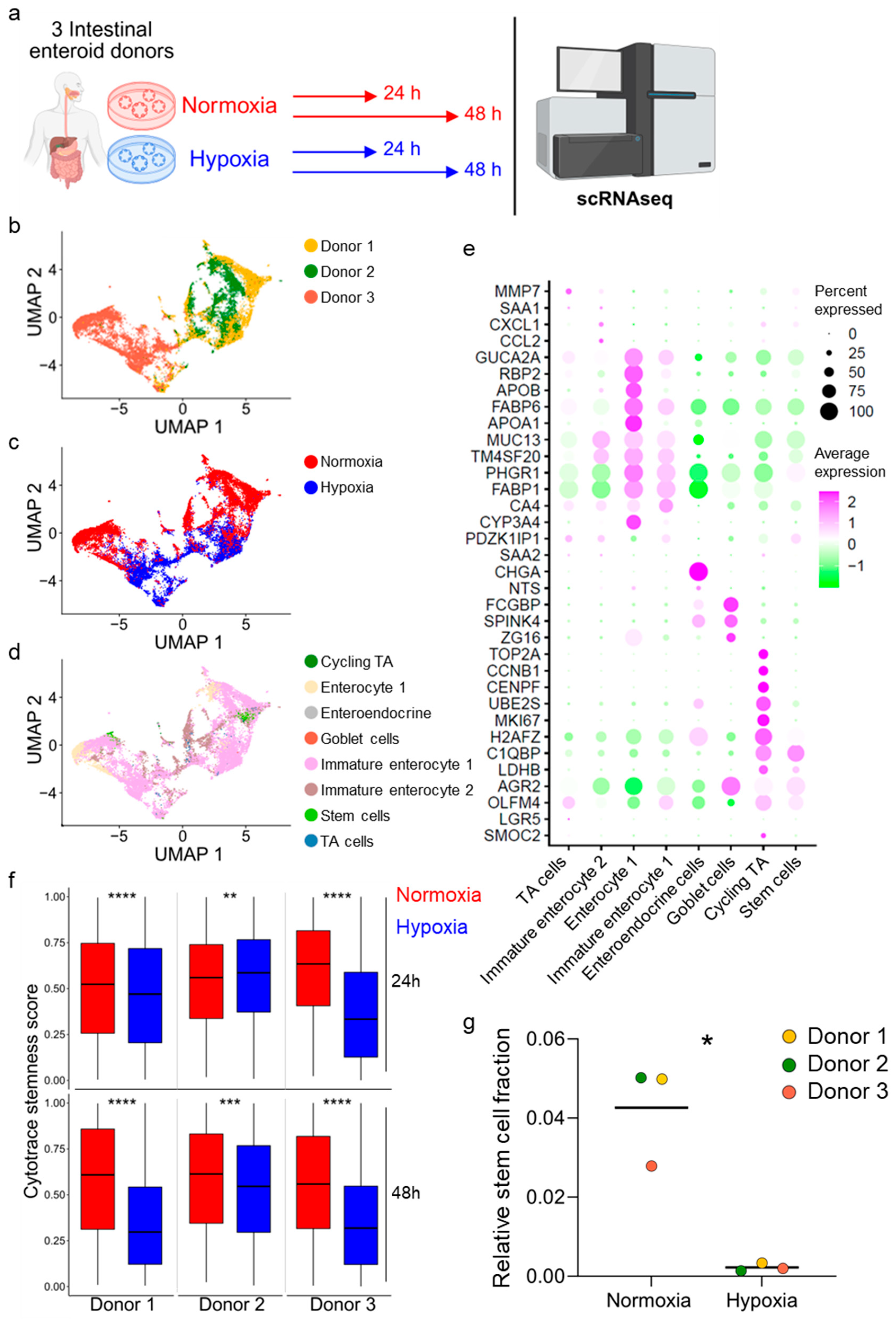
2.10. Single-Cell RNA Sequencing
2.11. Statistical Analysis, Data Visualization, and Illustrations
3. Results
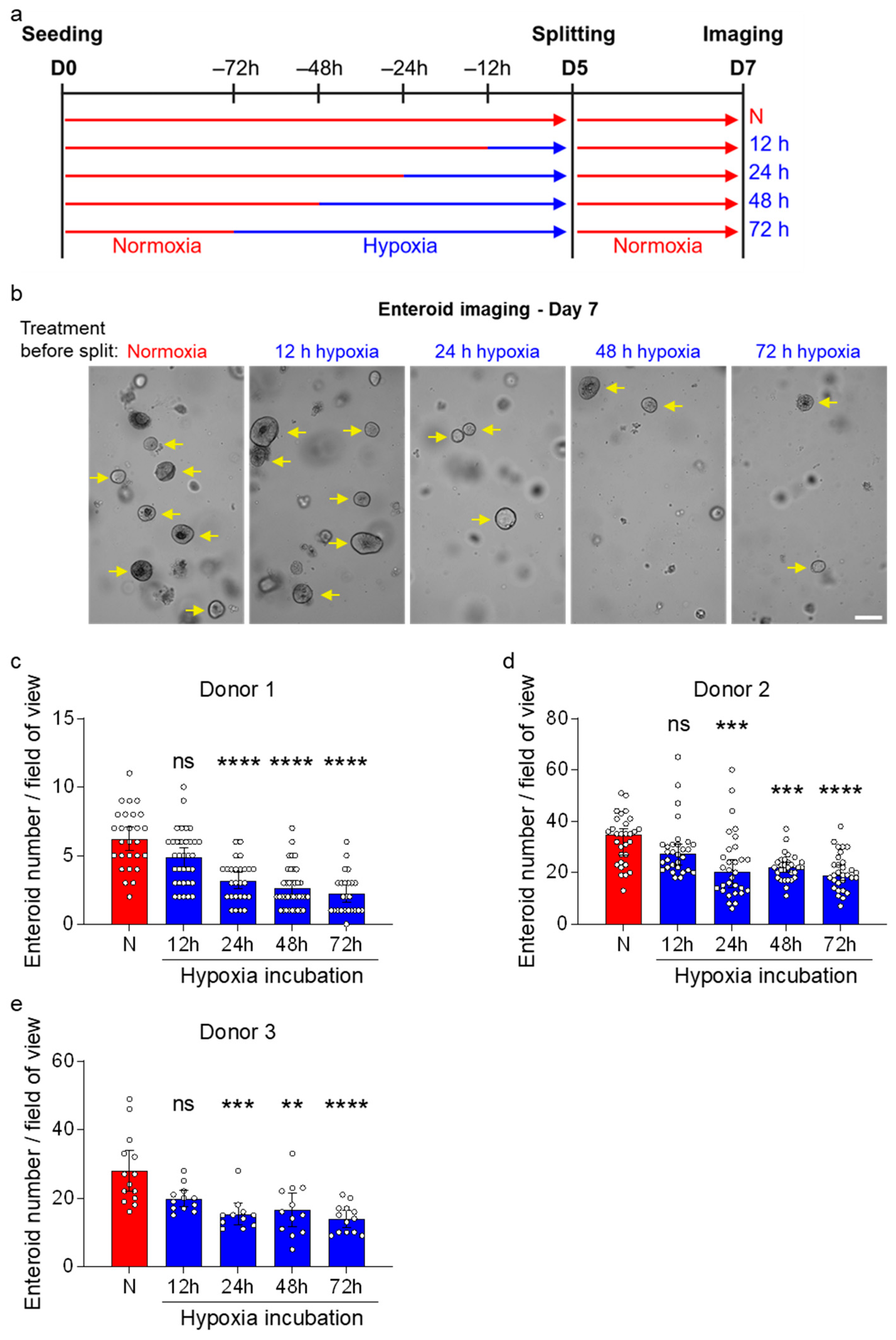
3.1. Hypoxia Impairs the Growth of Human Ileum-Derived Enteroids
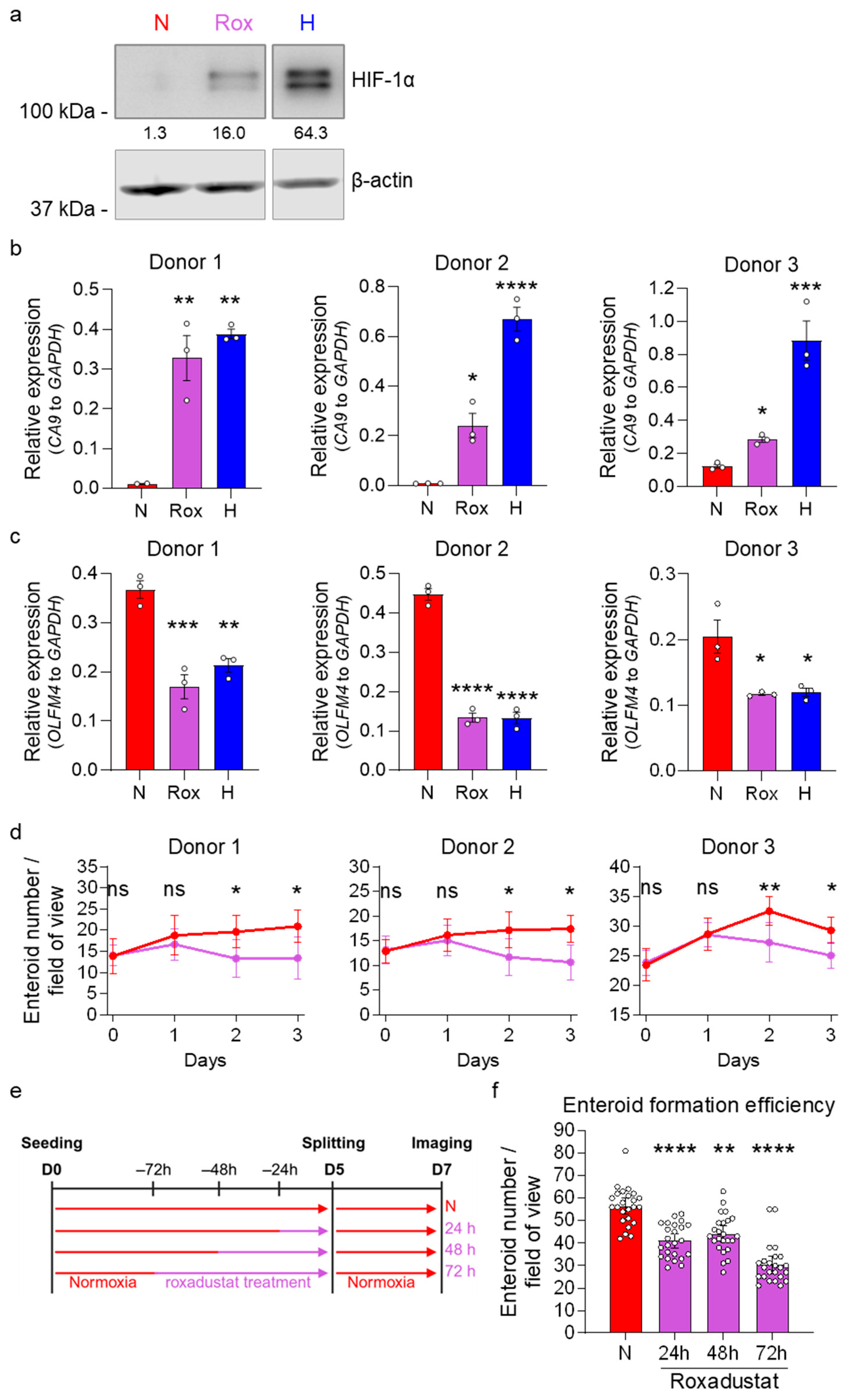
3.2. Stemness and Hypoxia Are Negatively Correlated in Ileum-Derived Enteroids
3.3. Hypoxia Induces Loss of Stem Cells in Human Ileum-Derived Enteroids
3.4. Enteroids Formation Efficiency of Human Ileum-Derived Enteroids Is Reduced with Longer Incubation in Hypoxia
3.5. HIF-1α Activity Reduces Stem Cell Activity in Normoxia
4. Discussion
4.1. Segment-Specific Response of ISCs to Hypoxia
4.2. Physiological Oxygen Gradient in Crypt Villus Structures and ISC Fate
4.3. Hypoxia, Metabolic Reprogramming, and ISCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMPs | Bone morphogenetic proteins |
| CA9 | Carbonic anhydrase 9 |
| CoCl2 | Cobalt chloride |
| GLUT1 | Glucose transporter 1 |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| HPRT1 | Hypoxanthine phosphoribosyltransferase 1 |
| IBD | Inflammatory bowel disease |
| ISCs | Intestinal stem cells |
| LDH | Lactate dehydrogenase |
| LGR5 | Leucine-rich repeat-containing G-protein coupled receptor 5 |
| MDS | Multi-dimensional scaling |
| OLFM4 | Olfactomedin 4 |
| OXPHOS | Oxidative phosphorylation |
| PHDs | Prolyl hydroxylases |
| PROGENy | Pathway RespOnsive GENes |
| qRT-PCR | Quantitative real-time PCR |
| Rox | Roxadustat |
| TA cells | Transit amplifying cells |
| TBP | TATA-box binding protein |
| TCA | Tricarboxylic acid |
| TGFβ | Transforming growth factor beta |
| UMAP | Uniform manifold approximation and projection |
| VEGF | Vascular endothelial growth factor |
| VHL | Von Hippel–Lindau |
References
- Crosnier, C.; Stamataki, D.; Lewis, J. Organizing Cell Renewal in the Intestine: Stem Cells, Signals and Combinatorial Control. Nat. Rev. Genet. 2006, 7, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, M.; Cheng, H. Clonal Analysis of Mouse Intestinal Epithelial Progenitors. Gastroenterology 1999, 116, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.S.; Aslam, U.; Ashcroft, D.M.; Rostami-Hodjegan, A. Meta-Analysis of the Turnover of Intestinal Epithelia in Preclinical Animal Species and Humans. Drug Metab. Dispos. 2014, 42, 2016–2022. [Google Scholar] [CrossRef]
- de Santa Barbara, P.; van den Brink, G.R.; Roberts, D.J. Development and Differentiation of the Intestinal Epithelium. CMLS Cell. Mol. Life Sci. 2003, 60, 1322–1332. [Google Scholar] [CrossRef]
- Moore, K.A.; Lemischka, I.R. Stem Cells and Their Niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Barker, N. Adult Intestinal Stem Cells: Critical Drivers of Epithelial Homeostasis and Regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef]
- Vermeulen, L.; Snippert, H.J. Stem Cell Dynamics in Homeostasis and Cancer of the Intestine. Nat. Rev. Cancer 2014, 14, 468–480. [Google Scholar] [CrossRef]
- Umar, S. Intestinal Stem Cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Regulation and Plasticity of Intestinal Stem Cells during Homeostasis and Regeneration. Development 2016, 143, 3639–3649. [Google Scholar] [CrossRef]
- de Sousa e Melo, F.; de Sauvage, F.J. Cellular Plasticity in Intestinal Homeostasis and Disease. Cell Stem Cell 2019, 24, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Gregorieff, A.; Clevers, H. Wnt Signaling in the Intestinal Epithelium: From Endoderm to Cancer. Genes Dev. 2005, 19, 877–890. [Google Scholar] [CrossRef]
- Farin, H.F.; Van Es, J.H.; Clevers, H. Redundant Sources of Wnt Regulate Intestinal Stem Cells and Promote Formation of Paneth Cells. Gastroenterology 2012, 143, 1518–1529.e7. [Google Scholar] [CrossRef]
- van Es, J.H.; Jay, P.; Gregorieff, A.; van Gijn, M.E.; Jonkheer, S.; Hatzis, P.; Thiele, A.; van den Born, M.; Begthel, H.; Brabletz, T.; et al. Wnt Signalling Induces Maturation of Paneth Cells in Intestinal Crypts. Nat. Cell Biol. 2005, 7, 381–386. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of Stem Cells in Small Intestine and Colon by Marker Gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Muñoz, J.; Stange, D.E.; Schepers, A.G.; van de Wetering, M.; Koo, B.; Itzkovitz, S.; Volckmann, R.; Kung, K.S.; Koster, J.; Radulescu, S.; et al. The Lgr5 Intestinal Stem Cell Signature: Robust Expression of Proposed Quiescent ‘+4’ Cell Markers. EMBO J. 2012, 31, 3079–3091. [Google Scholar] [CrossRef]
- Koo, B.-K.; Clevers, H. Stem Cells Marked by the R-Spondin Receptor LGR5. Gastroenterology 2014, 147, 289–302. [Google Scholar] [CrossRef]
- Haramis, A.-P.G.; Begthel, H.; van den Born, M.; van Es, J.; Jonkheer, S.; Offerhaus, G.J.A.; Clevers, H. De Novo Crypt Formation and Juvenile Polyposis on BMP Inhibition in Mouse Intestine. Science 2004, 303, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, C.; Li, V.S.W.; Chan, A.S.Y.; Zhang, J.; Ho, C.; Tsui, W.Y.; Chan, T.L.; Mifflin, R.C.; Powell, D.W.; Yuen, S.T.; et al. Gene Expression Patterns of Human Colon Tops and Basal Crypts and BMP Antagonists as Intestinal Stem Cell Niche Factors. Proc. Natl. Acad. Sci. USA 2007, 104, 15418–15423. [Google Scholar] [CrossRef] [PubMed]
- He, X.C.; Zhang, J.; Tong, W.-G.; Tawfik, O.; Ross, J.; Scoville, D.H.; Tian, Q.; Zeng, X.; He, X.; Wiedemann, L.M.; et al. BMP Signaling Inhibits Intestinal Stem Cell Self-Renewal through Suppression of Wnt–β-Catenin Signaling. Nat. Genet. 2004, 36, 1117–1121. [Google Scholar] [CrossRef]
- Fre, S.; Huyghe, M.; Mourikis, P.; Robine, S.; Louvard, D.; Artavanis-Tsakonas, S. Notch Signals Control the Fate of Immature Progenitor Cells in the Intestine. Nature 2005, 435, 964–968. [Google Scholar] [CrossRef]
- Clevers, H.C.; Bevins, C.L. Paneth Cells: Maestros of the Small Intestinal Crypts. Annu. Rev. Physiol. 2013, 75, 289–311. [Google Scholar] [CrossRef] [PubMed]
- VanDussen, K.L.; Carulli, A.J.; Keeley, T.M.; Patel, S.R.; Puthoff, B.J.; Magness, S.T.; Tran, I.T.; Maillard, I.; Siebel, C.; Kolterud, Å.; et al. Notch Signaling Modulates Proliferation and Differentiation of Intestinal Crypt Base Columnar Stem Cells. Development 2012, 139, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinet, L.; Rodilla, V.; Liu, Z.; Chen, S.; Koch, U.; Espinosa, L.; Kaestner, K.H.; Kopan, R.; Lewis, J.; Radtke, F. Dll1- and Dll4-Mediated Notch Signaling Are Required for Homeostasis of Intestinal Stem Cells. Gastroenterology 2011, 140, 1230–1240.e7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic Hypoxia and Oxygen Homeostasis in the Healthy Intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol.-Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte Metabolism Shapes the Gut Microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen Battle in the Gut: Hypoxia and Hypoxia-Inducible Factors in Metabolic and Inflammatory Responses in the Intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Kumar, T.; Pandey, R.; Chauhan, N.S. Hypoxia Inducible Factor-1α: The Curator of Gut Homeostasis. Front. Cell. Infect. Microbiol. 2020, 10, 227. [Google Scholar] [CrossRef]
- Walaas, G.A.; Gopalakrishnan, S.; Bakke, I.; Skovdahl, H.K.; Flatberg, A.; Østvik, A.E.; Sandvik, A.K.; Bruland, T. Physiological Hypoxia Improves Growth and Functional Differentiation of Human Intestinal Epithelial Organoids. Front. Immunol. 2023, 14, 1095812. [Google Scholar] [CrossRef]
- Rivera, K.R.; Bliton, R.J.; Burclaff, J.; Czerwinski, M.J.; Liu, J.; Trueblood, J.M.; Hinesley, C.M.; Breau, K.A.; Deal, H.E.; Joshi, S.; et al. Hypoxia Primes Human ISCs for Interleukin-Dependent Rescue of Stem Cell Activity. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 823–846. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; van Es, J.H.; van den Brink, S.; van Houdt, W.J.; Pronk, A.; van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, M.L.; Mukenhirn, M.; Muenchau, S.; Pervolaraki, K.; Kanaya, T.; Albrecht, D.; Odendall, C.; Hielscher, T.; Haucke, V.; Kagan, J.C.; et al. Asymmetric Distribution of TLR3 Leads to a Polarized Immune Response in Human Intestinal Epithelial Cells. Nat. Microbiol. 2020, 5, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R Package Rsubread Is Easier, Faster, Cheaper and Better for Alignment and Quantification of RNA Sequencing Reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Klinger, B.; Klünemann, M.; Sieber, A.; Uhlitz, F.; Sauer, S.; Garnett, M.J.; Blüthgen, N.; Saez-Rodriguez, J. Perturbation-Response Genes Reveal Signaling Footprints in Cancer Gene Expression. Nat. Commun. 2018, 9, 20. [Google Scholar] [CrossRef]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Weinstein, J.N.; Kamińska, B.; Huelsken, J.; Omberg, L.; Gevaert, O.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354.e15. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Steen, C.B.; Liu, C.L.; Alizadeh, A.A.; Newman, A.M. Profiling Cell Type Abundance and Expression in Bulk Tissues with CIBERSORTx. In Stem Cell Transcriptional Networks: Methods and Protocols; Kidder, B.L., Ed.; Springer: New York, NY, USA, 2020; pp. 135–157. ISBN 978-1-07-160301-7. [Google Scholar]
- Triana, S.; Stanifer, M.L.; Metz-Zumaran, C.; Shahraz, M.; Mukenhirn, M.; Kee, C.; Serger, C.; Koschny, R.; Ordonez-Rueda, D.; Paulsen, M.; et al. Single-Cell Transcriptomics Reveals Immune Response of Intestinal Cell Types to Viral Infection. Mol. Syst. Biol. 2021, 17, e9833. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated Analysis of Multimodal Single-Cell Data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of Immunity and Inflammation by Hypoxia in Immunological Niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Kimura, S.; Kitadai, Y.; Tanaka, S.; Kuwai, T.; Hihara, J.; Yoshida, K.; Toge, T.; Chayama, K. Expression of Hypoxia-Inducible Factor (HIF)-1α Is Associated with Vascular Endothelial Growth Factor Expression and Tumour Angiogenesis in Human Oesophageal Squamous Cell Carcinoma. Eur. J. Cancer 2004, 40, 1904–1912. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Beasley, N.J.P.; Watson, P.H.; Turner, K.J.; Pastorek, J.; Sibtain, A.; Wilson, G.D.; Turley, H.; Talks, K.L.; Maxwell, P.H.; et al. Hypoxia-Inducible Expression of Tumor-Associated Carbonic Anhydrases1. Cancer Res. 2000, 60, 7075–7083. [Google Scholar]
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of Glut1 MRNA by Hypoxia-Inducible Factor-1. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef]
- Jho, E.; Zhang, T.; Domon, C.; Joo, C.-K.; Freund, J.-N.; Costantini, F. Wnt/β-Catenin/Tcf Signaling Induces the Transcription of Axin2, a Negative Regulator of the Signaling Pathway. Mol. Cell. Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Colman, M.J.; Schewe, M.; Meerlo, M.; Stigter, E.; Gerrits, J.; Pras-Raves, M.; Sacchetti, A.; Hornsveld, M.; Oost, K.C.; Snippert, H.J.; et al. Interplay between Metabolic Identities in the Intestinal Crypt Supports Stem Cell Function. Nature 2017, 543, 424–427. [Google Scholar] [CrossRef]
- Kim, J.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Simsek, T.; Kocabas, F.; Zheng, J.; DeBerardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The Distinct Metabolic Profile of Hematopoietic Stem Cells Reflects Their Location in a Hypoxic Niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 Mediates Adaptation to Hypoxia by Actively Downregulating Mitochondrial Oxygen Consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Ferguson, A. Small Intestinal Epithelial Cell Kinetics and Protozoal Infection in Mice. Gastroenterology 1978, 74, 496–500. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Colgan, S.P.; Taylor, C.T. Hypoxia: An Alarm Signal during Intestinal Inflammation. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 281–287. [Google Scholar] [CrossRef]
- Pearson, A.D.; Eastham, E.J.; Laker, M.F.; Craft, A.W.; Nelson, R. Intestinal Permeability in Children with Crohn’s Disease and Coeliac Disease. BMJ 1982, 285, 20–21. [Google Scholar] [CrossRef]
- Xue, X.; Ramakrishnan, S.; Anderson, E.; Taylor, M.; Zimmermann, E.M.; Spence, J.R.; Huang, S.; Greenson, J.K.; Shah, Y.M. Endothelial PAS Domain Protein 1 Activates the Inflammatory Response in the Intestinal Epithelium to Promote Colitis in Mice. Gastroenterology 2013, 145, 831–841. [Google Scholar] [CrossRef]
- Xu, C.; Dong, W. Role of Hypoxia-Inducible Factor-1α in Pathogenesis and Disease Evaluation of Ulcerative Colitis. Exp. Ther. Med. 2016, 11, 1330–1334. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Cheng, C.-W.; Cao, A.Q.; Tripathi, S.; Mana, M.D.; Bauer-Rowe, K.E.; Abu-Remaileh, M.; Clavain, L.; Erdemir, A.; Lewis, C.A.; et al. Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 2018, 22, 769–778.e4. [Google Scholar] [CrossRef]
- Feron, O. Pyruvate into Lactate and Back: From the Warburg Effect to Symbiotic Energy Fuel Exchange in Cancer Cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef]
- Lan, X.; Qiu, P.; Mou, C. Hypoxia Impacts Small Intestinal Organoid Stemness and Differentiation. bioRxiv 2024. [Google Scholar] [CrossRef]
| Advanced DMEM/F12 (Thermo Fisher Scientific, Waltham, MA, USA #12634028) (1× GlutaMAX, 10 mM HEPES, 100 U/mL Penicillin, 100 μg/mL Streptomycin) | |
|---|---|
| 62% (v/v) | L-WRN cell-conditioned supernatant (Wnt-3A, R-spondin, Noggin) |
| 1× | B-27 supplement (Thermo Fisher Scientific, Waltham, MA, USA #17504001) |
| 1 mM | N-Acetyl-L-Cystein (Merck, Darmstadt, Germany #A9165) |
| 500 nM | A8301 (Merck, Darmstadt, Germany #SML0788) |
| 50 ng/mL | recombinant human FGF-basic (Peprotech, Waltham, MA, USA #10018B) |
| 25 ng/mL | mouse Noggin recombinant protein (Thermo Fisher Scientific, Waltham, MA, USA #25038) |
| 100 ng/mL | recombinant human IGF-1 (Fisher Scientific, Waltham, MA, USA #590908) |
| 10 nM | [Leu15]-Gastrin I human (Merck, Darmstadt, Germany #G9145) |
| 50 ng/mL | recombinant mouse EGF (Thermo Fisher Scientific, Waltham, MA, USA #PMG8041) |
| Advanced DMEM/F12 (Thermo Fisher Scientific, Waltham, MA, USA #12634028) (1× GlutaMAX, 10 mM HEPES, 100 U/mL Penicillin, 100 μg/mL Streptomycin) | |
|---|---|
| 10% (v/v) | HEK-R-spondin cell-conditioned supernatant |
| 1× | B-27 supplement (Thermo Fisher Scientific, Waltham, MA, USA #17504001) |
| 500 nM | A8301 (Merck, Darmstadt, Germany #SML0788) |
| 50 ng/mL | recombinant human FGF-basic (Peprotech, Waltham, MA, USA #10018B) |
| 50 ng/mL | mouse Noggin recombinant protein (Thermo Fisher Scientific, Waltham, MA, USA #25038) |
| 100 ng/mL | recombinant human IGF-1 (Fisher Scientific, Waltham, MA, USA #590908) |
| 10 nM | [Leu15]-Gastrin I human (Merck, Darmstadt, Germany #G9145) |
| 50 ng/mL | recombinant mouse EGF (Thermo Fisher Scientific, Waltham, MA, USA #PMG8041) |
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| AXIN2 | GTCTCTACCTCATTTCCCGAGAAC | CGAGATCAGCTCAGCTGCAA |
| CA9 | CATCCTAGCCCTGGTTTTTGG | GCTCACACCCCCTTTGGTT |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| GLUT1 | CTGCAGTTTGGCTACAACACTGGA | CCATAGCGGTGGACCCATGTCTG |
| HPRT1 | GCGTCGTGATTAGTGATG | GTCCATGAGGAATAAACACC |
| LGR5 | CCCTTCATTCAGTGCAGTGTT | AGCAGGTGTTCACAGGGTTT |
| OLFM4 | ACCTTTCCCGTGGACAGAGT | TGGACATATTCCCTCACTTTGGA |
| TBP | GCAGGTTCAAATCTCTGTCACC | AAGACAGGAGAGCTGCAACTC |
| VEGF | CTACCTCCACCATGCCAAGT | AGCTGCGCTGATAGACATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Uckeley, Z.M.; Kee, C.; Ramirez, C.; Karaluz, V.; Sharma, A.K.; Polanco, J.; Ortiz Martinez, F.D.; Mederos, C.I.; Jacobs, S.O.; Groose, I.J.; et al. Hypoxia Affects Stem Cell Fate in Patient-Derived Ileum Enteroids in a HIF-1α-Dependent Manner. Cells 2026, 15, 31. https://doi.org/10.3390/cells15010031
Uckeley ZM, Kee C, Ramirez C, Karaluz V, Sharma AK, Polanco J, Ortiz Martinez FD, Mederos CI, Jacobs SO, Groose IJ, et al. Hypoxia Affects Stem Cell Fate in Patient-Derived Ileum Enteroids in a HIF-1α-Dependent Manner. Cells. 2026; 15(1):31. https://doi.org/10.3390/cells15010031
Chicago/Turabian StyleUckeley, Zina M., Carmon Kee, Carlos Ramirez, Victoria Karaluz, Ashwini K. Sharma, Josmar Polanco, Freddie D. Ortiz Martinez, Christopher I. Mederos, Sorin O. Jacobs, Ingrid J. Groose, and et al. 2026. "Hypoxia Affects Stem Cell Fate in Patient-Derived Ileum Enteroids in a HIF-1α-Dependent Manner" Cells 15, no. 1: 31. https://doi.org/10.3390/cells15010031
APA StyleUckeley, Z. M., Kee, C., Ramirez, C., Karaluz, V., Sharma, A. K., Polanco, J., Ortiz Martinez, F. D., Mederos, C. I., Jacobs, S. O., Groose, I. J., Ramsden, J. M., Herrmann, C., Stanifer, M. L., & Boulant, S. (2026). Hypoxia Affects Stem Cell Fate in Patient-Derived Ileum Enteroids in a HIF-1α-Dependent Manner. Cells, 15(1), 31. https://doi.org/10.3390/cells15010031







