FAK-Activated Mucosal Healing Promotes Resistance to Reinjury
Highlights
- FAK activation accelerates ischemic ulcer healing, in part by enhancing angiogenesis.
- FAK activation during an initial injury reduces susceptibility to recurrent NSAID-induced intestinal injury.
- FAK activation may represent a novel therapeutic avenue for gastrointestinal injury.
- FAK activation may be especially valuable for patients requiring long-term NSAID therapy.
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Effects of a FAK Activator on Ulcer Healing
2.3. Histology
2.4. Immunofluorescence Staining
2.5. Cell Culture
2.6. Western Blotting
2.7. HUVECs Sprouting Assay
2.8. Cell Proliferation Assay
2.9. In Vitro Monolayer Wound Closure
2.10. Effects of FAK Activation During Initial NSAIDs Exposure on Subsequent NSAIDs-Induced Reinjury
2.11. Statistical Analysis
3. Results
3.1. FAK Activation Promoted Intestinal Mucosal Healing and Enhanced Both Blood Vessel Formation and Cell Proliferation in the Ulcer Bed and Ulcer Edge After 2 Days (Day 3) and 4 Days (Day 5) of Treatment in a Murine Model of Jejunal Ischemic Ulcers
3.2. ZN27 Activates FAK and Its Downstream Effector—ERK1/2 in HUVECs
3.3. ZN27 Increased the Number of Sprouts in HUVECs
3.4. FAK Activation Did Not Alter Proliferation but Stimulated Migration Through ERK1/2 Activation in HUVECs
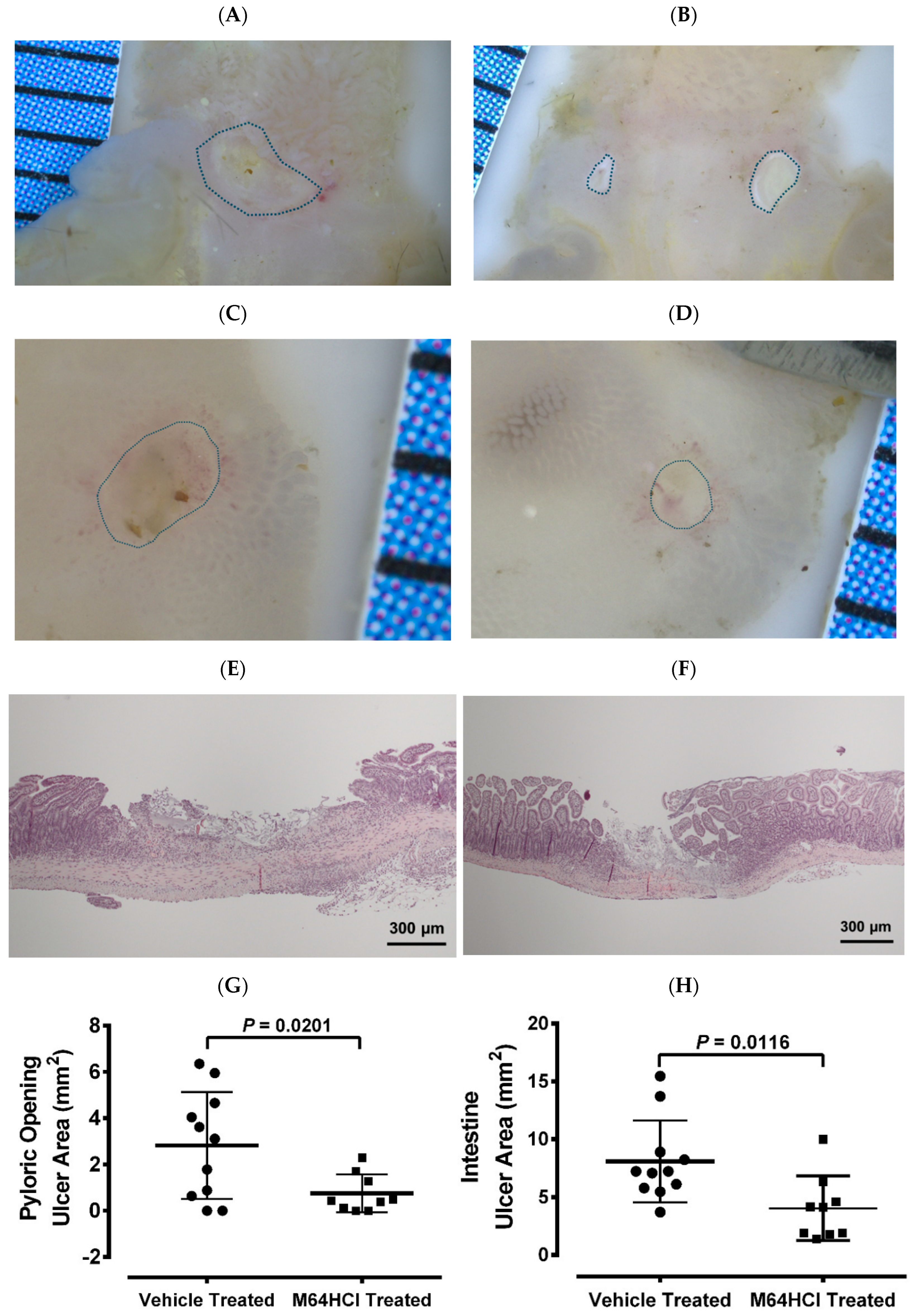
3.5. Mice Recovered from M64HCl-Treated, Indomethacin-Induced Intestinal Injury Showed Resistance to Subsequent Indomethacin Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131–134. [Google Scholar] [CrossRef]
- Fine, M. Quantifying the impact of NSAID-associated adverse events. Am. J. Manag. Care 2013, 19, s267–s272. [Google Scholar] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Sancarlo, D.; Addante, F.; Scarcelli, C.; Franceschi, M. Non-steroidal anti-inflammatory drug use in the elderly. Surg. Oncol. 2010, 19, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Laine, L. Gastrointestinal effects of NSAIDs and coxibs. J. Pain Symptom Manag. 2003, 25, S32–S40. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Lanas, A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res. Ther. 2013, 15, S3. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujiwara, Y.; Chan, F.K.L. Current knowledge on non-steroidal anti-inflammatory drug-induced small-bowel damage: A comprehensive review. J. Gastroenterol. 2020, 55, 481–495. [Google Scholar] [CrossRef]
- Guo, C.G.; Zhang, F.; Wu, J.T.; Cheung, K.S.; Li, B.; Law, S.Y.K.; Leung, W.K. Divergent trends of hospitalizations for upper and lower gastrointestinal bleeding based on population prescriptions of aspirin, proton pump inhibitors and Helicobacter pylori eradication therapy: Trends of upper and lower gastrointestinal bleeding. United Eur. Gastroenterol. J. 2021, 9, 543–551. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.H.; Park, C.H. Impact of proton pump inhibitors on the risk of small bowel or colorectal bleeding: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2023, 11, 861–873. [Google Scholar] [CrossRef]
- Lee, M.; Kim, M.; Cha, J.M. Risk of Lower Gastrointestinal Bleeding in Nonsteroidal Anti-inflammatory Drug (NSAID) and Proton Pump Inhibitor Users Compared with NSAID-Only Users: A Common Data Model Analysis. Gut Liver 2025, 19, 243–252. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, X.; Chen, P.R.; Li, Y.N.; Lv, X.H.; Yang, J.L. Proton Pump Inhibitors Increase the Risk of Nonsteroidal Anti-inflammatory Drug-Related Small-Bowel Injury: A Systematic Review with Meta-analysis. Clin. Transl. Gastroenterol. 2023, 14, e00588. [Google Scholar] [CrossRef]
- Tai, F.W.D.; McAlindon, M.E. NSAIDs and the small bowel. Curr. Opin. Gastroenterol. 2018, 34, 175–182. [Google Scholar] [CrossRef]
- Wallace, J.L. Mechanisms, prevention and clinical implications of nonsteroidal anti-inflammatory drug-enteropathy. World J. Gastroenterol. 2013, 19, 1861–1876. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Q.; Hou, H.; Zhang, W.; Li, M.; Chen, D.; Gu, Y.; Wang, B.; Hou, J.; Liu, Y.; et al. Gut Microbiota in NSAID Enteropathy: New Insights from Inside. Front. Cell Infect. Microbiol. 2021, 11, 679396. [Google Scholar] [CrossRef]
- Arakawa, T.; Watanabe, T.; Tanigawa, T.; Tominaga, K.; Fujiwara, Y.; Morimoto, K. Quality of ulcer healing in gastrointestinal tract: Its pathophysiology and clinical relevance. World J. Gastroenterol. 2012, 18, 4811–4822. [Google Scholar] [CrossRef]
- Pineton de Chambrun, G.; Peyrin-Biroulet, L.; Lémann, M.; Colombel, J.-F. Clinical implications of mucosal healing for the management of IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 15–29. [Google Scholar] [CrossRef]
- Somensi, L.B.; Costa, P.; Boeing, T.; Bolda Mariano, L.N.; de Gregório, E.; ATM, E.S.; Longo, B.; Locatelli, C.; de Souza, P.; Magalhães, C.G.; et al. Lupeol Stearate Accelerates Healing and Prevents Recurrence of Gastric Ulcer in Rodents. Evid. Based Complement. Altern. Med. Ecam 2022, 2022, 6134128. [Google Scholar] [CrossRef]
- Tarnawski, A.; Hollander, D.; Krause, W.J.; Dabros, W.; Stachura, J.; Gergely, H. “Healed” experimental gastric ulcers remain histologically and ultrastructurally abnormal. J. Clin. Gastroenterol. 1990, 12, S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E.; Jaakkimainen, L.; Bombardier, C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann. Intern. Med. 1991, 115, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Venerito, M.; Wex, T.; Malfertheiner, P. Nonsteroidal Anti-Inflammatory Drug-Induced Gastroduodenal Bleeding: Risk Factors and Prevention Strategies. Pharmaceuticals 2010, 3, 2225–2237. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yan, Y.; Song, B.; Zhu, S.; Mei, Q.; Wu, K. Focal adhesion kinase: From biological functions to therapeutic strategies. Exp. Hematol. Oncol. 2023, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Liang, Q.; Tong, R.; Huang, J.; Yang, X.; Xu, Y.; Wang, W.; Sun, M.; Shi, J. Recent progress on FAK inhibitors with dual targeting capabilities for cancer treatment. Biomed. Pharmacother. 2022, 151, 113116. [Google Scholar] [CrossRef]
- Su, Y.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) promotes cell migration and adhesion via focal adhesion kinase. J. Surg. Res. 2014, 189, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.D.; Sanders, M.A.; Gomez, R.; Hatfield, J.; VanderHeide, R.; Thamilselvan, V.; Zhang, J.; Walsh, M.F. Focal adhesion kinase protein levels in gut epithelial motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G491–G499. [Google Scholar] [CrossRef]
- Oncel, S.; Gupta, R.; Wang, Q.; Basson, M.D. ZINC40099027 Promotes Gastric Mucosal Repair in Ongoing Aspirin-Associated Gastric Injury by Activating Focal Adhesion Kinase. Cells 2021, 10, 908. [Google Scholar] [CrossRef]
- Wang, Q.; Gallardo-Macias, R.; Vomhof-DeKrey, E.E.; Gupta, R.; Golovko, S.A.; Golovko, M.Y.; Oncel, S.; Gurvich, V.J.; Basson, M.D. A novel drug-like water-soluble small molecule Focal Adhesion Kinase (FAK) activator promotes intestinal mucosal healing. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100147. [Google Scholar] [CrossRef]
- Wang, Q.; More, S.K.; Vomhof-DeKrey, E.E.; Golovko, M.Y.; Basson, M.D. Small molecule FAK activator promotes human intestinal epithelial monolayer wound closure and mouse ulcer healing. Sci. Rep. 2019, 9, 14669. [Google Scholar] [CrossRef]
- Liu, G.; Elsayed, A.A.R.; Kwantwi, L.B.; Gallardo-Macias, R.; Gurvich, V.J.; Basson, M.D. M64HCl, a focal adhesion kinase activator, promotes intestinal mucosal healing in rats. BMC Gastroenterol. 2025, 25, 347. [Google Scholar] [CrossRef]
- Rashmi; More, S.K.; Wang, Q.; Vomhof-DeKrey, E.E.; Porter, J.E.; Basson, M.D. ZINC40099027 activates human focal adhesion kinase by accelerating the enzymatic activity of the FAK kinase domain. Pharmacol. Res. Perspect. 2021, 9, e00737. [Google Scholar] [CrossRef] [PubMed]
- Tarnawski, A.S.; Ahluwalia, A.; Jones, M.K. Angiogenesis in gastric mucosa: An important component of gastric erosion and ulcer healing and its impairment in aging. J. Gastroenterol. Hepatol. 2014, 29, 112–123. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Sun, S.; Wu, H.J.; Guan, J.L. Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice. Sci. Rep. 2018, 8, 2550. [Google Scholar] [CrossRef]
- Peng, X.; Ueda, H.; Zhou, H.; Stokol, T.; Shen, T.L.; Alcaraz, A.; Nagy, T.; Vassalli, J.D.; Guan, J.L. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc. Res. 2004, 64, 421–430. [Google Scholar] [CrossRef]
- Shen, T.L.; Park, A.Y.; Alcaraz, A.; Peng, X.; Jang, I.; Koni, P.; Flavell, R.A.; Gu, H.; Guan, J.L. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 2005, 169, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Oncel, S.; Basson, M.D. Gut homeostasis, injury, and healing: New therapeutic targets. World J. Gastroenterol. 2022, 28, 1725–1750. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef]
- Cain, J.T.; Berosik, M.A.; Snyder, S.D.; Crawford, N.F.; Nour, S.I.; Schaubhut, G.J.; Darland, D.C. Shifts in the vascular endothelial growth factor isoforms result in transcriptome changes correlated with early neural stem cell proliferation and differentiation in mouse forebrain. Dev. Neurobiol. 2014, 74, 63–81. [Google Scholar] [CrossRef] [PubMed]
- More, S.K.; Vomhof-Dekrey, E.E.; Basson, M.D. ZINC4085554 inhibits cancer cell adhesion by interfering with the interaction of Akt1 and FAK. Oncol. Lett. 2019, 17, 5251–5260. [Google Scholar] [CrossRef]
- Bayless, K.J.; Kwak, H.I.; Su, S.C. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat. Protoc. 2009, 4, 1888–1898. [Google Scholar] [CrossRef]
- Basson, M.D.; Modlin, I.M.; Madri, J.A. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J. Clin. Investig. 1992, 90, 15–23. [Google Scholar] [CrossRef]
- Yamada, S.; Naito, Y.; Takagi, T.; Mizushima, K.; Hirai, Y.; Horie, R.; Fukumoto, K.; Inoue, K.; Harusato, A.; Yoshida, N.; et al. Reduced small-intestinal injury induced by indomethacin in interleukin-17A-deficient mice. J. Gastroenterol. Hepatol. 2011, 26, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; DeWispelaere, A.; Dastvan, F.; Osborne, W.R.; Blechner, C.; Windhorst, S.; Daum, G. Smooth Muscle-Alpha Actin Inhibits Vascular Smooth Muscle Cell Proliferation and Migration by Inhibiting Rac1 Activity. PLoS ONE 2016, 11, e0155726. [Google Scholar] [CrossRef]
- Elmaci, İ.; Altinoz, M.A.; Sari, R.; Bolukbasi, F.H. Phosphorylated Histone H3 (PHH3) as a Novel Cell Proliferation Marker and Prognosticator for Meningeal Tumors: A Short Review. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 627–631. [Google Scholar] [CrossRef]
- Oncel, S.; Basson, M.D. ZINC40099027 promotes monolayer circular defect closure by a novel pathway involving cytosolic activation of focal adhesion kinase and downstream paxillin and ERK1/2. Cell Tissue Res. 2022, 390, 261–279. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.H.; Shen, Y.; Lai, Y.; Liu, X.J. Laminar shear stress promotes endothelial cell migration and inhibits cell apoptosis in the presence of hydroxyurea. Cell. Mol. Biol. 2011, 57, 1550–1557. [Google Scholar]
- Graham, D.Y.; Opekun, A.R.; Willingham, F.F.; Qureshi, W.A. Visible small-intestinal mucosal injury in chronic NSAID users. Clin. Gastroenterol. Hepatol. 2005, 3, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Maiden, L.; Thjodleifsson, B.; Theodors, A.; Gonzalez, J.; Bjarnason, I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005, 128, 1172–1178. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr. Update on the Incidence and Prevalence of Inflammatory Bowel Disease in the United States. Gastroenterol. Hepatol. 2016, 12, 704–707. [Google Scholar]
- Hayashi, S.; Muraleedharan, C.K.; Oku, M.; Tomar, S.; Hogan, S.P.; Quiros, M.; Parkos, C.A.; Nusrat, A. Intestinal epithelial BLT1 promotes mucosal repair. JCI Insight 2022, 7, e162392. [Google Scholar] [CrossRef] [PubMed]
- Manieri, N.A.; Mack, M.R.; Himmelrich, M.D.; Worthley, D.L.; Hanson, E.M.; Eckmann, L.; Wang, T.C.; Stappenbeck, T.S. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J. Clin. Investig. 2015, 125, 3606–3618. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, X.; Tao, Y.; Zhu, Y.; Zhang, J.; Yu, X.; Guo, P.; Liu, S.; Wei, Z.; Dai, Y.; et al. Arctigenin promotes mucosal healing in ulcerative colitis through facilitating focal adhesion assembly and colonic epithelial cell migration via targeting focal adhesion kinase. Int. Immunopharmacol. 2024, 128, 111552. [Google Scholar] [CrossRef]
- Owen, K.A.; Abshire, M.Y.; Tilghman, R.W.; Casanova, J.E.; Bouton, A.H. FAK regulates intestinal epithelial cell survival and proliferation during mucosal wound healing. PLoS ONE 2011, 6, e23123. [Google Scholar] [CrossRef]
- Walsh, M.F.; Ampasala, D.R.; Hatfield, J.; Vander Heide, R.; Suer, S.; Rishi, A.K.; Basson, M.D. Transforming growth factor-beta stimulates intestinal epithelial focal adhesion kinase synthesis via Smad- and p38-dependent mechanisms. Am. J. Pathol. 2008, 173, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.J.; Carruth, J.A.; Basson, M.D.; Bloodgood, R.; Sumpio, B.E. Matrix-specific effect of endothelial control of smooth muscle cell migration. J. Vasc. Surg. 1996, 24, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Owen, C.R.; Sanders, M.A.; Turner, J.R.; Basson, M.D. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology 2006, 131, 1179–1189. [Google Scholar] [CrossRef]
- Zare, R.; Abdolsamadi, H.; Soleimani Asl, S.; Radi, S.; Bahrami, H.; Jamshidi, S. The bFGF Can Improve Angiogenesis in Oral Mucosa and Accelerate Wound Healing. Rep. Biochem. Mol. Biol. 2023, 11, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, T.; Wang, Y.; Yang, C.; Zhang, S.; Cao, X. Focal adhesion kinase signaling pathway is involved in mechanotransduction in MG-63 cells. Biochem. Biophys. Res. Commun. 2011, 410, 671–676. [Google Scholar] [CrossRef]
- Schaller, M.D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta 2001, 1540, 1–21. [Google Scholar] [CrossRef]
- Geng, K.; Wang, J.; Liu, P.; Tian, X.; Liu, H.; Wang, X.; Hu, C.; Yan, H. Electrical stimulation facilitates the angiogenesis of human umbilical vein endothelial cells through MAPK/ERK signaling pathway by stimulating FGF2 secretion. Am. J. Physiol. Cell Physiol. 2019, 317, C277–C286. [Google Scholar] [CrossRef]
- Kiwanuka, E.; Andersson, L.; Caterson, E.J.; Junker, J.P.; Gerdin, B.; Eriksson, E. CCN2 promotes keratinocyte adhesion and migration via integrin α5β1. Exp. Cell Res. 2013, 319, 2938–2946. [Google Scholar] [CrossRef]
- Escuin-Ordinas, H.; Li, S.; Xie, M.W.; Sun, L.; Hugo, W.; Huang, R.R.; Jiao, J.; de-Faria, F.M.; Realegeno, S.; Krystofinski, P.; et al. Cutaneous wound healing through paradoxical MAPK activation by BRAF inhibitors. Nat. Commun. 2016, 7, 12348. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, J.; Xu, R.; Zhang, Y.; Gu, L.; Dong, J.; Zhu, Y.; Zhou, R.; Zheng, L.; Zhang, X.; et al. Melatonin suppresses hypoxia-induced migration of HUVECs via inhibition of ERK/Rac1 activation. Int. J. Mol. Sci. 2014, 15, 14102–14121. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, H.Y.; Kim, S.D.; Shim, J.W.; Bae, Y.S. Lysophosphatidylglycerol stimulates chemotactic migration and tube formation in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2007, 363, 490–494. [Google Scholar] [CrossRef]
- Ou, D.; Wang, Q.; Huang, Y.; Zeng, D.; Wei, T.; Ding, L.; Li, X.; Zheng, Q.; Jin, Y. Co-culture with neonatal cardiomyocytes enhances the proliferation of iPSC-derived cardiomyocytes via FAK/JNK signaling. BMC Dev. Biol. 2016, 16, 11. [Google Scholar] [CrossRef]
- Pirone, D.M.; Liu, W.F.; Ruiz, S.A.; Gao, L.; Raghavan, S.; Lemmon, C.A.; Romer, L.H.; Chen, C.S. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 2006, 174, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Cham, C.M.; Chang, E.B. Epithelial wound healing in inflammatory bowel diseases: The next therapeutic frontier. Transl. Res. 2021, 236, 35–51. [Google Scholar] [CrossRef] [PubMed]
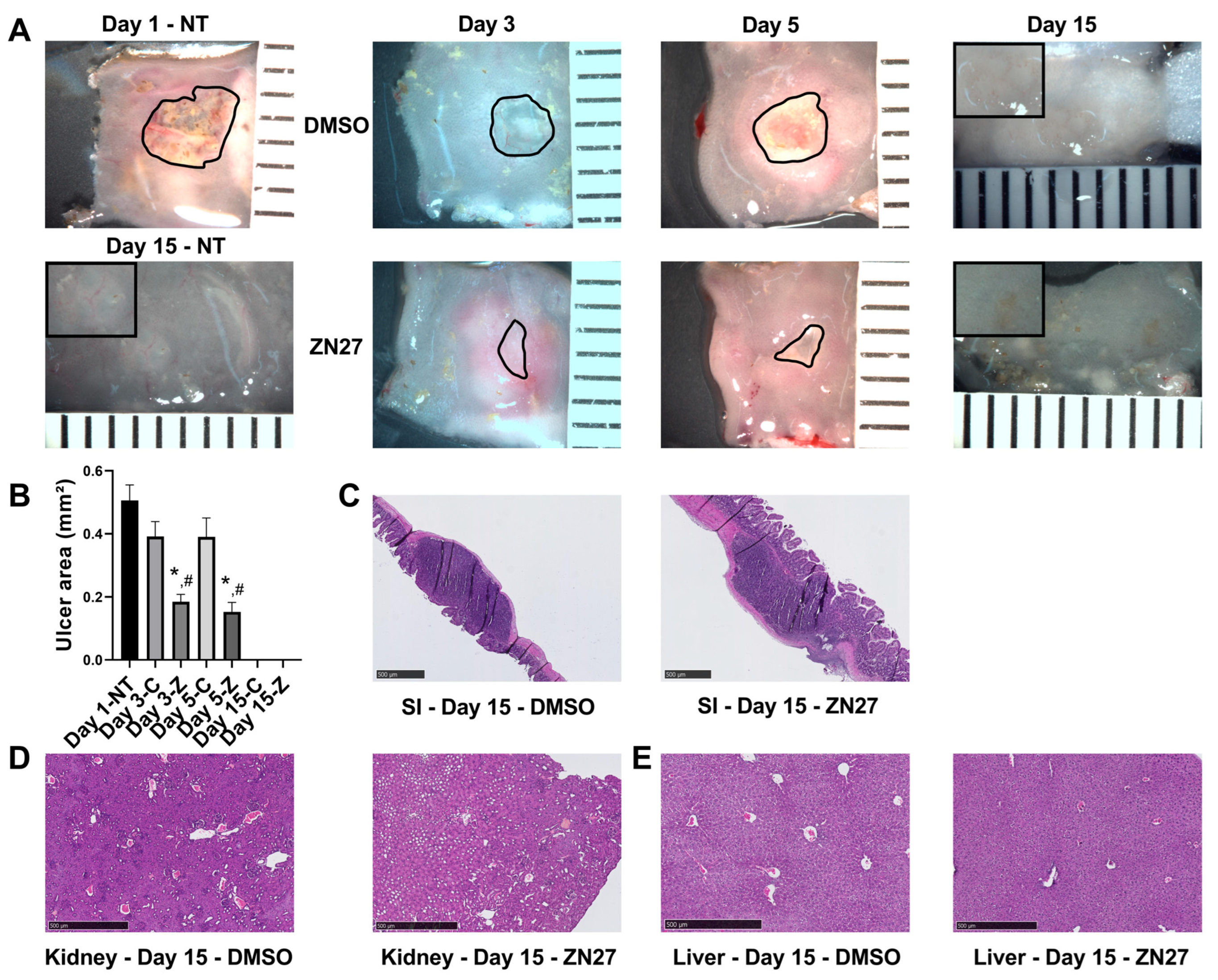
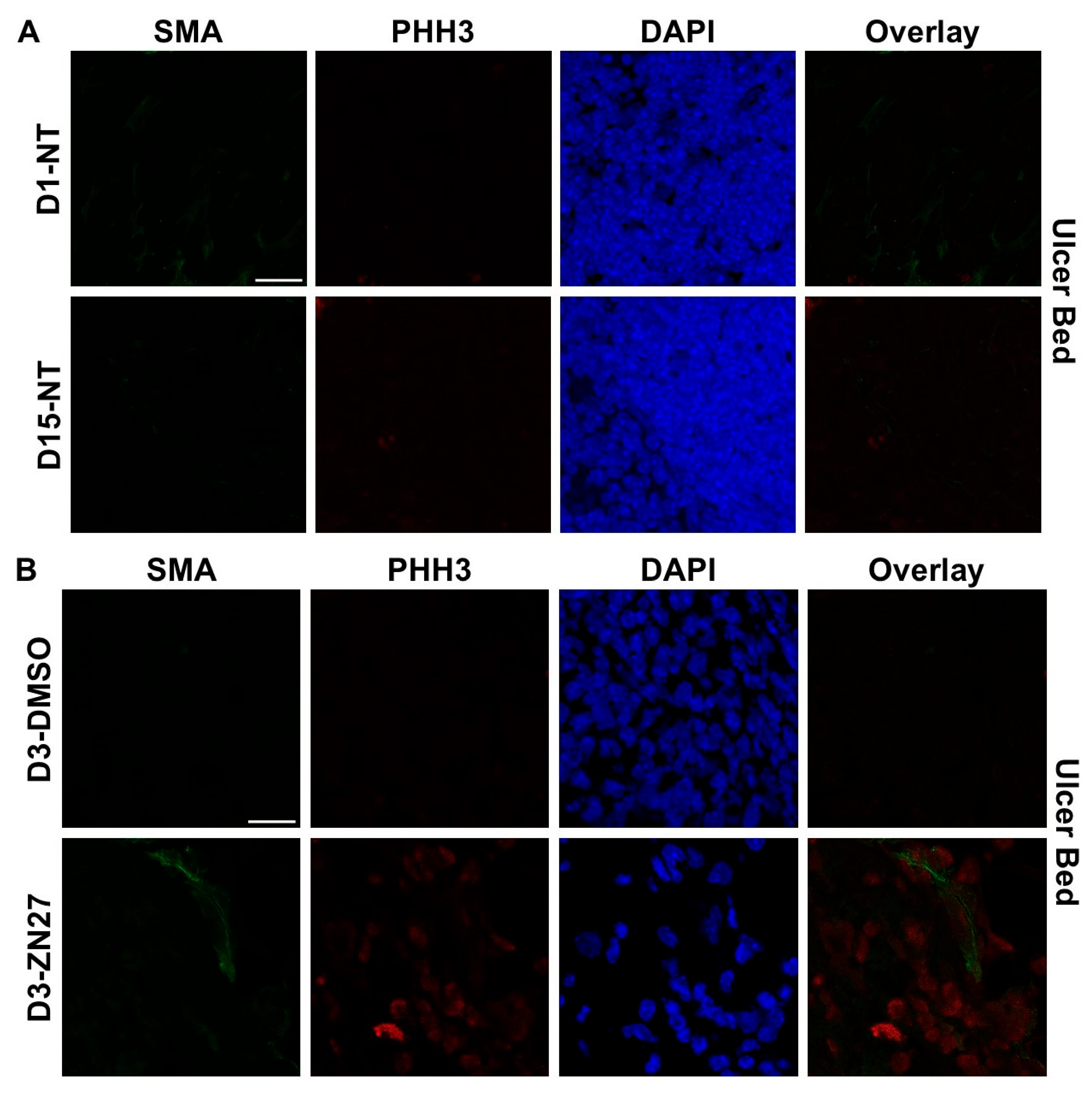
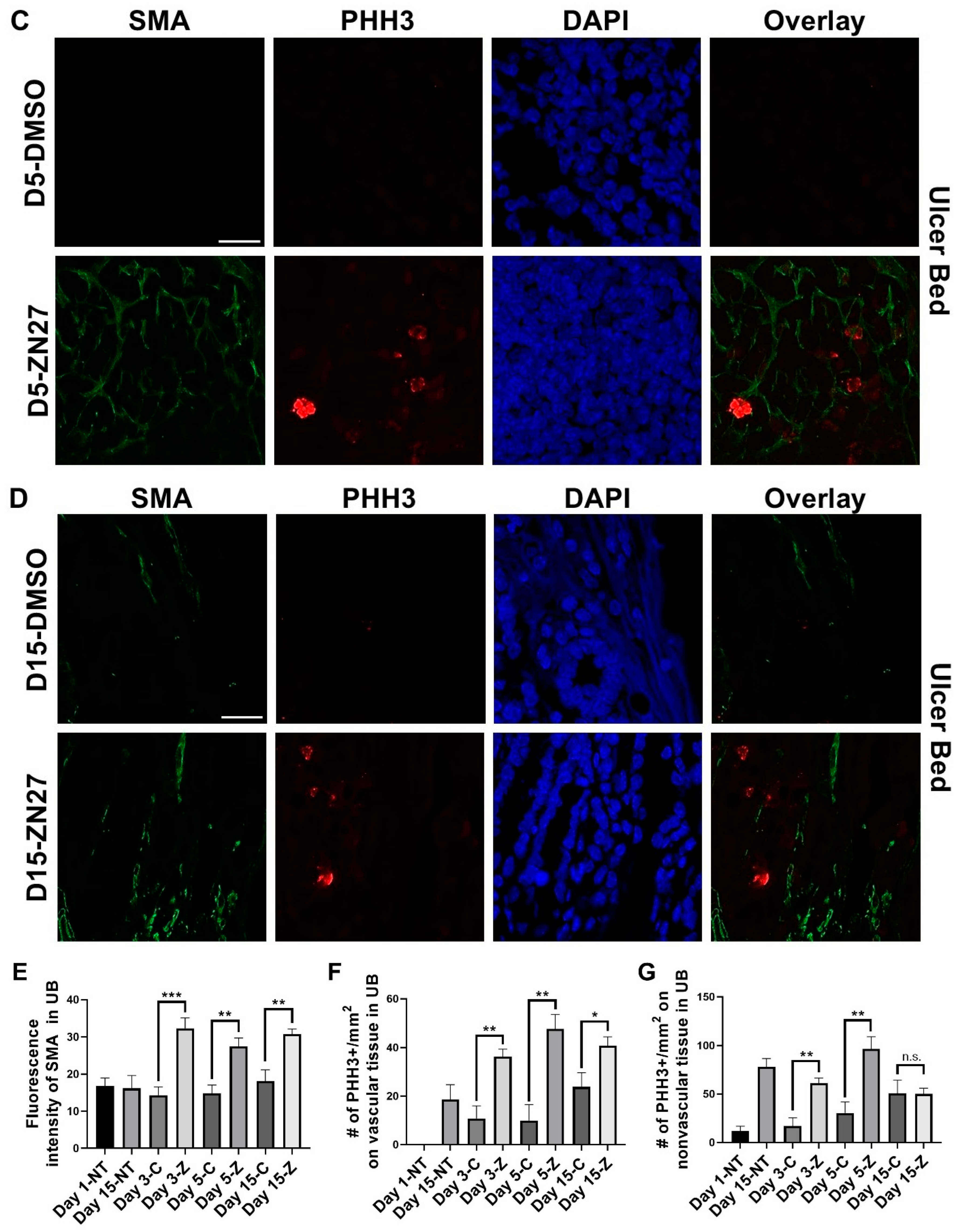
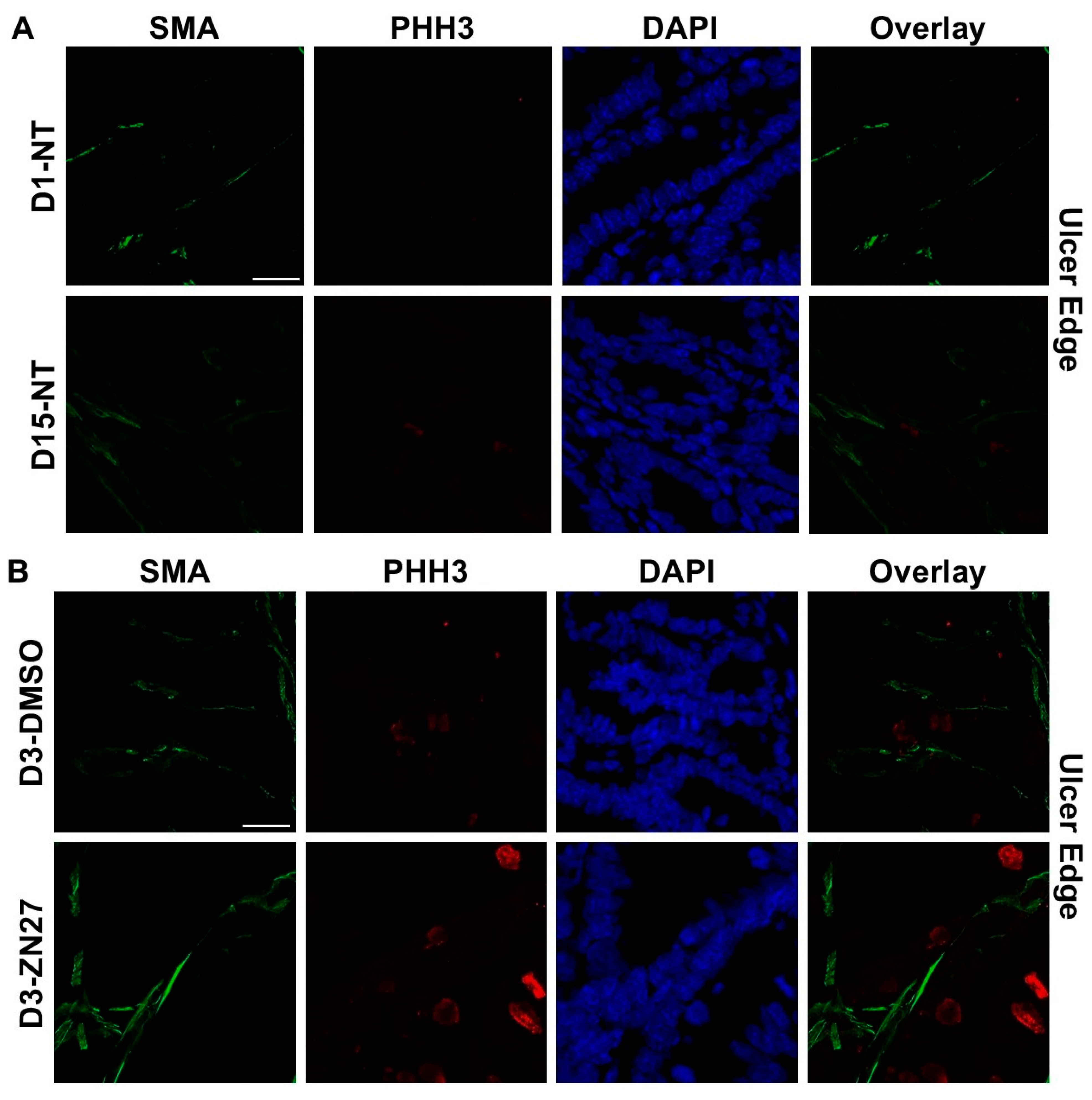
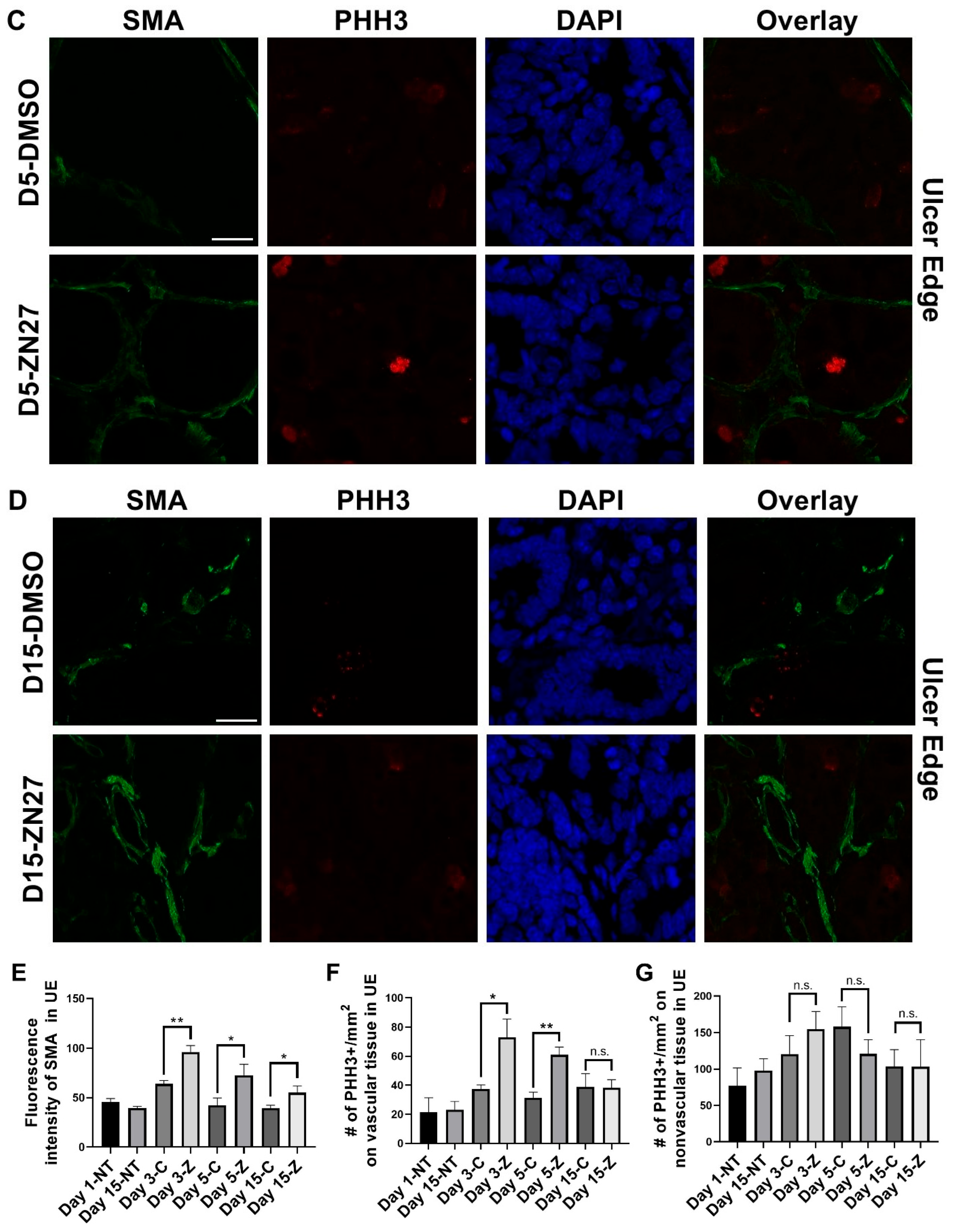
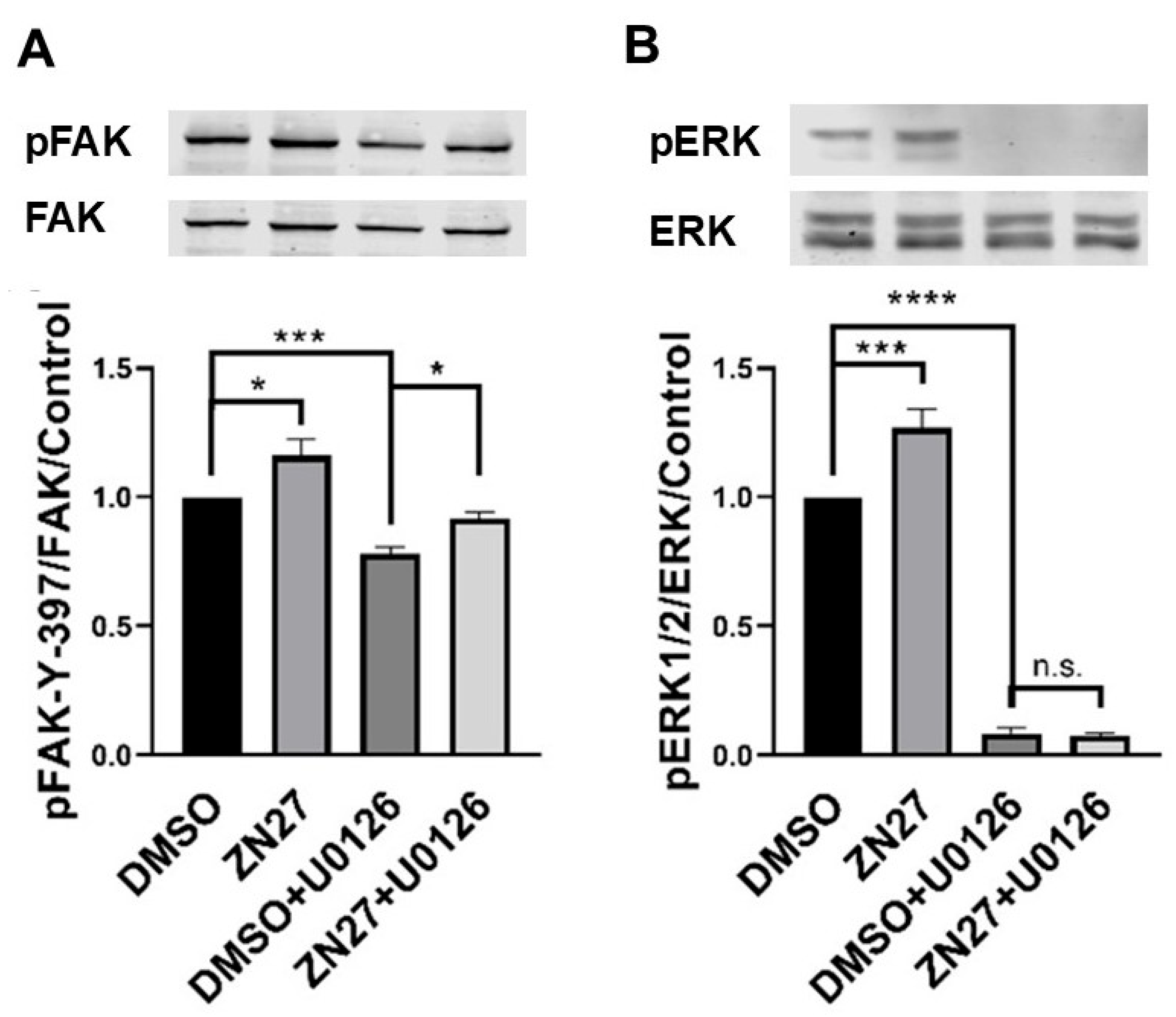
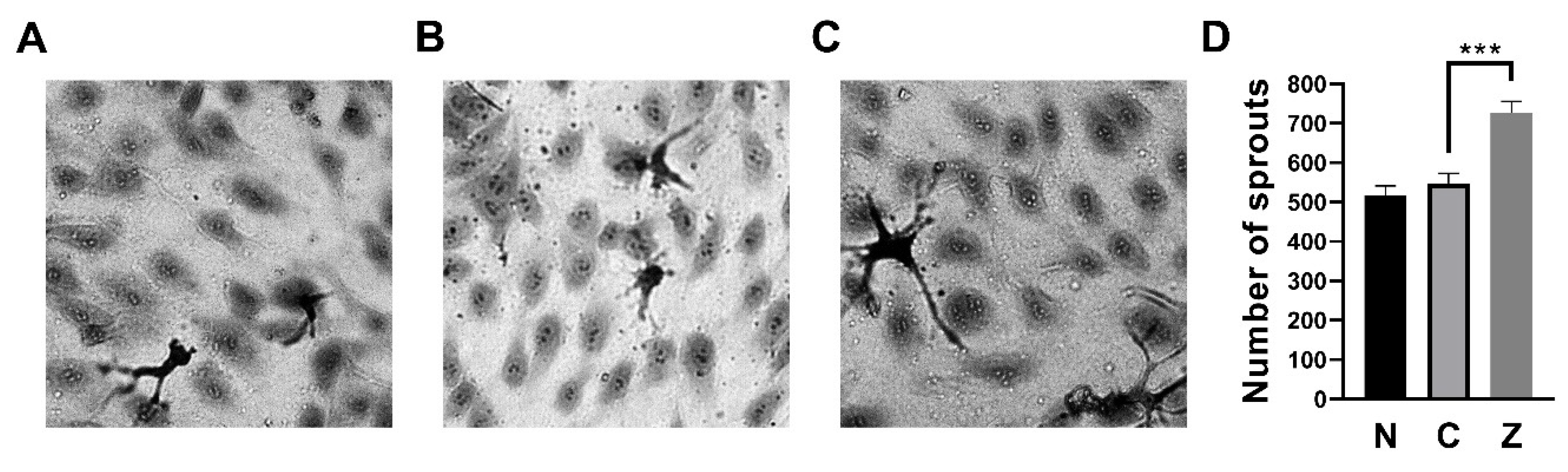
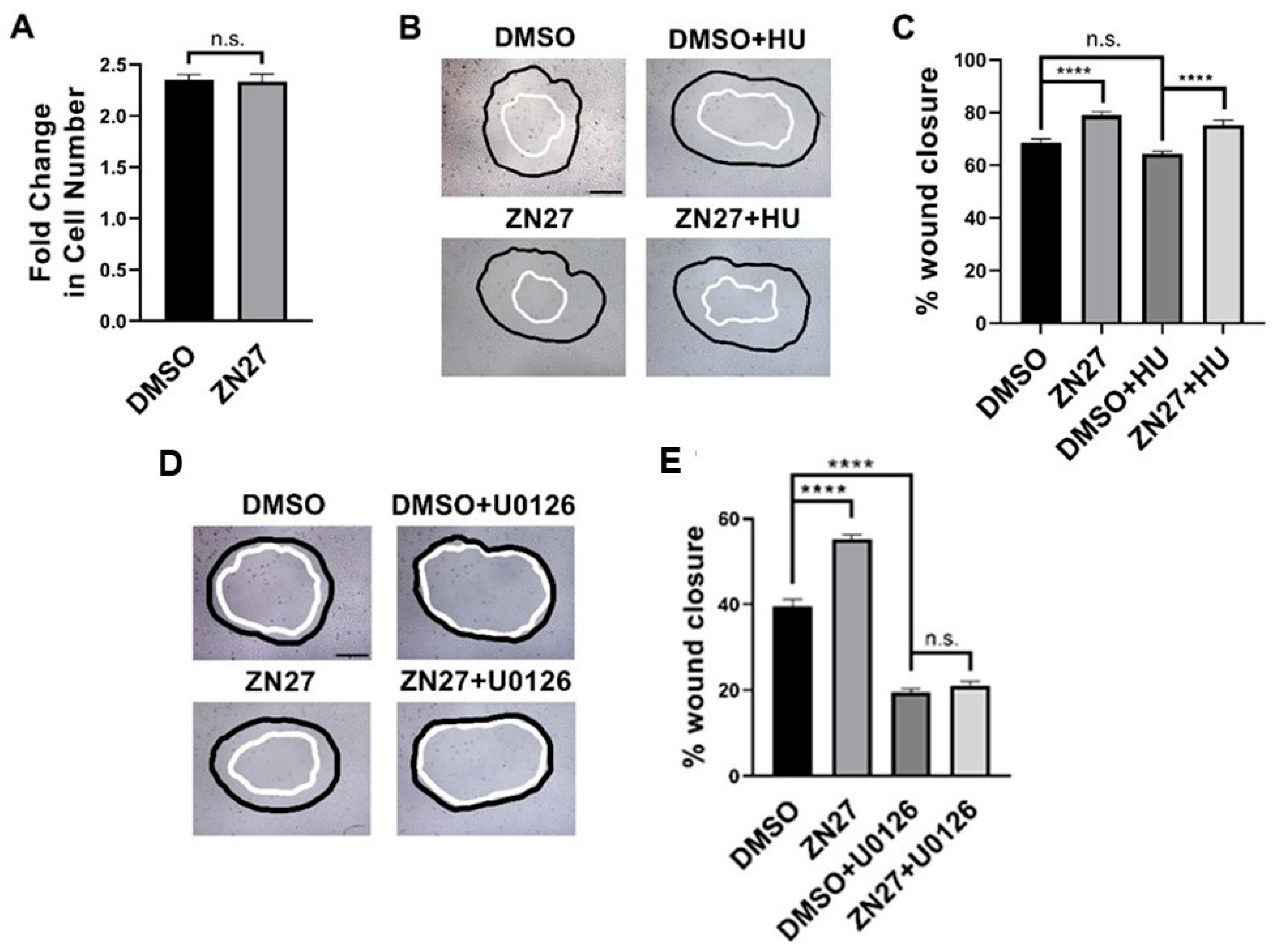
| Parameters | Normal Range | No Treatment (n = 3) | DMSO-Treated (n = 3) | ZN27-Treated (n = 3) |
|---|---|---|---|---|
| Serum creatinine | 0.06–16 mg/dL | 0.11 ± 0.02 | 0.14 ± 0.06 | 0.13 ± 0.03 |
| Serum ALT | 7.63–53.1 U/L | 8.80 ± 0.70 | 19.19 ± 1.37 | 17.95 ± 3.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Oncel, S.; Liu, G.; Kwantwi, L.; Vomhof-DeKrey, E.E.; Gallardo-Macias, R.; Gurvich, V.J.; Basson, M.D. FAK-Activated Mucosal Healing Promotes Resistance to Reinjury. Cells 2026, 15, 16. https://doi.org/10.3390/cells15010016
Oncel S, Liu G, Kwantwi L, Vomhof-DeKrey EE, Gallardo-Macias R, Gurvich VJ, Basson MD. FAK-Activated Mucosal Healing Promotes Resistance to Reinjury. Cells. 2026; 15(1):16. https://doi.org/10.3390/cells15010016
Chicago/Turabian StyleOncel, Sema, Guiming Liu, Louis Kwantwi, Emilie E. Vomhof-DeKrey, Ricardo Gallardo-Macias, Vadim J. Gurvich, and Marc D. Basson. 2026. "FAK-Activated Mucosal Healing Promotes Resistance to Reinjury" Cells 15, no. 1: 16. https://doi.org/10.3390/cells15010016
APA StyleOncel, S., Liu, G., Kwantwi, L., Vomhof-DeKrey, E. E., Gallardo-Macias, R., Gurvich, V. J., & Basson, M. D. (2026). FAK-Activated Mucosal Healing Promotes Resistance to Reinjury. Cells, 15(1), 16. https://doi.org/10.3390/cells15010016







