Optimization of In-Situ Exosome Enrichment Methodology On-a-Chip to Mimic Tumor Microenvironment Induces Cancer Stemness in Glioblastoma Tumor Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Estimating the Local Concentration of a Chemical Secreted from Tumor Cells Within the TME
2.1.2. Glucose Concentration and Cell Proliferation
2.1.3. Exosomes
2.2. Materials
2.3. Microfluidic Device Fabrication
2.4. Experimental Design
2.5. Cell Culture and Sample Preparation
2.6. Glucose Assessment
2.7. Tortuosity Index
2.8. Viability Assay
2.9. Scanning Electron Microscopy (SEM)
2.10. Atomic Force Microscopy (AFM)
2.11. Dynamic Light Scattering (DLS)
2.12. Immunocytochemistry (ICC) and Analyze Marker Expression
2.13. Real-Time RT-PCR
2.14. Enzyme-Linked Immunosorbent Assay (ELISA)
2.15. Statistical Analysis
3. Results
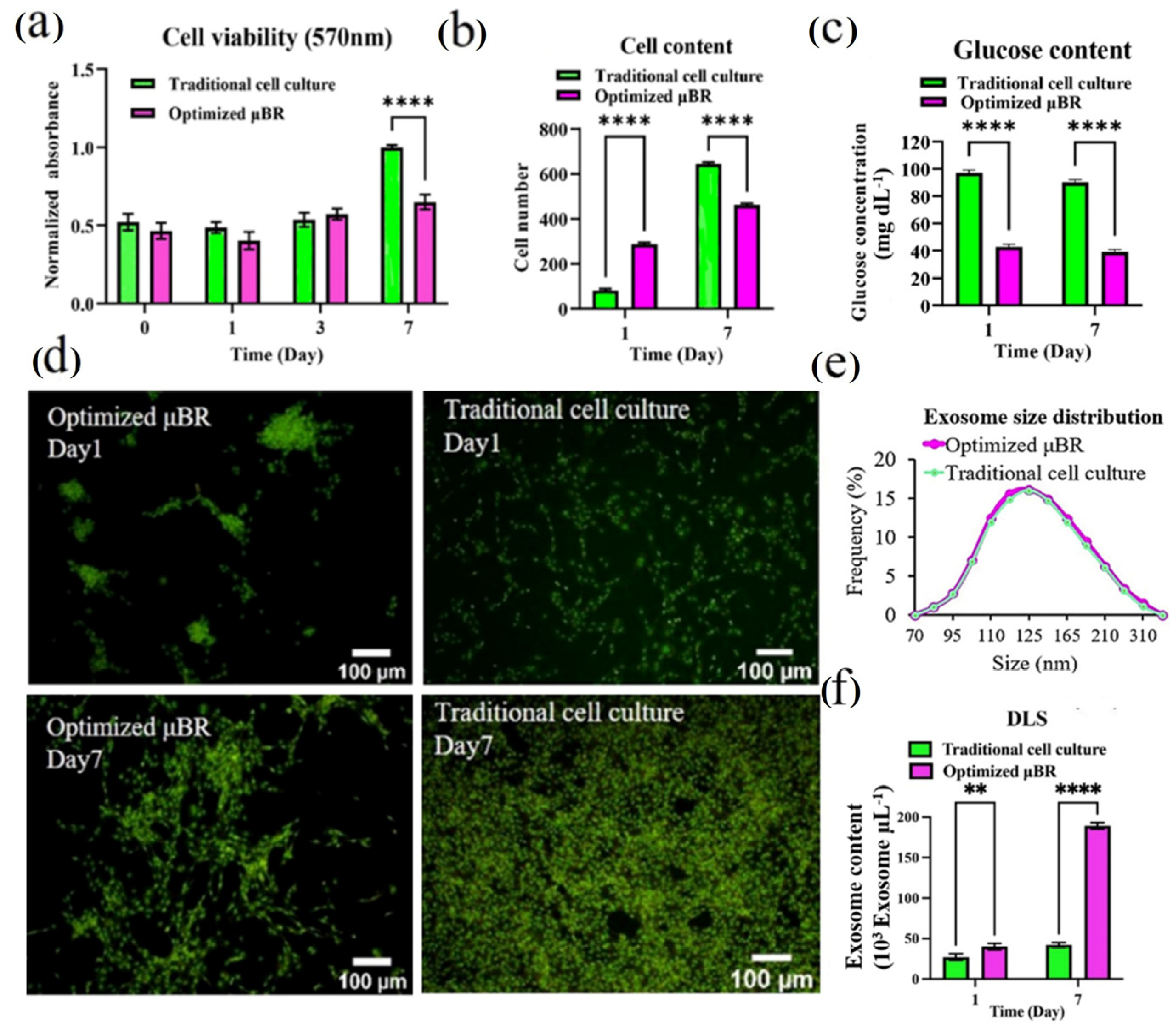
3.1. Culture Condition Characterization
3.2. Exosome Characterization
3.3. Cancer Model Optimization of TME Using RSM
3.4. Comparing the Behavior of Cells in Optimized Culture Conditions in μBR with Traditional Cell Culture
3.5. Glioblastoma Model On-a-Chip
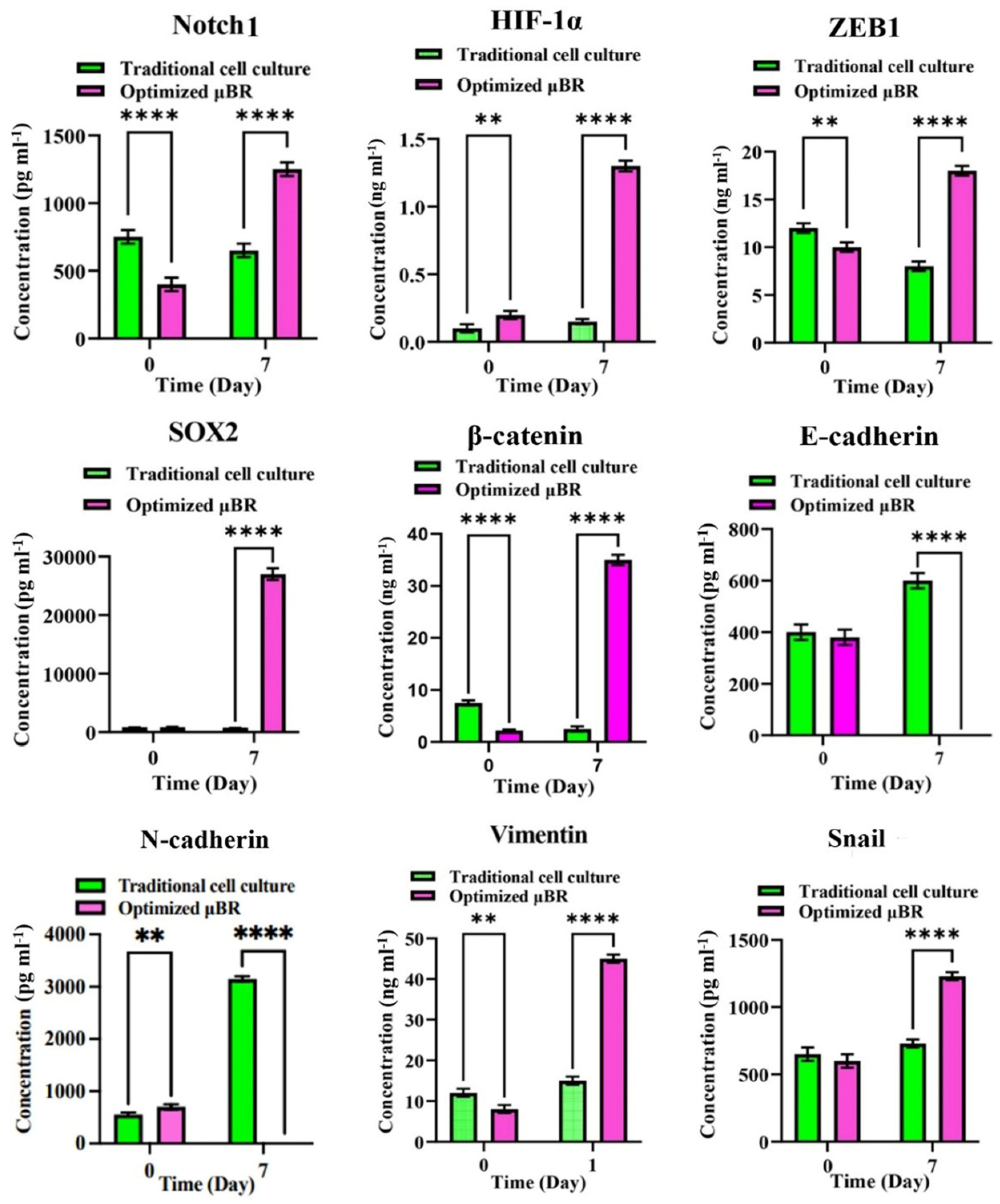
3.6. Epithelial-Mesenchymal-Transition Pathway Activation
3.7. EMT Pathway Is Triggered via a Variety of Axes
3.8. Enriched TME Stimulates HIF-1α Compensatory Pathway
3.9. Optimum Controlled Microenvironment Revives Neoplasm in the Cell Line
3.10. Mimicked TME in µBR Changes the Cancer Cell’s Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| TME | tumor microenvironment |
| µBR | microfluidic bioreactor |
| RSM | response surface methodology |
| EMT | epithelial-mesenchymal transition |
References
- Hicks, W.H.; Bird, C.E.; Traylor, J.I.; Shi, D.D.; El Ahmadieh, T.Y.; Richardson, T.E.; Mcbrayer, S.K.; Abdullah, K.G. Contemporary Mouse Models in Glioma Research. Cells 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and Function of Human Innate Immune Cells in a Humanized Mouse Model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef]
- Ramzy, G.M.; Koessler, T.; Ducrey, E.; McKee, T.; Ris, F.; Buchs, N.; Rubbia-Brandt, L.; Dietrich, P.Y.; Nowak-Sliwinska, P. Patient-Derived in Vitro Models for Drug Discovery in Colorectal Carcinoma. Cancers 2020, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yoshimura, K.; Sewastjanow-Silva, M.; Song, S.; Ajani, J.A. Challenges and Prospects of Patient-Derived Xenografts for Cancer Research. Cancers 2023, 15, 4352. [Google Scholar] [CrossRef]
- Thorel, L.; Perréard, M.; Florent, R.; Divoux, J.; Coffy, S.; Vincent, A.; Gaggioli, C.; Guasch, G.; Gidrol, X.; Weiswald, L.B.; et al. Patient-Derived Tumor Organoids: A New Avenue for Preclinical Research and Precision Medicine in Oncology. Exp. Mol. Med. 2024, 56, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Lê, H.; Deforges, J.; Hua, G.; Idoux-Gillet, Y.; Ponté, C.; Lindner, V.; Olland, A.; Falcoz, P.E.; Zaupa, C.; Jain, S.; et al. In Vitro Vascularized Immunocompetent Patient-Derived Model to Test Cancer Therapies. iScience 2023, 26, 108094. [Google Scholar] [CrossRef]
- Wan, L.; Neumann, C.A.; Leduc, P.R. Tumor-on-a-Chip for Integrating a 3D Tumor Microenvironment: Chemical and Mechanical Factors. Lab Chip 2020, 20, 873–888. [Google Scholar] [CrossRef]
- Mehta, P.; Rahman, Z.; ten Dijke, P.; Boukany, P.E. Microfluidics Meets 3D Cancer Cell Migration. Trends Cancer 2022, 8, 683–697. [Google Scholar] [CrossRef]
- Jouybar, M.; de Winde, C.M.; Wolf, K.; Friedl, P.; Mebius, R.E.; den Toonder, J.M.J. Cancer-on-Chip Models for Metastasis: Importance of the Tumor Microenvironment. Trends Biotechnol. 2024, 42, 431–448. [Google Scholar] [CrossRef]
- Cauli, E.; Polidoro, M.A.; Marzorati, S.; Bernardi, C.; Rasponi, M.; Lleo, A. Cancer-on-Chip: A 3D Model for the Study of the Tumor Microenvironment. J. Biol. Eng. 2023, 17, 53. [Google Scholar] [CrossRef]
- Farsani, H.Y.; Wutz, J.; DeVincentis, B.; Thomas, J.A.; Motevalian, S.P. Modeling Mass Transfer in Stirred Microbioreactors. Chem. Eng. Sci. 2022, 248, 117146. [Google Scholar] [CrossRef]
- Xie, Y.; Chindam, C.; Nama, N.; Yang, S.; Lu, M.; Zhao, Y.; Mai, J.D.; Costanzo, F.; Huang, T.J. Exploring Bubble Oscillation and Mass Transfer Enhancement in Acoustic-Assisted Liquid-Liquid Extraction with a Microfluidic Device. Sci. Rep. 2015, 5, 12572. [Google Scholar] [CrossRef]
- Leach, T.; Gandhi, U.; Reeves, K.D.; Stumpf, K.; Okuda, K.; Marini, F.C.; Walker, S.J.; Boucher, R.; Chan, J.; Cox, L.A.; et al. Development of a Novel Air—Liquid Interface Airway Tissue Equivalent Model for in Vitro Respiratory Modeling Studies. Sci. Rep. 2023, 13, 10137. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Song, J.M.; Yeom, E. Quantitative Study for Control of Air–Liquid Segmented Flow in a 3D-Printed Chip Using a Vacuum-Driven System. Sci. Rep. 2022, 12, 8986. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-esfahlan, R.; Seidi, K.; Ebrahimi-kalan, A. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 4, 59. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Mostafa, A.M.R.H.; Morton, J.P.; Hawinkels, L.J.A.C.; Prakash, J. Translating Complexity and Heterogeneity of Pancreatic Tumor: 3D in Vitro to in Vivo Models. Adv. Drug Deliv. Rev. 2021, 174, 265–293. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Bule, P.; Aguiar, S.I.; Aires-da-silva, F.; Nunes, J.; Dias, R.; Baranowska-bosiacka, I. Chemokine-Directed Tumor Microenvironment Modulation in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9804. [Google Scholar] [CrossRef]
- Cavallari, C.; Camussi, G.; Brizzi, M.F. Extracellular Vesicles in the Tumour Microenvironment: Eclectic Supervisors. Int. J. Mol. Sci. 2020, 21, 6768. [Google Scholar] [CrossRef]
- Maman, S.; Witz, I.P. A History of Exploring Cancer in Context. Nat. Rev. Cancer 2018, 18, 359–376. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Thomlinson, R.H. Hypoxia and Tumours. J. Clin. Pathol. 1977, 30, 105–113. [Google Scholar] [CrossRef]
- Macharia, L.W.; Wanjiru, C.M.; Mureithi, M.W.; Pereira, C.M.; Ferrer, V.P.; Moura-Neto, V. MicroRNAs, Hypoxia and the Stem-like State as Contributors to Cancer Aggressiveness. Front. Genet. 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Li, X.; Zhang, J.; Hu, Y.; Hu, B.; Shi, Y.; Sun, Y.; Hong, G. Conditioned Medium Mimicking the Tumor Microenvironment Augments Chemotherapeutic Resistance via Ataxia-Telangiectasia Mutated and Nuclear Factor-ΚB Pathways in Gastric Cancer Cells. Oncol. Rep. 2018, 40, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Basmaeil, Y.; Al Subayyil, A.; Abumaree, M.; Khatlani, T. Conditions Mimicking the Cancer Microenvironment Modulate the Functional Outcome of Human Chorionic Villus Mesenchymal Stem/Stromal Cells in Vitro. Front. Cell Dev. Biol. 2021, 9, 650125. [Google Scholar] [CrossRef]
- Xie, H.; Appelt, J.W.; Jenkins, R.W. Going with the Flow: Modeling the Tumor Microenvironment Using Microfluidic Technology. Cancers 2021, 13, 6052. [Google Scholar] [CrossRef]
- Devarasetty, M.; Dominijanni, A.; Herberg, S.; Shelkey, E.; Skardal, A.; Soker, S. Simulating the Human Colorectal Cancer Microenvironment in 3D Tumor-Stroma Co-Cultures in Vitro and in Vivo. Sci. Rep. 2020, 10, 9832. [Google Scholar] [CrossRef]
- Carter, E.P.; Roozitalab, R.; Gibson, S.V.; Grose, R.P. Tumour Microenvironment 3D-Modelling: Simplicity to Complexity and Back Again. Trends Cancer 2021, 7, 1033–1046. [Google Scholar] [CrossRef]
- Sariano, P.A.; Mizenko, R.R.; Shirure, V.S.; Brandt, A.K.; Nguyen, B.B.; Nesiri, C.; Shergill, B.S.; Brostoff, T.; Rocke, D.M.; Borowsky, A.D.; et al. Convection and Extracellular Matrix Binding Control Interstitial Transport of Extracellular Vesicles. J. Extracell. Vesicles 2023, 12, e12323. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.V.T.; Ye, S.; Gkouzioti, V.; van Wolferen, M.E.; Yengej, F.Y.; Melkert, D.; Siti, S.; de Jong, B.; Besseling, P.J.; Spee, B.; et al. A Human Kidney and Liver Organoid-Based Multi-Organ-on-a-Chip Model to Study the Therapeutic Effects and Biodistribution of Mesenchymal Stromal Cell-Derived Extracellular Vesicles. J. Extracell. Vesicles 2022, 11, 12280. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Jeong, D.; Kim, J.; Cho, S.; Jang, S.C.; Han, C.; Kang, J.Y.; Gho, Y.S.; Park, J. Microfluidic Fabrication of Cell-Derived Nanovesicles as Endogenous RNA Carriers. Lab Chip 2014, 14, 1261–1269. [Google Scholar] [CrossRef]
- Zhu, Q.; Heon, M.; Zhao, Z.; He, M. Microfluidic Engineering of Exosomes: Editing Cellular Messages for Precision Therapeutics. Lab Chip 2018, 18, 1690–1703. [Google Scholar] [CrossRef]
- Lyssiotis, C.A.; Kimmelman, A.C. Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol. 2017, 27, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G. Vander Understanding the Warburg Effect. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro: Selective Externalization of the Receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammonds, J.R.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of Exosomal Proteins, RNA and Lipids. Nucleic Acids Res. 2012, 40, 1241–1244. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Silvani, G.; Basirun, C.; Wu, H.; Mehner, C.; Poole, K.; Bradbury, P.; Chou, J. A 3D-Bioprinted Vascularized Glioblastoma-on-a-Chip for Studying the Impact of Simulated Microgravity as a Novel Pre-Clinical Approach in Brain Tumor Therapy. Adv. Ther. 2021, 4, 2100106. [Google Scholar] [CrossRef]
- Hussain, H.; Santhana Raj, L.; Ahmad, S.; Abd. Razak, M.F.; Wan Mohamud, W.N.; Bakar, J.; Ghazali, H.M. Determination of Cell Viability Using Acridine Orange/Propidium Iodide Dual-Spectrofluorometry Assay. Cogent Food Agric. 2019, 5, 1582398. [Google Scholar] [CrossRef]
- Shi, Z.; Liang, Y.J.; Chen, Z.S.; Wang, X.W.; Wang, X.H.; Ding, Y.; Chen, L.M.; Yang, X.P.; Fu, L.W. Reversal of MDR1/P-Glycoprotein-Mediated Multidrug Resistance by Vector-Based RNA Interference in Vitro and in Vivo. Cancer Biol. Ther. 2006, 5, 39–47. [Google Scholar] [CrossRef]
- Limlawan, P.; Marger, L.; Durual, S.; Vacharaksa, A. Delivery of MicroRNA-302a-3p by APTES Modified Hydroxyapatite Nanoparticles to Promote Osteogenic Differentiation in Vitro. BDJ Open 2023, 9, 8. [Google Scholar] [CrossRef]
- Yurtsever, A.; Yoshida, T.; Behjat, A.B.; Araki, Y.; Hanayama, R.; Fukuma, T. Structural and Mechanical Characteristics of Exosomes from Osteosarcoma Cells Explored. Nanoscale 2021, 13, 6661–6677. [Google Scholar] [CrossRef]
- Passos Gibson, V.; Tahiri, H.; Yang, C.; Phan, Q.T.; Banquy, X.; Hardy, P. Hyaluronan Decorated Layer-by-Layer Assembled Lipid Nanoparticles for MiR-181a Delivery in Glioblastoma Treatment. Biomaterials 2023, 302, 122341. [Google Scholar] [CrossRef]
- Shiri, F.; Feng, H.; Petersen, K.E.; Sant, H.; Bardi, G.T.; Schroeder, L.A.; Merchant, M.L.; Gale, B.K.; Hood, J.L. Separation of U87 Glioblastoma Cell-Derived Small and Medium Extracellular Vesicles Using Elasto-Inertial Flow Focusing (a Spiral Channel). Sci. Rep. 2022, 12, 6146. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Evenson, D.; Kapuscinski, J.; Melamed, M.R. Denaturation of RNA and DNA in Situ Induced by Acridine Orange. Exp. Cell Res. 1983, 148, 31–46. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Tanaka, F.; Morita, H.; Hiraki, A.; Hashimoto, S. Hypoxia-Induced HIF-1α and ZEB1 Are Critical for the Malignant Transformation of Ameloblastoma via TGF-β-Dependent EMT. Cancer Med. 2019, 8, 7822–7832. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Rahimpour, S.; Nesvick, C.L.; Zhang, X.; Ma, J.; Zhang, M.; Zhang, G.; Wang, L.; Yang, C.; Hong, C.S.; et al. Activation of Hypoxia Signaling Induces Phenotypic Transformation of Glioma Cells: Implications for Bevacizumab Antiangiogenic Therapy. Oncotarget 2015, 6, 11882–11893. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462. [Google Scholar] [CrossRef]
- Lu, Z.; Ghosh, S.; Wang, Z.; Hunter, T. Downregulation of Caveolin-1 Function by EGF Leads to the Loss of E-Cadherin, Increased Transcriptional Activity of β-Catenin, and Enhanced Tumor Cell Invasion. Cancer Cell 2003, 4, 499–515. [Google Scholar] [CrossRef]
- Ahmed, N.; Maines-Bandiera, S.; Quinn, M.A.; Unger, W.G.; Dedhar, S.; Auersperg, N.; Ahmed, S.; Maines-Bandiera, M.A.; Quinn, W.G.; Unger, S.; et al. Molecular Pathways Regulating EGF-Induced Epithelio-Mesenchymal Transition in Human Ovarian Surface Epithelium. Am. J. Physiol. Cell Physiol. 2006, 290, 1532–1542. [Google Scholar] [CrossRef]
- Oh, A.; Pardo, M.; Rodriguez, A.; Yu, C.; Nguyen, L.; Liang, O.; Chorzalska, A.; Dubielecka, P.M. NF-ΚB Signaling in Neoplastic Transition from Epithelial to Mesenchymal Phenotype. Cell Commun. Signal. 2023, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia Promotes Fibrogenesis in Vivo via HIF-1 Stimulation of Epithelial-to-Mesenchymal Transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, Y.L.; Liang, X.H. EMT: A New Vision of Hypoxia Promoting Cancer Progression. Cancer Biol. Ther. 2011, 11, 714–723. [Google Scholar] [CrossRef]
- Cooper, L.A.D.; Gutman, D.A.; Chisolm, C.; Appin, C.; Kong, J.; Rong, Y.; Kurc, T.; Van Meir, E.G.; Saltz, J.H.; Moreno, C.S.; et al. The Tumor Microenvironment Strongly Impacts Master Transcriptional Regulators and Gene Expression Class of Glioblastoma. Am. J. Pathol. 2012, 180, 2108–2119. [Google Scholar] [CrossRef]
- Afify, S.M.; Hassan, G.; Yan, T.; Seno, A.; Seno, M. Cancer Stem Cell Initiation by Tumor-Derived Extracellular Vesicles. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2022; Volume 2549, pp. 399–407. [Google Scholar]
- Sun, Z.; Wang, L.; Zhou, Y.; Dong, L.; Ma, W.; Lv, L.; Zhang, J.; Wang, X. Glioblastoma Stem Cell-Derived Exosomes Enhance Stemness and Tumorigenicity of Glioma Cells by Transferring Notch1 Protein. Cell. Mol. Neurobiol. 2020, 40, 767–784. [Google Scholar] [CrossRef]
- Comijn, J.; Comijn, J.; Berx, G.; Berx, G.; Vermassen, P.; Vermassen, P.; Verschueren, K.; Verschueren, K.; van Grunsven, L.; van Grunsven, L.; et al. The Two-Handed E Box Binding Zinc Finger Protein SIP1 Downregulates E-Cadherin and Induces Invasion. Mol. Cell 2001, 7, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, U.D.; Maciaczyk, D.; Doostkam, S.; Orr, B.A.; Simons, B.; Bogiel, T.; Reithmeier, T.; Prinz, M.; Schubert, J.; Niedermann, G.; et al. Activation of Canonical WNT/β-Catenin Signaling Enhances in Vitro Motility of Glioblastoma Cells by Activation of ZEB1 and Other Activators of Epithelial-to-Mesenchymal Transition. Cancer Lett. 2012, 325, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-ΚB Is Essential for Epithelial-Mesenchymal Transition and Metastasis in a Model of Breast Cancer Progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.V.; Conroy, S.; Tomar, T.; Eggens-Meijer, E.; Bhat, K.; Copray, S.; Walenkamp, A.M.E.; Boddeke, E.; Balasubramanyian, V.; Wagemakers, M.; et al. TGF-β Is an Inducer of ZEB1-Dependent Mesenchymal Transdifferentiation in Glioblastoma That Is Associated with Tumor Invasion. Cell Death Dis. 2014, 5, e1443-14. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.S.; Zhou, B.H.; Li, C.L.; Zhang, F.; Wang, X.F.; Zhang, G.; Bu, X.Z.; Cai, S.H.; Du, J. Epithelial-Mesenchymal Transition (EMT) Induced by TNF-α Requires AKT/GSK-3β-Mediated Stabilization of Snail in Colorectal Cancer. PLoS ONE 2013, 8, e56664. [Google Scholar] [CrossRef]

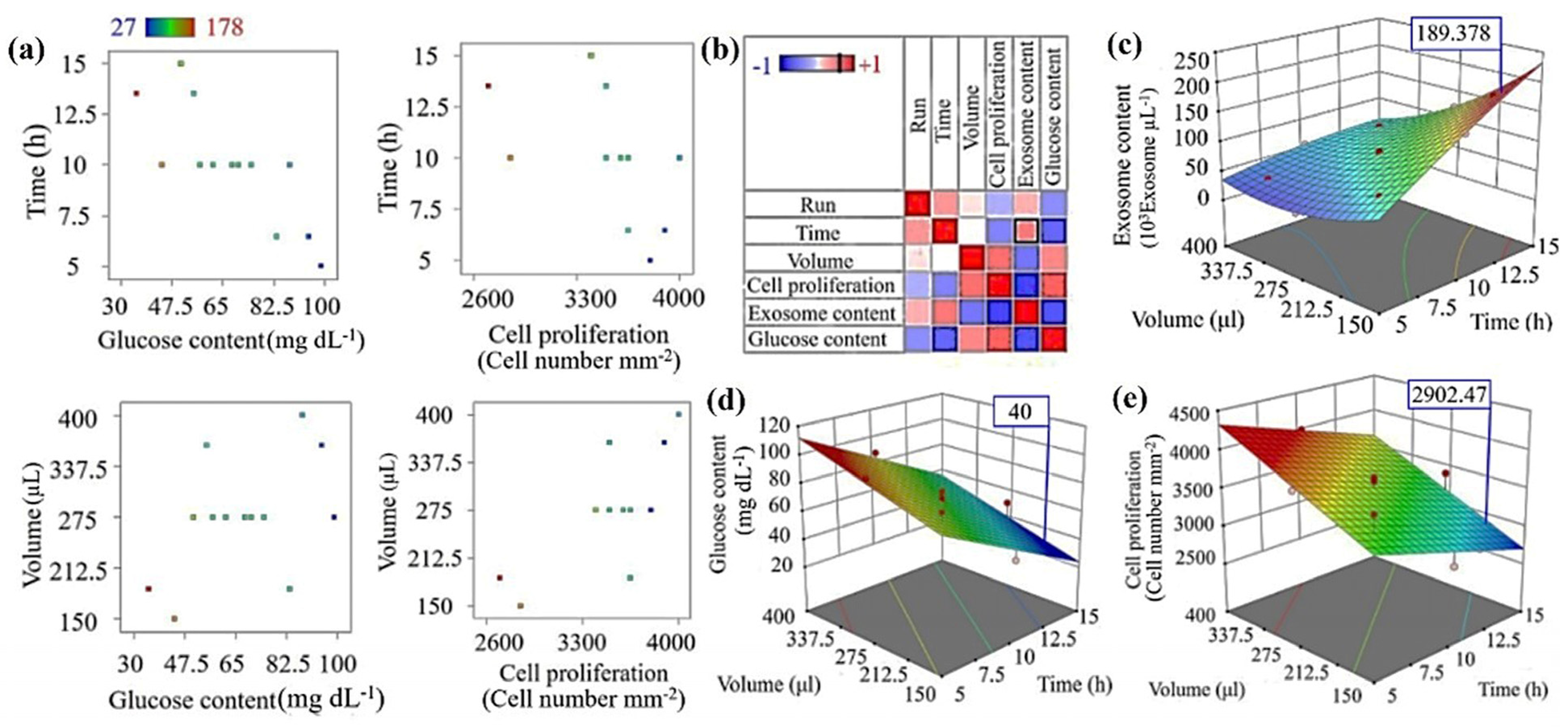




| Standard Order. | Variable | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| A: Time (h) | B: Volume (µL) | Experimental Values | Predictive Values | |||||
| Cell Proliferation (Cell Number/mm2) | Exosomes Content (103 Exosomes/µL) | Glucose Content (mg/dL) | Cell Proliferation (Cell Number/mm2) | Exosomes Content (103 Exosomes/µL) | Glucose Content (mg/dL) | |||
| 1 | 6.46 | 186 | 3650 | 75 | 83.6 | 3420 | 74.88 | 75.79 |
| 2 | 13.54 | 186 | 2700 | 178 | 35.2 | 2941 | 177.58 | 36.68 |
| 3 | 6.46 | 363 | 3900 | 38 | 94.6 | 4089 | 37.92 | 99.05 |
| 4 | 13.54 | 363 | 3500 | 72 | 55 | 3610 | 71.62 | 59.93 |
| 5 | 5 | 275 | 3800 | 27 | 99 | 3854 | 27.03 | 95.52 |
| 6 | 15 | 275 | 3400 | 123 | 50.6 | 3176 | 123.47 | 40.21 |
| 7 | 10 | 150 | 2850 | 156 | 44 | 3042 | 156.28 | 51.42 |
| 8 | 10 | 400 | 4000 | 55 | 88 | 3988 | 55.22 | 84.31 |
| 9 | 10 | 275 | 3650 | 85 | 70.4 | 3515 | 81.40 | 67.86 |
| 10 | 10 | 275 | 3500 | 80 | 68.2 | 3515 | 81.40 | 67.86 |
| 11 | 10 | 275 | 3600 | 82 | 61.6 | 3515 | 81.40 | 67.86 |
| 12 | 10 | 275 | 3500 | 77 | 74.8 | 3515 | 81.40 | 67.86 |
| 13 | 10 | 275 | 3650 | 83 | 57.2 | 3515 | 81.40 | 67.86 |
| Genes | Forward Primer Sequence (5′->3′) | Reverse Primer Sequence (5′->3′) |
|---|---|---|
| BAX | GGCCCTTTTGCTTCAGGGTT | GGAAAAAGACCTCTCGGGGG |
| BCL2 | GGTGAACTGGGGGAGGATTG | ATCACCAAGTGCACCTACCC |
| Ki-67 | TTTGGGTGCGACTTGACGAG | CGTCCAGCATGTTCTGAGGA |
| OCT-4 | CGCCGTATGAGTTCTGTGGG | CTGATCTGCTGCAGTGTGGGT |
| SOX2 | ATGGACAGTTACGCGCACAT | CGAGCTGGTCATGGAGTTGT |
| NF-κB | CGACAGCGGGGAAAGACAC | TGCCATTCTGAAGCTGGTGG |
| E-cadherin | GCTGGACCGAGAGAGTTTCC | CAAAATCCAAGCCCGTGGTG |
| N-cadherin | AAAGACCCATCCACGCTGAG | GCTCAAGGACCCCAAGGTG |
| Vimentin | GGACCAGCTAACCAACGACA | AAGGTCAAGACGTGCCAGAG |
| SNAIL | CGAGTGGTTCTTCTGCGCTA | GGGCTGCTGGAAGGTAAACT |
| ZEB1 | GGCGCAATAACGGAAAGGAAG | AGCCAGAATGGGAAAAGCGT |
| β-catenin | GGAGGAAGGTCTGAGGAGCA | AGGCTCCAGAAGCAGTCATC |
| STAT | CAGGAGCTGAAAAACCAGCAGT | GGGGATTCGGGGATAGAGGA |
| Notch1 | GCGAGGAAGATACGGAGTGG | GCCTTCCAGCCTGCCTTTTA |
| TGFβ | TGGTGGAAACCCACAACGAA | CGGTAGTGAACCCGTTGATG |
| EGF | CTGAATGTCCCCTGTCCCAC | TGCATTGACCCCAAGGTTGA |
| SMAD | TCACATCTCTCCCGTGCTGC | CATGCAGTGAGGCAATCGAC |
| β-actin | GGCATCCTCACCCTGAAGTA | AGGTGTGGTGCCAGATTTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saffar, S.; Ghiaseddin, A.; Irani, S.; Hamidieh, A.A. Optimization of In-Situ Exosome Enrichment Methodology On-a-Chip to Mimic Tumor Microenvironment Induces Cancer Stemness in Glioblastoma Tumor Model. Cells 2025, 14, 676. https://doi.org/10.3390/cells14090676
Saffar S, Ghiaseddin A, Irani S, Hamidieh AA. Optimization of In-Situ Exosome Enrichment Methodology On-a-Chip to Mimic Tumor Microenvironment Induces Cancer Stemness in Glioblastoma Tumor Model. Cells. 2025; 14(9):676. https://doi.org/10.3390/cells14090676
Chicago/Turabian StyleSaffar, Saleheh, Ali Ghiaseddin, Shiva Irani, and Amir Ali Hamidieh. 2025. "Optimization of In-Situ Exosome Enrichment Methodology On-a-Chip to Mimic Tumor Microenvironment Induces Cancer Stemness in Glioblastoma Tumor Model" Cells 14, no. 9: 676. https://doi.org/10.3390/cells14090676
APA StyleSaffar, S., Ghiaseddin, A., Irani, S., & Hamidieh, A. A. (2025). Optimization of In-Situ Exosome Enrichment Methodology On-a-Chip to Mimic Tumor Microenvironment Induces Cancer Stemness in Glioblastoma Tumor Model. Cells, 14(9), 676. https://doi.org/10.3390/cells14090676








