From Quiescence to Activation: The Reciprocal Regulation of Ras and Rho Signaling in Hepatic Stellate Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
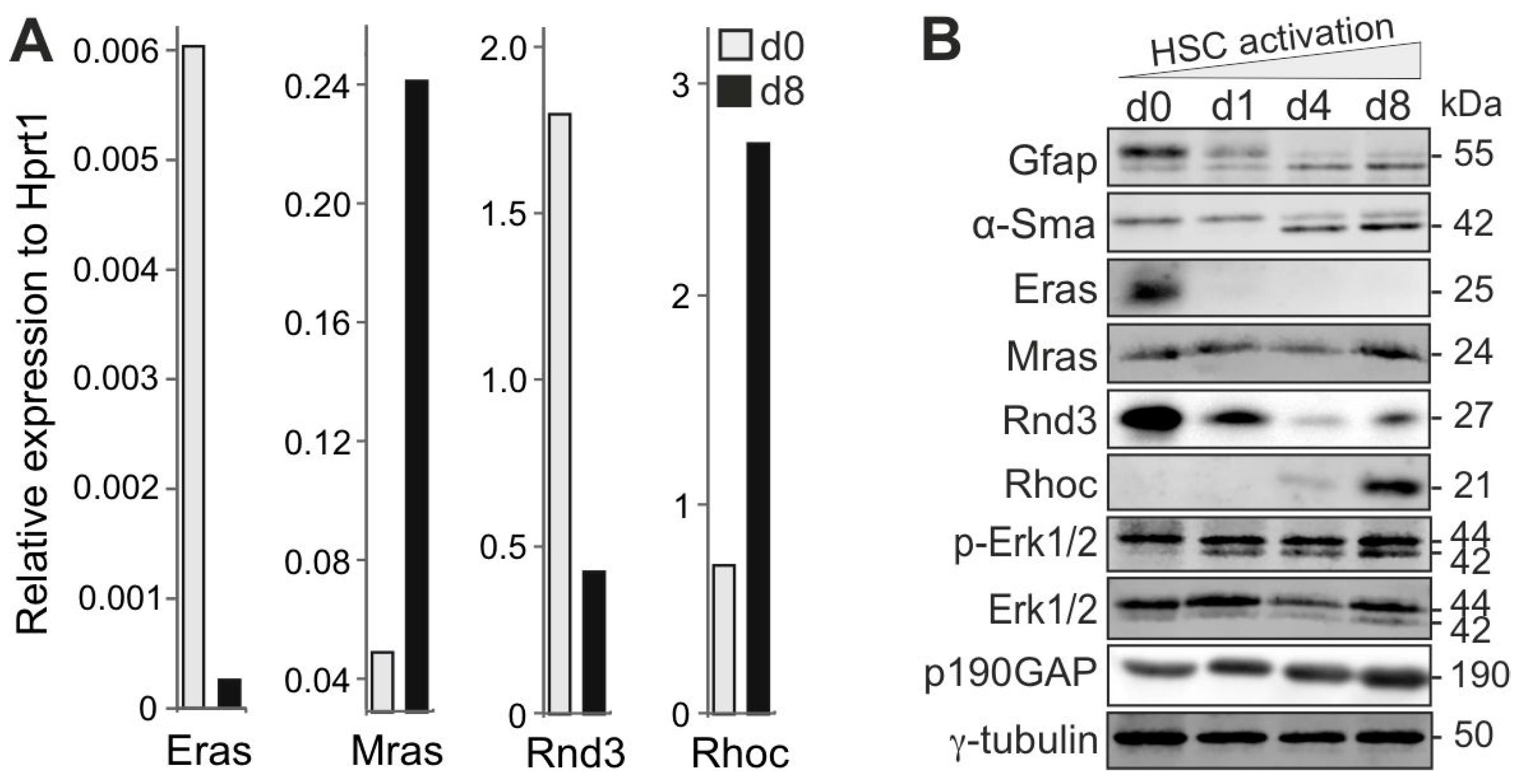
3.1. Reciprocal, Paralog-Specific Ras and Rho GTPases in HSC Fate Determination
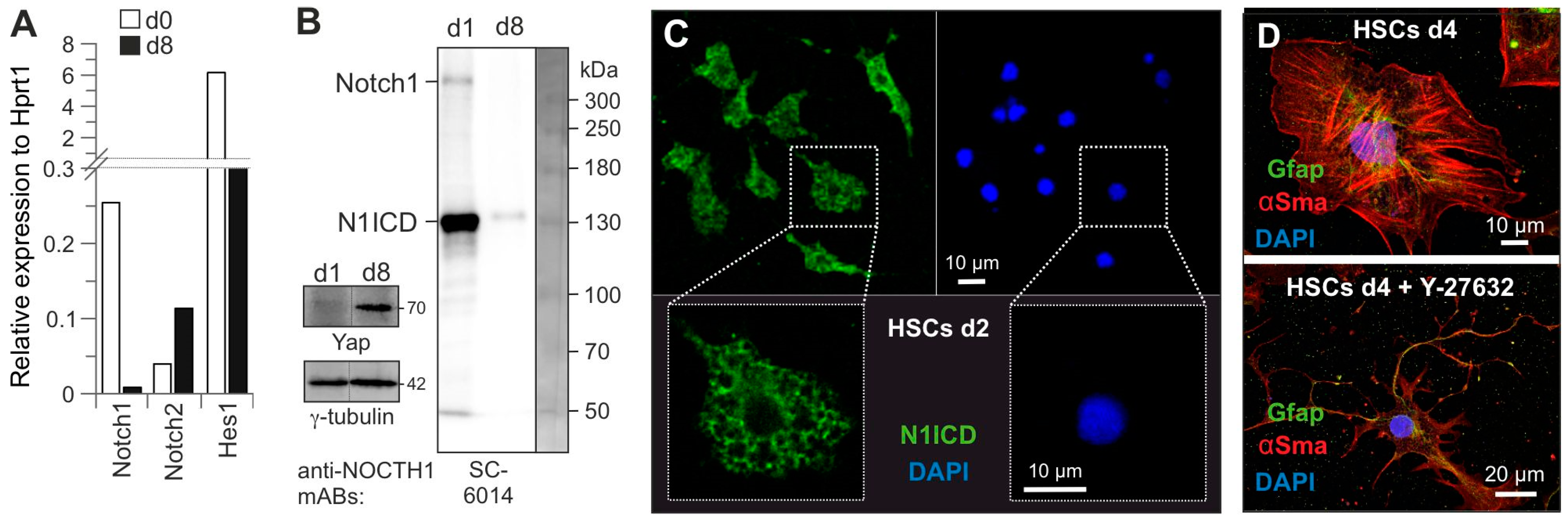
3.2. The Key Role of Cytosolic Notch1 and HSC Quiescence State
3.3. Rock a Key Determinant of HSC Activation
3.4. Role of Eras-Arg1-Polyamine Axis in HSC Homeostasis
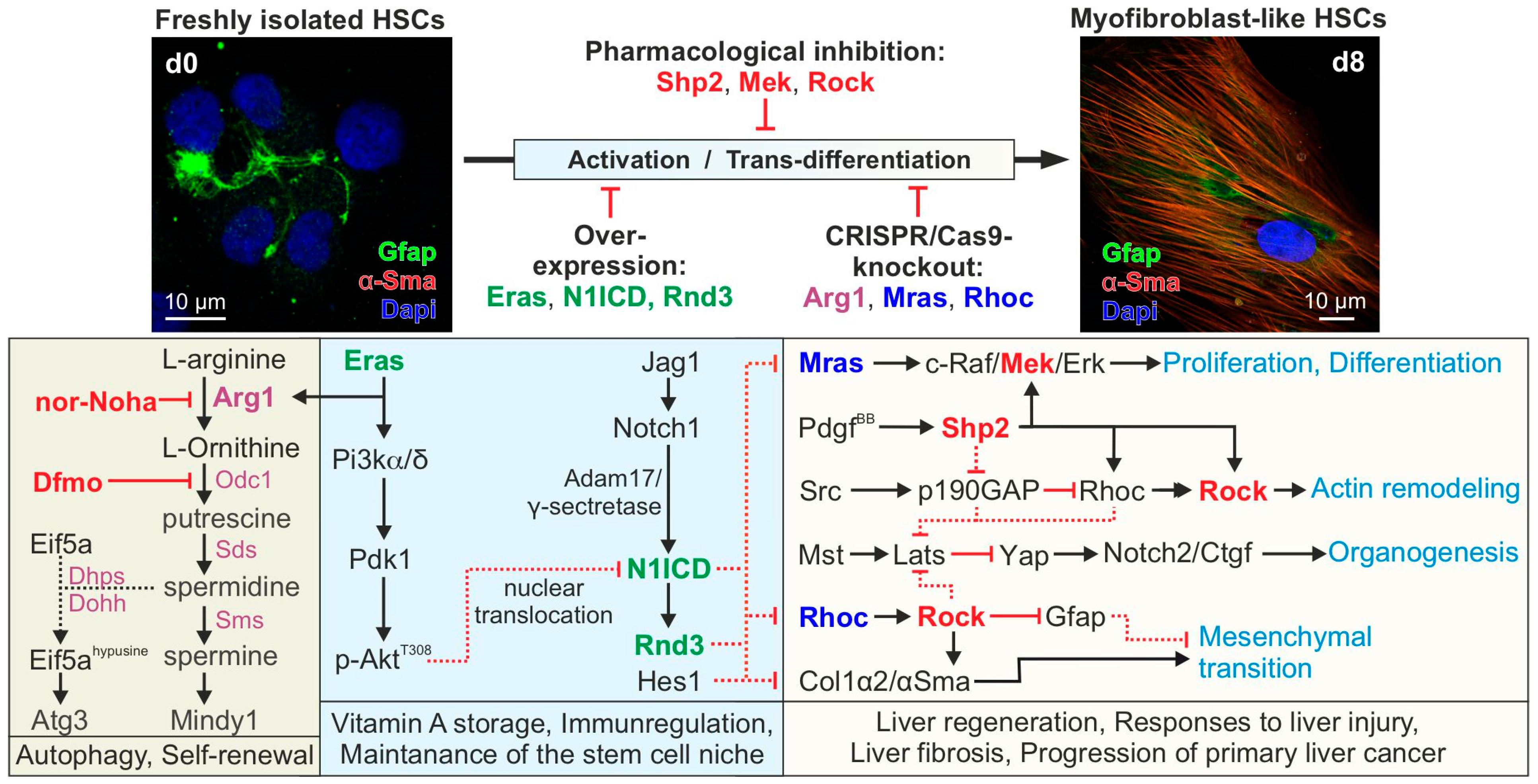
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Investig. 2013, 123, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei-Rad, S.; Nakhaeizadeh, H.; Götze, S.; Kordes, C.; Sawitza, I.; Hoffmann, M.J.; Franke, M.; Schulz, W.A.; Scheller, J.; Piekorz, R.P.; et al. The Role of Embryonic Stem Cell-expressed RAS (ERAS) in the Maintenance of Quiescent Hepatic Stellate Cells. J. Biol. Chem. 2016, 291, 8399–8413. [Google Scholar] [CrossRef]
- Lee, Y.; Leslie, J.; Yang, Y.; Ding, L. Hepatic stellate and endothelial cells maintain hematopoietic stem cells in the developing liver. J. Exp. Med. 2021, 218, e20200882. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka-Garbowska, K.; Galuppo, L.; Bourebaba, L. The Hepatic Stellate Cells (HSTCs) and Adipose-derived Mesenchymal Stem Cells (ASCs) Axis as a Potential Major Driver of Metabolic Syndrome—Novel Concept and Therapeutic Implications. Stem Cell Rev. Rep. 2022, 18, 1417–1422. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Badri, N. Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1079, 69–86. [Google Scholar] [CrossRef]
- Dias, D.O.; Kalkitsas, J.; Kelahmetoglu, Y.; Estrada, C.P.; Tatarishvili, J.; Holl, D.; Jansson, L.; Banitalebi, S.; Amiry-Moghaddam, M.; Ernst, A.; et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 2021, 12, 5501. [Google Scholar] [CrossRef]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Kordes, C.; Sawitza, I.; Götze, S.; Herebian, D.; Häussinger, D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J. Clin. Investig. 2014, 124, 5503–5515. [Google Scholar] [CrossRef] [PubMed]
- Kordes, C.; Sawitza, I.; Häussinger, D. Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells. Biochem. Biophys. Res. Commun. 2008, 367, 116–123. [Google Scholar] [CrossRef]
- Carloni, V.; Defranco, R.M.; Caligiuri, A.; Gentilini, A.; Sciammetta, S.C.; Baldi, E.; Lottini, B.; Gentilini, P.; Pinzani, M. Cell adhesion regulates platelet-derived growth factor-induced MAP kinase and PI-3 kinase activation in stellate cells. Hepatology 2002, 36, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Karaca, G.; Swiderska-Syn, M.; Michelotti, G.A.; Krüger, L.; Chen, Y.; Premont, R.T.; Choi, S.S.; Diehl, A.M. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology 2013, 58, 1801–1813. [Google Scholar] [CrossRef]
- Foglia, B.; Cannito, S.; Bocca, C.; Parola, M.; Novo, E. ERK Pathway in Activated, Myofibroblast-Like, Hepatic Stellate Cells: A Critical Signaling Crossroad Sustaining Liver Fibrosis. Int. J. Mol. Sci. 2019, 20, 2700. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Khalifa, M.O.; Gu, W.; Li, T.S. Hydrostatic pressure induces profibrotic properties in hepatic stellate cells via the RhoA/ROCK signaling pathway. FEBS Open Bio 2022, 12, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Yue, M.; Chen, H.; Sun, C.; Shao, Y.; Jin, Q.; Zhang, C.; Yu, G. YAP/TAZ as master regulators in liver regeneration and disease: Insights into mechanisms and therapeutic targets. Mol. Biol. Rep. 2024, 52, 78. [Google Scholar] [CrossRef]
- Pibiri, M.; Simbula, G. Role of the Hippo pathway in liver regeneration and repair: Recent advances. Inflamm. Regen. 2022, 42, 59. [Google Scholar] [CrossRef]
- Fukushima, M.; Nakamuta, M.; Kohjima, M.; Kotoh, K.; Enjoji, M.; Kobayashi, N.; Nawata, H. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int. 2005, 25, 829–838. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.Y.; Yang, C.Q.; Jiang, W. Effect of RhoA on transforming growth factor β1-induced rat hepatic stellate cell migration. Liver Int. 2012, 32, 1093–1102. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Pudewell, S.; Lissy, J.; Nakhaeizadeh, H.; Taha, M.S.; Akbarzadeh, M.; Rezaei Adariani, S.; Nakhaei-Rad, S.; Li, J.; Kordes, C.; Häussinger, D.; et al. Physical Interaction between Embryonic Stem Cell-Expressed Ras (ERas) and Arginase-1 in Quiescent Hepatic Stellate Cells. Cells 2022, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Labun, K.; Montague, T.G.; Krause, M.; Torres Cleuren, Y.N.; Tjeldnes, H.; Valen, E. CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019, 47, W171–W174. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.E.; Baldeosingh, R.; Lamm, R.; Patel, K.; Zhang, K.; Dominguez, D.A.; Kirton, K.J.; Shah, A.P.; Dang, H. Hepatic Stellate Cells and Hepatocarcinogenesis. Front. Cell Dev. Biol. 2020, 8, 709. [Google Scholar] [CrossRef]
- Takahashi, K.; Mitsui, K.; Yamanaka, S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature 2003, 423, 541–545. [Google Scholar] [CrossRef]
- Bravo-Cordero, J.J.; Sharma, V.P.; Roh-Johnson, M.; Chen, X.; Eddy, R.; Condeelis, J.; Hodgson, L. Spatial regulation of RhoC activity defines protrusion formation in migrating cells. J. Cell Sci. 2013, 126, 3356–3369. [Google Scholar] [CrossRef]
- Monaghan-Benson, E.; Wittchen, E.S.; Doerschuk, C.M.; Burridge, K. A Rnd3/p190RhoGAP pathway regulates RhoA activity in idiopathic pulmonary fibrosis fibroblasts. Mol. Biol. Cell 2018, 29, 2165–2175. [Google Scholar] [CrossRef]
- Zhu, Z.; Todorova, K.; Lee, K.K.; Wang, J.; Kwon, E.; Kehayov, I.; Kim, H.G.; Kolev, V.; Dotto, G.P.; Lee, S.W.; et al. Small GTPase RhoE/Rnd3 is a critical regulator of Notch1 signaling. Cancer Res. 2014, 74, 2082–2093. [Google Scholar] [CrossRef]
- Reister, S.; Kordes, C.; Sawitza, I.; Häussinger, D. The epigenetic regulation of stem cell factors in hepatic stellate cells. Stem Cells Dev. 2011, 20, 1687–1699. [Google Scholar] [CrossRef]
- Duncan, A.W.; Rattis, F.M.; DiMascio, L.N.; Congdon, K.L.; Pazianos, G.; Zhao, C.; Yoon, K.; Cook, J.M.; Willert, K.; Gaiano, N.; et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 2005, 6, 314–322. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Hui, Y.; Du, Y.; Chen, Z.; Feng, H.; Zhang, S.; Li, N.; Song, J.; Fang, Y.; et al. WNT/NOTCH Pathway Is Essential for the Maintenance and Expansion of Human MGE Progenitors. Stem Cell Rep. 2019, 12, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.J.; Li, X.G.; Wang, X.Q. Notch Signaling in Mammalian Intestinal Stem Cells: Determining Cell Fate and Maintaining Homeostasis. Curr. Stem Cell Res. Ther. 2019, 14, 583–590. [Google Scholar] [CrossRef]
- Moriyama, H.; Moriyama, M.; Isshi, H.; Ishihara, S.; Okura, H.; Ichinose, A.; Ozawa, T.; Matsuyama, A.; Hayakawa, T. Role of notch signaling in the maintenance of human mesenchymal stem cells under hypoxic conditions. Stem Cells Dev. 2014, 23, 2211–2224. [Google Scholar] [CrossRef]
- Bigas, A.; Porcheri, C. Notch and Stem Cells. Adv. Exp. Med. Biol. 2018, 1066, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.Q.; Ai, W.B.; Hu, Q.T.; Zhang, Q.J.; Wan, L.Y.; Wang, X.L.; Liu, C.B.; Wu, J.F. Hes1, an important gene for activation of hepatic stellate cells, is regulated by Notch1 and TGF-β/BMP signaling. World J. Gastroenterol. 2015, 21, 878–887. [Google Scholar] [CrossRef]
- Young, L.C.; Hartig, N.; Muñoz-Alegre, M.; Oses-Prieto, J.A.; Durdu, S.; Bender, S.; Vijayakumar, V.; Vietri Rudan, M.; Gewinner, C.; Henderson, S.; et al. An MRAS, SHOC2, and SCRIB Complex Coordinates ERK Pathway Activation with Polarity and Tumorigenic Growth. Mol. Cell 2013, 52, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Reymond, N.; Im, J.H.; Garg, R.; Cox, S.; Soyer, M.; Riou, P.; Colomba, A.; Muschel, R.J.; Ridley, A.J. RhoC and ROCKs regulate cancer cell interactions with endothelial cells. Mol. Oncol. 2015, 9, 1043–1055. [Google Scholar] [CrossRef]
- Komander, D.; Garg, R.; Wan, P.T.; Ridley, A.J.; Barford, D. Mechanism of multi-site phosphorylation from a ROCK-I:RhoE complex structure. EMBO J. 2008, 27, 3175–3185. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Görtzen, J.; Schierwagen, R.; Bierwolf, J.; Klein, S.; Uschner, F.E.; van der Ven, P.F.; Fürst, D.O.; Strassburg, C.P.; Laleman, W.; Pollok, J.M.; et al. Interplay of Matrix Stiffness and c-SRC in Hepatic Fibrosis. Front. Physiol. 2015, 6, 359. [Google Scholar] [CrossRef]
- Yasui, Y.; Amano, M.; Nagata, K.; Inagaki, N.; Nakamura, H.; Saya, H.; Kaibuchi, K.; Inagaki, M. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J. Cell Biol. 1998, 143, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Tamashiro, D.A.; Alarcon, V.B. Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Dev. Biol. 2014, 394, 142–155. [Google Scholar] [CrossRef]
- Shi, B.; Wang, W.; Ye, M.; Liang, M.; Yu, Z.; Zhang, Y.; Liu, Z.; Liang, X.; Ao, J.; Xu, F.; et al. Spermidine suppresses the activation of hepatic stellate cells to cure liver fibrosis through autophagy activator MAP1S. Liver Int. 2023, 43, 1307–1319. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Nishimura, K.; Zanelli, C.F.; Valentini, S.R. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 2010, 38, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Lubas, M.; Harder, L.M.; Kumsta, C.; Tiessen, I.; Hansen, M.; Andersen, J.S.; Lund, A.H.; Frankel, L.B. eIF5A is required for autophagy by mediating ATG3 translation. EMBO Rep. 2018, 19, e46072. [Google Scholar] [CrossRef]
- James, C.; Zhao, T.Y.; Rahim, A.; Saxena, P.; Muthalif, N.A.; Uemura, T.; Tsuneyoshi, N.; Ong, S.; Igarashi, K.; Lim, C.Y.; et al. MINDY1 Is a Downstream Target of the Polyamines and Promotes Embryonic Stem Cell Self-Renewal. Stem Cells 2018, 36, 1170–1178. [Google Scholar] [CrossRef]
- Kordes, C.; Sawitza, I.; Müller-Marbach, A.; Ale-Agha, N.; Keitel, V.; Klonowski-Stumpe, H.; Häussinger, D. CD133+ hepatic stellate cells are progenitor cells. Biochem. Biophys. Res. Commun. 2007, 352, 410–417. [Google Scholar] [CrossRef]
- Kordes, C.; Sawitza, I.; Götze, S.; Häussinger, D. Hepatic stellate cells support hematopoiesis and are liver-resident mesenchymal stem cells. Cell. Physiol. Biochem. 2013, 31, 290–304. [Google Scholar] [CrossRef]
- van Grunsven, L.A. 3D in vitro models of liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 133–146. [Google Scholar] [CrossRef]
- El Taghdouini, A.; Najimi, M.; Sancho-Bru, P.; Sokal, E.; van Grunsven, L.A. In vitro reversion of activated primary human hepatic stellate cells. Fibrogenesis Tissue Repair 2015, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Sakai-Sawada, K.; Niitsu, Y.; Tamura, Y. Vitamin A and insulin are required for the maintenance of hepatic stellate cell quiescence. Exp. Cell Res. 2016, 341, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Cottrill, K.A.; Annis, S.; Bhat, B.; Gochuico, B.R.; Osorio, J.C.; Rosas, I.; Haley, K.J.; Corey, K.E.; Chung, R.T.; et al. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci. Rep. 2015, 5, 18277. [Google Scholar] [CrossRef] [PubMed]
- Yanguas, S.C.; Cogliati, B.; Willebrords, J.; Maes, M.; Colle, I.; van den Bossche, B.; de Oliveira, C.; Andraus, W.; Alves, V.A.F.; Leclercq, I.; et al. Experimental models of liver fibrosis. Arch. Toxicol. 2016, 90, 1025–1048. [Google Scholar] [CrossRef]
- Sung, Y.C.; Liu, Y.C.; Chao, P.H.; Chang, C.C.; Jin, P.R.; Lin, T.T.; Lin, J.A.; Cheng, H.T.; Wang, J.; Lai, C.P.; et al. Combined delivery of sorafenib and a MEK inhibitor using CXCR4-targeted nanoparticles reduces hepatic fibrosis and prevents tumor development. Theranostics 2018, 8, 894–905. [Google Scholar] [CrossRef]
- Reichert, D.; Adolph, L.; Köhler, J.P.; Buschmann, T.; Luedde, T.; Häussinger, D.; Kordes, C. Improved Recovery from Liver Fibrosis by Crenolanib. Cells 2021, 10, 804. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhaei-Rad, S.; Pudewell, S.; Mirzaiebadizi, A.; Nouri, K.; Reichert, D.; Kordes, C.; Häussinger, D.; Ahmadian, M.R. From Quiescence to Activation: The Reciprocal Regulation of Ras and Rho Signaling in Hepatic Stellate Cells. Cells 2025, 14, 674. https://doi.org/10.3390/cells14090674
Nakhaei-Rad S, Pudewell S, Mirzaiebadizi A, Nouri K, Reichert D, Kordes C, Häussinger D, Ahmadian MR. From Quiescence to Activation: The Reciprocal Regulation of Ras and Rho Signaling in Hepatic Stellate Cells. Cells. 2025; 14(9):674. https://doi.org/10.3390/cells14090674
Chicago/Turabian StyleNakhaei-Rad, Saeideh, Silke Pudewell, Amin Mirzaiebadizi, Kazem Nouri, Doreen Reichert, Claus Kordes, Dieter Häussinger, and Mohammad Reza Ahmadian. 2025. "From Quiescence to Activation: The Reciprocal Regulation of Ras and Rho Signaling in Hepatic Stellate Cells" Cells 14, no. 9: 674. https://doi.org/10.3390/cells14090674
APA StyleNakhaei-Rad, S., Pudewell, S., Mirzaiebadizi, A., Nouri, K., Reichert, D., Kordes, C., Häussinger, D., & Ahmadian, M. R. (2025). From Quiescence to Activation: The Reciprocal Regulation of Ras and Rho Signaling in Hepatic Stellate Cells. Cells, 14(9), 674. https://doi.org/10.3390/cells14090674









