Enhanced Expression of Mitochondrial Magmas Protein in Ovarian Carcinomas: Magmas Inhibition Facilitates Antitumour Effects, Signifying a Novel Approach for Ovarian Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Ovarian Tumours from Patients
2.2.1. Ethics Approval
2.2.2. Collection of Ovarian Tumours
2.3. Immunohistochemical Analyses of Magmas in Ovarian Tumours
2.4. Ovarian Cancer Cell Lines
2.5. Immunofluorescence (IF) on Ovarian Cancer Cell Lines
2.6. Western Blot (WB)
2.7. Water-Soluble Tetrazolium Salts 1 (WST-1) Assay
2.8. Active Caspase 3/7 and Propidium Iodide Assays
2.9. TMRM Assay for the Measurement of Mitochondrial Membrane Potential
2.10. DCFDA Assay to Measure ROS Production
2.11. Superoxide Dismutase Assay (SOD)
2.12. Cell Bioenergetic Assay
2.13. Cell Proliferation and Migration Assays by xCELLigence
2.14. Methyl Thiazol Tetrazolium (MTT) Assay
2.15. RNA Extraction and qRT-PCR
2.16. Animal Experiments
Animal Ethics Statement
2.17. Statistical Analysis of the Data
3. Results
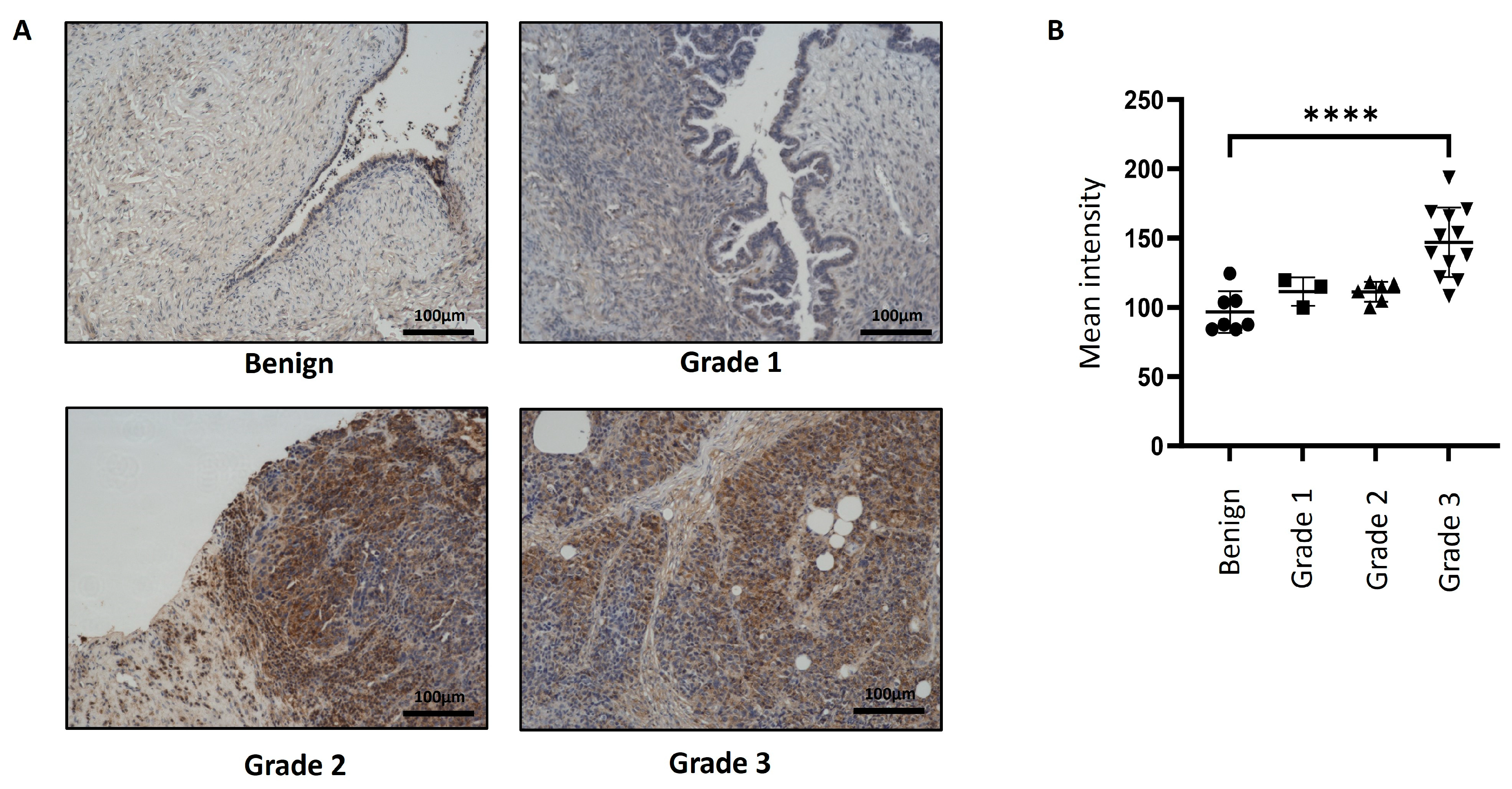
3.1. Expression of Magmas in Ovarian Carcinomas and Benign Ovarian Tumours
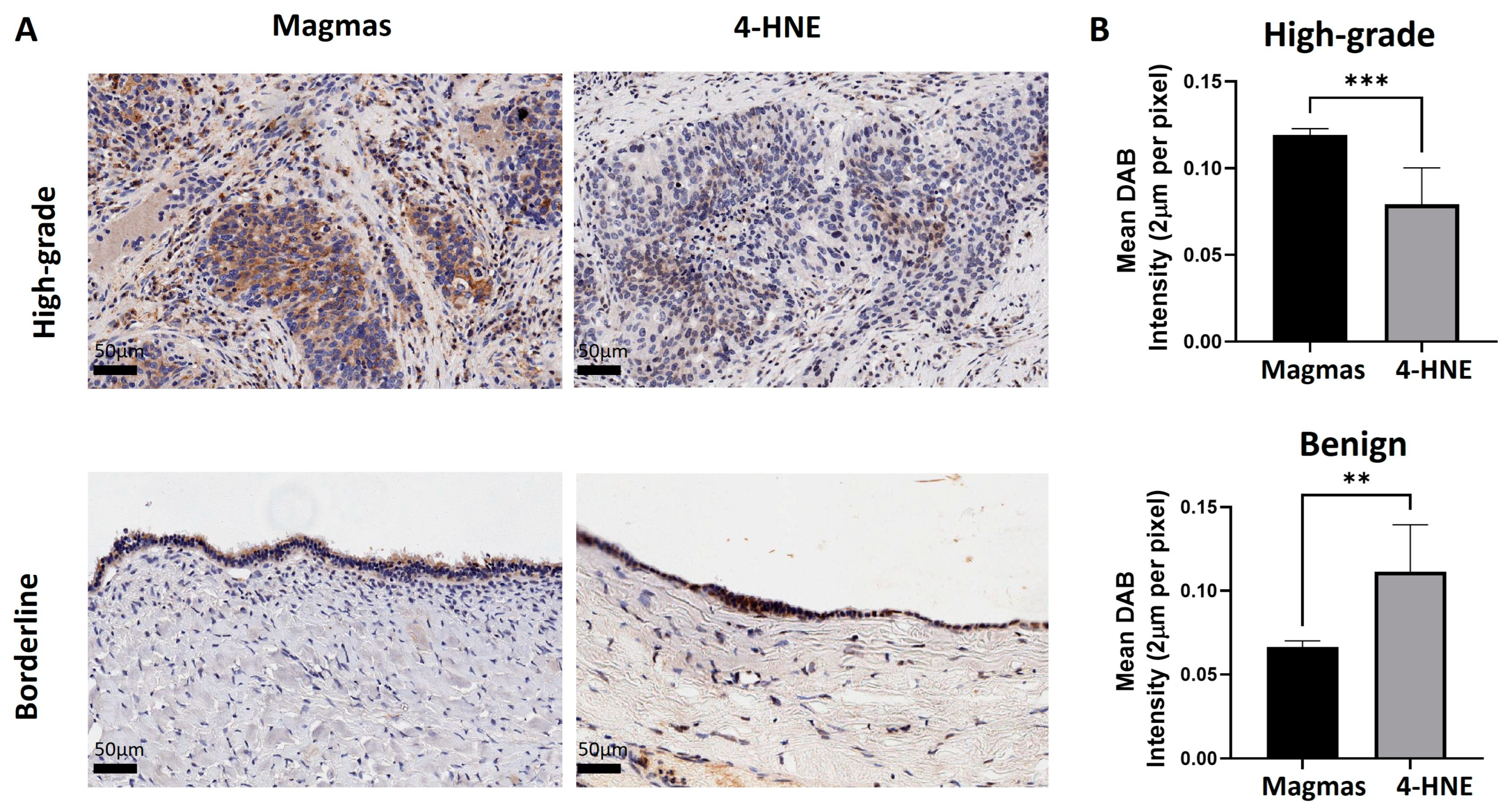
3.2. The Expression of Magmas Is Inversely Related to ROS Levels in Ovarian Tumours
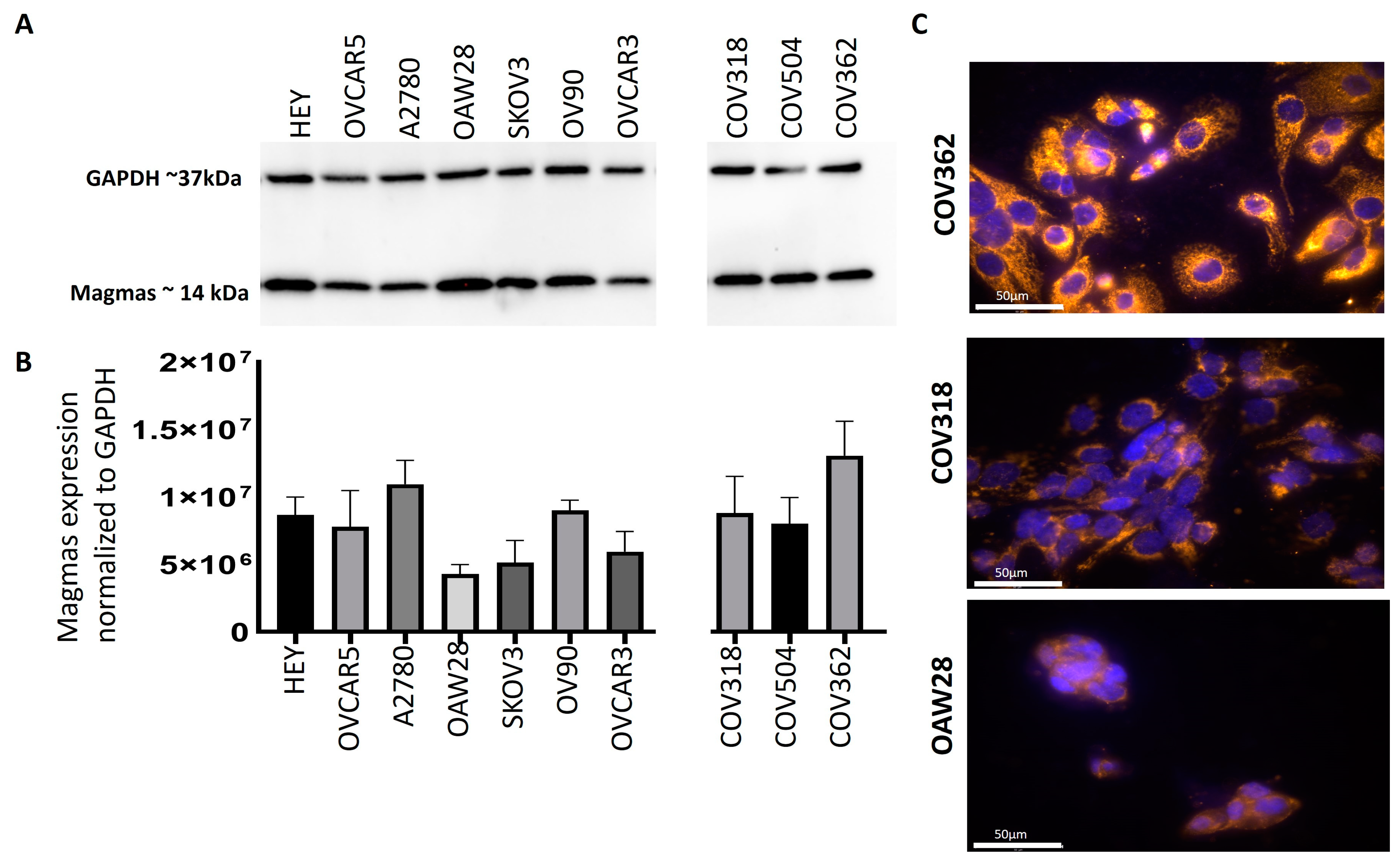
3.3. Magmas Was Expressed Variably in OC Cell Lines
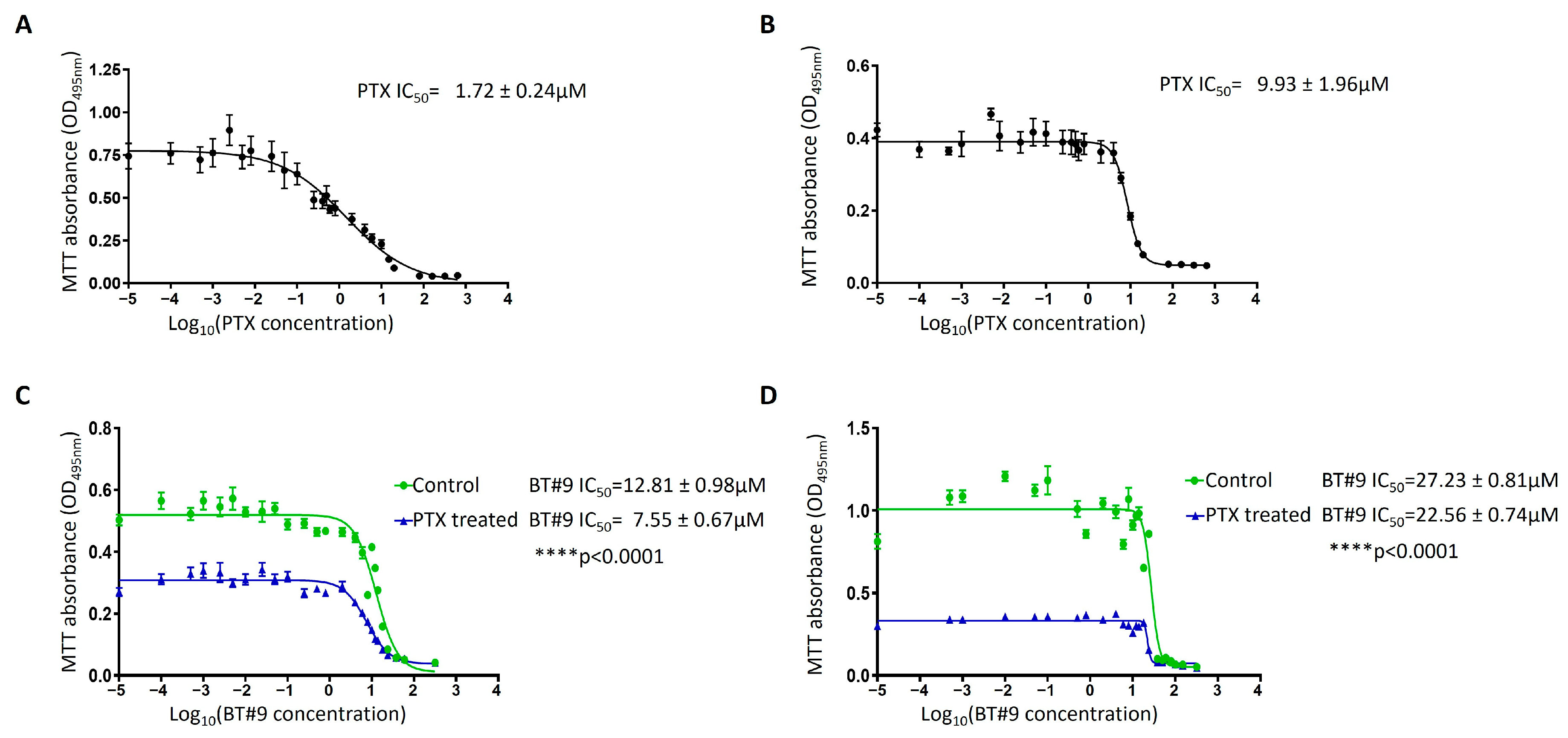
3.4. Determination of IC50 Values and the Effect of BT#9 on the Expression of Magmas in OC Cell Lines
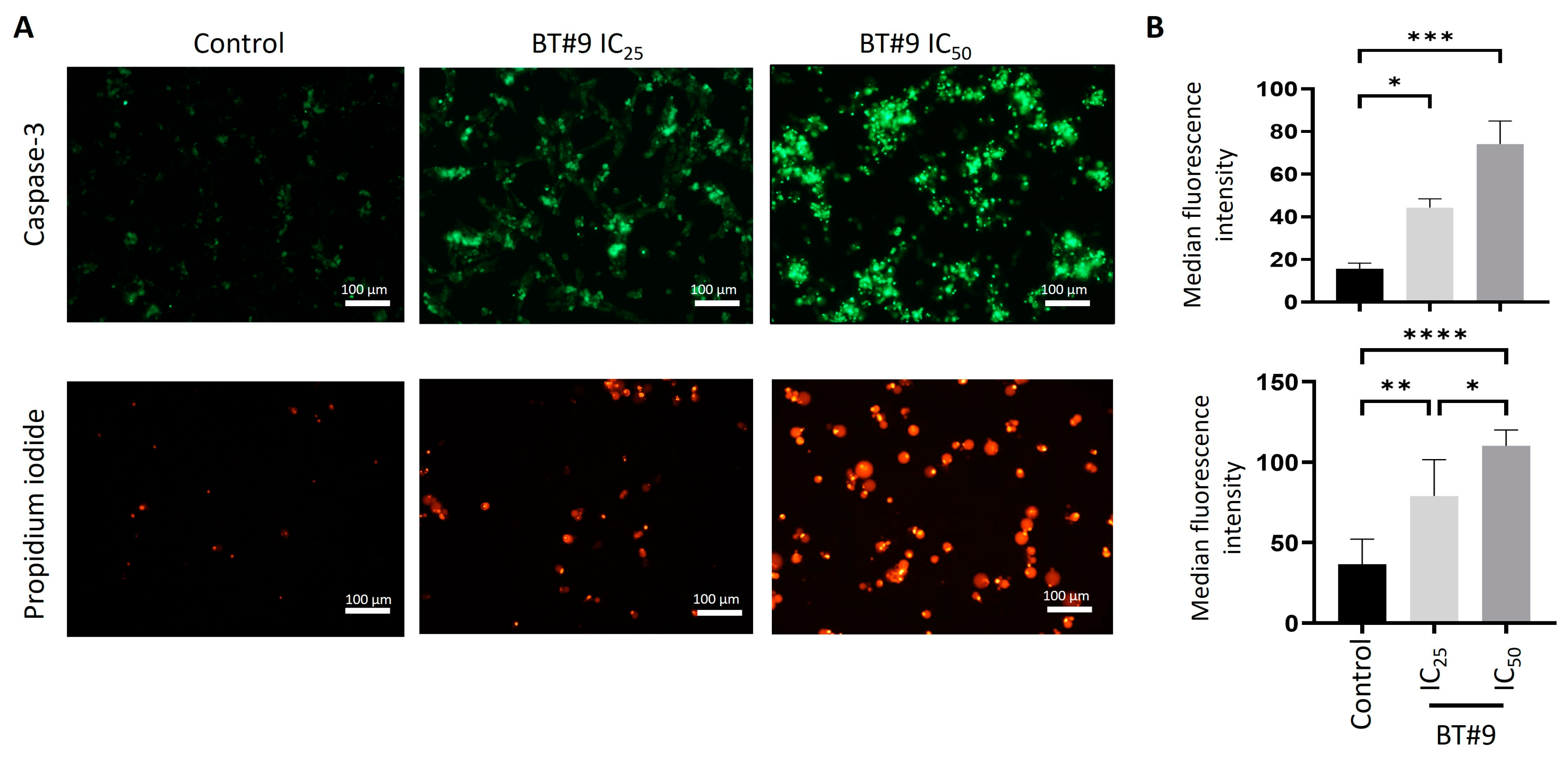
3.5. BT#9 Induces Both Apoptosis and Necrosis in OC Cell Lines
3.6. BT#9 Induces Loss of Mitochondrial Membrane Potential in OC Cell Lines: TMRM Assay
3.7. BT#9 Induced the Production of ROS Levels in OC Cell Lines: DCFDA Assay
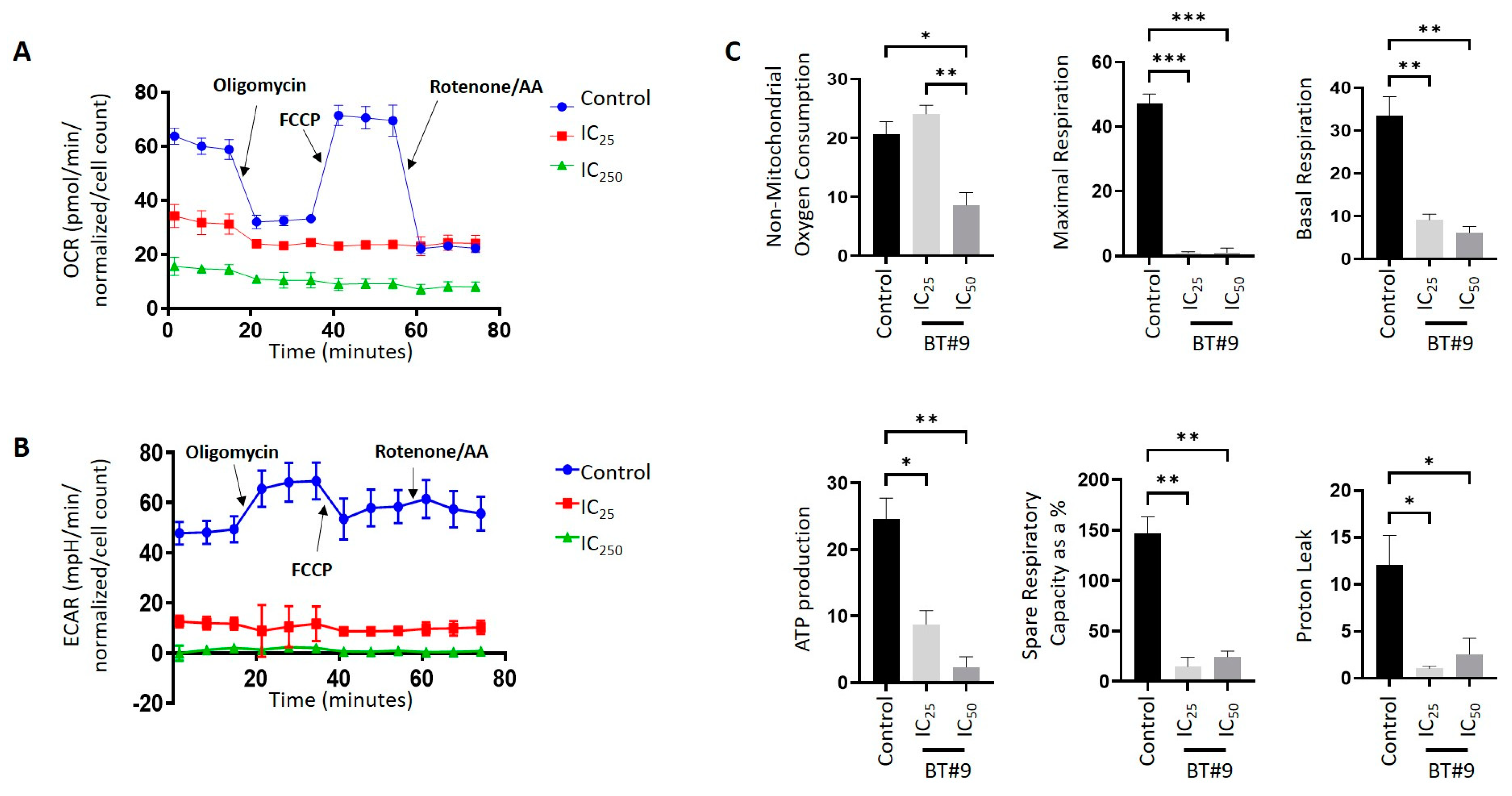
3.8. BT#9-Treated OC Cells Showed Lower Bioenergetics Compared to Their Untreated Control Cells
3.9. BT#9 Inhibits the Proliferation and Migration of OC Cell Lines
3.10. Chemosensitivity of the OC Cell Lines to Paclitaxel in Response to BT#9 Treatment
3.11. The Effect of BT#9 on HEY Cell-Induced Tumours in Mice: Tumour Burden and Survival Studies
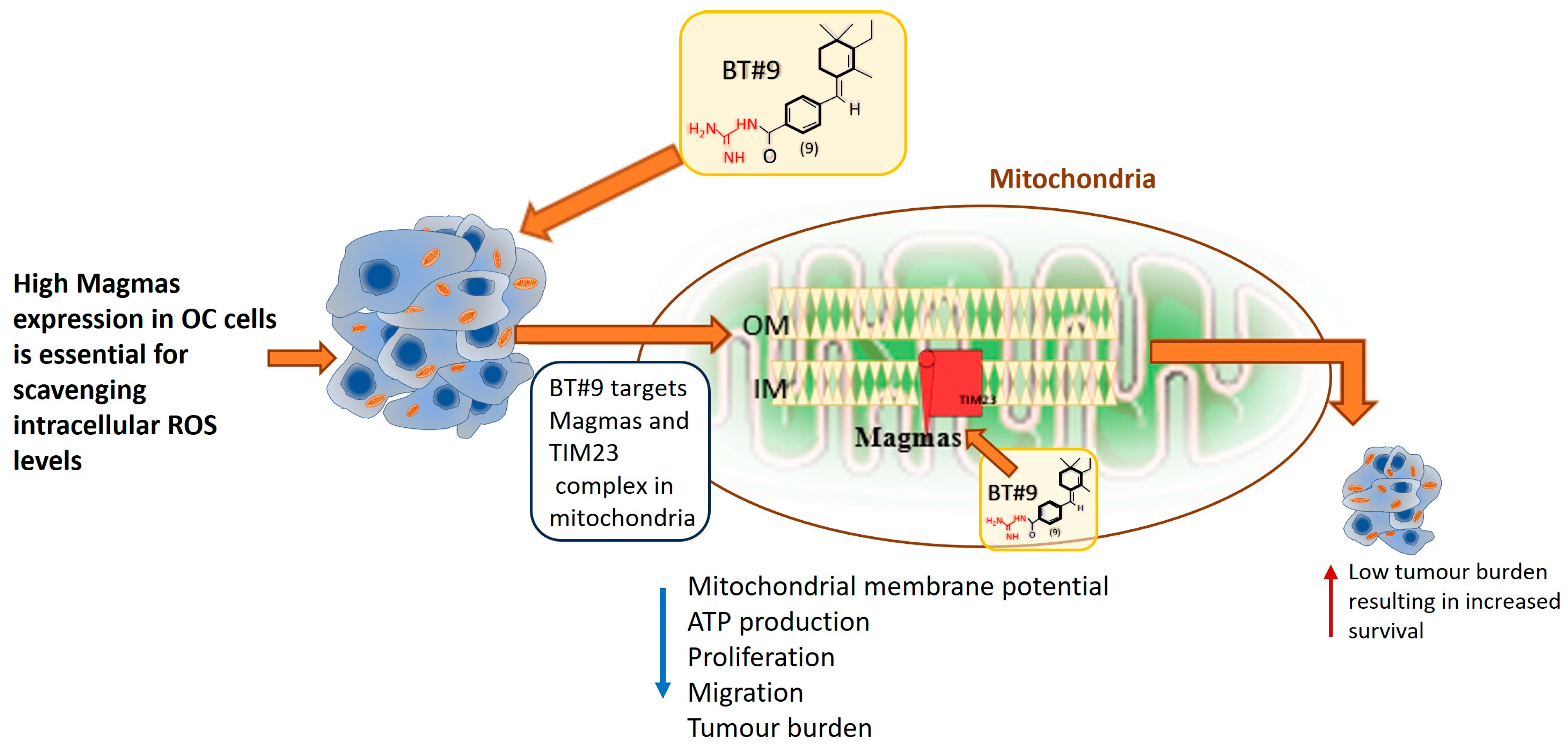
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OC | Ovarian cancer |
| ROS | Reactive oxygen species |
| 4-HNE | 4-Hydroxynonenol |
| PI | Propidium iodide |
| DCFDA | Dichlorodihydrofluorescein diacetate |
| TCA | Tricarboxylic cycle |
| FIGO | International Federation of Obstetrics and Gynaecology |
| HGOSC | High-grade serous ovarian carcinoma |
| PI3K | Phosphatidylinositol 3 kinase |
| TME | Tumour microenvironment |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| PAM | Pre-sequence translocated-associated motor subunit |
| TIM | Translocase of the inner membrane subunit |
| mthsp70 | Mitochondrial heat shock protein 70 |
| ETC | Electron transport chain |
| ACTH | Adrenocorticotrophic hormone |
| PKC | Protein kinase C |
| GH/PRL | Growth hormone/prolactin |
| HUVECs | Human umbilical vein endothelial cells |
| SOD | Superoxide dismutase |
| FDA | Food and Drug Administration |
| RWH | Royal Women’s Hospital |
| VCB | Victorian Cancer Biobank |
| DAB | 3,3′-diaminobenzidine |
| IHC | Immunohistochemistry |
| H&E | Hematoxylin and eosin |
| IF | Immunofluorescence |
| PBST | Phosphate-buffered saline with Tween 20 |
| BSA | Bovine serum albumin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SDS-PAGE | Sodium dodecyl-sulphate polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene difluoride |
| HRP | Horse radish peroxidase |
| RNS | Reactive nitrogen species |
| TBHP | Tert-butyl hydrogen peroxide |
| PTX | Paclitaxel |
| NHMRC | National Health and Medical Research Council |
| TEAB | Triethyl ammonium bicarbonate |
| EACR | Extracellular acidification rate |
| OCR | Oxygen consumption rate |
| OXPHOS | Mitochondrial oxidative phosphorylation |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kwolek, D.G.; Gerstberger, S.; Tait, S.; Qiu, J.M. Ovarian, Uterine, and Vulvovaginal Cancers: Screening, Treatment Overview, and Prognosis. Med. Clin. N. Am. 2023, 107, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Bast, R.C., Jr. Minireview: Human ovarian cancer: Biology, current management, and paths to personalizing therapy. Endocrinology 2012, 153, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef]
- Gavalas, N.G.; Liontos, M.; Trachana, S.P.; Bagratuni, T.; Arapinis, C.; Liacos, C.; Dimopoulos, M.A.; Bamias, A. Angiogenesis-related pathways in the pathogenesis of ovarian cancer. Int. J. Mol. Sci. 2013, 14, 15885–15909. [Google Scholar] [CrossRef]
- Mei, J.; Tian, H.; Huang, H.S.; Hsu, C.F.; Liou, Y.; Wu, N.; Zhang, W.; Chu, T.Y. Cellular models of development of ovarian high-grade serous carcinoma: A review of cell of origin and mechanisms of carcinogenesis. Cell Prolif. 2021, 54, e13029. [Google Scholar] [CrossRef]
- Rojas, V.; Hirshfield, K.M.; Ganesan, S.; Rodriguez-Rodriguez, L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int. J. Mol. Sci. 2016, 17, 2113. [Google Scholar] [CrossRef]
- Zhang, Y.; Qazi, S.; Raza, K. Differential expression analysis in ovarian cancer: A functional genomics and systems biology approach. Saudi J. Biol. Sci. 2021, 28, 4069–4081. [Google Scholar] [CrossRef]
- Roberto, A.; Radrezza, S.; Mosconi, P. Transparency in ovarian cancer clinical trial results: ClinicalTrials.gov versus PubMed, Embase and Google scholar. J. Ovarian Res. 2018, 11, 28. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Novak, C.; Horst, E.; Mehta, G. Review: Mechanotransduction in ovarian cancer: Shearing into the unknown. APL Bioeng. 2018, 2, 031701. [Google Scholar] [CrossRef] [PubMed]
- Hyler, A.R.; Baudoin, N.C.; Brown, M.S.; Stremler, M.A.; Cimini, D.; Davalos, R.V.; Schmelz, E.M. Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability. PLoS ONE 2018, 13, e0194170. [Google Scholar] [CrossRef]
- Compton, S.L.E.; Pyne, E.S.; Liu, L.; Guinan, J.; Shea, A.A.; Grieco, J.P.; Frisard, M.I.; Schmelz, E.M. Adaptation of metabolism to multicellular aggregation, hypoxia and obese stromal cell incorporation as potential measure of survival of ovarian metastases. Exp. Cell Res. 2021, 399, 112397. [Google Scholar] [CrossRef] [PubMed]
- Grieco, J.P.; Allen, M.E.; Perry, J.B.; Wang, Y.; Song, Y.; Rohani, A.; Compton, S.L.E.; Smyth, J.W.; Swami, N.S.; Brown, D.A.; et al. Progression-Mediated Changes in Mitochondrial Morphology Promotes Adaptation to Hypoxic Peritoneal Conditions in Serous Ovarian Cancer. Front. Oncol. 2020, 10, 600113. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Ronai, Z.A. Regulators of mitochondrial dynamics in cancer. Curr. Opin. Cell Biol. 2016, 39, 43–52. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Shukla, P.; Singh, K.K. The mitochondrial landscape of ovarian cancer: Emerging insights. Carcinogenesis 2021, 42, 663–671. [Google Scholar] [CrossRef]
- Shen, L.; Zhan, X. Mitochondrial Dysfunction Pathway Alterations Offer Potential Biomarkers and Therapeutic Targets for Ovarian Cancer. Oxid. Med. Cell Longev. 2022, 2022, 5634724. [Google Scholar] [CrossRef]
- Bindra, S.; McGill, M.A.; Triplett, M.K.; Tyagi, A.; Thaker, P.H.; Dahmoush, L.; Goodheart, M.J.; Ogden, R.T.; Owusu-Ansah, E.; Karan, R.K.; et al. Mitochondria in epithelial ovarian carcinoma exhibit abnormal phenotypes and blunted associations with biobehavioral factors. Sci. Rep. 2021, 11, 11595. [Google Scholar] [CrossRef]
- De Rasmo, D.; Cormio, A.; Cormio, G.; Signorile, A. Ovarian Cancer: A Landscape of Mitochondria with Emphasis on Mitochondrial Dynamics. Int. J. Mol. Sci. 2023, 24, 1224. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, H.; Cao, L.; Zhan, X. Quantitative analysis of the mitochondrial proteome in human ovarian carcinomas. Endocr. Relat. Cancer 2018, 25, 909–931. [Google Scholar] [CrossRef] [PubMed]
- Jubinsky, P.T.; Messer, A.; Bender, J.; Morris, R.E.; Ciraolo, G.M.; Witte, D.P.; Hawley, R.G.; Short, M.K. Identification and characterization of Magmas, a novel mitochondria-associated protein involved in granulocyte-macrophage colony-stimulating factor signal transduction. Exp. Hematol. 2001, 29, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Joshi, N.; Chittoor, B.; Samji, P.; D’Silva, P. Role of Magmas in protein transport and human mitochondria biogenesis. Hum. Mol. Genet. 2010, 19, 1248–1262. [Google Scholar] [CrossRef]
- Jubinsky, P.T.; Short, M.K.; Mutema, G.; Witte, D.P. Developmental expression of Magmas in murine tissues and its co-expression with the GM-CSF receptor. J. Histochem. Cytochem. 2003, 51, 585–596. [Google Scholar] [CrossRef]
- Li, Y.; Dudek, J.; Guiard, B.; Pfanner, N.; Rehling, P.; Voos, W. The presequence translocase-associated protein import motor of mitochondria. Pam16 functions in an antagonistic manner to Pam18. J. Biol. Chem. 2004, 279, 38047–38054. [Google Scholar] [CrossRef]
- Frazier, A.E.; Dudek, J.; Guiard, B.; Voos, W.; Li, Y.; Lind, M.; Meisinger, C.; Geissler, A.; Sickmann, A.; Meyer, H.E.; et al. Pam16 has an essential role in the mitochondrial protein import motor. Nat. Struct. Mol. Biol. 2004, 11, 226–233. [Google Scholar] [CrossRef]
- Schilke, B.A.; Hayashi, M.; Craig, E.A. Genetic analysis of complex interactions among components of the mitochondrial import motor and translocon in Saccharomyces cerevisiae. Genetics 2012, 190, 1341–1353. [Google Scholar] [CrossRef][Green Version]
- Van der Laan, M.; Chacinska, A.; Lind, M.; Perschil, I.; Sickmann, A.; Meyer, H.E.; Guiard, B.; Meisinger, C.; Pfanner, N.; Rehling, P. Pam17 is required for architecture and translocation activity of the mitochondrial protein import motor. Mol. Cell Biol. 2005, 25, 7449–7458. [Google Scholar] [CrossRef]
- Sinha, D.; Srivastava, S.; D’Silva, P. Functional Diversity of Human Mitochondrial J-proteins Is Independent of Their Association with the Inner Membrane Presequence Translocase. J. Biol. Chem. 2016, 291, 17345–17359. [Google Scholar] [CrossRef]
- Roy, S.; Short, M.K.; Stanley, E.R.; Jubinsky, P.T. Essential role of Drosophila black-pearl is mediated by its effects on mitochondrial respiration. FASEB J. 2012, 26, 3822–3833. [Google Scholar] [CrossRef] [PubMed]
- Priesnitz, C.; Bottinger, L.; Zufall, N.; Gebert, M.; Guiard, B.; van der Laan, M.; Becker, T. Coupling to Pam16 differentially controls the dual role of Pam18 in protein import and respiratory chain formation. Cell Rep. 2022, 39, 110619. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, P.R.; Schilke, B.; Hayashi, M.; Craig, E.A. Interaction of the J-protein heterodimer Pam18/Pam16 of the mitochondrial import motor with the translocon of the inner membrane. Mol. Biol. Cell 2008, 19, 424–432. [Google Scholar] [CrossRef]
- Tagliati, F.; Gentilin, E.; Buratto, M.; Mole, D.; degli Uberti, E.C.; Zatelli, M.C. Magmas, a gene newly identified as overexpressed in human and mouse ACTH-secreting pituitary adenomas, protects pituitary cells from apoptotic stimuli. Endocrinology 2010, 151, 4635–4642. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, D.; Saha, P.P.; Marthala, H.; D’Silva, P. Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis. 2014, 5, e1394. [Google Scholar] [CrossRef]
- Tagliati, F.; Gagliano, T.; Gentilin, E.; Minoia, M.; Mole, D.; Delgi Uberti, E.C.; Zatelli, M.C. Magmas overexpression inhibits staurosporine induced apoptosis in rat pituitary adenoma cell lines. PLoS ONE 2013, 8, e75194. [Google Scholar] [CrossRef]
- Yang, J.; Das, B.C.; Aljitawi, O.; Kumar, A.; Das, S.; Van Veldhuizen, P. Magmas Inhibition in Prostate Cancer: A Novel Target for Treatment-Resistant Disease. Cancers 2022, 14, 2732. [Google Scholar] [CrossRef] [PubMed]
- Di, K.; Lomeli, N.; Bota, D.A.; Das, B.C. Magmas inhibition as a potential treatment strategy in malignant glioma. J. Neurooncol 2019, 141, 267–276. [Google Scholar] [CrossRef]
- Tran, J.D.; Duran, A.D.; Tan, Q.W.; Almaguel, F.G.; De León, D. IGF-II regulates a critical mitochondrial protein, Magmas, with differential expression between African American and Non-Hispanic White women with TNBC. In Cancer Epidemiology, Biomarkers & Prevention; The American Association for Cancer Research: Philadelphia, PA, USA, 2023; p. C064. [Google Scholar]
- Durán, A.M.; Yoo, C.H.; Tran, J.D.; Santiago, K.; Mohammed, A.S.; Casiano, C.A.; Almaguel, F.A. Targeting mitochondrial protein Magmas increases sensitivity to docetaxel neuroendocrine prostate cancer cells. Cancer Res. 2023, 83 (Suppl. 7), 1754. [Google Scholar] [CrossRef]
- Ahmed, N.; Kadife, E.; Raza, A.; Short, M.; Jubinsky, P.T.; Kannourakis, G. Ovarian Cancer, Cancer Stem Cells and Current Treatment Strategies: A Potential Role of Magmas in the Current Treatment Methods. Cells 2020, 9, 719. [Google Scholar] [CrossRef]
- Mehawej, C.; Delahodde, A.; Legeai-Mallet, L.; Delague, V.; Kaci, N.; Desvignes, J.P.; Kibar, Z.; Capo-Chichi, J.M.; Chouery, E.; Munnich, A.; et al. The impairment of MAGMAS function in human is responsible for a severe skeletal dysplasia. PLoS Genet. 2014, 10, e1004311. [Google Scholar] [CrossRef] [PubMed]
- Jubinsky, P.T.; Short, M.K.; Ghanem, M.; Das, B.C. Design, synthesis, and biological activity of novel Magmas inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3479–3482. [Google Scholar] [CrossRef] [PubMed]
- Jubinsky, P.T. Small Molecule Magmas Inhibitors for the Treatment and Detection of Cancer. 2016. Available online: www.facebook.com/IACRFoundation (accessed on 10 April 2024).
- Escalona, R.M.; Kannourakis, G.; Findlay, J.K.; Ahmed, N. Expression of TIMPs and MMPs in Ovarian Tumors, Ascites, Ascites-Derived Cells, and Cancer Cell Lines: Characteristic Modulatory Response Before and After Chemotherapy Treatment. Front. Oncol. 2021, 11, 796588. [Google Scholar] [CrossRef] [PubMed]
- Karst, A.M.; Jones, P.M.; Vena, N.; Ligon, A.H.; Liu, J.F.; Hirsch, M.S.; Etemadmoghadam, D.; Bowtell, D.D.; Drapkin, R. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014, 74, 1141–1152. [Google Scholar] [CrossRef]
- Blayney, J.K.; Davison, T.; McCabe, N.; Walker, S.; Keating, K.; Delaney, T.; Greenan, C.; Williams, A.R.; McCluggage, W.G.; Capes-Davis, A.; et al. Prior knowledge transfer across transcriptional data sets and technologies using compositional statistics yields new mislabelled ovarian cell line. Nucleic Acids Res. 2016, 44, e137. [Google Scholar] [CrossRef]
- Lorenzi, P.L.; Reinhold, W.C.; Varma, S.; Hutchinson, A.A.; Pommier, Y.; Chanock, S.J.; Weinstein, J.N. DNA fingerprinting of the NCI-60 cell line panel. Mol. Cancer Ther. 2009, 8, 713–724. [Google Scholar] [CrossRef]
- Kwok, A.L.; Wong, O.G.; Wong, E.S.; Tsun, O.K.; Chan, K.K.; Cheung, A.N. Caution over use of ES2 as a model of ovarian clear cell carcinoma. J. Clin. Pathol. 2014, 67, 921–922. [Google Scholar] [CrossRef]
- Buick, R.N.; Pullano, R.; Trent, J.M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985, 45, 3668–3676. [Google Scholar]
- Fogh, J.; Trempe, G. New Human Tumor Cell Lines. In Human Tumor Cells in Vitro; Fogh, J., Ed.; Springer: Boston, MA, USA, 1975; pp. 115–159. [Google Scholar] [CrossRef]
- Langdon, S.P.; Lawrie, S.S. Establishment of Ovarian Cancer Cell Lines. Methods Mol. Med. Ovarian Cancer Methods Protoc. 2001, 39, 155–159. [Google Scholar]
- Escalona, R.M.; Chu, S.; Kadife, E.; Kelly, J.K.; Kannourakis, G.; Findlay, J.K.; Ahmed, N. Knock down of TIMP-2 by siRNA and CRISPR/Cas9 mediates diverse cellular reprogramming of metastasis and chemosensitivity in ovarian cancer. Cancer Cell Int. 2022, 22, 422. [Google Scholar] [CrossRef]
- Bilandzic, M.; Chu, S.; Farnworth, P.G.; Harrison, C.; Nicholls, P.; Wang, Y.; Escalona, R.M.; Fuller, P.J.; Findlay, J.K.; Stenvers, K.L. Loss of betaglycan contributes to the malignant properties of human granulosa tumor cells. Mol. Endocrinol. 2009, 23, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, K.; Luwor, R.B.; Zhu, H.; McNally, O.; Quinn, M.A.; Burns, C.J.; Thompson, E.W.; Findlay, J.K.; Ahmed, N. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer 2014, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Boughaba, J.A.; Chojnowski, G.; Christensen, M.E.; Waterhouse, N.J. Dead Cert: Measuring Cell Death. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Lane, A.N.; Higashi, R.M.; Fan, T.W. Metabolic reprogramming in tumors: Contributions of the tumor microenvironment. Genes. Dis. 2020, 7, 185–198. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef]
- Ul Fatima, N.; Ananthanarayanan, V. Mitochondrial movers and shapers: Recent insights into regulators of fission, fusion and transport. Curr. Opin. Cell Biol. 2023, 80, 102150. [Google Scholar] [CrossRef]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 2012, 942, 3–37. [Google Scholar] [CrossRef]
- Dier, U.; Shin, D.H.; Hemachandra, L.P.; Uusitalo, L.M.; Hempel, N. Bioenergetic analysis of ovarian cancer cell lines: Profiling of histological subtypes and identification of a mitochondria-defective cell line. PLoS ONE 2014, 9, e98479. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Venugopal, D.; Al-Dayan, N.; Ravinayagam, V.; Mohammed, A.A. Emerging insights into mitochondria-specific targeting and drug delivering strategies: Recent milestones and therapeutic implications. Saudi J. Biol. Sci. 2020, 27, 3581–3592. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, N.; van der Laan, M.; Hutu, D.P.; Rehling, P.; Pfanner, N. Sorting switch of mitochondrial presequence translocase involves coupling of motor module to respiratory chain. J. Cell Biol. 2007, 179, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, R.; Chen, R.; Liu, J. Altered Mitochondrial Dynamics, Biogenesis, and Functions in the Paclitaxel-Resistant Lung Adenocarcinoma Cell Line A549/Taxol. Med. Sci. Monit. 2020, 26, e918216. [Google Scholar] [CrossRef] [PubMed]
- Varbiro, G.; Veres, B.; Gallyas, F., Jr.; Sumegi, B. Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic. Biol. Med. 2001, 31, 548–558. [Google Scholar] [CrossRef]







| Cell Line | Origin | Growth Medium Used |
|---|---|---|
| HEY (ovarian papillary cystadenocarcinoma, peritoneal deposit) (Buick, Pullano, and Trent 1985) [50] | High-grade ovarian serous adenocarcinoma | RPMI + 10% FBS + 1% Penstrep |
| OVCAR5 (high-grade ovarian adenocarcinoma, derived from ascites, chemo-naïve) (Fogh and Trempe 1975 [51]; Langdon and Lawrie 2001) [52] | High-grade ovarian serous adenocarcinoma | RPMI + 10% FBS + 1% Penstrep |
| A2780 (sparse information on cell line, obtained from untreated primary tumour material, https://www.culturecollections.org.uk/nop/product/a2780) (accessed on 10 September 2024) | Ovarian endometrioid adenocarcinoma | DMEM + 10% FBS + 1% Penstrep |
| OAW28 (obtained from the ascites of a patient with ovarian cystadenocarcinoma, https://www.culturecollections.org.uk/nop/product/oaw28) (accessed on 10 September 2024) | High-grade ovarian serous adenocarcinoma | DMEM + 10% FBS + 1% Penstrep |
| SKOV3 (derived from the ascitic fluid of a patient with adenocarcinoma, https://www.culturecollections.org.uk/nop/product/sk-ov-3) (accessed on 10 September 2024) | Ovarian serous cystadenocarcinoma | RPMI + 10% FBS + 1% Penstrep |
| OV90 (epithelial-like cell isolated from an ovary of a patient with malignant papillary serous adenocarcinoma, https://www.atcc.org/products/crl-3585) (accessed on 10 September 2024) | Ovarian adenocarcinoma | RPMI + 10% FBS + 1% Penstrep |
| COV318 (epithelial serous carcinoma cell line obtained from peritoneal ascites, https://www.culturecollections.org.uk/nop/product/cov318) (accessed on 10 September 2024) | High-grade ovarian serous adenocarcinoma | DMEM + 10% FBS + 1% Penstrep |
| COV504 (human ovarian epithelial serous carcinoma cell line established from pleural effusion, https://www.culturecollections.org.uk/nop/product/cov504) (accessed on 10 September 2024) | Ovarian carcinoma | DMEM + 10% FBS + 1% Penstrep |
| COV362 (human epithelial endometrioid carcinoma cell line established from pleural effusion, https://www.culturecollections.org.uk/nop/product/cov362) (accessed on 10 September 2024) | High-grade ovarian serous adenocarcinoma | DMEM + 10% FBS + 1% Penstrep |
| OVCAR3 (epithelial cells obtained from malignant ascites of a patient with progressive adenocarcinoma of an ovary, https://www.atcc.org/products/htb-161) (accessed on 10 September 2024) | High-grade ovarian serous adenocarcinoma | DMEM + 10% FBS + 1% Penstrep |
| ES2 (human clear cell carcinoma isolated from the ovary of a patient with progressive clear cell carcinoma, https://www.atcc.org/products/crl-1978#) (accessed on 10 September 2024) | High-grade ovarian clear cell carcinoma | RPMI + 10% FBS + 1% Penstrep |
| FT282 (obtained from a normal fallopian tube of a donor, https://www.atcc.org/products/crl-3449) (accessed on 10 September 2024) | Normal fallopian tube cell line | DMEM-HAM’S F12 + 2% USG and 1% Penstrep |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Hoque, A.; Luwor, R.; Escalona, R.M.; Kelly, J.; Sharma, R.; Charchar, F.; Chu, S.; Short, M.K.; Jubinsky, P.T.; et al. Enhanced Expression of Mitochondrial Magmas Protein in Ovarian Carcinomas: Magmas Inhibition Facilitates Antitumour Effects, Signifying a Novel Approach for Ovarian Cancer Treatment. Cells 2025, 14, 655. https://doi.org/10.3390/cells14090655
Raza A, Hoque A, Luwor R, Escalona RM, Kelly J, Sharma R, Charchar F, Chu S, Short MK, Jubinsky PT, et al. Enhanced Expression of Mitochondrial Magmas Protein in Ovarian Carcinomas: Magmas Inhibition Facilitates Antitumour Effects, Signifying a Novel Approach for Ovarian Cancer Treatment. Cells. 2025; 14(9):655. https://doi.org/10.3390/cells14090655
Chicago/Turabian StyleRaza, Ali, Ashfaqul Hoque, Rodney Luwor, Ruth M. Escalona, Jason Kelly, Revati Sharma, Fadi Charchar, Simon Chu, Mary K. Short, Paul T. Jubinsky, and et al. 2025. "Enhanced Expression of Mitochondrial Magmas Protein in Ovarian Carcinomas: Magmas Inhibition Facilitates Antitumour Effects, Signifying a Novel Approach for Ovarian Cancer Treatment" Cells 14, no. 9: 655. https://doi.org/10.3390/cells14090655
APA StyleRaza, A., Hoque, A., Luwor, R., Escalona, R. M., Kelly, J., Sharma, R., Charchar, F., Chu, S., Short, M. K., Jubinsky, P. T., Kannourakis, G., & Ahmed, N. (2025). Enhanced Expression of Mitochondrial Magmas Protein in Ovarian Carcinomas: Magmas Inhibition Facilitates Antitumour Effects, Signifying a Novel Approach for Ovarian Cancer Treatment. Cells, 14(9), 655. https://doi.org/10.3390/cells14090655








