Abstract
Schizophrenia is a chronic, debilitating disorder with diverse symptomatology, including disorganised cognition and behaviour. Despite considerable research effort, we have only a limited understanding of the underlying brain dysfunction. A significant proportion of individuals with schizophrenia exhibit high levels of inflammation, and inflammation associated with maternal immune system activation is a risk factor for the disorder. In this review, we outline the potential role of inflammation in the disorder, with a particular focus on how cytokine release might affect the development and function of GABAergic interneurons. One consequence of this change in inhibitory control is a disruption in oscillatory processes in the brain. These changes disrupt the spatial and temporal synchrony of neural activity in the brain, which, by disturbing representations of time and space, may underlie some of the disorganisation symptoms observed in the disorder.
Keywords:
inflammation; IL-6; oscillations; GABA; schizophrenia; hippocampus; synchrony; theta; gamma; phase precession 1. Introduction
When immune system activation, and the resulting inflammatory response, occurs during critical periods of brain development, it can significantly influence the risk and progression of several conditions, such as autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), bipolar disorder, microcephaly, cerebral palsy, and schizophrenia [1]. While a considerable body of work describes how inflammation can alter brain connectivity, particularly in early development, it is less clear how these changes result in some of the complex symptomatology observed in diseases such as schizophrenia. This review will describe data that provide one possible link, with a particular interest in how inflammation-mediated changes in GABAergic mechanisms could disrupt the synchrony and coherence of spatial and temporal processing in the brain. Although the focus is on schizophrenia, the underlying mechanisms will likely overlap with several other neurodevelopmental conditions.
2. Evidence of Altered Inflammatory Responses in Individuals with Schizophrenia
Schizophrenia is a chronic, severe, disabling brain disease that is among the world’s top ten causes of long-term disability [2], affecting approximately 1% of the population across a lifetime. The symptoms of schizophrenia are typically separated into three broad categories: psychotic or positive symptoms, negative symptoms, and cognitive impairment [3]. The overt positive symptoms consist of hallucinations and delusions; the negative symptoms include problems with motivation and social interactions; while the cognitive symptoms comprise deficits in attention, memory, and executive function. The cognitive symptoms are recognised as a fundamental feature of the disorder, and their increased intensity is strongly related to negative social and vocational outcomes [4,5,6]. Current evidence indicates that schizophrenia results from a complex interplay of genetic, biological, and environmental factors, including epigenetic mechanisms that converge on shared pathways of molecular dysfunction. The primary risk factors appear to be subtle and accumulative, suggesting that initial events may trigger a developmental cascade of secondary events that progressively enhance schizophrenia risk. One risk factor that has received considerable attention in recent years is inflammation, and in particular, the effects of the cytokines that are secreted by the immune system as part of the inflammatory response to pathogens or injury, including to non-physical insults such as stress and depression [7,8].
Elevated cytokine levels have frequently been observed in the blood and cerebral spinal fluid of schizophrenia patients, including alterations in the inflammatory cytokine interleukin-6 (IL-6), Interleukin-1 beta (IL-1β), Tumour Necrosis Factor alpha (TNF-α), Interferon-gamma (IFN-γ), and the chemokine Monocyte Chemoattractant Protein-1 (MCP-1). This suggests that inflammatory processes may have a causal role in the disease or that they are a downstream consequence of the disorder. The fact that abnormalities in cytokine levels are also present in individuals experiencing their first psychosis episode and in the first-degree relatives of patients diagnosed with schizophrenia suggests that they may have a causal role [9,10]. Currently, some of the most robust cytokine findings are for IL-6, which has been described as a potential trait marker for schizophrenia in recent meta-analyses [11]. Higher IL-6 levels have been associated with higher scores on a childhood trauma questionnaire and with lower cognitive and social cognitive function [12]. Increases in IL-6 have also been associated with decreased executive function and poorer verbal learning and memory and attention processing [13]. In a recent meta-analysis, increased levels of a number of cytokines, including IL-6, were reported in individuals with either acute or chronic schizophrenia-spectrum disorder [14]. These data supported the results of earlier studies, which had described similar effects in neuroleptic naïve, first-episode psychosis patients [15]. Elevated cytokines are likely to underlie a number of downstream effects; for example, several studies have shown that IL-6 stimulates dopamine release, a key factor underlying positive symptoms, while dopamine itself exerts a reciprocal regulatory effect on inflammatory processes (for a review, see [16]). IL-6 levels have also been positively correlated with the negative and cognitive symptoms of schizophrenia, potentially via its effects on NMDA receptors and GABAergic interneurons [17].
3. The Role of Cytokines in the Aetiology and Development of Schizophrenia
Elevated cytokine levels during critical developmental periods, including in the prenatal environment, and during adolescence have been associated with the disorder and may contribute to the neurodevelopmental component of the disease [18]. Support for this hypothesis initially came from studies showing that an association exists between schizophrenia risk and increased exposure to pathogens or maternal adversity, such as being born during winter or spring, living in urban environments (particularly inner city neighbourhoods of low socio-economic status) or migrant communities, exposure to childhood trauma and social distress, and drug abuse, all factors that are associated with infection and/or inflammation [19,20]. Although many of these environmental risk factors can exert effects throughout childhood and early adulthood, most studies have identified two critical periods of development that are particularly sensitive to environmental insults—first, the pre- and perinatal period, and second, the adolescent period [18,21,22]. The idea that impacts during both of these periods might be important has been conceptualised as the two-hit hypothesis, whereby an initial event during early neurodevelopment (the first hit) induces subtle impairments that may not reach clinical significance by themselves but which instead predispose the individual to increased vulnerability to stressors occurring later in life (the second hit) [23]. In support of this proposal, evidence of broad cognitive and structural abnormalities among high-risk youth before the onset of psychosis suggests that a predisposing event has occurred early in development [24,25,26]. Furthermore, this hypothesis is supported by extensive epidemiological studies of schizophrenia patients and their families, which show that prenatal environmental factors such as famine or infection, particularly occurring during the first or second trimesters, are involved in the aetiology of the disorder [27,28,29,30,31,32,33]. For the infection risk factor, it has been demonstrated that it is the release of proinflammatory cytokines during the maternal immune response to pathogens, rather than prenatal exposure to the pathogens themselves, that triggers abnormal neurodevelopment in the foetus [34,35]. Maternal immune activation (MIA) studies show that inflammatory cytokines such as IL-6 can cross the placental barrier at critical moments of neurodevelopment [8,35,36,37]. Furthermore, maternal inflammation itself may indirectly trigger elevated levels of cytokines such as IL-6 in the placenta, without necessarily requiring transport across the placenta [38]. A number of recent papers review these MIA effects and how they are linked to schizophrenia and other disorders, e.g., [39,40,41,42].
Elevated cytokine levels in utero, occurring during key moments of gestation, can affect several developmental processes, including the proliferation, differentiation, and migration of neuronal subtypes to their appropriate locations, as well as the formation and connectivity of synapses [8,32,35]. This can lead to critical structural and functional abnormalities in the brain. For example, data from several different animal models have shown that MIA results in disrupted cytoarchitecture and molecular signalling pathways in MIA offspring, as well as behavioural abnormalities that match the symptomatic profile of schizophrenia [18,30,42]. In particular, elevated levels of cytokines induced by immune system activation during gestation have been shown to result in altered cortical layering [43] and neuronal signalling [44], which may enhance the effects of “second hits” [45,46] during later stages of development, such as adolescence.
Adolescence is generally defined as the time between the onset of puberty through to the mid-twenties, and is the period when widespread pruning of excess synapses occurs in the brain, with a subsequent myelination of surviving axons [47,48,49]. These processes underlie the maturation of the prefrontal cortex [50], a process that can continue into early adulthood [51]. Adolescence is, therefore, a critical developmental period when the ‘second hit’ may be most disruptive. Consistent with this, late adolescence is when the initial symptoms of psychosis are most likely to emerge, and numerous studies show reductions in cortical grey matter among high-risk adolescents who will then go on to develop symptoms of psychosis [52,53,54]. Recent studies have demonstrated how inflammation is associated with changes in several of the processes that are remodelling the brain during adolescence, for example, axon myelination [55] and synaptic pruning [56,57]. Cytokines such as TNF-α have also been shown to affect dopamine signalling by altering the function of dopamine receptors and the synthesis of dopamine itself. Since dopamine dysregulation is a hallmark of schizophrenia, this links inflammation to one of the prominent theories of the disease [58]. Cytokines have also been shown to interact with the glutamate system, which is involved in rapid communication in the brain, as well as in synaptic plasticity and cognition. For example, IL-1β and TNF-α can affect the function of the NMDA subtype of glutamate receptor, which is crucial for learning and memory. This interaction could contribute to cognitive deficits in schizophrenia and, furthermore, links inflammation to another prominent theory of the disorder [59].
4. How Might Inflammatory Cytokines Affect Brain Function?
One potential effect of MIA and the associated release of inflammatory cytokines is to activate microglia, the brain’s tissue-resident macrophages. These are cells that enter the brain early during neurodevelopment and that are long lived. As a result, changes in their activation patterns can have long-lasting consequences. While microglia can respond to infection or injury by initiating an inflammatory response and phagocytosing damaged neurons, so as to maintain CNS function, they also have an important role in the developing brain. Microglia have been shown to be involved in the development and maintenance of neural circuitry [60], with a role in guiding axons, pruning synapses, regulating myelination, and positioning interneurons [61,62]. Data from MIA models have shown that immune system activation during pregnancy has a number of different effects on microglial activation [63]. For example, in mice, MIA leads to a blunting in the microglial response in the adult offspring. This effect was associated with a decrease in the release probability of dopamine in the striatum, linking it to changes in a neurotransmitter system long associated with schizophrenia [64].
While disturbances in microglial activation could result in a general dysconnectivity in the developing brain, it is also clear that some impacts are specific to particular cell types. For example, Park et al. reported that, in culture, activated microglia affected developing cortical interneurons by impairing mitochondrial function, disrupting dendritic arborisation, synapse formation, and neurotransmitter release in these cells [65]. Inhibitory interneurons release the inhibitory transmitter gamma-aminobutyric acid (GABA) and are a key part of the microcircuitry of the cerebral cortex, with a primary function of limiting and shaping the activity of principal cells. Although there are a large number of different types of GABAergic interneurons in the brain [66,67,68], with a variety of associated characteristics [69], a considerable body of the schizophrenia research literature has tended to focus on the subset of inhibitory interneurons that are found throughout the brain and express the protein parvalbumin (PV) [70,71]. In the neocortex and hippocampus, most PV interneurons are capable of generating action potentials at a high rate due to their short actional potential duration and refractory period. These fast-spiking interneurons have projections onto pyramidal neurons, and because of their fast-firing characteristics, they are able to provide precise inhibitory control over principal cells via both feedforward and feedback connectivity.
The initial impetus for research into the role of GABAergic interneurons in schizophrenia originated in studies that showed a dysfunction in these neurons in postmortem analyses of patients with the disease [72]. These postmortem studies have consistently revealed reduced expression of GABA-related markers, such as the enzyme glutamic acid decarboxylase (GAD67) and parvalbumin (PV), in the prefrontal cortex of individuals with schizophrenia [73,74]. These deficits are particularly prominent in the fast-spiking PV-positive interneurons [75,76]. Consistent with a role for these GABAergic neurons in some of the cognitive functions that are disrupted in schizophrenia, magnetic resonance spectroscopy studies have reported correlations between symptom severity and cognitive deficits, and decreased GABA levels, in the brain of patients with schizophrenia [77,78,79].
5. How Inflammatory Cytokines Affect GABA
Previous research shows that inflammatory cytokines can affect GABAergic function in a number of ways. During critical periods of neurodevelopment, GABAergic interneurons undergo extensive maturation, including the establishment of synaptic connections; the acquisition of specific molecular markers, such as parvalbumin (PV); and the refinement of inhibitory circuits that regulate cortical excitability. Disruptions in these processes, mediated by inflammatory cytokines, can lead to enduring deficits in inhibitory neurotransmission and network dysfunction. Disrupting GABA signalling during early development can in turn alter cellular migration and cortical architecture [80]. The results of many animal-model studies show that maternal immune activation results in long-lasting deficits in GABAergic interneuron function, including reduced PV expression, paralleling findings in schizophrenia [81,82]. In particular, IL-6 has been implicated in reducing the expression of GAD67, the enzyme responsible for synthesising GABA, thereby diminishing inhibitory signalling during critical developmental windows [83]. Pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumour necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) have also been shown to modulate GABAergic signalling by altering GABA receptor expression, inhibiting GABA synthesis, and disrupting inhibitory synaptic transmission [84]. These developmental disruptions can lead to persistent deficits in cortical inhibition and cognitive function, mirroring the neuropathological and behavioural abnormalities observed in schizophrenia. If changes in GABAergic function occur early on in development, they may underlie some of the symptoms observed in the prodrome, before the initial psychotic episode [85], or in some of the neurological soft signs that are observed from early life and that are predictive of later development of the disorder [86,87]. Furthermore, these early changes in GABA may be a ‘first hit’ that increases vulnerability to a second hit that occurs later in development and triggers schizophrenia.
The effects of maternal immune activation on GABAergic interneuron development are dependent on a number of factors, including the timing of exposure relative to the stage of gestation [88,89]. For example within the hippocampus, effects on PV interneurons depend on an interaction between sex, time of exposure during gestation, and subregion [90], while markers for somatostatin receptor 2, a receptor found on interneurons that release the neurotransmitter somatostatin, are timing dependent in the cortex [91]. These temporal effects highlight the vulnerability of GABAergic interneurons to inflammatory insults during distinct phases of neurodevelopment. Furthermore, the interaction between cytokines and other developmental factors, such as oxidative stress and mitochondrial dysfunction, can exacerbate the impact on interneuron maturation [65,92,93].
The interplay between inflammation and GABAergic dysfunction is also evident in adults, as individuals with schizophrenia often exhibit elevated levels of peripheral and central inflammatory markers, which correlate with cognitive deficits and reduced GABAergic activity [94,95]. In the adult brain, TNF-α, for example, has been shown to modulate synaptic scaling by increasing the surface expression of excitatory AMPA receptors while simultaneously decreasing inhibitory GABAergic signalling, leading to an imbalance in neuronal excitability [96]. This cytokine-mediated disruption of synaptic homeostasis may contribute to the hyperexcitability and network dysregulation observed in conditions such as schizophrenia. Moreover, chronic exposure to inflammatory cytokines can induce oxidative stress and mitochondrial dysfunction in GABAergic interneurons, further compromising their ability to maintain an inhibitory tone [97].
6. How Changes to GABA Could Alter Neural Oscillations
How is it that changes in GABAergic function could produce the cognitive and behavioural effects observed in schizophrenia? One proposal is that GABAergic dysfunction results in an imbalance between excitatory and inhibitory neurotransmitter systems, which leads to downstream effects. For example, in one prominent hypothesis, schizophrenia is linked to a downregulation of NMDA-subtype excitatory glutamate receptors that are located on GABAergic interneurons. This change results in a decrease in the activity of these interneurons, which leads to hypofunction in the inhibitory component of the circuit [98]. As a result of the excitatory/inhibitory balance in the circuit shifting towards excitation, there are problems controlling the gain of information flow within brain circuits [99]. While this hyperexcitability hypothesis might seem to explain some of the positive symptoms of schizophrenia, such as hallucinations and delusions, further detail is required to account for the negative and cognitive symptoms of the disease. It is also unclear how these changes would produce a differential effect to that which occurs in other disorders such as epilepsy, where changes in the excitation/inhibition balance are also implicated [100]. While there are several other potential mechanisms via which changes in GABAergic function could produce the signs and symptoms of schizophrenia, for example, by modulating dopamine activity [101], for the purposes of this review, we will focus on a model where GABAergic dysfunction leads to compromised information storage and processing that is more subtle than can be explained by a simple hyperexcitability hypothesis. This proposal, which has been explored in some depth previously, is the hypothesis that changes in GABAergic function directly affect the brain’s ability to synchronise information across space and time. This would compromise many aspects of brain function, for example, the ability to integrate information from different sensory areas, the ability to detect cause-and-effect relationships, and difficulty in coherent motor planning, effects that are all evident in schizophrenia. A synchronisation problem has previously been linked to changes in the oscillatory mechanisms that underlie brain rhythms [102,103,104]. Rhythmic activity in the brain is organised into distinct frequency bands, particularly, the theta (~2–10 Hz), beta (~12–30 Hz), and gamma (~30–90 Hz) bands [105,106,107]. One function of this oscillatory activity is to synchronise neural activity, both within local regions and across wider brain networks [108]. For example, it is known that several cognitive and behavioural tasks associated with prefrontal and hippocampal regions require the precise spike timing of pyramidal neurons in relation to rhythmic oscillations of the local field potential [105,109,110,111]. Neural oscillations also have a role in parcellating neural processing into temporally constrained blocks [112,113] that may allow for the separation of distinct stages of processing [114].
A key factor that underlies the generation and modulation of many of these oscillations is the relationship between excitatory and inhibitory neural activity. Both experimental and computational research suggests that incoming neuronal activity interacts with local circuitry to generate fast oscillations, such as gamma- and the high-frequency ripples that are associated with memory consolidation in the hippocampus [115,116,117]. These excitatory/inhibitory interactions can be observed in a number of different brain areas. For example, PV interneurons have a critical role in synchronising gamma oscillations in the primary visual cortex [118], and gamma oscillations nested within theta-frequency activity in the medial entorhinal cortex are dependent on feedback inhibition [119], as is coherent activity in the hippocampus [115]. While gamma-frequency activity is associated with active information processing, theta-frequency activity in the hippocampus is associated with memory and learning [120]. Theta is also shaped by a series of excitatory and inhibitory events that occur at particular phases of the theta cycle [121,122,123], and this activity is under the control of GABAergic pacemaker neurons located in the medial septum [124]. Deletion of the ErbB4 receptor in fast-spiking PV interneurons leads to impairments in hippocampal–prefrontal synchrony at theta frequencies in a mouse model [125]. Critically, this receptor is a part of a signalling pathway that is critical for the development of neocortical and hippocampal inhibitory circuits [126]. Furthermore, it has also been linked to neuroinflammation and subsequent changes in theta and gamma oscillations [127].
7. Links Between Neural Oscillations and Schizophrenia
Given the important role of oscillatory activity in synchronising information across space and time in the brain, it is possible that changes in GABAergic function, as occurs in schizophrenia, could compromise this capability [128]. Consistent with this proposal, a number of studies have reported that schizophrenia is associated with a disruption of neuronal oscillations in several frequency bands [102,103,129,130]. Initial research showed that activity in the 40 Hz-frequency band, elicited by auditory stimulation (auditory steady-state responses; ASSRs), had reduced power and phase synchronisation in individuals with schizophrenia [131,132]. ASSR impairments were also evident in patients with first-episode psychosis, as well as participants at high risk for psychosis, indicating that these effects were unlikely to be a result of medication or psychosis itself, and may have existed for some time prior [133]. It was subsequently shown that individuals with schizophrenia exhibited reduced 40 Hz-range, EEG gamma band responses; delayed phase coherence; and reductions in interhemispheric coherence [134]. Again, several of these changes were shown to occur in individuals at risk for schizophrenia or psychosis [135,136]. Investigations of oscillations during speech and language processing also revealed changes in delta- and theta-frequency band synchrony between fronto-temporal regions during talking vs. listening in people with schizophrenia [137]. Hirvonen et al. also reported that patients with schizophrenia were characterised by a reduction in gamma band oscillation amplitudes and a pronounced deficit in large-scale synchronization across brain networks, such as between the visual and frontal cortex [138]. Patients with schizophrenia also display a reduction in theta power and diminished theta-phase coupling between the mPFC and the medial temporal lobe compared to healthy control participants, and this effect was correlated with both memory performance and abnormal GABAA receptor expression in the schizophrenia group. Similar findings have been observed in animal models of the disorder, suggesting that the theta rhythm in particular may be important for long-range functional connectivity between the prefrontal cortex and the hippocampus and, by extension, cognitive tasks that require memory or executive function [139,140]. Inflammation has been linked to these effects, consistent with a role in the development of schizophrenia via an impact on oscillations. For example, elevations of TNF-alpha were linked to blunted alpha- and beta-frequency oscillations, as measured by MEG, when participants were performing an abstract reasoning task [141]. In an animal model, Mamad et al. demonstrated that an inflammation-inducing interperitoneal injection of LPS resulted in a reduction of theta-frequency activity and an increase in lower-frequency delta activity in the hippocampus [142]. Similarly, Hirao et al. have shown that network oscillations are altered in brain slices obtained from the anterior cingulate cortex in LPS-treated mice, a preparation that isolates inflammatory effects on oscillatory mechanisms from changes in behaviour that themselves may result in altered oscillatory activity [143].
8. How Disruptions in Oscillatory Activity Could Lead to Disorganisation Symptoms
Disturbances in the synchronisation of neural activity, as might occur when oscillations are disrupted, may result in the disorganisation of experience and memory. In schizophrenia, this may be apparent in disorganised speech and thought, symptoms that have been proposed to reflect deficits in integration [144], disruptions in excitatory/inhibitory balance, and oscillatory dysconnectivity [145]. Changes in synchronisation may also underlie the temporal and sequence processing deficits that are well documented in schizophrenia. This includes disturbance in the judgement of temporal order and duration [146,147], predictive timing [148], transitive inference [149,150], and sequence learning [151,152,153]. Similar deficits have also been observed in first-degree relatives and other at-risk individuals during the prodromal phase [154], and they are independent of other cognitive impairments [146]. The ubiquity and early expression of these types of timing and sequencing deficits suggest that they may be a primary feature of the disorder, occurring prior to the first psychotic episode, and a potential trait marker for schizophrenia [155].
Although timing and sequencing processes are ubiquitous through the brain, many of the particular processes that are disturbed in schizophrenia have been linked to the hippocampus, particularly as they relate to episodic memory [156,157,158]. Previous evidence from both in vivo and post-mortem studies indicates that abnormalities in the hippocampus are a feature of schizophrenia and autism, in both cases disorders where risk has been linked to exposure to inflammation during neurodevelopment [159,160,161,162,163]. Studies of the hippocampus as it relates to both schizophrenia and autism have reported both structural and functional alterations in this region [159,160]. Consistent with this region having a role in timing and sequencing processes, disruptions in hippocampal activity have been suggested to have a role in the generation of disorganisation symptoms in schizophrenia [164,165].
As a model of the effect of inflammatory processes, maternal immune activation has been shown to impact both oscillatory processes in the hippocampus and their connections to other regions [166,167,168]. For example, studies using both the poly (I:C) and the methylazoxymethanol acetate (MAM) model have independently demonstrated changes in GAD67 expression and PV+ interneuron activity in hippocampal regions [169,170], and in both cases, these reductions were accompanied by aberrant neural synchrony between the hippocampus and medial prefrontal cortex. Furthermore, dysregulated oscillatory networks also appear to compromise the integrity of hippocampal projections to other subcortical areas, such as the LS, the striatum, and the ventral tegmental area [157,171,172,173]. These latter regions are important for dopaminergic modulation, and so disruptions to the coordinated spiking activity of upstream neurons are likely to exert effects on subcortical dopaminergic activity. In turn, striatal dopaminergic concentrations have been shown to strongly influence the synchronisation of GABAergic micro-circuits in a computational model, suggesting that the relationship might be reciprocal, and that dopamine might have a wider modulatory role in the maintenance of functional connectivity [174].
Previous work has associated changes in brain oscillations to disorganisation symptoms in schizophrenia [175]; however, it will be important to demonstrate mechanistically how this might lead to changes in the neural processing of information. One form of neural synchrony observed in the hippocampus (and elsewhere) that has been consistently linked to a neural mechanism underlying sequence learning for more than three decades [176,177] depends on the phase relationship between neuron firing and the ‘background’ local field potential and is known as phase precession. Here, the hippocampal theta rhythm serves as a reference signal, against which the phase of firing of hippocampal principal cells is related to the location of the animal in space.
Theta-phase precession was initially observed in CA1 “place” cells. When the firing of these cells was referenced to the underlying theta-frequency LFP oscillation, it was noted that the phase of firing changed systematically from later to earlier phases as an animal moved through that cell’s place field [176,177]. As a result, the firing phase of a cell, relative to the theta cycle, was shown to provide information about where the animal is located within a place field, over and above that of the firing rate code [157,178,179]. Critically, when several cells with overlapping place fields generate phase precession, the combined activity produces an emergent phenomenon known as a ‘theta sequence’ [180]. During a theta sequence, recently experienced episodes that have occurred at behavioural timescales are preserved and compressed into a single theta cycle (~120 ms). During this time-compressed period, synaptic plasticity mechanisms can operate to link the neurons representing that episode [176,181]. As a result, theta sequences have received considerable interest as a potential mechanism underlying sequential memory encoding and storage [113,176,182,183,184], a proposal that aligns with findings that the developmental emergence of theta sequences coincides with the maturation of hippocampal memory in rodents [185]. Phase precession and theta sequences have also been observed to occur in tasks that require goal planning and decision making [186,187,188], and in paradigms that do not include a spatial component, suggesting an involvement in processes outside the spatial domain [189,190,191,192]. Furthermore, several studies have reported phase precession in single-cell recordings in human participants [193]. In one recent example, this activity was linked to memory processes in human participants watching and remembering movie clips [194], with indications that the process may be associated with the encoding of event boundaries [195,196].
If theta sequences provide the biophysical scaffolding that supports the encoding and storage of temporally extended memories, perhaps via the construction of mental maps, an important component of both episodic memory and decision making [197], then a disruption of this system could result in disorganisation of thought, as observed in schizophrenia [106]. Since the phase of firing of hippocampal cells during phase precession is tightly regulated by phasic inputs [198], with PV+ interneurons playing a crucial role [125,169,170,184,199,200], can manipulations that trigger inflammation sufficient to have an effect on the development of these interneurons and their connections alter phase precession? One recent study has investigated whether phase precession is altered in the MIA model, and here it was shown that, compared to control animals, neurons in the hippocampal CA1 region of MIA animals displayed considerably more variation in the starting phase of the precession as the animal entered the cell’s place field [166]. Since phase precession otherwise continues normally, but with a random phase offset on each precession phase trajectory, there are major consequences for theta sequences [166], as they are reproduced in a disordered manner that does not match the sequential ordering of experience (Figure 1). While a change in phase precession in individuals with schizophrenia has not yet been described, it is of interest to note that there are alterations in the neural replay that appear to underlie the representation of sequential relationships [153].
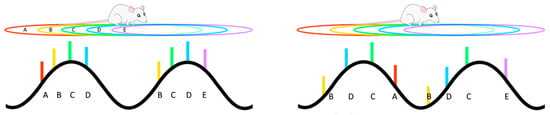
Figure 1.
As an animal moves along a trajectory (top left), multiple hippocampal place cells will be simultaneously active and responding to particular overlapping regions of space (their place fields A–E). At any particular moment, the currently active cells will fire (coloured bars) at a particular phase of the underlying theta rhythm (bottom left), with their firing phase being sequentially related to the order of the place fields. Maternal immune activation results in a disordering of this phase coding (bottom right), so that the phase sequence no longer matches the spatial sequence of place fields.
Hippocampal phase coding has been associated with the sequential integration of sound and odour cues [201], as well as internally generated states [202,203]; therefore, variation in the phase offset of precession in schizophrenia could potentially underlie the disintegration of experience and memory that is apparent in the disease [166]. An additional effect of increased starting phase variability is that the sequential spiking that occurs within each consecutive theta cycle is less clustered, and as a result, event sequences may overlap and merge [166]. This could contribute to disintegration of event boundaries [106,204], a phenomenon observed for both lower- and higher-order levels of processing among individuals with schizophrenia [205,206].
While we have described here how inflammation can lead to disorders of rhythmic activity, with a particular focus on the hippocampus, it is also worth noting that this region not only codes for space [207] but also for time. Investigations of ‘time’ cells, initially discovered in the hippocampus proper [208] and more recently in the medial entorhinal cortex [209], have shown that these cells represent the flow of time by firing at specific epochs during an episode, such that the population of neurons codes the entire temporal space of tasks that may last for many seconds [210]. Since timing and sequencing processes are disturbed in schizophrenia, and since these processes have previously been linked to the hippocampus [158,182,210], it will be of interest to explore whether hippocampal time cells are affected in the disorder. Changes in interneuron function may affect their activity, as it has been proposed that the mechanisms are dependent on recurrent neural connections that feed information back into a circuit with some delay [211]. The inclusion of delays in such circuits will likely depend on oscillations that provide some temporal distance between bursts of neural activity. Consistent with this model, time cell firing appears to be tied to rhythmic theta activity cycles [192]. These findings suggest that inflammation-mediated changes in GABAergic inhibition might be disruptive to time cell activity, and while time cells are difficult to examine in humans (although see [212]), they can be examined in detail in animal studies. At the present moment, however, there have been no investigations of these cells in an animal model of schizophrenia. This is clearly an avenue for future research.
9. Conclusions
Our ability to develop a sophisticated understanding of the biological basis of schizophrenia has been limited, in part, because of the complexity of the disorder. This has resulted in difficulties in linking changes in basic mechanisms, such as neurotransmitter systems, to high-level psychological symptoms [213]. These and related difficulties have meant that drug treatment strategies for schizophrenia have progressed only modestly over the last 40 years [213,214]. As a result, currently available treatments, while being somewhat effective against psychosis, are only efficacious in about half of patients, and often have little impact on the cognitive and negative symptoms [215]. This review has attempted to link initiating processes with mechanistic outcomes by focussing on the role of inflammation in disrupting inhibitory interneuron function in the brain and how this has consequences for the oscillatory activity that underlies timing, sequencing, and synchronisation in the brain (Figure 2). Disturbance in these latter processes has previously been proposed as a primary feature of schizophrenia and a potential trait marker for the disorder [155]. If changes in GABAergic interneuron function are an important mediator of the effects of inflammation on brain oscillations, then this transmitter system is an obvious target for therapeutic intervention. This can be attempted by manipulation of GABA systems directly or via modulation of the glutamatergic systems that activate interneurons [216,217]. Previous research in this area has, however, resulted in mixed results [218], with a need for better targeting of the errant systems, a project in which there is continued interest [219,220,221,222,223]. In this regard, the hippocampus will be a useful model system in this approach, as oscillating rhythms underlie much of the processing that occurs in this region. Furthermore, it has previously been implicated in schizophrenia [160,224,225,226], it is a region where disturbance could underlie some of the cognitive deficits observed in the disorder [227,228], and it is a location where defective GABAergic neurotransmission could be a critical factor in this dysfunction [162,219,229,230,231].
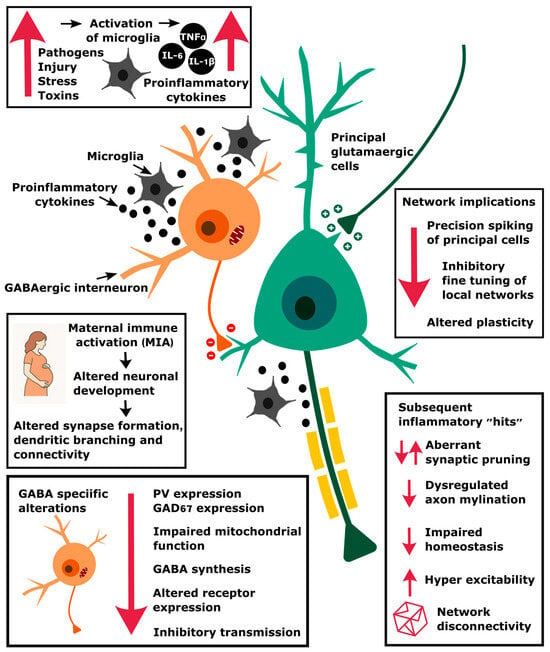
Figure 2.
An illustration of how inflammation, triggered by maternal immune activation and other stressors, can result in changes at the molecular level that compromise GABAergic function and network circuitry. This then affects the precision of spike timing, particularly during oscillatory activity, which alters plasticity and synchrony mechanisms.
Author Contributions
Both authors contributed equally to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Renewed Research Stay Fellowship for D.B. from the Alexander von Humboldt Stiftung, Germany.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the University of Otago, students, post-docs, technicians, and collaborators for their support. The grant number is NZL 1033125 HFST.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MIA | Maternal Immune Activation |
| GABA | gamma-aminobutyric acid |
| GAD67 | glutamate decarboxylase |
| NMDA | N-methyl-D-aspartate |
| PV | Pavalbumin |
References
- O’Donnell, K.; Meaney, M.J. Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis. Am. J. Psychiatry 2017, 174, 303–399. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The ICD-11 Classification of Mental and Behavioural Disorders-Clinical Description and Diagnostic Guidelines; WHO: Geneva, Switzerland, 2012; Available online: https://icd.who.int/en/ (accessed on 23 April 2025).
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Cognitive Deficits and Functional Outcome in Schizophrenia. Neuropsychiatr. Dis. Treat. 2006, 2, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F. Cognitive Impairment and Functional Outcome in Schizophrenia and Bipolar Disorder. J. Clin. Psychiatry 2006, 67 (Suppl. 9), 3–8; discussion 36–42. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Keefe, R.S.E.; McGuire, P.K. Cognitive Impairment in Schizophrenia: Aetiology, Pathophysiology, and Treatment. Mol. Psychiatry 2023, 28, 1902–1918. [Google Scholar] [CrossRef]
- Connor, T.J.; Leonard, B.E. Depression, Stress and Immunological Activation: The Role of Cytokines in Depressive Disorders. Life Sci. 1998, 62, 583–606. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J.; Yee, B.K. A Review of the Fetal Brain Cytokine Imbalance Hypothesis of Schizophrenia. Schizophr. Bull. 2009, 35, 959–972. [Google Scholar] [CrossRef]
- Martínez-Gras, I.; García-Sánchez, F.; Guaza, C.; Rodríguez-Jiménez, R.; Andrés-Esteban, E.; Palomo, T.; Rubio, G.; Borrell, J. Altered Immune Function in Unaffected First-Degree Biological Relatives of Schizophrenia Patients. Psychiatry Res. 2012, 200, 1022–1025. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef]
- Dawidowski, B.; Górniak, A.; Podwalski, P.; Lebiecka, Z.; Misiak, B.; Samochowiec, J. The Role of Cytokines in the Pathogenesis of Schizophrenia. J. Clin. Med. 2021, 10, 3849. [Google Scholar] [CrossRef]
- King, S.; Holleran, L.; Mothersill, D.; Patlola, S.; Rokita, K.; McManus, R.; Kenyon, M.; McDonald, C.; Hallahan, B.; Corvin, A.; et al. Early Life Adversity, Functional Connectivity and Cognitive Performance in Schizophrenia: The Mediating Role of IL-6. Brain Behav. Immun. 2021, 98, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. The Relationship between Inflammatory Biomarkers and Cognitive Dysfunction in Patients with Schizophrenia: A Systematic Review and Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 121, 110668. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.; Siskind, D.; Amft, M.; Wagner, E.; Yakimov, V.; Shih-Jung Liu, Z.; Walder, K.; Warren, N. Alteration Patterns of Peripheral Concentrations of Cytokines and Associated Inflammatory Proteins in Acute and Chronic Stages of Schizophrenia: A Systematic Review and Network Meta-Analysis. Lancet Psychiatry 2023, 10, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Manzanares-Teson, N.; Barnes, N.M. Cytokine Function in Medication-Naive First Episode Psychosis: A Systematic Review and Meta-Analysis. Schizophr. Res. 2014, 155, 101–108. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Barone, A.; Vellucci, L.; Benedetta, M.; Austin, M.; Iasevoli, F.; Ciccarelli, M. Linking Inflammation, Aberrant Glutamate-Dopamine Interaction, and Post-Synaptic Changes: Translational Relevance for Schizophrenia and Antipsychotic Treatment: A Systematic Review | Molecular Neurobiology. Mol. Neurobiol. 2022, 59, 6460–6501. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Zare-Shahabadi, A.; Rezaei, N. Cytokine Alterations in Schizophrenia: An Updated Review. Front. Psychiatry 2019, 10, 892. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. The Neurodevelopmental Hypothesis of Schizophrenia, Revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primer 2015, 1, 15067. [Google Scholar] [CrossRef]
- van Os, J.; Kenis, G.; Rutten, B.P.F. The Environment and Schizophrenia. Nature 2010, 468, 203–212. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Anderson, S.; Pettergrew, J.W. Is Schizophrenia Due to Excessive Synaptic Pruning in the Prefrontal Cortex? The Feinberg Hypothesis Revisited. J. Psychiatr. Res. 1994, 28, 239–265. [Google Scholar] [CrossRef]
- Rapoport, J.L.; Giedd, J.N.; Gogtay, N. Neurodevelopmental Model of Schizophrenia: Update 2012. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Maynard, T.M.; Sikich, L.; Lieberman, J.A.; LaMantia, A.-S. Neural Development, Cell-Cell Signaling, and the “Two-Hit” Hypothesis of Schizophrenia. Schizophr. Bull. 2001, 27, 457–476. [Google Scholar] [CrossRef]
- Cornblatt, B.A.; Lencz, T.; Smith, C.W.; Correll, C.U.; Auther, A.M.; Nakayama, E. The Schizophrenia Prodrome Revisited: A Neurodevelopmental Perspective. Schizophr. Bull. 2003, 29, 633–651. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Deste, G.; Smieskova, R.; Barlati, S.; Yung, A.R.; Howes, O.; Stieglitz, R.-D.; Vita, A.; McGuire, P.; Borgwardt, S. Cognitive Functioning in Prodromal Psychosis: A Meta-Analysis. Arch. Gen. Psychiatry 2012, 69, 562–571. [Google Scholar] [CrossRef]
- Merritt, K.; Luque Laguna, P.; Irfan, A.; David, A.S. Longitudinal Structural MRI Findings in Individuals at Genetic and Clinical High Risk for Psychosis: A Systematic Review. Front. Psychiatry 2021, 12, 62041. [Google Scholar] [CrossRef]
- Mednick, S.; Huttunen, M.O.; Machón, R.A. Prenatal Influenza Infections and Adult Schizophrenia. Schizophr. Bull. 1994, 20, 263–267. [Google Scholar] [CrossRef]
- Susser, E.; Neugebauer, R.; Hoek, H.W.; Brown, A.S.; Lin, S.; Labovitz, D.; Gorman, J.M. Schizophrenia After Prenatal Famine: Further Evidence. Arch. Gen. Psychiatry 1996, 53, 25–31. [Google Scholar] [CrossRef]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Babulas, V.P.; Susser, E.S. Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774–780. [Google Scholar] [CrossRef]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2009, 167, 261–280. [Google Scholar] [CrossRef]
- Brown, A.S.; Meyer, U. Maternal Immune Activation and Neuropsychiatric Illness: A Translational Research Perspective. Am. J. Psychiatry 2018, 175, 1073–1083. [Google Scholar] [CrossRef]
- Selemon, L.D.; Zecevic, N. Schizophrenia: A Tale of Two Critical Periods for Prefrontal Cortical Development. Transl. Psychiatry 2015, 5, e623. [Google Scholar] [CrossRef] [PubMed]
- Cheslack-Postava, K.; Brown, A.S. Prenatal Infection and Schizophrenia: A Decade of Further Progress. Schizophr. Res. 2022, 247, 7–15. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Jarskog, L.F. Exposure to Infection and Brain Development: Cytokines in the Pathogenesis of Schizophrenia. Schizophr. Res. 1997, 24, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Someya, T.; Nawa, H. Cytokine Hypothesis of Schizophrenia Pathogenesis: Evidence from Human Studies and Animal Models. Psychiatry Clin. Neurosci. 2010, 64, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef]
- Zaretsky, M.V.; Alexander, J.M.; Byrd, W.; Bawdon, R.E. Transfer of Inflammatory Cytokines Across the Placenta. Obstet. Gynecol. 2004, 103, 546. [Google Scholar] [CrossRef]
- Bermick, J.; Watson, S.; Lueschow, S.; McElroy, S.J. The Fetal Response to Maternal Inflammation Is Dependent upon Maternal IL-6 in a Murine Model. Cytokine 2023, 167, 156210. [Google Scholar] [CrossRef]
- Bergdolt, L.; Dunaevsky, A. Brain Changes in a Maternal Immune Activation Model of Neurodevelopmental Brain Disorders. Prog. Neurobiol. 2019, 175, 1–19. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal Acute and Chronic Inflammation in Pregnancy Is Associated with Common Neurodevelopmental Disorders: A Systematic Review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Meyer, U. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci. 2019, 42, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Niijima, M.; Kunisawa, K.; Teshigawara, T.; Kubota, H.; Fujigaki, S.; Fujigaki, H.; Yamamoto, Y.; Kim, H.-C.; Saito, K.; et al. Maternal Immune Activation Induces Neurodevelopmental Impairments of Adult Offspring through Alterations in Tryptophane-Kynurenine Pathway in the Placenta. Biochem. Biophys. Res. Commun. 2024, 737, 150922. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, M.; Pozzi, D.; Fossati, M.; Menna, E. Immune Synaptopathies: How Maternal Immune Activation Impacts Synaptic Function during Development. EMBO J. 2023, 42, e113796. [Google Scholar] [CrossRef] [PubMed]
- Cale, J.A.; Chauhan, E.J.; Cleaver, J.J.; Fusciardi, A.R.; McCann, S.; Waters, H.C.; Žavbi, J.; King, M.V. GABAergic and Inflammatory Changes in the Frontal Cortex Following Neonatal PCP plus Isolation Rearing, as a Dual-Hit Neurodevelopmental Model for Schizophrenia. Mol. Neurobiol. 2024, 61, 6968–6983. [Google Scholar] [CrossRef]
- Chamera, K.; Trojan, E.; Kotarska, K.; Szuster-Głuszczak, M.; Bryniarska, N.; Tylek, K.; Basta-Kaim, A. Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia. Int. J. Mol. Sci. 2021, 22, 1558. [Google Scholar] [CrossRef]
- Petanjek, Z.; Judaš, M.; Šimić, G.; Rašin, M.R.; Uylings, H.B.M.; Rakic, P.; Kostović, I. Extraordinary Neoteny of Synaptic Spines in the Human Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 13281–13286. [Google Scholar] [CrossRef]
- Zecevic, N.; Bourgeois, J.-P.; Rakic, P. Changes in Synaptic Density in Motor Cortex of Rhesus Monkey during Fetal and Postnatal Life. Dev. Brain Res. 1989, 50, 11–32. [Google Scholar] [CrossRef]
- Benes, F.M. Myelination of Cortical-Hippocampal Relays During Late Adolescence. Schizophr. Bull. 1989, 15, 585–593. [Google Scholar] [CrossRef]
- Caballero, A.; Granberg, R.; Tseng, K.Y. Mechanisms Contributing to Prefrontal Cortex Maturation during Adolescence. Neurosci. Biobehav. Rev. 2016, 70, 4–12. [Google Scholar] [CrossRef]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F.; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic Mapping of Human Cortical Development during Childhood through Early Adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef]
- Cannon, T.D.; Chung, Y.; He, G.; Sun, D.; Jacobson, A.; van Erp, T.G.M.; McEwen, S.; Addington, J.; Bearden, C.E.; Cadenhead, K.; et al. Progressive Reduction in Cortical Thickness as Psychosis Develops: A Multisite Longitudinal Neuroimaging Study of Youth at Elevated Clinical Risk. Biol. Psychiatry 2015, 77, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Pantelis, C.; Velakoulis, D.; McGorry, P.D.; Wood, S.J.; Suckling, J.; Phillips, L.J.; Yung, A.R.; Bullmore, E.T.; Brewer, W.; Soulsby, B.; et al. Neuroanatomical Abnormalities before and after Onset of Psychosis: A Cross-Sectional and Longitudinal MRI Comparison. Lancet 2003, 361, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ziermans, T.B.; Schothorst, P.F.; Schnack, H.G.; Koolschijn, P.C.M.P.; Kahn, R.S.; van Engeland, H.; Durston, S. Progressive Structural Brain Changes During Development of Psychosis. Schizophr. Bull. 2012, 38, 519–530. [Google Scholar] [CrossRef]
- van den Bosch, A.M.R.; Hümmert, S.; Steyer, A.; Ruhwedel, T.; Hamann, J.; Smolders, J.; Nave, K.-A.; Stadelmann, C.; Kole, M.H.P.; Möbius, W.; et al. Ultrastructural Axon–Myelin Unit Alterations in Multiple Sclerosis Correlate with Inflammation. Ann. Neurol. 2023, 93, 856–870. [Google Scholar] [CrossRef]
- Geloso, M.C.; D’Ambrosi, N. Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells 2021, 10, 686. [Google Scholar] [CrossRef]
- Khazaei, S.; Chen, C.C.L.; Andrade, A.F.; Kabir, N.; Azarafshar, P.; Morcos, S.M.; França, J.A.; Lopes, M.; Lund, P.J.; Danieau, G.; et al. Single Substitution in H3.3G34 Alters DNMT3A Recruitment to Cause Progressive Neurodegeneration. Cell 2023, 186, 1162–1178.e20. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Javitt, D.C.; Schoepp, D.; Kalivas, P.W.; Volkow, N.D.; Zarate, C.; Merchant, K.; Bear, M.F.; Umbricht, D.; Hajos, M.; Potter, W.Z.; et al. Translating Glutamate: From Pathophysiology to Treatment. Sci. Transl. Med. 2011, 3, 102mr2. [Google Scholar] [CrossRef]
- Mordelt, A.; de Witte, L.D. Microglia-Mediated Synaptic Pruning as a Key Deficit in Neurodevelopmental Disorders: Hype or Hope? Curr. Opin. Neurobiol. 2023, 79, 102674. [Google Scholar] [CrossRef]
- Mastenbroek, L.J.M.; Kooistra, S.M.; Eggen, B.J.L.; Prins, J.R. The Role of Microglia in Early Neurodevelopment and the Effects of Maternal Immune Activation. Semin. Immunopathol. 2024, 46, 1. [Google Scholar] [CrossRef]
- Santos, E.N.; Fields, R.D. Regulation of Myelination by Microglia. Sci. Adv. 2021, 7, eabk1131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jing, Y.; Zhang, H.; Bilkey, D.K.; Liu, P. Maternal Immune Activation Altered Microglial Immunoreactivity in the Brain of Postnatal Day 2 Rat Offspring. Synapse 2019, 73, e22072. [Google Scholar] [CrossRef]
- Hayes, L.N.; An, K.; Carloni, E.; Li, F.; Vincent, E.; Trippaers, C.; Paranjpe, M.; Dölen, G.; Goff, L.A.; Ramos, A.; et al. Prenatal Immune Stress Blunts Microglia Reactivity, Impairing Neurocircuitry. Nature 2022, 610, 327–334. [Google Scholar] [CrossRef]
- Park, G.-H.; Noh, H.; Shao, Z.; Ni, P.; Qin, Y.; Liu, D.; Beaudreault, C.P.; Park, J.S.; Abani, C.P.; Park, J.M.; et al. Activated Microglia Cause Metabolic Disruptions in Developmental Cortical Interneurons That Persist in Interneurons from Individuals with Schizophrenia. Nat. Neurosci. 2020, 23, 1352–1364. [Google Scholar] [CrossRef]
- Markram, H.; Toledo-Rodriguez, M.; Wang, Y.; Gupta, A.; Silberberg, G.; Wu, C. Interneurons of the Neocortical Inhibitory System. Nat. Rev. Neurosci. 2004, 5, 793–807. [Google Scholar] [CrossRef]
- Tzilivaki, A.; Tukker, J.J.; Maier, N.; Poirazi, P.; Sammons, R.P.; Schmitz, D. Hippocampal GABAergic Interneurons and Memory. Neuron 2023, 111, 3154–3175. [Google Scholar] [CrossRef]
- Wonders, C.P.; Anderson, S.A. The Origin and Specification of Cortical Interneurons. Nat. Rev. Neurosci. 2006, 7, 687–696. [Google Scholar] [CrossRef]
- McFarlan, A.R.; Chou, C.Y.C.; Watanabe, A.; Cherepacha, N.; Haddad, M.; Owens, H.; Sjöström, P.J. The Plasticitome of Cortical Interneurons. Nat. Rev. Neurosci. 2023, 24, 80–97. [Google Scholar] [CrossRef]
- Allami, P.; Yazdanpanah, N.; Rezaei, N. The Role of Neuroinflammation in PV Interneuron Impairments in Brain Networks; Implications for Cognitive Disorders. Rev. Neurosci. 2025. [Google Scholar] [CrossRef]
- Hijazi, S.; Smit, A.B.; van Kesteren, R.E. Fast-Spiking Parvalbumin-Positive Interneurons in Brain Physiology and Alzheimer’s Disease. Mol. Psychiatry 2023, 28, 4954–4967. [Google Scholar] [CrossRef]
- Birnbaum, R.; Weinberger, D.R. Genetic Insights into the Neurodevelopmental Origins of Schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Arion, D.; Unger, T.; Maldonado-Avilés, J.G.; Morris, H.M.; Volk, D.W.; Mirnics, K.; Lewis, D.A. Alterations in GABA-Related Transcriptome in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia. Mol. Psychiatry 2007, 13, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical Inhibitory Neurons and Schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef]
- Lewis, D.A.; Curley, A.A.; Glausier, J.R.; Volk, D.W. Cortical Parvalbumin Interneurons and Cognitive Dysfunction in Schizophrenia. Trends Neurosci. 2012, 35, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, T.; dos Santos Fabris, D.; de Oliveira, C.L.; Guimarães, F.S.; Gomes, F.V. Prefrontal and Hippocampal Parvalbumin Interneurons in Animal Models for Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2024, 50, 210–223. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Fish, K.N.; Lewis, D.A. GABA Neuron Alterations, Cortical Circuit Dysfunction and Cognitive Deficits in Schizophrenia. Neural Plast. 2011, 2011, 723184. [Google Scholar] [CrossRef]
- Nakahara, T.; Tsugawa, S.; Noda, Y.; Ueno, F.; Honda, S.; Kinjo, M.; Segawa, H.; Hondo, N.; Mori, Y.; Watanabe, H.; et al. Glutamatergic and GABAergic Metabolite Levels in Schizophrenia-Spectrum Disorders: A Meta-Analysis of 1H-Magnetic Resonance Spectroscopy Studies. Mol. Psychiatry 2022, 27, 744–757. [Google Scholar] [CrossRef]
- Reddy-Thootkur, M.; Kraguljac, N.V.; Lahti, A.C. The Role of Glutamate and GABA in Cognitive Dysfunction in Schizophrenia and Mood Disorders–A Systematic Review of Magnetic Resonance Spectroscopy Studies. Schizophr. Res. 2022, 249, 74–84. [Google Scholar] [CrossRef]
- Jahangir, M.; Zhou, J.-S.; Lang, B.; Wang, X.-P. GABAergic System Dysfunction and Challenges in Schizophrenia Research. Front. Cell Dev. Biol. 2021, 9, 663854. [Google Scholar] [CrossRef]
- Canetta, S.; Bolkan, S.; Padilla-Coreano, N.; Song, L.J.; Sahn, R.; Harrison, N.L.; Gordon, J.A.; Brown, A.; Kellendonk, C. Maternal Immune Activation Leads to Selective Functional Deficits in Offspring Parvalbumin Interneurons. Mol. Psychiatry 2016, 21, 956–968. [Google Scholar] [CrossRef]
- Rezaei, S.; Prévot, T.D.; Vieira, E.; Sibille, E. LPS-Induced Inflammation Reduces GABAergic Interneuron Markers and Brain-Derived Neurotrophic Factor in Mouse Prefrontal Cortex and Hippocampus. Brain Behav. Immun.-Health 2024, 38, 100761. [Google Scholar] [CrossRef] [PubMed]
- Labouesse, M.A.; Dong, E.; Grayson, D.R.; Guidotti, A.; Meyer, U. Maternal Immune Activation Induces GAD1 and GAD2 Promoter Remodeling in the Offspring Prefrontal Cortex. Epigenetics 2015, 10, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Pribiag, H.; Stellwagen, D. TNF-α Downregulates Inhibitory Neurotransmission through Protein Phosphatase 1-Dependent Trafficking of GABAA Receptors. J. Neurosci. 2013, 33, 15879–15893. [Google Scholar] [CrossRef]
- Klosterkötter, J.; Hellmich, M.; Steinmeyer, E.M.; Schultze-Lutter, F. Diagnosing Schizophrenia in the Initial Prodromal Phase. Arch. Gen. Psychiatry 2001, 58, 158–164. [Google Scholar] [CrossRef]
- Pieters, L.E.; Nadesalingam, N.; Walther, S.; van Harten, P.N. A Systematic Review of the Prognostic Value of Motor Abnormalities on Clinical Outcome in Psychosis. Neurosci. Biobehav. Rev. 2022, 132, 691–705. [Google Scholar] [CrossRef]
- Petrescu, C.; Petrescu, D.M.; Marian, G.; Focseneanu, B.E.; Iliuta, F.P.; Ciobanu, C.A.; Papacocea, S.; Ciobanu, A.M. Neurological Soft Signs in Schizophrenia, a Picture of the Knowledge in the Last Decade: A Scoping Review. Healthcare 2023, 11, 1471. [Google Scholar] [CrossRef]
- Vasistha, N.A.; Pardo-Navarro, M.; Gasthaus, J.; Weijers, D.; Müller, M.K.; García-González, D.; Malwade, S.; Korshunova, I.; Pfisterer, U.; von Engelhardt, J.; et al. Maternal Inflammation Has a Profound Effect on Cortical Interneuron Development in a Stage and Subtype-Specific Manner. Mol. Psychiatry 2020, 25, 2313–2329. [Google Scholar] [CrossRef]
- Nakamura, J.P.; Schroeder, A.; Gibbons, A.; Sundram, S.; Hill, R.A. Timing of Maternal Immune Activation and Sex Influence Schizophrenia-Relevant Cognitive Constructs and Neuregulin and GABAergic Pathways. Brain. Behav. Immun. 2022, 100, 70–82. [Google Scholar] [CrossRef]
- Gillespie, B.; Panthi, S.; Sundram, S.; Hill, R.A. The Impact of Maternal Immune Activation on GABAergic Interneuron Development: A Systematic Review of Rodent Studies and Their Translational Implications. Neurosci. Biobehav. Rev. 2024, 156, 105488. [Google Scholar] [CrossRef]
- Rahman, T.; Weickert, C.S.; Harms, L.; Meehan, C.; Schall, U.; Todd, J.; Hodgson, D.M.; Michie, P.T.; Purves-Tyson, T. Effect of Immune Activation during Early Gestation or Late Gestation on Inhibitory Markers in Adult Male Rats. Sci. Rep. 2020, 10, 1982. [Google Scholar] [CrossRef]
- Kann, O.; Papageorgiou, I.E.; Draguhn, A. Highly Energized Inhibitory Interneurons Are a Central Element for Information Processing in Cortical Networks. J. Cereb. Blood Flow Metab. 2014, 34, 1270–1282. [Google Scholar] [CrossRef]
- Behrens, M.M.; Sejnowski, T.J. Does Schizophrenia Arise from Oxidative Dysregulation of Parvalbumin-Interneurons in the Developing Cortex? Neuropharmacology 2009, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T.; Cryan, J.F.; Downer, E.J.; O’Leary, O.F. Inhibiting Neuroinflammation: The Role and Therapeutic Potential of GABA in Neuro-Immune Interactions. Brain. Behav. Immun. 2016, 54, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Herzig, K.-H.; Jokelainen, J.; Karhu, T.; Keinänen-Kiukaanniemi, S.; Järvelin, M.-R.; Veijola, J.; Viinamäki, H.; Tanskanen, P.; Jääskeläinen, E.; et al. Inflammation, Hippocampal Volume, and Cognition in Schizophrenia: Results from the Northern Finland Birth Cohort 1966. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 609–622. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic Scaling Mediated by Glial TNF-α. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Cuenod, M.; Steullet, P.; Cabungcal, J.-H.; Dwir, D.; Khadimallah, I.; Klauser, P.; Conus, P.; Do, K.Q. Caught in Vicious Circles: A Perspective on Dynamic Feed-Forward Loops Driving Oxidative Stress in Schizophrenia. Mol. Psychiatry 2022, 27, 1886–1897. [Google Scholar] [CrossRef]
- Coyle, J.T. The GABA-Glutamate Connection in Schizophrenia: Which Is the Proximate Cause? Biochem. Pharmacol. 2004, 68, 1507–1514. [Google Scholar] [CrossRef]
- Jardri, R.; Denève, S. Circular Inferences in Schizophrenia. Brain 2013, 136, 3227–3241. [Google Scholar] [CrossRef]
- Righes Marafiga, J.; Vendramin Pasquetti, M.; Calcagnotto, M.E. GABAergic Interneurons in Epilepsy: More than a Simple Change in Inhibition. Epilepsy Behav. 2021, 121, 106935. [Google Scholar] [CrossRef]
- Purves-Tyson, T.D.; Brown, A.M.; Weissleder, C.; Rothmond, D.A.; Shannon Weickert, C. Reductions in Midbrain GABAergic and Dopamine Neuron Markers Are Linked in Schizophrenia. Mol. Brain 2021, 14, 96. [Google Scholar] [CrossRef]
- Hirano, Y.; Uhlhaas, P.J. Current Findings and Perspectives on Aberrant Neural Oscillations in Schizophrenia. Psychiatry Clin. Neurosci. 2021, 75, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. Abnormal Neural Oscillations and Synchrony in Schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. Oscillations and Neuronal Dynamics in Schizophrenia: The Search for Basic Symptoms and Translational Opportunities. Biol. Psychiatry 2015, 77, 1001–1009. [Google Scholar] [CrossRef]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006; ISBN 978-0-19-804125-2. [Google Scholar]
- Lisman, J.; Buzsaki, G. A Neural Coding Scheme Formed by the Combined Function of Gamma and Theta Oscillations. Schizophr. Bull. 2008, 34, 974–980. [Google Scholar] [CrossRef]
- Singer, W. Neuronal Synchrony: A Versatile Code for the Definition of Relations? Neuron 1999, 24, 49–65. [Google Scholar] [CrossRef]
- Engel, A.K.; Singer, W. Temporal Binding and the Neural Correlates of Sensory Awareness. Trends Cogn. Sci. 2001, 5, 16–25. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroglu, C.; Karakaş, S.; Schürmann, M. Gamma, Alpha, Delta, and Theta Oscillations Govern Cognitive Processes. Int. J. Psychophysiol. 2001, 39, 241–248. [Google Scholar] [CrossRef]
- Buzsáki, G.; Geisler, C.; Henze, D.A.; Wang, X.-J. Interneuron Diversity Series: Circuit Complexity and Axon Wiring Economy of Cortical Interneurons. Trends Neurosci. 2004, 27, 186–193. [Google Scholar] [CrossRef]
- Mann, E.O.; Paulsen, O. Role of GABAergic Inhibition in Hippocampal Network Oscillations. Trends Neurosci. 2007, 30, 343–349. [Google Scholar] [CrossRef]
- Belluscio, M.A.; Mizuseki, K.; Schmidt, R.; Kempter, R.; Buzsáki, G. Cross-Frequency Phase-Phase Coupling between θ and γ Oscillations in the Hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 423–435. [Google Scholar] [CrossRef]
- Jaramillo, J.; Kempter, R. Phase Precession: A Neural Code Underlying Episodic Memory? Curr. Opin. Neurobiol. 2017, 43, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; Bodelón, C.; Wyble, B.P. A Proposed Function for Hippocampal Theta Rhythm: Separate Phases of Encoding and Retrieval Enhance Reversal of Prior Learning. Neural Comput. 2002, 14, 793–817. [Google Scholar] [CrossRef] [PubMed]
- Adaikkan, C.; Joseph, J.; Foustoukos, G.; Wang, J.; Polygalov, D.; Boehringer, R.; Middleton, S.J.; Huang, A.J.Y.; Tsai, L.-H.; McHugh, T.J. Silencing CA1 Pyramidal Cells Output Reveals the Role of Feedback Inhibition in Hippocampal Oscillations. Nat. Commun. 2024, 15, 2190. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Hippocampal Sharp Wave-Ripple: A Cognitive Biomarker for Episodic Memory and Planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef]
- Colgin, L.L. Rhythms of the Hippocampal Network. Nat. Rev. Neurosci. 2016, 17, 239–249. [Google Scholar] [CrossRef]
- Onorato, I.; Tzanou, A.; Schneider, M.; Uran, C.; Broggini, A.C.; Vinck, M. Distinct Roles of PV and Sst Interneurons in Visually Induced Gamma Oscillations. Cell Rep. 2025, 44, 115385. [Google Scholar] [CrossRef]
- Pastoll, H.; Solanka, L.; van Rossum, M.C.W.; Nolan, M.F. Feedback Inhibition Enables Theta-Nested Gamma Oscillations and Grid Firing Fields. Neuron 2013, 77, 141–154. [Google Scholar] [CrossRef]
- Herweg, N.A.; Solomon, E.A.; Kahana, M.J. Theta Oscillations in Human Memory. Trends Cogn. Sci. 2020, 24, 208–227. [Google Scholar] [CrossRef]
- Brankačk, J.; Stewart, M.; Fox, S.E. Current Source Density Analysis of the Hippocampal Theta Rhythm: Associated Sustained Potentials and Candidate Synaptic Generators. Brain Res. 1993, 615, 310–327. [Google Scholar] [CrossRef]
- Buzsáki, G. Theta Oscillations in the Hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef]
- Fox, S.E. Membrane Potential and Impedance Changes in Hippocampal Pyramidal Cells during Theta Rhythm. Exp. Brain Res. 1989, 77, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Hangya, B.; Borhegyi, Z.; Szilágyi, N.; Freund, T.F.; Varga, V. GABAergic Neurons of the Medial Septum Lead the Hippocampal Network during Theta Activity. J. Neurosci. 2009, 29, 8094–8102. [Google Scholar] [CrossRef] [PubMed]
- del Pino, I.; García-Frigola, C.; Dehorter, N.; Brotons-Mas, J.R.; Alvarez-Salvado, E.; Martínez de Lagrán, M.; Ciceri, G.; Gabaldón, M.V.; Moratal, D.; Dierssen, M.; et al. Erbb4 Deletion from Fast-Spiking Interneurons Causes Schizophrenia-like Phenotypes. Neuron 2013, 79, 1152–1168. [Google Scholar] [CrossRef] [PubMed]
- Batista-Brito, R.; Majumdar, A.; Nuño, A.; Ward, C.; Barnes, C.; Nikouei, K.; Vinck, M.; Cardin, J.A. Developmental Loss of ErbB4 in PV Interneurons Disrupts State-Dependent Cortical Circuit Dynamics. Mol. Psychiatry 2023, 28, 3133–3143. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Zhou, Z.; Liu, P.; Yang, J.; Ji, M. Dysfunction of NRG1/ErbB4 Signaling in the Hippocampus Might Mediate Long-Term Memory Decline After Systemic Inflammation. Mol. Neurobiol. 2023, 60, 3210–3226. [Google Scholar] [CrossRef]
- Van Derveer, A.B.; Bastos, G.; Ferrell, A.D.; Gallimore, C.G.; Greene, M.L.; Holmes, J.T.; Kubricka, V.; Ross, J.M.; Hamm, J.P. A Role for Somatostatin-Positive Interneurons in Neuro-Oscillatory and Information Processing Deficits in Schizophrenia. Schizophr. Bull. 2021, 47, 1385–1398. [Google Scholar] [CrossRef]
- Günther, A.; Hanganu-Opatz, I.L. Neuronal Oscillations: Early Biomarkers of Psychiatric Disease? Front. Behav. Neurosci. 2022, 16, 1038981. [Google Scholar] [CrossRef]
- Williams, S.; Boksa, P. Gamma Oscillations and Schizophrenia. J. Psychiatry Neurosci. 2010, 35, 75–77. [Google Scholar] [CrossRef]
- Krishnan, G.P.; Vohs, J.L.; Hetrick, W.P.; Carroll, C.A.; Shekhar, A.; Bockbrader, M.A.; O’Donnell, B.F. Steady State Visual Evoked Potential Abnormalities in Schizophrenia. Clin. Neurophysiol. 2005, 116, 614–624. [Google Scholar] [CrossRef]
- Thuné, H.; Recasens, M.; Uhlhaas, P.J. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-Analysis. JAMA Psychiatry 2016, 73, 1145–1153. [Google Scholar] [CrossRef]
- Grent-‘t-Jong, T.; Gajwani, R.; Gross, J.; Gumley, A.I.; Krishnadas, R.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Uhlhaas, P.J. 40-Hz Auditory Steady-State Responses Characterize Circuit Dysfunctions and Predict Clinical Outcomes in Clinical High-Risk for Psychosis Participants: A Magnetoencephalography Study. Biol. Psychiatry 2021, 90, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.M.; Nestor, P.G.; Niznikiewicz, M.A.; Salisbury, D.F.; Shenton, M.E.; McCarley, R.W. Abnormal Neural Synchrony in Schizophrenia. J. Neurosci. 2003, 23, 7407–7411. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.L.; Roach, B.J.; Wiley, K.; Loewy, R.L.; Ford, J.M.; Mathalon, D.H. Reduced Amplitude of Low-Frequency Brain Oscillations in the Psychosis Risk Syndrome and Early Illness Schizophrenia. Neuropsychopharmacology 2016, 41, 2388–2398. [Google Scholar] [CrossRef]
- Ramyead, A.; Kometer, M.; Studerus, E.; Koranyi, S.; Ittig, S.; Gschwandtner, U.; Fuhr, P.; Riecher-Roessler, A. Aberrant Current Source-Density and Lagged Phase Synchronization of Neural Oscillations as Markers for Emerging Psychosis. Schizophr. Bull. 2015, 41, 919–929. [Google Scholar] [CrossRef]
- Ford, J.M.; Mathalon, D.H.; Whitfield, S.; Faustman, W.O.; Roth, W.T. Reduced Communication between Frontal and Temporal Lobes during Talking in Schizophrenia. Biol. Psychiatry 2002, 51, 485–492. [Google Scholar] [CrossRef]
- Hirvonen, J.; Wibral, M.; Palva, J.M.; Singer, W.; Uhlhaas, P.; Palva, S. Whole-Brain Source-Reconstructed MEG-Data Reveal Reduced Long-Range Synchronization in Chronic Schizophrenia. eNeuro 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Dickerson, D.D.; Wolff, A.R.; Bilkey, D.K. Abnormal Long-Range Neural Synchrony in a Maternal Immune Activation Animal Model of Schizophrenia. J. Neurosci. 2010, 30, 12424–12431. [Google Scholar] [CrossRef]
- Sigurdsson, T.; Stark, K.L.; Karayiorgou, M.; Gogos, J.A.; Gordon, J.A. Impaired Hippocampal–Prefrontal Synchrony in a Genetic Mouse Model of Schizophrenia. Nature 2010, 464, 763–767. [Google Scholar] [CrossRef]
- Dietz, S.M.; Schantell, M.; Spooner, R.K.; Sandal, M.E.; Mansouri, A.; Arif, Y.; Okelberry, H.J.; John, J.A.; Glesinger, R.; May, P.E.; et al. Elevated CRP and TNF-α Levels Are Associated with Blunted Neural Oscillations Serving Fluid Intelligence. Brain. Behav. Immun. 2023, 114, 430–437. [Google Scholar] [CrossRef]
- Mamad, O.; Islam, M.N.; Cunningham, C.; Tsanov, M. Differential Response of Hippocampal and Prefrontal Oscillations to Systemic LPS Application. Brain Res. 2018, 1681, 64–74. [Google Scholar] [CrossRef]
- Hirao, A.; Hojo, Y.; Murakami, G.; Ito, R.; Hashizume, M.; Murakoshi, T.; Uozumi, N. Effects of Systemic Inflammation on the Network Oscillation in the Anterior Cingulate Cortex and Cognitive Behavior. PLoS ONE 2024, 19, e0302470. [Google Scholar] [CrossRef] [PubMed]
- Hardy-Baylé, M.-C.; Sarfati, Y.; Passerieux, C. The Cognitive Basis of Disorganization Symptomatology in Schizophrenia and Its Clinical Correlates: Toward a Pathogenetic Approach to Disorganization. Schizophr. Bull. 2003, 29, 459–471. [Google Scholar] [CrossRef]
- Palaniyappan, L. Dissecting the Neurobiology of Linguistic Disorganisation and Impoverishment in Schizophrenia. Semin. Cell Dev. Biol. 2022, 129, 47–60. [Google Scholar] [CrossRef]
- Ciullo, V.; Spalletta, G.; Caltagirone, C.; Jorge, R.E.; Piras, F. Explicit Time Deficit in Schizophrenia: Systematic Review and Meta-Analysis Indicate It Is Primary and Not Domain Specific. Schizophr. Bull. 2016, 42, 505–518. [Google Scholar] [CrossRef]
- Thoenes, S.; Oberfeld, D. Meta-Analysis of Time Perception and Temporal Processing in Schizophrenia: Differential Effects on Precision and Accuracy. Clin. Psychol. Rev. 2017, 54, 44–64. [Google Scholar] [CrossRef]
- Ciullo, V.; Piras, F.; Vecchio, D.; Banaj, N.; Coull, J.T.; Spalletta, G. Predictive Timing Disturbance Is a Precise Marker of Schizophrenia. Schizophr. Res. Cogn. 2018, 12, 42–49. [Google Scholar] [CrossRef]
- Titone, D.; Ditman, T.; Holzman, P.S.; Eichenbaum, H.; Levy, D.L. Transitive Inference in Schizophrenia: Impairments in Relational Memory Organization. Schizophr. Res. 2004, 68, 235–247. [Google Scholar] [CrossRef]
- Onwuameze, O.E.; Titone, D.; Ho, B.-C. Transitive Inference Deficits in Unaffected Biological Relatives of Schizophrenia Patients. Schizophr. Res. 2016, 175, 64–71. [Google Scholar] [CrossRef][Green Version]
- Pedersen, A.; Siegmund, A.; Ohrmann, P.; Rist, F.; Rothermundt, M.; Suslow, T.; Arolt, V. Reduced Implicit and Explicit Sequence Learning in First-Episode Schizophrenia. Neuropsychologia 2008, 46, 186–195. [Google Scholar] [CrossRef]
- Siegert, R.J.; Weatherall, M.; Bell, E.M. Is Implicit Sequence Learning Impaired in Schizophrenia? A Meta-Analysis. Brain Cogn. 2008, 67, 351–359. [Google Scholar] [CrossRef]
- Nour, M.M.; Liu, Y.; Arumuham, A.; Kurth-Nelson, Z.; Dolan, R.J. Impaired Neural Replay of Inferred Relationships in Schizophrenia. Cell 2021, 184, 4315–4328.e17. [Google Scholar] [CrossRef]
- Dickinson, D.; Ramsey, M.E.; Gold, J.M. Overlooking the Obvious: A Meta-Analytic Comparison of Digit Symbol Coding Tasks and Other Cognitive Measures in Schizophrenia. Arch. Gen. Psychiatry 2007, 64, 532–542. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Nopoulos, P.; O’Leary, D.S.; Miller, D.D.; Wassink, T.; Flaum, M. Defining the Phenotype of Schizophrenia: Cognitive Dysmetria and Its Neural Mechanisms. Biol. Psychiatry 1999, 46, 908–920. [Google Scholar] [CrossRef]
- Eichenbaum, H. Memory: Organization and Control. Annu. Rev. Psychol. 2017, 68, 19–45. [Google Scholar] [CrossRef]
- Tingley, D.; Buzsáki, G. Transformation of a Spatial Map across the Hippocampal-Lateral Septal Circuit. Neuron 2018, 98, 1229–1242.e5. [Google Scholar] [CrossRef]
- Meck, W.H.; Church, R.M.; Matell, M.S. Hippocampus, Time, and Memory—A Retrospective Analysis. Behav. Neurosci. 2013, 127, 642–654. [Google Scholar] [CrossRef]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal Contributions to Social and Cognitive Deficits in Autism Spectrum Disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Harrison, P.J. The Hippocampus in Schizophrenia: A Review of the Neuropathological Evidence and Its Pathophysiological Implications. Psychopharmacology 2004, 174, 151–162. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate Immune Dysfunction and Neuroinflammation in Autism Spectrum Disorder (ASD). Brain. Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef]
- Knight, S.; McCutcheon, R.; Dwir, D.; Grace, A.A.; O’Daly, O.; McGuire, P.; Modinos, G. Hippocampal Circuit Dysfunction in Psychosis. Transl. Psychiatry 2022, 12, 344. [Google Scholar] [CrossRef]
- Stone, W.S.; Iguchi, L. Do Apparent Overlaps between Schizophrenia and Autistic Spectrum Disorders Reflect Superficial Similarities or Etiological Commonalities? N. Am. J. Med. Sci. 2011, 4, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Krajcovic, B.; Fajnerova, I.; Horacek, J.; Kelemen, E.; Kubik, S.; Svoboda, J.; Stuchlik, A. Neural and Neuronal Discoordination in Schizophrenia: From Ensembles through Networks to Symptoms. Acta Physiol. 2019, 226, e13282. [Google Scholar] [CrossRef] [PubMed]
- Olypher, A.V. Cognitive Disorganization in Hippocampus: A Physiological Model of the Disorganization in Psychosis. J. Neurosci. 2006, 26, 158–168. [Google Scholar] [CrossRef]
- Speers, L.J.; Cheyne, K.R.; Cavani, E.; Hayward, T.; Schmidt, R.; Bilkey, D.K. Hippocampal Sequencing Mechanisms Are Disrupted in a Maternal Immune Activation Model of Schizophrenia Risk. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 6954–6965. [Google Scholar] [CrossRef]
- Munn, R.G.K.; Wolff, A.; Speers, L.J.; Bilkey, D.K. Disrupted Hippocampal Synchrony Following Maternal Immune Activation in a Rat Model. Hippocampus 2023, 33, 995–1008. [Google Scholar] [CrossRef]
- Speers, L.J.; Bilkey, D.K. Disorganization of Oscillatory Activity in Animal Models of Schizophrenia. Front. Neural Circuits 2021, 15, 108. [Google Scholar] [CrossRef]
- Dickerson, D.D.; Overeem, K.A.; Wolff, A.R.; Williams, J.M.; Abraham, W.C.; Bilkey, D.K. Association of Aberrant Neural Synchrony and Altered GAD67 Expression Following Exposure to Maternal Immune Activation, a Risk Factor for Schizophrenia. Transl. Psychiatry 2014, 4, e418. [Google Scholar] [CrossRef]
- Lodge, D.J.; Behrens, M.M.; Grace, A.A. A Loss of Parvalbumin-Containing Interneurons Is Associated with Diminished Oscillatory Activity in an Animal Model of Schizophrenia. J. Neurosci. 2009, 29, 2344–2354. [Google Scholar] [CrossRef]
- Lansink, C.S.; Goltstein, P.M.; Lankelma, J.V.; McNaughton, B.L.; Pennartz, C.M. Hippocampus Leads Ventral Striatum in Replay of Place-Reward Information. PLoS Biol. 2009, 7, e1000173. [Google Scholar] [CrossRef]
- Luo, A.H.; Tahsili-Fahadan, P.; Wise, R.A.; Lupica, C.R.; Aston-Jones, G. Linking Context with Reward: A Functional Circuit from Hippocampal CA3 to Ventral Tegmental Area. Science 2011, 333, 353–357. [Google Scholar] [CrossRef]
- Speers, L.J.; Schmidt, R.; Bilkey, D.K. Aberrant Phase Precession of Lateral Septal Cells in a Maternal Immune Activation Model of Schizophrenia Risk May Disrupt the Integration of Location with Reward. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 4187–4201. [Google Scholar] [CrossRef]
- Humphries, M.D.; Wood, R.; Gurney, K. Dopamine-Modulated Dynamic Cell Assemblies Generated by the GABAergic Striatal Microcircuit. Neural Netw. 2009, 22, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, L.E.; Brookes, M.J.; Rathnaiah, M.; Katshu, M.Z.U.H.; Koelewijn, L.; Williams, G.; Kumar, J.; Walters, J.T.R.; Seedat, Z.A.; Palaniyappan, L.; et al. Motor-Related Oscillatory Activity in Schizophrenia According to Phase of Illness and Clinical Symptom Severity. NeuroImage Clin. 2021, 29, 102524. [Google Scholar] [CrossRef]
- Skaggs, W.E.; McNaughton, B.L.; Wilson, M.A.; Barnes, C.A. Theta Phase Precession in Hippocampal Neuronal Populations and the Compression of Temporal Sequences. Hippocampus 1996, 6, 149–172. [Google Scholar] [CrossRef]
- O’Keefe, J.; Recce, M.L. Phase Relationship between Hippocampal Place Units and the EEG Theta Rhythm. Hippocampus 1993, 3, 317–330. [Google Scholar] [CrossRef]
- Huxter, J.; Burgess, N.; O’Keefe, J. Independent Rate and Temporal Coding in Hippocampal Pyramidal Cells. Nature 2003, 425, 828–832. [Google Scholar] [CrossRef]
- Jensen, O.; Lisman, J.E. Position Reconstruction from an Ensemble of Hippocampal Place Cells: Contribution of Theta Phase Coding. J. Neurophysiol. 2000, 83, 2602–2609. [Google Scholar] [CrossRef]
- Foster, D.J.; Wilson, M.A. Hippocampal Theta Sequences. Hippocampus 2007, 17, 1093–1099. [Google Scholar] [CrossRef]
- Dan, Y.; Poo, M.-M. Spike Timing-Dependent Plasticity of Neural Circuits. Neuron 2004, 44, 23–30. [Google Scholar] [CrossRef]
- Buzsáki, G.; Tingley, D. Space and Time: The Hippocampus as a Sequence Generator. Trends Cogn. Sci. 2018, 22, 853–869. [Google Scholar] [CrossRef]
- Dragoi, G.; Buzsáki, G. Temporal Encoding of Place Sequences by Hippocampal Cell Assemblies. Neuron 2006, 50, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Drieu, C.; Zugaro, M. Hippocampal Sequences During Exploration: Mechanisms and Functions. Front. Cell. Neurosci. 2019, 13, 232. [Google Scholar] [CrossRef]
- Muessig, L.; Lasek, M.; Varsavsky, I.; Cacucci, F.; Wills, T.J. Coordinated Emergence of Hippocampal Replay and Theta Sequences during Post-Natal Development. Curr. Biol. 2019, 29, 834–840.e4. [Google Scholar] [CrossRef]
- Wikenheiser, A.M.; Redish, A.D. Hippocampal Theta Sequences Reflect Current Goals. Nat. Neurosci. 2015, 18, 289–294. [Google Scholar] [CrossRef]
- Gupta, A.S.; van der Meer, M.A.A.; Touretzky, D.S.; Redish, A.D. Segmentation of Spatial Experience by Hippocampal Theta Sequences. Nat. Neurosci. 2012, 15, 1032–1039. [Google Scholar] [CrossRef]
- Johnson, A.; Redish, A.D. Neural Ensembles in CA3 Transiently Encode Paths Forward of the Animal at a Decision Point. J. Neurosci. 2007, 27, 12176–12189. [Google Scholar] [CrossRef]
- Pastalkova, E.; Itskov, V.; Amarasingham, A.; Buzsaki, G. Internally Generated Cell Assembly Sequences in the Rat Hippocampus. Science 2008, 321, 1322–1327. [Google Scholar] [CrossRef]
- Cei, A.; Girardeau, G.; Drieu, C.; Kanbi, K.E.; Zugaro, M. Reversed Theta Sequences of Hippocampal Cell Assemblies during Backward Travel. Nat. Neurosci. 2014, 17, 719–724. [Google Scholar] [CrossRef]
- Lenck-Santini, P.-P.; Fenton, A.A.; Muller, R.U. Discharge Properties of Hippocampal Neurons during Performance of a Jump Avoidance Task. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 6773–6786. [Google Scholar] [CrossRef]
- Shimbo, A.; Izawa, E.-I.; Fujisawa, S. Scalable Representation of Time in the Hippocampus. Sci. Adv. 2021, 7, eabd7013. [Google Scholar] [CrossRef]
- Qasim, S.E.; Fried, I.; Jacobs, J. Phase Precession in the Human Hippocampus and Entorhinal Cortex. Cell 2021, 184, 3242–3255.e10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yebra, M.; Schjetnan, A.G.P.; Patel, K.; Katz, C.N.; Kyzar, M.; Mosher, C.P.; Kalia, S.K.; Chung, J.M.; Reed, C.M.; et al. Theta Phase Precession Supports Memory Formation and Retrieval of Naturalistic Experience in Humans. Nat. Hum. Behav. 2024, 8, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Bilkey, D.K.; Jensen, C. Neural Markers of Event Boundaries. Top. Cogn. Sci. 2019, 13, 28–141. [Google Scholar] [CrossRef] [PubMed]
- Kurby, C.A.; Zacks, J.M. Segmentation in the Perception and Memory of Events. Trends Cogn. Sci. 2008, 12, 72–79. [Google Scholar] [CrossRef]
- Kaplan, R.; Schuck, N.W.; Doeller, C.F. The Role of Mental Maps in Decision-Making. Trends Neurosci. 2017, 40, 256–259. [Google Scholar] [CrossRef]
- Sloin, H.E.; Spivak, L.; Levi, A.; Gattegno, R.; Someck, S.; Stark, E. Local Activation of CA1 Pyramidal Cells Induces Theta-Phase Precession. Science 2024, 383, 551–558. [Google Scholar] [CrossRef]
- Royer, S.; Zemelman, B.V.; Losonczy, A.; Kim, J.; Chance, F.; Magee, J.C.; Buzsáki, G. Control of Timing, Rate and Bursts of Hippocampal Place Cells by Dendritic and Somatic Inhibition. Nat. Neurosci. 2012, 15, 769–775. [Google Scholar] [CrossRef]
- Ducharme, G.; Lowe, G.C.; Goutagny, R.; Williams, S. Early Alterations in Hippocampal Circuitry and Theta Rhythm Generation in a Mouse Model of Prenatal Infection: Implications for Schizophrenia. PLoS ONE 2012, 7, e29754. [Google Scholar] [CrossRef]
- Terada, S.; Sakurai, Y.; Nakahara, H.; Fujisawa, S. Temporal and Rate Coding for Discrete Event Sequences in the Hippocampus. Neuron 2017, 94, 1248–1262.e4. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishida, H.; Redish, A.D.; Lauwereyns, J. Theta Phase Shift in Spike Timing and Modulation of Gamma Oscillation: A Dynamic Code for Spatial Alternation during Fixation in Rat Hippocampal Area CA1. J. Neurophysiol. 2014, 111, 1601–1614. [Google Scholar] [CrossRef]
- Wang, Y.; Romani, S.; Lustig, B.; Leonardo, A.; Pastalkova, E. Theta Sequences Are Essential for Internally Generated Hippocampal Firing Fields. Nat. Neurosci. 2015, 18, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Radvansky, G.A.; Zacks, J.M. Event Boundaries in Memory and Cognition. Curr. Opin. Behav. Sci. 2017, 17, 133–140. [Google Scholar] [CrossRef]
- Zalla, T.; Verlut, I.; Franck, N.; Puzenat, D.; Sirigu, A. Perception of Dynamic Action in Patients with Schizophrenia. Psychiatry Res. 2004, 128, 39–51. [Google Scholar] [CrossRef]
- Coffman, B.A.; Haigh, S.M.; Murphy, T.K.; Salisbury, D.F. Event-Related Potentials Demonstrate Deficits in Acoustic Segmentation in Schizophrenia. Schizophr. Res. 2016, 173, 109–115. [Google Scholar] [CrossRef]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Clarendon Press: New York, NY, USA; Oxford University Press: Oxford, UK, 1978; ISBN 0-19-857206-9. [Google Scholar]
- MacDonald, C.J.; Lepage, K.Q.; Eden, U.T.; Eichenbaum, H. Hippocampal “Time Cells” Bridge the Gap in Memory for Discontiguous Events. Neuron 2011, 71, 737–749. [Google Scholar] [CrossRef]
- Heys, J.G.; Dombeck, D.A. Evidence for a Subcircuit in Medial Entorhinal Cortex Representing Elapsed Time during Immobility. Nat. Neurosci. 2018, 21, 1574–1582. [Google Scholar] [CrossRef]
- Eichenbaum, H. Time Cells in the Hippocampus: A New Dimension for Mapping Memories. Nat. Rev. Neurosci. 2014, 15, 732–744. [Google Scholar] [CrossRef]
- Banquet, J.-P.; Gaussier, P.; Cuperlier, N.; Hok, V.; Save, E.; Poucet, B.; Quoy, M.; Wiener, S.I. Time as the Fourth Dimension in the Hippocampus. Prog. Neurobiol. 2021, 199, 101920. [Google Scholar] [CrossRef]
- Reddy, L.; Zoefel, B.; Possel, J.K.; Peters, J.; Dijksterhuis, D.E.; Poncet, M.; van Straaten, E.C.W.; Baayen, J.C.; Idema, S.; Self, M.W. Human Hippocampal Neurons Track Moments in a Sequence of Events. J. Neurosci. 2021, 41, 6714–6725. [Google Scholar] [CrossRef]
- Carpenter, W.T.; Davis, J.M. Another View of the History of Antipsychotic Drug Discovery and Development. Mol. Psychiatry 2012, 17, 1168–1173. [Google Scholar] [CrossRef]
- Girgis, R.R.; Zoghbi, A.W.; Javitt, D.C.; Lieberman, J.A. The Past and Future of Novel, Non-Dopamine-2 Receptor Therapeutics for Schizophrenia: A Critical and Comprehensive Review. J. Psychiatr. Res. 2018, 108, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Belforte, J.E.; Zsiros, V.; Sklar, E.R.; Jiang, Z.; Yu, G.; Li, Y.; Quinlan, E.M.; Nakazawa, K. Postnatal NMDA Receptor Ablation in Corticolimbic Interneurons Confers Schizophrenia-like Phenotypes. Nat. Neurosci. 2010, 13, 76–83. [Google Scholar] [CrossRef]
- Gordon, J.A. Testing the Glutamate Hypothesis of Schizophrenia. Nat. Neurosci. 2010, 13, 2–4. [Google Scholar] [CrossRef]
- Howes, O.D.; Bukala, B.R.; Beck, K. Schizophrenia: From Neurochemistry to Circuits, Symptoms and Treatments. Nat. Rev. Neurol. 2024, 20, 22–35. [Google Scholar] [CrossRef]
- Uliana, D.L.; Lisboa, J.R.F.; Gomes, F.V.; Grace, A.A. The Excitatory-Inhibitory Balance as a Target for the Development of Novel Drugs to Treat Schizophrenia. Biochem. Pharmacol. 2024, 228, 116298. [Google Scholar] [CrossRef]
- Kaar, S.J.; Nottage, J.F.; Angelescu, I.; Marques, T.R.; Howes, O.D. Gamma Oscillations and Potassium Channel Modulation in Schizophrenia: Targeting GABAergic Dysfunction. Clin. EEG Neurosci. 2024, 55, 203–213. [Google Scholar] [CrossRef]
- Ventriglio, A.; Bellomo, A.; Ricci, F.; Magnifico, G.; Rinaldi, A.; Borraccino, L.; Piccininni, C.; Cuoco, F.; Gianfelice, G.; Fornaro, M.; et al. New Pharmacological Targets for the Treatment of Schizophrenia: A Literature Review. Curr. Top. Med. Chem. 2021, 21, 1500–1516. [Google Scholar] [CrossRef]
- Chiou, L.-C.; Sieghart, W. IUPHAR Review: Alpha6-Containing GABAA Receptors–Novel Targets for the Treatment of Schizophrenia. Pharmacol. Res. 2025, 213, 107613. [Google Scholar] [CrossRef]
- Howes, O.D.; Dawkins, E.; Lobo, M.C.; Kaar, S.J.; Beck, K. New Drug Treatments for Schizophrenia: A Review of Approaches to Target Circuit Dysfunction. Biol. Psychiatry 2024, 96, 638–650. [Google Scholar] [CrossRef]
- Singh, S.; Khushu, S.; Kumar, P.; Goyal, S.; Bhatia, T.; Deshpande, S.N. Evidence for Regional Hippocampal Damage in Patients with Schizophrenia. Neuroradiology 2018, 60, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.A.; Stan, A.D.; Wagner, A.D. The Hippocampal Formation in Schizophrenia. Am. J. Psychiatry 2010, 167, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R. Cell Biology of the Hippocampal Formation in Schizophrenia. Biol. Psychiatry 1999, 45, 395–402. [Google Scholar] [CrossRef]
- Boyer, P.; Phillips, J.L.; Rousseau, F.L.; Ilivitsky, S. Hippocampal Abnormalities and Memory Deficits: New Evidence of a Strong Pathophysiological Link in Schizophrenia. Brain Res. Rev. 2007, 54, 92–112. [Google Scholar] [CrossRef]
- Tamlyn, D.; McKenna, P.J.; Mortimer, A.M.; Lund, C.E.; Hammond, S.; Baddeley, A.D. Memory Impairment in Schizophrenia: Its Extent, Affiliations and Neuropsychological Character. Psychol. Med. 1992, 22, 101–115. [Google Scholar] [CrossRef]
- Steiner, J.; Brisch, R.; Schiltz, K.; Dobrowolny, H.; Mawrin, C.; Krzyżanowska, M.; Bernstein, H.-G.; Jankowski, Z.; Braun, K.; Schmitt, A.; et al. GABAergic System Impairment in the Hippocampus and Superior Temporal Gyrus of Patients with Paranoid Schizophrenia: A Post-Mortem Study. Schizophr. Res. 2016, 177, 10–17. [Google Scholar] [CrossRef]
- Heckers, S.; Konradi, C. GABAergic Mechanisms of Hippocampal Hyperactivity in Schizophrenia. Schizophr. Res. 2015, 167, 4–11. [Google Scholar] [CrossRef]
- Fujikawa, R.; Yamada, J.; Jinno, S. Subclass Imbalance of Parvalbumin-Expressing GABAergic Neurons in the Hippocampus of a Mouse Ketamine Model for Schizophrenia, with Reference to Perineuronal Nets. Schizophr. Res. 2021, 229, 80–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).