Abstract
Extracellular vesicles (EVs) are membrane-bound cargoes secreted by normal and pathological cells. Through their protein, nucleic acid, and lipid cargoes, EVs mediate several cellular processes, such as cell–cell communication, cell development, immune response, and tissue repair. Most importantly, through their enzyme cargo, EVs mediate pathophysiological processes, including the pathogenesis of cancer. In this review, we enumerate several enzymes secreted in EVs (EV enzyme cargo) from cells and patient clinical samples of breast and prostate cancers and detail their contributions to the progression and survival of both cancers. Findings in this review reveal that the EV enzyme cargo could exert cell progression functions via adhesion, proliferation, migration, invasion, and metastasis. The EV enzyme cargo might also influence cell survival functions of chemoresistance, radioresistance, angiogenesis, cell death inhibition, cell colony formation, and immune evasion. While the current literature provides evidence of the possible contributions of the EV enzyme cargo to the progression and survival mechanisms of breast and prostate cancers, future studies are required to validate that these effects are modified by EVs and provide insights into the clinical applications of the EV enzyme cargo in breast and prostate cancer.
Keywords:
extracellular; vesicles; proteins; enzymes; breast; prostate; cancer; tumor; progression; survival 1. Introduction
Enzymes are proteins that catalyze various biological reactions in living organisms [1]. Given their ubiquitous nature, enzymes are involved in both physiological and pathological processes [2,3], including the pathogenesis of various cancers, such as breast and prostate cancer [4,5]. According to recent epidemiological data from the United States, breast and prostate cancers are the most frequently diagnosed cancers among women and men, respectively [6], and the world [7]. In Australia, breast and prostate cancers are the second and first most frequently diagnosed cancers, respectively [8]. Given these statistics, breast and prostate cancers seem to have similar trends in terms of their high frequencies among women and men globally. Moreover, while the breast and prostate serve different purposes, malignancies that develop in both tissues are similar in that they (1) depend on hormones [9,10], (2) portray similar genetic underpinnings [11,12], (3) frequently exhibit large proportions of indolent disease [13], and (4) respond to similar therapy, such as endocrine therapy [14]. These similarities justify the simultaneous focus of this review on breast and prostate cancers.
Extracellular vesicles (EVs) are 30–1000 nm in diameter cargo deliverers secreted by almost every cell in the body in both the normal and pathologic state [15]. Although there are continuously evolving classifications, EVs are broadly classified according to their size and biogenesis into ectosomes (100–1000 nm) and exosomes (30–150 nm) [16]. However, the usage of the terms “exosomes” and “ectosomes” has recently been discouraged by the International Society for Extracellular Vesicles (ISEV) due to insufficient evidence to support their biogenesis [16]. Therefore, the term “EV” has been established by ISEV as the adopted nomenclature for both exosomes and ectosomes. According to ISEV 2023 guidelines, EVs are now characterized based on size (small EVs: <200 nm and large EVs: >200 nm), density (low, medium, and high), and biochemistry (protein markers) [16].
EVs carry cargoes of a wide range of important bioactive molecules, including lipids, proteins (such as cytoskeletal proteins, enzymes, cytoplasmic proteins, and RNA-binding proteins), RNAs (mRNAs, tRNAs, miRNAs, DNAs, and other RNAs), and DNAs (such as mtDNA, dsDNA, and ssDNA) [17] (Figure 1).
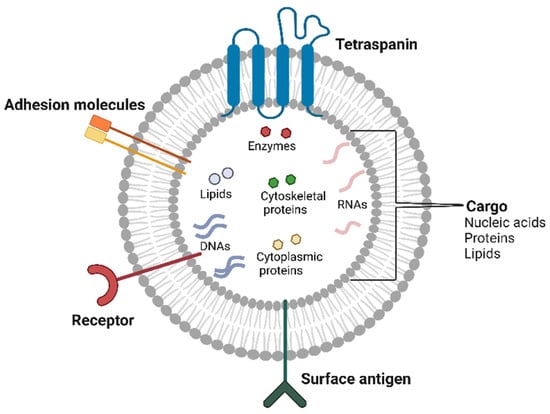
Figure 1.
Components of extracellular vesicles. EVs are membrane-bound carriers of nucleic acids, proteins, and lipids. EVs are structurally composed of specific proteins, including receptors, surface antigens, adhesion molecules, and tetraspanins. The image was created with BioRender.com (accessed on 14 January 2025).
Given their ability to transfer molecules that influence gene expression, EVs play vital roles in cell–cell communication, cell development, immune response, and tissue repair [18]. Additionally, several studies have reported certain differences in EVs of certain pathological cells compared to their corresponding normal cells [18]. These differences may include higher secretion rates of EVs, alterations in the expression of EV structural proteins, and functional differences in specific EV content (e.g., proteins and nucleic acids). Since cancers remodel their microenvironment by transferring various molecules (including enzymes) between their cells via secretion and uptake of EVs [19], there is a need to investigate the contributions of these molecules to the pathogenesis of cancers. This would provide a basis for exploring their diagnostic, prognostic, and therapeutic relevance to various cancers, including breast and prostate cancers. Therefore, in this review, we provide an overview of enzymes in the pathogenesis of breast and prostate cancer. Most importantly, we discuss the contributions of enzymes secreted in EVs associated with breast and prostate cancers to the progression and survival of both cancers.
2. Roles of Enzymes in the Pathogenesis of Breast and Prostate Cancers
Enzymes have been implicated in the pathogenesis of breast and prostate cancers through their contribution to various malignant processes in both cancers (Figure 2).
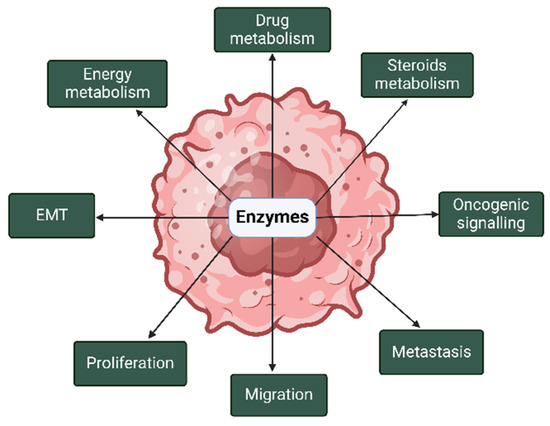
Figure 2.
Roles of enzymes in the pathogenesis of breast and prostate cancers. In breast and prostate cancers, enzymes are implicated in metabolism, oncogenic signaling, proliferation, and epithelial–mesenchymal transition (EMT), among others. The image was created with BioRender.com (accessed on 14 January 2025).
In breast cancer, there is a marked increase in the activities of glycolytic enzymes, such as pyruvate kinase (PK), hexokinase (HK), enolase, phosphofructokinase (PFK), and aldolase, as compared to normal breast tissues [20]. Similarly, in prostate cancer, there is an increase in the activities of PK, HK, and PFK as compared to normal prostate tissues [21]. This indicates a pivotal role for these glycolytic enzymes in the supply of energy to drive the progression of breast and prostate cancers, as explained by the Warburg effect [22,23], which could be harnessed as a treatment strategy for both cancers [24,25,26].
In the metabolism of drugs and xenobiotics in breast and prostate cancers, several enzymes have been implicated. In breast cancer, there is an increased expression of UDP-glucuronosyltransferases [27] and various cytochrome P450 (CYP) enzymes, such as CYP2A6 and CYP1B1, as compared to normal breast tissues [28]. Similarly, the upregulated expression of various CYP enzymes, such as CYP27A1 and CYP2R1, in prostate cancer has been documented [29]. The expression pattern of these drugs and xenobiotics metabolizing enzymes offer opportunities for the development of personalized treatments for both breast and prostate cancers [28,29]. Given that breast and prostate cancers are both driven by sex steroid hormones [9], the involvement of sex steroid metabolizing enzymes, such as 17β-hydroxysteroid dehydrogenases (17βHSDs), in the pathogenesis of both cancers has been documented [30,31,32,33,34,35,36]. The 17βHSD family of enzymes catalyzes the conversion (oxidation/reduction) of hydroxyl groups in the 17β position of the backbones of steroids [37]. This conversion by 17βHSDs can lead to various outcomes, including the production of the active form of estrogen and androgen (estradiol and testosterone, respectively), which drive breast and prostate cancers, respectively [38]. Moreover, 17βHSD types 1 and 2 have been shown to predict patient outcomes for breast cancer [39,40], whereas 17βHSD type 3 has been shown to offer therapeutic opportunities for prostate cancer treatment [41].
In the progression of breast and prostate cancers, several enzymes have been found to act as either tumor suppressors or tumor promoters. A typical example of such enzymes is the silent information regulator 2 (sir2) family of proteins (sirtuins). Sirtuins are classified as class III histone deacetylases that transfer an acetyl group to the ribose moiety of adenosine diphosphate (ADP), producing 2-O-acetyl-ADP-ribose (o-AADPR) and NAM, a feedback inhibitor of sirtuins [42]. Seven sirtuins (SIRT1–7) have been identified in mammals [43], which function as NAD-dependent deacetylases (SIRT1–3 and SIRT5–7) and ADP-ribosyl transferases (SIRT4 and 6) [44]. In our recent review [44], we extensively revealed that sirtuins modulate various molecular targets to mediate tumor-suppressing and/or tumor-promoting effects, thus offering therapeutic opportunities against both cancers. Furthermore, cystathionine β-synthase and cystathionine γ-lyase are hydrogen sulfide (H2S)-producing enzymes whose constitutive upregulation in breast and prostate cancers [45,46] has been shown to promote the aggressiveness of breast and prostate cancers through increased production of H2S [47,48]. H2S has been shown to significantly drive several oncogenic signaling pathways, including the JAK/STAT, Ras/Raf/MEK/ERK, and PI3K/AKT/mTOR signaling pathways [47].
Another class of enzymes implicated in the aggressiveness of breast and prostate cancers is the hyaluronan synthases (HASs), which comprise three isoforms (HAS1–3) that differ by their expression patterns, enzymatic activities, and subcellular locations [49,50]. HASs are responsible for the biosynthesis of hyaluronan (HA), an extracellular matrix molecule composed of a large glycosaminoglycan polymer. Studies have reported an increased expression of HASs and HA in breast and prostate cancers, which suggests the potential diagnostic and prognostic relevance of HASs in both cancers [51,52,53,54]. As a mechanism, HA promotes the aggressiveness of breast and prostate cancers via the induction of epithelial–mesenchymal transition (EMT) to drive migration and metastasis of both cancers [53,55]. Moreover, EMT has been found to confer migratory capacity, radio/chemoresistance, invasiveness, and resistance to apoptotic cell death in cancers [56]. Based on these findings, HASs offer opportunities for the identification of potential drug targets for the treatment of breast and prostate cancers [57].
Additionally, the role of proteases in breast and prostate cancers has been extensively investigated [58,59,60,61,62]. For instance, matrix metalloproteinases (MMPs) have been found to promote proliferation and metastasis of both breast and prostate cancers [63,64,65,66]. Also, protein kinases have been found to play pivotal roles in breast and prostate cancers [67,68,69,70]. A typical example is protein kinase D, which has been shown to promote metastasis of breast and prostate cancers [71,72,73]. Overall, enzymes are indicated in the pathogenesis of breast and prostate cancers. Therefore, in this review, we explore the cell progression and survival functions of enzymes secreted in EVs associated with breast and prostate cancers.
3. Cell Progression and Survival Functions of Enzymes Secreted in EVs Associated with Breast and Prostate Cancers
Perhaps the main relevance of metabolic reprogramming in cancers is embodied by “reprogrammed enzymes” that exhibit malignant functions [74,75,76,77] in addition to their canonical functions as biological catalysts. This enzyme reprogramming may involve the selective upregulation or downregulation of enzymes either via alteration in gene expression [78] or suggestively via uptake of EVs containing enzymes and/or their modulators from neighboring cells. Moreover, the selective inclusion of enzymes in EVs may serve to transport certain enzymes between cells, which can influence pathophysiological processes, including the pathogenesis of cancer [79]. A crucial example is the discovery that EVs from cancer cells can selectively include purinergic enzymes (e.g., ectonucleotidases) in their cargo to influence cancer progression [80].
Although cancer progression has been previously described as a combination of genetic and epigenetic signals that confer a growth advantage to cancers [81], it is also described as a learning process that ensures the survival of cancers [82]. Based on the above descriptions, it is apparent that the pathogenesis of cancer involves the synergistic function of two broad, mutually connected signals: cell survival and cell progression (Figure 3).
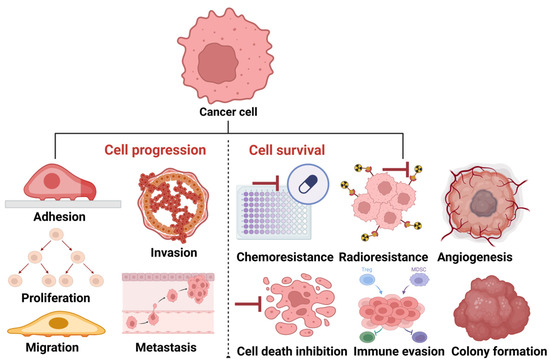
Figure 3.
Progression and survival signals in cancer. To progress, cancer cells turn on tumor progression signals, such as adhesion, proliferation, migration, invasion, and metastasis. To survive, cancer cells turn on tumor adaptative signals, such as chemo/radioresistance, angiogenesis, colony formation, and cell death inhibition. The image was created with BioRender.com (accessed on 20 March 2025).
Since tumor functions, such as adhesion, proliferation, migration, invasion, and metastasis, are mechanisms supporting cancer cell progression [83], we opine that tumor adaptative functions, such as chemoresistance, radioresistance, angiogenesis, cell death inhibition, colony formation, and immune suppression (evasion), support cancer cell survival. Therefore, this review explores the contributions of enzymes secreted in EVs to these cancer cell progression and survival mechanisms.
Using protein-based and/or mRNA-based methods, studies included in this review have identified and quantified enzymes in EVs from breast/prostate cancer cells and clinical samples (plasma and urine) from patients with breast/prostate cancer. However, most of these studies are faced with limitations of non-uniformity in the methods of isolating EVs, and the lack of enzyme activity assays for verifying the active form of identified enzymes. Barring these limitations, we enumerate several enzymes detected in EVs associated with breast and prostate cancers and discuss their malignant functions in terms of their contributions to the progression and survival of both cancers (summarized in Table 1 for breast cancer and Table 2 for prostate cancer).

Table 1.
Malignant functions of enzymes secreted in EVs associated with breast cancer.

Table 2.
Malignant functions of enzymes secreted in EVs associated with prostate cancer.
3.1. Enzymes Secreted in EVs Associated with Both Breast and Prostate Cancers
3.1.1. Adenosine Triphosphate (ATP) Citrate Lyase (ACLY)
ACLY has been identified in EVs associated with cancers of the breast [84] and prostate [99]. ACLY expression is upregulated in both breast [110] and prostate [111,112] cancers. ACLY has been reported as a cell survival factor in both breast and prostate cancers. For instance, silencing of ACLY by siRNA significantly decreased cell viability and increased apoptosis of MCF-7 breast cancer cells [110]. Consistent with this finding, Velez et al. demonstrated that ACLY inhibition by a combination of bempedoic acid (BA) and palbociclib decreases the viability of MDA-MB-231 breast cancer cells and that ACLY inhibition by BA induces apoptosis in MDA-MB-231 breast cancer cells via elevation of the apoptotic markers cPARP and p-c-Jun [113]. Moreover, the inhibition of ACLY has also been found to induce apoptotic cell death in other cancers [114]. On the other hand, Gao et al. showed that pharmacologic inhibition of ACLY by hydroxycitrate tribasic induces apoptosis of PC-3 and LNCaP prostate cancer cells [115]. The authors further demonstrated that ACLY inhibition by siRNAs enhances the apoptosis of PC-3 and LNCaP prostate cancer cells induced by Cucurbitacin B [115] and that Cucurbitacin B downregulates the expression of ACLY in PC-3 and LNCaP prostate cancer cells [115]. The inhibition of apoptosis by ACLY is at least in part due to the inhibition of ROS, as shown by Migita et al. through siRNA inhibition of ACLY in LNCaP prostate cancer cells [116]. Moreover, it has been previously demonstrated in earlier studies that ACLY knockdown by siRNAs inhibits cell cycle progression and induces apoptosis of PC-3M prostate cancer cells [117]. Substantiating these findings, the tumor suppressor miR-22 [118] was found to mediate its anticancer effect by inhibiting ACLY activity in PC-3 prostate cancer cells [112]. Together, these findings indicate that ACLY contributes to the survival of breast and prostate cancers via cell death inhibition. However, it would be interesting to investigate the contributions of ACLY to the progression of both cancers.
3.1.2. Enolase (ENO)
Among the ENO isoforms, ENO1 and ENO2 have been identified in EVs associated with cancers of the breast [85,86,87] and prostate [99]. Consistent with this finding, upregulated expressions of ENO1 and ENO2 have been reported in both breast [119,120] and prostate [121,122] cancers. There is limited information regarding the relevance of ENO to the progression and survival of breast and prostate cancers. Notwithstanding, Tu et al. [123] established that induced expression of ENO1 by 4-hydroxy-tamoxifen (4-OHT) confers 4-OHT drug-induced resistance in MC7 breast cancer cells via induction of the ER/NFkB pathway [123]. Chen et al. showed that the ENO1 inhibitor HuL227 downregulates tumor growth, angiogenesis, and recruitment of CCR2+ inflammatory monocytes in the PC-3 subcutaneous xenograft model of prostate cancer [124]. This suggests that ENO1 contributes to the survival of breast cancer via chemoresistance while contributing to the survival of prostate cancer via angiogenesis and immune evasion. The contributions of ENO2 to the survival of breast and prostate cancers need to be investigated.
In another study, inhibition of ENO1 by HuL227 halted inflammation-induced migration of PC-3 prostate cancer cells and reduced CCL2 or TGFβ-enhanced migration of PC-3 and DU145 prostate cancer cells [124]. This indicates a role for ENO1 in the progression of prostate cancer via the promotion of migration. Studies are required to determine the contributions of ENO1 to the progression of breast cancer. Although there is a paucity of data on the relevance of ENO2 to the progression of breast and prostate cancers, ENO2 mRNA was found to be elevated in breast cancer lymph node metastases compared to primary breast tumors [125], which may suggest its role in breast cancer progression. Similarly, ENO2 expression was also upregulated in the estrogen receptor-positive subset of 36 invasive ductal breast carcinomas compared to non-tumor counterparts [126]. Also, Zhou et al. opined in their systematic review of five studies that ENO2 contributes to the metastasis of prostate cancer [122]. Given the limited information, more studies are required to investigate the malignant functions of ENO1 and ENO2 in both cancers before a conclusion can be reached.
3.1.3. Fatty Acid Synthase (FASN)
FASN has been identified in EVs associated with breast [87] and prostate [99] cancers. It is also highly expressed in cancers of the breast [127,128,129] and prostate [130,131]. In the study by Menendez et al., FASN inhibition by the mycotoxin cerulenin sensitizes SK-BR-3 breast cancer cells to the cytotoxic effect of the anticancer drug docetaxel via downregulation of HER-2/neu oncogene expression [127]. Also, FASN inhibition by either siRNA or exogenous inhibitor increases cisplatin-induced apoptotic cell death in MDA-MB-231 breast cancer cells [128]. To corroborate this finding, Schroeder et al. demonstrated that FASN inhibition by the FASN inhibitor C75 induces apoptotic cell death in BT-474, MCF-7, MDA-MB-231 breast cancer cells by promoting ROS-dependent mitochondrial cytochrome c release and upregulating pro-apoptotic factors, such as BIM, NOXA, and PUMA [129]. In an earlier study by Jin et al., FASN inhibition by lapatinib or C75 was found to inhibit the invasion of SK-BR-3 and BT-474 breast cancer cells [123]. Using LNCaP-LN3 prostate cancer cells, Oh et al. demonstrated that FASN inhibition by FAS inhibitors (TVB-3166, GSK2194069, and Fasnall) results in metabolic alterations in [132], which may negatively affect the cancer’s metabolic reprogramming and survival. Additionally, FASN inhibition by triclosan or siRNAs induced apoptosis of LNCaP prostate cancer cells [133,134]. Moreover, studies have demonstrated that FASN inhibition by C75 sensitizes PC-3 and LNCaP prostate cancer cells to the apoptotic and growth-inhibiting effects of radiotherapy [135,136]. Corroborating these findings, Huang et al. reported a downregulated expression of FASN in patients with prostate cancer who were treated with androgen deprivation therapy and chemotherapy [137], thus suggesting FASN as a molecular target against prostate cancer. Together, the above findings indicate that FASN contributes to the survival of breast and prostate cancers via chemoresistance and cell death inhibition.
Furthermore, Li et al. demonstrated that FASN inhibition by cerulenin results in decreased migration of MCF-7-MEK5 breast cancer cells [138]. Corroborating this finding, Xu et al. demonstrated that FASN inhibition by cerulenin decreases the migration of SK-BR-3 breast cancer cells [125]. Also, upregulated FASN expression and activities were detected in brain metastasis of breast cancer, which was evidenced by the detection of FASN mRNA and lipid products, such as monosaturated fatty acids (e.g., triacylglycerols), in brain metastasis of BT-474 breast cancer cells in mice [126]. Moreover, a recent study demonstrated that FASN inhibition by TVB-2640 slows migration and reduces invasion of MDA-MB-231 breast cancer cells and its brain metastatic variant MDA-MB-231BR [139]. Using LNCaP and C4-2 prostate cancer cells, Huang et al. showed that FASN inhibition by siRNAs and cerulenin decreased the proliferation of prostate cancer [137]. Also, Yoshii et al. demonstrated that FASN inhibition by siRNAs reduces the proliferation and migration of LNCaP prostate cancer cells [140]. A recent study by De Piano et al. also demonstrated that FASN inhibition by siRNAs results in decreased migration of 1542 and PC-3 prostate cancer cells [141]. Together, the above studies indicate that FASN contributes to the progression of breast cancer via the promotion of migration, invasion, and metastasis while contributing to the progression of prostate cancer via the promotion of proliferation and migration.
3.1.4. Focal Adhesion Kinase (FAK)
FAK has been identified in EVs associated with cancers of the breast [88] and prostate [100]. Consistent with this, upregulated FAK expression has been reported in both breast [142] and prostate [143] cancers. In a study by Li-Hui et al., it was demonstrated that the downregulation of FAK results in the loss of cellular adhesion and induces the apoptosis of BT474 breast cancer cells [144]. Corroborating this finding, Vita et al. demonstrated that inhibition of FAK downregulates the AKT and ERK1/2 survival pathways and induces the apoptosis of BT-20 breast cancer cells [145]. Another earlier study by Satoh et al. demonstrated that FAK inhibition by antisense oligonucleotides sensitizes ZR-75-1, MDA-MB-231, and MCF-7 breast cancer cells to the cytotoxic effects of camptothecin [146], a plant-derived anticancer agent [147]. Also, the inhibition of FAK by the FAK inhibitor PF87 was reported to sensitize BT-474 and MDA-361 breast cancer cells to the cytotoxic effects of the anti-Her2+ breast cancer agent trastuzumab [148]. In mice infected with PC-3 prostate cancer cells, FAK inhibition by shRNA prevented the growth of prostate tumors via induction of apoptosis [149]. Corroborating this finding, another study demonstrated that FAK inhibition by co-administration of defactinib and docetaxel reduces the viability of docetaxel-sensitive and resistant PC-3 and DU145 prostate cancer cells, inhibits the growth of PC-3 xenografts, and induces apoptosis in patient-derived prostate tumor explants [150]. Moreover, Johnson et al. demonstrated that overexpression of FAK promotes cell survival of PC-3 prostate cancer cells via an increase of clonogenic activity (colony formation) [151]. Together, these studies indicate that FAK contributes to the survival of breast and prostate cancers via chemoresistance and cell death inhibition.
FAK inhibition by PF878 was shown to suppress migration but not the proliferation of BT-474 and MDA-361 breast cancer cells [148]. Similarly, inhibition of FAK was also shown to reduce cell–matrix attachment (cell adhesion) and migration of endocrine-resistant variants of MCF-7 breast cancer cells [152]. Also, Sumitomo et al. demonstrated that migration of LNCaP prostate cancer cells was halted following the inhibition of FAK by neutral endopeptidase (NEP) [153]. NEP is a surface enzyme of prostatic epithelial cells that inactivates neuropeptides involved in androgen-independent prostate cancer progression [154]. Consistent with this, Lacoste et al. demonstrated that bombesin, a substrate of NEP, induces motility of PC-3 prostate cancer cells via activation (tyrosine phosphorylation) of FAK [155]. Moreover, phosphorylation of FAK at tyrosine 861 residue has been reported to enhance the migration of PC-3 prostate cancer cells [156]. Additionally, several proteins (such as S100A9 and RAB11A) implicated in the proliferation, invasion, migration, and metastasis of PC-3, VCaP, and DU145 prostate cancer cells have been found to mediate their malignant functions via FAK [157,158]. Overall, the above studies indicate that FAK contributes to the progression of breast cancer via the promotion of adhesion and migration while contributing to the progression of prostate cancer via the promotion of proliferation, invasion, migration, and metastasis.
3.1.5. Pyruvate Kinase (PK)
Among the PK isoforms, PKM2 has been identified in EVs associated with breast [86,87] and prostate [99,101] cancers. Also, PKM2 is highly expressed in breast [159,160] and prostate [161,162] cancers. Overexpression of PKM2 has been associated with chemosensitivity to epirubicin and 5-fluorouracil in patients with breast cancer [163]. In contrast, there is evidence that chemotherapeutic drugs, including TAM and lapatinib, exert their cytotoxic effects against breast cancer by inhibiting PKM2 [164,165,166]. Additionally, a combination of PKM2 inhibitors (gliotoxin and shikonin) was found to sensitize high-density MDA-MB-231 breast cancer cells to the cytotoxic effect of vincristine, which was substantiated by increased apoptosis of the breast cancer cells [167]. Also, Dey et al. demonstrated that PKM2 knockdown by siRNAs induces autophagic cell death of DU145 prostate cancer cells via inhibition of the Akt/mTOR signaling [161]. The authors further demonstrated that PKM2 knockdown by siRNAs alters metabolism and inhibits viability and colony formation of DU145 prostate cancer cells [161]. Moreover, it has been previously noted that silencing of PKM2 by siRNAs decreases colony formation of PC-3 and LNCaP prostate cancer cells [168]. Corroborating these findings, a recent study by Jiang et al. revealed that PKM2 knockdown by a novel PKM2 inhibitor, compound 3h, induces apoptotic and autophagic cell death of LNCaP prostate cancer cells [169]. Together, these studies indicate that PKM2 contributes to the survival of breast cancer via chemoresistance and cell death inhibition while contributing to the survival of prostate cancer via cell death inhibition and colony formation.
Furthermore, the Knockdown of PKM2 by specific siRNAs was reported to suppress growth and migration as well as induce G2/M phase cell cycle arrest of MDA-MB-231 and HCC1937 breast cancer cells [159]. In another study, the knockdown of PKM2 by siRNA suppressed EMT and consequently downregulated migration and invasion of MDA-MB-231 and MCF-7 breast cancer cells [170]. Also, Guo et al. demonstrated that PKM2 silencing by siRNAs inhibits migration, invasion, and EMT of DU145 and PC-3 prostate cancer cells [162]. As a mechanism, the authors further established that PKM2 overexpression promotes migration, invasion, and metastasis of DU145 and PC-3 prostate cancer cells via the ERK1/2/c-Jun/Cox-2 signaling pathway [162]. Together, these findings suggest that PKM2 contributes to the progression of breast cancer via the promotion of migration and invasion while contributing to the progression of prostate cancer via the promotion of migration, invasion, and metastasis.
3.2. Enzymes Secreted in EVs Associated with Breast Cancer (with No Current Evidence in Prostate Cancer)
3.2.1. Phosphoglycerate Kinase 1 (PGK1)
While PGK1 mRNA is identified in EVs associated with breast cancer [86], it is overexpressed in breast cancer [171,172]. There is limited information on the relevance of PGK1 to breast cancer cell survival and progression. Notwithstanding, a functional study by Deyuan et al. demonstrated that PGK1 knockdown by lentivirus-mediated transfection inhibits invasion and reverses EMT of MDA-MB-231 and MCF-7 breast cancer cells [173], thus suggesting PGK1 is a cell progression factor in breast cancer. Moreover, an earlier study by Dan et al. demonstrated that 17β-HSD5 knockdown-induced overexpression of PGK1 inhibits apoptosis and promotes cell viability and proliferation of MCF-7 breast cancer cells [174], indicating PGK1 as a cell survival and progression factor in breast cancer. In addition, correlational studies involving patients with breast cancer revealed an association of PGK1 with paclitaxel resistance [175] and cell survival factors, such as HIF-1α [173] and immune checkpoints [176], although these need to be further investigated in future studies.
3.2.2. Phosphoglycerate Mutase 1 (PGAM1)
Consistent with the expression of PGAM1 mRNA in EVs associated with breast cancer [86], there is an upregulated expression of PGAM1 in breast cancer [177,178,179]. In a study by Zhang et al., it was reported that the knockdown of PGAM1 in 4T1, MCF-7, and MDA-MB-231 breast cancer cells resulted in a marked reduction in M2 polarization, migration, and interleukin-10 (IL-10) production of macrophages [179]. M2 macrophages have been reported to act as immunoregulators by suppressing inflammatory responses via the production of anti-inflammatory cytokines, such as IL-10 [180]. Corroborating the above findings, another study by Zhang et al. revealed that PGAM1 suppression in triple-negative breast cancer (TNBC) synergizes with anti–PD–1 immunotherapy to diminish breast cancer cell survival [178]. Zhang et al. also demonstrated that the depletion of PGAM1 by shRNA reduces the migration of MDA-MB-231 breast cancer cells [181]. Supporting this finding, a recent study demonstrated that the depletion of PGAM1 by siRNA inhibited the proliferation, invasion, migration, and EMT of MDA-MB-231 and MCF-7 breast cancer cells [177]. Overall, these findings indicate that PGAM1 is a cell survival factor via immune cell suppression and a cell progression factor via the promotion of proliferation, invasion, migration, and EMT in breast cancer.
3.2.3. Glucose-6-Phosphate Dehydrogenase (G6PDH)
G6PDH has been identified in EVs associated with breast cancer [87]. It is also consistently overexpressed in breast cancer [182,183]. Using MCF-7 breast cancer cells, Luigi et al. demonstrated that G6PDH overexpression reduces autophagic cell death, as evidenced by the decreased expression of autophagosome formation markers, such as LAMP1, p62, and LC-3 [182]. The authors further demonstrated that G6PDH overexpression induces MCF-7 breast cancer cell resistance to lapatinib [182], a tyrosine kinase inhibitor used in the treatment of patients with breast cancer [184]. Consistent with these findings, using MCF-7 and MDA-MB-231 breast cancer cells, Yin et al. demonstrated that G6PDH inhibition by 6-aminonicotinamide caused a marked increase in the production of reactive oxygen species (ROS), inducing autophagic cell death [183]. These findings suggest that G6PDH promotes breast cancer cell survival via cell death inhibition and chemoresistance [185]. The role of G6PDH in breast cancer cell progression is yet to be investigated.
3.2.4. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH)
GAPDH has been identified in EVs associated with breast cancer [86,87,89]. Consistent with its expression in the EVs, GAPDH is also overexpressed in breast cancer [186]. Although there is a paucity of information regarding the relevance of GAPDH to breast cancer cell survival, studies have established that the enzyme is largely involved in the adaptation of breast cancer to hypoxia. For instance, Yasuki et al. demonstrated that hypoxia induces the upregulation of GAPDH in MCF-7 breast cancer cells and that this hypoxia-induced expression of GAPDH is mediated by hypoxia-inducible factor (HIF)-1α. HIF-1α is a master regulator of how cancer cells respond to hypoxia and is crucial for cancer cell survival as it activates the transcription of genes involved in resistance to radiation therapy, chemotherapy, and angiogenesis [187]. Moreover, angiogenesis is important for the supply of oxygen and nutrients to tumors for their continuous growth and survival [145]. Therefore, given that GAPDH expression is upregulated by HIF-1α, future studies are required to investigate the relevance of the HIF-1α/GAPDH axis to breast cancer cell survival in terms of radioresistance, chemoresistance, and angiogenesis. In addition, future studies are needed to determine the relevance of GAPDH in the proliferation, migration, invasion, and metastasis of breast cancer cells since GAPDH expression is found to be associated with breast cancer cell proliferation and tumor aggression in patients with breast cancer [186].
3.2.5. A Disintegrin and Metalloproteinases (ADAM) 9 and 10
Among the ADAM isoforms, ADAM9 and ADAM10 have been identified in EVs associated with breast cancer [90,91,92]. Consistent with this, ADAM9 and ADAM10 are overexpressed in breast cancer [188,189,190,191]. Silencing ADAM9 by siRNA inhibited the migration of BT-549 breast cancer cells [192] and the invasion of MDA-MB-231 breast cancer cells [193]. Corroborating this finding, a recent study by Song et al. demonstrated that the depletion of ADAM9 expression by shRNA inhibits the proliferation, migration, and invasion of MCF-7 and MDA-MB-231 breast cancer cells [189]. The authors further demonstrated that depletion of ADAM9 impedes growth and increases radiosensitivity and apoptosis of MCF-7 and MDA-MB-231 breast cancer cells [189]. Moreover, a mechanistic study revealed that ADAM9 promotes the progression of MDA-MB-231 breast cancer cells by activating the AKT/NF-κB pathway [188], an oncogenic signaling pathway implicated in many cancers [194]. Together, these findings establish that ADAM9 contributes to breast cancer cell survival via cell death inhibition and radioresistance while contributing to breast cancer cell progression via the promotion of proliferation, migration, and invasion.
Inhibition of ADAM10 by INCB8765 was reported to improve trastuzumab response against BT-474 and SK-BR-3 breast cancer cells [195]. Also, ADAM10 knockdown by siRNA in MDA-MB-231 breast cancer cells induces apoptosis and reduces the IC50 value of the anticancer drugs paclitaxel and adriamycin (ADR) [190]. In further analysis, it was demonstrated that ADAM10 inhibits apoptosis and confers chemoresistance in MDA-MB-231 breast cancer cells via upregulation of CD44 and PrPc proteins [190]. CD44 promotes chemoresistance in cancers [196], while PrPc protects cells against oxidative stress [197]. Moreover, low-level oxidative stress activates cell survival signals, while high levels induce apoptotic cell death [198]. Furthermore, Mullooly et al. demonstrated that ADAM10 knockdown by siRNA or GI254023X reduced the migration and invasion of BT-20, MDA-MB-231, and MDA-MB-453 breast cancer cells [191]. Corroborating this finding, Tsang et al. demonstrated that ADAM10 knockdown by siRNA inhibited the proliferation and migration of MDA-MB-231 breast cancer cells [199]. Overall, these findings indicate that ADAM10 supports breast cancer cell survival via cell death inhibition and chemoresistance while supporting breast cancer cell progression via the promotion of proliferation, migration, and invasion.
3.2.6. Matrix Metalloproteinase 1 (MMP1)
Among the several existing MMPs, MMP1 has been identified in EVs associated with breast cancer [93,94]. MMP1 is also overexpressed in breast cancer [200,201]. Knockdown of MMP-1 by shRNA was reported to significantly inhibit proliferation, migration, and invasion while increasing the apoptosis of MCF-7 and MDA-MB-231 breast cancer cells [200]. Moreover, an earlier study by Liu et al. demonstrated that the silencing of MMP1 inhibits the invasiveness of brain metastatic variants of MDA-MB 231 breast cancer and reduces the brain and lung metastases of breast cancer in nude mice [202]. Also, Xin et al. demonstrated that MMP1 overexpression promotes bone metastases of MCF-7 and MDA-MB-435 breast cancer in nude mice [203]. Furthermore, MMP1 upregulation was reported to potentiate tamoxifen (TAM) resistance in TAM-resistant MCF-7 breast cancer cells and increase tumor growth of TAM-resistant MCF-7 breast cancer in a xenograft mouse model [204]. Additionally, MMP1 upregulation was reported to enhance multi-drug resistance to MCF-7 breast cancer cells, as evidenced by the increase of the IC50 values of ADR, vincristine, and paclitaxel in MCF-7 breast cancer cells overexpressing MMP1 [205]. Together, these findings indicate that MMP1 contributes to breast cancer cell survival via chemoresistance while contributing to breast cancer cell progression via the promotion of invasion and metastasis.
3.2.7. Peroxiredoxin (PRDX) 1 and 2
Among the PRDX isoforms, PRDX1 and PRDX2 have been identified in EVs associated with breast cancer [95]. Also, PRDX 1 and PRDX2 are highly expressed in breast cancer [206,207]. Knockdown of PRDX 1 was reported to potentiate doxorubicin (DOX)-induced apoptosis of MCF-7 [208] and MDA-MB-231 breast cancer cells [209]. Another study demonstrated that PRDX1 knockdown results in oxidative stress-induced suppression of ERα in T-47D and ZR-75-1 breast cancer cells [210] and sensitizes MCF-7, ZR-75-1, T-47D, MDA-MB-231, HCC-1806, and SK-BR-3 breast cancer cells to the cytotoxic effects of oxidative stress-inducing agents [206,211,212]. Similarly, PRDX2 knockdown sensitizes the lung metastatic variant of MDA-MB-435 breast cancer cells to oxidative stress [213] and sensitizes MCF-7 breast cancer cells to DOX-induced apoptosis [208] and ionizing radiation-induced cytotoxicity [214]. Overall, these studies suggest that PRDX1 and PRDX2 promote breast cancer cell survival via induction of radio/chemoresistance and inhibition of oxidative stress-induced cell death. Future studies are required to understand the relevance of both isoforms of the enzyme to breast cancer cell progression.
3.2.8. Sirtuin 1 and 6 (SIRT1 and SIRT6)
Among the sirtuins, SIRT1 and SIRT6 have been identified in EVs associated with breast cancer [84]. Also, in breast cancer, there is an overexpression of SIRT1 [215] and SIRT6 [216]. In our recent review, we discussed the dual roles of both SIRT1 and SIRT6 in breast cancer, highlighting the tumor-suppressing and tumor-promoting effects of SIRT1 and SIRT6 in breast cancer [44]. Given the dual roles of both SIRT1 and SIRT6 in breast cancer, functional studies involving the introduction of EVs expressing SIRT1 and SIRT6 to breast cancer cells are required to determine the definitive roles of both enzymes in breast cancer. Notwithstanding, recent studies have supported the breast cancer-promoting effects of SIRT1 and SIRT6. For instance, Sahoo et al. demonstrated that SIRT1 inhibition by sirtinol, CHIC-35, or EX527 decreases the proliferation and migration of MCF-7 breast cancer cells [217], thus suggesting SIRT1 as a cell progression factor in breast cancer. The authors further demonstrated that the knockdown of SIRT1 expression decreases the viability and colony formation of MCF-7 breast cancer cells [217], thus suggesting SIRT1 as a cell survival factor in breast cancer. Also, Andreani et al. demonstrated that SIRT6 overexpression promotes migration, invasion, and lung metastasis in the Delta16HER2 mice model of breast cancer [218], thus suggesting SIRT6 as a cell progression factor in breast cancer. Although the authors further demonstrated that SIRT6 overexpression protects Delta16HER2 breast tumor cells against oxidative DNA damage [218], the contributions of the enzyme to specific breast cancer cell survival mechanisms were not investigated. Thus, more studies are required to validate the current findings and address the research gaps before a conclusion can be reached.
3.2.9. Ras Homolog A and C (RhoA and RhoC) GTPases
RhoA and RhoC GTPases have been identified in EVs associated with breast cancer [96]. Consistent with this, upregulated expressions of RhoA and RhoC have been reported in breast cancer [219,220,221]. Knockdown of both RhoA and RhoC GTPases by siRNA was reported to inhibit the proliferation and invasion of MDA-MB-231 breast cancer cells [222]. Similarly, RhoA and RhoC knockdown by siRNA was also reported to reduce invasion, motility, and monolayer growth rate in SUM149 and MDA-MB-231 breast cancer cells [223]. Also, Xu et al. demonstrated that RhoC knockdown by siRNA inhibits viability, migration, and invasion but increases apoptosis and induces cell cycle arrest of SUM149 and SUM190 breast cancer cells [224]. The authors further demonstrated that the inhibition of the proliferation and invasion of SUM149 and SUM190 breast cancer cells by RhoC knockdown was via the increase of KAI1 (a tumor metastasis suppressor) and decrease of CXCR4 (a tumor progression promoter) and MMP9 [224]. Conversely, a recent study by Kalpana et al. demonstrated that RhoA expression suppresses the invasion of BT-20 and MDA-MB-231 breast cancer cells and reduces the lung and lymph node metastasis of breast cancer in mice [225]. The authors further demonstrated that RhoA suppresses invasion by reducing the expression of the tumor progression chemokines CCR5 and CXCR4 [225]. The opposing effects of RhoA GTPase as compared to RhoC GTPases may indicate the independence of both GTPases in breast cancer progression, which needs to be investigated in future studies. Also, while it is established that RhoC is relevant to breast cancer survival (via cell death inhibition) and progression (via the promotion of proliferation, invasion, and metastasis), data accumulated so far only reveals the relevance of RhoA to breast cancer cell progression. Thus, future studies are required to investigate the role of RhoA GTPase in breast cancer cell survival.
3.2.10. Indoleamine-2,3-Dioxygenase (IDO)
IDO has been identified in EVs associated with breast cancer [97]. It is also consistently overexpressed in breast cancer [226,227,228,229]. Inhibition of IDO by epacadostat has been reported to induce apoptosis of MDA-MB-231 breast cancer cells [227]. Also, IDO knockdown by siRNA sensitized MCF-7 breast cancer cells to the cytotoxic effect of TAM via downregulation of IL-6. IL-6 is a TME cytokine involved in cancer chemoresistance [230]. Moreover, the upregulated expression of IDO was found to be correlated with chemoresistance in breast cancer [231]. There is an indication that IDO interacts with immune cells to promote immune evasion of breast cancer [232]. For instance, correlational studies involving patients with breast cancer have revealed that the upregulation of IDO expression is positively correlated with the increase of infiltrated regulatory T cells in situ [233]. These findings suggest that IDO is a cell survival factor in breast cancer via chemoresistance, immune cell suppression, and cell death inhibition. Furthermore, in BALB/c mice inoculated with 4T1/IDO1+ breast cancer cells, an increase in tumor growth and lung metastases was reported [234]. Also, 4T1/IDO1+ breast cancer cells showed an increased proliferation in BALB/c mice compared to 4T1/IDO1− breast cancer cells [234]. This indicates that IDO is an important factor in breast cancer cell progression. Given the limited information, studies using human breast cancer cells are required to validate the tumor progressive role of IDO in breast cancer.
3.2.11. Tissue Transglutaminase (TTG)
TTG has been detected in EVs associated with breast cancer [98], while overexpressed in breast cancer [235,236]. Downregulation of TTG expression was found to sensitize MDA-MB-231 and MCF-7 breast cancer cells to DOX [237,238] and MCF-7 to ADR [239]. In addition, TTG downregulation was found to induce apoptosis of MCF-7 breast cancer cells [239]. Corroborating this finding, a recent study demonstrated that a novel TTG inhibitor, AA9, induces apoptosis of MDA-MB-231 and MCF-7 breast cancer cells [240]. Furthermore, Mangala et al. demonstrated that breast cancer cells with induced (MDA-MB-231 cells) and constitutive (MDA231/cl.16 cells) high expression of TTG are characterized by increased migrative and invasive properties [241]. Corroborating this finding, He et al. demonstrated that inhibition of TTG by shRNA results in the downregulation of EMT via an increase of E-cadherin expression and a decrease of vimentin expression in MDA-MB-231 breast cancer cells. Substantiating this finding, Seyoung et al. demonstrated that the GTP binding activity of TTG expression induces EMT via downregulation of miRNA-205 to promote bone metastasis of MCF-7 breast cancer cells in mice [242]. Moreover, using weakly migratory MDA-MB-231 breast cancer cells, Schwager et al. demonstrated that TTG knockdown by shRNA reduces metastasis, while TTG overexpression increases metastasis [243]. From the above findings, it is apparent that TTG supports breast cancer cell survival via chemoresistance and cell death inhibition while supporting breast cancer cell progression via the promotion of migration, invasion, and metastasis.
3.3. Enzymes Secreted in EVs Associated with Prostate Cancer (with No Current Evidence in Breast Cancer)
3.3.1. Src Kinase (SK)
EVs associated with prostate cancer have been found to contain SK [100]. Consistent with this, an upregulated expression of SK has also been noted in prostate cancer [244,245]. Irwin et al. found that SK loss in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice results in reduced microscopic metastasis of prostate cancer to the lung, liver, and pelvic lymph nodes [246]. This may occur through SK synergizing with androgen receptor (AR) of the prostate via upregulation of MMP9 and induction of EMT to contribute to the induction of invasive adenocarcinoma [247]. Moreover, MMP9 activity has been detected in EVs from the NB26 tumorigenic prostate cell line created by chemical mutagenesis [248], although this needs to be validated by determining the activity in EVs secreted by cells originating from actual prostate tumors. Furthermore, it has been previously established that SK potentiates androgen receptor transactivation and invasion of C4-2 prostate cancer cells [249,250]. Goc et al. also demonstrated that SK promotes growth, proliferation, and metastasis of prostate cancer in vitro (PC-3 cells) and in vivo (TRAMP mice) via activation of glycogen synthase 3 (GS3) [251]. GS3 has been implicated in several cellular functions, including energy metabolism, insulin and growth factor signaling, apoptosis, and cell division [252]. Corroborating these findings, studies investigating the effect of SK inhibition have confirmed the pivotal role of this enzyme in the promotion of prostate cancer cell progression [253,254]. Together, the above studies suggest SK as a cell progression factor in prostate cancer via the promotion of proliferation and metastasis.
Furthermore, SK has also been implicated in the survival of prostate cancer. For instance, Wu et al. demonstrated that inhibition of SK by saracatinib induces autophagy and apoptosis of PC-3 and LNCaP prostate cancer cells [255]. Additionally, the authors demonstrated that the inhibition of autophagy by 3-Methyladenine and chloroquine enhances SK inhibitor-induced apoptosis of PC-3 prostate cancer cells [255]. Findings from the above study suggest SK is an important cell survival factor of prostate cancer via cell death inhibition. Notwithstanding, more studies are required to validate the survival functions of SK before a conclusion can be reached.
3.3.2. AKT1 Kinase
EVs associated with prostate cancer have been found to contain AKT1 [102]. Also, AKT1 is overexpressed in prostate cancer [256,257]. Graff et al. demonstrated that AKT1 expression is elevated in bone and soft tissue metastasis of prostate cancer compared to normal prostate tissues [257], thus indicating a role for AKT1 in the progression of prostate cancer. Supporting this, Goc et al. demonstrated that TRAMP cells with constitutive expression of AKT1 exhibit an increased migration, while TRAMP cells with dominant-negative AKT1 expression exhibit a reduced migration [258]. As a mechanism, the authors demonstrated that AKT1 activation promotes migration of PC-3 prostate cancer cells via activation of integrin [258]. Integrin αvβ3 is a cell surface receptor that participates in tumorigenesis, angiogenesis, EMT, stemness, bone metastasis, immune escape, drug resistance, and metabolic reprogramming [259]. Given the paucity of information, more studies are required to determine the mechanistic role of AKT1 in the progression and survival of prostate cancer, particularly considering the increased rate of AKT1 gene mutation in prostate cancer [260].
3.3.3. TMPRSS2 Serine Protease
TMPRSS2 has been identified in EVs associated with prostate cancer [103,104,105]. Consistently, TMPRSS2 is upregulated in prostate cancer [261,262,263]. Lucas et al. demonstrated that high expression of TMPRSS2 in metastatic prostate cancer is induced by androgens [264]. The authors further showed that TMPRSS2 promotes invasion and metastasis of prostate cancer in TRAMP mice [264]. Similarly, Chun-Jung et al. demonstrated that silencing of TMPRSS2 by shRNA significantly halted dihydrotestosterone-induced invasion of LNCaP prostate cancer cells [265]. The authors further demonstrated that TMPRSS2 promotes the growth of LNCaP prostate cancer in nude mice and that TMPRSS2 promotes invasion of LNCaP prostate cancer via proteolytic activation of matriptase [265]. Matriptase is a serine protease that promotes prostate cancer invasion [266]. Moreover, it has been previously reported that TMPRSS2 proteolytic activity is crucial for prostate cancer metastasis [267]. To corroborate these findings, a recent study by Chun-Jung et al. demonstrated that overexpression of TMPRSS2 significantly increases the invasion of LNCaP, PC-3, and DU145 prostate cancer cells [268]. The authors further demonstrated that TMPRSS2 inhibition by hepatocyte growth factor activator inhibitor-2 abrogates the TMPRSS2-induced invasion of the prostate cancer cells and that activated matriptase levels correlate with TMPRSS2 expression in LNCaP and VCaP prostate cancer cells [268]. Together, these studies indicate that TMPRSS2 proteolytically activates its substrates (such as matriptase) to promote prostate cancer progression via an increase in tumor growth, invasion, and metastasis. Future studies are required to investigate the contributions of TMPRSS2 to the survival of prostate cancer.
3.3.4. Transglutaminase-4 (TGM4)
EVs associated with prostate cancer have been found to express TGM4 [104]. Consistent with this, TGM4 expression is upregulated in prostate cancer [269,270]. In contrast, downregulated expressions of TGM4 have also been reported in certain prostate cancer cells [270]. This may suggest a wide range of TGM4 expressions in prostate cancer. Knockdown of TGM4 by ribozyme transgene reduced adhesion, motility, and invasiveness of CAHPV-10 prostate cancer cells, while forced expression of TGM4 increased adhesion, motility, and invasion of PC-3 prostate cancer cells [270,271,272]. Moreover, TGM4 overexpression in PC-3 prostate cancer cells reversed the adhesion, growth, and migration inhibitory effect of melanoma differentiation-associated gene-7/interleukin-24 (MDA-7/IL-24) [273]. MDA-7/IL-24 is a multifunctional cancer-killing cytokine [274]. Corroborating these findings, TGM4 overexpression increased migration of PC-3, PZ-HPV-7, and DU145 prostate cancer cells, while TGM4 knockdown by ribozyme transgene reduced EMT of CAHPV-10 prostate cancer cells [275]. Together, these findings suggest TGM4 as a cell progression factor in prostate cancer via the promotion of adhesion, growth, migration, invasion, and EMT. However, the role of TGM4 in the survival of prostate cancer needs to be investigated, particularly considering its opposing effect on MDA-7/IL-24.
3.3.5. Six-Transmembrane Epithelial Antigen of Prostate (STEAP) 1 and 2
Among the STEAP isoforms, STEAP1 and STEAP2 have been identified in EVs associated with prostate cancer [104,107]. Consistent with this, the two STEAP isoforms are overexpressed in prostate cancer [276,277,278]. There is limited information on the relevance of STEAP1 and STEAP2 to the progression and survival of prostate cancer. Notwithstanding, Sandra et al. demonstrated that knockdown of STEAP1 by siRNA reduces viability and proliferation and downregulates survival pathways (such as AKT and ERK signaling) of LNCaP and C4-2B prostate cancer cells [279]. Surprisingly, the authors further demonstrated that STEAP1 knockdown by siRNA reversed the sensitivity of LNCap prostate cancer cells to the apoptotic effects of paclitaxel and cabazitaxel [279]. These findings suggest STEAP1 is an important survival factor in prostate cancer via chemoresistance and cell death inhibition. Future studies are required to investigate the contribution of STEAP1 to the progression of prostate cancer. On the other hand, Jones et al. demonstrated that STEAP2 knockdown by CRISPR/Cas9 reduces proliferation, migration, and invasion of LNCaP and C4-2B prostate cancer cells [280]. Corroborating this finding, Burnell et al. demonstrated that STEAP2 knockdown by siRNA reduces proliferation, migration, and invasion of PC-3 and LNCaP prostate cancer cells [281]. While there are no studies on the relevance of STEAP2 to prostate cancer survival, the above studies suggest STEAP2 as a cell progression factor in prostate cancer via the promotion of proliferation, migration, and invasion.
3.3.6. Hyaluronidase 1 (HYAL1)
Among the HYAL isoforms, HYAL1 has been identified in EVs associated with prostate cancer [108,109]. Also, HYAL1 has been reported to be overexpressed in prostate cancer [282]. Demonstrating the relevance of HYAL1 to the progression of prostate cancer, Kovar et al. demonstrated that overexpression of HYAL1 induced lymph node metastasis in an orthotopic mouse model of prostate cancer [283]. Additionally, McAtee et al. demonstrated that overexpression of HYAL1 increased the proliferation and motility of 22Rv1 prostate cancer cells [109]. Together, these studies suggest HYAL1 as a cell progression factor in prostate cancer via the promotion of proliferation, motility, and metastasis. Notwithstanding, future studies are required to validate the current findings and investigate the relevance of HYAL1 to prostate cancer cell survival before a conclusion can be reached on its mechanistic relevance.
4. Clinical Potential of EVs in Breast and Prostate Cancers
EVs have been shown to induce the production of factors that create a favorable microenvironment for tumorigenesis [284]. As a result, research is now focused on investigating the potential of EVs and their molecules in the diagnosis, prognosis, and treatment of cancer (Figure 4). While research on the clinical potential of EVs in breast and prostate cancers is ongoing [285,286,287], it is pertinent to highlight some promising outcomes of existing research in the field. For instance, in breast cancer treatment, it has been demonstrated in vitro that EVs could serve as delivery vessels for various anticancer drugs, such as DOX and ADR, to improve targeting efficiency and reduce side effects [288]. Likely, this approach could also be effective in the treatment of prostate cancer.
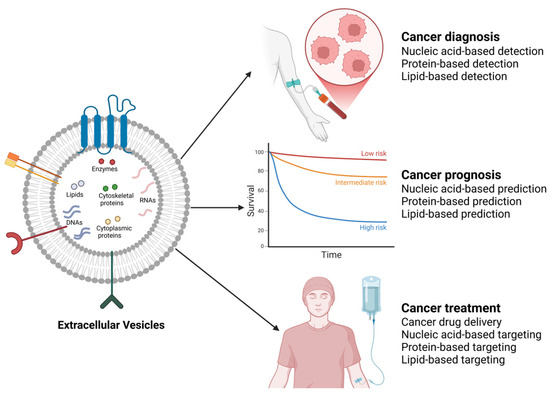
Figure 4.
Clinical potential of cancer-associated EVs. By harnessing EVs through various approaches guided by their cargo (nucleic acid-based, protein-based, and lipid-based approaches), the diagnosis, prognosis, and treatment of various cancers, including breast and prostate cancers, could be achieved. The image was created with BioRender.com (accessed on 17 February 2025).
Additionally, as demonstrated in a study using EVs from mesenchymal stem cells to transfer miR-145 into T-47D breast cancer cells to inhibit T-47D breast cancer metastasis [289], functional small RNAs (e.g., siRNAs and miRNAs) can be packaged into EVs to downregulate target genes involved in the progression of both breast and prostate cancers. Furthermore, in a study by Huang et al., engineered EVs were used as in situ dendritic cells-primed vaccine to boost antitumor immunity in mice with orthotopic TNBC [290], thus suggesting the potential of engineered EVs in the treatment of breast cancer. The use of engineered EVs with antitumor functions could also be explored in the treatment of prostate cancer.
Given the potential of tumor-derived EVs in the discovery of novel cancer biomarkers, studies have explored the potential of EV molecules in the diagnosis and prognosis of breast cancer [291]. Perhaps one of the most significant breakthroughs in this area of research is the development of a microfluidic chip-based EV mRNA sensor for the detection of HER2-positive breast cancer via quantitation of EV ERBB2 in the blood [292]. Other promising EV biomarkers for breast cancer include miR-200a, miR-200c, and miR-205 [293]. In the diagnosis and prognosis of prostate cancer, miRNA-196a-5p and miRNA-501-3p in EVs isolated from urine were found to be diagnostic markers for patients with advanced prostate cancer [294], while miRNA-1290 and miRNA-375 were associated with poor prognosis of patients with castrate-resistant prostate cancer [295]. Other promising EV biomarkers for prostate cancer include miR-107, miR-375, and PCA-3 [293].
Notwithstanding, to fully explore the clinical potential of EVs in the diagnosis, prognosis, and treatment of breast and prostate cancers, future studies are required to identify, quantitate, and functionally analyze cell survival and progression molecules in EVs secreted by breast and prostate cancers. For instance, the expression pattern of functionally relevant EV molecules (such as enzymes) needs to be determined in EVs derived from breast and prostate cancers to ascertain their usefulness in the early diagnosis of both cancers [296]. Most importantly, breast and prostate cancer-derived EVs with high or low expression of enzymes can be incorporated into breast and prostate cells, respectively, to determine their contribution to cell survival and progression. This will provide clues for the development of therapeutic strategies against both cancers. Specifically, the enzyme cargo of EVs from breast and prostate cancers could be targeted as part of the therapy against both cancers by (1) modifying the enzyme(s) in the EVs, (2) preventing its release into both breast and prostate cancer cells, and (3) interfering with its contributions to survival and progression of both cancers via targeted inhibition of the enzyme(s). However, a major setback to the clinical application of EVs is the lack of standardized methods for EV isolation, characterization, and downstream analyses [297]. This has resulted in potential contamination of EV particles by non-EV particles, thus negatively affecting the reproducibility, reliability, and clinical translation of findings from EV research [298]. To circumvent this challenge, transparent reporting, interlaboratory comparison studies, and standardization of pre-analytical procedures have been recommended, among other strategies for improving the reliability and clinical translation of EV research [298].
5. Conclusions
EVs provide valuable clues to unraveling the pathogenesis of various cancers. In their cargoes, EVs include both catalytic (e.g., enzymes) and non-catalytic (e.g., non-catalytic proteins and nucleic acids) molecules that create a microenvironment for tumorigenesis. Several enzymes that are constitutively upregulated in breast and prostate cancers have been identified in EVs secreted by both cancers. In this review, we discussed the functional relevance of enzymes in EVs associated with breast and prostate cancers, emphasizing their relevance to the progression and survival of both cancers. Among the enzymes discussed, ACLY, ENO, FASN, FAK, and PK are found in EVs from both breast and prostate cancers and contribute to the progression and survival of both cancers. Key mechanisms of cell progression influenced by these enzymes are adhesion, proliferation, migration, invasion, and metastasis, whereas mechanisms of cell survival influenced are chemoresistance, radioresistance, angiogenesis, cell death inhibition, cell colony formation, and immune evasion. Given this information, future studies are required to validate the current findings, address the research gaps, and explore the usefulness of these EV enzymes in the diagnosis, prognosis, and treatment of breast and prostate cancers.
Author Contributions
Conceptualization, C.I.O. and J.W.; writing—original draft preparation, C.I.O. and N.K.K.; writing—review and editing, C.I.O., J.W., and C.J.S.; supervision, J.W. and C.J.S.; funding acquisition, C.I.O., J.W., and C.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a University of Newcastle (Australia) Vice Chancellor’s Post-Graduate Scholarship awarded to Cosmos Ifeanyi Onyiba and a grant from Crestani Scholarships Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wei, M.; Jia, M.; Cao, M. Involvement of Various Enzymes in the Physiology and Pathogenesis of Streptococcus suis. Vet. Sci. 2020, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.G.N.d.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 714–770. [Google Scholar] [CrossRef] [PubMed]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N.K. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Woodward, J.K.; Holen, I.; Coleman, R.E.; Buttle, D.J. The roles of proteolytic enzymes in the development of tumour-induced bone disease in breast and prostate cancer. Bone 2007, 41, 912–927. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- The Australian Institute of Health and Welfare. Cancer Data in Australia; AIHW: Canberra, Australia, 2022. [Google Scholar]
- Risbridger, G.P.; Davis, I.D.; Birrell, S.N.; Tilley, W.D. Breast and prostate cancer: More similar than different. Nat. Rev. Cancer 2010, 10, 205–212. [Google Scholar] [CrossRef]
- Michael, P.; Roversi, G.; Brown, K.; Sharifi, N. Adrenal Steroids and Resistance to Hormonal Blockade of Prostate and Breast Cancer. Endocrinology 2023, 164, bqac218. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Chen, B. Breast and prostate cancer: Familial associations. Nat. Rev. Cancer 2010, 10, 523. [Google Scholar] [CrossRef]
- Chen, Y.; Sadasivan, S.M.; She, R.; Datta, I.; Taneja, K.; Chitale, D.; Gupta, N.; Davis, M.B.; Newman, L.A.; Rogers, C.G.; et al. Breast and prostate cancers harbor common somatic copy number alterations that consistently differ by race and are associated with survival. BMC Med. Genom. 2020, 13, 116. [Google Scholar] [CrossRef]
- Spratt, D.E.; Jagsi, R. Breast and Prostate Cancer: Lessons to Be Shared. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Lange, P.H. Chapter 19—Breast and Prostate Cancers: A Comparison of Two Endocrinologic Malignancies. In Prostate Cancer, 2nd ed.; Mydlo, J.H., Godec, C.J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 157–165. [Google Scholar] [CrossRef]
- Chitti, S.V.; Gummadi, S.; Kang, T.; Shahi, S.; Marzan, A.L.; Nedeva, C.; Sanwlani, R.; Bramich, K.; Stewart, S.; Petrovska, M.; et al. Vesiclepedia 2024: An extracellular vesicles and extracellular particles repository. Nucleic Acids Res. 2024, 52, D1694–D1698. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Yue, M.; Hu, S.; Sun, H.; Tuo, B.; Jia, B.; Chen, C.; Wang, W.; Liu, J.; Liu, Y.; Sun, Z.; et al. Extracellular vesicles remodel tumor environment for cancer immunotherapy. Mol. Cancer 2023, 22, 203. [Google Scholar] [CrossRef]
- Hennipman, A.; Smits, J.; van Oirschot, B.; van Houwelingen, J.C.; Rijksen, G.; Neyt, J.P.; van Unnik, J.A.M.; Staal, G.E.J. Glycolytic Enzymes in Breast Cancer, Benign Breast Disease and Normal Breast Tissue. Tumor Biol. 2009, 8, 251–263. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Li, X.; Peng, S.; Li, J.; Yan, W.; Cui, Y.; Xiao, H.; Wen, X. Increased expression of glycolytic enzymes in prostate cancer tissues and association with Gleason scores. Int. J. Clin. Exp. Pathol. 2017, 10, 11080–11089. [Google Scholar]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Kim, S.K.; Jung, W.H.; Koo, J.S. Differential Expression of Enzymes Associated with Serine/Glycine Metabolism in Different Breast Cancer Subtypes. PLoS ONE 2014, 9, e101004. [Google Scholar] [CrossRef]
- Plymate, S.R.; Sprenger, C.; Haffner, M.C. Starving lethal prostate cancer by targeting heat shock proteins and glycolytic enzymes. Cell Rep. Med. 2022, 3, 100493. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Chelakkot, V.S.; Shin, Y.; Song, K. Modulating Glycolysis to Improve Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 2606. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ma, H.; Wang, J.; Huang, M.; Fu, D.; Qin, L.; Yin, Q. Energy metabolism pathways in breast cancer progression: The reprogramming, crosstalk, and potential therapeutic targets. Transl. Oncol. 2022, 26, 101534. [Google Scholar] [CrossRef]
- Starlard-Davenport, A.; Lyn-Cook, B.; Radominska-Pandya, A. Novel identification of UDP-glucuronosyltransferase 1A10 as an estrogen-regulated target gene. Steroids 2008, 73, 139–147. [Google Scholar] [CrossRef]
- Li, Y.; Steppi, A.; Zhou, Y.; Mao, F.; Miller, P.C.; He, M.M.; Zhao, T.; Sun, Q.; Zhang, J. Tumoral expression of drug and xenobiotic metabolizing enzymes in breast cancer patients of different ethnicities with implications to personalized medicine. Sci. Rep. 2017, 7, 4747. [Google Scholar] [CrossRef]
- Chen, T.C.; Sakaki, T.; Yamamoto, K.; Kittaka, A. The Roles of Cytochrome P450 Enzymes in Prostate Cancer Development and Treatment. Anticancer. Res. 2012, 32, 291–298. [Google Scholar]
- Honma, N.; Takubo, K.; Sawabe, M.; Arai, T.; Akiyama, F.; Sakamoto, G.; Utsumi, T.; Yoshimura, N.; Harada, N. Estrogen-Metabolizing Enzymes in Breast Cancers from Women over the Age of 80 Years. J. Clin. Endocrinol. Metab. 2006, 91, 607–613. [Google Scholar] [CrossRef]
- Pasqualini, J.R.; Chetrite, G.S. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J. Steroid Biochem. Mol. Biol. 2005, 93, 221–236. [Google Scholar] [CrossRef]
- Pasqualini, J.R. Breast cancer and steroid metabolizing enzymes: The role of progestogens. Maturitas 2009, 65 (Suppl. S1), S17–S21. [Google Scholar] [CrossRef]
- Myutan, K.; Mohamed, S.; Kefah, M. Oestrogen-Synthesising Enzymes and Breast Cancer. Anticancer. Res. 2009, 29, 1095. [Google Scholar]
- Suzuki, T.; Miki, Y.; Nakamura, Y.; Moriya, T.; Ito, K.; Ohuchi, N.; Sasano, H. Sex steroid-producing enzymes in human breast cancer. Endocr. Relat. Cancer 2005, 12, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Soronen, P.; Laiti, M.; Törn, S.; Härkönen, P.; Patrikainen, L.; Li, Y.; Pulkka, A.; Kurkela, R.; Herrala, A.; Kaija, H.; et al. Sex steroid hormone metabolism and prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Suzuki, T.; Nakabayashi, M.; Endoh, M.; Sakamoto, K.; Mikami, Y.; Moriya, T.; Ito, A.; Takahashi, S.; Yamada, S.; et al. In situ androgen producing enzymes in human prostate cancer. Endocr. Relat. Cancer 2005, 12, 101–107. [Google Scholar] [CrossRef]
- McNamara, K.M.; Sasano, H. The role of 17βHSDs in breast tissue and breast cancers. Mol. Cell. Endocrinol. 2019, 489, 32–44. [Google Scholar] [CrossRef]
- Mindnich, R.; Möller, G.; Adamski, J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2004, 218, 7–20. [Google Scholar] [CrossRef]
- Collin, L.J.; Ulrichsen, S.P.; Ahern, T.P.; Goodman, M.; McCullough, L.E.; Waller, L.A.; Bang Christensen, K.; Damkier, P.; Hamilton-Dutoit, S.; Lauridsen, K.L.; et al. 17β-Hydroxysteroid dehydrogenase 1:2 and breast cancer recurrence: A Danish population-based study. Acta Oncol. 2020, 59, 329–333. [Google Scholar] [CrossRef]
- Hilborn, E.; Stål, O.; Jansson, A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: With a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 2017, 8, 30552. [Google Scholar]
- Ning, X.; Yang, Y.; Deng, H.; Zhang, Q.; Huang, Y.; Su, Z.; Fu, Y.; Xiang, Q.; Zhang, S. Development of 17β-hydroxysteroid dehydrogenase type 3 as a target in hormone-dependent prostate cancer therapy. Steroids 2017, 121, 10–16. [Google Scholar] [CrossRef]
- Denu, J.M. The Sir 2 family of protein deacetylases. Curr. Opin. Chem. Biol. 2005, 9, 431–440. [Google Scholar] [CrossRef]
- Vassilopoulos, A.; Fritz, K.S.; Petersen, D.R.; Gius, D. The human sirtuin family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Onyiba, C.I.; Scarlett, C.J.; Weidenhofer, J. The Mechanistic Roles of Sirtuins in Breast and Prostate Cancer. Cancers 2022, 14, 5118. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gai, J.W.; Wang, Y.; Jin, H.F.; Du, J.B.; Jin, J. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology 2012, 79, 483.e481–485. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kawahara, B.; Gupta, D.; Tsai, R.; Khachatryan, M.; Roy-Chowdhuri, S.; Bose, S.; Yoon, A.; Faull, K.; Farias-Eisner, R.; et al. Role of cystathionine β-synthase in human breast Cancer. Free Radic. Biol. Med. 2015, 86, 228–238. [Google Scholar] [CrossRef]
- Nafea, H.; Youness, R.A.; Dawoud, A.; Khater, N.; Manie, T.; Abdel-Kader, R.; Bourquin, C.; Szabo, C.; Gad, M.Z. Dual targeting of H(2)S synthesizing enzymes; cystathionine β-synthase and cystathionine γ-lyase by miR-939-5p effectively curbs triple negative breast cancer. Heliyon 2023, 9, e21063. [Google Scholar] [CrossRef]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.-Q.; Chen, H.-J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.-Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef]
- Siiskonen, H.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K. Hyaluronan synthase 1: A mysterious enzyme with unexpected functions. Front. Immunol. 2015, 6, 43. [Google Scholar] [CrossRef]
- Auvinen, P.; Rilla, K.; Tumelius, R.; Tammi, M.; Sironen, R.; Soini, Y.; Kosma, V.M.; Mannermaa, A.; Viikari, J.; Tammi, R. Hyaluronan synthases (HAS1-3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res. Treat. 2014, 143, 277–286. [Google Scholar] [CrossRef]
- Li, P.; Xiang, T.; Li, H.; Li, Q.; Yang, B.; Huang, J.; Zhang, X.; Shi, Y.; Tan, J.; Ren, G. Hyaluronan synthase 2 overexpression is correlated with the tumorigenesis and metastasis of human breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 12101–12114. [Google Scholar]
- Karousou, E.; Parnigoni, A.; Moretto, P.; Passi, A.; Viola, M.; Vigetti, D. Hyaluronan in the Cancer Cells Microenvironment. Cancers 2023, 15, 798. [Google Scholar] [CrossRef]
- Simpson, M.A. Concurrent expression of hyaluronan biosynthetic and processing enzymes promotes growth and vascularization of prostate tumors in mice. Am. J. Pathol. 2006, 169, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gao, F.; Han, Z.; Xu, X.; Underhill, C.B.; Zhang, L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res. 2001, 61, 5207–5214. [Google Scholar] [PubMed]
- Velesiotis, C.; Vasileiou, S.; Vynios, D.H. A guide to hyaluronan and related enzymes in breast cancer: Biological significance and diagnostic value. FEBS J. 2019, 286, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, P.; Maddela, J.J.; Mani, S.A. Spatial and Temporal Relationship between Epithelial–Mesenchymal Transition (EMT) and Stem Cells in Cancer. Clin. Chem. 2024, 70, 190–205. [Google Scholar] [CrossRef]
- Karalis, T. Targeting Hyaluronan Synthesis in Cancer: A Road Less Travelled. Biologics 2023, 3, 402–414. [Google Scholar] [CrossRef]
- Eatemadi, A.; Aiyelabegan, H.T.; Negahdari, B.; Mazlomi, M.A.; Daraee, H.; Daraee, N.; Eatemadi, R.; Sadroddiny, E. Role of protease and protease inhibitors in cancer pathogenesis and treatment. Biomed. Pharmacother. 2017, 86, 221–231. [Google Scholar] [CrossRef]
- Tagirasa, R.; Yoo, E. Role of Serine Proteases at the Tumor-Stroma Interface. Front. Immunol. 2022, 13, 832418. [Google Scholar] [CrossRef]
- Kim, S. TMPRSS4, a type II transmembrane serine protease, as a potential therapeutic target in cancer. Exp. Mol. Med. 2023, 55, 716–724. [Google Scholar] [CrossRef]
- Navasatli, S.A.; Vahdati, S.N.; Arjmand, T.F.; Behboudi, H. New insight into the role of the ADAM protease family in breast carcinoma progression. Heliyon 2024, 10, e24805. [Google Scholar] [CrossRef]
- Koistinen, H.; Kovanen, R.-M.; Hollenberg, M.D.; Dufour, A.; Radisky, E.S.; Stenman, U.-H.; Batra, J.; Clements, J.; Hooper, J.D.; Diamandis, E.; et al. The roles of proteases in prostate cancer. IUBMB Life 2023, 75, 493–513. [Google Scholar] [CrossRef]
- Park, K.C.; Dharmasivam, M.; Richardson, D.R. The Role of Extracellular Proteases in Tumor Progression and the Development of Innovative Metal Ion Chelators that Inhibit their Activity. Int. J. Mol. Sci. 2020, 21, 6805. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J. Matrix metalloproteinases as therapeutic targets in breast cancer. Front. Oncol. 2023, 12, 1108695. [Google Scholar] [CrossRef] [PubMed]
- Escaff, S.; Fernández, J.M.; González, L.O.; Suárez, A.; González-Reyes, S.; González, J.M.; Vizoso, F.J. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br. J. Cancer 2010, 102, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Liao, C.-H.; Huang, W.-C.; Chang, W.-S.; Wu, H.-C.; Hsu, S.-W.; Chen, K.-Y.; Wang, Z.-H.; Hsia, T.-C.; Bau, D.-T.; et al. Association of Matrix Metalloproteinase-2 Genotypes With Prostate Cancer Risk. Anticancer. Res. 2023, 43, 343–349. [Google Scholar] [CrossRef]
- Luo, J.; Zou, H.; Guo, Y.; Tong, T.; Ye, L.; Zhu, C.; Deng, L.; Wang, B.; Pan, Y.; Li, P. SRC kinase-mediated signaling pathways and targeted therapies in breast cancer. Breast Cancer Res. 2022, 24, 99. [Google Scholar] [CrossRef]
- García-Aranda, M.; Redondo, M. Protein Kinase Targets in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 2543. [Google Scholar] [CrossRef]
- Miller, K.J.; Asim, M. Unravelling the Role of Kinases That Underpin Androgen Signalling in Prostate Cancer. Cells 2022, 11, 952. [Google Scholar] [CrossRef]
- Bagheri, S.; Rahban, M.; Bostanian, F.; Esmaeilzadeh, F.; Bagherabadi, A.; Zolghadri, S.; Stanek, A. Targeting Protein Kinases and Epigenetic Control as Combinatorial Therapy Options for Advanced Prostate Cancer Treatment. Pharmaceutics 2022, 14, 515. [Google Scholar] [CrossRef]
- Gali, A.; Bijnsdorp, I.V.; Piersma, S.R.; Pham, T.V.; Gutiérrez-Galindo, E.; Kühnel, F.; Tsolakos, N.; Jimenez, C.R.; Hausser, A.; Alexopoulos, L.G. Protein kinase D drives the secretion of invasion mediators in triple-negative breast cancer cell lines. iScience 2024, 27, 108958. [Google Scholar] [CrossRef]
- Roy, A.; Prasad, S.; Chen, Y.; Chao, Y.; Liu, Y.; Zhao, J.; Wang, Q.J. Protein Kinase D2 and D3 Promote Prostate Cancer Cell Bone Metastasis by Positively Regulating Runx2 in a MEK/ERK1/2–Dependent Manner. Am. J. Pathol. 2023, 193, 624–637. [Google Scholar] [CrossRef]
- Durand, N.; Borges, S.; Storz, P. Functional and therapeutic significance of protein kinase D enzymes in invasive breast cancer. Cell. Mol. Life Sci. 2015, 72, 4369–4382. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Fingleton, B. Non-canonical roles for metabolic enzymes and intermediates in malignant progression and metastasis. Clin. Exp. Metastasis 2019, 36, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qi, Y.; Yang, W. The Noncanonical Functions of Metabolites in Tumor Progression. Metabolites 2024, 14, 171. [Google Scholar] [CrossRef]
- Shegay, P.V.; Shatova, O.P.; Zabolotneva, A.A.; Shestopalov, A.V.; Kaprin, A.D. Moonlight functions of glycolytic enzymes in cancer. Front. Mol. Biosci. 2023, 10, 1076138. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y. Nonmetabolic functions of metabolic enzymes in cancer development. Cancer Commun. 2018, 38, 63. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, Y.; Yang, W.; Han, Y.; Chen, Y.; Oyang, L.; Lin, J.; et al. Metabolic reprogramming and epigenetic modifications in cancer: From the impacts and mechanisms to the treatment potential. Exp. Mol. Med. 2023, 55, 1357–1370. [Google Scholar] [CrossRef]
- Berumen Sánchez, G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular vesicles: Mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal. 2021, 19, 104. [Google Scholar] [CrossRef]
- Winzer, R.; Nguyen, D.H.; Schoppmeier, F.; Cortesi, F.; Gagliani, N.; Tolosa, E. Purinergic enzymes on extracellular vesicles: Immune modulation on the go. Front. Immunol. 2024, 15, 1362996. [Google Scholar] [CrossRef]
- Garraway, L.A.; Lander, E.S. Lessons from the cancer genome. Cell 2013, 153, 17–37. [Google Scholar] [CrossRef]
- Shomar, A.; Barak, O.; Brenner, N. Cancer progression as a learning process. iScience 2022, 25, 103924. [Google Scholar] [CrossRef]
- Albitre, A.; Reglero, C.; González-Muñoz, T.; Penela, P. The stress connection in cancer: The adrenergic fuelling of breast tumors. Curr. Opin. Physiol. 2023, 36, 100720. [Google Scholar] [CrossRef]
- Minic, Z.; Hüttmann, N.; Poolsup, S.; Li, Y.; Susevski, V.; Zaripov, E.; Berezovski, M.V. Phosphoproteomic Analysis of Breast Cancer-Derived Small Extracellular Vesicles Reveals Disease-Specific Phosphorylated Enzymes. Biomedicines 2022, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Dalla, P.V.; Santos, J.; Milthorpe, B.K.; Padula, M.P. Selectively Packaged Proteins in Breast Cancer Extracellular Vesicles Involved in Metastasis. Int. J. Mol. Sci. 2020, 21, 4990. [Google Scholar] [CrossRef]
- Minic, Z.; Li, Y.; Hüttmann, N.; Uppal, G.K.; D’Mello, R.; Berezovski, M.V. Lysine Acetylome of Breast Cancer-Derived Small Extracellular Vesicles Reveals Specific Acetylation Patterns for Metabolic Enzymes. Biomedicines 2023, 11, 1076. [Google Scholar] [CrossRef]
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the specificity of tumor cell derived exosomes with tumor cells In Vitro. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 2954–2965. [Google Scholar] [CrossRef]
- Galindo-Hernandez, O.; Villegas-Comonfort, S.; Candanedo, F.; González-Vázquez, M.C.; Chavez-Ocaña, S.; Jimenez-Villanueva, X.; Sierra-Martinez, M.; Salazar, E.P. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch. Med. Res. 2013, 44, 208–214. [Google Scholar] [CrossRef]
- Pallares-Rusiñol, A.; Moura, S.L.; Martí, M.; Pividori, M.I. Electrochemical Genosensing of Overexpressed GAPDH Transcripts in Breast Cancer Exosomes. Anal. Chem. 2023, 95, 2487–2495. [Google Scholar] [CrossRef]
- Green, T.M.; Alpaugh, M.L.; Barsky, S.H.; Rappa, G.; Lorico, A. Breast Cancer-Derived Extracellular Vesicles: Characterization and Contribution to the Metastatic Phenotype. Biomed. Res. Int. 2015, 2015, 634865. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Biol. 2019, 2, 325. [Google Scholar] [CrossRef]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tao, Z.; Chen, Y.; Lin, S.; Zhu, M.; Ji, W.; Liu, X.; Li, T.; Hu, X. Exosomal MMP-1 transfers metastasis potential in triple-negative breast cancer through PAR1-mediated EMT. Breast Cancer Res. Treat. 2022, 193, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, G.; Albanese, N.N.; Di Cara, G.; Gygax, D.; Vittorelli, M.L.; Pucci-Minafra, I. Proteomic Analysis of Exosome-like Vesicles Derived from Breast Cancer Cells. Anticancer. Res. 2012, 32, 847–860. [Google Scholar]
- Schlienger, S.; Campbell, S.; Claing, A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol. Biol. Cell 2014, 25, 17–29. [Google Scholar] [CrossRef]
- Isla Larrain, M.T.; Rabassa, M.E.; Lacunza, E.; Barbera, A.; Cretón, A.; Segal-Eiras, A.; Croce, M.V. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumor Biol. 2014, 35, 6511–6519. [Google Scholar] [CrossRef]
- Antonyak, M.A.; Li, B.; Boroughs, L.K.; Johnson, J.L.; Druso, J.E.; Bryant, K.L.; Holowka, D.A.; Cerione, R.A. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4852–4857. [Google Scholar] [CrossRef]
- Hosseini-Beheshti, E.; Pham, S.; Adomat, H.; Li, N.; Tomlinson Guns, E.S. Exosomes as biomarker enriched microvesicles: Characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol. Cell Proteom. 2012, 11, 863–885. [Google Scholar] [CrossRef]
- DeRita, R.M.; Zerlanko, B.; Singh, A.; Lu, H.; Iozzo, R.V.; Benovic, J.L.; Languino, L.R. c-Src, Insulin-Like Growth Factor I Receptor, G-Protein-Coupled Receptor Kinases and Focal Adhesion Kinase are Enriched Into Prostate Cancer Cell Exosomes. J. Cell Biochem. 2017, 118, 66–73. [Google Scholar] [CrossRef]
- Dai, J.; Escara-Wilke, J.; Keller, J.M.; Jung, Y.; Taichman, R.S.; Pienta, K.J.; Keller, E.T. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 2019, 216, 2883–2899. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Spinelli, C.; Reis-Sobreiro, M.; Cavallini, L.; You, S.; Zandian, M.; Li, X.; Mishra, R.; Chiarugi, P.; Adam, R.M.; et al. MYC Mediates Large Oncosome-Induced Fibroblast Reprogramming in Prostate Cancer. Cancer Res. 2017, 77, 2306–2317. [Google Scholar] [CrossRef]
- Lázaro-Ibáñez, E.; Lunavat, T.R.; Jang, S.C.; Escobedo-Lucea, C.; Oliver-De La Cruz, J.; Siljander, P.; Lötvall, J.; Yliperttula, M. Distinct prostate cancer-related mRNA cargo in extracellular vesicle subsets from prostate cell lines. BMC Cancer 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Sequeiros, T.; Rigau, M.; Chiva, C.; Montes, M.; Garcia-Grau, I.; Garcia, M.; Diaz, S.; Celma, A.; Bijnsdorp, I.; Campos, A.; et al. Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer. Oncotarget 2017, 8, 4960–4976. [Google Scholar] [CrossRef]
- Casanova-Salas, I.; Aguilar, D.; Cordoba-Terreros, S.; Agundez, L.; Brandariz, J.; Herranz, N.; Mas, A.; Gonzalez, M.; Morales-Barrera, R.; Sierra, A.; et al. Circulating tumor extracellular vesicles to monitor metastatic prostate cancer genomics and transcriptomic evolution. Cancer Cell 2024, 42, 1301–1312.e1307. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Capasso, C.; Del Prete, S.; Di Raimo, R.; Falchi, M.; Angelini, D.F.; Sciarra, A.; Maggi, M.; Supuran, C.T.; et al. Plasmatic exosomes from prostate cancer patients show increased carbonic anhydrase IX expression and activity and low pH. J. Enzym. Inhib. Med. Chem. 2020, 35, 280–288. [Google Scholar] [CrossRef]
- Khanna, K.; Salmond, N.; Lynn, K.S.; Leong, H.S.; Williams, K.C. Clinical significance of STEAP1 extracellular vesicles in prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 802–811. [Google Scholar] [CrossRef]
- McAtee, C.O.; Booth, C.; Elowsky, C.; Zhao, L.; Payne, J.; Fangman, T.; Caplan, S.; Henry, M.D.; Simpson, M.A. Prostate tumor cell exosomes containing hyaluronidase Hyal1 stimulate prostate stromal cell motility by engagement of FAK-mediated integrin signaling. Matrix Biol. 2019, 78, 165–179. [Google Scholar] [CrossRef]
- McAtee, C.O.; Berkebile, A.R.; Elowsky, C.G.; Fangman, T.; Barycki, J.J.; Wahl, J.K.; Khalimonchuk, O.; Naslavsky, N.; Caplan, S.; Simpson, M.A. Hyaluronidase Hyal1 Increases Tumor Cell Proliferation and Motility through Accelerated Vesicle Trafficking. J. Biol. Chem. 2015, 290, 13144–13156. [Google Scholar] [CrossRef]
- Wang, D.; Yin, L.; Wei, J.; Yang, Z.; Jiang, G. ATP citrate lyase is increased in human breast cancer, depletion of which promotes apoptosis. Tumor Biol. 2017, 39, 1010428317698338. [Google Scholar] [CrossRef]
- Shah, S.; Carriveau, W.J.; Li, J.; Campbell, S.L.; Kopinski, P.K.; Lim, H.W.; Daurio, N.; Trefely, S.; Won, K.J.; Wallace, D.C.; et al. Targeting ACLY sensitizes castration-resistant prostate cancer cells to AR antagonism by impinging on an ACLY-AMPK-AR feedback mechanism. Oncotarget 2016, 7, 43713–43730. [Google Scholar] [CrossRef]
- Xin, M.; Qiao, Z.; Li, J.; Liu, J.; Song, S.; Zhao, X.; Miao, P.; Tang, T.; Wang, L.; Liu, W.; et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: Evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget 2016, 7, 44252–44265. [Google Scholar] [CrossRef]
- Velez, B.C.; Petrella, C.P.; DiSalvo, K.H.; Cheng, K.; Kravtsov, R.; Krasniqi, D.; Krucher, N.A. Combined inhibition of ACLY and CDK4/6 reduces cancer cell growth and invasion. Oncol. Rep. 2023, 49, 32. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, Q.; Zhao, C.; Li, J.; Yu, Z.; Zhu, Q. ATP citrate lyase inhibitor triggers endoplasmic reticulum stress to induce hepatocellular carcinoma cell apoptosis via p-eIF2α/ATF4/CHOP axis. J. Cell Mol. Med. 2021, 25, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Islam, M.S.; Tian, J.; Lui, V.W.Y.; Xiao, D. Inactivation of ATP citrate lyase by Cucurbitacin B: A bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014, 349, 15–25. [Google Scholar] [CrossRef]
- Migita, T.; Okabe, S.; Ikeda, K.; Igarashi, S.; Sugawara, S.; Tomida, A.; Taguchi, R.; Soga, T.; Seimiya, H. Inhibition of ATP Citrate Lyase Induces an Anticancer Effect via Reactive Oxygen Species: AMPK as a Predictive Biomarker for Therapeutic Impact. Am. J. Pathol. 2013, 182, 1800–1810. [Google Scholar] [CrossRef]
- Zaidi, N.; Royaux, I.; Swinnen, J.V.; Smans, K. ATP Citrate Lyase Knockdown Induces Growth Arrest and Apoptosis through Different Cell- and Environment-Dependent Mechanisms. Mol. Cancer Ther. 2012, 11, 1925–1935. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Ding, M.; Zhang, H.; Xu, X.; Tang, J. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (Review). Int. J. Oncol. 2017, 50, 345–355. [Google Scholar] [CrossRef]
- Tu, S.-H.; Chang, C.-C.; Chen, C.-S.; Tam, K.-W.; Wang, Y.-J.; Lee, C.-H.; Lin, H.-W.; Cheng, T.-C.; Huang, C.-S.; Chu, J.-S.; et al. Increased expression of enolase α in human breast cancer confers tamoxifen resistance in human breast cancer cells. Breast Cancer Res. Treat. 2010, 121, 539–553. [Google Scholar] [CrossRef]
- Cancemi, P.; Buttacavoli, M.; Roz, E.; Feo, S. Expression of Alpha-Enolase (ENO1), Myc Promoter-Binding Protein-1 (MBP-1) and Matrix Metalloproteinases (MMP-2 and MMP-9) Reflect the Nature and Aggressiveness of Breast Tumors. Int. J. Mol. Sci. 2019, 20, 3952. [Google Scholar] [CrossRef]
- Santiago, K.R.; Duran, A.M.; Casiano, C.A.; Almaguel, F.G.; Das, B. Abstract C063: Enolase-1 as a candidate theranostics target for neuroendocrine prostate cancer. Cancer Epidemiol. Biomark. Prev. 2023, 32, C063. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, F.; Richards, G.O.; Wang, N. ENO2, a Glycolytic Enzyme, Contributes to Prostate Cancer Metastasis: A Systematic Review of Literature. Cancers 2024, 16, 2503. [Google Scholar] [CrossRef]
- Jin, Q.; Yuan, L.X.; Boulbes, D.; Baek, J.M.; Wang, Y.N.; Gomez-Cabello, D.; Hawke, D.H.; Yeung, S.C.; Lee, M.H.; Hortobagyi, G.N.; et al. Fatty acid synthase phosphorylation: A novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 2010, 12, R96. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Yuan, T.-T.; Chuang, C.-F.; Huang, Y.-T.; Chung, I.-C.; Huang, W.-C. A Novel Enolase-1 Antibody Targets Multiple Interacting Players in the Tumor Microenvironment of Advanced Prostate Cancer. Mol. Cancer Ther. 2022, 21, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, T.; Dong, L.; Li, T.; Xue, H.; Gao, B.; Ding, X.; Wang, H.; Li, H. Fatty acid synthase promotes breast cancer metastasis by mediating changes in fatty acid metabolism. Oncol. Lett. 2021, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.B.; Ali, A.; Luengo, A.; Kodack, D.P.; Deik, A.; Abbott, K.L.; Bezwada, D.; Blanc, L.; Prideaux, B.; Jin, X.; et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2021, 2, 414–428. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R.; Colomer, R. Inhibition of Tumor-associated Fatty Acid Synthase Hyperactivity Induces Synergistic Chemosensitization of HER-2/neu-Overexpressing Human Breast Cancer Cells to Docetaxel (taxotere). Breast Cancer Res. Treat. 2004, 84, 183–195. [Google Scholar] [CrossRef]
- Al-Bahlani, S.; Al-Lawati, H.; Al-Adawi, M.; Al-Abri, N.; Al-Dhahli, B.; Al-Adawi, K. Fatty acid synthase regulates the chemosensitivity of breast cancer cells to cisplatin-induced apoptosis. Apoptosis 2017, 22, 865–876. [Google Scholar] [CrossRef]
- Schroeder, B.; Vander Steen, T.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z.; Silva, F.M.; Regan, K.; Cuyàs, E.; Meng, X.W.; Verdura, S.; et al. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis. 2021, 12, 977. [Google Scholar] [CrossRef]
- Rossi, S.; Graner, E.; Febbo, P.; Weinstein, L.; Bhattacharya, N.; Onody, T.; Bubley, G.; Balk, S.; Loda, M. Fatty Acid Synthase Expression Defines Distinct Molecular Signatures in Prostate Cancer1. Mol. Cancer Res. 2003, 1, 707–715. [Google Scholar]
- Shah, U.S.; Dhir, R.; Gollin, S.M.; Chandran, U.R.; Lewis, D.; Acquafondata, M.; Pflug, B.R. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Human. Pathol. 2006, 37, 401–409. [Google Scholar] [CrossRef]
- Oh, J.E.; Jung, B.H.; Park, J.; Kang, S.; Lee, H. Deciphering Fatty Acid Synthase Inhibition-Triggered Metabolic Flexibility in Prostate Cancer Cells through Untargeted Metabolomics. Cells 2020, 9, 2447. [Google Scholar] [CrossRef]
- Sadowski, M.C.; Pouwer, R.H.; Gunter, J.H.; Lubik, A.A.; Quinn, R.J.; Nelson, C.C. The fatty acid synthase inhibitor triclosan: Repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget 2014, 5, 9362. [Google Scholar] [PubMed]
- De Schrijver, E.; Brusselmans, K.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Silencing of the Fatty Acid Synthase Gene by RNA Interference Inhibits Growth and Induces Apoptosis of LNCaP Prostate Cancer Cells. In Hormonal Carcinogenesis IV; Li, J.J., Li, S.A., Llombart-Bosch, A., Eds.; Springer US: Boston, MA, USA, 2005; pp. 350–356. [Google Scholar] [CrossRef]
- Rae, C.; Haberkorn, U.; Babich, J.W.; Mairs, R.J. Inhibition of Fatty Acid Synthase Sensitizes Prostate Cancer Cells to Radiotherapy. Radiat. Res. 2015, 184, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.; Fragkoulis, G.I.; Chalmers, A.J. Cytotoxicity and Radiosensitizing Activity of the Fatty Acid Synthase Inhibitor C75 Is Enhanced by Blocking Fatty Acid Uptake in Prostate Cancer Cells. Adv. Radiat. Oncol. 2020, 5, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Koizumi, A.; Narita, S.; Inoue, T.; Tsuchiya, N.; Nakanishi, H.; Numakura, K.; Tsuruta, H.; Saito, M.; Satoh, S.; et al. Diet-induced alteration of fatty acid synthase in prostate cancer progression. Oncogenesis 2016, 5, e195. [Google Scholar] [CrossRef]
- Li, J.; Dong, L.; Wei, D.; Wang, X.; Zhang, S.; Li, H. Fatty Acid Synthase Mediates the Epithelial-Mesenchymal Transition of Breast Cancer Cells. Int. J. Biol. Sci. 2014, 10, 171–180. [Google Scholar] [CrossRef]
- Serhan, H.A.; Bao, L.; Cheng, X.; Qin, Z.; Liu, C.-J.; Heth, J.A.; Udager, A.M.; Soellner, M.B.; Merajver, S.D.; Morikawa, A.; et al. Targeting fatty acid synthase in preclinical models of TNBC brain metastases synergizes with SN-38 and impairs invasion. NPJ Breast Cancer 2024, 10, 43. [Google Scholar] [CrossRef]
- Yoshii, Y.; Furukawa, T.; Oyama, N.; Hasegawa, Y.; Kiyono, Y.; Nishii, R.; Waki, A.; Tsuji, A.B.; Sogawa, C.; Wakizaka, H.; et al. Fatty Acid Synthase Is a Key Target in Multiple Essential Tumor Functions of Prostate Cancer: Uptake of Radiolabeled Acetate as a Predictor of the Targeted Therapy Outcome. PLoS ONE 2013, 8, e64570. [Google Scholar] [CrossRef]
- De Piano, M.; Manuelli, V.; Zadra, G.; Loda, M.; Muir, G.; Chandra, A.; Morris, J.; Van Hemelrijck, M.; Wells, C.M. Exploring a role for fatty acid synthase in prostate cancer cell migration. Small GTPases 2021, 12, 265–272. [Google Scholar] [CrossRef]
- Ganguly, K.K.; Sen, T.; Mandal, S.; Biswas, J.; Chatterjee, A. Studies on Focal Adhesion Kinase in Human Breast Cancer Tissue. J. Cancer Ther. 2012, 3, 7–19. [Google Scholar]
- Rovin, J.D.; Frierson, H.F., Jr.; Ledinh, W.; Parsons, J.T.; Adams, R.B. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate 2002, 53, 124–132. [Google Scholar] [CrossRef]
- Xu, L.-H.; Yang, X.; Bradham, C.A.; Brenner, D.A.; Baldwin, A.S., Jr.; Craven, R.J.; Cance, W.G. The Focal Adhesion Kinase Suppresses Transformation-associated, Anchorage-independent Apoptosis in Human Breast Cancer Cells: Involvement of Death Receptor-Related Signaling Pathways. J. Biol. Chem. 2000, 275, 30597–30604. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Beviglia, L.; Xu, L.-H.; Earp, H.S., III; Craven, R.; Cance, W. Dual Inhibition of Focal Adhesion Kinase and Epidermal Growth Factor Receptor Pathways Cooperatively Induces Death Receptor-mediated Apoptosis in Human Breast Cancer Cells. J. Biol. Chem. 2002, 277, 38978–38987. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.H.; Surmacz, T.A.; Nyormoi, O.; Whitacre, C.M. Inhibition of focal adhesion kinase by antisense oligonucleotides enhances the sensitivity of breast cancer cells to camptothecins. Biocell 2003, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Danta, C.C.; Sahu, A.N. Chapter 16—Naturally occurring anticancer drugs. In Medicinal Chemistry of Chemotherapeutic Agents; Acharya, P.C., Kurosu, M., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 539–588. [Google Scholar] [CrossRef]
- Lazaro, G.; Smith, C.; Goddard, L.; Jordan, N.; McClelland, R.; Barrett-Lee, P.; Nicholson, R.I.; Hiscox, S. Targeting focal adhesion kinase in ER+/HER2+ breast cancer improves trastuzumab response. Endocr. Relat. Cancer 2013, 20, 691–704. [Google Scholar] [CrossRef]
- Rovin, J.D.; Ledinh, W.; Parsons, T.J.; Adams, R.B. Inhibition of focal adhesion kinase prevents growth of prostate tumors in mice. J. Am. Coll. Surg. 2000, 191, S87. [Google Scholar] [CrossRef]
- Lin, H.-M.; Lee, B.Y.; Castillo, L.; Spielman, C.; Grogan, J.; Yeung, N.K.; Kench, J.G.; Stricker, P.D.; Haynes, A.-M.; Centenera, M.M.; et al. Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate 2018, 78, 308–317. [Google Scholar] [CrossRef]
- Johnson, T.R.; Khandrika, L.; Kumar, B.; Venezia, S.; Koul, S.; Chandhoke, R.; Maroni, P.; Donohue, R.; Meacham, R.B.; Koul, H.K. Focal adhesion kinase controls aggressive phenotype of androgen-independent prostate cancer. Mol. Cancer Res. 2008, 6, 1639–1648. [Google Scholar] [CrossRef]
- Hiscox, S.; Barnfather, P.; Hayes, E.; Bramble, P.; Christensen, J.; Nicholson, R.I.; Barrett-Lee, P. Inhibition of focal adhesion kinase suppresses the adverse phenotype of endocrine-resistant breast cancer cells and improves endocrine response in endocrine-sensitive cells. Breast Cancer Res. Treat. 2011, 125, 659–669. [Google Scholar] [CrossRef]
- Sumitomo, M.; Shen, R.; Walburg, M.; Dai, J.; Geng, Y.; Navarro, D.; Boileau, G.; Papandreou, C.N.; Giancotti, F.G.; Knudsen, B.; et al. Neutral endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. J. Clin. Investig. 2000, 106, 1399–1407. [Google Scholar] [CrossRef]
- Albrecht, M.; Gillen, S.; Wilhelm, B.; Doroszewicz, J.; Aumüller, G. Expression, Localization and Activity of Neutral Endopeptidase in Cultured Cells of Benign Prostatic Hyperplasia and Prostate Cancer. J. Urol. 2002, 168, 336–342. [Google Scholar] [CrossRef]
- Lacoste, J.; Aprikian, A.G.; Chevalier, S. Focal adhesion kinase is required for bombesin-induced prostate cancer cell motility. Mol. Cell. Endocrinol. 2005, 235, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, T.; Varkaris, A.S.; Parikh, N.U.; Song, J.H.; Cheng, C.-J.; Schweppe, R.E.; Alexander, S.; Davis, J.W.; Troncoso, P.; Friedl, P.; et al. Yes-mediated phosphorylation of focal adhesion kinase at tyrosine 861 increases metastatic potential of prostate cancer cells. Oncotarget 2015, 6, 10175. [Google Scholar] [PubMed]
- Chen, W.; Wang, J. RAB11A Promotes Cell Malignant Progression and Tumor Formation of Prostate Cancer via Activating FAK/AKT Signaling Pathway. Evid.-Based Complement. Altern. Med. 2023, 2023, 5885387. [Google Scholar] [CrossRef]
- Lv, Z.; Li, W.; Wei, X. S100A9 promotes prostate cancer cell invasion by activating TLR4/NF-κB/integrin β1/FAK signaling. OncoTargets Ther. 2020, 13, 6443–6452. [Google Scholar] [CrossRef]
- Ma, C.; Zu, X.; Liu, K.; Bode, A.M.; Dong, Z.; Liu, Z.; Kim, D.J. Knockdown of Pyruvate Kinase M Inhibits Cell Growth and Migration by Reducing NF-kB Activity in Triple-Negative Breast Cancer Cells. Mol. Cells 2019, 42, 628–636. [Google Scholar] [CrossRef]
- Jiang, K.; He, B.; Lai, L.; Chen, Q.; Liu, Y.; Guo, Q.; Wang, Q. Cyclosporine A inhibits breast cancer cell growth by downregulating the expression of pyruvate kinase subtype M2. Int. J. Mol. Med. 2012, 30, 302–308. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Sachan, R.; Park, J.H.; Ahn, M.Y.; Yoon, K.; Lee, J.; Kim, N.D.; Kim, I.S.; Lee, B.M.; et al. PKM2 Knockdown Induces Autophagic Cell Death via AKT/mTOR Pathway in Human Prostate Cancer Cells. Cell Physiol. Biochem. 2019, 52, 1535–1552. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Li, G.; Lai, X.; Gu, R.; Xu, W.; Chen, H.; Xing, Z.; Chen, L.; Qian, J.; et al. Pyruvate Kinase M2 Promotes Prostate Cancer Metastasis Through Regulating ERK1/2-COX-2 Signaling. Front. Oncol. 2020, 10, 544288. [Google Scholar] [CrossRef]
- Lin, Y.; Lv, F.; Liu, F.; Guo, X.; Fan, Y.; Gu, F.; Gu, J.; Fu, L. High Expression of Pyruvate Kinase M2 is Associated with Chemosensitivity to Epirubicin and 5-Fluorouracil in Breast Cancer. J. Cancer 2015, 6, 1130–1139. [Google Scholar] [CrossRef]
- Guan, M.; Tong, Y.; Guan, M.; Liu, X.; Wang, M.; Niu, R.; Zhang, F.; Dong, D.; Shao, J.; Zhou, Y. Lapatinib Inhibits Breast Cancer Cell Proliferation by Influencing PKM2 Expression. Technol. Cancer Res. Treat. 2018, 17, 1533034617749418. [Google Scholar] [CrossRef]
- Ji, F.; Guo, B.; Wang, N.; Zhong, C.; Huang, L.; Huang, Y.; Wei, L.; Su, M.; Jiang, Y.; Jin, Q.; et al. Pyruvate kinase M2 interacts with mammalian sterile 20-like kinase 1 and inhibits tamoxifen-induced apoptosis in human breast cancer cells. Tumor Biol. 2017, 39, 1010428317692251. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Pulugu, P.; Singh, A.A.; Chatterjee, D.R.; Baviskar, S.; Vyas, H.; Behera, S.K.; Srivastava, A.; Kumar, H.; Shard, A. Mechanistic Investigation of Thiazole-Based Pyruvate Kinase M2 Inhibitor Causing Tumor Regression in Triple-Negative Breast Cancer. J. Med. Chem. 2024, 67, 3339–3357. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, Y.; Lee, J.-S.; Park, J.H.; Shin, J.-K.; Han, J.-H.; Kim, H.S.; Yoon, S. Combination Treatment Using Pyruvate Kinase M2 Inhibitors for the Sensitization of High-Density Triple-negative Breast Cancer Cells. Vivo 2022, 36, 2105–2115. [Google Scholar] [CrossRef]
- Hasan, D.; Gamen, E.; Abu Tarboush, N.; Ismail, Y.; Pak, O.; Azab, B. PKM2 and HIF-1α regulation in prostate cancer cell lines. PLoS ONE 2018, 13, e0203745. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, X.; Jeong, T.; Kang, J.Y.; Park, J.H.; Kim, I.S.; Kim, H.S. Novel Specific Pyruvate Kinase M2 Inhibitor, Compound 3h, Induces Apoptosis and Autophagy through Suppressing Akt/mTOR Signaling Pathway in LNCaP Cells. Cancers 2022, 15, 265. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, L.; Chen, Y.; Zhou, C.; Wang, X.; Wang, D.; Liu, Z. PKM2 Promotes Breast Cancer Progression by Regulating Epithelial Mesenchymal Transition. Anal. Cell. Pathol. 2020, 2020, 8396023. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, X.; Yang, R.; Wei, X.; Yan, R.; Jiang, Y.; Shen, W. Expression Characteristics and Significant Prognostic Values of PGK1 in Breast Cancer. Front. Mol. Biosci. 2021, 8, 695420. [Google Scholar] [CrossRef]
- Chen, J.Y.; Li, J.D.; He, R.Q.; Huang, Z.G.; Chen, G.; Zou, W. Bibliometric analysis of phosphoglycerate kinase 1 expression in breast cancer and its distinct upregulation in triple-negative breast cancer. World J. Clin. Oncol. 2024, 15, 867–894. [Google Scholar] [CrossRef]
- Fu, D.; He, C.; Wei, J.; Zhang, Z.; Luo, Y.; Tan, H.; Ren, C. PGK1 is a Potential Survival Biomarker and Invasion Promoter by Regulating the HIF-1α–Mediated Epithelial-Mesenchymal Transition Process in Breast Cancer. Cell. Physiol. Biochem. 2018, 51, 2434–2444. [Google Scholar] [CrossRef]
- Xu, D.; Aka, J.A.; Wang, R.; Lin, S.X. 17beta-hydroxysteroid dehydrogenase type 5 is negatively correlated to apoptosis inhibitor GRP78 and tumor-secreted protein PGK1 and modulates breast cancer cell viability and proliferation. J. Steroid Biochem. Mol. Biol. 2017, 171, 270–280. [Google Scholar] [CrossRef]
- Sun, S.; Liang, X.; Zhang, X.; Liu, T.; Shi, Q.; Song, Y.; Jiang, Y.; Wu, H.; Jiang, Y.; Lu, X.; et al. Phosphoglycerate kinase-1 is a predictor of poor survival and a novel prognostic biomarker of chemoresistance to paclitaxel treatment in breast cancer. Br. J. Cancer 2015, 112, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bai, Y.; Gao, Y.; Li, D.; Chen, L.; Zhou, C.; Feng, M.; Chen, X.; Jin, W.; Cao, Y. Systematic Analysis Uncovers Associations of PGK1 with Prognosis and Immunological Characteristics in Breast Cancer. Dis. Markers 2021, 2021, 7711151. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, R.; Wang, M.; Liu, T.; Zhou, Q.; Zhang, D.; Wang, J.; Shen, M.; Ren, X.; Sun, Q. PGAM1 regulation of ASS1 contributes to the progression of breast cancer through the cAMP/AMPK/CEBPB pathway. Mol. Oncol. 2022, 16, 2843–2860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, M.; Wang, W.; Ma, S.; Yu, W.; Ren, X.; Sun, Q. PGAM1 suppression remodels the tumor microenvironment in triple-negative breast cancer and synergizes with anti–PD-1 immunotherapy. J. Leukoc. Biol. 2024, 116, 579–588. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, M.; Ma, S.; Liu, M.; Yu, W.; Zhang, X.; Liu, T.; Liu, S.; Ren, X.; Sun, Q. Phosphoglycerate mutase 1 promotes breast cancer progression through inducing immunosuppressive M2 macrophages. Cancer Gene Ther. 2024, 31, 1018–1033. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, N.; Sun, W.; Li, X.; Liu, B.; Xie, Z.; Qu, J.; Xu, J.; Yang, X.; Su, Y.; et al. Phosphoglycerate mutase 1 promotes cancer cell migration independent of its metabolic activity. Oncogene 2017, 36, 2900–2909. [Google Scholar] [CrossRef]
- Mele, L.; la Noce, M.; Paino, F.; Regad, T.; Wagner, S.; Liccardo, D.; Papaccio, G.; Lombardi, A.; Caraglia, M.; Tirino, V.; et al. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J. Exp. Clin. Cancer Res. 2019, 38, 160. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, F.; Zhang, Y.; Lin, Z.; Yang, J.; Han, X.; Feng, Y.; Pei, X.; Li, F.; Liu, Q.; et al. Targeting glucose-6-phosphate dehydrogenase by 6-AN induces ROS-mediated autophagic cell death in breast cancer. Febs. J. 2023, 290, 763–779. [Google Scholar] [CrossRef]
- Jhaveri, K.D.; Wanchoo, R.; Sakhiya, V.; Ross, D.W.; Fishbane, S. Adverse Renal Effects of Novel Molecular Oncologic Targeted Therapies: A Narrative Review. Kidney Int. Rep. 2017, 2, 108–123. [Google Scholar] [CrossRef]
- Zhen, X.; Zhang, M.; Hao, S.; Sun, J. Glucose-6-phosphate dehydrogenase and transketolase: Key factors in breast cancer progression and therapy. Biomed. Pharmacother. 2024, 176, 116935. [Google Scholar] [CrossRef]
- Révillion, F.; Pawlowski, V.; Hornez, L.; Peyrat, J.P. Glyceraldehyde-3-phosphate dehydrogenase gene expression in human breast cancer. Eur. J. Cancer 2000, 36, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Cho, W.C.S.; Ma, V.; Cheuk, W.; So, Y.K.; Wong, S.C.C.; Zhang, M.; Li, C.; Sun, Y.; Zhang, H.; et al. ADAM9 Mediates Triple-Negative Breast Cancer Progression via AKT/NF-κB Pathway. Front. Med. 2020, 7, 214. [Google Scholar] [CrossRef]
- Song, P.; Wu, J.; Chen, J.; Wang, F.; Chen, J.; Wang, G. Knockdown of circ-ADAM9 inhibits malignant phenotype and enhances radiosensitivity in breast cancer cells via acting as a sponge for miR-383-5p. Strahlenther. Und Onkol. 2023, 199, 78–89. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, L.; Li, X.; Lu, A.; Hou, C.; Wu, Q.; Hu, X.; Zhou, Z.; Chen, Z.; Tang, F. ADAM10 is involved in the oncogenic process and chemo-resistance of triple-negative breast cancer via regulating Notch1 signaling pathway, CD44 and PrPc. Cancer Cell Int. 2021, 21, 32. [Google Scholar] [CrossRef]
- Mullooly, M.; McGowan, P.M.; Kennedy, S.A.; Madden, S.F.; Crown, J.; O’ Donovan, N.; Duffy, M.J. ADAM10: A new player in breast cancer progression? Br. J. Cancer 2015, 113, 945–951. [Google Scholar] [CrossRef]
- Fry, J.L.; Toker, A. Secreted and Membrane-Bound Isoforms of Protease ADAM9 Have Opposing Effects on Breast Cancer Cell Migration. Cancer Res. 2010, 70, 8187–8198. [Google Scholar] [CrossRef]
- Micocci, K.C.; Martin, A.C.B.M.; Montenegro, C.d.F.; Durante, A.C.; Pouliot, N.; Cominetti, M.R.; Selistre-de-Araujo, H.S. ADAM9 silencing inhibits breast tumor cell invasion In Vitro. Biochimie 2013, 95, 1371–1378. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Feldinger, K.; Generali, D.; Kramer-Marek, G.; Gijsen, M.; Ng, T.B.; Wong, J.H.; Strina, C.; Cappelletti, M.; Andreis, D.; Li, J.-L.; et al. ADAM10 mediates trastuzumab resistance and is correlated with survival in HER2 positive breast cancer. Oncotarget 2014, 5, 6633. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Scialò, C.; Legname, G. Chapter Twelve—The role of the cellular prion protein in the uptake and toxic signaling of pathological neurodegenerative aggregates. In Progress in Molecular Biology and Translational Science; Legname, G., Ed.; Academic Press: San Diego, CA, USA, 2020; Volume 175, pp. 297–323. [Google Scholar]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Lee, M.A.; Chan, T.-H.; Li, J.; Ni, Y.-B.; Shao, Y.; Chan, S.-K.; Cheungc, S.-Y.; Lau, K.-F.; Tse, G.M.K. Proteolytic cleavage of amyloid precursor protein by ADAM10 mediates proliferation and migration in breast cancer. EBiomedicine 2018, 38, 89–99. [Google Scholar] [CrossRef]
- Wang, Q.M.; Lv, L.; Tang, Y.; Zhang, L.; Wang, L.F. MMP-1 is overexpressed in triple-negative breast cancer tissues and the knockdown of MMP-1 expression inhibits tumor cell malignant behaviors In Vitro. Oncol. Lett. 2019, 17, 1732–1740. [Google Scholar] [CrossRef]
- Mohammadian, H.; Sharifi, R.; Rezanezhad Amirdehi, S.; Taheri, E.; Babazadeh Bedoustani, A. Matrix metalloproteinase MMP1 and MMP9 genes expression in breast cancer tissue. Gene Rep. 2020, 21, 100906. [Google Scholar] [CrossRef]
- Liu, H.; Kato, Y.; Erzinger, S.A.; Kiriakova, G.M.; Qian, Y.; Palmieri, D.; Steeg, P.S.; Price, J.E. The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer 2012, 12, 583. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Q.; Hu, G.; Van Poznak, C.; Fleisher, M.; Reiss, M.; Massagué, J.; Kang, Y. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes. Dev. 2009, 23, 1882–1894. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, J.E.; Baek, M.; Kim, H.; Ji, H.W.; Yun, S.H.; Jeong, D.; Ham, J.; Park, S.; Lu, X.; et al. Matrix Metalloproteinase-1 (MMP1) Upregulation through Promoter Hypomethylation Enhances Tamoxifen Resistance in Breast Cancer. Cancers 2022, 14, 1232. [Google Scholar] [CrossRef]
- Shen, C.-J.; Kuo, Y.-L.; Chen, C.-C.; Chen, M.-J.; Cheng, Y.-M. MMP1 expression is activated by Slug and enhances multi-drug resistance (MDR) in breast cancer. PLoS ONE 2017, 12, e0174487. [Google Scholar] [CrossRef]
- Bajor, M.; Zych, A.O.; Graczyk-Jarzynka, A.; Muchowicz, A.; Firczuk, M.; Trzeciak, L.; Gaj, P.; Domagala, A.; Siernicka, M.; Zagozdzon, A.; et al. Targeting peroxiredoxin 1 impairs growth of breast cancer cells and potently sensitises these cells to prooxidant agents. Br. J. Cancer 2018, 119, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Tehan, L.; Taparra, K.; Phelan, S. Peroxiredoxin overexpression in MCF-7 breast cancer cells and regulation by cell proliferation and oxidative stress. Cancer Investig. 2013, 31, 374–384. [Google Scholar] [CrossRef]
- McDonald, C.; Muhlbauer, J.; Perlmutter, G.; Taparra, K.; Phelan, S.A. Peroxiredoxin proteins protect MCF-7 breast cancer cells from doxorubicin-induced toxicity. Int. J. Oncol. 2014, 45, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Lian, X.-D.; Lee, S.-J.; Li, W.-L.; Sun, H.-N.; Jin, M.-H.; Kwon, T. Regulatory effect of peroxiredoxin 1 (PRDX1) on doxorubicin-induced apoptosis in triple negative breast cancer cells. Appl. Biol. Chem. 2022, 65, 63. [Google Scholar] [CrossRef]
- O’Leary, P.C.; Terrile, M.; Bajor, M.; Gaj, P.; Hennessy, B.T.; Mills, G.B.; Zagozdzon, A.; O’Connor, D.P.; Brennan, D.J.; Connor, K.; et al. Peroxiredoxin-1 protects estrogen receptor α from oxidative stress-induced suppression and is a protein biomarker of favorable prognosis in breast cancer. Breast Cancer Res. 2014, 16, R79. [Google Scholar] [CrossRef]
- Fiskus, W.; Coothankandaswamy, V.; Chen, J.; Ma, H.; Ha, K.; Saenz, D.T.; Krieger, S.S.; Mill, C.P.; Sun, B.; Huang, P.; et al. SIRT2 Deacetylates and Inhibits the Peroxidase Activity of Peroxiredoxin-1 to Sensitize Breast Cancer Cells to Oxidant Stress-Inducing Agents. Cancer Res. 2016, 76, 5467–5478. [Google Scholar] [CrossRef]
- Spínola-Lasso, E.; Montero, J.C.; Jiménez-Monzón, R.; Estévez, F.; Quintana, J.; Guerra, B.; Elokely, K.M.; León, F.; del Rosario, H.; Fernández-Pérez, L.; et al. Chemical-proteomics Identify Peroxiredoxin-1 as an Actionable Target in Triple-negative Breast Cancer. Int. J. Biol. Sci. 2023, 19, 1731–1747. [Google Scholar] [CrossRef]
- Stresing, V.; Baltziskueta, E.; Rubio, N.; Blanco, J.; Arriba, M.; Valls, J.; Janier, M.; Clézardin, P.; Sanz-Pamplona, R.; Nieva, C.; et al. Peroxiredoxin 2 specifically regulates the oxidative and metabolic stress response of human metastatic breast cancer cells in lungs. Oncogene 2013, 32, 724–735. [Google Scholar] [CrossRef]
- Wang, T.; Tamae, D.; LeBon, T.; Shively, J.E.; Yen, Y.; Li, J.J. The Role of Peroxiredoxin II in Radiation-Resistant MCF-7 Breast Cancer Cells. Cancer Res. 2005, 65, 10338–10346. [Google Scholar] [CrossRef]
- Jin, X.; Wei, Y.; Xu, F.; Zhao, M.; Dai, K.; Shen, R.; Yang, S.; Zhang, N. SIRT1 promotes formation of breast cancer through modulating Akt activity. J. Cancer 2018, 9, 2012–2023. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.; Zhang, X. The promoter methylation status and mRNA expression levels of CTCF and SIRT6 in sporadic breast cancer. DNA Cell Biol. 2014, 33, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kumari, S.; Pulipaka, S.; Chandra, Y.; Kotamraju, S. SIRT1 promotes doxorubicin-induced breast cancer drug resistance and tumor angiogenesis via regulating GSH-mediated redox homeostasis. Mol. Carcinog. 2024, 63, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Andreani, C.; Bartolacci, C.; Persico, G.; Casciaro, F.; Amatori, S.; Fanelli, M.; Giorgio, M.; Galié, M.; Tomassoni, D.; Wang, J.; et al. SIRT6 promotes metastasis and relapse in HER2-positive breast cancer. Sci. Rep. 2023, 13, 22000. [Google Scholar] [CrossRef]
- Nicknam, A.; Khojasteh Pour, S.; Hashemnejad, M.A.; Hussen, B.M.; Safarzadeh, A.; Eslami, S.; Taheri, M.; Ghafouri-Fard, S.; Jamali, E. Expression analysis of Rho GTPase-related lncRNAs in breast cancer. Pathol.-Res. Pract. 2023, 244, 154429. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Busch, H.; Boerries, M.; Brummer, T.; Timme, S.; Lassmann, S.; Aktories, K.; Schmidt, G. Specific role of RhoC in tumor invasion and metastasis. Oncotarget 2017, 8, 87364. [Google Scholar] [CrossRef]
- Kleer, C.G.; van Golen, K.L.; Zhang, Y.; Wu, Z.-F.; Rubin, M.A.; Merajver, S.D. Characterization of RhoC Expression in Benign and Malignant Breast Disease: A Potential New Marker for Small Breast Carcinomas with Metastatic Ability. Am. J. Pathol. 2002, 160, 579–584. [Google Scholar] [CrossRef]
- Pillé, J.Y.; Denoyelle, C.; Varet, J.; Bertrand, J.R.; Soria, J.; Opolon, P.; Lu, H.; Pritchard, L.L.; Vannier, J.P.; Malvy, C.; et al. Anti-RhoA and Anti-RhoC siRNAs Inhibit the Proliferation and Invasiveness of MDA-MB-231 Breast Cancer Cells In Vitro and In Vivo. Mol. Ther. 2005, 11, 267–274. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Z.F.; Rosenthal, D.T.; Rhee, E.M.; Merajver, S.D. Characterization of the roles of RHOC and RHOA GTPases in invasion, motility, and matrix adhesion in inflammatory and aggressive breast cancers. Cancer 2010, 116, 2768–2782. [Google Scholar] [CrossRef]
- Xu, X.D.; Shen, H.B.; Zhu, L.; Lu, J.Q.; Zhang, L.; Luo, Z.Y.; Wu, Y.Q. Anti-RhoC siRNAs inhibit the proliferation and invasiveness of breast cancer cells via modulating the KAI1, MMP9, and CXCR4 expression. Onco Targets Ther. 2017, 10, 1827–1834. [Google Scholar] [CrossRef]
- Kalpana, G.; Figy, C.; Yeung, M.; Yeung, K.C. Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci. Rep. 2019, 9, 16351. [Google Scholar] [CrossRef]
- Asghar, K.; Loya, A.; Rana, I.A.; Tahseen, M.; Ishaq, M.; Farooq, A.; Bakar, M.A.; Masood, I. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manag. Res. 2019, 11, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Bilir, C.; Eskiler, G.G.; Bilir, F. The cytotoxic effects of indoleamine 2, 3-dioxygenase inhibitors on triple negative breast cancer cells upon tumor necrosis factor α stimulation. J. Cancer Res. Ther. 2023, 19, S74–S80. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhu, S.; Li, M.; Li, F.; Wei, F.; Liu, J.; Ren, X. High Indoleamine 2,3-Dioxygenase Is Correlated With Microvessel Density and Worse Prognosis in Breast Cancer. Front. Immunol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Xu, M.; Hu, J.; Feng, N.; Deng, H.; Lu, C.; Huang, T. Indoleamine 2,3-dioxygenase 1 regulates breast cancer tamoxifen resistance through interleukin-6/signal transducer and activator of transcription. Toxicol. Appl. Pharmacol. 2022, 440, 115921. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, L.; Liu, J.; Li, F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother. Pharmacol. 2020, 85, 77–93. [Google Scholar] [CrossRef]
- Sarangi, P. Role of indoleamine 2, 3-dioxygenase 1 in immunosuppression of breast cancer. Cancer Pathog. Ther. 2024, 2, 246–255. [Google Scholar] [CrossRef]
- Yu, J.; Sun, J.; Wang, S.E.; Li, H.; Cao, S.; Cong, Y.; Liu, J.; Ren, X. Upregulated Expression of Indoleamine 2, 3-Dioxygenase in Primary Breast Cancer Correlates with Increase of Infiltrated Regulatory T Cells In Situ and Lymph Node Metastasis. J. Immunol. Res. 2011, 2011, 469135. [Google Scholar] [CrossRef]
- Levina, V.; Su, Y.; Gorelik, E. Immunological and Nonimmunological Effects of Indoleamine 2,3-Dioxygenase on Breast Tumor Growth and Spontaneous Metastasis Formation. J. Immunol. Res. 2012, 2012, 173029. [Google Scholar] [CrossRef]
- Xu, D.; Xu, N.; Sun, L.; Yang, Z.; He, M.; Li, Y. TG2 as a novel breast cancer prognostic marker promotes cell proliferation and glycolysis by activating the MEK/ERK/LDH pathway. BMC Cancer 2022, 22, 1267. [Google Scholar] [CrossRef]
- Hettasch, J.M.; Bandarenko, N.; Burchette, J.L.; Lai, T.S.; Marks, J.R.; Haroon, Z.A.; Peters, K.; Dewhirst, M.W.; Iglehart, J.D.; Greenberg, C.S. Tissue transglutaminase expression in human breast cancer. Lab. Investig. 1996, 75, 637–645. [Google Scholar] [PubMed]
- Mehta, K.; Fok, J.; Miller, F.R.; Koul, D.; Sahin, A.A. Prognostic Significance of Tissue Transglutaminase in Drug Resistant and Metastatic Breast Cancer. Clin. Cancer Res. 2004, 10, 8068–8076. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.F.; Mangala, L.S.; Mehta, K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 2006, 25, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Wang, X.H.; Hua, Y.T.; Zhang, Y.Z.; Han, Y.; Yang, Z.L. The tissue transglutaminase: A potential target regulating MDR in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6175–6184. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrari, E.; Terrazzan, A.; Brugnoli, F.; Spisni, A.; Taccioli, C.; Aguiari, G.; Trentini, A.; Volinia, S.; Keillor, J.W.; et al. Metabolic characterisation of transglutaminase 2 inhibitor effects in breast cancer cell lines. FEBS J. 2023, 290, 5411–5433. [Google Scholar] [CrossRef]
- Mangala, L.S.; Fok, J.Y.; Zorrilla-Calancha, I.R.; Verma, A.; Mehta, K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 2007, 26, 2459–2470. [Google Scholar] [CrossRef]
- Seo, S.; Moon, Y.; Choi, J.; Yoon, S.; Jung, K.H.; Cheon, J.; Kim, W.; Kim, D.; Lee, C.H.; Kim, S.W.; et al. The GTP binding activity of transglutaminase 2 promotes bone metastasis of breast cancer cells by downregulating microRNA-205. Am. J. Cancer Res. 2019, 9, 597–607. [Google Scholar]
- Schwager, S.C.; Young, K.M.; Hapach, L.A.; Carlson, C.M.; Mosier, J.A.; McArdle, T.J.; Wang, W.; Schunk, C.; Jayathilake, A.L.; Bates, M.E.; et al. Weakly migratory metastatic breast cancer cells activate fibroblasts via microvesicle-Tg2 to facilitate dissemination and metastasis. Elife 2022, 11, e74433. [Google Scholar] [CrossRef]
- Tatarov, O.; Mitchell, T.J.; Seywright, M.; Leung, H.Y.; Brunton, V.G.; Edwards, J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin. Cancer Res. 2009, 15, 3540–3549. [Google Scholar] [CrossRef]
- Xu, W.; Allbritton, N.; Lawrence, D.S. Src Kinase Regulation in Progressively Invasive Cancer. PLoS ONE 2012, 7, e48867. [Google Scholar] [CrossRef]
- Gelman, I.H.; Peresie, J.; Eng, K.H.; Foster, B.A. Differential Requirement for Src Family Tyrosine Kinases in the Initiation, Progression, and Metastasis of Prostate Cancer. Mol. Cancer Res. 2014, 12, 1470–1479. [Google Scholar] [CrossRef]
- Cai, H.; Babic, I.; Wei, X.; Huang, J.; Witte, O.N. Invasive Prostate Carcinoma Driven by c-Src and Androgen Receptor Synergy. Cancer Res. 2011, 71, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, J.S.; Bond, D.R.; Jankowski, H.; Goldie, B.J.; Burchell, R.; Naudin, C.; Smith, N.D.; Scarlett, C.J.; Larsen, M.R.; Dun, M.D.; et al. Extracellular vesicles with altered tetraspanin CD9 and CD151 levels confer increased prostate cell motility and invasion. Sci. Rep. 2018, 8, 8822. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Siddiqui, I.A.; Hafeez, B.B.; Baniahmad, A.; Mukhtar, H. Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene 2008, 27, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Wang, J.; Qin, M.; Gao, L.; Holtz, R.; Vessella, R.L.; Leach, R.W.; Gelman, I.H. Src promotes castration-recurrent prostate cancer through androgen receptor-dependent canonical and non-canonical transcriptional signatures. Oncotarget 2016, 8, 10324. [Google Scholar]
- Goc, A.; Al-Husein, B.A.-H.; Katsanevas, K.; Steinbach, A.; Lou, U.; Sabbineni, H.; DeRemer, D.L.; Somanath, P.R. Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression In Vitro and In Vivo. Oncotarget 2014, 5, 775. [Google Scholar]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef]
- Chang, Y.M.; Bai, L.; Liu, S.; Yang, J.C.; Kung, H.J.; Evans, C.P. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene 2008, 27, 6365–6375. [Google Scholar] [CrossRef]
- Yang, C.-C.; Fazli, L.; Loguercio, S.; Zharkikh, I.; Aza-Blanc, P.E.; Gleave, M.; Wolf, D.A. Downregulation of c-SRC kinase CSK promotes castration resistant prostate cancer and pinpoints a novel disease subclass. Oncotarget 2015, 6, 22060. [Google Scholar]
- Wu, Z.; Chang, P.-C.; Yang, J.C.; Chu, C.-Y.; Wang, L.-Y.; Chen, N.-T.; Ma, A.-H.; Desai, S.J.; Lo, S.H.; Evans, C.P.; et al. Autophagy Blockade Sensitizes Prostate Cancer Cells towards Src Family Kinase Inhibitors. Genes. Cancer 2010, 1, 40–49. [Google Scholar] [CrossRef]
- Le Page, C.; Koumakpayi, I.H.; Alam-Fahmy, M.; Mes-Masson, A.M.; Saad, F. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br. J. Cancer 2006, 94, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.R.; Konicek, B.W.; McNulty, A.M.; Wang, Z.; Houck, K.; Allen, S.; Paul, J.D.; Hbaiu, A.; Goode, R.G.; Sandusky, G.E.; et al. Increased AKT Activity Contributes to Prostate Cancer Progression by Dramatically Accelerating Prostate Tumor Growth and Diminishing p27Kip1 Expression. J. Biol. Chem. 2000, 275, 24500–24505. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Liu, J.; Byzova, T.V.; Somanath, P.R. Akt1 mediates prostate cancer cell microinvasion and chemotaxis to metastatic stimuli via integrin β3 affinity modulation. Br. J. Cancer 2012, 107, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Dong, B.; He, X.; Qiu, Z.; Zhang, J.; Zhang, M.; Liu, H.; Pang, X.; Cui, Y. The challenges and opportunities of αvβ3-based therapeutics in cancer: From bench to clinical trials. Pharmacol. Res. 2023, 189, 106694. [Google Scholar] [CrossRef]
- Alasmar, A.; Al-Alami, Z.; Zein, S.; Al-Smadi, A.; Al Bashir, S.; Alorjani, M.S.; Al-Zoubi, R.M.; Al Zoubi, M. Novel Mutations in AKT1 Gene in Prostate Cancer Patients in Jordan. Curr. Issues Mol. Biol. 2024, 46, 9856–9866. [Google Scholar] [CrossRef]
- Vaarala, M.H.; Porvari, K.; Kyllönen, A.; Vihko, P. Differentially expressed genes in two LNCaP prostate cancer cell lines reflecting changes during prostate cancer progression. Lab. Investig. 2000, 80, 1259–1268. [Google Scholar] [CrossRef]
- Vaarala, M.H.; Porvari, K.; Kyllönen, A.; Lukkarinen, O.; Vihko, P. The TMPRSS2 gene encoding transmembrane serine protease is overexpressed in a majority of prostate cancer patients: Detection of mutated TMPRSS2 form in a case of aggressive disease. Int. J. Cancer 2001, 94, 705–710. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, M.S.; Lucht, A.; Chou, F.P.; Huang, W.; Havighurst, T.C.; Kim, K.; Wang, J.K.; Antalis, T.M.; Johnson, M.D.; et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am. J. Pathol. 2010, 176, 2986–2996. [Google Scholar] [CrossRef]
- Lucas, J.M.; Heinlein, C.; Kim, T.; Hernandez, S.A.; Malik, M.S.; True, L.D.; Morrissey, C.; Corey, E.; Montgomery, B.; Mostaghel, E.; et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014, 4, 1310–1325. [Google Scholar] [CrossRef]
- Ko, C.-J.; Huang, C.-C.; Lin, H.-Y.; Juan, C.-P.; Lan, S.-W.; Shyu, H.-Y.; Wu, S.-R.; Hsiao, P.-W.; Huang, H.-P.; Shun, C.-T.; et al. Androgen-Induced TMPRSS2 Activates Matriptase and Promotes Extracellular Matrix Degradation, Prostate Cancer Cell Invasion, Tumor Growth, and Metastasis. Cancer Res. 2015, 75, 2949–2960. [Google Scholar] [CrossRef]
- Tsai, C.H.; Teng, C.H.; Tu, Y.T.; Cheng, T.S.; Wu, S.R.; Ko, C.J.; Shyu, H.Y.; Lan, S.W.; Huang, H.P.; Tzeng, S.F.; et al. HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene 2014, 33, 4643–4652. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R. TMPRSS2 promotes metastasis through proteolysis. Nat. Rev. Urol. 2014, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-J.; Hsu, T.-W.; Wu, S.-R.; Lan, S.-W.; Hsiao, T.-F.; Lin, H.-Y.; Lin, H.-H.; Tu, H.-F.; Lee, C.-F.; Huang, C.-C.; et al. Inhibition of TMPRSS2 by HAI-2 reduces prostate cancer cell invasion and metastasis. Oncogene 2020, 39, 5950–5963. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bujanda, Z.A.; Obradovic, A.; Nirschl, T.R.; Crowley, L.; Macedo, R.; Papachristodoulou, A.; O’Donnell, T.; Laserson, U.; Zarif, J.C.; Reshef, R.; et al. TGM4: An immunogenic prostate-restricted antigen. J. Immunother. Cancer 2021, 9, e001649. [Google Scholar] [CrossRef]
- Davies, G.; Ablin, R.J.; Mason, M.D.; Jiang, W.G. Expression of the prostate transglutaminase (TGase-4) in prostate cancer cells and its impact on the invasiveness of prostate cancer. J. Exp. Ther. Oncol. 2007, 6, 257–264. [Google Scholar]
- Jiang, W.G.; Ye, L.; Ablin, R.J.; Kynaston, H.G.; Mason, M.D. The prostate transglutaminase, TGase-4, coordinates with the HGFL/MSP-RON system in stimulating the migration of prostate cancer cells. Int. J. Oncol. 2010, 37, 413–418. [Google Scholar] [CrossRef]
- Jiang, W.G.; Ye, L.; Sanders, A.J.; Ruge, F.; Kynaston, H.G.; Ablin, R.J.; Mason, M.D. Prostate transglutaminase (TGase-4, TGaseP) enhances the adhesion of prostate cancer cells to extracellular matrix, the potential role of TGase-core domain. J. Transl. Med. 2013, 11, 269. [Google Scholar] [CrossRef]
- Ablin, R.J.; Kynaston, H.G.; Mason, M.D.; Jiang, W.G. Prostate transglutaminase (TGase-4) antagonizes the anti-tumour action of MDA-7/IL-24 in prostate cancer. J. Transl. Med. 2011, 9, 49. [Google Scholar] [CrossRef]
- Menezes, M.E.; Bhatia, S.; Bhoopathi, P.; Das, S.K.; Emdad, L.; Dasgupta, S.; Dent, P.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. MDA-7/IL-24: Multifunctional cancer killing cytokine. Adv. Exp. Med. Biol. 2014, 818, 127–153. [Google Scholar] [CrossRef]
- Ablin, R.J.; Owen, S.; Jiang, W.G. Prostate Transglutaminase (TGase-4) Induces Epithelial-to-Mesenchymal Transition in Prostate Cancer Cells. Anticancer. Res. 2017, 37, 481–487. [Google Scholar] [CrossRef]
- Gomes, I.M.; Santos, C.R.; Maia, C.J. Expression of STEAP1 and STEAP1B in prostate cell lines, and the putative regulation of STEAP1 by post-transcriptional and post-translational mechanisms. Genes. Cancer 2014, 5, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Morrison, K.; Etessami, S.; An, Z.; Morrison, K.J.; Perez-Villar, J.J.; Raitano, A.B.; Jia, X.-C.; Gudas, J.M.; Kanner, S.B.; et al. Monoclonal Antibodies to Six-Transmembrane Epithelial Antigen of the Prostate-1 Inhibit Intercellular Communication In Vitro and Growth of Human Tumor Xenografts In Vivo. Cancer Res. 2007, 67, 5798–5805. [Google Scholar] [CrossRef] [PubMed]
- Burnell, S.E.A.; Spencer-Harty, S.; Howarth, S.; Bodger, O.; Kynaston, H.; Morgan, C.; Doak, S.H. Utilisation of the STEAP protein family in a diagnostic setting may provide a more comprehensive prognosis of prostate cancer. PLoS ONE 2019, 14, e0220456. [Google Scholar] [CrossRef]
- Rocha, S.M.; Nascimento, D.; Coelho, R.S.; Cardoso, A.M.; Passarinha, L.A.; Socorro, S.; Maia, C.J. STEAP1 Knockdown Decreases the Sensitivity of Prostate Cancer Cells to Paclitaxel, Docetaxel and Cabazitaxel. Int. J. Mol. Sci. 2023, 24, 6643. [Google Scholar] [CrossRef]
- Jones, L.A.; Conway, G.E.; Nguyen-Chi, A.; Burnell, S.; Jenkins, G.J.; Conlan, R.S.; Doak, S.H. Investigating STEAP2 as a potential therapeutic target for the treatment of aggressive prostate cancer. Cell. Mol. Biol. 2023, 69, 179–187. [Google Scholar] [CrossRef]
- Burnell, S.E.A.; Spencer-Harty, S.; Howarth, S.; Bodger, O.; Kynaston, H.; Morgan, C.; Doak, S.H. STEAP2 Knockdown Reduces the Invasive Potential of Prostate Cancer Cells. Sci. Rep. 2018, 8, 6252. [Google Scholar] [CrossRef]
- Patel, S.; Turner, P.R.; Stubberfield, C.; Barry, E.; Rohlff, C.R.; Stamps, A.; Tyson, K.; Terrett, J.; Box, G.; Eccles, S.; et al. Hyaluronidase gene profiling and role of HYAL-1 overexpression in an orthotopic model of prostate cancer. Int. J. Cancer 2002, 97, 416–424. [Google Scholar] [CrossRef]
- Kovar, J.L.; Johnson, M.A.; Volcheck, W.M.; Chen, J.; Simpson, M.A. Hyaluronidase Expression Induces Prostate Tumor Metastasis in an Orthotopic Mouse Model. Am. J. Pathol. 2006, 169, 1415–1426. [Google Scholar] [CrossRef]
- Reale, A.; Khong, T.; Spencer, A. Extracellular Vesicles and Their Roles in the Tumor Immune Microenvironment. J. Clin. Med. 2022, 11, 6892. [Google Scholar] [CrossRef]
- Hamed, M.A.; Wasinger, V.; Wang, Q.; Graham, P.; Malouf, D.; Bucci, J.; Li, Y. Prostate cancer-derived extracellular vesicles metabolic biomarkers: Emerging roles for diagnosis and prognosis. J. Control. Release 2024, 371, 126–145. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, H.; Cui, Y.; Xing, J.; Wang, W.; Chen, J.; Wang, S.; Yang, Z. Extracellular vesicles for breast cancer diagnosis and therapy. Extracell. Vesicle 2024, 3, 100039. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Sheykhhasan, M.; Kalhor, N.; Sheikholeslami, A.; Dolati, M.; Amini, E.; Fazaeli, H. Exosomes of Mesenchymal Stem Cells as a Proper Vehicle for Transfecting miR-145 into the Breast Cancer Cell Line and Its Effect on Metastasis. BioMed Res. Int. 2021, 2021, 5516078. [Google Scholar] [CrossRef]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, A.; de Miguel-Pérez, D.; Ortega, F.G.; García-Puche, J.L.; Robles-Fernández, I.; Exposito, J.; Martorell-Marugan, J.; Carmona-Sáez, P.; Garrido-Navas, M.D.C.; Rolfo, C.; et al. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019, 21, 21. [Google Scholar] [CrossRef]
- Lim, J.; Kang, B.; Son, H.Y.; Mun, B.; Huh, Y.-M.; Rho, H.W.; Kang, T.; Moon, J.; Lee, J.-J.; Seo, S.B.; et al. Microfluidic device for one-step detection of breast cancer-derived exosomal mRNA in blood using signal-amplifiable 3D nanostructure. Biosens. Bioelectron. 2022, 197, 113753. [Google Scholar] [CrossRef]
- Bamankar, S.; Londhe, V.Y. The Rise of Extracellular Vesicles as New Age Biomarkers in Cancer Diagnosis: Promises and Pitfalls. Technol. Cancer Res. Treat. 2023, 22, 15330338221149266. [Google Scholar] [CrossRef]
- Rodríguez, M.; Bajo-Santos, C.; Hessvik, N.P.; Lorenz, S.; Fromm, B.; Berge, V.; Sandvig, K.; Linē, A.; Llorente, A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer 2017, 16, 156. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F.; et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015, 67, 33–41. [Google Scholar] [CrossRef]
- Andre, M.; Caobi, A.; Miles, J.S.; Vashist, A.; Ruiz, M.A.; Raymond, A.D. Diagnostic potential of exosomal extracellular vesicles in oncology. BMC Cancer 2024, 24, 322. [Google Scholar] [CrossRef]
- Dudzik, D.; Macioszek, S.; Struck-Lewicka, W.; Kordalewska, M.; Buszewska-Forajta, M.; Waszczuk-Jankowska, M.; Wawrzyniak, R.; Artymowicz, M.; Raczak-Gutknecht, J.; Siluk, D.; et al. Perspectives and challenges in extracellular vesicles untargeted metabolomics analysis. TrAC Trends Anal. Chem. 2021, 143, 116382. [Google Scholar] [CrossRef]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: From exosomes to microvesicles. Cardiovasc. Res. 2022, 119, 45–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).