Impact of Secondhand Smoke and E-Cigarette Exposure on Placental Apoptotic and Growth-Regulatory Proteins in Mouse Pregnancy
Abstract
:1. Introduction
2. Methods
2.1. Animal and Tissue Preparation
2.2. Apoptotic Molecular Analyses
2.3. Statistical Analysis
3. Results
3.1. Pro-Apoptotic Markers (BAD, Cytochrome c, and FAS/FASL)
3.2. Anti-Apoptotic Markers (BCL-2, cIAP-2, HSP27, HSP70, Survivin, and XIAP)
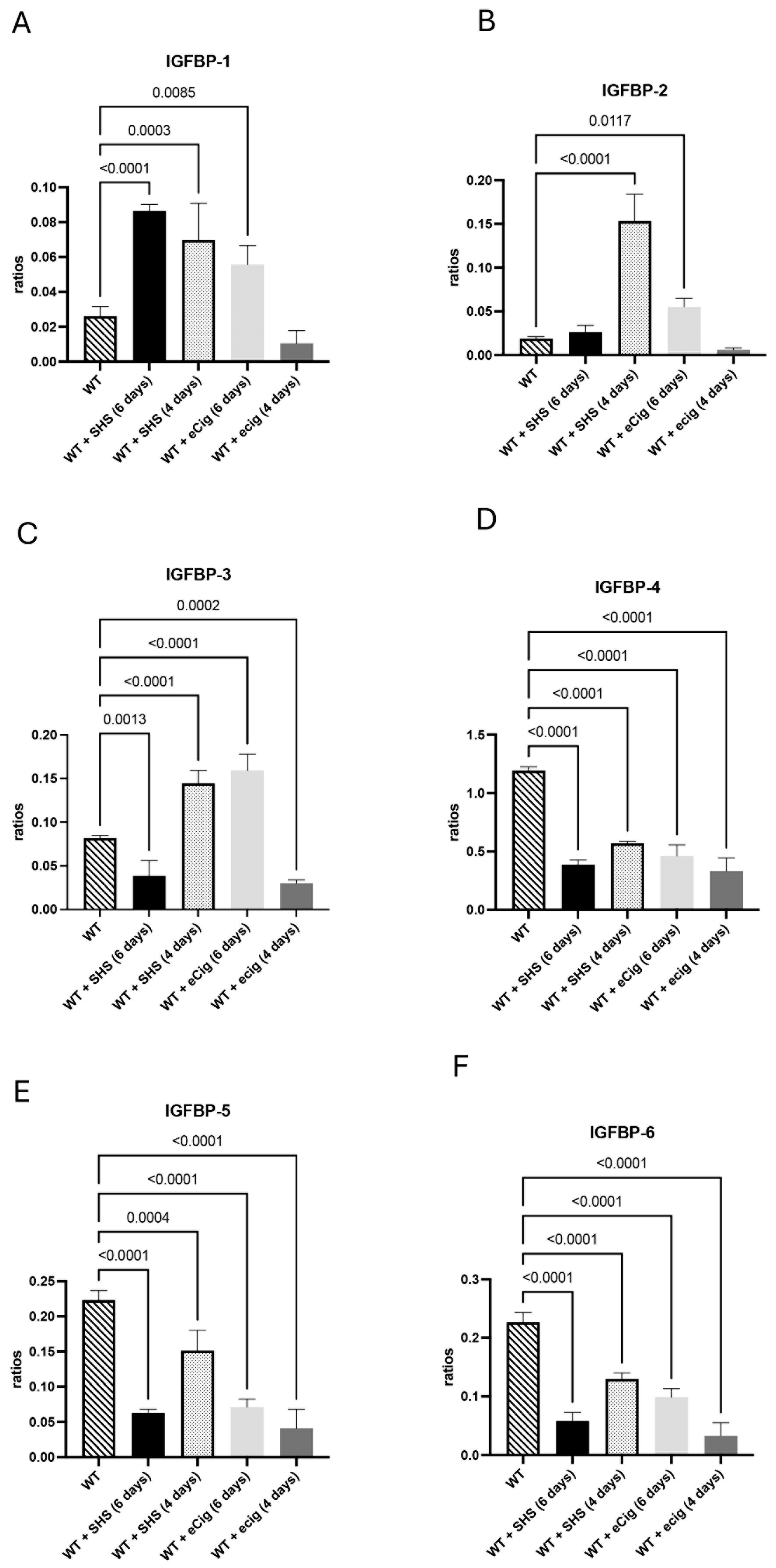
3.3. IGFBP Family and Growth Regulation
3.4. Cell Cycle and Growth Inhibition Markers (p27 and IGF-1)
4. Discussion
4.1. Pro-Apoptotic Markers
4.2. Anti-Apoptotic Markers
4.3. IGFBP Family and Growth Regulation
4.4. Cell Cycle and Growth Inhibition Markers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Latest advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef]
- Chen, B.; Longtine, M.S.; Sadovsky, Y.; Nelson, D.M. Hypoxia downregulates p53 but induces apoptosis and enhances expression of BAD in cultures of human syncytiotrophoblasts. Am. J. Physiol. Cell Physiol. 2010, 299, C968–C976. [Google Scholar] [CrossRef]
- Gicquel, C.; Le Bouc, Y. Hormonal regulation of fetal growth. Horm. Res. 2006, 65 (Suppl. S3), 28–33. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M. Tobacco, and pregnancy: An overview of exposures and effects. Birth Defects Res. C Embryo Today 2008, 84, 1–15. [Google Scholar] [CrossRef]
- Holloway, A.C.; Salomon, A.; Soares, M.J.; Garnier, V.; Raha, S.; Sergent, F.; Nicholson, C.J.; Feige, J.J.; Benharouga, M.; Alfaidy, N. Characterization of the adverse effects of nicotine on placental development: In vivo and in vitro studies. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E443–E456. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.N.; Cooper, C.; Broberg, E.; Robertson, P.; Clarke, D.; Pickett, B.E.; Bikman, B.; Reynolds, P.R.; Arroyo, J.A. Different Lengths of Gestational Exposure to Secondhand Smoke or e-Cigarette Vapor Induce the Development of Placental Disease Symptoms. Cells 2024, 13, 1009. [Google Scholar] [CrossRef]
- Yu, J.; Duan, Y.; Lu, Q.; Chen, M.; Ning, F.; Ye, Y.; Lu, S.; Ou, D.; Sha, X.; Gan, X.; et al. Cytochrome c oxidase IV isoform 1 (COX4-1) regulates trophoblast cell proliferation, migration, and invasion via modulating mitochondrial function. Placenta 2024, 151, 48–58. [Google Scholar] [CrossRef]
- Roh, C.R.; Lee, J.W.; Kang, B.H.; Yang, S.H.; Kim, B.G.; Bae, D.S.; Kim, J.H.; Lee, J.H. Differential expressions of Fas and Fas ligand in human placenta. J. Korean Med. Sci. 2002, 17, 213–216. [Google Scholar] [CrossRef]
- Selvakumaran, M.; Lin, H.K.; Miyashita, T.; Wang, H.G.; Krajewski, S.; Reed, J.C.; Hoffman, B.; Liebermann, D. Immediate early up-regulation of bax expression by p53 but not TGF beta 1: A paradigm for distinct apoptotic pathways. Oncogene 1994, 9, 1791–1798. [Google Scholar]
- Biswas, D.D.; Martin, R.K.; Brown, L.N.; Mockenhaupt, K.; Gupta, A.S.; Surace, M.J.; Tharakan, A.; Yester, J.W.; Bhardwaj, R.; Conrad, D.H.; et al. Cellular inhibitor of apoptosis 2 (cIAP2) restricts neuroinflammation during experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2022, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Concannon, C.G.; Gorman, A.M.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Molvarec, A.; Tamasi, L.; Losonczy, G.; Madach, K.; Prohaszka, Z.; Rigo, J., Jr. Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress. Chaperones 2010, 15, 237–247. [Google Scholar] [CrossRef]
- Muschol-Steinmetz, C.; Friemel, A.; Kreis, N.N.; Reinhard, J.; Yuan, J.; Louwen, F. Function of survivin in trophoblastic cells of the placenta. PLoS ONE 2013, 8, e73337. [Google Scholar] [CrossRef]
- Mejia, J.F.; Hirschi, K.M.; Tsai, K.Y.F.; Long, M.G.; Tullis, B.C.; Bitter, E.E.K.; Bikman, B.T.; Reynolds, P.R.; Arroyo, J.A. Differential placental ceramide levels during gestational diabetes mellitus (GDM). Reprod. Biol. Endocrinol. 2019, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.L.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; Corey, K.E.; Chung, R.T.; Lee, H.; Kleiner, D.E.; et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2021, 106, e520–e533. [Google Scholar] [CrossRef]
- Unek, G.; Ozmen, A.; Mendilcioglu, I.; Simsek, M.; Korgun, E.T. The expression of cell cycle-related proteins PCNA, Ki67, p27 and p57 in normal and preeclamptic human placentas. Tissue Cell 2014, 46, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Gratton, R.J.; Asano, H.; Han, V.K. The regional expression of insulin-like growth factor II (IGF-II) and insulin-like growth factor binding protein-1 (IGFBP-1) in the placentae of women with pre-eclampsia. Placenta 2002, 23, 303–310. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- DiFederico, E.; Genbacev, O.; Fisher, S.J. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am. J. Pathol. 1999, 155, 293–301. [Google Scholar] [CrossRef]
- Dekker, G.A.; Sibai, B.M. Early detection of preeclampsia. Am. J. Obstet. Gynecol. 1991, 165, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Mor, G. The Role of Apoptosis in the Regulation of Trophoblast Survival and Differentiation during Pregnancy. Endocr. Rev. 2005, 26, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Hagymasi, A.T.; Dempsey, J.P.; Srivastava, P.K. Heat-Shock Proteins. Curr. Protoc. 2022, 2, e592. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef]
- Talia, C.; Connolly, L.; Fowler, P.A. The insulin-like growth factor system: A target for endocrine disruptors? Environ. Int. 2021, 147, 106311. [Google Scholar] [CrossRef]
- Abbastabar, M.; Kheyrollah, M.; Azizian, K.; Bagherlou, N.; Tehrani, S.S.; Maniati, M.; Karimian, A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: A double-edged sword protein. DNA Repair 2018, 69, 63–72. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, L.; Kirkham, M.N.; Shin, M.; Bikman, B.T.; Reynolds, P.R.; Arroyo, J.A. Impact of Secondhand Smoke and E-Cigarette Exposure on Placental Apoptotic and Growth-Regulatory Proteins in Mouse Pregnancy. Cells 2025, 14, 453. https://doi.org/10.3390/cells14060453
Beck L, Kirkham MN, Shin M, Bikman BT, Reynolds PR, Arroyo JA. Impact of Secondhand Smoke and E-Cigarette Exposure on Placental Apoptotic and Growth-Regulatory Proteins in Mouse Pregnancy. Cells. 2025; 14(6):453. https://doi.org/10.3390/cells14060453
Chicago/Turabian StyleBeck, Logan, Madison N. Kirkham, Marley Shin, Benjamin T. Bikman, Paul R. Reynolds, and Juan A. Arroyo. 2025. "Impact of Secondhand Smoke and E-Cigarette Exposure on Placental Apoptotic and Growth-Regulatory Proteins in Mouse Pregnancy" Cells 14, no. 6: 453. https://doi.org/10.3390/cells14060453
APA StyleBeck, L., Kirkham, M. N., Shin, M., Bikman, B. T., Reynolds, P. R., & Arroyo, J. A. (2025). Impact of Secondhand Smoke and E-Cigarette Exposure on Placental Apoptotic and Growth-Regulatory Proteins in Mouse Pregnancy. Cells, 14(6), 453. https://doi.org/10.3390/cells14060453








