The Recovery of Epidermal Proliferation Pattern in Human Skin Xenograft
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Skin Xenotransplantation
2.2. Primary Keratinocyte Isolation and Culture
2.3. Flow Cytometry
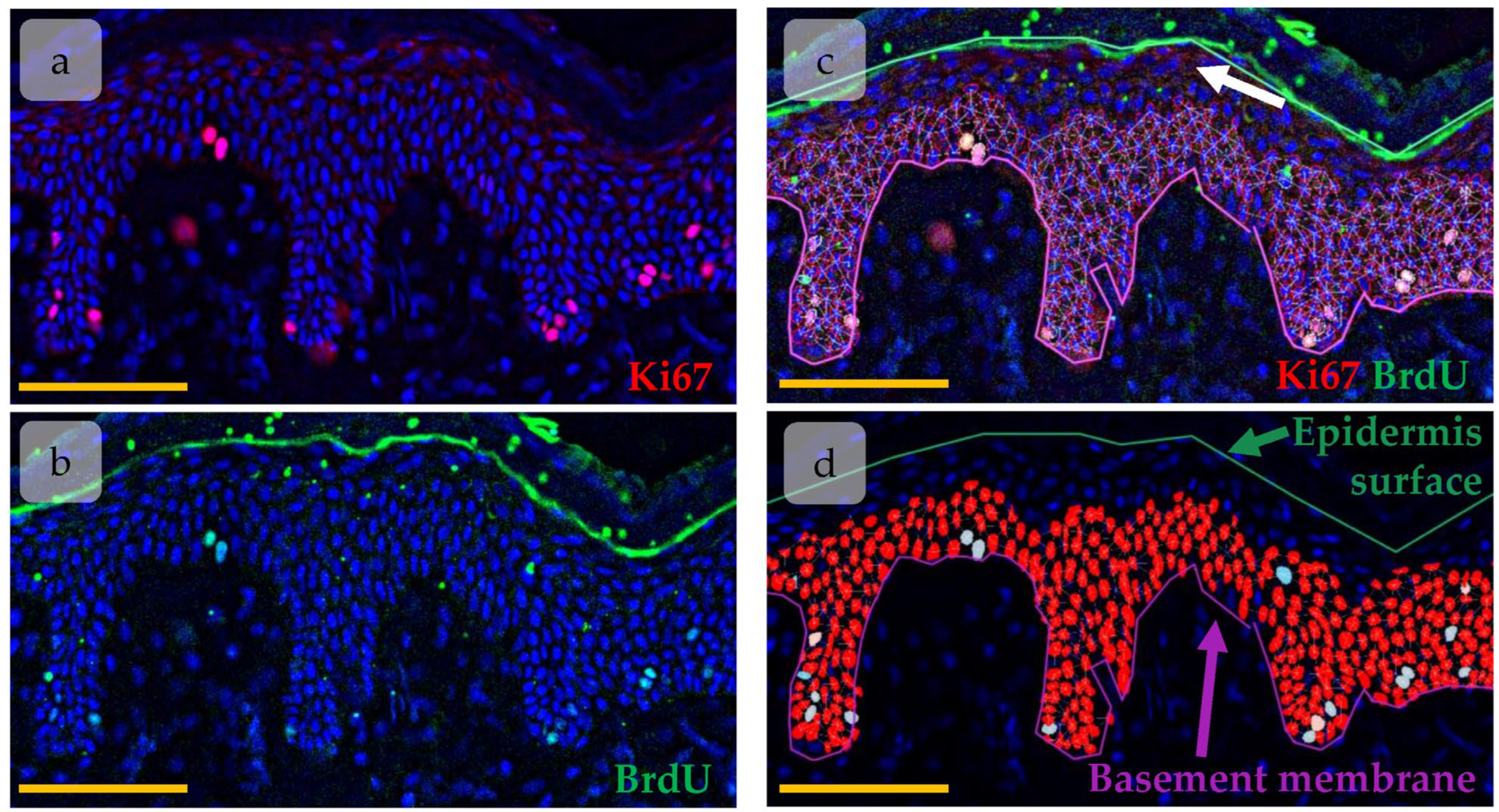
2.4. Immunohistochemical Staining and Imaging
2.5. Image Analysis
2.6. Statistical and Cluster Analysis
2.7. Single Cell Data Analysis
3. Results
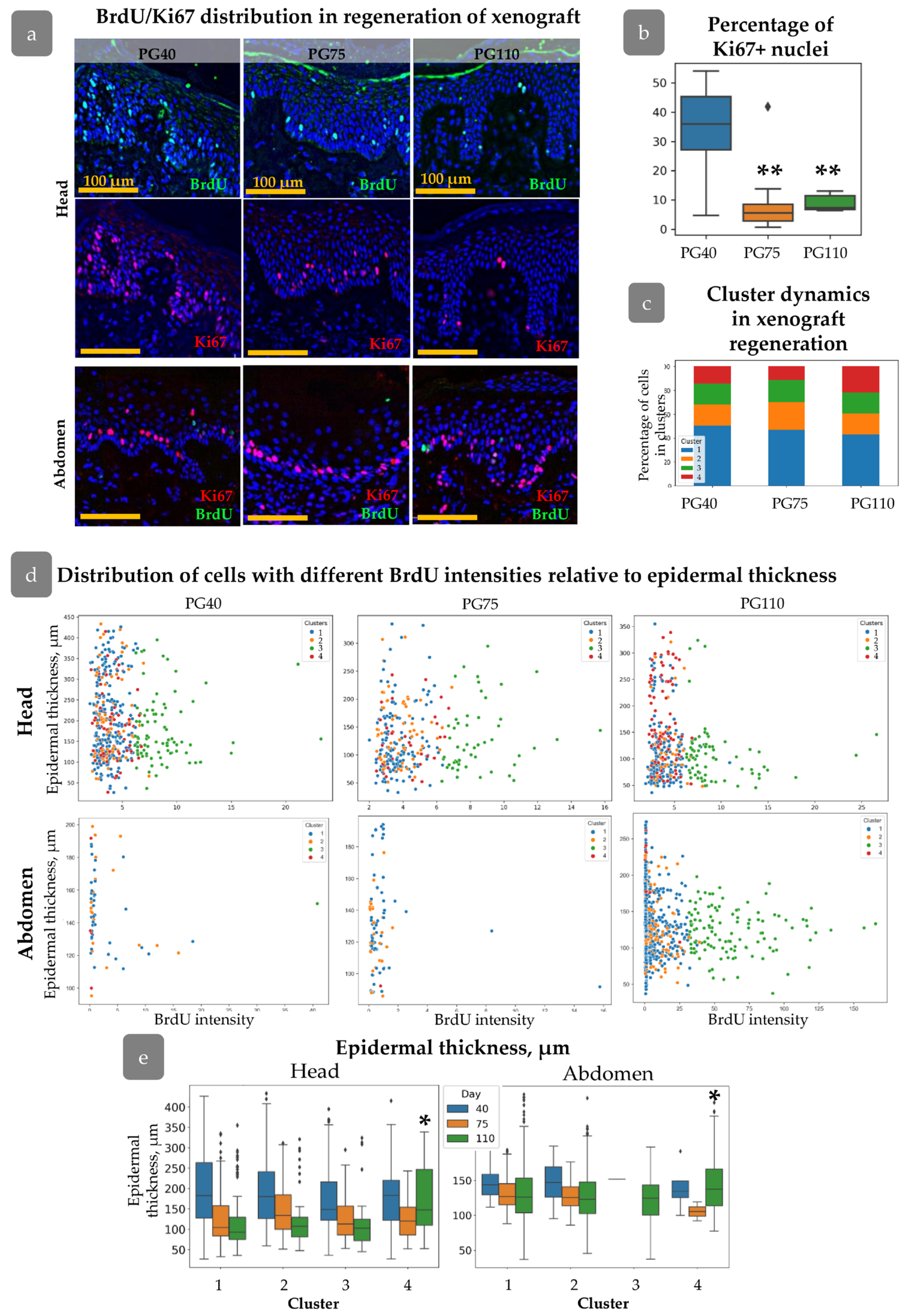
3.1. Regeneration of Human Skin Xenograft
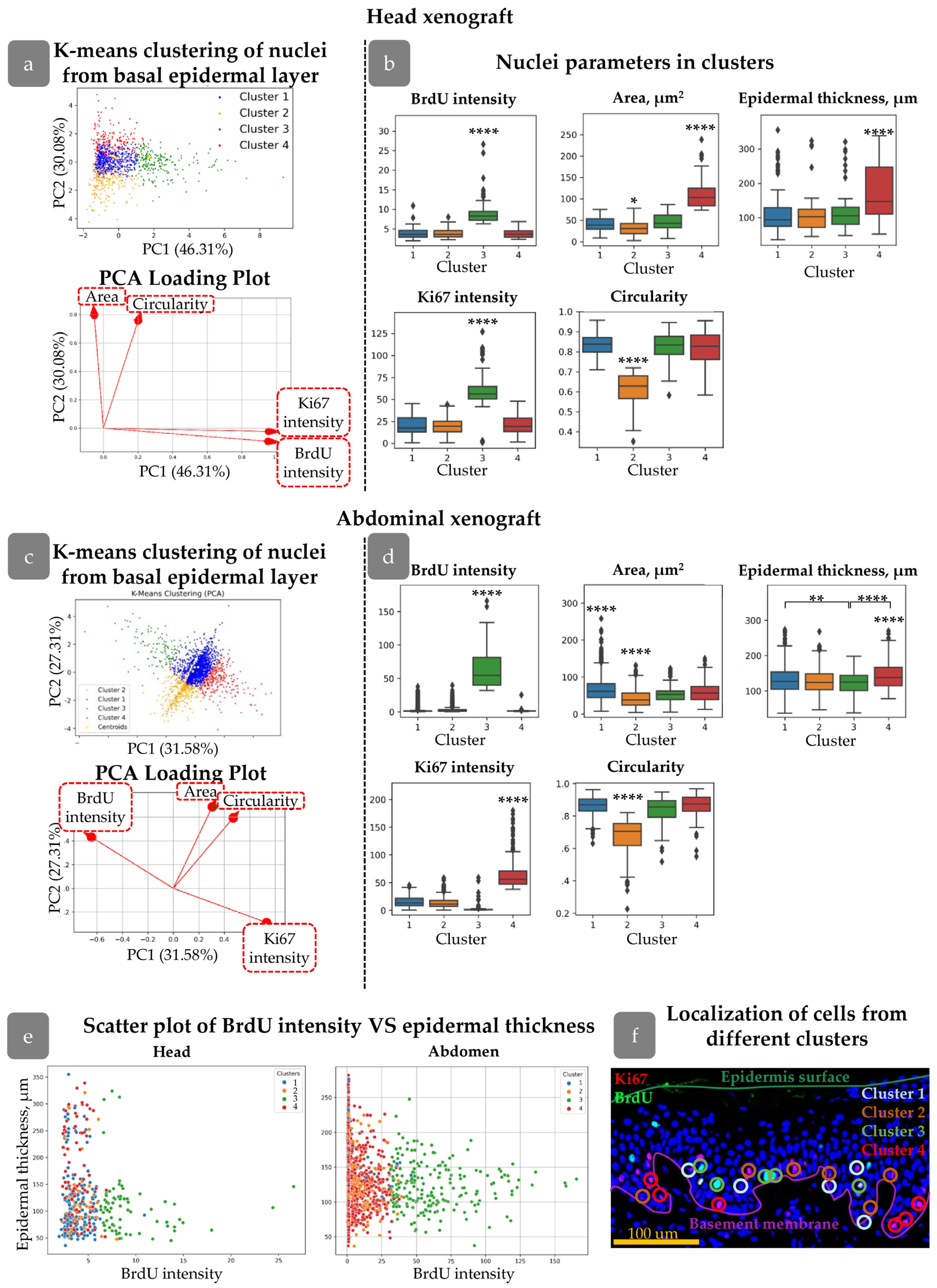
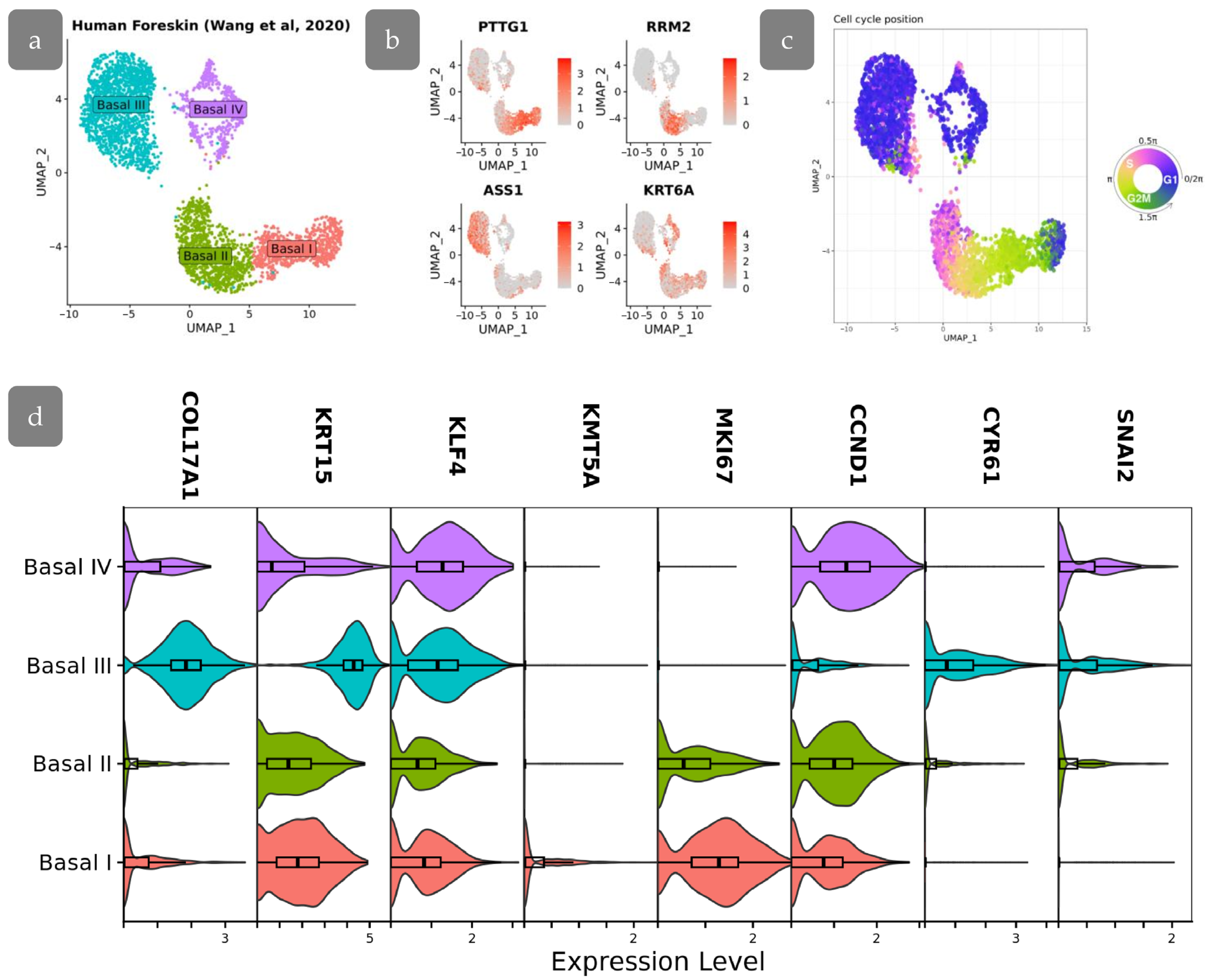
3.2. Clustering of Proliferative Cells in Epidermal Basal Layer
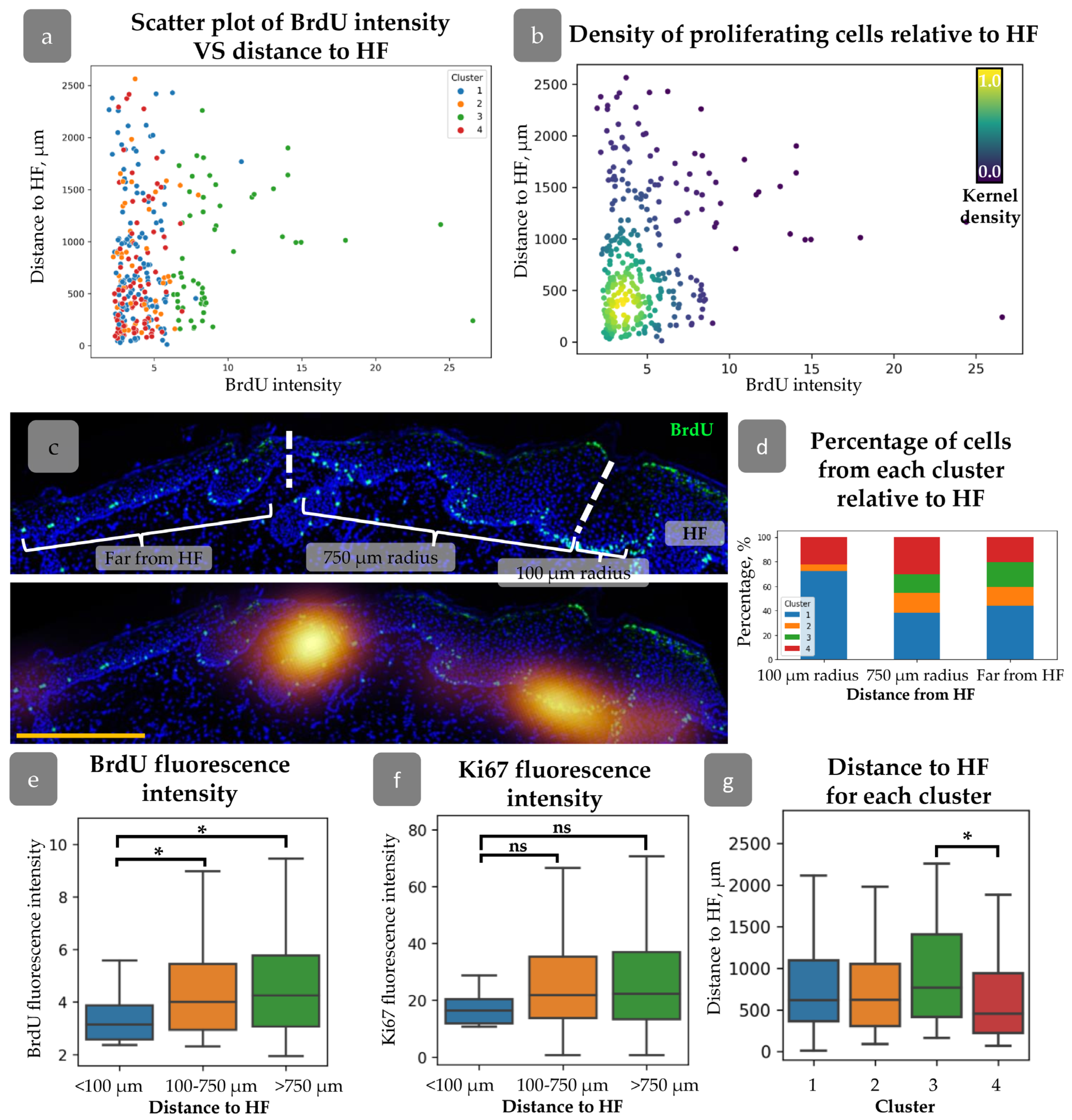
3.3. HFs Influence on Proliferating Cell Distribution
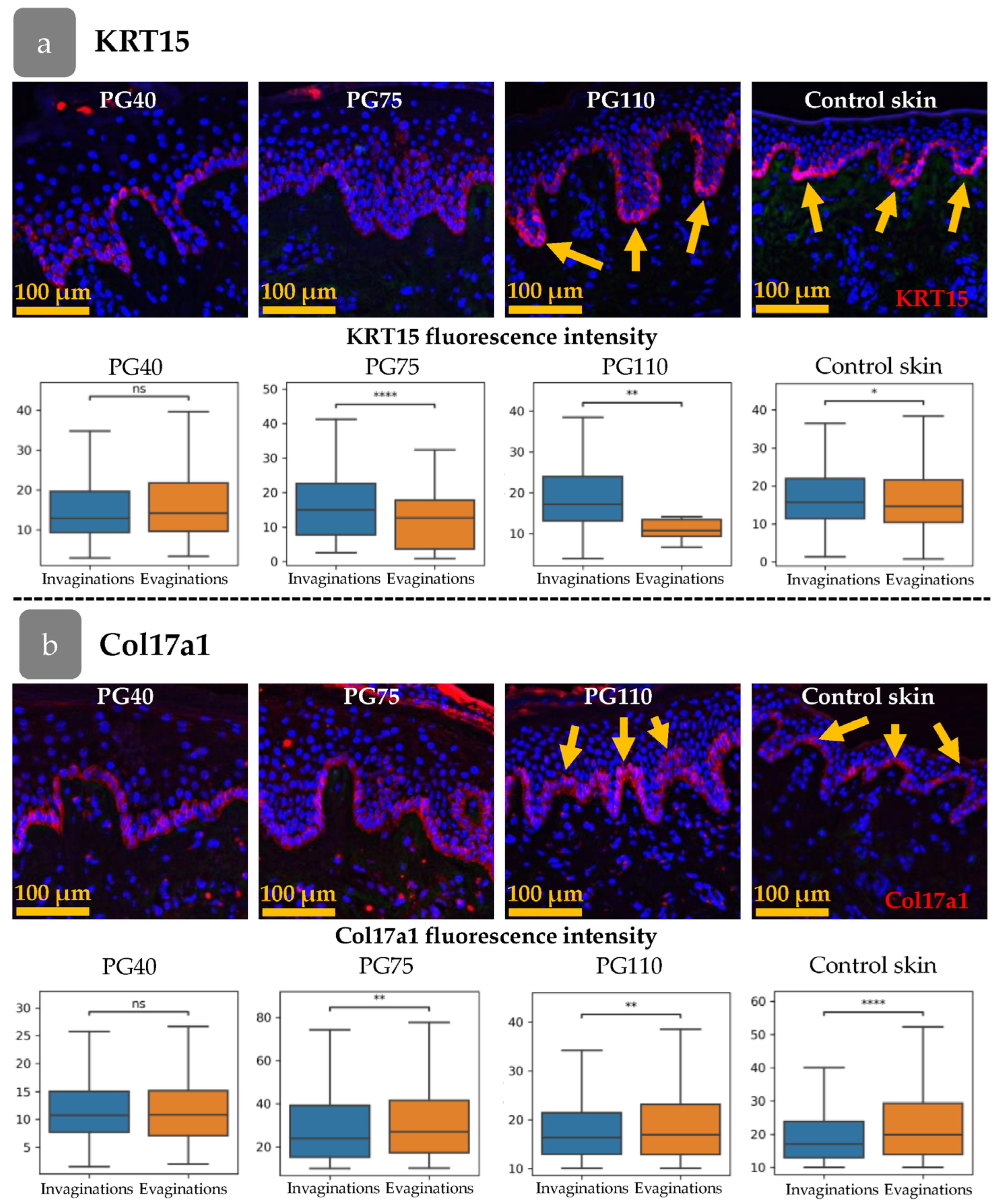
3.4. Xenograft Regeneration
3.5. Distribution of Cell Clusters During Xenograft Regeneration
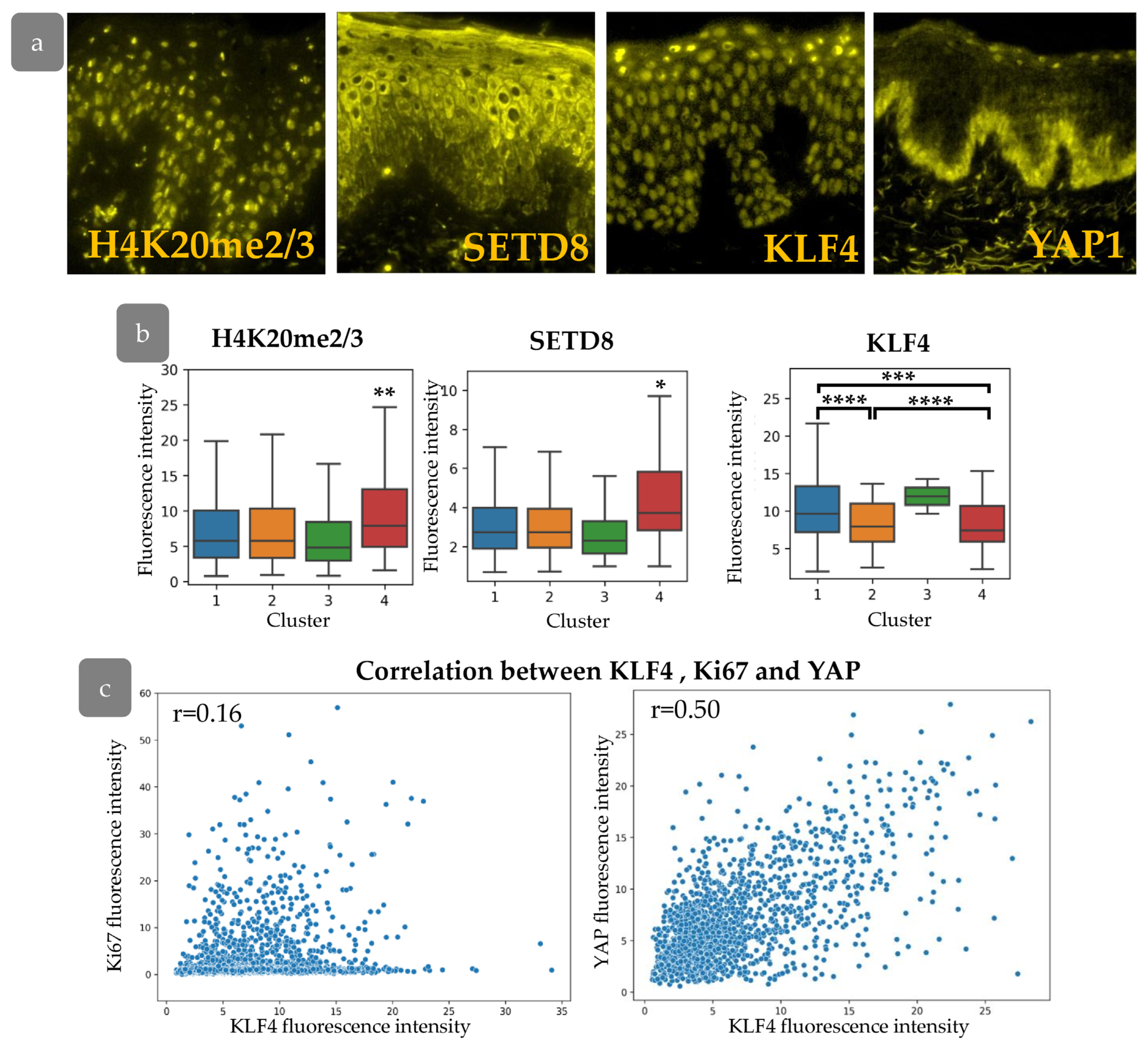
3.6. Cluster Definition
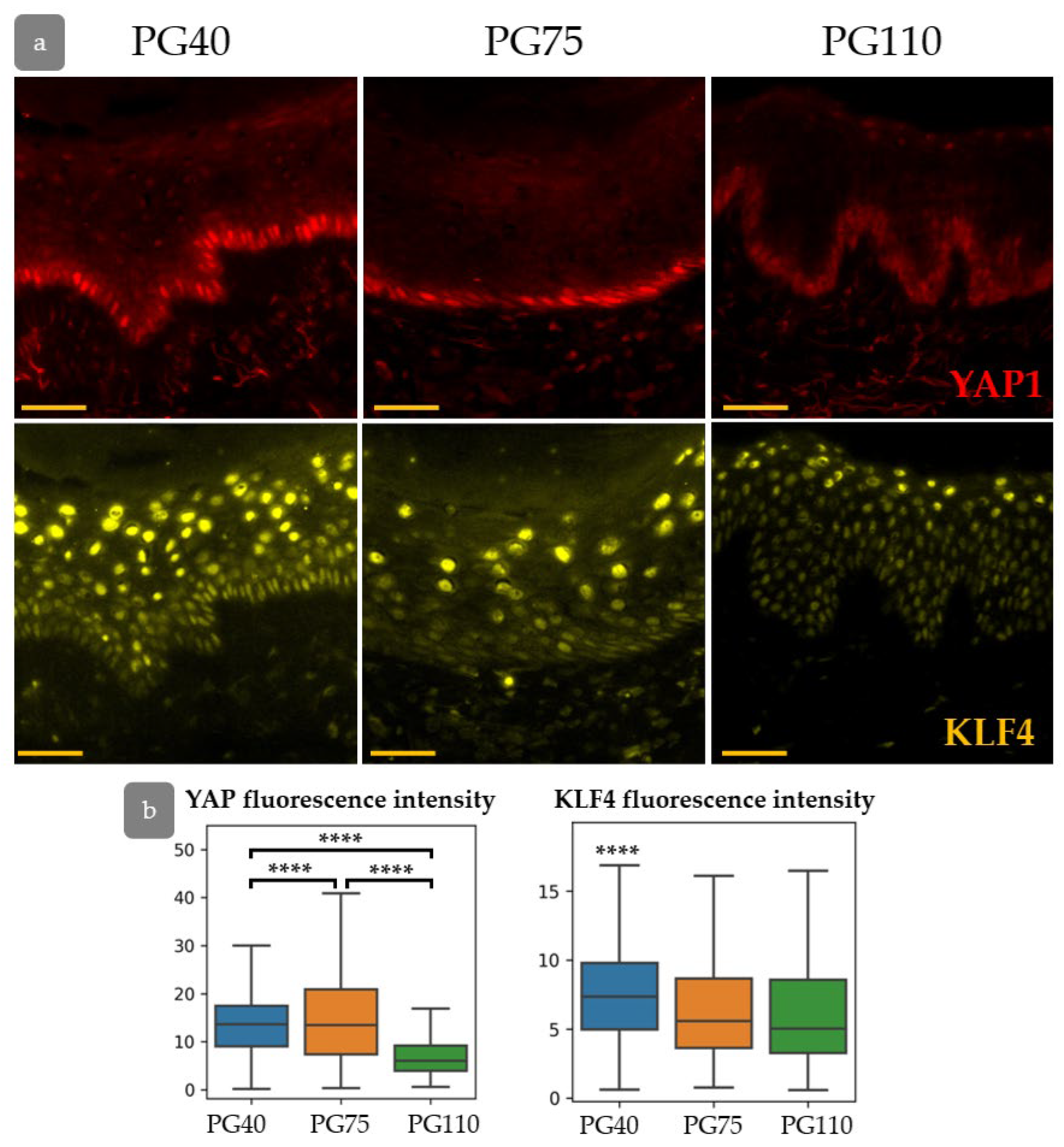
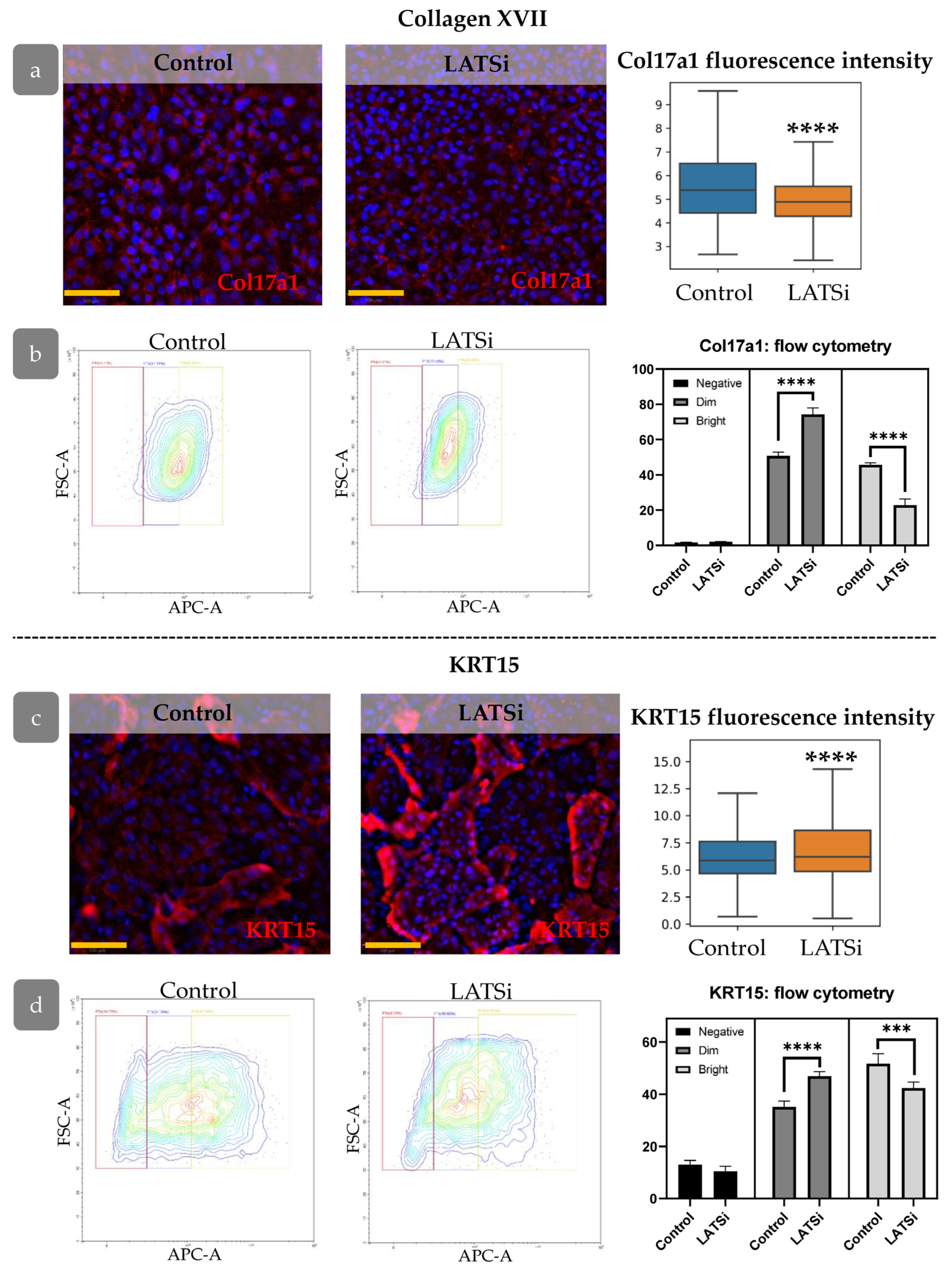
3.7. YAP Influence on Cell Proliferation Dynamics
3.8. Transcriptional Differences in Proliferating Cell Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potten, C.S. The Epidermal Proliferative Unit: The Possible Role of the Central Basal Cell. Cell Tissue Kinet 1974, 7, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-Retaining Cells Reside in the Bulge Area of Pilosebaceous Unit: Implications for Follicular Stem Cells, Hair Cycle, and Skin Carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.; Doupé, D.P.; Klein, A.M.; Winton, D.J.; Simons, B.D.; Jones, P.H. A Single Type of Progenitor Cell Maintains Normal Epidermis. Nature 2007, 446, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Doupé, D.P.; Klein, A.M.; Simons, B.D.; Jones, P.H. The Ordered Architecture of Murine Ear Epidermis Is Maintained by Progenitor Cells with Random Fate. Dev. Cell 2010, 18, 317–323. [Google Scholar] [CrossRef]
- Piedrafita, G.; Kostiou, V.; Wabik, A.; Colom, B.; Fernandez-Antoran, D.; Herms, A.; Murai, K.; Hall, B.A.; Jones, P.H. A Single-Progenitor Model as the Unifying Paradigm of Epidermal and Esophageal Epithelial Maintenance in Mice. Nat. Commun. 2020, 11, 1429. [Google Scholar] [CrossRef]
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohée, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct Contribution of Stem and Progenitor Cells to Epidermal Maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef]
- Sada, A.; Jacob, F.; Leung, E.; Wang, S.; White, B.S.; Shalloway, D.; Tumbar, T. Defining the Cellular Lineage Hierarchy in the Interfollicular Epidermis of Adult Skin. Nat. Cell Biol. 2016, 18, 619–631. [Google Scholar] [CrossRef]
- Ghuwalewala, S.; Lee, S.A.; Jiang, K.; Baidya, J.; Chovatiya, G.; Kaur, P.; Shalloway, D.; Tumbar, T. Binary Organization of Epidermal Basal Domains Highlights Robustness to Environmental Exposure. EMBO J. 2022, 41, e110488. [Google Scholar] [CrossRef]
- Barrandon, Y.; Green, H. Three Clonal Types of Keratinocyte with Different Capacities for Multiplication. Proc. Natl. Acad. Sci. USA 1987, 84, 2302–2306. [Google Scholar] [CrossRef]
- Hirose, T.; Kotoku, J.; Toki, F.; Nishimura, E.K.; Nanba, D. Label-Free Quality Control and Identification of Human Keratinocyte Stem Cells by Deep Learning-Based Automated Cell Tracking. Stem Cells 2021, 39, 1091–1100. [Google Scholar] [CrossRef]
- Schlüter, H.; Paquet-Fifield, S.; Gangatirkar, P.; Li, J.; Kaur, P. Functional Characterization of Quiescent Keratinocyte Stem Cells and Their Progeny Reveals a Hierarchical Organization in Human Skin Epidermis. Stem Cells 2011, 29, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Alhattab, D.; Jafar, H.; Alzubide, M.; Sharar, N.; Bdour, S.; Awidi, A. Differential Marker Expression between Keratinocyte Stem Cells and Their Progeny Generated from a Single Colony. Int. J. Mol. Sci. 2021, 22, 10810. [Google Scholar] [CrossRef]
- Webb, A.; Li, A.; Kaur, P. Location and Phenotype of Human Adult Keratinocyte Stem Cells of the Skin. Differentiation 2004, 72, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.T.; Kaur, P. Dermal Contributions to Human Interfollicular Epidermal Architecture and Self-Renewal. Int. J. Mol. Sci. 2015, 16, 28098–28107. [Google Scholar] [CrossRef]
- Wang, S.; Drummond, M.L.; Guerrero-Juarez, C.F.; Tarapore, E.; MacLean, A.L.; Stabell, A.R.; Wu, S.C.; Gutierrez, G.; That, B.T.; Benavente, C.A.; et al. Single Cell Transcriptomics of Human Epidermis Identifies Basal Stem Cell Transition States. Nat. Commun. 2020, 11, 4239. [Google Scholar] [CrossRef]
- Cherkashina, O.L.; Morgun, E.I.; Rippa, A.L.; Kosykh, A.V.; Alekhnovich, A.V.; Stoliarzh, A.B.; Terskikh, V.V.; Vorotelyak, E.A.; Kalabusheva, E.P. Blank Spots in the Map of Human Skin: The Challenge for Xenotransplantation. IJMS 2023, 24, 12769. [Google Scholar] [CrossRef] [PubMed]
- Kappes, U.; Schliemann-Willers, S.; Bankova, L.; Heinemann, C.; Fischer, T.; Ziemer, M.; Schubert, H.; Norgauer, J.; Fluhr, J.; Elsner, P. The Quality of Human Skin Xenografts on SCID Mice: A Noninvasive Bioengineering Approach. Br. J. Dermatol. 2004, 151, 971–976. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Shen, A.H.; Griffin, M.; Mascharak, S.; Adem, S.; Deleon, N.M.D.; Ngaage, L.M.; Longaker, M.T.; Wan, D.C.; Lorenz, H.P. A Novel Xenograft Model Demonstrates Human Fibroblast Behavior During Skin Wound Repair and Fibrosis. Adv. Wound Care 2022, 11, 455–465. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Li, K.; Jiang, X.; Zhang, J.; Liu, H. A Highly Simulated Scar Model Developed by Grafting Human Thin Split-Thickness Skin on Back of Nude Mouse: The Remodeling Process, Histological Characteristics of Scars. Biochem. Biophys. Res. Commun. 2020, 526, 744–750. [Google Scholar] [CrossRef]
- Lloyd, M.G.; Smith, N.A.; Tighe, M.; Travis, K.L.; Liu, D.; Upadhyaya, P.K.; Kinchington, P.R.; Chan, G.C.; Moffat, J.F. A Novel Human Skin Tissue Model To Study Varicella-Zoster Virus and Human Cytomegalovirus. J. Virol. 2020, 94, 1082–1102. [Google Scholar] [CrossRef]
- Maldonado, A.A.; Cristóbal, L.; Martín-López, J.; Mallén, M.; García-Honduvilla, N.; Buján, J. A Novel Model of Human Skin Pressure Ulcers in Mice. PLoS ONE 2014, 9, e109003. [Google Scholar] [CrossRef]
- Moffat, J.F.; Stein, M.D.; Kaneshima, H.; Arvin, A.M. Tropism of Varicella-Zoster Virus for Human CD4+ and CD8+ T Lymphocytes and Epidermal Cells in SCID-Hu Mice. J. Virol. 1995, 69, 5236. [Google Scholar] [CrossRef]
- Yang, D.Y.; Li, S.R.; Wu, J.L.; Chen, Y.Q.; Li, G.; Bi, S.; Dai, X. Establishment of a Hypertrophic Scar Model by Transplanting Full-Thickness Human Skin Grafts onto the Backs of Nude Mice. Plast. Reconstr. Surg. 2007, 119, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, M.; Kwan, P.; Ding, J.; Anderson, C.C.; Honardoust, D.; Goekjian, S.; Tredget, E.E. A Nude Mouse Model of Hypertrophic Scar Shows Morphologic and Histologic Characteristics of Human Hypertrophic Scar. Wound Repair Regen. 2013, 21, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Caspary, F.; Elliott, G.; Navé, B.T.; Verzaal, P.; Rohrbach, M.; Das, P.K.; Nagelkerken, L.; Nieland, J.D. A New Therapeutic Approach to Treat Psoriasis by Inhibition of Fatty Acid Oxidation by Etomoxir. Br. J. Dermatol. 2005, 153, 937–944. [Google Scholar] [CrossRef]
- Chang, T.; Sun, L.; Wang, Y.; Wang, D.; Li, W.; Li, C.; Gao, T.; Liu, Y.; Wang, G. Inhibition of Keratin 17 Expression with Antisense and RNAi Strategies: Exploring Novel Therapy for Psoriasis. Exp. Dermatol. 2011, 20, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Genderen, J.; Wolthuis, O.L.; Ariens, A.T.; Ericson, A.C.; Datema, R. The Effects of Topical Foscarnet in a New Model of Herpes Simplex Skin Infection. J. Antimicrob. Chemother. 1987, 20, 547–556. [Google Scholar] [CrossRef]
- Sasaki-Iwaoka, H.; Taguchi, K.; Okada, Y.; Imamura, E.; Kubo, S.; Furukawa, S.; Morokata, T. AS2762900-00, a Potent Anti-Human IL-23 Receptor Monoclonal Antibody, Prevents Epidermal Hyperplasia in a Psoriatic Human Skin Xenograft Model. Eur. J. Pharmacol. 2019, 843, 190–198. [Google Scholar] [CrossRef]
- Ghazizadeh, S.; Taichman, L.B. Organization of Stem Cells and Their Progeny in Human Epidermis. J. Investig. Dermatol. 2005, 124, 367–372. [Google Scholar] [CrossRef]
- Cherkashina, O.L.; Kosykh, A.V.; Morgun, E.I.; Rippa, A.L.; Tsitrina, A.A.; Oettinger, A.P.; Chermnykh, E.S.; Kalabusheva, E.P.; Vorotelyak, E.A. Modeling the Regeneration of Human Skin and Hair Follicles in a Full-Thickness Xenograft. Russ. J. Dev. Biol. 2022, 53, 217–220. [Google Scholar] [CrossRef]
- Kalabusheva, E.P.; Rippa, A.L.; Tsitrina, A.A.; Pinto, A.; Terskikh, A.V.; Chermnykh, E.S.; Vorotelyak, E.A. Xenotransplantation of a Full-Layer Human Skin Strip as a Model for Studying Skin Regeneration and the Hair Follicle Cycle. Russ. J. Dev. Biol. 2020, 52, 42–52. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-Convex Polygons. arXiv 2018, arXiv:1806.03535. [Google Scholar]
- Zheng, S.C.; Stein-O’Brien, G.; Augustin, J.J.; Slosberg, J.; Carosso, G.A.; Winer, B.; Shin, G.; Bjornsson, H.T.; Goff, L.A.; Hansen, K.D. Universal Prediction of Cell-Cycle Position Using Transfer Learning. Genome Biol. 2022, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.; Neufeld, Z.; Cerone, L.; Wong, H.Y.; Hodgson, S.; Livet, J.; Khosrotehrani, K. Bimodal Behaviour of Interfollicular Epidermal Progenitors Regulated by Hair Follicle Position and Cycling. EMBO J. 2016, 35, 2658–2670. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Fisher, A.G.; Watt, F.M. Epidermal Stem Cells Are Defined by Global Histone Modifications That Are Altered by Myc-Induced Differentiation. PLoS ONE 2007, 2, e763. [Google Scholar] [CrossRef]
- Driskell, I.; Oda, H.; Blanco, S.; Nascimento, E.; Humphreys, P.; Frye, M. The Histone Methyltransferase Setd8 Acts in Concert with C-Myc and Is Required to Maintain Skin. EMBO J. 2012, 31, 616–629. [Google Scholar] [CrossRef]
- Takawa, M.; Cho, H.-S.; Hayami, S.; Toyokawa, G.; Kogure, M.; Yamane, Y.; Iwai, Y.; Maejima, K.; Ueda, K.; Masuda, A.; et al. Histone Lysine Methyltransferase SETD8 Promotes Carcinogenesis by Deregulating PCNA Expression. Cancer Res. 2012, 72, 3217–3227. [Google Scholar] [CrossRef]
- Fortunel, N.O.; Chadli, L.; Coutier, J.; Lemaître, G.; Auvré, F.; Domingues, S.; Bouissou-Cadio, E.; Vaigot, P.; Cavallero, S.; Deleuze, J.-F.; et al. KLF4 Inhibition Promotes the Expansion of Keratinocyte Precursors from Adult Human Skin and of Embryonic-Stem-Cell-Derived Keratinocytes. Nat. Biomed. Eng. 2019, 3, 985–997. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Yu, F.; Yu, T.; Liu, C.; Huang, S.; Wang, T.C.; Ai, W. Deficiency of the Kruppel-like Factor KLF4 Correlates with Increased Cell Proliferation and Enhanced Skin Tumorigenesis. Carcinogenesis 2012, 33, 1239–1246. [Google Scholar] [CrossRef]
- Yuan, Y.; Park, J.; Feng, A.; Awasthi, P.; Wang, Z.; Chen, Q.; Iglesias-Bartolome, R. YAP1/TAZ-TEAD Transcriptional Networks Maintain Skin Homeostasis by Regulating Cell Proliferation and Limiting KLF4 Activity. Nat. Commun. 2020, 11, 1472. [Google Scholar] [CrossRef]
- Akladios, B.; Mendoza-Reinoso, V.; Samuel, M.S.; Hardeman, E.C.; Khosrotehrani, K.; Key, B.; Beverdam, A. Epidermal YAP2-5SA-ΔC Drives β-Catenin Activation to Promote Keratinocyte Proliferation in Mouse Skin In Vivo. J. Investig. Dermatol. 2017, 137, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 Acts Downstream of α-Catenin to Control Epidermal Proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Reinoso, V.; Beverdam, A. Epidermal YAP Activity Drives Canonical WNT16/β-Catenin Signaling to Promote Keratinocyte Proliferation in Vitro and in the Murine Skin. Stem Cell Res. 2018, 29, 15–23. [Google Scholar] [CrossRef]
- Walko, G.; Woodhouse, S.; Pisco, A.O.; Rognoni, E.; Liakath-Ali, K.; Lichtenberger, B.M.; Mishra, A.; Telerman, S.B.; Viswanathan, P.; Logtenberg, M.; et al. A Genome-Wide Screen Identifies YAP/WBP2 Interplay Conferring Growth Advantage on Human Epidermal Stem Cells. Nat. Commun. 2017, 8, 14744. [Google Scholar] [CrossRef]
- Yuan, J.P.; Wang, L.W.; Qu, A.P.; Chen, J.M.; Xiang, Q.M.; Chen, C.; Sun, S.-R.; Pang, D.-W.; Liu, J.; Li, Y. Quantum Dots-Based Quantitative and in Situ Multiple Imaging on Ki67 and Cytokeratin to Improve Ki67 Assessment in Breast Cancer. PLoS ONE 2015, 10, e0122734. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 Is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Shen, Z.; Sun, L.; Liu, Z.; Li, M.; Cao, Y.; Han, L.; Wang, J.; Wu, X.; Sang, S. Rete Ridges: Morphogenesis, Function, Regulation, and Reconstruction. Acta Biomater. 2023, 155, 19–34. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, Z.; Sun, L.; Li, M.; Han, L.; Wang, J.; Wu, X.; Sang, S. Constructing Epidermal Rete Ridges Using Composite Hydrogel to Enhance Multiple Signaling Pathways for the Maintenance of Epidermal Stem Cell Niche. Acta Biomater. 2023, 169, 273–288. [Google Scholar] [CrossRef]
- Jang, I.; Kim, J.-H.; Kim, M.; Andre Marinho, P.; Nho, Y.; Kang, S.; Yun, W.-S.; Shim, J.-H.; Jin, S. Three-Dimensional Bioprinting of in Vitro Full-Thickness Skin Model Incorporating the Rete Ridge Structure. IJB 2024, 10, 3961. [Google Scholar] [CrossRef]
- Gdula, M.R.; Poterlowicz, K.; Mardaryev, A.N.; Sharov, A.A.; Peng, Y.; Fessing, M.Y.; Botchkarev, V.A. Remodeling of Three-Dimensional Organization of the Nucleus during Terminal Keratinocyte Differentiation in the Epidermis. J. Investig. Dermatol. 2013, 133, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Kim, M.-S.; Chung, B.M.; Leahy, D.J.; Coulombe, P.A. Structural Basis for Heteromeric Assembly and Perinuclear Organization of Keratin Filaments. Nat. Struct. Mol. Biol. 2012, 19, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ai, W. Function of KLF4 in Stem Cell Biology. In Pluripotent Stem Cells; Bhartiya, D., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1192-4. [Google Scholar]
- Kaufmann, R.; Mielke, V.; Reimann, J.; Klein, C.E.; Sterry, W. Cellular and Molecular Composition of Human Skin in Long-term Xenografts on SCID Mice. Exp. Dermatol. 1993, 2, 209–216. [Google Scholar] [CrossRef]
- Viswanathan, P.; Guvendiren, M.; Chua, W.; Telerman, S.B.; Liakath-Ali, K.; Burdick, J.A.; Watt, F.M. Mimicking the Topography of the Epidermal–Dermal Interface with Elastomer Substrates. Integr. Biol. 2016, 8, 21–29. [Google Scholar] [CrossRef]
- Pankratova, M.D.; Riabinin, A.A.; Butova, E.A.; Selivanovskiy, A.V.; Morgun, E.I.; Ulianov, S.V.; Vorotelyak, E.A.; Kalabusheva, E.P. YAP/TAZ Signalling Controls Epidermal Keratinocyte Fate. IJMS 2024, 25, 12903. [Google Scholar] [CrossRef]
- Grzelak, E.M.; Elshan, N.G.R.D.; Shao, S.; Bulos, M.L.; Joseph, S.B.; Chatterjee, A.K.; Chen, J.J.; Nguyên-Trân, V.; Schultz, P.G.; Bollong, M.J. Pharmacological YAP Activation Promotes Regenerative Repair of Cutaneous Wounds. Proc. Natl. Acad. Sci. USA 2023, 120, e2305085120. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dong, Y.; Xu, X.; Shen, Y.; Huang, Z.; Yu, Y.; Liu, Z.; Gong, W.; Zhang, S.; Zheng, Y.; et al. Fibroblast Growth Factor 10 Protects against UVB-induced Skin Injury by Activating the ERK/YAP Signalling Pathway. Cell Prolif. 2022, 55, e13315. [Google Scholar] [CrossRef]
- Zhang, H.; Pasolli, H.A.; Fuchs, E. Yes-Associated Protein (YAP) Transcriptional Coactivator Functions in Balancing Growth and Differentiation in Skin. Proc. Natl. Acad. Sci. USA 2011, 108, 2270–2275. [Google Scholar] [CrossRef]
- Pibiri, M.; Simbula, G. Role of the Hippo Pathway in Liver Regeneration and Repair: Recent Advances. Inflamm. Regen. 2022, 42, 59. [Google Scholar] [CrossRef]
- Leng, J.; Wang, C.; Liang, Z.; Qiu, F.; Zhang, S.; Yang, Y. An Updated Review of YAP: A Promising Therapeutic Target against Cardiac Aging? Int. J. Biol. Macromol. 2024, 254, 127670. [Google Scholar] [CrossRef]
- Xue, Y.; Lyu, C.; Taylor, A.; Van Ee, A.; Kiemen, A.; Choi, Y.; Khavanian, N.; Henn, D.; Lee, C.; Hwang, L.; et al. Mechanical Tension Mobilizes Lgr6+ Epidermal Stem Cells to Drive Skin Growth. Sci. Adv. 2022, 8, eabl8698. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Mistiaen, Z.; Elbediwy, A.; Vanyai, H.; Cotton, J.; Stamp, G.; Nye, E.; Spencer-Dene, B.; Thomas, G.J.; Mao, J.; Thompson, B. YAP Drives Cutaneous Squamous Cell Carcinoma Formation and Progression. eLife 2018, 7, e33304. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Silvis, M.R.; Honaker, Y.; Lien, W.-H.; Arron, S.T.; Vasioukhin, V. αE-Catenin Inhibits a Src–YAP1 Oncogenic Module That Couples Tyrosine Kinases and the Effector of Hippo Signaling Pathway. Genes Dev. 2016, 30, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Ikeda, K.; Fujishiro, M.; Yoshida, Y.; Hirai, T.; Tsushima, H.; Miyashita, T.; Morimoto, S.; Suga, Y.; Takamori, K.; et al. Connective Tissue Growth Factor Neutralization Aggravates the Psoriasis Skin Lesion: The Analysis of Psoriasis Model Mice and Patients. Ann. Dermatol. 2018, 30, 47. [Google Scholar] [CrossRef]
- Kiwanuka, E.; Hackl, F.; Caterson, E.J.; Junker, J.P.E.; Gerdin, B.; Nowinski, D.; Eriksson, E. Connective Tissue Growth Factor Is Expressed by Hyperproliferative Keratinocytes during Reepithelialization of Full Thickness Wounds. J. Am. Coll. Surg. 2011, 213, S90. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhai, T.; Li, H.; Li, H.; Huo, R.; Shen, B.; Wang, B.; Chen, X.; Li, N.; et al. CCN1 Promotes IL-1β Production in Keratinocytes by Activating P38 MAPK Signaling in Psoriasis. Sci. Rep. 2017, 7, 43310. [Google Scholar] [CrossRef]
- Shehata, W.A.; Maraee, A.H.; Abdo, E.E.; Hemida, A.S. Role of CYR61 in Psoriatic Lesional and Perilesional Skin: A Clinical and Immunohistochemical Study. J. Cosmet. Dermatol. 2021, 20, 2981–2988. [Google Scholar] [CrossRef]
- Ou, L.; Shi, Y.; Dong, W.; Liu, C.; Schmidt, T.J.; Nagarkatti, P.; Nagarkatti, M.; Fan, D.; Ai, W. Kruppel-Like Factor KLF4 Facilitates Cutaneous Wound Healing by Promoting Fibrocyte Generation from Myeloid-Derived Suppressor Cells. J. Investig. Dermatol. 2015, 135, 1425–1434. [Google Scholar] [CrossRef]
- Xu, D.; Chen, P.; Zheng, P.; Yin, F.; Cheng, Q.; Zhou, Z.; Xie, H.; Li, J.; Ni, J.; Wang, Y.; et al. KLF4 Initiates Sustained YAP Activation to Promote Renal Fibrosis in Mice after Ischemia-Reperfusion Kidney Injury. Acta Pharmacol. Sin. 2021, 42, 436–450. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Malara, M.M.; Baumann, M.E.; McFarland, K.L.; Supp, D.M.; Powell, H.M. Laser Micropatterning Promotes Rete Ridge Formation and Enhanced Engineered Skin Strength without Increased Inflammation. Bioengineering 2023, 10, 861. [Google Scholar] [CrossRef]
- Giangreco, A.; Goldie, S.J.; Failla, V.; Saintigny, G.; Watt, F.M. Human Skin Aging Is Associated with Reduced Expression of the Stem Cell Markers Β1 Integrin and MCSP. J. Investig. Dermatol. 2010, 130, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Liu, Y.; Yang, Z.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem Cells in the Hair Follicle Bulge Contribute to Wound Repair but Not to Homeostasis of the Epidermis. Nat. Med. 2005, 11, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Guerrero-Juarez, C.F.; Ito, M.; Li, Y.R.; Dedhia, P.H.; Zheng, Y.; Shao, M.; Gay, D.L.; Ramos, R.; Hsi, T.-C.; et al. Regeneration of Fat Cells from Myofibroblasts during Wound Healing. Science 2017, 355, 748–752. [Google Scholar] [CrossRef]
- Nuutila, K. Hair Follicle Transplantation for Wound Repair. Adv. Wound Care 2021, 10, 153–163. [Google Scholar] [CrossRef]
- Plotczyk, M.; Jiménez, F.; Limbu, S.; Boyle, C.J.; Ovia, J.; Almquist, B.D.; Higgins, C.A. Anagen Hair Follicles Transplanted into Mature Human Scars Remodel Fibrotic Tissue. NPJ Regen. Med. 2023, 8, 1. [Google Scholar] [CrossRef]
- Jia, J.; Li, C.; Yang, J.; Wang, X.; Li, R.; Luo, S.; Li, Z.; Liu, J.; Liu, Z.; Zheng, Y. Yes-Associated Protein Promotes the Abnormal Proliferation of Psoriatic Keratinocytes via an Amphiregulin Dependent Pathway. Sci. Rep. 2018, 8, 14513. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, N.; Zheng, Y.; Mo, X.; Zhang, Y.; Ye, S.; Liu, J.; Yan, F.; Li, H.; Chen, D. RAS-association Domain Family 1A Regulates the Abnormal Cell Proliferation in Psoriasis via Inhibition of Yes-associated Protein. J. Cell. Mol. Medi 2021, 25, 5070–5081. [Google Scholar] [CrossRef]
- Okamoto, K.; Ando, T.; Izumi, H.; Kobayashi, S.S.; Shintani, T.; Gutkind, J.S.; Yanamoto, S.; Miyauchi, M.; Kajiya, M. AXL Activates YAP through the EGFR-LATS1/2 Axis and Confers Resistance to EGFR-Targeted Drugs in Head and Neck Squamous Cell Carcinoma. Oncogene 2023, 42, 2869–2877. [Google Scholar] [CrossRef]
- Mickle, M.; Adhikary, G.; Shrestha, S.; Xu, W.; Eckert, R.L. VGLL4 Inhibits YAP1/TEAD Signaling to Suppress the Epidermal Squamous Cell Carcinoma Cancer Phenotype. Mol. Carcinog. 2021, 60, 497–507. [Google Scholar] [CrossRef]
- Maglic, D.; Schlegelmilch, K.; Dost, A.F.; Panero, R.; Dill, M.T.; Calogero, R.A.; Camargo, F.D. YAP-TEAD Signaling Promotes Basal Cell Carcinoma Development via a c-JUN/AP1 Axis. EMBO J. 2018, 37, e98642. [Google Scholar] [CrossRef]
- Quan, T.; Xu, Y.; Qin, Z.; Robichaud, P.; Betcher, S.; Calderone, K.; He, T.; Johnson, T.M.; Voorhees, J.J.; Fisher, G.J. Elevated YAP and Its Downstream Targets CCN1 and CCN2 in Basal Cell Carcinoma. Am. J. Pathol. 2014, 184, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Salinas Parra, N.; Chen, Q.; Iglesias-Bartolome, R. Oncogenic Hedgehog-Smoothened Signaling Depends on YAP1–TAZ/TEAD Transcription to Restrain Differentiation in Basal Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 65–76.e7. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, L.; Secone Seconetti, A.; De Santis, G.; Pellacani, G.; Hirsch, T.; Rothoeft, T.; Teig, N.; Pellegrini, G.; Bauer, J.W.; De Luca, M. Laminin 332-Dependent YAP Dysregulation Depletes Epidermal Stem Cells in Junctional Epidermolysis Bullosa. Cell Rep. 2019, 27, 2036–2049.e6. [Google Scholar] [CrossRef]
- Shanmugam, V.K.; Tassi, E.; Schmidt, M.O.; Mcnish, S.; Baker, S.; Attinger, C.; Wang, H.; Shara, N.; Wellstein, A. Utility of a Human–Mouse Xenograft Model and in Vivo Near-infrared Fluorescent Imaging for Studying Wound Healing. Int. Wound J. 2015, 12, 699. [Google Scholar] [CrossRef]
- Sparks, H.D.; Mandla, S.; Vizely, K.; Rosin, N.; Radisic, M.; Biernaskie, J. Application of an Instructive Hydrogel Accelerates Re-Epithelialization of Xenografted Human Skin Wounds. Sci. Rep. 2022, 12, 14233. [Google Scholar] [CrossRef]
- Theocharidis, G.; Yuk, H.; Roh, H.; Wang, L.; Mezghani, I.; Wu, J.; Kafanas, A.; Contreras, M.; Sumpio, B.; Li, Z.; et al. A Strain-Programmed Patch for the Healing of Diabetic Wounds. Nat. Biomed. Eng. 2022, 6, 1118–1133. [Google Scholar] [CrossRef]
- Cristóbal, L.; Ortega, M.A.; Asúnsolo, Á.; Romero, B.; Álvarez-Mon, M.; Buján, J.; Maldonado, A.A.; García-Honduvilla, N. Human Skin Model for Mimic Dermal Studies in Pathology with a Clinical Implication in Pressure Ulcers. Histol. Histopathol. 2018, 33, 959–970. [Google Scholar] [CrossRef]
- Gilhar, A.; David, M.; Ullmann, Y.; Berkutski, T.; Kalish, R.S. T-Lymphocyte Dependence of Psoriatic Pathology in Human Psoriatic Skin Grafted to SCID Mice. J. Investig. Dermatol. 1997, 109, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.S.; Brent, L.; Valdimarsson, H.; Powles, A.V.; Al-Imara, L.; Walker, M.; Fry, L. Is Epidermal Cell Proliferation in Psoriatic Skin Grafts on Nude Mice Driven by T-Cell Derived Cytokines? Br. J. Dermatol. 1992, 126, 105–110. [Google Scholar] [CrossRef]
- Yamao, M.; Inamatsu, M.; Okada, T.; Ogawa, Y.; Ishida, Y.; Tateno, C.; Yoshizato, K. Restorative Effect of Hair Follicular Dermal Cells on Injured Human Hair Follicles in a Mouse Model. Exp. Dermatol. 2015, 24, 225–227. [Google Scholar] [CrossRef]
- Lorenz, H.P.; Longaker, M.T.; Perkocha, L.A.; Jennings, R.W.; Harrison, M.R.; Adzick, N.S. Scarless Wound Repair: A Human Fetal Skin Model. Development 1992, 114, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Demarchez, M.; Hartmann, D.J.; Prunieras, M. An Immunohistological Study of the Revascularization Process in Human Skin Transplanted onto the Nude Mouse. Transplantation 1987, 43, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Klicznik, M.M.; Morawski, P.A.; Höllbacher, B.; Varkhande, S.R.; Motley, S.J.; Kuri-Cervantes, L.; Goodwin, E.; Rosenblum, M.D.; Long, S.A.; Brachtl, G.; et al. Human CD4+CD103+ Cutaneous Resident Memory T Cells Are Found in the Circulation of Healthy Individuals. Sci. Immunol. 2019, 4, eaav8995. [Google Scholar] [CrossRef] [PubMed]




| Antibody | Cat. № | Dilution |
|---|---|---|
| Primary rat anti-BrdU | Abcam, ab6326 | 1:250 |
| Primary rabbit anti-Ki67 | Abcam, ab16667 | 1:200 |
| Primary rabbit anti-KRT14 | Abcam, ab181595 | 1:1000 |
| Primary mouse anti-KRT10 | Thermo Fisher Scientific, MA1-06319 | 1:200 |
| Primary rabbit anti-KRT15 | Abcam, ab52816 | 1:100 |
| Primary rabbit anti-Col17a1 | Novus Biologicals, NBP2-38686 | 1:100 |
| Primary rabbit anti-PDGFRa | Abcam, ab203491 | 1:500 |
| Primary goat anti-SM22a | Abcam, ab10135 | 1:100 |
| Primary mouse anti-histone H4 (di methyl K20, tri methyl K20) | Abcam, ab78517 | 1:400 |
| Primary mouse anti-KMT5A/SETD8 | Abcam, ab3798 | 1:100 |
| Primary goat anti-KLF4 | R&D Systems, Minneapolis, MN, USA, AF3640 | 1:15 |
| Primary rabbit anti-YAP | Abcam, ab52771 | 1:100 |
| Secondary goat anti-rat IgG, Alexa 488 | Invitrogen, San Diego, CA, USA, A-11006 | 1:1000 |
| Secondary donkey anti-rabbit IgG, Alexa 546 | Invitrogen, A10040 | 1:1000 |
| Secondary goat anti-rabbit IgG, Alexa 405 | Invitrogen, AB_221605 | 1:1000 |
| Secondary chicken anti-rabbit IgG, Alexa 488 | Invitrogen, A-21441 | 1:1000 |
| Secondary donkey anti-mouse IgG, Alexa 555 | Invitrogen, A32773 | 1:1000 |
| Secondary donkey anti-goat IgG, Alexa 546 | Invitrogen, A11056 | 1:1000 |
| Secondary goat anti-mouse IgG, Alexa 660 | Invitrogen, A21055 | 1:1000 |
| Secondary chicken anti-goat IgG, Alexa 647 | Invitrogen, A21469 | 1:1000 |
| Anti-human nuclei Alexa 488 conjugate | Sigma-Aldrich, MAB1281A4 | 1:100 |
| Cluster | BrdU Intensity | Ki67 Intensity | Nuclei Area | Nuclei Circularity | Localization |
|---|---|---|---|---|---|
| 1 | Low | Low | Medium | Round | Mixed |
| 2 | Low | Low | Small | Elongated | Mixed |
| 3 | High | High/Low | Medium | Round | Inter-ridges |
| 4 | Low | Low/High | Large | Round | Rete ridges |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherkashina, O.; Tsitrina, A.; Abolin, D.; Morgun, E.; Kosykh, A.; Sabirov, M.; Vorotelyak, E.; Kalabusheva, E. The Recovery of Epidermal Proliferation Pattern in Human Skin Xenograft. Cells 2025, 14, 448. https://doi.org/10.3390/cells14060448
Cherkashina O, Tsitrina A, Abolin D, Morgun E, Kosykh A, Sabirov M, Vorotelyak E, Kalabusheva E. The Recovery of Epidermal Proliferation Pattern in Human Skin Xenograft. Cells. 2025; 14(6):448. https://doi.org/10.3390/cells14060448
Chicago/Turabian StyleCherkashina, Olga, Alexandra Tsitrina, Danila Abolin, Elena Morgun, Anastasiya Kosykh, Marat Sabirov, Ekaterina Vorotelyak, and Ekaterina Kalabusheva. 2025. "The Recovery of Epidermal Proliferation Pattern in Human Skin Xenograft" Cells 14, no. 6: 448. https://doi.org/10.3390/cells14060448
APA StyleCherkashina, O., Tsitrina, A., Abolin, D., Morgun, E., Kosykh, A., Sabirov, M., Vorotelyak, E., & Kalabusheva, E. (2025). The Recovery of Epidermal Proliferation Pattern in Human Skin Xenograft. Cells, 14(6), 448. https://doi.org/10.3390/cells14060448







