Myostatin Mutation Enhances Bovine Myogenic Differentiation through PI3K/AKT/mTOR Signalling via Removing DNA Methylation of RACK1
Abstract
1. Introduction
2. Methods
2.1. Animal and Muscle Tissue Collection
2.2. WGBS Library Preparation, Sequencing, Quality Analysis, and Mapping
2.3. Identification of Differentially Methylated Regions (DMRs) and the GO Enrichment Analyses of WGBS
2.4. Pyrosequencing Assay
2.5. Bovine Skeletal Muscle Satellite Cells Resuscitation and Induction of Differentiation Culture, Cell Transfection Assay, and Immunofluorescence Staining
2.6. Extraction of Total Cellular RNA, Synthesis of the First Strand of cDNA and qRT-PCR Assay
2.7. Western Blot Assay and CCK-8 Analysis
2.8. Ch-IP Experiments and Co-IP Experiments
2.9. Hydroxymethylcytosine (5hmC) Hydroxylase TET Activity Assay (ELISA Assay)
2.10. Construction of Overexpression Vector and Synthesis of siRNA
2.11. Bioinformatics Analysis
2.12. Statistical Analysis
3. Results
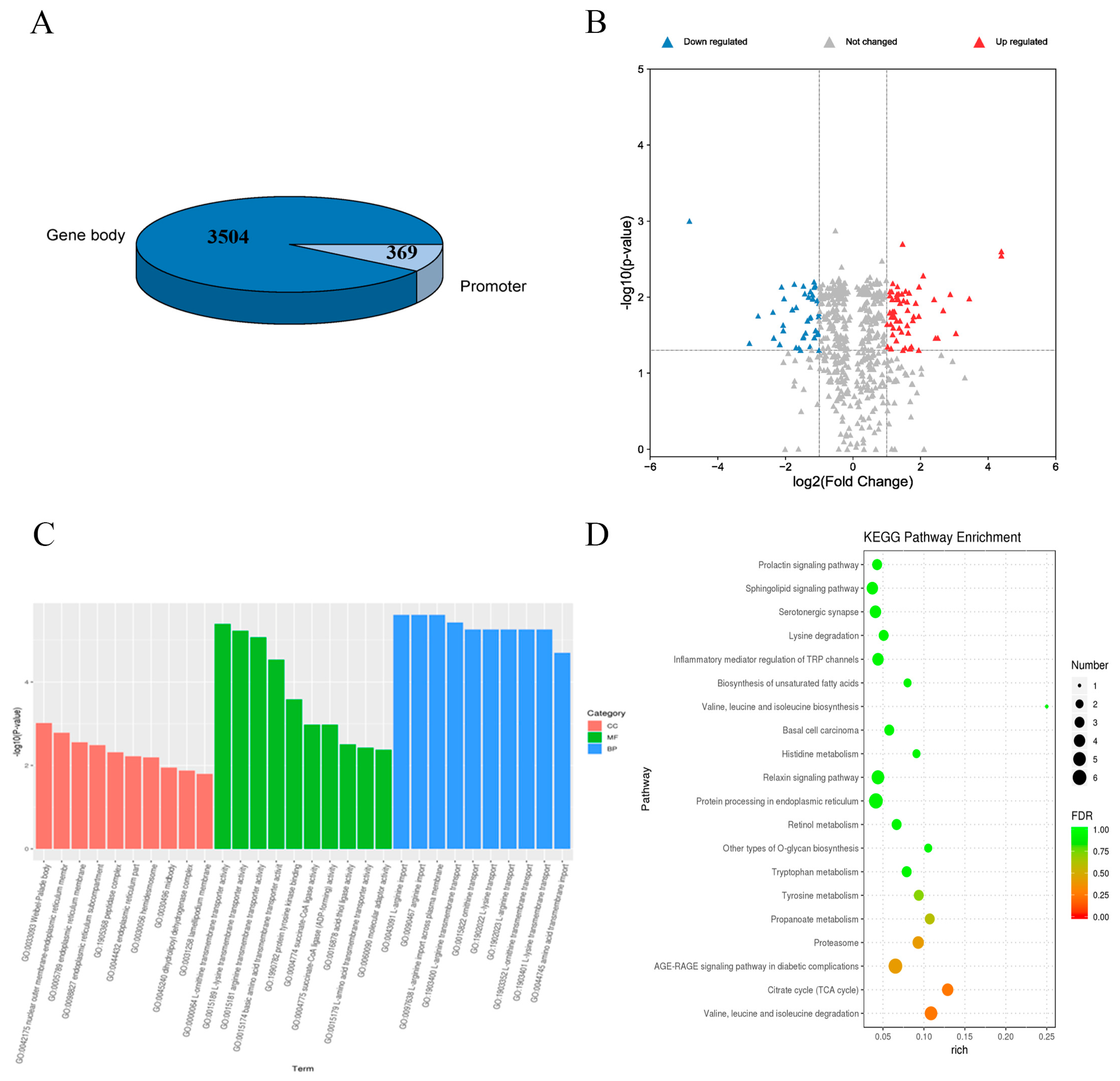
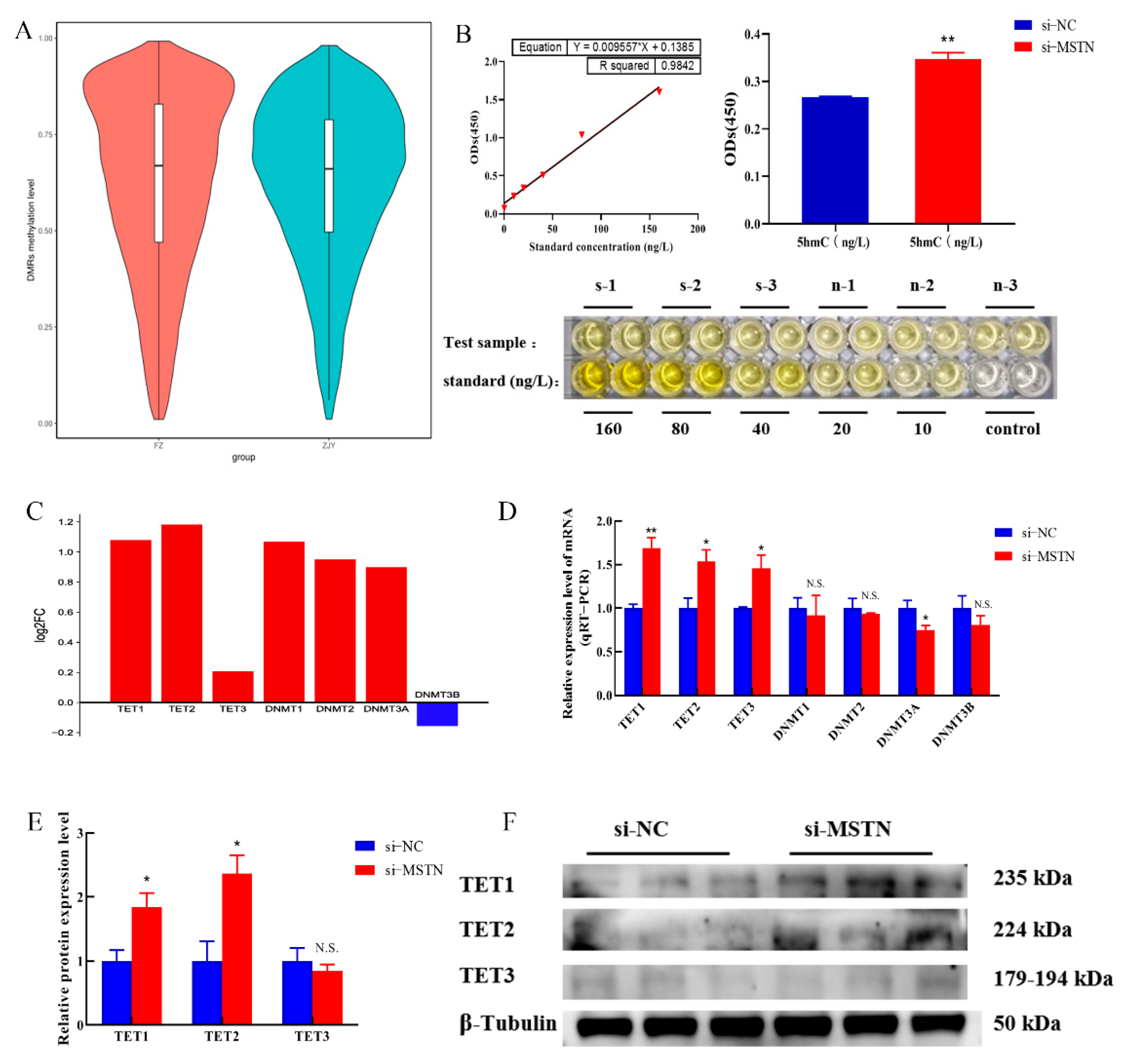
3.1. Genome-Wide DNA Methylation Profiling and Data Accuracy Validation
3.2. Global DNA Demethylation Patterns Resulting from Myostatin Deletion
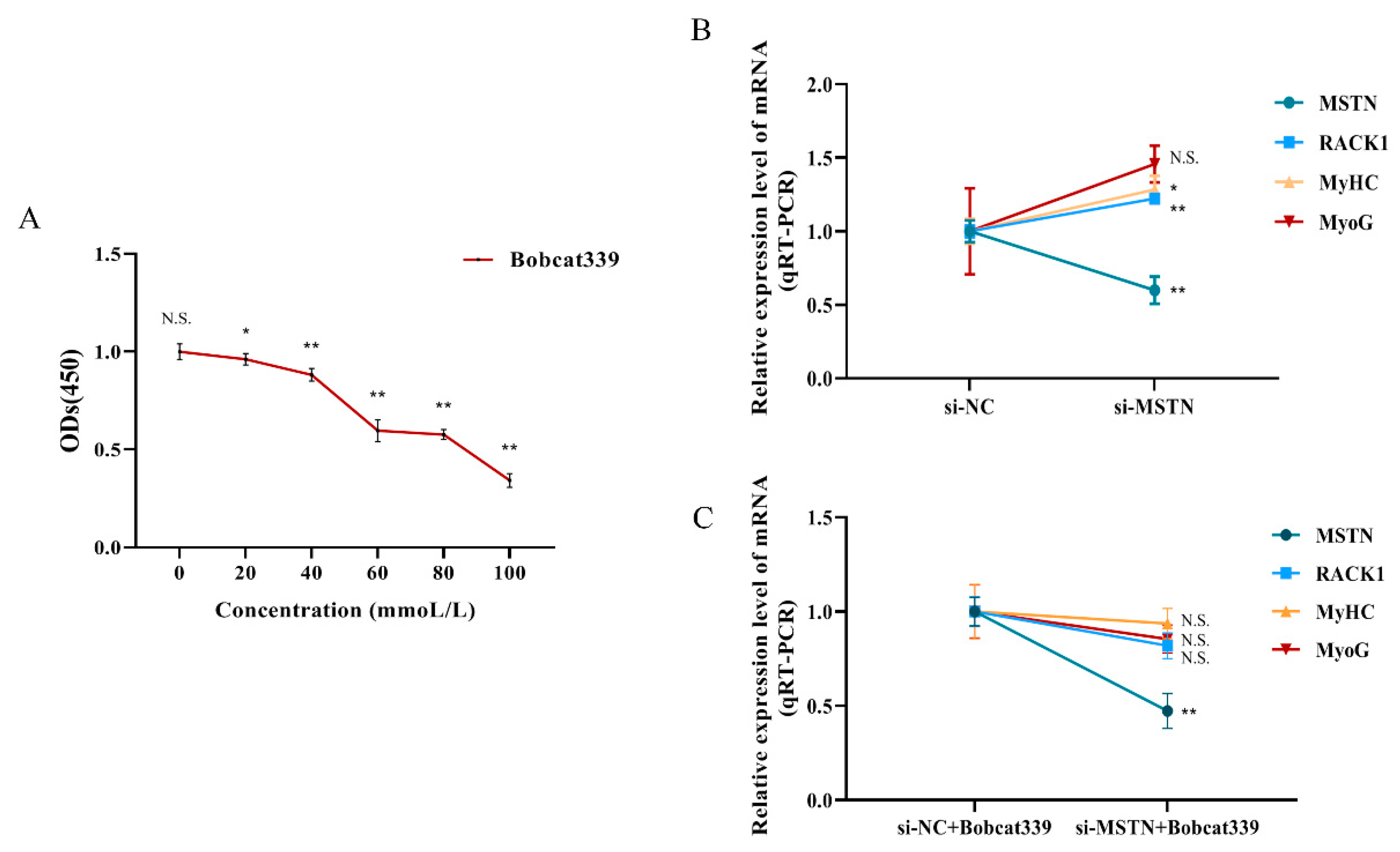
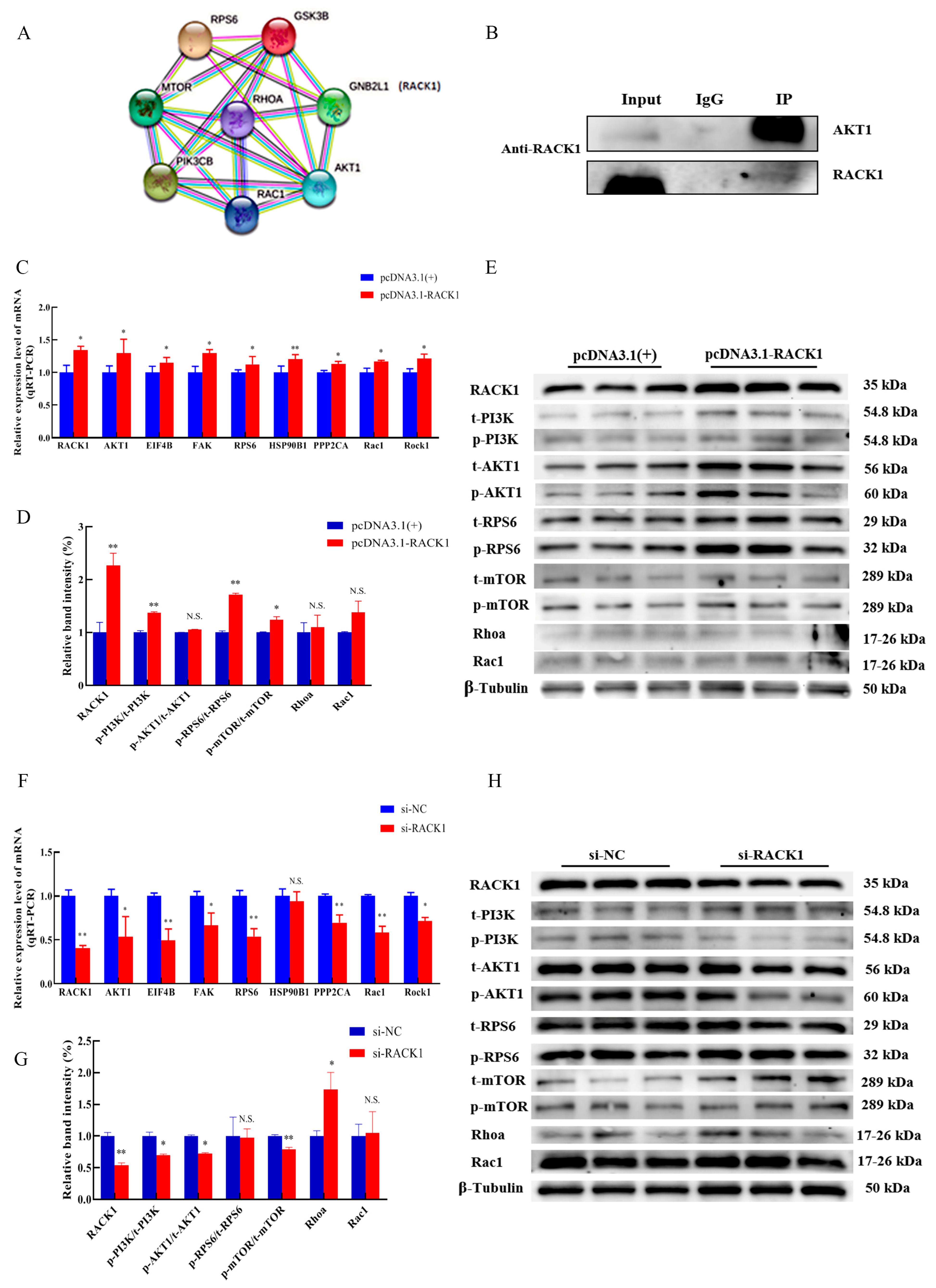
3.3. Differentially Methylated Regions (DMRs) of the RACK1 Gene
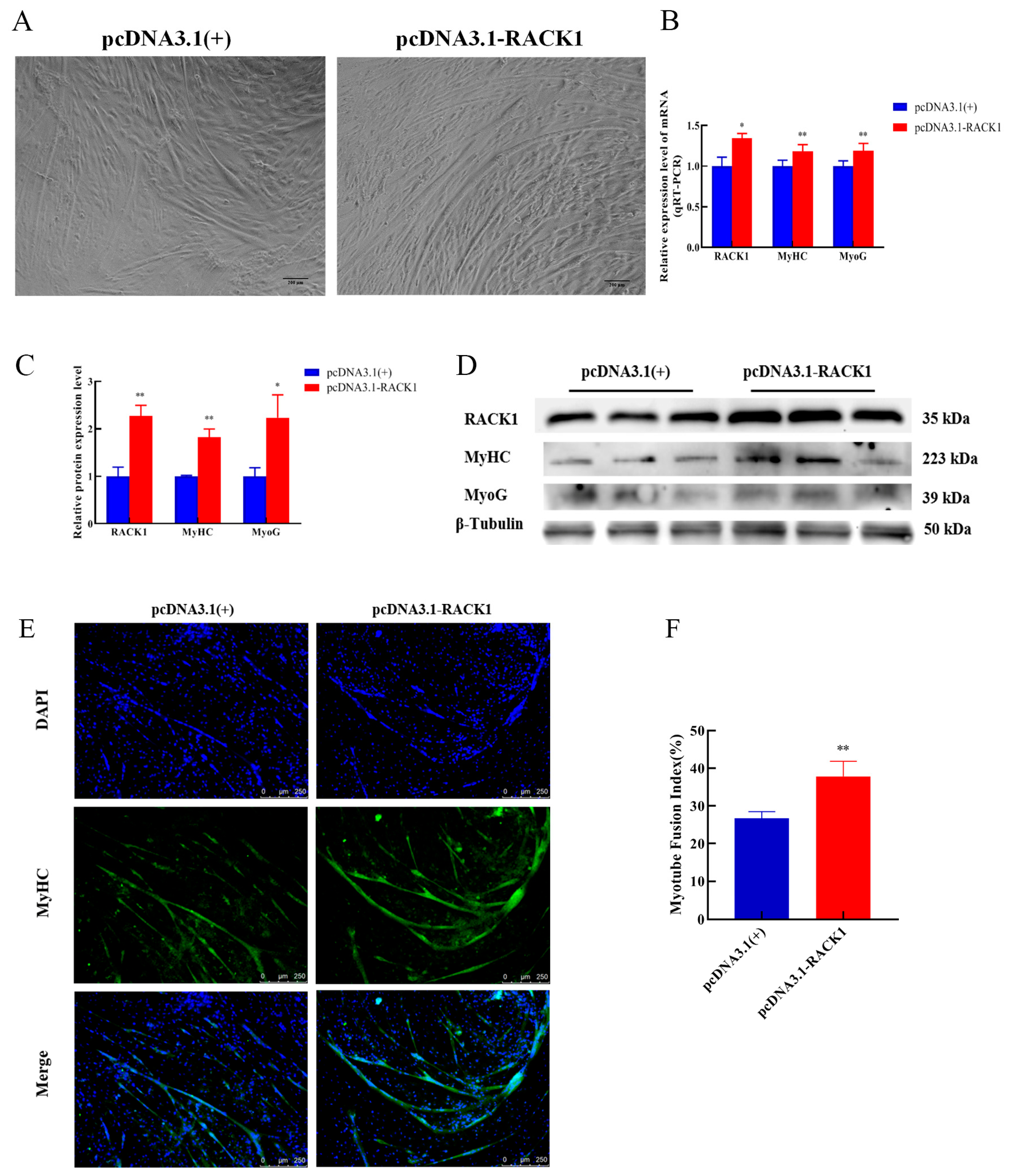
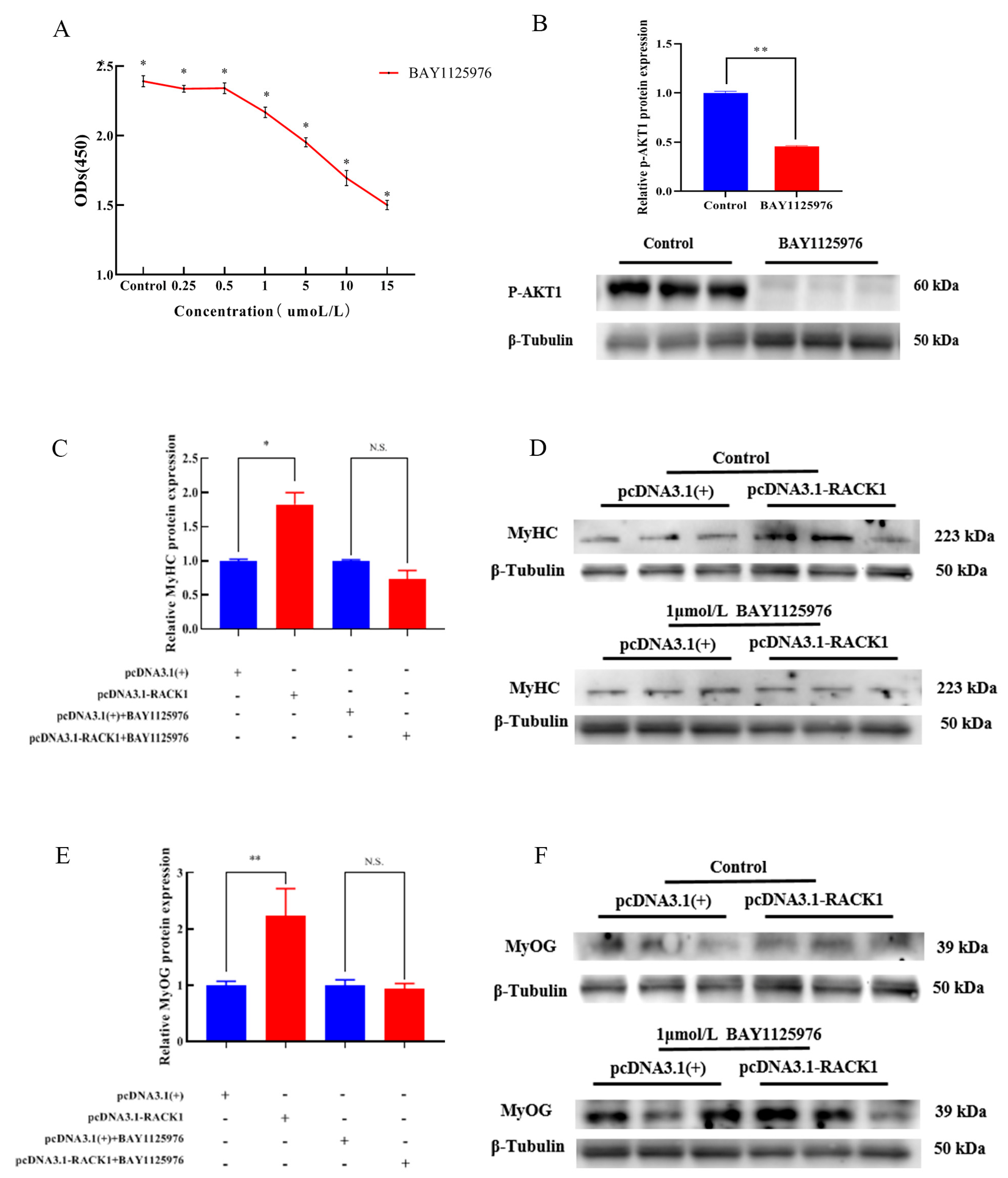
3.4. Impact of RACK1 Overexpression on Myogenic Differentiation of Bovine Skeletal Muscle
3.5. Impact of RACK1 on the PI3K/AKT/mTOR Pathway
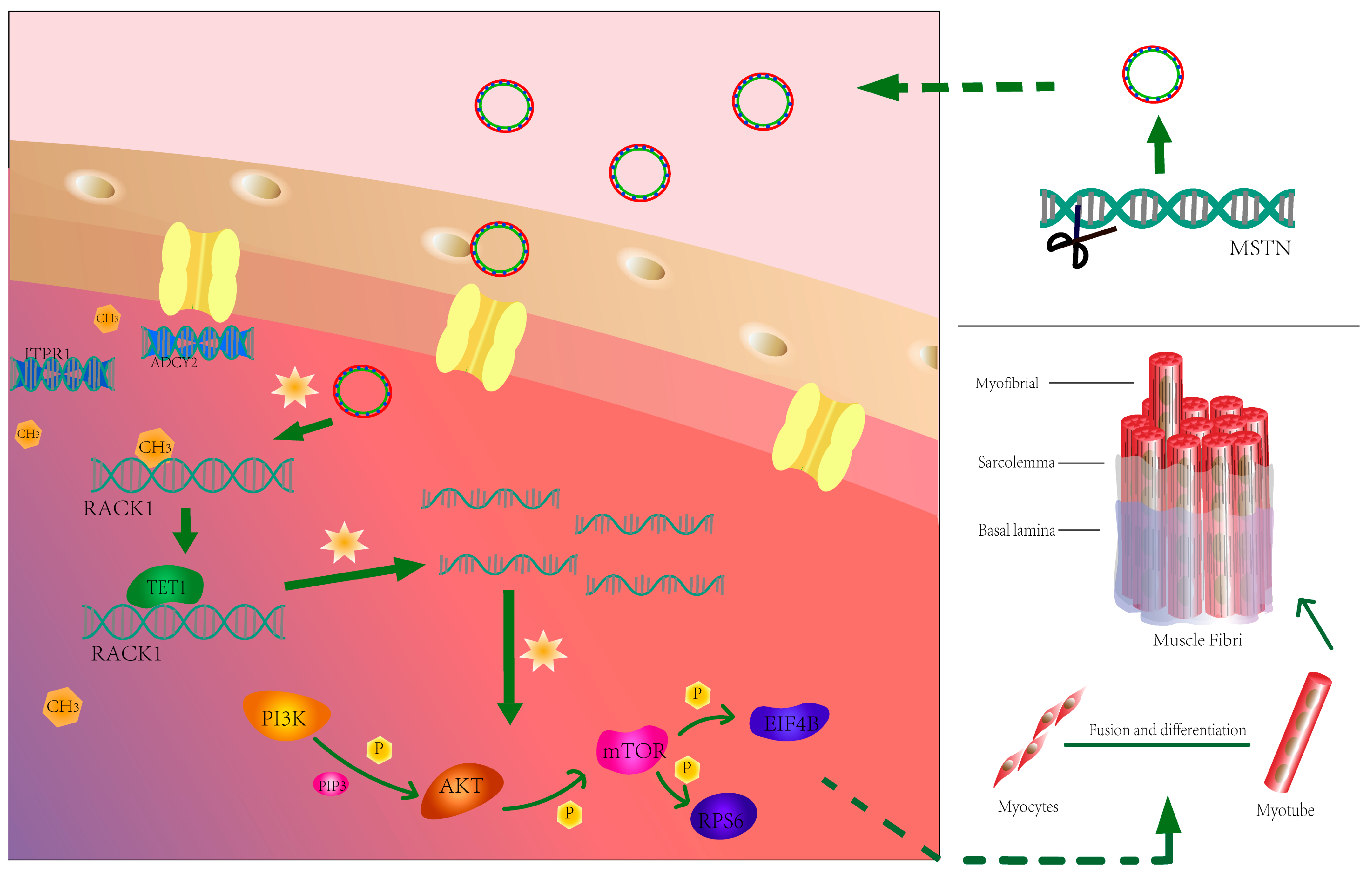
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Allen, M.D.; Grummitt, C.G.; Hilcenko, C.; Min, S.Y.; Tonkin, L.M.; Johnson, C.M.; Freund, S.M.; Bycroft, M.; Warren, A.J. Solution structure of the nonmethyl-CpG-binding CXXC domain of the leukaemia-associated MLL histone methyltransferase. EMBO J. 2006, 25, 4503–4512. [Google Scholar] [CrossRef]
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef]
- Bochtler, M.; Kolano, A.; Xu, G.L. DNA demethylation pathways: Additional players and regulators. BioEssays 2016, 39, 1. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Chen, P.; Cheng, B.; Cheng, Y. RACKI induces chemotherapy resistance in esophageal carcinoma by upregulating the PI3K/AKT pathway and Bcl-2 expression. Oncotargets Ther. 2018, 11, 211–220. [Google Scholar] [CrossRef]
- Carrió, E.; Magli, A.; Muñoz, M.; Peinado, M.A.; Perlingeiro, R.; Suelves, M. Muscle cell identity requires Pax7-mediated lineage-specific DNA demethylation. BMC Biol. 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Ceco, E.; Weinberg, S.E.; Chandel, N.S.; Sznajder, J.I. Metabolism and skeletal muscle homeostasis in lung disease. Am. J. Respir. Cell Mol. Biol. 2017, 57, 28–34. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Chen, M.-M.; Zhao, Y.-P.; Zhao, Y.; Deng, S.-L.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef]
- Consortium, U.P. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Dalton, S.R.; Bellacosa, A. DNA demethylation by TDG. Epigenomics 2012, 4, 459–467. [Google Scholar] [CrossRef]
- El Shafey, N.; Guesnon, M.; Simon, F.; Deprez, E.; Cosette, J.; Stockholm, D.; Scherman, D.; Bigey, P.; Kichler, A. Inhibition of the myostatin/Smad signaling pathway by short decorin-derived peptides. Exp. Cell Res. 2016, 341, 187–195. [Google Scholar] [CrossRef]
- Gao, L.; Yang, M.; Wei, Z.; Gu, M.; Li, G. MSTN Mutant Promotes Myogenic Differentiation by Increasing Demethylase TET1 Expression via the SMAD2/SMAD3 Pathway. Int. J. Biol. Sci. 2020, 16, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Outeiral, V.; De La Parte, C.; Fidalgo, M.; Guallar, D. The Complexity of TET2 Functions in Pluripotency and Development. Front. Cell Dev. Biol. 2021, 8, 1861. [Google Scholar] [CrossRef]
- Gong, X.; Tang, H.; Yang, K. PER1 suppresses glycolysis and cell proliferation in oral squamous cell carcinoma via the PER1/RACK1/PI3K signaling complex. Cell Death Dis. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Gu, T.-P.; Guo, F.; Yang, H.; Wu, H.-P.; Xu, G.-F.; Liu, W.; Xie, Z.-G.; Shi, L.; He, X.; Jin, S.-G. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 2011, 477, 606–610. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Huang, D.; Sherman, B.; Lempicki, R. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Iqbal, K.; Jin, S.-G.; Pfeifer, G.P.; Szabó, P.E. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl. Acad. Sci. USA 2011, 108, 3642–3647. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, Q.; Ye, J.; Wang, X.; Yang, W.; Zhu, D. Myostatin induces p300 degradation to silence cyclin D1 expression through the PI3K/PTEN/Akt pathway. Cell. Signal. 2008, 20, 1452–1458. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Kiely, P.A.; Sant, A.; O’connor, R. RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J. Biol. Chem. 2002, 277, 22581–22589. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Gao, L.; Niu, Y.; Gongpan, P.; Xu, Y.; Li, Y.; Xiong, W. RACK1 is required for adipogenesis. Am. J. Physiol.-Cell Physiol. 2016, 311, C831–C836. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, K.; Saitou, M. Germ cell reprogramming. Curr. Top. Dev. Biol. 2019, 135, 91–125. [Google Scholar] [PubMed]
- Lee, S.-J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef] [PubMed]
- Lessard, S.J.; Macdonald, T.L.; Pathak, P.; Han, M.S.; Coffey, V.G.; Edge, J.; Rivas, D.A.; Hirshman, M.F.; Davis, R.J.; Goodyear, L.J. JNK regulates muscle remodeling via myostatin/SMAD inhibition. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J. DNA demethylation pathways: Recent insights. Genet. Epigenetics 2013, 5, S12143. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef]
- Liu, W.; Wu, G.; Xiong, F.; Chen, Y. Advances in the DNA methylation hydroxylase TET1. Biomark. Res. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Miao, M.; Guo, Y.; Hu, D.; Zeng, Y.; Li, X.; Zhang, L.; Ding, X.; Guo, H. MSTN gene-edited bovine muscle transcriptome sequencing and bioinformatics analysis. China Anim. Husb. Vet. Med. 2021, 48, 11. [Google Scholar]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2012, 38, 23–38. [Google Scholar] [CrossRef]
- Onodera, A.; González-Avalos, E.; Lio, C.; Georges, R.O.; Rao, A. Roles of TET and TDG in DNA demethylation in proliferating and non-proliferating immune cells. Genome Biol. 2021, 22, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Paivandy, A.; Grujic, M.; Rafati, N.; Pejler, G. DNA demethylation regulates gene expression in IgE-activated mouse mast cells. Allergy 2020, 75, 1780–1783. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Gong, P.-G.; Li, J.-B.; Cai, L.-M.; Yang, L.; Liu, Y.-Y.; Yao, K.-T.; Li, X. The important role of the receptor for activated C kinase 1 (RACK1) in nasopharyngeal carcinoma progression. J. Transl. Med. 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pompura, S.L.; Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. 2018, 103, 1065–1076. [Google Scholar] [CrossRef]
- Retamales, A.; Zuloaga, R.; Valenzuela, C.; Gallardo-Escarate, C.; Molina, A.; Valdés, J. Insulin-like growth factor-1 suppresses the Myostatin signaling pathway during myogenic differentiation. Biochem. Biophys. Res. Commun. 2015, 464, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Bogdanovic, O. TET enzymes, DNA demethylation and pluripotency. Biochem. Soc. Trans. 2019, 47, BST20180606. [Google Scholar] [CrossRef] [PubMed]
- Senesi, P.; Luzi, L.; Montesano, A.; Terruzzi, I. DNA demethylation enhances myoblasts hypertrophy during the late phase of myogenesis activating the IGF-I pathway. Endocrine 2014, 47, 244–254. [Google Scholar] [CrossRef][Green Version]
- Von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. STRING: Known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005, 33 (Suppl. 1), D433–D437. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Yu, M.; Yu, Y.; Hu, P. Muscle regeneration controlled by a designated DNA dioxygenase. Cell Death Dis. 2021, 12, 535. [Google Scholar] [CrossRef]
- Wossidlo, M.; Nakamura, T.; Lepikhov, K.; Marques, C.J.; Zakhartchenko, V.; Boiani, M.; Arand, J.; Nakano, T.; Reik, W.; Walter, J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat. Commun. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia. Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, Y.-M.; Sun, B.; Zhong, F.-J.; Yang, L.-Y. GNA14’s interaction with RACK1 inhibits hepatocellular carcinoma progression through reducing MAPK/JNK and PI3K/AKT signaling pathway. Carcinogenesis 2021, 42, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, R.; Yu, J.; Wu, X. DNA Demethylation: Where Genetics Meets Epigenetics. Curr. Pharm. Des. 2014, 20, 1625–1631. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Liu, Y.; Wang, W.; Liu, Y. Knockdown of RACK1 suppresses MyoG and MHC gene expression in C2C12 cells. Chin. J. Anim. Sci. 2017, 53, 106–111. [Google Scholar]
- Zhang, X.; Nie, Y.; Cai, S.; Ding, S.; Fu, B.; Wei, H.; Chen, L.; Liu, X.; Liu, M.; Yuan, R. Earlier demethylation of myogenic genes contributes to embryonic precocious terminal differentiation of myoblasts in miniature pigs. FASEB J. 2019, 33, 9638–9655. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, Q.Q.; Li, J.W.; Zhang, Y.M.; An, X.R.; Hou, J. Ten-Eleven Translocation-2 (Tet2) Is Involved in Myogenic Differentiation of Skeletal Myoblast Cells in Vitro. Sci. Rep. 2017, 7, 43539. [Google Scholar] [CrossRef]


| Groups | Sample | Clean Base (Gb) | Clean Reads | GC (%) | Q30 (%) | Mapped (%) | Bisulfite Conversion Rate (%) | Total_mC (%) |
|---|---|---|---|---|---|---|---|---|
| Wild type | F81199 | 155.23 | 1,037,577,664 | 22.77 | 90.56 | 76.30 | 98.66 | 0.29 |
| F81208 | 156.91 | 1,049,963,834 | 22.93 | 89.09 | 77.00 | 98.84 | 0.37 | |
| F81209 | 156.12 | 1,044,003,946 | 22.64 | 90.70 | 76.60 | 98.69 | 0.32 | |
| F81210 | 155.08 | 1,036,046,942 | 22.92 | 88.50 | 76.90 | 98.85 | 0.34 | |
| F81214 | 155.68 | 1,041,440,172 | 22.99 | 88.34 | 76.50 | 98.82 | 0.36 | |
| MSTN+/−-Edited | Z61004 | 156.31 | 1,046,166,382 | 23.17 | 87.40 | 75.68 | 99.20 | 0.36 |
| Z61020 | 155.89 | 1,042,125,536 | 23.24 | 90.11 | 74.25 | 98.76 | 0.37 | |
| Z61023 | 141.55 | 960,468,910 | 30.65 | 87.61 | 59.35 | 99.20 | 0.27 | |
| Z61117 | 151.37 | 1,016,262,024 | 23.29 | 88.51 | 75.24 | 99.20 | 0.34 | |
| Z61128 | 155.40 | 1,038,158,924 | 22.15 | 87.24 | 76.42 | 99.20 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xia, X.; Wang, Q.; Hu, D.; Zhang, L.; Li, X.; Ding, X.; Guo, H.; Guo, Y. Myostatin Mutation Enhances Bovine Myogenic Differentiation through PI3K/AKT/mTOR Signalling via Removing DNA Methylation of RACK1. Cells 2023, 12, 59. https://doi.org/10.3390/cells12010059
Zhao Y, Xia X, Wang Q, Hu D, Zhang L, Li X, Ding X, Guo H, Guo Y. Myostatin Mutation Enhances Bovine Myogenic Differentiation through PI3K/AKT/mTOR Signalling via Removing DNA Methylation of RACK1. Cells. 2023; 12(1):59. https://doi.org/10.3390/cells12010059
Chicago/Turabian StyleZhao, Yiping, Xiaoxia Xia, Qiaomeng Wang, Debao Hu, Linlin Zhang, Xin Li, Xiangbin Ding, Hong Guo, and Yiwen Guo. 2023. "Myostatin Mutation Enhances Bovine Myogenic Differentiation through PI3K/AKT/mTOR Signalling via Removing DNA Methylation of RACK1" Cells 12, no. 1: 59. https://doi.org/10.3390/cells12010059
APA StyleZhao, Y., Xia, X., Wang, Q., Hu, D., Zhang, L., Li, X., Ding, X., Guo, H., & Guo, Y. (2023). Myostatin Mutation Enhances Bovine Myogenic Differentiation through PI3K/AKT/mTOR Signalling via Removing DNA Methylation of RACK1. Cells, 12(1), 59. https://doi.org/10.3390/cells12010059





