Pyruvate Regulates the Expression of DLAT to Promote Follicular Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Experiments
2.2. Cell Lines, Culture, and Treatment
2.3. Follicles Culture
2.4. Hematoxylin and Eosin (H & E)
2.5. TUNEL Assay
2.6. RNA and RT-qPCR
2.7. Western Blot
2.8. Co-Immunoprecipitation (Co-IP)
2.9. EdU Assay
2.10. Flow Cytometry
2.11. Plasmids
2.12. Statistical Analyses
3. Results
3.1. Pyruvate Promotes Follicular Growth in Mice
3.2. Pyruvate Promotes the Proliferation and Inhibits the Apoptosis of GCs
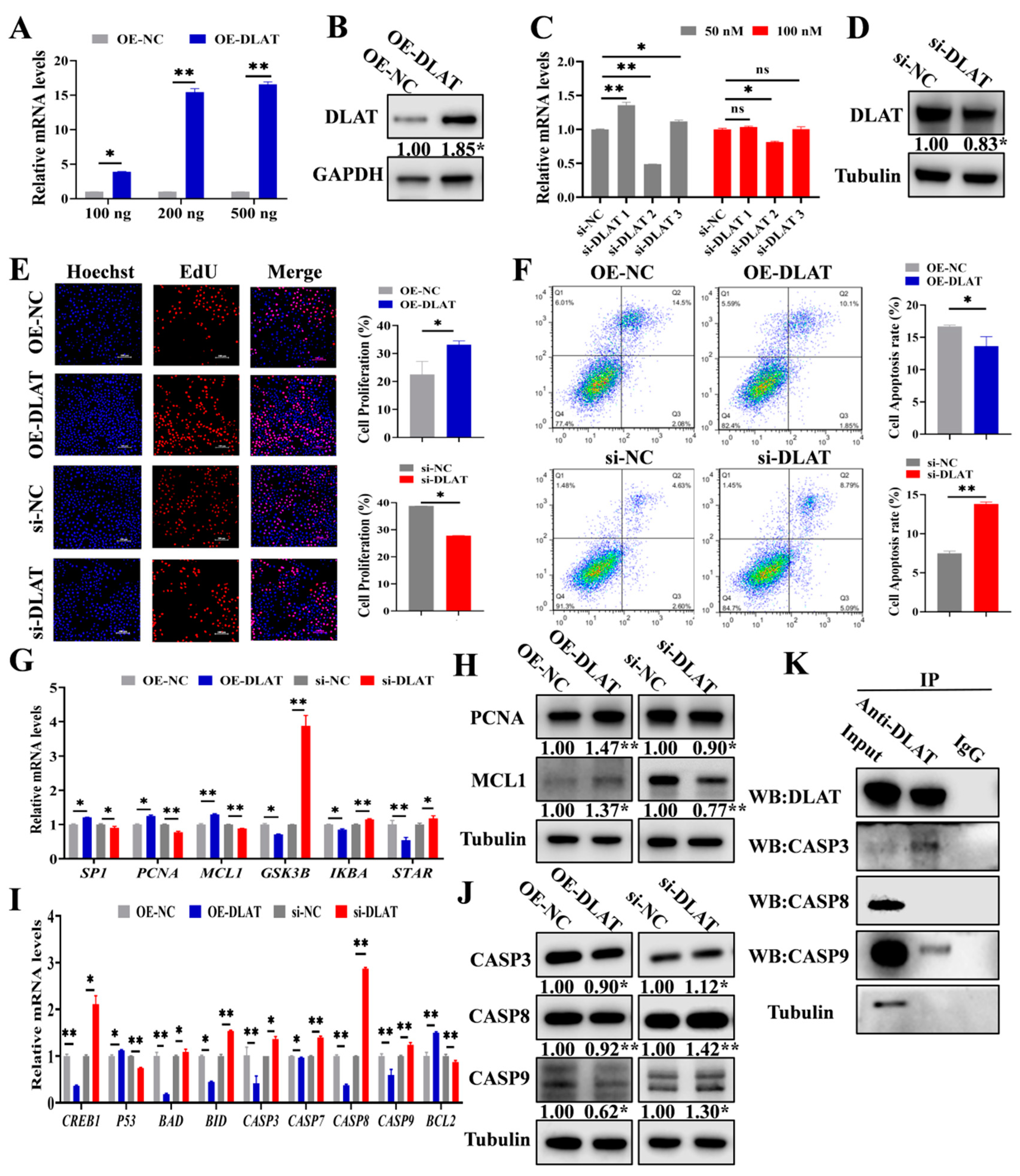
3.3. DLAT Accelerates GC Proliferation While Attenuating Apoptosis
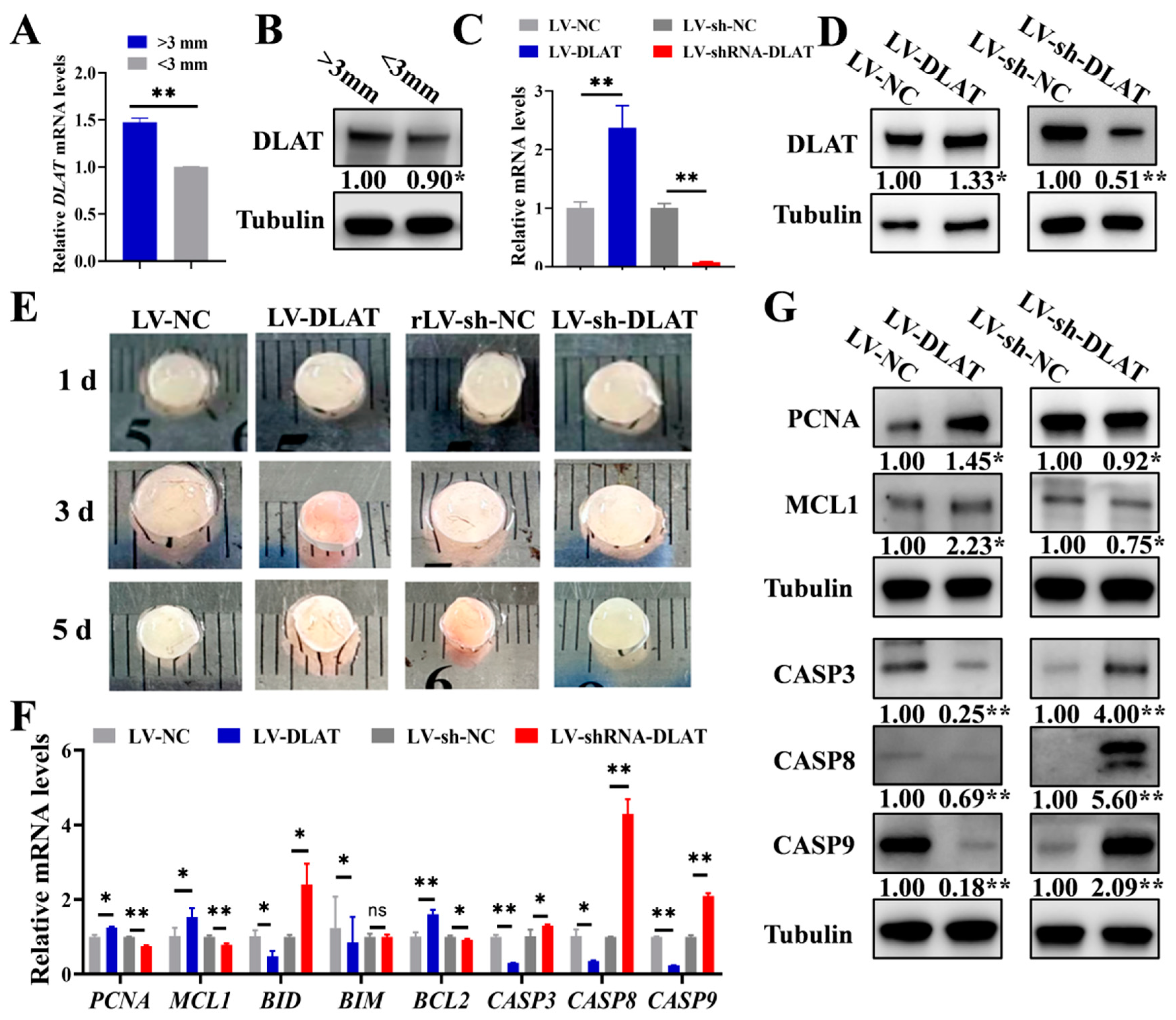
3.4. DLAT Promotes the Growth of Porcine Follicles
3.5. DLAT Stimulates Follicular Growth in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Peng, X.; Mei, S. Autophagy in Ovarian Follicular Development and Atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Black, J.L.; Erickson, B.H. Oogenesis and ovarian development in the prenatal pig. Anat. Rec. 1968, 161, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.; Li, N.; Bai, G.; Pan, X.; Zhang, Z.; Zhang, H.; Li, J.; Yuan, X. DNA methylation mediated RSPO2 to promote follicular development in mammals. Cell Death Dis. 2021, 12, 653. [Google Scholar] [CrossRef]
- McGee, E.A.; Hsueh, A.J.W. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef]
- Archilia, E.C.; Bello, C.; Batalha, I.M.; Wulstein, K.; Enriquez, C.; Schütz, L.F. Effects of follicle-stimulating hormone, insulin-like growth factor 1, fibroblast growth factor 2, and fibroblast growth factor 9 on sirtuins expression and histone deacetylase activity in bovine granulosa cells. Theriogenology 2023, 210, 1–8. [Google Scholar] [CrossRef]
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef]
- Li, N.; Zhou, Y.; Cai, J.; Wang, Y.; Zhou, X.; Hu, M.; Li, Y.; Zhang, H.; Li, J.; Cai, B.; et al. A novel trans-acting lncRNA of ACTG1 that induces the remodeling of ovarian follicles. Int. J. Biol. Macromol. 2023, 242, 125170. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.; Pan, X.; Quan, H.; He, B.; Li, Y.; Bai, G.; Li, N.; Zhang, Z.; Zhang, H.; et al. DNMT1-mediated lncRNA IFFD controls the follicular development via targeting GLI1 by sponging miR-370. Cell Death Differ. 2022, 30, 576–588. [Google Scholar] [CrossRef]
- Safdar, M.; Liang, A.; Rajput, S.A.; Abbas, N.; Zubair, M.; Shaukat, A.; Rehman, A.U.; Jamil, H.; Guo, Y.; Ullah, F.; et al. Orexin-A Regulates Follicular Growth, Proliferation, Cell Cycle and Apoptosis in Mouse Primary Granulosa Cells via the AKT/ERK Signaling Pathway. Molecules 2021, 26, 5635. [Google Scholar] [CrossRef]
- Wang, W.; Craig, Z.R.; Basavarajappa, M.S.; Hafner, K.S.; Flaws, J.A. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles1. Biol. Reprod. 2012, 87, 152. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.F.; Sanderson, N.; Quirke, L.D.; Lawrence, S.B.; Juengel, J.L. Association between antral follicle count and reproductive measures in New Zealand lactating dairy cows maintained in a pasture-based production system. Theriogenology 2016, 85, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Mossa, F.; Walsh, S.W.; Berry, D.P.; Carter, F.; Lonergan, P.; Smith, G.W.; Ireland, J.J.; Evans, A.C.O. Low numbers of ovarian follicles >/=3 mm in diameter are associated with low fertility in dairy cows. J. Dairy Sci. 2012, 95, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boucret, L.; de la Barca, J.-M.C.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Updat. 2016, 22, 725–743. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, Y.L.; Ji, S.Y.; Zhao, L.W.; Shang, W.N.; Li, D.; Chen, Z.; Tong, C.; Fan, H.Y. Mitochondrial Function Regulated by Mitoguardin-1/2 Is Crucial for Ovarian Endocrine Functions and Ovulation. Endocrinology 2017, 158, 3988–3999. [Google Scholar] [CrossRef]
- Doctor, A.; Spinella, P. Effect of processing and storage on red blood cell function in vivo. Semin. Perinatol. 2012, 36, 248–259. [Google Scholar] [CrossRef]
- Golias, T.; Kery, M.; Radenkovic, S.; Papandreou, I. Microenvironmental control of glucose metabolism in tumors by regulation of pyruvate dehydrogenase. Int. J. Cancer 2019, 144, 674–686. [Google Scholar] [CrossRef]
- Korotchkina, L.G.; Yang, H.-S.; Tirosh, O.; Packer, L.; Patel, M.S. Protection by thiols of the mitochondrial complexes from 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2001, 30, 992–999. [Google Scholar] [CrossRef]
- Nagaraj, R.; Sharpley, M.S.; Chi, F.; Braas, D.; Zhou, Y.; Kim, R.; Clark, A.T.; Banerjee, U. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell 2017, 168, 210–223.e11. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Han, R.; Kuang, T.; Min, C.; Wang, H.; Zhao, Y.; Wang, J.; Yang, L.; Che, D. Effects of pyruvate on early embryonic development and zygotic genome activation in pigs. Theriogenology 2022, 189, 77–85. [Google Scholar] [CrossRef]
- Liu, N.; Wu, Y.-G.; Lan, G.-C.; Sui, H.-S.; Ge, L.; Wang, J.-Z.; Liu, Y.; Qiao, T.-W.; Tan, J.-H. Pyruvate prevents aging of mouse oocytes. Reproduction 2009, 138, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.L.; Zhu, S.; Zhang, J.; Wen, J.; Yuan, H.-J.; Pan, L.-Z.; Luo, M.-J.; Tan, J.-H. Glucose metabolism during in vitro maturation of mouse oocytes: An study using RNA interference. J. Cell Physiol. 2018, 233, 6952–6964. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cao, Y.; Jiang, Y.; Wei, Y.; Liu, H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox Biol. 2018, 18, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Latham, K.E.; Wigglesworth, K.; McMenamin, M.; Eppig, J.J. Stage-dependent effects of oocytes and growth differentiation factor 9 on mouse granulosa cell development: Advance programming and subsequent control of the transition from preantral secondary follicles to early antral tertiary follicles. Biol. Reprod. 2004, 70, 1253–1262. [Google Scholar] [CrossRef]
- Gilchrist, R.; Ritter, L.; Armstrong, D. Oocyte–somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004, 82–83, 431–446. [Google Scholar] [CrossRef]
- Roche, T.E.; Hiromasa, Y.; Turkan, A.; Gong, X.; Peng, T.; Yan, X.; Kasten, S.A.; Bao, H.; Dong, J. Essential roles of lipoyl domains in the activated function and control of pyruvate dehydrogenase kinases and phosphatase isoform 1. Eur. J. Biochem. 2003, 270, 1050–1056. [Google Scholar] [CrossRef]
- Reed, L.J.; Hackert, M.L. Structure-function relationships in dihydrolipoamide acyltransferases. J. Biol. Chem. 1990, 265, 8971–8974. [Google Scholar] [CrossRef]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The Pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, H.; Shao, B.; Zhang, S.; Pall, H.; Peixoto, R.D.; Mok, S.R.; Zhu, G. Glycolysis-related gene dihydrolipoamide acetyltransferase promotes poor prognosis in hepatocellular carcinoma through the Wnt/β-catenin and PI3K/Akt signaling pathways. Ann. Transl. Med. 2022, 10, 1240. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, R.; Yang, S.; Zhu, J. Cuproptosis-related gene DLAT is a biomarker of the prognosis and immune microenvironment of gastric cancer and affects the invasion and migration of cells. Cancer Med. 2024, 13, e70012. [Google Scholar] [CrossRef]
- Yang, Q.; Zeng, S.; Liu, W. Roles of cuproptosis-related gene DLAT in various cancers: A bioinformatic analysis and preliminary verification on pro-survival autophagy. PeerJ 2023, 11, e15019. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Roche, T.E. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990, 4, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Roche, T.E.; Baker, J.C.; Yan, X.; Hiromasa, Y.; Gong, X.; Peng, T.; Dong, J.; Turkan, A.; Kasten, S.A. Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog. Nucleic. Acid Res. Mol. Biol. 2001, 70, 33–75. [Google Scholar]
- Steeves, T.E.; Gardner, D.K. Metabolism of glucose, pyruvate, and glutamine during the maturation of oocytes derived from pre-pubertal and adult cows. Mol. Reprod. Dev. 1999, 54, 92–101. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Harris, S.E.; Leese, H.J.; Gosden, R.G.; Picton, H.M. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol. Reprod. Dev. 2009, 76, 231–238. [Google Scholar] [CrossRef]
- Sugiura, K.; Su, Y.-Q.; Diaz, F.J.; Pangas, S.A.; Sharma, S.; Wigglesworth, K.; O’Brien, M.J.; Matzuk, M.M.; Shimasaki, S.; Eppig, J.J. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 2007, 134, 2593–2603. [Google Scholar] [CrossRef]
- Cinco, R.; Digman, M.A.; Gratton, E.; Luderer, U. Spatial Characterization of Bioenergetics and Metabolism of Primordial to Preovulatory Follicles in Whole Ex Vivo Murine Ovary. Biol. Reprod. 2016, 95, 129. [Google Scholar] [CrossRef]
- Bao, S.; Yin, T.; Liu, S. Ovarian aging: Energy metabolism of oocytes. J. Ovarian Res. 2024, 17, 118. [Google Scholar] [CrossRef]
- Makanji, Y.; Tagler, D.; Pahnke, J.; Shea, L.D.; Woodruff, T.K. Hypoxia-mediated carbohydrate metabolism and transport promote early-stage murine follicle growth and survival. Am. J. Physiol. Metab. 2014, 306, E893–E903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Wang, Z.; Zheng, N.; Yuan, F.; Li, B.; Li, X.; Deng, L.; Lin, M.; Chen, X.; et al. Enhanced glycolysis in granulosa cells promotes the activation of primordial follicles through mTOR signaling. Cell Death Dis. 2022, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, J.C.; Chandele, A.; Chopde, B.A.; Shastry, P. Sodium pyruvate protects against H2O2 mediated apoptosis in human neuroblastoma cell line-SK-N-MC. J. Chem. Neuroanat. 2003, 26, 109–118. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Liu, X.; Zhou, X.; Wang, A.; Lai, Y.; Zhang, J.; Li, Y.; Chen, S. Sustained release of miR-21 carried by mesenchymal stem cell-derived exosomes from GelMA microspheres inhibits ovarian granulosa cell apoptosis in premature ovarian insufficiency. Mater. Today Bio 2025, 31, 101469. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, F.; Yue, S.; Li, J.; Li, A.; Liu, Y.; Liang, J.; Gao, Y.; Xue, S. miR-17-5p-Mediated RNA Activation Upregulates KPNA2 Expression and Inhibits High-Glucose-Induced Apoptosis of Sheep Granulosa Cells. Int. J. Mol. Sci. 2025, 26, 943. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- van den Hurk, R.; Zhao, J. Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 2005, 63, 1717–1751. [Google Scholar] [CrossRef]
- Trounson, A.; Anderiesz, C.; Jones, G. Maturation of human oocytes in vitro and their developmental competence. Reproduction 2001, 121, 51–75. [Google Scholar] [CrossRef]
- Otoi, T.; Yamamoto, K.; Koyama, N.; Tachikawa, S.; Suzuki, T. Bovine oocyte diameter in relation to developmental competence. Theriogenology 1997, 48, 769–774. [Google Scholar] [CrossRef]
- Fraser, H.M. Regulation of the ovarian follicular vasculature. Reprod. Biol. Endocrinol. 2006, 4, 18. [Google Scholar] [CrossRef]
- Dompe, C.; Kulus, M.; Stefanska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B.; et al. Human Granulosa Cells-Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Z.; Li, W.; Zhang, L.; Zhou, J.; Sun, M.; Zhou, J.; Yang, M.; Zhang, X.; Wang, H.; et al. The FSH-HIF-1alpha-VEGF Pathway Is Critical for Ovulation and Oocyte Health but Not Necessary for Follicular Growth in Mice. Endocrinology 2020, 161, bqaa038. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Momoi, T. Caspases involved in ER stress-mediated cell death. J. Chem. Neuroanat. 2004, 28, 101–105. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Pan, G.; Ni, J.; Wei, Y.-F.; Yu, G.-L.; Gentz, R.; Dixit, V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277, 815–818. [Google Scholar] [CrossRef]
- Fearnhead, H.O.; Rodriguez, J.; Govek, E.-E.; Guo, W.; Kobayashi, R.; Hannon, G.; Lazebnik, Y.A. Oncogene-dependent apoptosis is mediated by caspase-9. Proc. Natl. Acad. Sci. USA 1998, 95, 13664–13669. [Google Scholar] [CrossRef]
- Kwintkiewicz, J.; Nishi, Y.; Yanase, T.; Giudice, L.C. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ. Health Persp. 2010, 118, 400–406. [Google Scholar] [CrossRef]
- Hu, X.; Lu, J.; Ding, C.; Li, J.; Zou, Q.; Xia, W.; Qian, C.; Li, H.; Huang, B. The N6-methyladenosine landscape of ovarian development and aging highlights the regulation by RNA stability and chromatin state. Aging Cell 2025, 24, e14376. [Google Scholar] [CrossRef]



| Gene Name | Primer Sequences (5′ → 3′) |
|---|---|
| Bcl2 (mouse) | F: CCTGTGGATGACTGAGTACCTG |
| R: AGCCAGGAGAAATCAAACAGAGG | |
| Casp3 (mouse) | F: GGAGTCTGACTGGAAAGCCGAA |
| R: CTTCTGGCAAGCCATCTCCTCA | |
| Casp7 (mouse) | F: CCGTCCACAATGACTGCTCTTG |
| R: CCCGTAAATCAGGTCCTCTTCC | |
| Casp8 (mouse) | F: GCTGACTTTCTGCTGGGGAT |
| R: GACATCGCTCTCTCAGGCTC | |
| Casp9 (mouse) | F: GCTGTGTCAAGTTTGCCTACCC |
| R: CCAGAATGCCATCCAAGGTCTC | |
| p53 (mouse) | F: GGCAGACTTTTCGCCACAG |
| R: GATGATGGTAAGGATAGGTCGG | |
| Bim (mouse) | F: GGAGATACGGATTGCACAGGAG |
| R: CTCCATACCAGACGGAAGATAAAG | |
| Creb1 (mouse) | F: CACAGACCACTGATGGACAGCA |
| R: AGGACGCCATAACAACTCCAGG | |
| Bax (mouse) | F: AGGATGCGTCCACCAAGAAGCT |
| R: TCCGTGTCCACGTCAGCAATCA | |
| Pcna (mouse) | F: CAAGTGGAGAGCTTGGCAATGG |
| R: GCAAACGTTAGGTGAACAGGCTC | |
| Mcl1 (mouse) | F: AGCTTCATCGAACCATTAGCAGAA |
| R: CCTTCTAGGTCCTGTACGTGGA | |
| Star (mouse) | F: GTGCTTCATCCACTGGCTGGAA |
| R: GTCTGCGATAGGACCTGGTTGA | |
| Sp1 (mouse) | F: CTCCAGACCATTAACCTCAGTGC |
| R: CACCACCAGATCCATGAAGACC | |
| Gapdh (mouse) | F: GGACTCATGACCACGGTCCAT |
| R: TCAGATCCACAACCGACACGT | |
| Dlat (mouse) | F: CTGAGTGAAGGAGACTTGCTGG |
| R: TCCCAGAGGAACATCCCTTGTG |
| Gene Name | Primer Sequences (5′ → 3′) |
|---|---|
| BCL2 (human) | F: ATCGCCCTGTGGATGACTGAGT |
| R: GCCAGGAGAAATCAAACAGAGGC | |
| CASP3 (human) | F: GGAAGCGAATCAATGGACTCTGG |
| R: GCATCGACATCTGTACCAGACC | |
| CASP7 (human) | F: CGGAACAGACAAAGATGCCGAG |
| R: AGGCGGCATTTGTATGGTCCTC | |
| CASP8 (human) | F: GCTGACTTTCTGCTGGGGAT |
| R: GACATCGCTCTCTCAGGCTC | |
| CASP9 (human) | F: GTTTGAGGACCTTCGACCAGCT |
| R: CAACGTACCAGGAGCCACTCTT | |
| P53 (human) | F: CCTCAGCATCTTATCCGAGTGG |
| R: TGGATGGTGGTACAGTCAGAGC | |
| BIM (human) | F: CAAGAGTTGCGGCGTATTGGAG |
| R: ACACCAGGCGGACAATGTAACG | |
| CREB1 (human) | F: GACCACTGATGGACAGCAGATC |
| R: GAGGATGCCATAACAACTCCAGG | |
| BAX (human) | F: TCAGGATGCGTCCACCAAGAAG |
| R: TGTGTCCACGGCGGCAATCATC | |
| IKBA (human) | F: TCCACTCCATCCTGAAGGCTAC |
| R: CAAGGACACCAAAAGCTCCACG | |
| GSK3B (human) | F: CCGACTAACACCACTGGAAGCT |
| R: AGGATGGTAGCCAGAGGTGGAT | |
| PCNA (human) | F: CAAGTAATGTCGATAAAGAGGAGG |
| R: GTGTCACCGTTGAAGAGAGTGG | |
| MCL1 (human) | F: CCAAGAAAGCTGCATCGAACCAT |
| R: CAGCACATTCCTGATGCCACCT | |
| STAR (human) | F: TACGTGGCTACTCAGCATCGAC |
| R: TCAACACCTGGCTTCAGAGGCA | |
| SP1 (human) | F: ACGCTTCACACGTTCGGATGAG |
| R: TGACAGGTGGTCACTCCTCATG | |
| GAPDH (human) | F: TGTTCGTCATGGGTGTGAAC |
| R: ATGGCATGGACTGTGGTCAT | |
| DLAT (human) | F: TTGATGTCAGTGTTGCGGTCAGTAC |
| R: GTGGCTGTAGTTTACCCTCTCTTGC |
| Gene Name | Primer Sequences (5′ → 3′) |
|---|---|
| BCL2 (pig) | F: GAGTTCGGTGGGGTCATGTG |
| R: TACAGCTCCACAAAGGCATCC | |
| CASP3 (pig) | F: AAGAAAACAGCATTACCCTCCTTG |
| R: TTCAGAGGGGACTGCTGTAGA | |
| CASP8 (pig) | F: CTCTGCCTACAGGGTCATGC |
| R: AGGATGGCCCTCTTCTCCAT | |
| CASP9 (pig) | F: AACTTCTGCCATGAGTCGGG |
| R: CTGGCCTTGGCAGTCAGG | |
| BIM (pig) | F: ATCCTCCCTGCTGTCTCGAT |
| R: AAGAAAACAGCATTACCCTCCTTG | |
| BID (pig) | F: ACGAGCGCATCACAAACCTA |
| R: GCCTCCTGGCTCTCAGAATC | |
| PCNA (pig) | F: AAGAGGAGGAAGCAGTTACCA |
| R: TCATCTTCGATCTTGGGAGCC | |
| MCL1 (pig) | F: GGAAGGCGTTAGAGACCCTG |
| R: GTCACAATCCTGCCCCAGTT | |
| GAPDH (pig) | F: GGACTCATGACCACGGTCCAT |
| R: TCAGATCCACAACCGACACGT | |
| DLAT (pig) | F: AGCAGCACGACTAGAGGGTATGG |
| R: TACCAGCGGCGGTTAGGAGAC |
| Gene Name | Primer Sequences (5′ → 3′) |
|---|---|
| DLAT | F: GGATCCCCCGCATCAGAAGGTTCCAT |
| R: GAATTCTCAGTGTGACCTGGGAGAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Guo, Y.; Huang, E.; Lu, J.; Wang, T.; Shi, Y.; Lv, M.; Chen, Y.; Li, S.; Yuan, X.; et al. Pyruvate Regulates the Expression of DLAT to Promote Follicular Growth. Cells 2025, 14, 444. https://doi.org/10.3390/cells14060444
Zhang L, Guo Y, Huang E, Lu J, Wang T, Shi Y, Lv M, Chen Y, Li S, Yuan X, et al. Pyruvate Regulates the Expression of DLAT to Promote Follicular Growth. Cells. 2025; 14(6):444. https://doi.org/10.3390/cells14060444
Chicago/Turabian StyleZhang, Liuhong, Yixuan Guo, Enyuan Huang, Jianing Lu, Tiantian Wang, Yonghua Shi, Meng Lv, Yongcai Chen, Shuo Li, Xiaolong Yuan, and et al. 2025. "Pyruvate Regulates the Expression of DLAT to Promote Follicular Growth" Cells 14, no. 6: 444. https://doi.org/10.3390/cells14060444
APA StyleZhang, L., Guo, Y., Huang, E., Lu, J., Wang, T., Shi, Y., Lv, M., Chen, Y., Li, S., Yuan, X., & Li, J. (2025). Pyruvate Regulates the Expression of DLAT to Promote Follicular Growth. Cells, 14(6), 444. https://doi.org/10.3390/cells14060444






