Abstract
The EFR3 (Eighty-Five Requiring 3) protein and its homologs are rather poorly understood eukaryotic plasma membrane peripheral proteins. They belong to the armadillo-like family of superhelical proteins. In higher vertebrates two paralog genes, A and B were found, each expressing at least 2–3 protein isoforms. EFR3s are involved in several physiological functions, mostly including phosphatidyl inositide phosphates, e.g., phototransduction (insects), GPCRs, and insulin receptors regulated processes (mammals). Mutations in the EFR3A were linked to several types of human disorders, i.e., neurological, cardiovascular, and several tumors. Structural data on the atomic level indicate the extended superhelical rod-like structure of the first two-thirds of the molecule with a typical armadillo repeat motif (ARM) in the N-terminal part and a triple helical motif in its C-terminal part. EFR3s’ best-known molecular function is anchoring the giant phosphatidylinositol 4-kinase A complex to the plasma membrane crucial for cell signaling, also linked directly to the KRAS mutant oncogenic function. Another function connected to the newly uncovered interaction of EFR3A with flotillin-2 may be the participation of the former in the organization and regulation of the membrane raft domain. This review presents EFR3A as an intriguing subject of future studies.
1. Introduction
The EFR3A (Eighty-Five Requiring, homolog A) protein and its paralogs and orthologs are rather poorly understood eukaryotic plasma membrane (PM) peripheral proteins. In eukaryotic organisms, the genes encoding yeast EFR3 homologs belong to a group of genes called the armadillo-like superfamily ARMH, also called ARM-like, encoding highly helical and superhelical proteins. This superfamily is characterized by a domain consisting of a multi-helical motif composed of two curved layers of α-helices arranged in a right-handed superhelix, in which the repeats forming this structure are arranged around a common axis. (see below, Section 4).
The yeast EFR3 (YMR212C) gene ortholog was originally discovered and named as one of seven Pho 85 (nonessential CDK) Requiring (efr) loci, efr1–3 and 5–8 [1]. It appeared orthologous to D. melanogaster, the rolling blackout (rbo, stmA-PF; stambhA; cmp 44E) involved in phototransduction and synaptic vesicular traffic [2,3]. Moreover, EFR3 ortholog genes can be found in lower metazoan organisms such as Cnidaria, e.g., coral Stylophora pistillata, and Nematoda, e.g., Caenorhabditis elegans [4]. In Chordata, including Urochordata (tunicates), Cephalochordata (Lancelets), and Cyclostomata (Lampreys), orthologs resembling homolog B in all remaining vertebrates can be found [5].
In all vertebrates (excluding Cyclostomata), two homolog genes and proteins, A and B, were found, each expressing at least 2–3 experimentally confirmed protein isoforms as well as several predicted ones (Figure 1A) that may mean that the first two paralogs might have appeared in cartilagous fish around 400 mya. In humans, two homolog genes located in chromosomes 8 and 2 encode proteins EFR3A and EFR3B, respectively. Overall homology between these two gene products is rather moderate and reaches, for example, for isoforms 1 of each protein, 64% identity and 78% overall similarity. As can be seen, isoforms 2 of either homolog lack an N-terminal sequence containing 3–4 cysteine residues, which are the target of palmitoylation (see Figure 1B and the text below). It is interesting that in yeast EFR3, a small hydrophobic three methionine and one phenylalanine residue cluster (5-MRMMF-9) can be observed (see Figure 1C). Constituting a permanent hydrophobic patch, it might participate in the interaction with the plasma membrane.
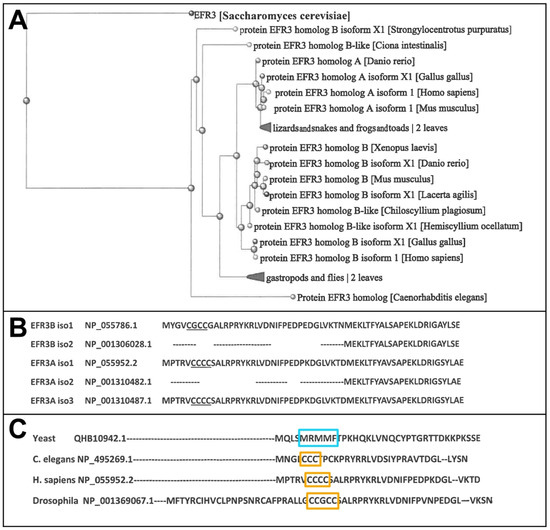
Figure 1.
Evolution of EFR3 proteins. (A). Simplified phylogenic tree of EFR3 prepared with NIH BLASTp software (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins&PROGRAM=blastp&BLAST_PROGRAMS, accessed on 15 December 2024). The more detailed phylogenic tree can be found under (Ensemble GeneTree ENSGT00390000002143) [6]. (B). Sequence comparison of EFR3A isoforms. Isoforms 2 of both EFR3A and EFR3B lack an N-terminal sequence containing a C-cluster (undelined). (C). Three methionine residues in yeast EFR3 (blue frame) may have preceded N-terminal cysteine cluster (3–4 residues) in metazoan orthologs (orange frames) starting from Nematoda. Analyses were performed by using Clustal Omega web software [7].
Moreover, Noack et al., while looking for possible EFR3 homologs in Arabidopsis via sequence alignments found four potential proteins that they called the EFR of plants (EFOP) 1–4 which belong to the class of ARMH superfamily. They found several structural and functional similarities between fungal and animal EFR3 and plant proteins [8].
The EFR3A gene in Homo sapiens is located on chromosome 8 (8q24.22) and spans 109,550 nt. The gene comprises up to 40 exons with 11 transcription variants, including 8 alternatively spliced [9]. According to this source, the EFR3A gene was found to be expressed to rather high levels, i.e., exceeding 4.3 times the average gene expression in normal and pathological human tissues. In turn, the gene encoding EFR3B is located on the short arm of chromosome 2, spans 117,036 nt, and contains up to 33 exons, the product of which is eight different mRNA molecules, including four representing alternatively spliced transcripts. Two isoforms of 817 and 787 amino acid residues long (isoform 1 and 2, respectively) have been listed in humans [10]. This homolog is expressed at the average level in various organs, tissues, and cells of primates and humans [9].
2. Physiological Roles of EFR3A
2.1. Drosophila Melanogaster Phototransduction
Phospholipase C (PLC)-dependent opening of transient receptor potential (TRP) and TRP-like (TRPL) channels mediate phototransduction in flies [11,12]. PLCβ cleaves PI(4,5)P2 into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which results in the activation of the channels mentioned above and triggers an electrical signal in response to light stimulus [13]. As the PLC activity is very high, a feedback inhibition mechanism involving Ca2+ and PKC maintaining the levels of PI(4)P and PI(4,5)P2 is suggested [14].
Reducing phosphatidylinositol 4-kinase PI4KIIIα (PI4KA) levels by knockdown or using PI4KA mutants results in decreased levels of PM PI(4)P and PI(4,5)P2 and altered light responses of Drosophila photoreceptors. Depletion of the Drosophila Efr3 (rbo) and tetratricopeptide repeat domain 7 (TTC7), which attach PI4KAα to the PM, also results in reduced PM PI(4)P and PI(4,5)P2 levels, and an impaired light response [15,16].
Phosphoinositides are implicated in the vesicular traffic. Data on Drosophila rbo mutants may confirm this hypothesis, although they indicate some differences between neuronal and non-neuronal vesicular traffic [17].
2.2. EFR3 Proteins Affect GPCR Responsiveness by Regulating Receptor Phosphorylation
A and B paralogs of EFR3 were found to be involved in the GPCR receptor signaling cascades [18]. The AT1R (angiotensin II receptor type 1) belongs to seven transmembrane helical G-protein-coupled receptors (GPCRs). Activation of the AT1R regulates various physiological events in different tissues, namely, vasoconstriction, aldosterone release, renal sodium reabsorption, facilitation of adrenergic transmission, vascular smooth muscle cells (VSMC) hypertrophy, and cardiac myocyte hyperplasia (for a review, see e.g., [19,20,21]). One of the several and best-known signaling pathways of the AT1R is the activation of phospholipase C via heterotrimeric Gq/11 protein (human Gα11 encoded by the GNA11 gene) leading to the generation of two second messengers: DAG and IP3. The first one activates the calcium-dependent protein kinase C (PKC), which phosphorylates several proteins, among them myosin light chain kinase or PP2Ac (a protein phosphatase), which dephosphorylates AKT and inactivates it leading to the death-receptor-independent apoptosis [22]. The second product of PI(4,5)P2 hydrolysis binds to IP3 receptors in the endoplasmic reticulum; ligand-gated Ca2+ channels are responsible for this ion release from this organelle functioning as intracellular calcium stores [23].
EFR3 proteins are thought to localize phosphatidylinositol 4-kinase (PI4KA) to the PM (see below). Bojjireddy et al. [18], while silencing EFR3A and/or EFR3B gene expression, found that after AngII stimulation in knockdown (KnD) cells, no reduction in PI(4)P and PI(4,5)P2 pools were observed, which suggests that EFR3s do not directly affect the phosphoinositide pool in the PM since PI4K still can provide sufficient amounts of these phosphoinositides in the PM. However, they observed a decreased plateau phase in Ca2+ levels following stimulation of AT1 with angiotensin II, opposite to control cells. It appeared that in the EFR3 KnD cells, the AT1 receptor is hyperphosphorylated, which the authors consider responsible for its uncoupling from the Gq11 alpha subunit after stimulation. However, it still maintains its ability to be internalized. In their opinion, in EFR3 KnD cells, a defect in returning the receptors from their desensitized state occurred [18]. The same authors found a similar, although less pronounced, effect on the endogenous adrenergic β-receptors after stimulating EFR3 KnD HEK cells with isoproterenol, measuring cAMP production. However, there are no detailed data on the molecular mechanism governing the regulation of phosphorylation of GPCRs in which EFR3 proteins are involved.
2.3. EFR3A and Insulin-Mediated Dispersal of GLUT4
Vital for glucose homeostasis in animal organisms is insulin-regulated glucose transport into muscle and adipose cells. It depends on GLUT4 (glucose transporter type 4), an integral, 12-transmembrane domain protein responsible for the facilitated diffusion of glucose into cells. Insulin binding to its receptor triggers a phosphorylation cascade involving PI3K, AKT, and mTORC2 that results in several vesicular traffic events leading to the delivery of GLUT4 to the PM (recent review see [24]). Failure to target GLUT4 in the PM results in insulin resistance and type 2 diabetes [25]. In unstimulated adipocytes, most of the GLUT4 pool resides in the intracellular vesicular compartment, including the TGN (trans-Golgi network), endosomal compartment, and insulin-responding vesicles (IRV) [26]. The latter fuse with the PM in a Rab13-dependent manner [27]. Apart from insulin-regulated delivery of GLUT4-bearing vesicles to the PM, insulin plays a role in the regulation of GLUT4 dynamics in the PM. Stenkula et al. [28] suggest that in the PM, GLUT4 occurs as freely diffusing monomers and stable domains containing GLUT4 clusters, which result from fusion with ”selective retention”. According to their model, insulin causes exocytosis of GLUT4-bearing vesicles, leading to monomeric GLUT4 in the PM, which transports glucose into the cell and above-mentioned domains containing GLUT4 clusters. The generation of monomeric GLUT4 outweighs selective retention by 30 times. Retrograde clathrin-mediated endocytosis also takes place from the preexisting clusters. Therefore, the above-mentioned clusters are the hub between functional monomers and the re-internalization of GLUT4.
Ectopically expressed human GLUT4, although distributed to the plasma membrane, did not support the growth of a yeast strain lacking hexose transporters unless this strain had a mutation in the EFR3 gene (fgy1-1) [29,30,31]. These results pointed the researchers’ attention to the role of EFR3 protein and PI4KA in regulating insulin-stimulated glucose transport and GLUT4 dispersal in 3T3-L1 adipocytes. EFR3A, a dominant paralog expressed in these cells (500-fold higher mRNA level when compared to EFR3B) [32], is suggested to function as a key regulator of GLUT4 dispersal in the PM. Knockdown of EFR3A or PI4KA impairs insulin-stimulated glucose transport in adipocytes. Knockdown of EFR3A expression reduces insulin-mediated spreading of GLUT4 in the PM. These data may shed light on the participation of the EFR3A–PI4KA axis in the regulation of GLUT4 function and the role of genes encoding this multiprotein complex in the regulation of GLUT4 function and possibly type 2 diabetes [33].
2.4. Brain-Specific EfrA Knockout Promotes Hippocampal Neurogenesis in Mice
Brain-specific deletion of Efr3a revealed enhanced hippocampal neurogenesis in adult mice. It appeared that in Efr3a KO mice, hippocampus newborn neurons were characterized by extended survival and decreased levels of apoptosis. The probable mechanism is connected with the increased levels of the expression of brain-derived neurotrophic factor (BDNF) and tropomyosin-related kinase B TrkB (TrkB)-encoding genes that govern signaling pathways controlling survival, and first of all, the AKT pathway [34].
Other reports suggest that the EFR3A protein may also play a key role in the irreversible spiral ganglion (SGCs) degeneration in the cochlea. Observation of expression of the mouse Efr3a gene during drug (kanamycin and furosemide)-induced hair cell loss and spiral ganglion degeneration indicated a temporal increase in Efr3a expression [35]. Further studies using Efr3a knockdown mice and mice overexpressing this gene led to the conclusion that loss of Efr3a expression may lead to a delay in hair cell loss and spiral ganglion degeneration. The molecular mechanism underlying this effect relates to the abovementioned increased expression of BDNF signaling pathway genes [36,37].
3. Association of EFR3A Protein with Disease States
Data concerning the involvement of the EFR3A gene and/or protein in human pathology are scarce. However, the literature indicates the involvement of this gene in several types of human disorders, i.e., neurological, cardiovascular, and several tumors.
Neurological disorders include autism spectrum disorders (ASD), in which six somatic, nonsynonymous mutations in the EFR3A coding sequence were observed twice more frequently in patients than in control subjects, as was found in a large cohort exome and Sanger sequencing-based study. The fact that the EFR3A expression pattern is shared with ASD-associated genes i.e., synaptic genes and PI(4,5)P2 phosphatase substantiates the correlation between EFR3A and ASD [38]. EFR3A was found among the fourteen genes as the features of primary glioma, allowing the stratification of these patients into low- and high-risk groups [39]. EFR3A was also found to be engaged in essential tremor (ET), a common neurological disorder. Analysis of publicly available and the authors’ own RNAseq data indicated EFR3A among seven genes whose expression pattern was abnormally changed, i.e., EFR3A was upregulated [21]. Other reports suggest that the EFR3 protein may also play a key role in spiral ganglion cell (SGCs) degeneration in the cochlea [36,37]. Irreversible sensorineural hearing loss is caused by irreversible damage to cochlear hair cells and subsequent progressive degeneration of spiral ganglion neurons (SGNs).
Interestingly, EFR3A is associated with the development of Alzheimer’s disease. The knockout of Efr3a in the mice CA3 area of the hippocampus led to Aβ (Amyloid β)-induced depletion of PI(4,5)P2. Moreover, selectively deleting Efr3a at the presynaptic site in CA1 pyramidal neurons leads to improved cognitive function and memory in the APP/PS1 mice model of Alzheimer’s disease [40]. On the other hand, the depletion of highly enriched isoform Efr3b in the CA2/CA3 hippocampal area of pyramidal neurons (PN) results in excitability and social novelty recognition deficits in mice [41].
There are also reports linking EFR3A with cardiovascular diseases, in particular with coronary artery disease (CAD), as EFR3A was found to be one of three genes regulated by miR-367. EFR3A and CD69 were significantly upregulated while FBXW7 was downregulated in the sera of these patients. CD69 encodes the transmembrane Ca2+-dependent lectin-type receptor, and FBXW7 encodes the F-box protein (containing the F-box, an approximately 40 amino acid residue motif), which constitutes one of the four subunits of SCFs (SKP1-cullin-F-box), the ubiquitin ligase complex. Further experiments on human aortic endothelial cells led to the conclusion that EFR3A and FBXW7 may participate in the regulation of NF-κB-activated inflammatory pathways, possibly via modulation of mTOR/AKT pathways [42].
A whole exome and targeted sequencing study in patients with acute coronary syndromes (ACS) subjected to antiplatelet therapy (after artery opening) revealed that a single nucleotide polymorphism (SNP) in the EFR3A loci (rs4736529) was among SNPs in eight genes showing strong associations with 18-month major adverse cardiovascular events (MACE) [43].
Data on the importance of EFR3A and its product in neoplastic diseases concern colorectal, pancreatic ductal, nasopharyngeal carcinomas, and brain tumors. In the adenoma case, a novel missense variant in the EFR3A gene (chr8: 133057465; p.G390E) was found. However, this was not validated in the cohort of 288 colorectal cancer (CRC) cases. It was not determined whether this variant was a rare nonsignificant mutation in adenoma or was a low-frequency change overlooked by Sanger sequencing [44].
Analysis of the genetic alterations in EFR3A in human neoplasms (cBioportal cancer genomics datasets) revealed that pancreatic ductal adenocarcinoma (PDAC) exhibits the highest EFR3A amplification frequency (almost 12% of cases) compared to, e.g., nonsmall cell lung cancer (NSCLC) and colorectal cancer (~5%). Patients with EFR3A gene alterations (mainly amplifications) presented lower overall survival (~26%) and reduced by ~53% disease-free period compared to subjects with near-normal EFR3A expression levels [45]. The increased expression level of EFR3A was observed mainly in subjects with mutations in the KRAS gene, which is observed in 85% of PDAC patients [46,47]. When EFR3A gene HEK-HT cells harboring KRASG12V were disrupted, a drastic decrease in PI(4)P, phosphatidylserine, and KRAS levels at the PM was observed, which resulted in reduced RAS signaling. The effect was even stronger after both EFR3A and EFR3B genes were targeted with sgRNA [47]. The authors suggested the PI4KA pathway as a possible drug target in KRAS mutation-positive PDAC cases and proposed using, e.g., simeprevir, a known antiviral drug, or C7, PI4KA inhibitors [45,47].
circEFR3A (hsa_circ_0135761) was detected among the top dysregulated circRNAs in nasopharyngeal carcinoma (NPC) cases [48]. Further studies confirmed the overexpression of circEFR3A in human NPC cell lines and indicated that circEFR3A promotes the expression of EFR3A. The silencing of circEFR3A had an anti-oncogenic effect, which was manifested in increased apoptosis and decreased cell proliferation [49]. The putative role of the oncogenic circEFR3A in the EFR3A expression regulation may become a drug target for NPC. However, the limitations concerning circRNA research are still valid [50]. As mentioned above, EFR3A may also be involved in brain tumors. The study which utilized the data from the CRISPR-Cas9 lethality screens [51] performed on patient-derived glioma stem cells indicated a dependency on EFR3A at least in a single mesenchymal GSC-0131 line. Moreover, further analysis of these cell lines suggested a strong genetic redundancy between EFR3A and EFR3B in these tumors [6].
4. EFR3 Structural Features: Atomic Structure and Domain Organization
Examples of detailed models and experimental atomic structures of EFR3 proteins available in the Protein Data Bank are shown in Figure 2A–C. The crystal structure of the EFR3 protein was first proposed by Wu et al. in 2014 that was performed on the yeast, Saccharomyces cerevisiae protein, more precisely on the N-terminal fragment of the EFR3 protein (EFR3-N8–562) [52] except for the disordered loop containing residues 217–232 at 3.2 Å resolution (Figure 2C, PDB ID: 4N5A) by using SAD (single-wavelength anomalous dispersion method) with selenomethionine-substituted crystals for phasing. From their studies, it is known that the N-terminal fragment EFR3-N8-562 is predominantly α-helical, in which the first ~150 residues are folded in 8 helices (H1–H8), being similar ARM or HEAT motifs, and forming a superhelical VHS (Vps-27, Hrs and STAM) domain [53,54,55,56,57]. Residues 48–144 form an ARM (armadillo) motif [58], which consists of a right-handed superhelix formed of several α-helices, a characteristic feature of the EFR3 protein family. In contrast, the remaining part of the molecule comprises 19 alpha-helices being HEAT motifs. The entire molecule of EFR3-N8–562 forms an extended 120 nm rod stabilized via hydrophobic interactions of the helices of one repeat making contact with their counterparts in the neighboring repeats, which results in forming a continuous α-α-superhelix [59]. A conserved, basic patch at an N-terminal part of the molecule (VHS domain) was proven to be responsible for interactions with phosphatidyl inositols [52]. The authors suggest that these interactions are responsible for the attachment of EFR3 and its complexes with other proteins to the PM. Another basic patch occurs near the middle part of the studied molecule, by the loops joining helices 11–18. The abovementioned studies allowed the application of a sophisticated Alpha-Fold software to propose structural models of vertebrate EFR3 molecules (for example, the human EFR3A structural model is shown in Figure 2A (AlphaFold DB: AF-Q14156-F1) and EFR3B structure is shown in Figure 2B (AlphaFold DB: AF-Q9Y2G0-F1). As can be seen, the obtained models are remarkably similar to the original template molecule (see Figure 2D). This indicates the remarkable level of conservation of the secondary and tertiary structures, while only the N-terminal region (residues 11–109 EFR3 of yeast and 14–111 of human EFR3A) molecule shows the substantial degree of amino acid residue sequence identity (23%) and similarity (58%).
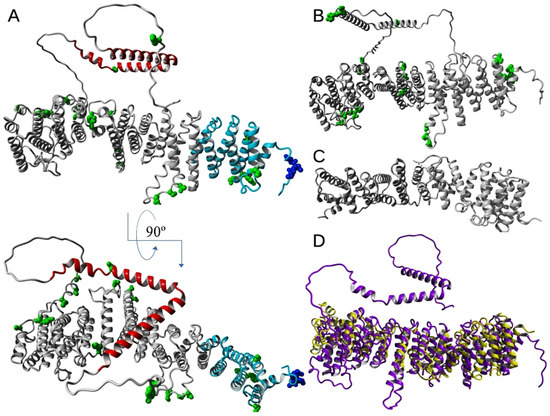
Figure 2.
Structural models of EFR3 proteins. Computed structure model of (A) human EFR3A (AlphaFold DB: AF-Q14156-F1), cysteine residues marked with blue, phosphorylation sites in green, fragment comprising first 150 amino acid residues at N-terminus marked with cyan, C-terminal part of the protein in red and (B) EFR3B (AlphaFold DB: AF-Q9Y2G0-F1), phosphorylation sites in green [60]. Experimental structure (C) of Saccharomyces cerevisiae EFR3 (PDB ID: 4N5A) [52]. Overlay (D) of structures presented in A (magenta) and C (yellow). Structures rendered with YASARA [61].
From the first structural studies, it was proposed that the C-terminal part of the EFR3 molecule and its metazoan orthologs form mostly random coil structures [62,63]. However, cryo-EM, HDX-MS, and recent mutational studies of Suresh et al. [64] showed that the conserved middle C-terminal region of the EFR3A molecule (residues 724–787) forms a V-shaped structure composed of three α-helices which allows interaction with its partner proteins that allows PI4K interaction with the PM (see below Section 6.1 and Figure 3).
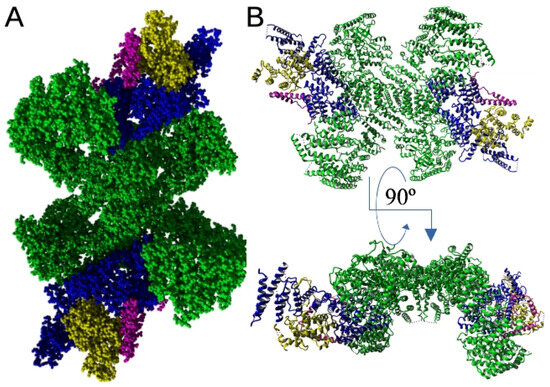
Figure 3.
Full atom (A) and secondary structure (B) representation of PI4KA complex bound to C-terminus of EFR3A as determined via electron microscopy with [64] 3.65 Å resolution (PDB ID: 9BAX). Green, two molecules of PI4Kalpha that form central tandem; blue, TTC7B; yellow, FAM126; magenta, C-terminus of EFR3A. Structures rendered with YASARA [61].
5. Post-Translational Modifications
5.1. S-Palmitoylation
S-palmitoylation is a well-known post-translational modification catalyzed mainly by enzymes representing a zinc-finger DHHC motif-containing protein family that is supposed to be responsible for numerous proteins’ interaction with a membrane bilayer and, therefore, subcellular localization of proteins in eukaryotic cells. S-palmitoylation could also be responsible for the stabilization of protein conformation, permitting protein–protein interactions. It is also thought to play a role in several crucial signaling pathways [65,66]. The palmitoylation process of some proteins was found to be engaged in human pathologies, therefore, palmitoylation inhibitors are considered potential therapeutics [67].
Vertebrate EFR3A is known to be palmitoylated. In the N-terminal region, a cluster of 3–4 cysteine residues that are strong candidates for palmitoylation is easily detected with several in silico predicting tools, e.g., CSS-PALM [68], (see Figure 1C and Figure 2A) or found in the proteomic palmitoylation database [69]. EFR3B also has a triple cysteine motif (C5, C7, and C8), a target of palmitoylation. An identical/similar motif is present in C. elegans and D. melanogaster orthologs of EFR3. Both proteins were found to be metabolically radiolabeled with [3H]palmitate [18]. The arrangement of palmitoylated groups in EFR3B affects the interactions between EFR3B and the transmembrane protein, TMEM150A [70]. Interestingly, the same authors found that this post-translational modification, in contrast to the other palmitoylated proteins is stable, i.e., in the case of EFR3B, it was stable over several hours. Similarly, our attempt to pharmacologically inhibit EFR3A palmitoylation with 2-bromo-palmitate (2-BrP) in HeLa cells, even for 48 h treatment was unsuccessful (Trybus et al.—unpublished data). Data from mutational experiments indicate that the PM localization of both EFR3A and EFR3B depends on the presence of a palmitoylation motif at the N-terminal region of the proteins [18,71]. The latter authors also reported that a short 37 N-terminal amino acid residue fragment of EFR3A can target GFP protein to the membrane. This construct is retained in the Golgi network, suggesting that other regions of this protein are responsible for further transport to the PM [18]. On the other hand, data on yeast EFR3 protein suggest stable PM localization despite the absence of an N-terminal ~140 amino acid residue segment containing 3–4 cysteine palmitoylation motif. We have to keep in mind that there are isoforms of EFR3A and EFR3B (isoforms 2) that do not have this cysteine motif (see Figure 1B). So far, we are unaware of existing experimental data on the localization of these isoforms in the PM.
5.2. Phosphorylation
According to the data reported in the PhosphoSitePlus database, 19 phosphorylation sites can be found in the EFR3A, including three tyrosine residues. At least six S/T residues are present within the short region between S680 and S738, including S694, detected as phosphorylated in numerous reports [72] and S738 in the helical region interacting with TTC7B and FAM126A (see above and [64]). In yeast, phosphorylation was found to inhibit the binding of Ypp-1 (ortholog of TTC7) and, as a consequence, the recruitment of yeast PI4K homolog, Stt4, to the PM [52]. It should be mentioned that although EFR3 orthologs and paralogs differ in the exact phosphorylation sites, one can see some similarities. Namely, certain regions of both molecules contain residues that are the target of phosphorylation, i.e., residues 219–224 in EFR3A and residues 212–216 in EFR3B and in the C-terminus residues 680–738 in paralog A and 689–723 in paralog B, while the other phosphorylation sites are not conserved [72] (see also Figure 2). In yeast EFR3, phosphorylation sites are located mostly within the C-terminal region, i.e., 23 of 25 S/T/Y residues are located downstream of position 580. The phospho-cluster seems much wider than in vertebrates as 19 S/T/Y residues are located between residues 611 and 771 (Figure 2C).
5.3. Other Post-Translational Modifications
Both EFR3A and EFR3B are subject to post-translational modifications such as ubiquitination at K residues. In the PhosphoSitePlus database [72], the number of sites for this modification varies, from 9 out of 11 K residues in EFR3A to 3 out of 8 K residues in EFR3B. There are no available experimental data concerning the particular function of this modification. Also, there are no experimental data on the function of acetylation of three out of eight K residues that were found to be a target of acetylation in EFR3B and a single K residue detected in EFR3A [72].
6. Functional Complexes
The interaction network of human EFR3 proteins has not been explored in detail. The STRING database shows only several experimentally confirmed partner proteins (Figure 4) for EFR3A. There are indications of numerous other interactions, but they still await further confirmation [73]. Interesting results were obtained using BioID proximity assay technology (review [74]). Using this technique on the osteosarcoma cell line U2OS to study the proteome of focal adhesions, Dong et al. found EFR3A as a new partner for kindlin-2, a stress fiber and focal adhesion integrin regulating protein [75]. Using a similar approach, Counter’s team discovered EFR3A as a KRAS oncogenic (G12V) variant protein partner. Their further experiments documented the in vitro interaction of these proteins and that KO of EFR3A and EFR3B or PI4KA reduced cell proliferation signaling and colony formation of cultured cancer cells accompanied by reduced oncogenic KRAS localization at the PM due to the reduced content of PI(4)P and phosphatidylserine (PS). Moreover, they showed the reduction of KRAS-dependent cancer cell proliferation via PI4KA inhibition as Compound 7 (C7, PMID: 24366037) or Simeprevir, an anti-Hepatitis C virus (known as PI4KA inhibitors) mislocalized KRAS from the PM, indicating the potentiality of these compounds to be repurposed towards anti-cancer drugs [47].
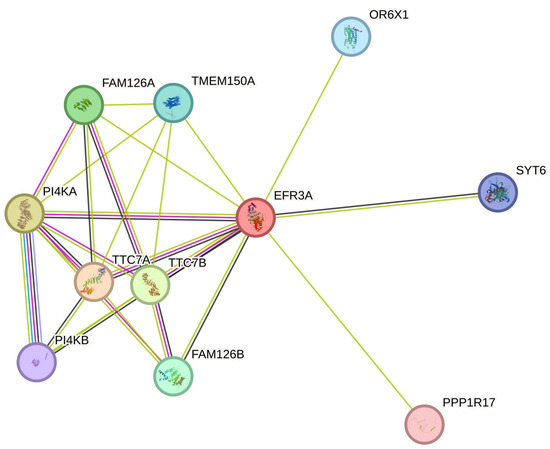
Figure 4.
EFR3A known interactions—simplified interactome from String database [73]. Line color code as in the String database, i.e., blue-green, data from the curated database; magenta, experimentally determined; green, red, and dark blue, gene neighborhood, gene fusions, gene co-occurrence, respectively light green, text mining; black, co-expression; and blue, protein homology. For other details, see the String database.
6.1. PI4K Anchoring in the Plasma Membrane
The best known and the most important function of the yeast Efr3 protein (or EFR3A/B in humans) is the PM anchorage of phosphatidylinositol-4 kinase (PI4KA in humans or Stt4 in yeast), the key enzyme of the PM phosphoinositide metabolism [76,77,78]. It was found that EFR3A, together with other proteins (see below), forms a PI4KA/Stt4 membrane-anchoring complex [18]. Thus, one of the best-known roles of EFR3 proteins is participation in the metabolism of phosphatidylinositol phosphates, in particular phosphatidylinositol 4-phosphate (PI(4)P), which is one of the most important membrane regulatory phospholipids.
Phosphatidyl inositol phosphates play a fundamental role in cellular signaling, being involved in the regulation of growth and proliferation, migration, and survival (for recent review, see e.g., [79]). Examples of known signaling pathways are numerous, starting from phosphatidyl inositol (3,4,5)-trisphosphate (PI(3,4,5)P3) and phosphatidyl inositol (4,5)-bisphosphate (PI(4,5P)2) that are the sources of diacylglycerol DAG and inositol trisphosphate IP3 to PI(3,4)P2 engaged in growth factor, mTOR, and AKT signaling to various PIP derivatives engaged in endocytosis. Phosphatidyl inositol phosphates are most abundant in the PM but are also present in the Golgi complex, the endosomal, and the lysosomal system [80,81]. Both yeast and higher eukaryotic PI4Kinases are large proteins with a subunit molecular weight of 214 kDa (1900 amino acid residues in yeast) [82] and 240 kDa (2102 amino acid residues in humans) [83]. This enzyme was found to be essential for yeast cell survival, but its deletion was rescuable at 1 M sorbitol, according to its discoverer group [84]. However, other researchers working with different strain backgrounds found this enzyme essential under all conditions [85]. On the other hand, in C. albicans, PI(4)P and, thus, PI4Kinase were found not necessary for survival but appeared indispensable for cell wall synthesis and infectivity [86].
The tetratricopeptide repeat domain 7 (TTC7A/B, [87,88]) are 858 and 848 amino acid residue proteins encoded by two paralog genes, located in chromosomes 2 and 14, respectively. Tetratricopeptide (TPR) domains are 34 amino acid residue repeating units forming two antiparallel α-helices separated by a turn that forms a structure resembling a spiraling staircase [87,88]. TTC7 (similar to yeast equivalent, Ypp1) is responsible for maintaining the integrity of the complex and is thought to be responsible for the correct folding of the complex [62,89]. Mutations in TTC7A have been associated with the rare hereditary human disease, combined immunodeficiency with multiple intestinal atresias (CID-MIA) [90,91,92,93,94]. Mutations in TTC7B have also been inferred in the molecular basis of several disorders, e.g., hypomyelinating leukoencephalopathy [95].
Proteomic analysis of TCC7 protein partners showed that FAM126A/B, also known as hyccin, or HYCC1 (hyccin PI4KA lipid kinase complex subunit 1) belongs to the complex [62]. FAM 126A, a 521 amino acid residue protein, is present only in higher eukaryotes, and there is no ortholog protein/gene in S. cerevisiae. Mutations in the gene encoding this protein resulting in the absence or presence of dysfunctional HYCC1 were reported to be connected with hypomyelination and congenital cataracts. Patients lacking the FAM126A protein and with L53P and C57R substitutions showed a reduction in the levels of PI4KA and its adapter proteins TTC7A/B and EFR3A. This suggests the disorganization of the complex in the absence of FAM126A. A decrease in PI(4)P was also observed in fibroblasts from hypomyelination and congenital cataract patients [62,96,97]. It should be noted that the homologous gene, FAM126B/HYCC2, encodes the other member of the PI4 kinase complex paralog protein, but there are no experimental details apart from the expression profile. However, a recent study on colorectal cancer indicates the loss of expression of FAM126A in 7% of cases due to promoter hypermethylation. These cancer cells become dependent on FAM126B expression [98].
As was mentioned, PI4KA/Stt4 in eukaryotic cells is anchored to the PM by the evolutionarily conserved giant protein complex containing TTC7A/B (or Ypp1), FAM126A [62], and EFR3A/B (EFR3 in yeast) [89]. Data from cryo-EM analysis of the structure (at 3.6 Å resolution) showed that 700 kDa super-assembly is formed of heterotrimers assembled from PI4KA, TTC7, and FAM126 [62,99]. The complex (also called complex I) has a flat surface perfectly adapted to the PM bilayer interface. Catalytic sites of the kinase are directed towards PM-localized PI [63]. It is suggested that interaction between PI4KA and TTC7/FAM126 helps to stabilize the conformation of the PI4KA and to enable the formation of an interaction surface with the membrane, including the kinase active sites. The conserved common surface in TTC7/FAM126 parallel to the PI4KA membrane interaction surface serves as a docking site for the abovementioned C-terminally located, V-shaped domain of EFR3, which is responsible for PM attachment of the complex [63,64,89] (Figure 3).
There is also an alternative way to connect PI4KA to PM via complex II. Namely, this is mediated by TMEM150, a conserved mammalian ortholog of the yeast Sfk1 protein belonging to the TMEM150/FRAG1/DRAM family, which was found to substitute TTC7 without changing the localization of PI4KA or EFR3A in the PM [100]. However, attachment to TMEM150, at least in the case of EFR3B homolog, depends on its palmitoylation pattern. More specifically, the form of EFR3B triple palmitoylated on residues C5, C7, and C8 or double palmitoylated on residues C5 and C7 or C8 forms complex I occurring in the lo region and is responsible for the synthesis of PI(4)P and PI(4,5)P2 in these domains. Double palmitoylation of EFR3B at residues C7 and C8 enables its interaction with TMEM150A and the formation of complex II and the associated relocation of TMEM150A from lo to ld and the synthesis of PI(4)P and PI(4,5)P2 in the ld PM regions [70]. A similar mechanism likely governs switching between alternative protein complexes and, therefore, PM domains by EFR3A, but it remains to be discovered.
6.2. EFRA Possibly Plays a Role as Membrane Raft Organizer—Interaction with Flotillin-2
PMs of living cells are characterized by several physicochemical parameters, e.g., phase state, mobility of component molecules, and mechanical properties. The phase state behavior of the PM is characterized by lateral heterogeneity as was demonstrated via a variety of biophysical and biochemical methods. The existence of membrane (lipid) rafts is one of the best-known forms of this heterogeneity first proposed by Simons and Ikonen in 1997 [101]. The basic assemblies, called resting-state rafts, are cholesterol-enriched, more ordered domains that are small (~20 nm in diameter), dynamic (Ʈ1/2 ~1 s), and contain a set of characteristic proteins (for reviews, see e.g., [102,103,104]). The specific set of membrane proteins, including membrane proteins belonging to the SPFH family (stomatin/prohibitin/flotillin/HflK), such as raft scaffold proteins flotillin-1 and flotillin-2, stomatin, or stomatin-like protein, seem to be permanent components of membrane rafts [105]. These proteins share a common feature: they associate with raft domains, possibly through cholesterol-binding and/or oligomerization [106,107]. Besides the mentioned proteins, rafts also include other palmitoylated and transmembrane proteins and GPI-anchored proteins (for a review, see [108,109]). In general, raft domain(s) are engaged in many physiological processes underlying various functions of organisms, such as numerous signal transduction pathways [110,111], several innate and adaptive immune responses, and well-known host-pathogen interactions (e.g., [112,113]), neoplasia (e.g., [114,115,116,117]), and other pathologies, in particular neuropathologies such as atherosclerosis and Alzheimer’s disease [118,119].
Results of our work on the lateral membrane organization support the idea that the underlying molecular mechanism of resting state raft organization is, to a large extent, protein–protein interactions [102,120], particularly those involving flotillins and other palmitoylated proteins. We have previously found that MPP1 (membrane palmitoylated protein 1, for review, see [121]) is involved in erythroid cells’ membrane raft organization and regulation in a palmitoylation-dependent manner. Based on experiments on erythroid cell line HEL with MPP1 KnD, on erythrocyte membranes and recombinant proteins, and using a variety of biochemical and biophysical methods, we discovered that MPP1 may play a key role in resting state membrane raft organization and regulation in erythroid cells. This conclusion was based on three major sets of observations. Firstly, pharmacological inhibition of palmitoylation or KnD of the MPP1 gene led to the decreased membrane order and changed membrane probe mobility [122,123,124]. Secondly, MPP1-knockdown affected the activation of MAP-kinase signaling via raft-dependent tyrosine kinase receptors, particularly from insulin receptors [122,125]. The signal inhibition occurred at the level of H-Ras, as GDP-to-GTP exchange upon insulin treatment was not observed. Moreover, FLIM-FRET microscopy revealed impaired interaction of H-Ras with Raf (effector) in insulin-treated MPP1 KnD cells [118]. Thirdly, MPP1 in the erythrocyte membrane was found to bind the raft-marker proteins, flotillin-1 and flotillin-2 [126]. These proteins are found in high molecular-weight complexes obtained after chemical cross-linking in native erythrocyte membranes, and their presence was shown to be independent of the well-known protein 4.1-spectrin-actin-based complexes of MPP1. These interactions were confirmed via co-immunoprecipitation, pull-down, overlay, and proximity assays. Moreover, our kinetics studies, by using the surface plasmon resonance (SPR) method, indicate high affinity binding to flotillin-1 (KD = 18.1 ± 2.4 nM) and flotillin-2 (KD = 24.8 ± 6 nM). Further studies on recombinant domains of MPP1 binding to flotillins indicate that a short sequence of ~40 amino acid residues between domains D5 and SH3 may be responsible for these interactions [127].
On the other hand, MPP1 is not expressed at high levels in most of the cell lines of mammalian origin we examined [120]. Our recent studies on HeLa cells were inspired by the idea that other protein(s) may play a role in resting state raft organization and regulation via interaction with raft scaffolding proteins, which are flotillins [128]. We first used a pull-down approach in which flotillin-2 was immobilized on a Sepharose resin and identified DRM (detergent-resistant membrane) fraction-associated proteins via the MS/MS technique. DRMs are considered a biochemical representation of membrane rafts, a kind of “read-out of whether a protein is likely to associate with rafts” [129]. Among several proteins bound to the resin, EFR3A appeared as a strong putative flotillin-2 partner. This result was confirmed via immunodetection of EFR3A and co-immunoprecipitation. The same pull-down results were obtained on several other cells, e.g., LNCaP and PC-3. EFR3A was present in the DRM fraction, and this presence was cholesterol-dependent. Further experiments indicated a substantial decrease in PM order and change in membrane raft probe mobility as revealed via FLIM and svFCS of living HeLa cells with an EFR3A KnD. Moreover, changes in membrane fluidity were observed also in the case of giant PM vesicles (GPMVs), which are supposed to be membrane skeleton and cytoskeleton proteins-free [130]. Moreover, a role of raft-associated EFR3A in cell signaling could be expected, as the EGF receptor (EGFR) function is known to depend on raft association [131] and depends on the EFR3A cellular status. Indeed, cells with a decreased level of that protein showed disturbed phosphorylation of EGFR and PLCγ1 (phospholipase Cγ1) upon stimulation with EGF. A similar effect was observed upon cholesterol depletion via treatment of wild-type HeLa cells with MβCD [128]. In light of the above-referred study, we may suggest that another role of EFR3A was uncovered. Namely, EFR3A may induce flotillin(s) and other protein and lipid components’ oligomerization and, as a result, resting state raft formation (Figure 5). However, we should note that further studies should be carried out to build a complete picture in light of the current discovery of flotillin structure in the membrane environment [132]. The interaction of flotillins with EFR3A in vitro should be characterized, and reconstitution studies to confirm the effect of the protein complex on the physical properties of the membrane should provide more evidence to support our model.
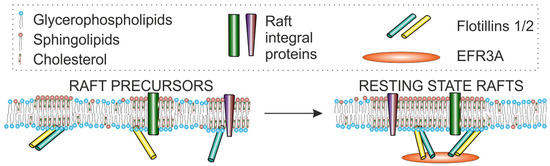
Figure 5.
The interaction of EFR3A with flotillins may play a key role in the organization and regulation of resting state rafts. Reprinted from ref. [128].
7. Concluding Remarks and Prospects
This review summarizes the information regarding the still poorly understood EFR3A protein and its encoding gene. Most data relate to the role of this protein as an element of the PI4KA complex responsible for its anchoring to the plasma membrane. However, it is interesting what the role of isoform 2 of both EFR3 paralog proteins is, as they neither have hydrophobic cluster residues nor are palmitoylated. Data from studies using the BioID technique encourage hypothesizing on diverse physiological roles, especially those related to the role of EFR3A interactions with the mutated KRAS protein. It seems that the data from our laboratory regarding the role of this protein in the organization and regulation of membrane rafts open a new field of research. When it comes to the major functions of this protein in the cell, many questions arise, and the main one seems to be whether the formation of the PI4KA docking complex in the plasmalemma is related to the function of the “raft organizer”. However, we need to wait for further research as the experimental data are still slightly incomplete.
Author Contributions
M.T. wrote the first draft; A.H.-J. discussed ideas and edited sequential versions of the manuscript; A.C. resources, writing-review and editing, visualization, project administration; A.F.S. funding, writing and editing the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre, Poland, projects No. 2020/37/B/NZ1/01106.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACS | acute coronary syndromes |
| ARM | armadillo |
| ASD | autism spectrum disorders |
| AT1R | angiotensin II receptor type 1 |
| BDNF | brain-derived neurotrophic factor |
| CAD | coronary artery disease |
| CID-MIA | combined immunodeficiency with multiple intestinal atresias |
| CRC | colorectal cancer |
| DAG | diacylglycerol |
| DRM | detergent-resistant membrane |
| EFR3A | eighty-five requiring 3A |
| GPMVs | giant PM vesicles |
| IRV | insulin-responding vesicles |
| NPC | nasopharyngeal carcinoma |
| PDAC | pancreatic ductal adenocarcinoma |
| PI(3,4,5)P3 | phosphatidyl inositol (3,4,5)-trisphosphate |
| PI(4,5P)2 | phosphatidyl inositol (4,5)-bisphosphate |
| PI4KA | phosphatidylinositol 4-kinase alpha |
| PLCγ1 | phospholipase Cγ1 |
| PM | plasma membrane |
| SAD | single-wavelength anomalous dispersion |
| SNP | single nucleotide polymorphism |
| TRP | transient receptor potential |
| TTC7 | tetratricopeptide repeat domain 7 |
References
- Lenburg, M.E.; O’Shea, E.K. Genetic evidence for a morphogenetic function of the Saccharomyces cerevisiae Pho85 cyclin-dependent kinase. Genetics 2001, 157, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.L.; Dockendorff, T.C.; Jongens, T.A. Clonal analysis of cmp44E, which encodes a conserved putative transmembrane protein, indicates a requirement for cell viability in Drosophila. Dev. Genet. 1998, 23, 264–274. [Google Scholar] [CrossRef]
- Huang, F.D.; Woodruff, E.; Mohrmann, R.; Broadie, K. Rolling blackout is required for synaptic vesicle exocytosis. J. Neurosci. 2006, 26, 2369–2379. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/protein/ (accessed on 15 January 2025).
- Available online: https://www.ebi.ac.uk/interpro/protein/UniProt/Q14156/entry/,pfam/#table (accessed on 20 December 2024).
- Hoellerbauer, P.; Biery, M.C.; Arora, S.; Rao, Y.; Girard, E.J.; Mitchell, K.; Dighe, P.; Kufeld, M.; Kuppers, D.A.; Herman, J.A.; et al. Functional genomic analysis of adult and pediatric brain tumor isolates. bioRxiv 2023. [Google Scholar] [CrossRef]
- The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Available online: https://www.ebi.ac.uk/jdispatcher/msa/clustalo (accessed on 4 November 2024).
- Noack, L.C.; Bayle, V.; Armengot, L.; Rozier, F.; Mamode-Cassim, A.; Stevens, F.D.; Caillaud, M.C.; Munnik, T.; Mongrand, S.; Pleskot, R.; et al. A nanodomain-anchored scaffolding complex is required for the function and localization of phosphatidylinositol 4-kinase alpha in plants. Plant Cell 2022, 34, 302–332. [Google Scholar] [CrossRef] [PubMed]
- Thierry-Mieg, D.; Thierry-Mieg, J. AceView: A comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006, 7 (Suppl. S1), S12. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/gene/22979 (accessed on 15 January 2025).
- Liu, C.H.; Bollepalli, M.K.; Long, S.V.; Asteriti, S.; Tan, J.; Brill, J.A.; Hardie, R.C. Genetic dissection of the phosphoinositide cycle in Drosophila photoreceptors. J. Cell Sci. 2018, 131, jcs214478. [Google Scholar] [CrossRef]
- Hardie, R.C.; Juusola, M. Phototransduction in Drosophila. Curr. Opin. Neurobiol. 2015, 34, 37–45. [Google Scholar] [CrossRef]
- Montell, C. Drosophila visual transduction. Trends Neurosci. 2012, 35, 356–363. [Google Scholar] [CrossRef]
- Hardie, R.C.; Liu, C.H.; Randall, A.S.; Sengupta, S. In vivo tracking of phosphoinositides in Drosophila photoreceptors. J. Cell Sci. 2015, 128, 4328–4340. [Google Scholar] [CrossRef]
- Balakrishnan, S.S.; Basu, U.; Shinde, D.; Thakur, R.; Jaiswal, M.; Raghu, P. Regulation of PI4P levels by PI4KIIIalpha during G-protein-coupled PLC signaling in Drosophila photoreceptors. J. Cell Sci. 2018, 131, jcs217257. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ncbi.nlm.nih.gov/datasets/gene/id/23167 (accessed on 20 December 2024).
- Vijayakrishnan, N.; Phillips, S.E.; Broadie, K. Drosophila rolling blackout displays lipase domain-dependent and -independent endocytic functions downstream of dynamin. Traffic 2010, 11, 1567–1578. [Google Scholar] [CrossRef]
- Bojjireddy, N.; Guzman-Hernandez, M.L.; Reinhard, N.R.; Jovic, M.; Balla, T. EFR3s are palmitoylated plasma membrane proteins that control responsiveness to G-protein-coupled receptors. J. Cell Sci. 2015, 128, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Gironacci, M.M.; Bruna-Haupt, E. Unraveling the crosstalk between renin-angiotensin system receptors. Acta Physiol. 2024, 240, e14134. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.F.; Sun, Y.L.; Hamet, P.; Inagami, T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001, 11, 165–180. [Google Scholar] [CrossRef]
- Nadel, G.; Yao, Z.; Hacohen-Lev-Ran, A.; Wainstein, E.; Maik-Rachline, G.; Ziv, T.; Naor, Z.; Admon, A.; Seger, R. Phosphorylation of PP2Ac by PKC is a key regulatory step in the PP2A-switch-dependent AKT dephosphorylation that leads to apoptosis. Cell Commun. Signal 2024, 22, 154. [Google Scholar] [CrossRef]
- Streb, H.; Irvine, R.F.; Berridge, M.J.; Schulz, I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 1983, 306, 67–69. [Google Scholar] [CrossRef]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- Bogan, J.S. Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 2012, 81, 507–532. [Google Scholar] [CrossRef]
- Geiser, A.; Foylan, S.; Tinning, P.W.; Bryant, N.J.; Gould, G.W. GLUT4 dispersal at the plasma membrane of adipocytes: A super-resolved journey. Biosci. Rep. 2023, 43, BSR20230946. [Google Scholar] [CrossRef]
- Sun, Y.; Jaldin-Fincati, J.; Liu, Z.; Bilan, P.J.; Klip, A. A complex of Rab13 with MICAL-L2 and alpha-actinin-4 is essential for insulin-dependent GLUT4 exocytosis. Mol. Biol. Cell 2016, 27, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Stenkula, K.G.; Lizunov, V.A.; Cushman, S.W.; Zimmerberg, J. Insulin controls the spatial distribution of GLUT4 on the cell surface through regulation of its postfusion dispersal. Cell Metab. 2010, 12, 250–259. [Google Scholar] [CrossRef]
- Wieczorke, R.; Dlugai, S.; Krampe, S.; Boles, E. Characterisation of mammalian GLUT glucose transporters in a heterologous yeast expression system. Cell Physiol. Biochem. 2003, 13, 123–134. [Google Scholar] [CrossRef]
- Boles, E.; Oreb, M. A Growth-Based Screening System for Hexose Transporters in Yeast. Methods Mol. Biol. 2018, 1713, 123–135. [Google Scholar] [PubMed]
- Schmidl, S.; Tamayo Rojas, S.A.; Iancu, C.V.; Choe, J.Y.; Oreb, M. Functional Expression of the Human Glucose Transporters GLUT2 and GLUT3 in Yeast Offers Novel Screening Systems for GLUT-Targeting Drugs. Front. Mol. Biosci. 2020, 7, 598419. [Google Scholar] [CrossRef]
- Koester, A.M.; Geiser, A.; Laidlaw, K.M.E.; Morris, S.; Cutiongco, M.F.A.; Stirrat, L.; Gadegaard, N.; Boles, E.; Black, H.L.; Bryant, N.J.; et al. EFR3 and phosphatidylinositol 4-kinase IIIalpha regulate insulin-stimulated glucose transport and GLUT4 dispersal in 3T3-L1 adipocytes. Biosci. Rep. 2022, 42, BSR20221181. [Google Scholar] [CrossRef] [PubMed]
- Koester, A.M.; Geiser, A.; Bowman, P.R.T.; van de Linde, S.; Gadegaard, N.; Bryant, N.J.; Gould, G.W. GLUT4 translocation and dispersal operate in multiple cell types and are negatively correlated with cell size in adipocytes. Sci. Rep. 2022, 12, 20535. [Google Scholar] [CrossRef]
- Qian, Q.; Liu, Q.; Zhou, D.; Pan, H.; Liu, Z.; He, F.; Ji, S.; Wang, D.; Bao, W.; Liu, X.; et al. Brain-specific ablation of Efr3a promotes adult hippocampal neurogenesis via the brain-derived neurotrophic factor pathway. FASEB J. 2017, 31, 2104–2113. [Google Scholar] [CrossRef]
- Nie, C.; Hu, H.; Shen, C.; Ye, B.; Wu, H.; Xiang, M. Expression of EFR3A in the mouse cochlea during degeneration of spiral ganglion following hair cell loss. PLoS ONE 2015, 10, e0117345. [Google Scholar] [CrossRef]
- Hu, H.; Ma, Y.; Ye, B.; Wang, Q.; Yang, T.; Lv, J.; Shi, J.; Wu, H.; Xiang, M. The role of Efr3a in age-related hearing loss. Neuroscience 2017, 341, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ye, B.; Zhang, L.; Wang, Q.; Liu, Z.; Ji, S.; Liu, Q.; Lv, J.; Ma, Y.; Xu, Y.; et al. Efr3a Insufficiency Attenuates the Degeneration of Spiral Ganglion Neurons after Hair Cell Loss. Front. Mol. Neurosci. 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.R.; Pirruccello, M.; Cheng, F.; Kang, H.J.; Fernandez, T.V.; Baskin, J.M.; Choi, M.; Liu, L.; Ercan-Sencicek, A.G.; Murdoch, J.D.; et al. Rare deleterious mutations of the gene EFR3A in autism spectrum disorders. Mol. Autism 2014, 5, 31. [Google Scholar] [CrossRef]
- Zhao, K.; Bai, X.; Wang, X.; Cao, Y.; Zhang, L.; Li, W.; Wang, S. Insight on the hub gene associated signatures and potential therapeutic agents in epilepsy and glioma. Brain Res. Bull. 2023, 199, 110666. [Google Scholar] [CrossRef]
- He, Y.; Wei, M.; Wu, Y.; Qin, H.; Li, W.; Ma, X.; Cheng, J.; Ren, J.; Shen, Y.; Chen, Z.; et al. Amyloid beta oligomers suppress excitatory transmitter release via presynaptic depletion of phosphatidylinositol-4,5-bisphosphate. Nat. Commun. 2019, 10, 1193. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; Yang, E.; Zhang, Y.; Qian, Q.; Li, X.; Huang, F.; Sun, B. Efr3b is essential for social recognition by modulating the excitability of CA2 pyramidal neurons. Proc. Natl. Acad. Sci. USA 2024, 121, e2314557121. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, C.; Jin, X.; Li, X.; Wu, D. Mir-367 is downregulated in coronary artery disaese and its overexpression exerts anti-inflammatory effect via inhibition of the NF-κB-activated inflammatory pathway. Int. J. Clin. Exp. Patholol. 2017, 10, 4047–4057. [Google Scholar]
- Liu, X.H.; Xu, H.; Geng, Q.; Mak, W.-H.; Ling, F.; Su, Z.; Yang, F.; Zhang, T.; Chen, J.; Yang, H.; et al. New genetic variants associated with major adverse cardiovascular events in patients with acute coronary syndromes and treated with clopidogrel and aspirin. Pharmacogen. J. 2021, 21, 664–672. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, L.; Zheng, L.; Ge, W.; Li, D.; Zhang, Y.; Hu, X.; Gao, Z.; Xu, J.; Huang, Y.; et al. Exome capture sequencing of adenoma reveals genetic alterations in multiple cellular pathways at the early stage of colorectal tumorigenesis. PLoS ONE 2013, 8, e53310. [Google Scholar] [CrossRef]
- Adhikari, H.; Kattan, W.E.; Kumar, S.; Zhou, P.; Hancock, J.F.; Counter, C.M. Oncogenic KRAS is dependent upon an EFR3A-PI4KA signaling axis for potent tumorigenic activity. Nat. Commun. 2021, 12, 5248. [Google Scholar] [CrossRef]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kattan, W.E.; Liu, J.; Montufar-Solis, D.; Liang, H.; Brahmendra Barathi, B.; van der Hoeven, R.; Zhou, Y.; Hancock, J.F. Components of the phosphatidylserine endoplasmic reticulum to plasma membrane transport mechanism as targets for KRAS inhibition in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2114126118. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Jiang, Q.; Liu, L.; Li, S.; Zhou, Q.; Huang, F.; Liu, Z. Circular RNA Expression Profiles in Nasopharyngeal Carcinoma by Sequence Analysis. Front. Oncol. 2020, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Xia, B. Circular RNA EFR3A promotes nasopharyngeal carcinoma progression through modulating the miR-654-3p/EFR3A axis. Cell. Mol. Biol. 2023, 69, 111–117. [Google Scholar] [CrossRef]
- Conn, V.M.; Chinnaiyan, A.M.; Conn, S.J. Circular RNA in cancer. Nat. Rev. Cancer 2024. online ahead of print. [Google Scholar]
- Toledo, C.M.; Ding, Y.; Hoellerbauer, P.; Davis, R.J.; Basom, R.; Girard, E.J.; Lee, E.; Corrin, P.; Hart, T.; Bolouri, H.; et al. Genome-wide CRISPR-Cas9 Screens Reveal Loss of Redundancy between PKMYT1 and WEE1 in Glioblastoma Stem-like Cells. Cell Rep. 2015, 13, 2425–2439. [Google Scholar] [CrossRef]
- Wu, X.; Chi, R.J.; Baskin, J.M.; Lucast, L.; Burd, C.G.; De Camilli, P.; Reinisch, K.M. Structural insights into assembly and regulation of the plasma membrane phosphatidylinositol 4-kinase complex. Dev. Cell 2014, 28, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Beach, B.M.; Hurley, J.H. Structure of the VHS domain of human Tom1 (target of myb 1): Insights into interactions with proteins and membranes. Biochemistry 2000, 39, 11282–11290. [Google Scholar] [CrossRef]
- Boal, F.; Mansour, R.; Gayral, M.; Saland, E.; Chicanne, G.; Xuereb, J.M.; Marcellin, M.; Burlet-Schiltz, O.; Sansonetti, P.J.; Payrastre, B.; et al. TOM1 is a PI5P effector involved in the regulation of endosomal maturation. J. Cell Sci. 2015, 128, 815–827. [Google Scholar] [CrossRef]
- Mao, Y.; Nickitenko, A.; Duan, X.; Lloyd, T.E.; Wu, M.N.; Bellen, H.; Quiocho, F.A. Crystal structure of the VHS and FYVE tandem domains of Hrs, a protein involved in membrane trafficking and signal transduction. Cell 2000, 100, 447–456. [Google Scholar] [CrossRef]
- Lohi, O.; Poussu, A.; Mao, Y.; Quiocho, F.; Lehto, V.P. VHS domain—A longshoreman of vesicle lines. FEBS Lett. 2002, 513, 19–23. [Google Scholar] [CrossRef]
- Andrade, M.A.; Petosa, C.; O’Donoghue, S.I.; Muller, C.W.; Bork, P. Comparison of ARM and HEAT protein repeats. J. Mol. Biol. 2001, 309, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://pfam.xfam.org/ (accessed on 25 November 2024).
- Kippert, F.; Gerloff, D.L. Highly sensitive detection of individual HEAT and ARM repeats with HHpred and COACH. PLoS ONE 2009, 4, e7148. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 25 November 2024).
- Krieger, E.; Vriend, G. YASARA View—Molecular graphics for all devices—From smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Wu, X.; Christiano, R.; Oh, M.S.; Schauder, C.M.; Gazzerro, E.; Messa, M.; Baldassari, S.; Assereto, S.; Biancheri, R.; et al. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat. Cell Biol. 2016, 18, 132–138. [Google Scholar] [CrossRef]
- Lees, J.A.; Zhang, Y.; Oh, M.S.; Schauder, C.M.; Yu, X.; Baskin, J.M.; Dobbs, K.; Notarangelo, L.D.; De Camilli, P.; Walz, T.; et al. Architecture of the human PI4KIIIalpha lipid kinase complex. Proc. Natl. Acad. Sci. USA 2017, 114, 13720–13725. [Google Scholar] [CrossRef]
- Suresh, S.; Shaw, A.L.; Pemberton, J.G.; Scott, M.K.; Harris, N.J.; Parson, M.A.H.; Jenkins, M.L.; Rohilla, P.; Alvarez-Prats, A.; Balla, T.; et al. Molecular basis for plasma membrane recruitment of PI4KA by EFR3. Sci. Adv. 2024, 10, eadp6660. [Google Scholar] [CrossRef] [PubMed]
- Korycka, J.; Lach, A.; Heger, E.; Boguslawska, D.M.; Wolny, M.; Toporkiewicz, M.; Augoff, K.; Korzeniewski, J.; Sikorski, A.F. Human DHHC proteins: A spotlight on the hidden player of palmitoylation. Eur. J. Cell Biol. 2012, 91, 107–117. [Google Scholar] [CrossRef]
- Tabaczar, S.; Czogalla, A.; Podkalicka, J.; Biernatowska, A.; Sikorski, A.F. Protein palmitoylation: Palmitoyltransferases and their specificity. Exp. Biol. Med. 2017, 242, 1150–1157.Efr3. [Google Scholar] [CrossRef]
- Pei, S.; Piao, H.L. Exploring Protein S-Palmitoylation: Mechanisms, Detection, and Strategies for Inhibitor Discovery. ACS Chem. Biol. 2024, 19, 1868–1882. [Google Scholar] [CrossRef]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. CSS-Palm 2.0: An updated software for palmitoylation sites prediction. Protein Eng. Des. Sel. 2008, 21, 639–644. [Google Scholar] [CrossRef]
- Blanc, M.; David, F.P.A.; van der Goot, F.G. SwissPalm 2: Protein S-Palmitoylation Database. Methods Mol. Biol. 2019, 2009, 203–214. [Google Scholar] [PubMed]
- Batrouni, A.G.; Bag, N.; Phan, H.T.; Baird, B.A.; Baskin, J.M. A palmitoylation code controls PI4KIIIalpha complex formation and PI(4,5)P2 homeostasis at the plasma membrane. J. Cell Sci. 2022, 135, jcs259365. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, F.; Baskin, J.M.; Chung, J.; Tanner, L.B.; Shui, G.; Lee, S.Y.; Pirruccello, M.; Hao, M.; Ingolia, N.T.; Wenk, M.R.; et al. PtdIns4P synthesis by PI4KIIIalpha at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 2012, 199, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Available online: https://string-db.org/ (accessed on 20 December 2024).
- Roux, K.J.; Kim, D.I.; Burke, B.; May, D.G. BioID: A Screen for Protein-Protein Interactions. Curr. Protoc. Protein Sci. 2018, 91, 19.23.1–19.23.15. [Google Scholar] [CrossRef]
- Dong, J.M.; Tay, F.P.; Swa, H.L.; Gunaratne, J.; Leung, T.; Burke, B.; Manser, E. Proximity biotinylation provides insight into the molecular composition of focal adhesions at the nanometer scale. Sci. Signal 2016, 9, rs4. [Google Scholar] [CrossRef]
- Falkenburger, B.H.; Jensen, J.B.; Dickson, E.J.; Suh, B.C.; Hille, B. Phosphoinositides: Lipid regulators of membrane proteins. J. Physiol. 2010, 588, 3179–3185. [Google Scholar] [CrossRef]
- Katan, M.; Cockcroft, S. Phosphatidylinositol(4,5)bisphosphate: Diverse functions at the plasma membrane. Essays Biochem. 2020, 64, 513–531. [Google Scholar]
- Audhya, A.; Emr, S.D. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2002, 2, 593–605. [Google Scholar] [CrossRef]
- Posor, Y.; Jang, W.; Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 2022, 23, 797–816. [Google Scholar] [CrossRef]
- Ray, J.; Sapp, D.G.; Fairn, G.D. Phosphatidylinositol 3,4-bisphosphate: Out of the shadows and into the spotlight. Curr. Opin. Cell Biol. 2024, 88, 102372. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.R.; Machner, M.P.; Balla, T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 2014, 205, 113–126. [Google Scholar] [CrossRef]
- Salazar, A.N.; Gorter de Vries, A.R.; van den Broek, M.; Wijsman, M.; de la Torre Cortes, P.; Brickwedde, A.; Brouwers, N.; Daran, J.G.; Abeel, T. Nanopore sequencing enables near-complete de novo assembly of Saccharomyces cerevisiae reference strain CEN.PK113-7D. FEMS Yeast Res. 2017, 17, fox074. [Google Scholar] [CrossRef]
- Wong, K.; Cantley, L.C. Cloning and characterization of a human phosphatidylinositol 4-kinase. J. Biol. Chem. 1994, 269, 28878–28884. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohya, Y.; Goebl, M.; Nakano, A.; Anraku, Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Cutler, N.S.; Heitman, J.; Cardenas, M.E. STT4 is an essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. J. Biol. Chem. 1997, 272, 27671–27677. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodas, R.; Labbaoui, H.; Orange, F.; Solis, N.; Zaragoza, O.; Filler, S.G.; Bassilana, M.; Arkowitz, R.A. Plasma Membrane Phosphatidylinositol-4-Phosphate Is Not Necessary for Candida albicans Viability yet Is Key for Cell Wall Integrity and Systemic Infection. mBio 2021, 13, e0387321. [Google Scholar] [CrossRef]
- Scheufler, C.; Brinker, A.; Bourenkov, G.; Pegoraro, S.; Moroder, L.; Bartunik, H.; Hartl, F.U.; Moarefi, I. Structure of TPR domain-peptide complexes: Critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 2000, 101, 199–210. [Google Scholar] [CrossRef]
- Izert, M.A.; Szybowska, P.E.; Gorna, M.W.; Merski, M. The Effect of Mutations in the TPR and Ankyrin Families of Alpha Solenoid Repeat Proteins. Front. Bioinform. 2021, 1, 696368. [Google Scholar] [CrossRef]
- Baird, D.; Stefan, C.; Audhya, A.; Weys, S.; Emr, S.D. Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3. J. Cell Biol. 2008, 183, 1061–1074. [Google Scholar] [CrossRef]
- Agarwal, N.S.; Northrop, L.; Anyane-Yeboa, K.; Aggarwal, V.S.; Nagy, P.L.; Demirdag, Y.Y. Tetratricopeptide repeat domain 7A (TTC7A) mutation in a newborn with multiple intestinal atresia and combined immunodeficiency. J. Clin. Immunol. 2014, 34, 607–610. [Google Scholar] [CrossRef]
- Jardine, S.; Dhingani, N.; Muise, A.M. TTC7A: Steward of Intestinal Health. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Sharafian, S.; Alimadadi, H.; Shahrooei, M.; Gharagozlou, M.; Aghamohammadi, A.; Parvaneh, N. A Novel TTC7A Deficiency Presenting With Combined Immunodeficiency and Chronic Gastrointestinal Problems. J. Investig. Allergol. Clin. Immunol. 2018, 28, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Yang, S.; Guo, R.; Fu, L.; Zhang, L.; Guo, W.; Du, J.; He, J.; Ren, Q.; Hao, C.; et al. A Novel Homozygous TTC7A Missense Mutation Results in Familial Multiple Intestinal Atresia and Combined Immunodeficiency. Front. Immunol. 2021, 12, 759308. [Google Scholar] [CrossRef]
- Dannheim, K.; Ouahed, J.; Field, M.; Snapper, S.; Raphael, B.P.; Glover, S.C.; Bishop, P.R.; Bhesania, N.; Kamin, D.; Thiagarajah, J.; et al. Pediatric Gastrointestinal Histopathology in Patients with Tetratricopeptide Repeat Domain 7A (TTC7A) Germline Mutations: A Rare Condition Leading to Multiple Intestinal Atresias, Severe Combined Immunodeficiency, and Congenital Enteropathy. Am. J. Surg. Pathol. 2022, 46, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Hypomyelinating Leukoencephalopathy. Available online: https://www.malacards.org/card/hypomyelinating_leukoencephalopathy (accessed on 10 January 2025).
- Gazzerro, E.; Baldassari, S.; Giacomini, C.; Musante, V.; Fruscione, F.; La Padula, V.; Biancheri, R.; Scarfi, S.; Prada, V.; Sotgia, F.; et al. Hyccin, the molecule mutated in the leukodystrophy hypomyelination and congenital cataract (HCC), is a neuronal protein. PLoS ONE 2012, 7, e32180. [Google Scholar] [CrossRef]
- Traverso, M.; Yuregir, O.O.; Mimouni-Bloch, A.; Rossi, A.; Aslan, H.; Gazzerro, E.; Baldassari, S.; Fruscione, F.; Minetti, C.; Zara, F.; et al. Hypomyelination and congenital cataract: Identification of novel mutations in two unrelated families. Eur. J. Paediatr. Neurol. 2013, 17, 108–111. [Google Scholar] [CrossRef]
- Li, S.; Han, T. Frequent loss of FAM126A expression in colorectal cancer results in selective FAM126B dependency. iScience 2024, 27, 109646. [Google Scholar] [CrossRef]
- Dornan, G.L.; Dalwadi, U.; Hamelin, D.J.; Hoffmann, R.M.; Yip, C.K.; Burke, J.E. Probing the Architecture, Dynamics, and Inhibition of the PI4KIIIalpha/TTC7/FAM126 Complex. J. Mol. Biol. 2018, 430, 3129–3142. [Google Scholar] [CrossRef]
- Chung, J.; Nakatsu, F.; Baskin, J.M.; De Camilli, P. Plasticity of PI4KIIIalpha interactions at the plasma membrane. EMBO Rep. 2015, 16, 312–320. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Yuan, Z.; Hansen, S.B. Cholesterol Regulation of Membrane Proteins Revealed by Two-Color Super-Resolution Imaging. Membranes 2023, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.G.N.; Kusumi, A. Refinement of Singer-Nicolson fluid-mosaic model by microscopy imaging: Lipid rafts and actin-induced membrane compartmentalization. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184093. [Google Scholar] [CrossRef] [PubMed]
- Saldana-Villa, A.K.; Lara-Lemus, R. The Structural Proteins of Membrane Rafts, Caveolins and Flotillins, in Lung Cancer: More Than Just Scaffold Elements. Int. J. Med. Sci. 2023, 20, 1662–1670. [Google Scholar] [CrossRef]
- Neumann-Giesen, C.; Falkenbach, B.; Beicht, P.; Claasen, S.; Luers, G.; Stuermer, C.A.; Herzog, V.; Tikkanen, R. Membrane and raft association of reggie-1/flotillin-2: Role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J. 2004, 378, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef]
- Suzuki, K.G.N. Single-Molecule Imaging of Ganglioside Probes in Living Cell Plasma Membranes. Methods Mol. Biol. 2023, 2613, 215–227. [Google Scholar]
- Komatsuya, K.; Kikuchi, N.; Hirabayashi, T.; Kasahara, K. The Regulatory Roles of Cerebellar Glycosphingolipid Microdomains/Lipid Rafts. Int. J. Mol. Sci. 2023, 24, 5566. [Google Scholar] [CrossRef]
- Ruzzi, F.; Cappello, C.; Semprini, M.S.; Scalambra, L.; Angelicola, S.; Pittino, O.M.; Landuzzi, L.; Palladini, A.; Nanni, P.; Lollini, P.L. Lipid rafts, caveolae, and epidermal growth factor receptor family: Friends or foes? Cell Commun. Signal 2024, 22, 489. [Google Scholar] [CrossRef]
- Isik, O.A.; Cizmecioglu, O. Rafting on the Plasma Membrane: Lipid Rafts in Signaling and Disease. Adv. Exp. Med. Biol. 2023, 1436, 87–108. [Google Scholar] [PubMed]
- Shouman, S.; El-Kholy, N.; Hussien, A.E.; El-Derby, A.M.; Magdy, S.; Abou-Shanab, A.M.; Elmehrath, A.O.; Abdelwaly, A.; Helal, M.; El-Badri, N. SARS-CoV-2-associated lymphopenia: Possible mechanisms and the role of CD147. Cell Commun. Signal 2024, 22, 349. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Temerozo, J.R.; Pereira-Dutra, F.S.; Ferreira, A.C.; Mattos, M.; Goncalves, B.S.; Sacramento, C.Q.; Palhinha, L.; Cunha-Fernandes, T.; Dias, S.S.G.; et al. Simvastatin Downregulates the SARS-CoV-2-Induced Inflammatory Response and Impairs Viral Infection Through Disruption of Lipid Rafts. Front. Immunol. 2022, 13, 820131. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, N.; Li, H.F.; Gu, J.; Zhang, C.J.; Liao, D.F.; Qin, L. The lipid rafts in cancer stem cell: A target to eradicate cancer. Stem Cell Res. Ther. 2022, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Erazo-Oliveras, A.; Munoz-Vega, M.; Salinas, M.L.; Wang, X.; Chapkin, R.S. Dysregulation of cellular membrane homeostasis as a crucial modulator of cancer risk. FEBS J. 2024, 291, 1299–1352. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef]
- Hryniewicz-Jankowska, A.; Augoff, K.; Biernatowska, A.; Podkalicka, J.; Sikorski, A.F. Membrane rafts as a novel target in cancer therapy. Biochim. Biophys. Acta 2014, 1845, 155–165. [Google Scholar] [CrossRef]
- Ferrer, I. Amyloid-beta Pathology Is the Common Nominator Proteinopathy of the Primate Brain Aging. J. Alzheimers Dis. 2024, 100, S153–S164. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Matveichuk, O.V.; Fronk, J.; Ciesielska, A. Flotillins: At the Intersection of Protein S-Palmitoylation and Lipid-Mediated Signaling. Int. J. Mol. Sci. 2020, 21, 2283. [Google Scholar] [CrossRef]
- Trybus, M.; Niemiec, L.; Biernatowska, A.; Hryniewicz-Jankowska, A.; Sikorski, A.F. MPP1-based mechanism of resting state raft organization in the plasma membrane. Is it a general or specialized mechanism in erythroid cells? Folia Histochem. Cytobiol. 2019, 57, 43–55. [Google Scholar] [CrossRef]
- Chytla, A.; Gajdzik-Nowak, W.; Olszewska, P.; Biernatowska, A.; Sikorski, A.F.; Czogalla, A. Not Just Another Scaffolding Protein Family: The Multifaceted MPPs. Molecules 2020, 25, 4954. [Google Scholar] [CrossRef] [PubMed]
- Biernatowska, A.; Podkalicka, J.; Majkowski, M.; Hryniewicz-Jankowska, A.; Augoff, K.; Kozak, K.; Korzeniewski, J.; Sikorski, A.F. The role of MPP1/p55 and its palmitoylation in resting state raft organization in HEL cells. Biochim. Biophys. Acta 2013, 1833, 1876–1884. [Google Scholar] [CrossRef]
- Podkalicka, J.; Biernatowska, A.; Majkowski, M.; Grzybek, M.; Sikorski, A.F. MPP1 as a Factor Regulating Phase Separation in Giant Plasma Membrane-Derived Vesicles. Biophys. J. 2015, 108, 2201–2211. [Google Scholar] [CrossRef]
- Biernatowska, A.; Wojtowicz, K.; Trombik, T.; Sikorski, A.F.; Czogalla, A. MPP1 Determines the Mobility of Flotillins and Controls the Confinement of Raft-Associated Molecules. Cells 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Podkalicka, J.; Biernatowska, A.; Olszewska, P.; Tabaczar, S.; Sikorski, A.F. The microdomain-organizing protein MPP1 is required for insulin-stimulated activation of H-Ras. Oncotarget 2018, 9, 18410–18421. [Google Scholar] [CrossRef]
- Biernatowska, A.; Augoff, K.; Podkalicka, J.; Tabaczar, S.; Gajdzik-Nowak, W.; Czogalla, A.; Sikorski, A.F. MPP1 directly interacts with flotillins in erythrocyte membrane—Possible mechanism of raft domain formation. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2203–2212. [Google Scholar] [CrossRef]
- Biernatowska, A.; Olszewska, P.; Grzymajlo, K.; Drabik, D.; Kraszewski, S.; Sikorski, A.F.; Czogalla, A. Molecular characterization of direct interactions between MPP1 and flotillins. Sci. Rep. 2021, 11, 14751. [Google Scholar] [CrossRef] [PubMed]
- Trybus, M.; Hryniewicz-Jankowska, A.; Wojtowicz, K.; Trombik, T.; Czogalla, A.; Sikorski, A.F. EFR3A: A new raft domain organizing protein? Cell Mol. Biol. Lett. 2023, 28, 86. [Google Scholar] [CrossRef]
- Brown, D.A. Analysis of raft affinity of membrane proteins by detergent-insolubility. Methods Mol. Biol. 2007, 398, 9–20. [Google Scholar]
- Sezgin, E.; Kaiser, H.J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; MacKinnon, R. Structure of the flotillin complex in a native membrane environment. Proc. Natl. Acad. Sci. USA 2024, 121, e2409334121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).