Long-Term Regulation of IL-17 Expression in Pacific Oyster Hemocytes by mGluR5 Through the Phosphoinositide Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Microorganisms
2.2. Experimental Treatments and Sample Collection
2.3. Quantitative RT-PCR Analysis
2.4. Protein Abundance Analysis Using Western Blotting
2.5. Hemocyte Typing Analysis
2.6. Hemocyte Apoptosis Detection
2.7. Glutamate Content Measurement
2.8. Analysis of PKC Activity and Signaling Molecules
2.9. Statistical Analysis
3. Results
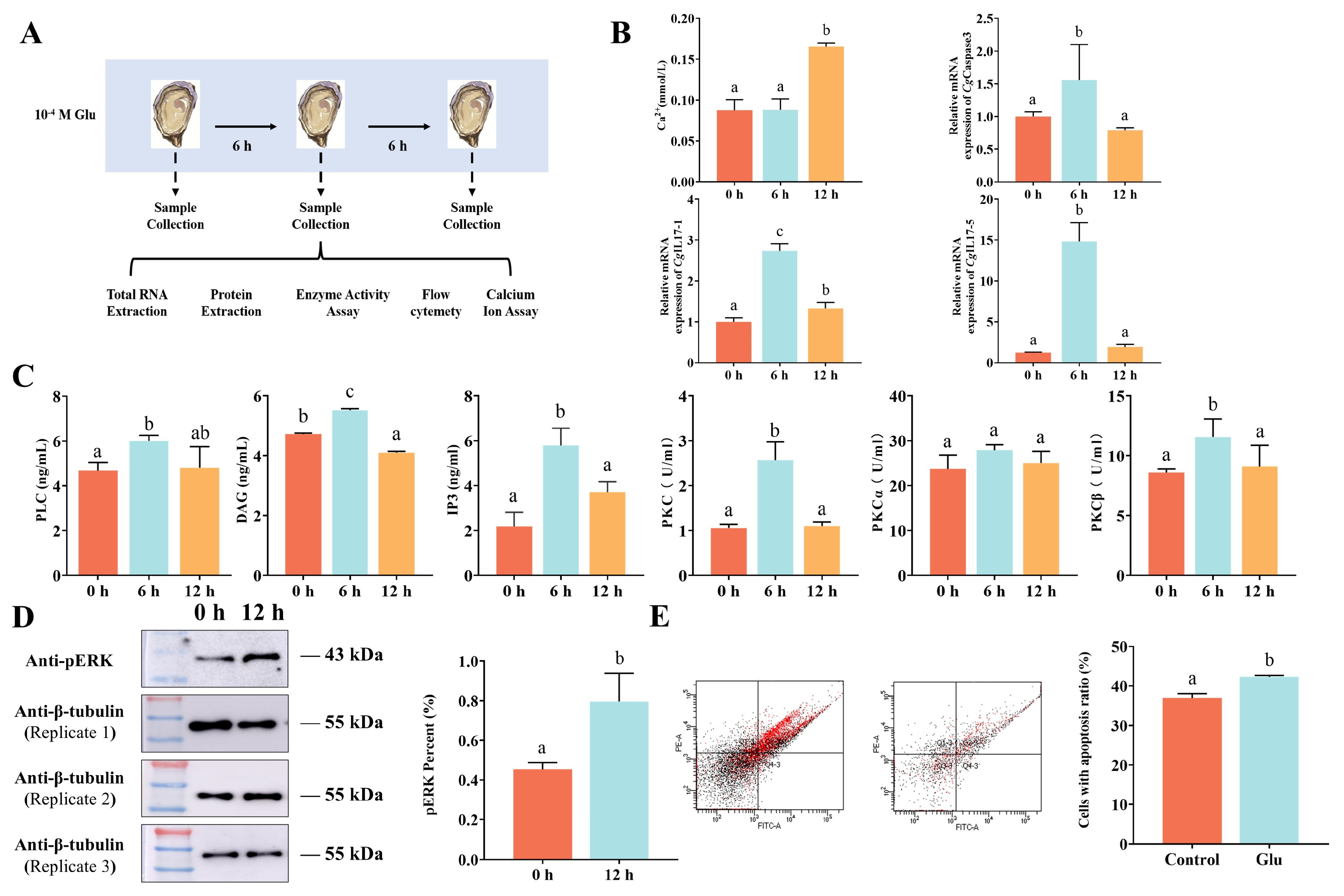
3.1. Response of CgmGluR5 to V. splendidus and Glu Treatment
3.2. Response of CgmGluR5 to Glu Treatment
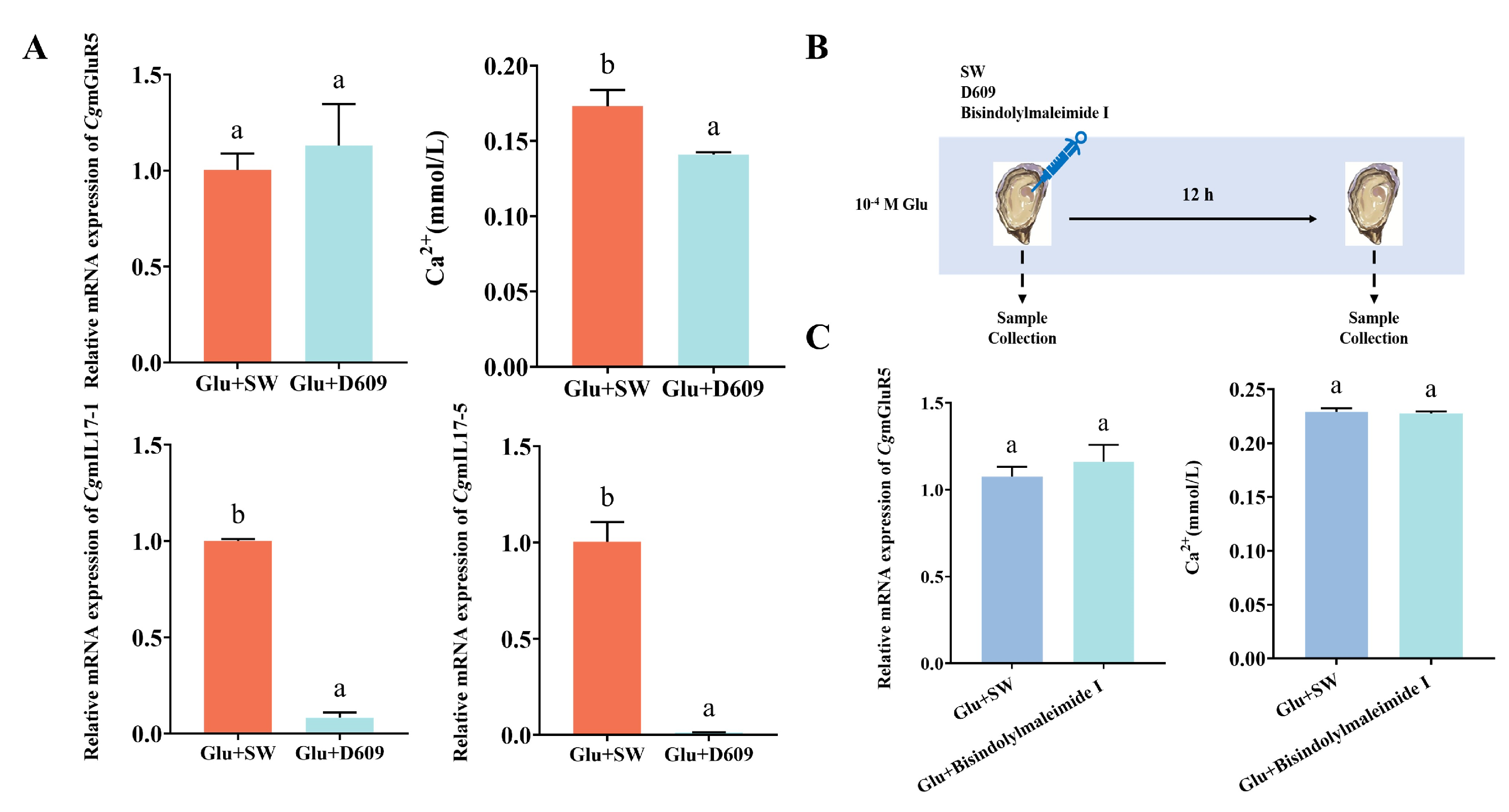
3.3. CgmGluR5 Regulation of IL-17 Through PLC and PKC
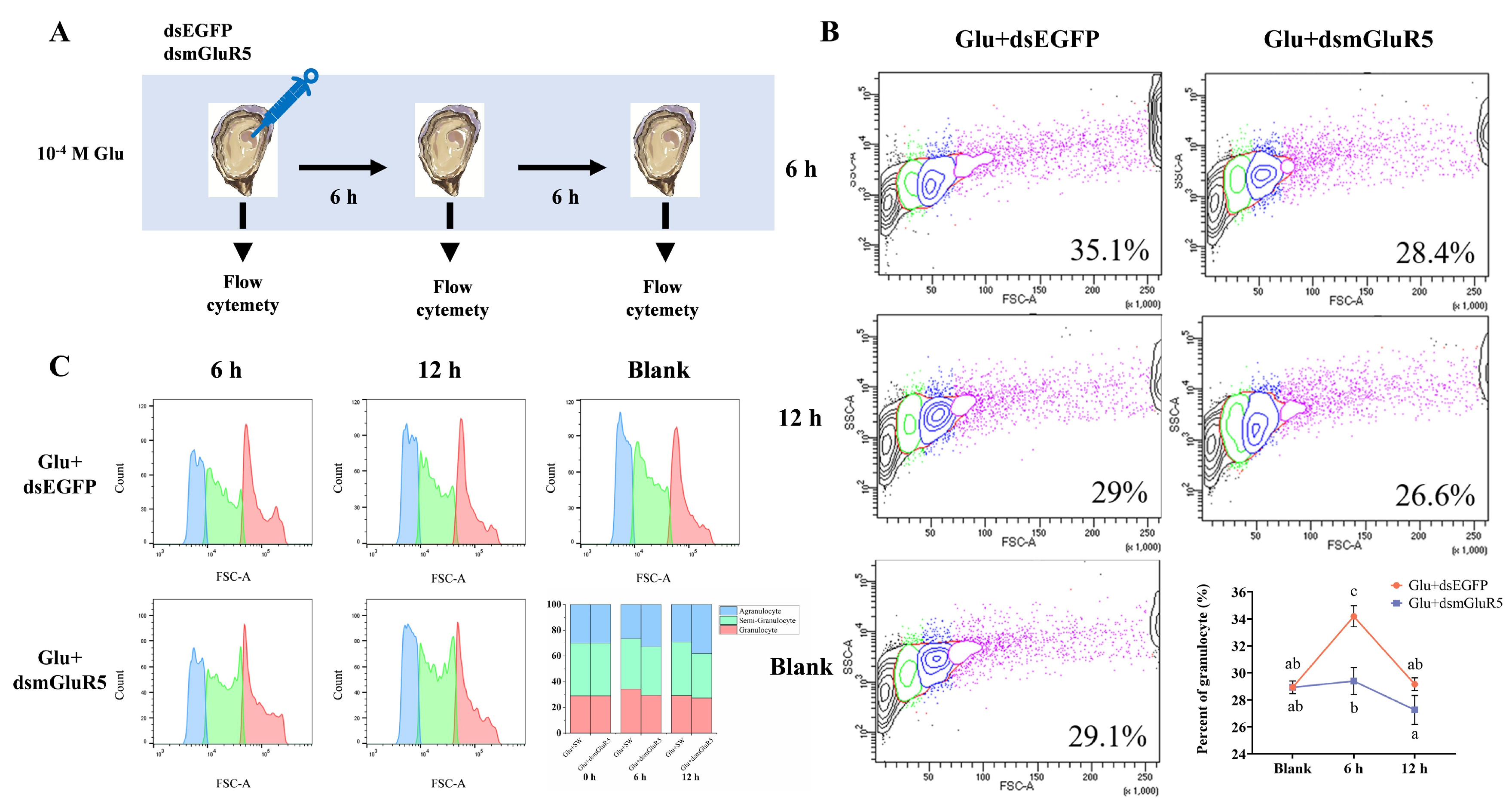
3.4. Modulation of Hemocyte Subpopulation Proportions by CgmGluR5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Deckx, N.; Lee, W.-P.; Berneman, Z.N.; Cools, N. Neuroendocrine immunoregulation in multiple sclerosis. J. Immunol. Res. 2013, 2013, 705232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Si, Y.; Zhang, L.; Wen, X.; Yang, C.; Wang, L.; Song, L. Involvement of Metabotropic Glutamate Receptors in regulation of immune response in the Pacific Oyster Crassostrea gigas. Fish Shellfish. Immunol. 2024, 151, 109709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiao, Q.; Yang, P.; Chen, X.; Zhang, J.; Zhao, B.; Zheng, P.; Liu, Y. Metabotropic glutamate receptor 5 promotes proliferation of human neural stem/progenitor cells with activation of mitogen-activated protein kinases signaling pathway in vitro. Neuroscience 2011, 192, 185–194. [Google Scholar] [CrossRef]
- Ibrahim, K. Role of vesicular glutamate transporter 3 and optineurin in metabotropic glutamate receptor 5 signaling. Ph.D. Thesis, Université d’Ottawa/University of Ottawa, Ottawa, ON, Canada, 2023. [Google Scholar]
- Sharma, D.; Parameswaran, N. Multifaceted role of β-arrestins in inflammation and disease. Genes Immun. 2015, 16, 499–513. [Google Scholar] [CrossRef]
- Koda, S.; Hu, J.; Ju, X.; Sun, G.; Shao, S.; Tang, R.-X.; Zheng, K.-Y.; Yan, J. The role of glutamate receptors in the regulation of the tumor microenvironment. Front. Immunol. 2023, 14, 1123841. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Stoica, B.; Loane, D.J.; Riccio, A.; Davis, M.I.; Faden, A.I. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 2009, 57, 550–560. [Google Scholar] [CrossRef]
- Shigetomi, E.; Saito, K.; Sano, F.; Koizumi, S. Aberrant calcium signals in reactive astrocytes: A key process in neurological disorders. Int. J. Mol. Sci. 2019, 20, 996. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cao, X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef]
- Huang, X.-D.; Zhang, H.; He, M.-X. Comparative and evolutionary analysis of the interleukin 17 gene family in invertebrates. PLoS ONE 2015, 10, e0132802. [Google Scholar] [CrossRef]
- Dragon, S.; Saffar, A.S.; Shan, L.; Gounni, A.S. IL-17 attenuates the anti-apoptotic effects of GM-CSF in human neutrophils. Mol. Immunol. 2008, 45, 160–168. [Google Scholar] [CrossRef]
- Witowski, J.; Ksiązek, K.; Warnecke, C.; Kuźlan, M.; Korybalska, K.; Tayama, H.; Wiśniewska-Elnur, J.; Pawlaczyk, K.; Trómińska, J.; Bręborowicz, A. Role of mesothelial cell-derived granulocyte colony-stimulating factor in interleukin-17-induced neutrophil accumulation in the peritoneum. Kidney Int. 2007, 71, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, Y.; Xiang, Z.; Tong, Y.; Qu, F.; Yu, Z. Genomic characterization and expression analysis of five novel IL-17 genes in the Pacific oyster, Crassostrea gigas. Fish Shellfish. Immunol. 2014, 40, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, L.; Si, X.; Zhang, X.; Guo, B.; Liao, Z.; Yan, X.; Qi, P. Exploring the Role of a Novel Interleukin-17 Homolog from Invertebrate Marine Mussel Mytilus coruscus in Innate Immune Response: Is Negative Regulation by Mc-Novel_miR_145 the Key? Int. J. Mol. Sci. 2023, 24, 5928. [Google Scholar] [CrossRef]
- Guo, X.; He, Y.; Zhang, L.; Lelong, C.; Jouaux, A. Immune and stress responses in oysters with insights on adaptation. Fish Shellfish. Immunol. 2015, 46, 107–119. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Song, L. The oyster immunity. Dev. Comp. Immunol. 2018, 80, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Gomez-Chiarri, M.; Castillo, M.G.; Figueras, A.; Fiorito, G.; Moreira, R.; Novoa, B.; Pallavicini, A.; Ponte, G.; Roumbedakis, K. Immunity in molluscs: Recognition and effector mechanisms, with a focus on bivalvia. In Advances in Comparative Immunology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 225–341. [Google Scholar]
- Liu, R.; Qiu, L.; Yu, Z.; Zi, J.; Yue, F.; Wang, L.; Zhang, H.; Teng, W.; Liu, X.; Song, L. Identification and characterisation of pathogenic Vibrio splendidus from Yesso scallop (Patinopecten yessoensis) cultured in a low temperature environment. J. Invertebr. Pathol. 2013, 114, 144–150. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Sun, J.; Li, Y.; Wu, S.; Li, Q.; Dong, M.; Wang, L.; Song, L. CgDM9CP-5-integrin-MAPK pathway regulates the production of CgIL-17s and Cgdefensins in the pacific oyster, Crassostrea gigas. J. Immunol. 2023, 210, 245–258. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Wang, S.; Liu, J.; Dan, F.; Yang, F.; Hong, S.; Liu, N.; Zeng, Y.; Huang, K. The Syvn1 inhibits neuronal cell ferroptosis by activating Stat3/Gpx4 axis in rat with spinal cord injury. Cell Prolif. 2024, 57, e13658. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, H.; Liu, Z.; Jia, Z.; Qiu, L.; Song, L. The granulocytes are the main immunocompetent hemocytes in Crassostrea gigas. Dev. Comp. Immunol. 2017, 67, 221–228. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Leng, J.; Wang, W.; Li, Y.; Yang, C.; Sun, J.; Wang, L.; Song, L. CgCaspase-3 activates the translocation of CgGSDME in haemocytes of Pacific oyster Crassostrea gigas. Fish Shellfish. Immunol. 2022, 131, 757–765. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Liu, Z.; Li, Y.; Wang, L.; Song, L. The glutaminase (CgGLS-1) mediates anti-bacterial immunity by prompting cytokine synthesis and hemocyte apoptosis in Pacific oyster Crassostrea gigas. Sci. Rep. 2021, 11, 1281. [Google Scholar] [CrossRef]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the immune system: A focus on cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef]
- Pacheco, R.; Oliva, H.; Martinez-Navío, J.M.; Climent, N.; Ciruela, F.; Gatell, J.M.; Gallart, T.; Mallol, J.; Lluis, C.; Franco, R. Glutamate released by dendritic cells as a novel modulator of T cell activation. J. Immunol. 2006, 177, 6695–6704. [Google Scholar] [CrossRef]
- Pacheco, R.; Riquelme, E.; Kalergis, A.M. Emerging evidence for the role of neurotransmitters in the modulation of T cell responses to cognate ligands. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem. -Cent. Nerv. Syst. Agents) 2010, 10, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Ganor, Y.; Levite, M. Glutamate in the immune system: Glutamate receptors in immune cells, potent effects, endogenous production and involvement in disease. In Nerve-Driven Immunity: Neurotransmitters and Neuropeptides in the Immune System; Springer: Berlin/Heidelberg, Germany, 2012; pp. 121–161. [Google Scholar]
- Shi, S.; Chen, T.; Zhao, M. The crosstalk between neurons and glia in methamphetamine-induced neuroinflammation. Neurochem. Res. 2022, 47, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Li, M.; Liu, Y.; Qu, C.; Wang, L.; Song, L. A novel JNK is involved in immune response by regulating IL expression in oyster Crassostrea gigas. Fish Shellfish. Immunol. 2018, 79, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, L. Inflammation and its mechanism in molluscs: A review. J. Dalian Fish. Univ. 2023, 38, 369–379. [Google Scholar]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Qu, T.; Tang, X.; Li, L.; Zhang, G. Conservation and divergence of mitochondrial apoptosis pathway in the Pacific oyster, Crassostrea gigas. Cell Death Dis. 2017, 8, e2915. [Google Scholar] [CrossRef]
- Hua, F.; Li, K.; Shang, S.; Wang, F.; Hu, Z. Immune signaling and autophagy regulation. In Autophagy: Biology and Diseases: Basic Science; Springer: Berlin/Heidelberg, Germany, 2019; pp. 551–593. [Google Scholar]
- Gao, X.; Xu, X.; Pang, J.; Zhang, C.; Ding, J.; Peng, X.; Liu, Y.; Cao, J. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol. Res. 2007, 56, 559. [Google Scholar] [CrossRef]
- Allam, B.; Raftos, D. Immune responses to infectious diseases in bivalves. J. Invertebr. Pathol. 2015, 131, 121–136. [Google Scholar] [CrossRef]
- De la Ballina, N.R.; Maresca, F.; Cao, A.; Villalba, A. Bivalve haemocyte subpopulations: A review. Front. Immunol. 2022, 13, 826255. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, J.; Wu, Z.; Lian, X.; Han, S.; Huang, S.; Yang, C.; Wang, L.; Song, L. AP-1 regulates the expression of IL17-4 and IL17-5 in the pacific oyster Crassostrea gigas. Fish Shellfish. Immunol. 2020, 97, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, W.; Fan, S.; Li, J.; Li, Q.; Wu, S.; Wang, L.; Song, L. The receptor CgIL-17R1 expressed in granulocytes mediates the CgIL-17 induced haemocytes proliferation in Crassostrea gigas. Dev. Comp. Immunol. 2022, 131, 104376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, F.; Liu, Y.; Ma, B.; Chen, X.; Zhu, K.; Shi, Y.; Wei, T.; Xing, Y.; Gao, Y. Activation of type 5 metabotropic glutamate receptor promotes the proliferation of rat retinal progenitor cell via activation of the PI-3-K and MAPK signaling pathways. Neuroscience 2016, 322, 138–151. [Google Scholar] [CrossRef]
- Jansson, L.C.; Åkerman, K.E. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J. Neural Transm. 2014, 121, 819–836. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.; Li, D.; Ren, W.; Zhang, X.; Wang, L.; Song, L. Long-Term Regulation of IL-17 Expression in Pacific Oyster Hemocytes by mGluR5 Through the Phosphoinositide Pathway. Cells 2025, 14, 438. https://doi.org/10.3390/cells14060438
Si Y, Li D, Ren W, Zhang X, Wang L, Song L. Long-Term Regulation of IL-17 Expression in Pacific Oyster Hemocytes by mGluR5 Through the Phosphoinositide Pathway. Cells. 2025; 14(6):438. https://doi.org/10.3390/cells14060438
Chicago/Turabian StyleSi, Yiran, Deliang Li, Wenjing Ren, Xueshu Zhang, Lingling Wang, and Linsheng Song. 2025. "Long-Term Regulation of IL-17 Expression in Pacific Oyster Hemocytes by mGluR5 Through the Phosphoinositide Pathway" Cells 14, no. 6: 438. https://doi.org/10.3390/cells14060438
APA StyleSi, Y., Li, D., Ren, W., Zhang, X., Wang, L., & Song, L. (2025). Long-Term Regulation of IL-17 Expression in Pacific Oyster Hemocytes by mGluR5 Through the Phosphoinositide Pathway. Cells, 14(6), 438. https://doi.org/10.3390/cells14060438






