The Role of Hypoxia-Inducible Factor-1α (HIF-1α) in the Progression of Ovarian Cancer: Perspectives on Female Infertility
Abstract
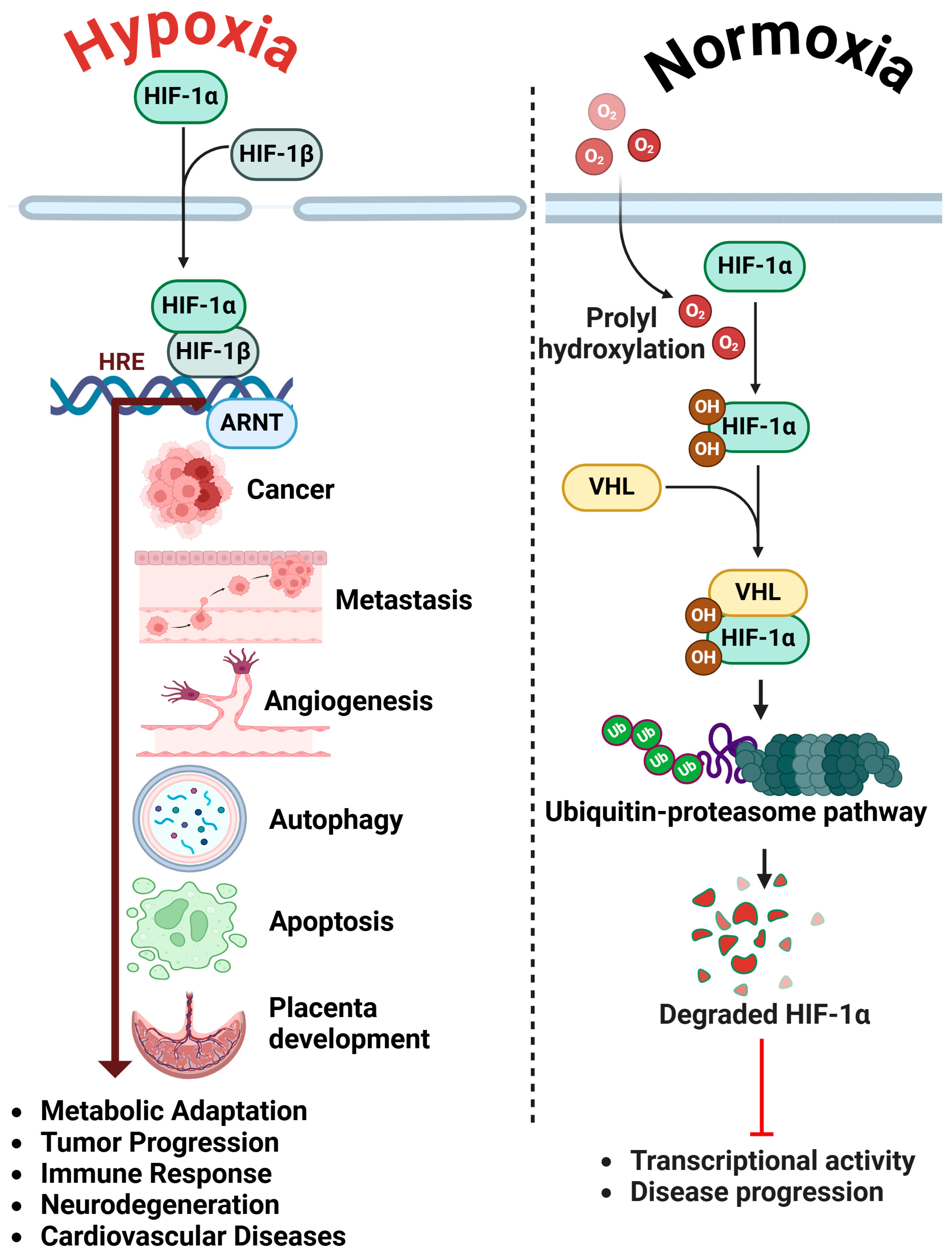
1. Introduction
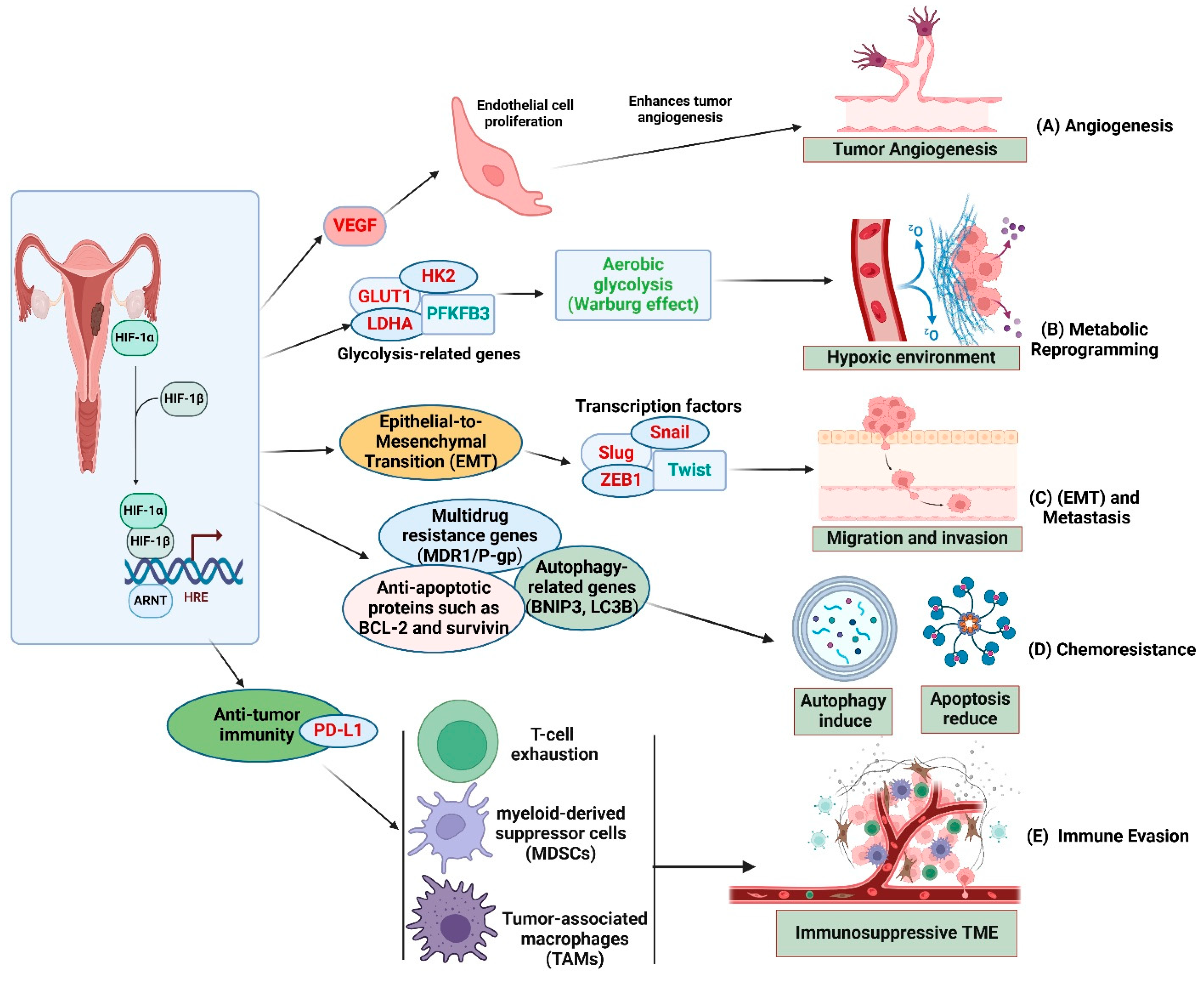
2. HIF-1α in Ovarian Cancer Progression
2.1. Angiogenesis and Metabolic Reprogramming
2.2. Epithelial-to-Mesenchymal Transition (EMT)
2.3. Resistance to Therapy
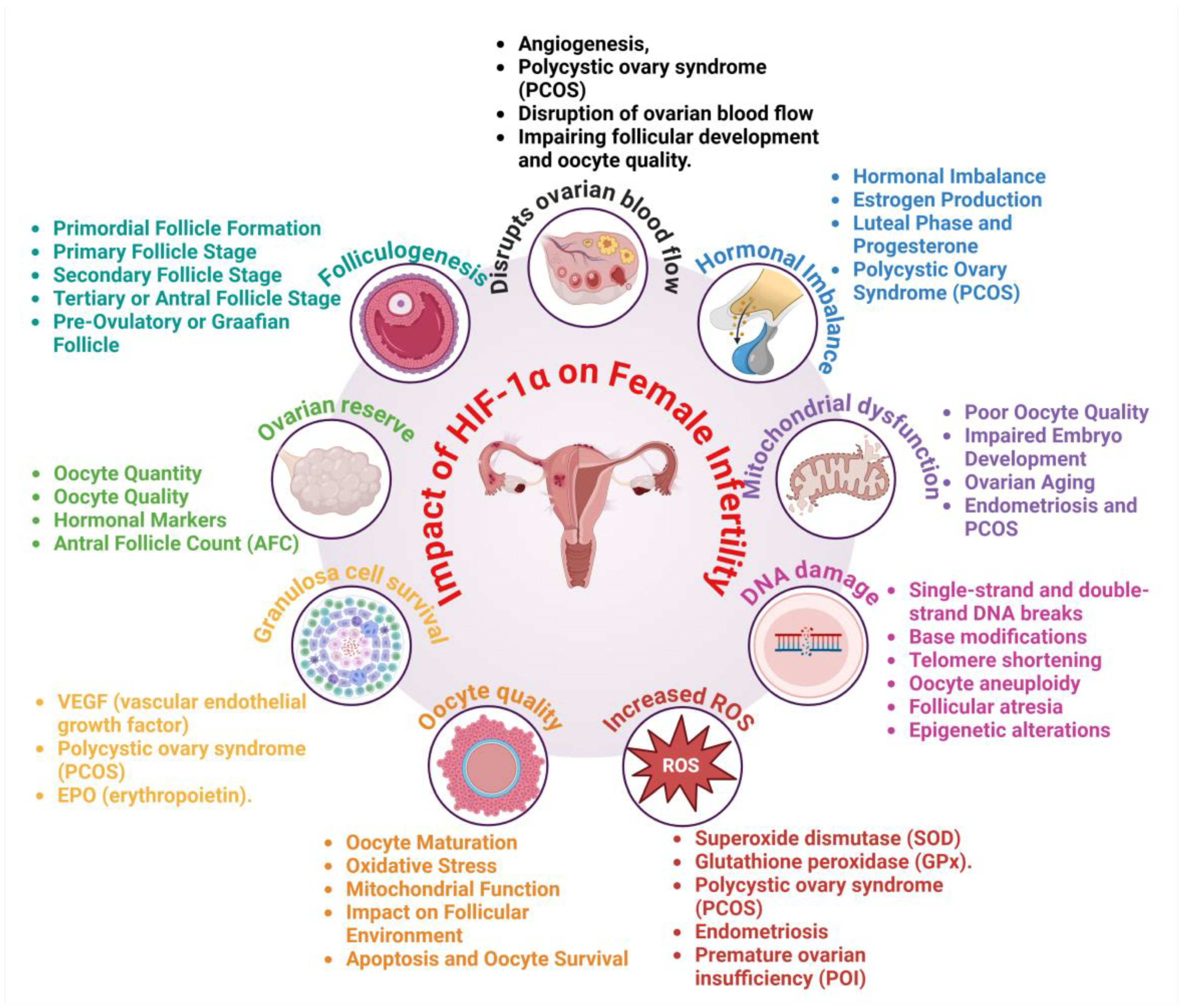
3. Impact of HIF-1α on Female Infertility
3.1. Disruption of Ovarian Function and Follicular Development
3.2. Oxidative Stress and Chronic Inflammation
3.3. Hormonal Imbalance and Vascular Abnormalities
4. Clinical Implications and Potential Therapeutic Approaches
4.1. Inhibitors of HIF-1α
| HIF-1α Inhibitor | Mechanism of Action | Potential Benefits for Ovarian Cancer | Potential Benefits for Female Infertility | Commercial Availability | Ref. |
|---|---|---|---|---|---|
| PX-478 | Directly inhibits HIF-1α transcription and protein accumulation | Suppresses tumor angiogenesis, reduces therapy resistance | Improves ovarian vascular function, prevents follicular atresia | Yes | [56] |
| Acriflavine | Disrupts HIF-1α dimerization, blocking its transcriptional activity | Inhibits cancer progression and metastasis | Reduces hypoxia-induced ovarian dysfunction | Yes (Clinical use for infections) | [57] |
| Echinomycin | Binds to HIF-1 DNA binding sites, preventing target gene expression | Reduces hypoxia-driven tumor growth and survival | Protect ovarian follicles from oxidative damage | No (Investigational) | [58] |
| Digoxin | Downregulates HIF-1α protein levels via inhibition of its synthesis | Decreases cancer cell proliferation and vascular abnormalities | Improves ovarian microcirculation, enhances hormonal balance | Yes (FDA-approved cardiac drug) | [59] |
| 2-Methoxyestradiol (2-ME) | Destabilizes HIF-1α protein and inhibits its transcription | Reduces angiogenesis and tumor progression | Restores hormonal equilibrium and ovarian function | No (Investigational) | [60] |
| YC-1 | Inhibits HIF-1α synthesis and blocks downstream VEGF signaling | Suppresses tumor growth and hypoxia adaptation | Reduces inflammatory damage to ovarian tissues | No (Investigational) | [61] |
| LW6 | Promotes HIF-1α degradation via the proteasome pathway | Decreases in hypoxia-induced drug resistance in ovarian cancer | Protect ovarian cells from stress-induced apoptosis | No (Investigational) | [62] |
| PX-12 | Inhibits thioredoxin, preventing HIF-1α stabilization under hypoxia | Increases chemotherapy sensitivity, prevents metastasis | Restores oxidative balance in ovarian tissues | No (Investigational) | [63] |
| KRIBB11 | Blocks HIF-1α transcriptional activation and nuclear localization | Reduces cancer cell invasion and therapy resistance | Improves ovarian follicular survival by limiting hypoxic stress | No (Investigational) | [17] |
| Bortezomib | Inhibits the proteasome, leading to degradation of HIF-1α | Prevents tumor adaptation to hypoxia, enhances drug efficacy | Enhances ovarian cell viability and prevents premature ovarian failure | Yes (FDA-approved for multiple myeloma) | [64] |
4.2. Antioxidant Therapy
4.3. Anti-Inflammatory Agents
| Anti-Inflammatory Agent | Mechanism of Action | Potential Benefits for Ovarian Cancer | Potential Benefits for Female Infertility | Limitations | Ref. |
|---|---|---|---|---|---|
| Curcumin | Inhibits NF-κB and HIF-1α signaling, reducing inflammation and oxidative stress | Suppresses tumor growth, reduces angiogenesis | Protects ovarian follicles, improves oocyte quality | Poor Stability, Toxicity and Biocompatibility | [74] |
| Resveratrol | Downregulates HIF-1α and cytokine production, reducing hypoxia-driven inflammation | Inhibits metastasis, enhances chemotherapy sensitivity | Preserves ovarian function, delays ovarian aging | Stability and Aggregation, Blood–Brain Barrier (BBB) Penetration | [75] |
| Aspirin | Blocks COX-2 and prostaglandins, reducing chronic inflammation | Decreases cancer cell proliferation, lowers risk of metastasis | Improves ovarian blood flow, reduces endometriosis-related infertility | Rapid Clearance, Limited Targeting Efficiency, Poor Stability, | [76] |
| Melatonin | Acts as an antioxidant and anti-inflammatory agent, reducing IL-6 and TNF-α levels | Inhibits HIF-1α-mediated tumor progression | Enhances ovarian reserve, protects against oxidative stress | Limited Targeting Efficiency, Regulatory Challenges, Rapid Clearance and Short Half-Life | [77] |
| Sulforaphane | NF-κB and HIF-1α, reducing inflammatory cytokines | Prevents tumor progression and therapy resistance | Improves ovarian microenvironment, enhances fertility potential | Stability and Aggregation, Cost of Production, Toxicity and Biocompatibility | [78] |
| Omega-3 Fatty Acids | Reduces pro-inflammatory cytokines and oxidative stress | Suppresses tumor growth, improves immune response | Enhances ovarian response, reduces inflammation-induced infertility | Poor Targeting Efficiency, Short Circulation Half-Life | [79] |
| Quercetin | Inhibits HIF-1α and TNF-α, preventing hypoxia-driven inflammation | Reduces cancer progression and EMT | Protects ovarian follicles from inflammatory damage | Stability and Aggregation, Blood–Brain Barrier (BBB) Penetration, Limited Targeting Efficiency | [80] |
| N-Acetylcysteine (NAC) | Boosts glutathione levels, reduces oxidative stress and inflammation | Increases chemotherapy efficacy, protects normal cells | Prevents ovarian fibrosis, restores hormonal balance | Toxicity Concerns, Biodegradability and Clearance, Scalability and Cost | [81] |
| Boswellia Serrata (Frankincense) | Inhibits 5-LOX and NF-κB, reducing inflammatory responses | Lowers tumor-related inflammation, prevents metastasis | Supports ovarian function, reducing inflammatory stress on reproductive tissues | Manufacturing Challenges, Biocompatibility, Limited Targeting | [82] |
| Gingerol (from Ginger) | Suppresses COX-2, TNF-α, and IL-6, reducing systemic inflammation | Inhibits ovarian cancer progression, enhances chemotherapy response | Reduces inflammation-related ovarian dysfunction, improves reproductive outcomes | Limited Tissue Penetration, Possible Drug Resistance, Toxicity, Regulatory Challenge | [83] |
4.4. Nanoparticle-Based Drug Delivery
| No. | Nanoparticle-Based Drug | Type of Nanoparticle | Mechanism of Action | Potential Benefits for Ovarian Cancer | Potential Benefits for Female Infertility | References |
|---|---|---|---|---|---|---|
| 1 | HIF-1α siRNA Nanoparticles | Lipid-based | Silences HIF-1α expression, reducing tumor hypoxia | Inhibits tumor progression, enhances therapy response | Protects ovarian function by reducing hypoxia-induced damage | [88] |
| 2 | PX-478-Loaded Nanoparticles | Polymeric | HIF-1α inhibitor, blocks transcriptional activity | Suppresses angiogenesis, EMT, and drug resistance | Improves ovarian vascularization, reduces oxidative stress | [61] |
| 3 | Acriflavine-Encapsulated Nanoparticles | Liposomal | Disrupts HIF-1α dimerization, blocking its function | Inhibits tumor growth and metastasis | Prevents HIF-1α-induced follicular atresia | [57] |
| 4 | Curcumin Nanoparticles | Polymeric | Anti-inflammatory, inhibits HIF-1α and NF-κB signaling | Reduces inflammation, enhances chemotherapy efficacy | Protect ovarian follicles from oxidative stress | [89] |
| 5 | Resveratrol-Loaded Nanoparticles | Lipid-based | Antioxidant, modulates HIF-1α and mitochondrial function | Inhibits cancer cell proliferation, reduces therapy resistance | Improves oocyte quality and ovarian reserve | [90] |
| 6 | Melatonin-Conjugated Nanoparticles | Solid lipid | Antioxidant, stabilizes mitochondrial function, downregulates HIF-1α | Reduces hypoxia-induced cancer aggressiveness | Protects ovarian tissue from ROS and apoptosis | [77] |
| 7 | N-Acetylcysteine (NAC) Nanoparticles | Polymeric | Restores redox balance, inhibits HIF-1α signaling | Increases chemosensitivity, prevents tumor relapse | Enhances ovarian function and fertility outcomes | [91] |
| 8 | Doxorubicin-Loaded Nanoparticles | Liposomal | Chemotherapy drug, enhances drug delivery to hypoxic tumors | Targets hypoxic cancer cells more effectively | Reduces off-target toxicity to ovarian tissue | [92] |
| 9 | HIF-1α Inhibitor-Conjugated Gold Nanoparticles | Gold-based | Enhances targeted inhibition of HIF-1α in tumors | Suppresses angiogenesis and tumor hypoxia | Prevents premature ovarian aging by improving blood flow | [17] |
| 10 | Selenium Nanoparticles | Metal-based | Antioxidant, regulates oxidative stress and HIF-1α pathways | Protects against cancer progression, boosts immune response | Enhances ovarian follicle survival and hormonal balance | [93] |
5. Limitations and Future Directions
5.1. Research Gaps and Emerging Trends
5.2. Integrating Cancer Treatment with Fertility Preservation Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Xing, C.; Deng, Y.; Ye, C.; Peng, H. HIF-1α signaling: Essential roles in tumorigenesis and implications in targeted therapies. Genes Dis. 2024, 11, 234–251. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Moradi, S.Z.; Faraji, F.; Kooshki, L.; Webber, K.; Bishayee, A. Modulation of hypoxia-inducible factor-1 signaling pathways in cancer angiogenesis, invasion, and metastasis by natural compounds: A comprehensive and critical review. Cancer Metastasis Rev. 2024, 43, 501–574. [Google Scholar] [CrossRef] [PubMed]
- Muttiah, B.; Muhammad Fuad, N.D.; Jaafar, F.; Abdullah, N.A.H. Extracellular Vesicles in Ovarian Cancer: From Chemoresistance Mediators to Therapeutic Vectors. Biomedicines 2024, 12, 1806. [Google Scholar] [CrossRef]
- Dai, W.; Guo, R.; Na, X.; Jiang, S.; Liang, J.; Guo, C.; Fang, Y.; Na, Z.; Li, D. Hypoxia and the endometrium: An indispensable role for HIF-1α as therapeutic strategies. Redox Biol. 2024, 73, 103205. [Google Scholar] [CrossRef]
- Kumari, P.; Bharti, V.K.; Kumar, K.; Sharma, I. Hypobaric hypoxia affects the reproductive physiology of dairy cattle. Anim. Reprod. Update 2023, 3, 6–17. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Fu, J.-C.; Hong, Z.-L.; Zeng, D.-F.; Guo, C.-Q.; Li, P.; Wu, J.-X. Psychological stressors involved in the pathogenesis of premature ovarian insufficiency and potential intervention measures. Gynecol. Endocrinol. 2024, 40, 2360085. [Google Scholar] [CrossRef]
- Zhao, H.; Wong, R.J.; Stevenson, D.K. The impact of hypoxia in early pregnancy on placental cells. Int. J. Mol. Sci. 2021, 22, 9675. [Google Scholar] [CrossRef]
- Chiang, Y.-T.; Seow, K.-M.; Chen, K.-H. The pathophysiological, genetic, and hormonal changes in preeclampsia: A systematic review of the molecular mechanisms. Int. J. Mol. Sci. 2024, 25, 4532. [Google Scholar] [CrossRef]
- Shabir, S.; Gill, P.K. Global scenario on ovarian cancer–Its dynamics, relative survival, treatment, and epidemiology. Adesh Univ. J. Med. Sci. Res. 2020, 2, 17–25. [Google Scholar] [CrossRef]
- Konishi, I.; Abiko, K.; Hayashi, T.; Koshiyama, M.; Matsumura, N.; Baba, T.; Yamanoi, K.; Yamaguchi, K.; Hamanishi, J.; Mandai, M. Ovarian Cancer Screening: Where Do We Stand Now? Acad. Oncol. 2025, 2. [Google Scholar] [CrossRef]
- Tavares, V.; Marques, I.S.; Melo, I.G.d.; Assis, J.; Pereira, D.; Medeiros, R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. Int. J. Mol. Sci. 2024, 25, 1845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, L.; Yu, Y.; Zang, J.; Zhang, L.; Guo, R.-B.; Li, S.-T.; Cheng, L.; Li, X.-T.; Chen, Y.-Q. Tumor Microenvironment Responsive Key Nanomicelles for Effective Against Invasion and Metastasis in Ovarian Cancer Using Mice Model. Int. J. Nanomed. 2025, 20, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Manian, M.; Taherian, M.; Nickho, H.; Emami Nejad, A.; Shaverdi, S. Hypoxia, Stem Cells and Cancer Stem Cells. In Cancer Stem Cells and Cancer Therapy; Springer: Berlin/Heidelberg, Germany, 2025; pp. 29–114. [Google Scholar]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef]
- Zou, Z.; Luo, T.; Wang, X.; Wang, B.; Li, Q. Exploring the interplay between triple-negative breast cancer stem cells and tumor microenvironment for effective therapeutic strategies. J. Cell. Physiol. 2024, 239, e31278. [Google Scholar] [CrossRef]
- Yu, X.; Xu, J.; Song, B.; Zhu, R.; Liu, J.; Liu, Y.F.; Ma, Y.J. The role of epigenetics in women’s reproductive health: The impact of environmental factors. Front. Endocrinol. 2024, 15, 1399757. [Google Scholar] [CrossRef]
- Wang, X.; Du, Z.-w.; Xu, T.-m.; Wang, X.-j.; Li, W.; Gao, J.-l.; Li, J.; Zhu, H. HIF-1α is a rational target for future ovarian cancer therapies. Front. Oncol. 2021, 11, 785111. [Google Scholar] [CrossRef]
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.C.; Wang, L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2022, 80, 379–390. [Google Scholar] [CrossRef]
- Fang, J.; Yan, L.; Shing, Y.; Moses, M.A. HIF-1α-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 2001, 61, 5731–5735. [Google Scholar]
- Zhou, W.; Zeng, T.; Chen, J.; Tang, X.; Yuan, Y.; Hu, D.; Zhang, Y.; Li, Y.; Zou, J. Aberrant angiogenic signaling pathways: Accomplices in ovarian cancer progression and treatment. Cell. Signal. 2024, 120, 111240. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α) and Cancer: Mechanisms of Tumor Hypoxia and Therapeutic Targeting. Cureus 2024, 16, e70700. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Cavalcante, G.C.; Soares-Souza, G.B. Exploring Aerobic Energy Metabolism in Breast Cancer: A Mutational Profile of Glycolysis and Oxidative Phosphorylation. Int. J. Mol. Sci. 2024, 25, 2585. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lin, X.; Wang, J.; Zhou, Z.; Chen, S.; Chen, G. Advances of HIF-1α/glycolysis axis in non-small cell lung cancer. Oncol. Rep. 2024, 51, 55. [Google Scholar] [CrossRef]
- Yao, W.; Wang, Z.; Ma, H.; Lin, Y.; Liu, X.; Li, P.; He, X. Epithelial-mesenchymal plasticity (EMP) in wound healing: Exploring EMT mechanisms, regulatory network, and therapeutic opportunities. Heliyon 2024, 10, e34269. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, N.; Nag, S.; Bhattacharya, B.; Alexiou, A.; Mirgh, D.; Mukherjee, D.; Adhikari, M.D.; Anand, K.; Muthusamy, R.; Gorai, S. Clinical impact of epithelial–mesenchymal transition for cancer therapy. Clin. Transl. Discov. 2024, 4, e260. [Google Scholar] [CrossRef]
- Tam, S.Y.; Wu, V.W.; Law, H.K. Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1α and beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef]
- Ucaryilmaz Metin, C.; Ozcan, G. The HIF-1α as a potent inducer of the hallmarks in gastric cancer. Cancers 2022, 14, 2711. [Google Scholar] [CrossRef]
- Yong, L.; Tang, S.; Yu, H.; Zhang, H.; Zhang, Y.; Wan, Y.; Cai, F. The role of hypoxia-inducible factor-1 alpha in multidrug-resistant breast cancer. Front. Oncol. 2022, 12, 964934. [Google Scholar] [CrossRef]
- Qannita, R.A.; Alalami, A.I.; Harb, A.A.; Aleidi, S.M.; Taneera, J.; Abu-Gharbieh, E.; El-Huneidi, W.; Saleh, M.A.; Alzoubi, K.H.; Semreen, M.H. Targeting hypoxia-inducible Factor-1 (HIF-1) in Cancer: Emerging therapeutic strategies and pathway regulation. Pharmaceuticals 2024, 17, 195. [Google Scholar] [CrossRef]
- Li, W.-N.; Wu, M.-H.; Tsai, S.-J. Hypoxia and reproductive health: The role of hypoxia in the development and progression of endometriosis. Reproduction 2021, 161, F19–F31. [Google Scholar] [CrossRef]
- Pereira, M.; Matuszewska, K.; Jamieson, C.; Petrik, J. Characterizing endocrine status, tumor hypoxia and immunogenicity for therapy success in epithelial ovarian cancer. Front. Endocrinol. 2021, 12, 772349. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, L.; Tang, W.; Xiong, J.; Chen, D.; Dai, Y.; Wu, C.; Wei, S.; Dai, J.; Wu, M.; et al. Ovarian microenvironment: Challenges and opportunities in protecting against chemotherapy-associated ovarian damage. Hum. Reprod. Update 2024, 30, 614–647. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.; Brown, H.M.; Kind, K.L.; Russell, D.L. The Ovarian Antral Follicle: Living on the Edge of Hypoxia or Not? Biol Reprod. 2015, 92, 153. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Pan, C.; Zhang, C. Unraveling the complexity of follicular fluid: Insights into its composition, function, and clinical implications. J. Ovarian Res. 2024, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Cleary, M.P. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor. Rev. 2017, 38, 80–97. [Google Scholar] [CrossRef]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of vascular endothelial growth factor (VEGF) in human embryo implantation: Clinical implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Zhu, X.-T.; Zhang, S.-N.; Ma, Y.-F.; Han, Y.-H.; Jiang, Y.; Zhang, Y.-H. Premature ovarian insufficiency: A review on the role of oxidative stress and the application of antioxidants. Front. Endocrinol. 2023, 14, 1172481. [Google Scholar] [CrossRef]
- Baldini, G.M.; Ferri, D.; Malvasi, A.; Laganà, A.S.; Vimercati, A.; Dellino, M.; Baldini, D.; Trojano, G. Genetic Abnormalities of Oocyte Maturation: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 13002. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Shandley, L.M.; Fothergill, A.; Spencer, J.B.; Mertens, A.C.; Cottrell, H.N.; Howards, P.P. Impact of cancer treatment on risk of infertility and diminished ovarian reserve in women with polycystic ovary syndrome. Fertil. Steril. 2018, 109, 516–525.e511. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, M.; Xia, S.; Zeng, F.; Liu, Q. Systematic and comprehensive insights into HIF-1 stabilization under normoxic conditions: Implications for cellular adaptation and therapeutic strategies in cancer. Cell Mol Biol Lett. 2025, 30, 2. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wang, J.; Lu, X.; Guo, Z.; Lv, S.; Sun, Z.; Gao, T.; Gao, F.; Yuan, J. Mitochondrial Quality Control in Ovarian Function: From Mechanisms to Therapeutic Strategies. Reprod. Sci. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, D.; He, J.; Luo, T.; Liu, Y.; Xue, Y.; Huang, J.; Zheng, L.; Li, J. TRIM28-Mediated Excessive Oxidative Stress Induces Cellular Senescence in Granulosa Cells and Contributes to Premature Ovarian Insufficiency In Vitro and In Vivo. Antioxidants 2024, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Shi, H.; Ye, J.; Qi, X. Exploring Strategies to Prevent and Treat Ovarian Cancer in Terms of Oxidative Stress and Antioxidants. Antioxidants 2025, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Chen, Y.; Singla, R.K.; Cao, D.; Shen, B. Pro-inflammatory Cytokines and CXC Chemokines as Game-Changer in Age-Associated Prostate Cancer and Ovarian Cancer: Insights from Preclinical and Clinical Studies’ Outcomes. Pharmacol. Res. 2024, 204, 107213. [Google Scholar] [CrossRef]
- Hoffmann-Młodzianowska, M.; Maksym, R.B.; Pucia, K.; Kuciak, M.; Mackiewicz, A.; Kieda, C. Endometriosis development in relation to hypoxia: A murine model study. Mol. Med. 2024, 30, 195. [Google Scholar] [CrossRef]
- Oală, I.E.; Mitranovici, M.-I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R. Endometriosis and the role of pro-inflammatory and anti-inflammatory cytokines in pathophysiology: A narrative review of the literature. Diagnostics 2024, 14, 312. [Google Scholar] [CrossRef]
- Goldsammler, M.; Merhi, Z.; Buyuk, E. Role of hormonal and inflammatory alterations in obesity-related reproductive dysfunction at the level of the hypothalamic-pituitary-ovarian axis. Reprod. Biol. Endocrinol. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Wu, G.; Li, C.; Tao, J.; Liu, Z.; Li, X.; Zang, Z.; Fu, C.; Wei, J.; Yang, Y.; Zhu, Q. FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1α–AMPK–GLUT1 signaling pathway. J. Biol. Chem. 2022, 298, 101830. [Google Scholar] [CrossRef]
- Neykova, K.; Tosto, V.; Giardina, I.; Tsibizova, V.; Vakrilov, G. Endometrial receptivity and pregnancy outcome. J. Matern.-Fetal Neonatal Med. 2022, 35, 2591–2605. [Google Scholar] [CrossRef]
- Shaw, S.; Gidugu, H.; Bhaumik, G.; Reddy, M.P.K.; Panjwani, U.; Ghosh, D. Anti-Mullerian hormone and macrophage migration inhibitory factor determine the reproductive health of Ladakhi women residing at 3,500 m. High. Alt. Med. Biol. 2021, 22, 317–326. [Google Scholar] [CrossRef]
- Fu, X.; Shi, L.; Liu, P.; Jiao, Y.; Guo, S.; Chen, Q.; Zheng, Q.; Chen, X.; Wang, Y. Expression and clinical significance of HIF-1α in follicular fluid and granulosa cells in infertile PCOS patients. Reprod. Sci. 2023, 30, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Zhang, K.; Zhao, X.; Tao, H.-P.; Jia, G.-X.; Fang, Y.-G.; Hou, Y.-P.; Yang, Q.-E. Fetal hypoxia exposure induces Hif1a activation and autophagy in adult ovarian granulosa cells. Biol. Reprod. 2024, 111, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.R.; Zanolla, K.; Spritzer, P.M.; Reis, F.M. Assisted reproductive technology in the presence of polycystic ovary syndrome: Current evidence and knowledge gaps. Endocr. Pract. 2024, 30, 64–69. [Google Scholar] [CrossRef]
- Garlisi, B.; Lauks, S.; Aitken, C.; Ogilvie, L.M.; Lockington, C.; Petrik, D.; Eichhorn, J.S.; Petrik, J. The complex tumor microenvironment in ovarian cancer: Therapeutic challenges and opportunities. Curr. Oncol. 2024, 31, 3826–3844. [Google Scholar] [CrossRef]
- Welsh, S.; Williams, R.; Kirkpatrick, L.; Paine-Murrieta, G.; Powis, G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1α. Mol. Cancer Ther. 2004, 3, 233–244. [Google Scholar] [CrossRef]
- Piorecka, K.; Kurjata, J.; Stanczyk, W.A. Acriflavine, an Acridine Derivative for Biomedical Application: Current State of the Art. J. Med. Chem. 2022, 65, 11415–11432. [Google Scholar] [CrossRef]
- Kong, D.; Park, E.J.; Stephen, A.G.; Calvani, M.; Cardellina, J.H.; Monks, A.; Fisher, R.J.; Shoemaker, R.H.; Melillo, G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005, 65, 9047–9055. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, D.Z.; Tan, Y.S.; Lee, K.; Gao, P.; Ren, Y.R.; Rey, S.; Hammers, H.; Chang, D.; Pili, R. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19579–19586. [Google Scholar] [CrossRef]
- Becker, C.M.; Rohwer, N.; Funakoshi, T.; Cramer, T.; Bernhardt, W.; Birsner, A.; Folkman, J.; D’Amato, R.J. 2-Methoxyestradiol inhibits hypoxia-inducible factor-1α and suppresses growth of lesions in a mouse model of endometriosis. Am. J. Pathol. 2008, 172, 534–544. [Google Scholar] [CrossRef]
- Bui, B.P.; Nguyen, P.L.; Lee, K.; Cho, J. Hypoxia-inducible factor-1: A novel therapeutic target for the management of cancer, drug resistance, and cancer-related pain. Cancers 2022, 14, 6054. [Google Scholar] [CrossRef]
- Lee, K.; Kang, J.E.; Park, S.-K.; Jin, Y.; Chung, K.-S.; Kim, H.-M.; Lee, K.; Kang, M.R.; Lee, M.K.; Song, K.B. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1α via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 2010, 80, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Coon, A.; Baker, A.F.; Powis, G. Antitumor agent PX-12 inhibits HIF-1α protein levels through an Nrf2/PMF-1-mediated increase in spermidine/spermine acetyl transferase. Cancer Chemother. Pharmacol. 2011, 68, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Chao, A.; Tsai, C.; Chuang, W.; Huang, W.; Chen, G.; Lin, C.; Wang, T.; Wang, H.; Lai, C. Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation. Cell Death Dis. 2014, 5, e1510. [Google Scholar] [CrossRef]
- Sun, S.; Wang, P.; Sun, S.; Liang, X. Applications of micro/nanotechnology in ultrasound-based drug delivery and therapy for tumor. Curr. Med. Chem. 2021, 28, 525–547. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Mozafaryan, M.J.; Rezaei, P.; Masoudpoor, F.; Bahri, A.; Samini, M.; Farkhondeh, T.; Pourhanifeh, M.H.; Samarghandian, S. Resveratrol in the Treatment of Gynecological Cancer: Mechanisms and Therapeutic Potential. Curr. Med. Chem. 2025. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, S.J.; Lee, C.; Yim, G.W.; Kim, J.W.; Kim, H.S. Hypoxia-induced maspin expression affects the prognosis of ovarian clear cell carcinoma. Vivo 2022, 36, 212–220. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef]
- Guo, Y.M.; Sun, T.C.; Wang, H.P.; Chen, X. Research progress of melatonin (MT) in improving ovarian function: A review of the current status. Aging 2021, 13, 17930. [Google Scholar] [CrossRef]
- Savant, S.S.; Sriramkumar, S.; O’Hagan, H.M. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, J.W.; Gilkes, D.M. Tumor hypoxia as an enhancer of inflammation-mediated metastasis: Emerging therapeutic strategies. Target. Oncol. 2018, 13, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Feng, Y.; Zheng, X.; Sun, L.; Wasan, H.S.; Ruan, S.; Shen, M. Resveratrol and its analogs: Potent agents to reverse epithelial-to-mesenchymal transition in tumors. Front. Oncol. 2021, 11, 644134. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Zare, H.; Shafabakhsh, R.; Reiter, R.J.; Asemi, Z. Melatonin is a potential inhibitor of ovarian cancer: Molecular aspects. J. Ovarian Res. 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Pastorek, M.; Simko, V.; Takacova, M.; Barathova, M.; Bartosova, M.; Hunakova, L.; Sedlakova, O.; Hudecova, S.; Krizanova, O.; Dequiedt, F. Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy. Int. J. Oncol. 2015, 47, 51–60. [Google Scholar] [CrossRef]
- Freitas, R.D.; Campos, M.M. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Shahveghar Asl, Z.; Parastouei, K.; Eskandari, E. The effects of N-acetylcysteine on ovulation and sex hormones profile in women with polycystic ovary syndrome: A systematic review and meta-analysis. Br. J. Nutr. 2023, 130, 202–210. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Gelovani, J.G.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by downregulating inflammatory, proliferative, invasive and angiogenic biomarkers. Int. J. Cancer 2012, 130, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Ayustaningwarno, F.; Anjani, G.; Ayu, A.M.; Fogliano, V. A critical review of Ginger’s (Zingiber officinale) antioxidant, anti-inflammatory, and immunomodulatory activities. Front. Nutr. 2024, 11, 1364836. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.Z.; Dias, M.M.; Ropelle, E.R.; Osório-Costa, F.; Rossato, F.A.; Vercesi, A.E.; Saad, M.J.; Carvalheira, J.B. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin. Cancer Res. 2011, 17, 3993–4005. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, S.; Hancerliogullari, N.; Guney, G.; Gozukucuk, M.; Caydere, M.; Guney, S.S.; Tokmak, A.; Ustun, Y. Does the addition of metformin to carboplatin treatment decreases ovarian reserve damage associated with carboplatin usage? J. Ovarian Res. 2023, 16, 184. [Google Scholar] [CrossRef]
- Mills, G.B. Mechanisms underlying chemoprevention of ovarian cancer. Clin. Cancer Res. 2002, 8, 7–10. [Google Scholar]
- Baandrup, L.; Faber, M.T.; Christensen, J.; Jensen, A.; Andersen, K.K.; Friis, S.; Kjaer, S.K. Nonsteroidal anti-inflammatory drugs and risk of ovarian cancer: Systematic review and meta-analysis of observational studies. Acta Obs. Gynecol. Scand. 2013, 92, 245–255. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, T.; Wu, B.; Zhang, X. Insights into the therapeutic potential of hypoxia-inducible factor-1α small interfering RNA in malignant melanoma delivered via folate-decorated cationic liposomes. Int. J. Nanomed. 2016, 11, 991–1002. [Google Scholar]
- Mundekkad, D.; Cho, W. Applications of curcumin and its nanoforms in the treatment of cancer. Pharmaceutics 2023, 15, 2223. [Google Scholar] [CrossRef]
- Battaglia, R.; Caponnetto, A.; Caringella, A.; Cortone, A.; Ferrara, C.; Smirni, S.; Iannitti, R.; Purrello, M.; D’amato, G.; Fioretti, B. Resveratrol treatment induces Mito-miRNome modification in follicular fluid from aged women with a poor prognosis for in vitro fertilization cycles. Antioxidants 2022, 11, 1019. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Wang, H.; Xu, H.; Sheng, Y.; Lian, F. Role of N-acetylcysteine treatment in women with advanced age undergoing IVF/ICSI cycles: A prospective study. Front. Med. 2022, 9, 917146. [Google Scholar] [CrossRef]
- Perche, F.; Patel, N.R.; Torchilin, V.P. Accumulation and toxicity of antibody-targeted doxorubicin-loaded PEG–PE micelles in ovarian cancer cell spheroid model. J. Control. Release 2012, 164, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, O.E.; Ahmed, Y.H.; Mekkawy, A.M.; Mahmoud, M.Y.; Elbargeesy, G. The ameliorative effect of selenium-loaded chitosan nanoparticles against silver nanoparticles-induced ovarian toxicity in female albino rats. J. Ovarian Res. 2025, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Spivak-Kroizman, T.; Venturini, S.; Welsh, S.; Williams, R.R.; Kirkpatrick, D.L.; Powis, G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1α. Mol. Cancer Ther. 2008, 7, 90–100. [Google Scholar] [CrossRef]
- Chau, N.-M.; Rogers, P.; Aherne, W.; Carroll, V.; Collins, I.; McDonald, E.; Workman, P.; Ashcroft, M. Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1α induction in response to hypoxic stress and growth factors. Cancer Res. 2005, 65, 4918–4928. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Abir, R.; Ben-Aharon, I.; Garor, R.; Yaniv, I.; Ash, S.; Stemmer, S.; Ben-Haroush, A.; Freud, E.; Kravarusic, D.; Sapir, O. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum. Reprod. 2016, 31, 750–762. [Google Scholar] [CrossRef]
- Al-Shahat, A.; Hulail, M.A.; Soliman, N.M.; Khamis, T.; Fericean, L.M.; Arisha, A.H.; Moawad, R.S. Melatonin mitigates cisplatin-induced ovarian dysfunction via altering steroidogenesis, inflammation, apoptosis, oxidative stress, and PTEN/PI3K/Akt/mTOR/AMPK signaling pathway in female rats. Pharmaceutics 2022, 14, 2769. [Google Scholar] [CrossRef]
- Luo, J.; Wang, H.; Chen, J.; Wei, X.; Feng, J.; Zhang, Y.; Zhou, Y. The Application of Drugs and Nano-Therapies Targeting Immune Cells in Hypoxic Inflammation. Int. J. Nanomed. 2024, 19, 3441–3459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Jalouli, M.; Bhajan, S.K.; Al-Zharani, M.; Harrath, A.H. The Role of Hypoxia-Inducible Factor-1α (HIF-1α) in the Progression of Ovarian Cancer: Perspectives on Female Infertility. Cells 2025, 14, 437. https://doi.org/10.3390/cells14060437
Rahman MA, Jalouli M, Bhajan SK, Al-Zharani M, Harrath AH. The Role of Hypoxia-Inducible Factor-1α (HIF-1α) in the Progression of Ovarian Cancer: Perspectives on Female Infertility. Cells. 2025; 14(6):437. https://doi.org/10.3390/cells14060437
Chicago/Turabian StyleRahman, Md Ataur, Maroua Jalouli, Sujay Kumar Bhajan, Mohammed Al-Zharani, and Abdel Halim Harrath. 2025. "The Role of Hypoxia-Inducible Factor-1α (HIF-1α) in the Progression of Ovarian Cancer: Perspectives on Female Infertility" Cells 14, no. 6: 437. https://doi.org/10.3390/cells14060437
APA StyleRahman, M. A., Jalouli, M., Bhajan, S. K., Al-Zharani, M., & Harrath, A. H. (2025). The Role of Hypoxia-Inducible Factor-1α (HIF-1α) in the Progression of Ovarian Cancer: Perspectives on Female Infertility. Cells, 14(6), 437. https://doi.org/10.3390/cells14060437








