Blockade of HSP70 Improves Vascular Function in a Mouse Model of Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Model and Experimental Design
2.3. Ex Vivo Assessment of Vascular Function in Thoracic Aortas Using Wire Myography
2.3.1. Cumulative Concentration–Response Curves
2.3.2. Time–Force Curves
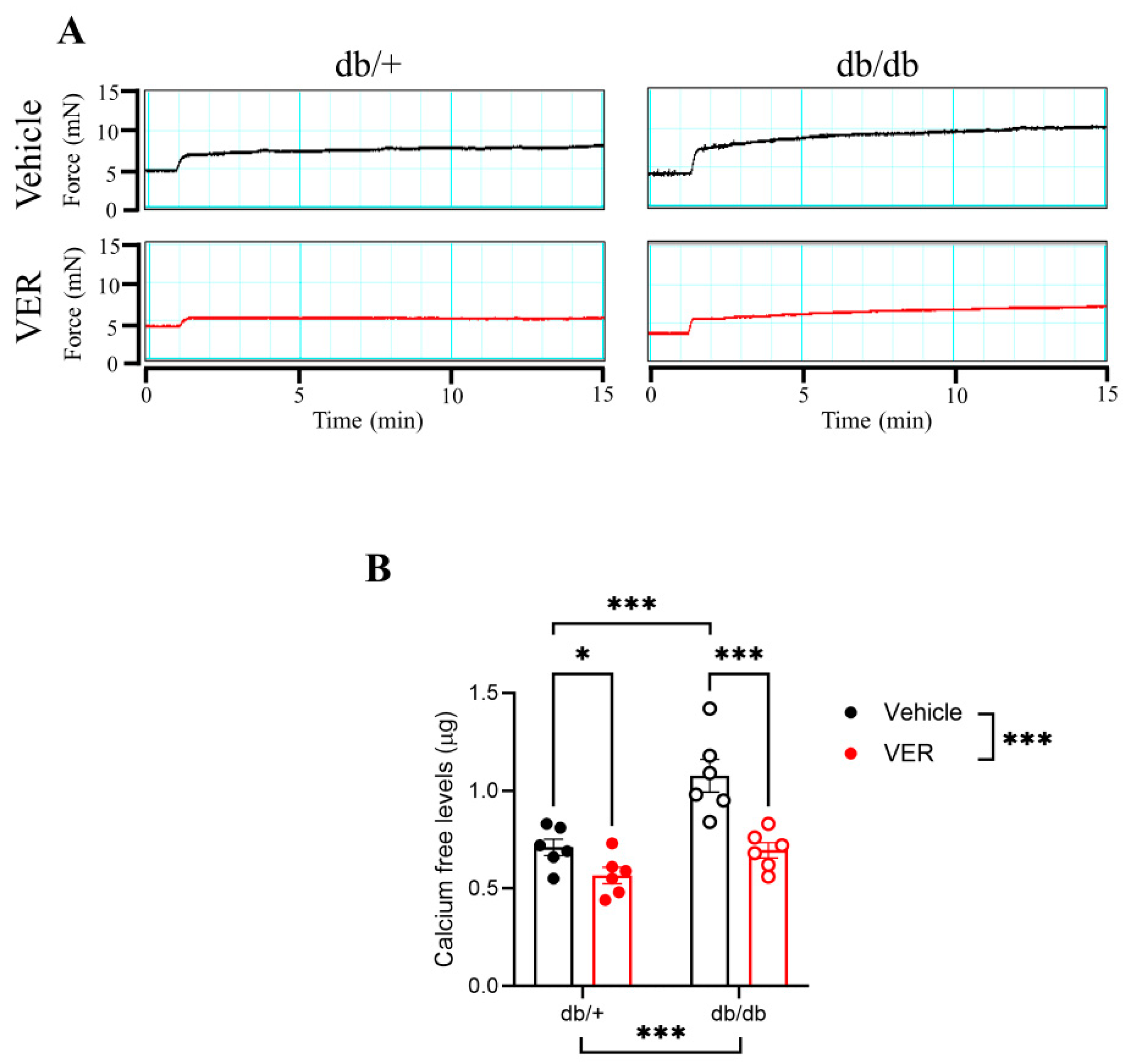
2.3.3. Calcium Protocol
2.4. Immunofluorescence for HSP70
2.5. Western Blotting for HSP70
2.6. Determination of Levels of Free Calcium
2.7. Statistical Analysis
3. Results
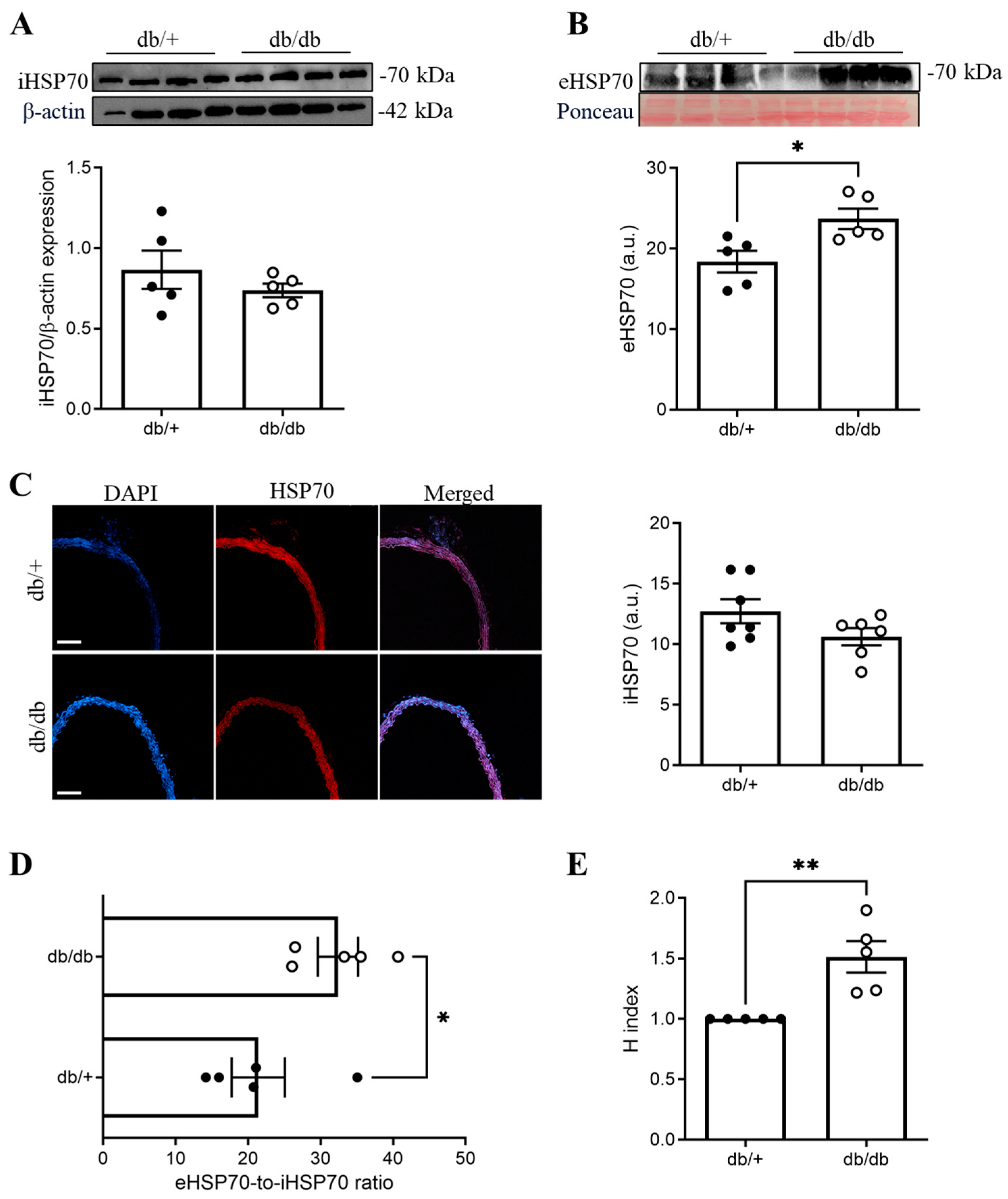
3.1. The eHSP70-to-iHSP70 Ratio Was Disrupted in the Aortas of db/db Mice
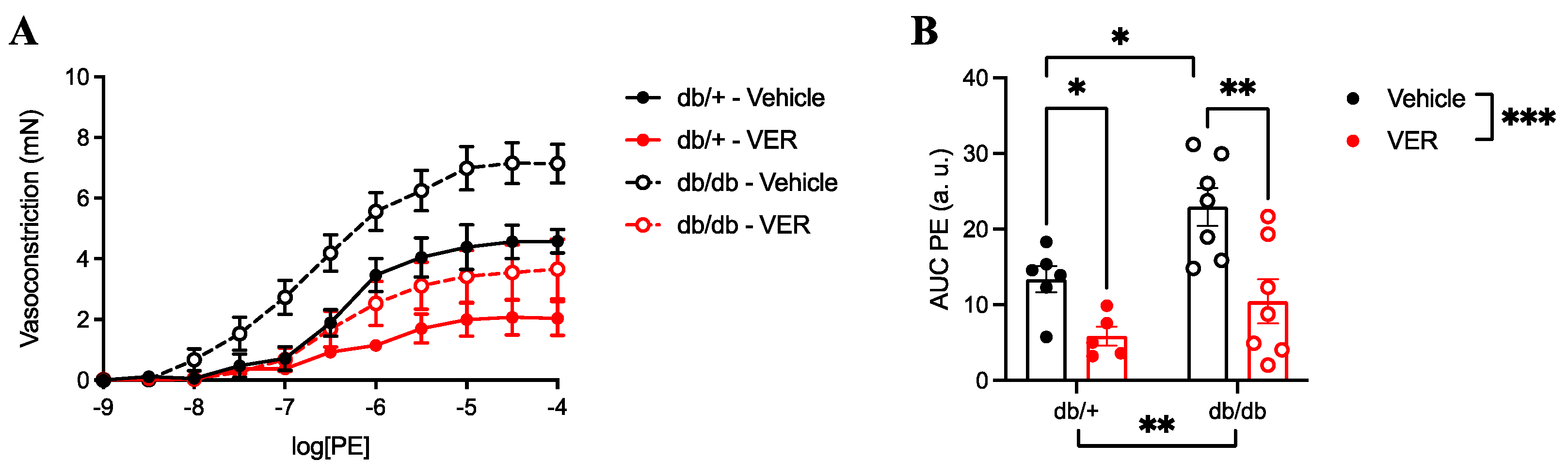
3.2. Vasoconstriction to PE Was Increased in the Aortas of db/db Mice and Reduced by HSP70 Inhibition
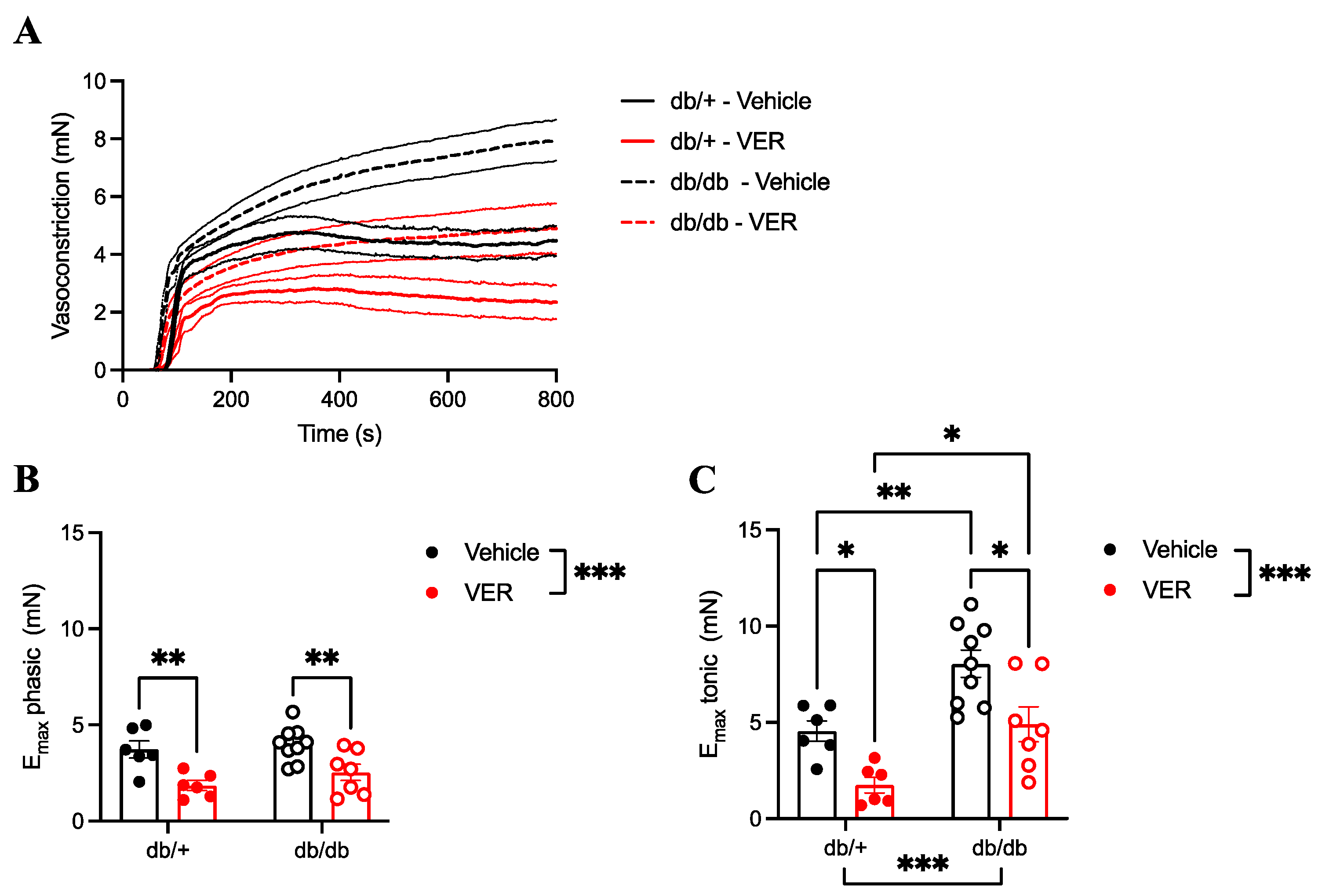
3.3. Increased Vasoconstriction to PE in the Aortas of db/db Mice Only Happened in the Tonic Part of the Curve and Was Prevented by HSP70 Inhibition
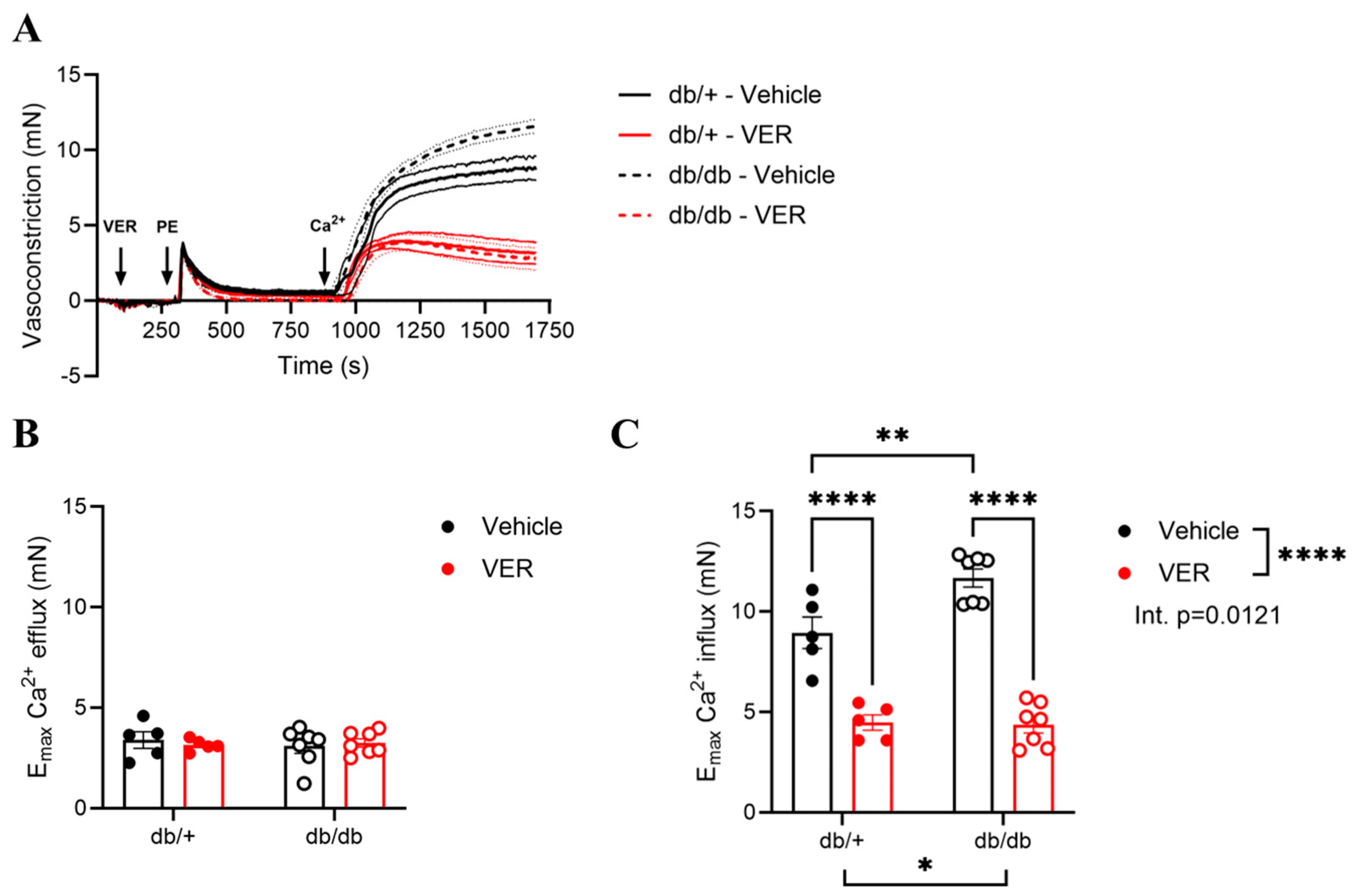
3.4. Increased Vasoconstriction to PE in the Aortas of db/db Mice Was Associated with Changes in Calcium Handling via HSP70
3.5. Calcium-Free Levels Were Increased in the Aortas of db/db Mice and Dependent on HSP70
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Holman, N.; Young, B.; Gadsby, R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet. Med. 2015, 32, 1119–1120. [Google Scholar] [CrossRef]
- Bruno, G.; Runzo, C.; Cavallo-Perin, P.; Merletti, F.; Rivetti, M.; Pinach, S.; Novelli, G.; Trovati, M.; Cerutti, F.; Pagano, G.; et al. Incidence of Type 1 and Type 2 Diabetes in Adults Aged 30–49 Years: The population-based registry in the province of Turin, Italy. Diabetes Care 2005, 28, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Sallam, N.; Fisher, A.; Golbidi, S.; Laher, I. Weight and inflammation are the major determinants of vascular dysfunction in the aortae of db/db mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2011, 383, 483–492. [Google Scholar] [CrossRef]
- Lemmey, H.A.L.; Ye, X.; Ding, H.C.; Triggle, C.R.; Garland, C.J.; Dora, K.A. Hyperglycaemia disrupts conducted vasodilation in the resistance vasculature of db/db mice. Vascul. Pharmacol. 2018, 103–105, 29–35. [Google Scholar] [CrossRef]
- Sallam, N.A.; Laher, I. Redox Signaling and Regional Heterogeneity of Endothelial Dysfunction in db/db Mice. Int. J. Mol. Sci. 2020, 21, 6147. [Google Scholar] [CrossRef]
- Beck, L.; Su, J.; Comerma-Steffensen, S.; Pinilla, E.; Carlsson, R.; Hernanz, R.; Sheykhzade, M.; Danielsen, C.C.; Simonsen, U. Endothelial Dysfunction and Passive Changes in the Aorta and Coronary Arteries of Diabetic db/db Mice. Front. Physiol. 2020, 11, 667. [Google Scholar] [CrossRef]
- Fernández-Velasco, M.; Ruiz-Hurtado, G.; Gómez, A.M.; Rueda, A. Ca2+ handling alterations and vascular dysfunction in diabetes. Calcium Diabetes 2014, 56, 397–407. [Google Scholar] [CrossRef]
- Moreno-Salgado, A.; Coyotl-Santiago, N.; Moreno-Vazquez, R.; Lopez-Teyssier, M.; Garcia-Carrasco, M.; Moccia, F.; Berra-Romani, R. Alterations of the Ca2+ clearing mechanisms by type 2 diabetes in aortic smooth muscle cells of Zucker diabetic fatty rat. Front. Physiol. 2023, 14, 1200115. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, X.-Y.; Kuang, S.-J.; Zhou, M.-Y.; Zhang, L.; Zeng, Z.; Liu, L.; Wu, F.-L.; Zhang, M.-Z.; Mai, L.-P.; et al. Abnormal Ca2+ handling contributes to the impairment of aortic smooth muscle contractility in Zucker diabetic fatty rats. J. Mol. Cell. Cardiol. 2020, 141, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Harris, K.H.; MacLeod, K.M. Enhanced Contractile Responses of Arteries from Diabetic Rats to α1-Adrenoceptor Stimulation in the Absence and Presence of Extracellular Calcium. J. Cardiovasc. Pharmacol. 1990, 16, 239–248. [Google Scholar] [CrossRef]
- Nobe, K.; Sakai, Y.; Maruyama, Y.; Momose, K. Hyper-reactivity of diacylglycerol kinase is involved in the dysfunction of aortic smooth muscle contractility in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2002, 136, 441–451. [Google Scholar] [CrossRef]
- Fleischhacker, E.; Esenabhalu, V.E.; Spitaler, M.; Holzmann, S.; Skrabal, F.; Koidl, B.; Kostner, G.M.; Graier, W.F. Human diabetes is associated with hyperreactivity of vascular smooth muscle cells due to altered subcellular Ca2+ distribution. Diabetes 1999, 48, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Okon, E.B.; Szado, T.; Laher, I.; McManus, B.; van Breemen, C. Augmented Contractile Response of Vascular Smooth Muscle in a Diabetic Mouse Model. J. Vasc. Res. 2004, 40, 520–530. [Google Scholar] [CrossRef]
- Bakker, W.; Eringa, E.C.; Sipkema, P.; van Hinsbergh, V.W.M. Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009, 335, 165–189. [Google Scholar] [CrossRef]
- Yang, J.; Gourley, G.R.; Gilbertsen, A.; Chen, C.; Wang, L.; Smith, K.; Namenwirth, M.; Yang, L. High Glucose Levels Promote Switch to Synthetic Vascular Smooth Muscle Cells via Lactate/GPR81. Cells 2024, 13, 236. [Google Scholar] [CrossRef]
- Fransen, P.; Van Hove, C.E.; Leloup, A.J.A.; Martinet, W.; De Meyer, G.R.Y.; Lemmens, K.; Bult, H.; Schrijvers, D.M. Dissecting out the Complex Ca2+-Mediated Phenylephrine-Induced Contractions of Mouse Aortic Segments. PLoS ONE 2015, 10, e0121634. [Google Scholar] [CrossRef]
- Karaki, H.; Ozaki, H. Relationship Between Cytosolic Calcium Level and Contractile Tension in Vascular Smooth Muscle. In Essential Hypertension 2; Aoki, K., Ed.; Springer: Tokyo, Japan, 1989; pp. 95–110. [Google Scholar] [CrossRef]
- Karaki, H.; Kubota, H.; Urakawa, N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur. J. Pharmacol. 1979, 56, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Dinh Cat, A.; Callera, G.E.; Friederich-Persson, M.; Sanchez, A.; Dulak-Lis, M.G.; Tsiropoulou, S.; Montezano, A.C.; He, Y.; Briones, A.M.; Jaisser, F.; et al. Vascular dysfunction in obese diabetic db/db mice involves the interplay between aldosterone/mineralocorticoid receptor and Rho kinase signaling. Sci. Rep. 2018, 8, 2952. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, B.E.; Watras, J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature 1988, 336, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Aihara, K.; Honda, H.; Inazu, M. Calcium mobilization and phosphatidylinositol turnover in vas deferens smooth muscle of diabetic rats. Eur. J. Pharmacol. 1989, 162, 475–481. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Priviero, F.; Tostes, R.C.; Webb, R.C.; Nunes, K.P. Dissecting the interaction between HSP70 and vascular contraction: Role of Ca2+ handling mechanisms. Sci. Rep. 2021, 11, 1420. [Google Scholar] [CrossRef]
- Piercy, V.; Taylor, S.G. A Comparison of Spasmogenic and Relaxant Responses in Aortae from C57/BL/KsJ Diabetic Mice with Those from Their Non-Diabetic Litter Mates. Pharmacology 1998, 56, 267–275. [Google Scholar] [CrossRef]
- Khazaei, M.; Moien-Afshari, F.; Kieffer, T.; Laher, I. Effect of exercise on augmented aortic vasoconstriction in the db/db mouse model of type-II diabetes. Physiol. Res. 2007, 57, 847–856. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Priviero, F.; Webb, R.C.; Nunes, K.P. Increased eHSP70-to-iHSP70 ratio disrupts vascular responses to calcium and activates the TLR4-MD2 complex in type 1 diabetes. Life Sci. 2022, 310, 121079. [Google Scholar] [CrossRef]
- Krause, M.; Bock, P.M.; Takahashi, H.K.; Homem De Bittencourt, P.I., Jr.; Newsholme, P. The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin. Sci. 2015, 128, 789–803. [Google Scholar] [CrossRef]
- Krause, M.; Heck, T.G.; Bittencourt, A.; Scomazzon, S.P.; Newsholme, P.; Curi, R.; Homem de Bittencourt, P.I., Jr. The chaperone balance hypothesis: The importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm. 2015, 2015, 249205. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Faustino, J.; de Lima, M.E.; Menezes, R.; Nunes, K.P. Unveiling the Interplay between the TLR4/MD2 Complex and HSP70 in the Human Cardiovascular System: A Computational Approach. Int. J. Mol. Sci. 2019, 20, 3121. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Webb, R.C.; Nunes, K.P. Toll-Like Receptor 4 and Heat-Shock Protein 70: Is it a New Target Pathway for Diabetic Vasculopathies? Curr. Drug Targets 2019, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Garamvölgyi, Z.; Prohászka, Z.; Rigó, J.; Kecskeméti, A.; Molvarec, A. Increased circulating heat shock protein 70 (HSPA1A) levels in gestational diabetes mellitus: A pilot study. Cell Stress Chaperones 2015, 20, 575–581. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Morteza, A.; Khajeali, L.; Esteghamati, A.; Khalilzadeh, O.; Asgarani, F.; Outeiro, T.F. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones 2010, 15, 959–964. [Google Scholar] [CrossRef]
- Burke, S.J.; Batdorf, H.M.; Burk, D.H.; Noland, R.C.; Eder, A.E.; Boulos, M.S.; Karlstad, M.D.; Jason Collier, J. db/db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. J. Diabetes Res. 2017, 2017, 8503754. [Google Scholar] [CrossRef] [PubMed]
- Wyse, B.M.; Dulin, W.E. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia 1970, 6, 268–273. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. The ARRIVE guidelines animal research: Reporting in vivo experiments. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.; Nunes, K.P. An additional physiological role for HSP70: Assistance of vascular reactivity. Life Sci. 2020, 256, 117986. [Google Scholar] [CrossRef]
- Diller, T.; Thompson, J.; Steer, B. Biological validation of a novel process and product for quantitating western blots. J. Biotechnol. 2021, 326, 52–60. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Suarez, J.; Hu, Y.; McDonough, P.M.; Boer, C.; Dix, D.J.; Dillmann, W.H. Deletion of the Inducible 70-kDa Heat Shock Protein Genes in Mice Impairs Cardiac Contractile Function and Calcium Handling Associated with Hypertrophy. Circulation 2006, 113, 2589–2597. [Google Scholar] [CrossRef]
- Senf, S.M.; Howard, T.M.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS ONE 2013, 8, e62687. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.; Mendoza, V.O.; Rastogi, S.; Nunes, K.P. New insights into the role and therapeutic potential of HSP70 in diabetes. Pharmacol. Res. 2022, 178, 106173. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, B.; Chen, X.; Liu, J.; Guan, Y.; Ren, J. Abnormalities of Sarcoplasmic Reticulum Ca2+ Mobilization in Aortic Smooth Muscle Cells from Streptozotocin-Induced Diabetic Rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 568–573. [Google Scholar] [CrossRef]
- Berra-Romani, R.; Guzmán-Silva, A.; Vargaz-Guadarrama, A.; Flores-Alonso, J.C.; Alonso-Romero, J.; Treviño, S.; Sánchez-Gómez, J.; Coyotl-Santiago, N.; García-Carrasco, M.; Moccia, F. Type 2 Diabetes Alters Intracellular Ca2+ Handling in Native Endothelium of Excised Rat Aorta. Int. J. Mol. Sci. 2020, 21, 250. [Google Scholar] [CrossRef]
- Velmurugan, G.V.; White, C. Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsP3R calcium release channels. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H124–H134. [Google Scholar] [CrossRef]
- Inoue, R.; Okada, T.; Onoue, H.; Hara, Y.; Shimizu, S.; Naitoh, S.; Ito, Y.; Mori, Y. The Transient Receptor Potential Protein Homologue TRP6 Is the Essential Component of Vascular α1-Adrenoceptor–Activated Ca2+-Permeable Cation Channel. Circ. Res. 2001, 88, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Daskoulidou, N.; Zeng, B.; Berglund, L.M.; Jiang, H.; Chen, G.-L.; Kotova, O.; Bhandari, S.; Ayoola, J.; Griffin, S.; Atkin, S.L.; et al. High glucose enhances store-operated calcium entry by upregulating ORAI/STIM via calcineurin-NFAT signalling. J. Mol. Med. 2015, 93, 511–521. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Mendoza, V.O.; Priviero, F.; Webb, R.C.; Nunes, K.P. Age-Related Decline in Vascular Responses to Phenylephrine Is Associated with Reduced Levels of HSP70. Biomolecules 2022, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.; Priviero, F.; Webb, R.C.; Nunes, K.P. Impaired HSP70 Expression in the Aorta of Female Rats: A Novel Insight Into Sex-Specific Differences in Vascular Function. Front. Physiol. 2021, 12, 666696. [Google Scholar] [CrossRef]
- Williamson, D.S.; Borgognoni, J.; Clay, A.; Daniels, Z.; Dokurno, P.; Drysdale, M.J.; Foloppe, N.; Francis, G.L.; Graham, C.J.; Howes, R.; et al. Novel Adenosine-Derived Inhibitors of 70 kDa Heat Shock Protein, Discovered Through Structure-Based Design. J. Med. Chem. 2009, 52, 1510–1513. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Kim, M.; Baek, W.; Kim, I. Effect of heat shock on the vascular contractility in isolated rat aorta. J. Pharmacol. Toxicol. Methods 1999, 42, 171–174. [Google Scholar] [CrossRef]
- Kizub, I.; Pavlova, O.; Johnson, C.; Soloviev, A.; Zholos, A. Rho kinase and protein kinase C involvement in vascular smooth muscle myofilament calcium sensitization in arteries from diabetic rats. Br. J. Pharmacol. 2010, 159, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, G.; Juretić, N.; Jaimovich, E.; Riveros, N. Membrane depolarization induces calcium-dependent upregulation of Hsp70 and Hmox-1 in skeletal muscle cells. Am. J. Physiol.-Cell Physiol. 2009, 297, C581–C590. [Google Scholar] [CrossRef] [PubMed]
- Sriram, M.; Osipiuk, J.; Freeman, B.; Morimoto, R.; Joachimiak, A. Human Hsp70 molecular chaperone binds two calcium ions within the ATPase domain. Structure 1997, 5, 403–414. [Google Scholar] [CrossRef]
- Chatterjee, M.; Andrulis, M.; Stuhmer, T.; Muller, E.; Hofmann, C.; Steinbrunn, T.; Heimberger, T.; Schraud, H.; Kressmann, S.; Einsele, H.; et al. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica 2013, 98, 1132–1141. [Google Scholar] [CrossRef]
- Ambrose, A.J.; Chapman, E. Function, Therapeutic Potential, and Inhibition of Hsp70 Chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Seike, T.; Oikawa, S.; Kobayashi, H.; Kido, H.; Yanagi, M.; Yamamiya, D.; Li, S.; Boontem, P.; Mizukoshi, E. Hsp70.1 carbonylation induces lysosomal cell death for lifestyle-related diseases. Front. Mol. Biosci. 2023, 9, 1063632. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670. [Google Scholar] [CrossRef]
| Group | Body Weight (g) | Glucose Levels (mg/dL) | Abdominal Circumference (cm) |
|---|---|---|---|
| db/+ | 31.2 ± 1.3 | 140.4 ± 12.4 | 9.5 ± 0.07 |
| Db/db | 54.8 ± 1.9 * | 314.4 ± 29.4 * | 13.3 ± 0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa Mendoza, V.; de Oliveira, A.A.; Nunes, K.P. Blockade of HSP70 Improves Vascular Function in a Mouse Model of Type 2 Diabetes. Cells 2025, 14, 424. https://doi.org/10.3390/cells14060424
Ochoa Mendoza V, de Oliveira AA, Nunes KP. Blockade of HSP70 Improves Vascular Function in a Mouse Model of Type 2 Diabetes. Cells. 2025; 14(6):424. https://doi.org/10.3390/cells14060424
Chicago/Turabian StyleOchoa Mendoza, Valentina, Amanda Almeida de Oliveira, and Kenia Pedrosa Nunes. 2025. "Blockade of HSP70 Improves Vascular Function in a Mouse Model of Type 2 Diabetes" Cells 14, no. 6: 424. https://doi.org/10.3390/cells14060424
APA StyleOchoa Mendoza, V., de Oliveira, A. A., & Nunes, K. P. (2025). Blockade of HSP70 Improves Vascular Function in a Mouse Model of Type 2 Diabetes. Cells, 14(6), 424. https://doi.org/10.3390/cells14060424









