Post-Secretion Processes and Modification of Extracellular Vesicles
Abstract
1. Introduction
2. Alteration of the EV Surface
2.1. Alteration of Lipid Bilayers
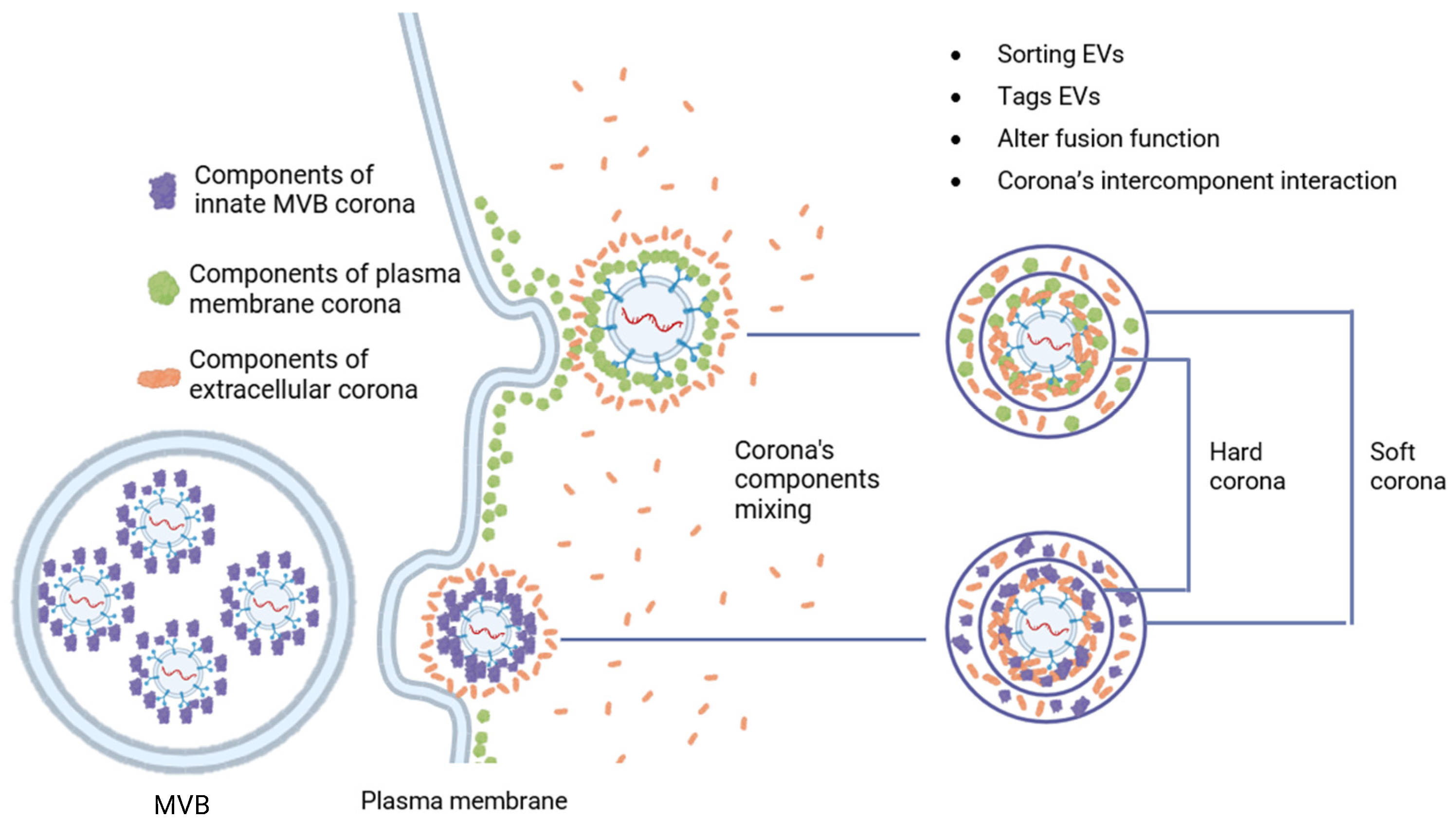
2.2. Formation of a Biomolecular Corona on the Surface of EV
2.2.1. Post-Translational Modifications of Components
2.2.2. Formation of Multimolecular Machine
3. Intervesicular Extracellular Interactions
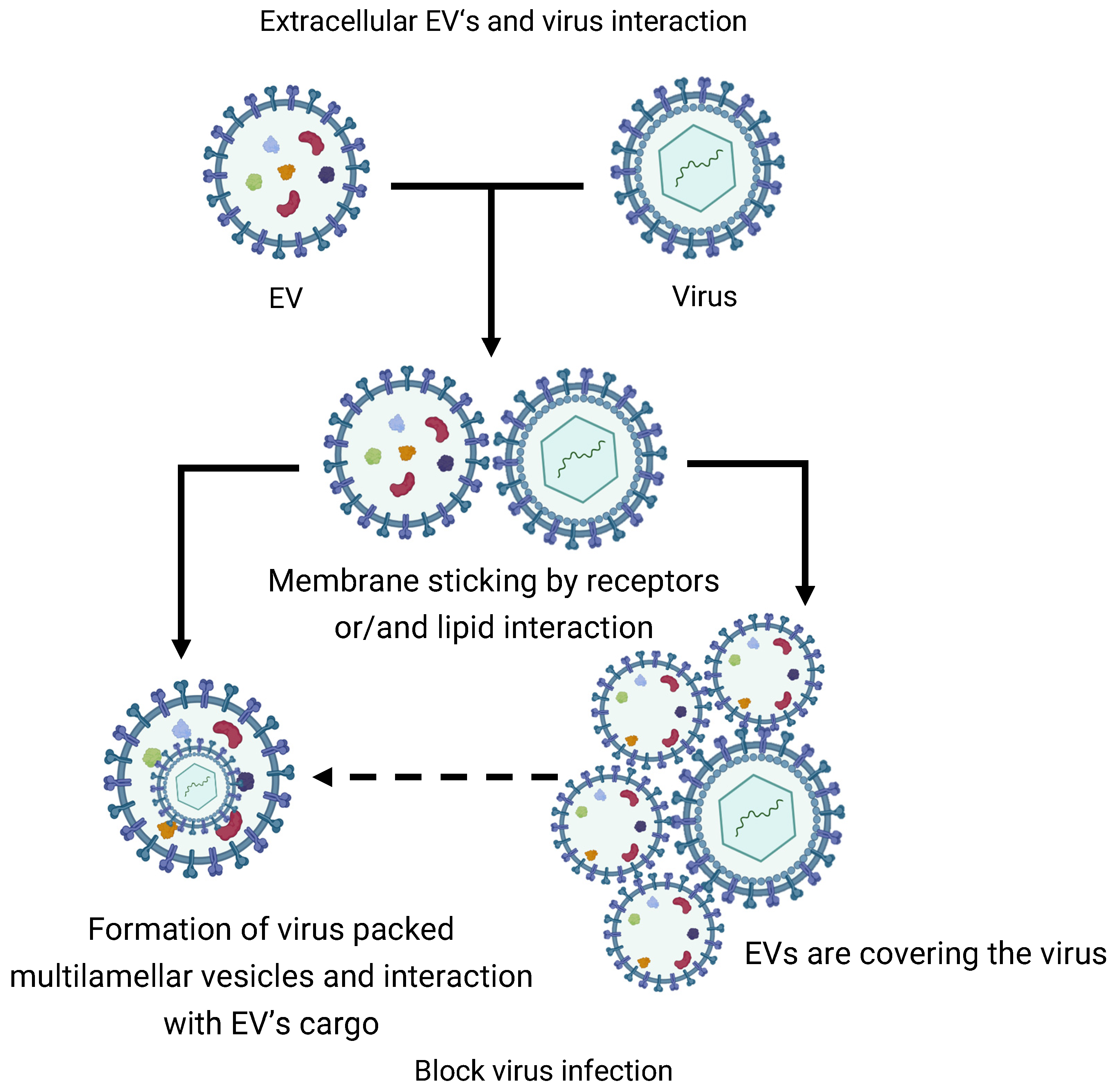
3.1. Mutual EVs Sticking
3.2. EVs Extracellular Fusion
4. Volume Alteration and Deformation of the EV
5. Attachment of the EV to Extracellular Matrix
6. Extracellular Disrupt of the EV
7. Therapeutic Application of EVs
8. PSPMs’ Contribution to the Optimization of Protocols for Working with EV
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| AQP | aquaporin |
| BMC | biomolecular corona |
| COP2 | Coat Protein Complex II |
| COX2 | cyclooxygenase-2 |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOPS | 1,2-dioleoyl-sn-glycero-3-phospho-L-serine |
| ECM | extracellular matrix |
| ECS | extracellular space |
| EV | extracellular vesicles |
| GUV | giant unilamellar vesicles |
| HIV | human immunodeficiency virus |
| LUV | large unilamellar vesicles |
| MBV | matrix bound vesicles |
| mEV-IC | medium-sized extracellular vesicle-containing immune complex |
| MFHE | membrane fusion-based hybrid exosomes |
| MMP | matrix mettaloproteinase |
| MSC | mesenchymal stem cell |
| MV | matrix vesicles |
| MVB | multivesicular bodies |
| MβCD | methyl-β-cyclodextrin |
| NF-kB | nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| PAI-1 | Plasminogen Activator Inhibitor 1 |
| PGE2 | prostaglandin E2 |
| PS | phosphatidylserine |
| PSMP | post-secretion processes and modifications |
| SEC | size-exclusion chromatography |
| SUV | small unilamellar vesicles |
| TFPI | Tissue Factor Pathway Inhibitor |
| TGF-beta | transforming growth factor beta |
| TNAP | tissue-non-specific alkaline phosphatase |
| ZIKV | Zika virus |
References
- Liu, S.; Wu, X.; Chandra, S.; Lyon, C.; Ning, B.; Jiang, L.; Fan, J.; Hu, T.Y. Extracellular Vesicles: Emerging Tools as Therapeutic Agent Carriers. Acta Pharm. Sin. B 2022, 12, 3822–3842. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Urban, S.K.; Lukacs-Kornek, V.; Żekanowska, E.; Kornek, M. Large Extracellular Vesicles: Have We Found the Holy Grail of Inflammation? Front. Immunol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Wang, D.; Yu, L. Migrasome Biogenesis: When Biochemistry Meets Biophysics on Membranes. Trends Biochem. Sci. 2024, 49, 829–840. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of Exosome versus Small Ectosome Secretion Revealed by Live Intracellular Tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Jansen, F.H.; Krijgsveld, J.; Van Rijswijk, A.; Van Den Bemd, G.-J.; Van Den Berg, M.S.; Van Weerden, W.M.; Willemsen, R.; Dekker, L.J.; Luider, T.M.; Jenster, G. Exosomal Secretion of Cytoplasmic Prostate Cancer Xenograft-Derived Proteins. Mol. Cell. Proteom. 2009, 8, 1192–1205. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Van Engeland, M.; Nieland, L.J.W.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, C.P.M. Annexin V-Affinity Assay: A Review on an Apoptosis Detection System Based on Phosphatidylserine Exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Zhao, X.; Lei, Y.; Zheng, J.; Peng, J.; Li, Y.; Yu, L.; Chen, Y. Identification of Markers for Migrasome Detection. Cell Discov. 2019, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Sadovsky, Y. The Biology of Extracellular Vesicles: The Known Unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef] [PubMed]
- Hrabetova, S.; Cognet, L.; Rusakov, D.A.; Nägerl, U.V. Unveiling the Extracellular Space of the Brain: From Super-Resolved Microstructure to In Vivo Function. J. Neurosci. 2018, 38, 9355–9363. [Google Scholar] [CrossRef]
- O’Brien, K.; Ughetto, S.; Mahjoum, S.; Nair, A.V.; Breakefield, X.O. Uptake, Functionality, and Re-Release of Extracellular Vesicle-Encapsulated Cargo. Cell Rep. 2022, 39, 110651. [Google Scholar] [CrossRef]
- Mecham, R.P. (Ed.) The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-16554-2. [Google Scholar]
- Ghadami, S.; Dellinger, K. The Lipid Composition of Extracellular Vesicles: Applications in Diagnostics and Therapeutic Delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef]
- Fam, T.K.; Klymchenko, A.S.; Collot, M. Recent Advances in Fluorescent Probes for Lipid Droplets. Materials 2018, 11, 1768. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.M.; Schwarzmann, G.; Möbius, W.; Hoernschemeyer, J.; Slot, J.-W.; Geuze, H.J.; Stoorvogel, W. Proteomic and Biochemical Analyses of Human B Cell-Derived Exosomes. J. Biol. Chem. 2003, 278, 10963–10972. [Google Scholar] [CrossRef]
- Sapoń, K.; Mańka, R.; Janas, T.; Janas, T. The Role of Lipid Rafts in Vesicle Formation. J. Cell Sci. 2023, 136, jcs260887. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding Microvesicles: Artefacts No More. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An Emerging Focus on Lipids in Extracellular Vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Doktorova, M.; Symons, J.L.; Levental, I. Structural and Functional Consequences of Reversible Lipid Asymmetry in Living Membranes. Nat. Chem. Biol. 2020, 16, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Segawa, K.; Nagata, S. An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Wang, H.; Wang, L.; Xiao, F.; Guo, Z.; Zhang, H. Surface Phosphatidylserine Is Responsible for the Internalization on Microvesicles Derived from Hypoxia-Induced Human Bone Marrow Mesenchymal Stem Cells into Human Endothelial Cells. PLoS ONE 2016, 11, e0147360. [Google Scholar] [CrossRef]
- Gong, Y.-N.; Guy, C.; Crawford, J.C.; Green, D.R. Biological Events and Molecular Signaling Following MLKL Activation during Necroptosis. Cell Cycle 2017, 16, 1748–1760. [Google Scholar] [CrossRef]
- Steck, T.L.; Lange, Y. Transverse Distribution of Plasma Membrane Bilayer Cholesterol: Picking Sides. Traffic 2018, 19, 750–760. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Bun, P.; Huveneers, S.; Van Niel, G.; Pegtel, D.M.; Verweij, F.J. Real-Time Imaging of Multivesicular Body–Plasma Membrane Fusion to Quantify Exosome Release from Single Cells. Nat. Protoc. 2020, 15, 102–121. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Angelini, D.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental pH and Exosome Levels Interplay in Human Cancer Cell Lines of Different Histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef]
- Bonsergent, E.; Lavieu, G. Content Release of Extracellular Vesicles in a Cell-free Extract. FEBS Lett. 2019, 593, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Morandi, M.I.; Busko, P.; Ozer-Partuk, E.; Khan, S.; Zarfati, G.; Elbaz-Alon, Y.; Abou Karam, P.; Napso Shogan, T.; Ginini, L.; Gil, Z.; et al. Extracellular Vesicle Fusion Visualized by Cryo-Electron Microscopy. PNAS Nexus 2022, 1, pgac156. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as New Vesicular Lipid Transporters Involved in Cell–Cell Communication and Various Pathophysiologies. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Staneva, G.; Seigneuret, M.; Conjeaud, H.; Puff, N.; Angelova, M.I. Making a Tool of an Artifact: The Application of Photoinduced Lo Domains in Giant Unilamellar Vesicles to the Study of Lo/Ld Phase Spinodal Decomposition and Its Modulation by the Ganglioside GM1. Langmuir 2011, 27, 15074–15082. [Google Scholar] [CrossRef]
- Kim, S.Y.; Bondar, A.-N.; Wimley, W.C.; Hristova, K. pH-Triggered Pore-Forming Peptides with Strong Composition-Dependent Membrane Selectivity. Biophys. J. 2021, 120, 618–630. [Google Scholar] [CrossRef]
- Angelova, M.I.; Bitbol, A.-F.; Seigneuret, M.; Staneva, G.; Kodama, A.; Sakuma, Y.; Kawakatsu, T.; Imai, M.; Puff, N. pH Sensing by Lipids in Membranes: The Fundamentals of pH-Driven Migration, Polarization and Deformations of Lipid Bilayer Assemblies. Biochim. Biophys. Acta BBA Biomembr. 2018, 1860, 2042–2063. [Google Scholar] [CrossRef]
- Kodama, A.; Sakuma, Y.; Imai, M.; Oya, Y.; Kawakatsu, T.; Puff, N.; Angelova, M.I. Migration of Phospholipid Vesicles in Response to OH− Stimuli. Soft Matter 2016, 12, 2877–2886. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Protein Corona and Exosomes: New Challenges and Prospects. Cell Commun. Signal. 2023, 21, 64. [Google Scholar] [CrossRef]
- Tóth, E.Á.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a Protein Corona on the Surface of Extracellular Vesicles in Blood Plasma. J. Extracell. Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef]
- Wolf, M.; Poupardin, R.W.; Ebner-Peking, P.; Andrade, A.C.; Blöchl, C.; Obermayer, A.; Gomes, F.G.; Vari, B.; Maeding, N.; Eminger, E.; et al. A Functional Corona around Extracellular Vesicles Enhances Angiogenesis, Skin Regeneration and Immunomodulation. J. Extracell. Vesicles 2022, 11, e12207. [Google Scholar] [CrossRef]
- Papafilippou, L.; Nicolaou, A.; Kendall, A.C.; Camacho-Muñoz, D.; Hadjidemetriou, M. The Lipidomic Profile of the Nanoparticle-Biomolecule Corona Reflects the Diversity of Plasma Lipids. Nanoscale 2023, 15, 11038–11051. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. Opportunities and Challenges in Studying the Extracellular Vesicle Corona. Nat. Cell Biol. 2022, 24, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Németh, A.; Orgovan, N.; Sódar, B.W.; Osteikoetxea, X.; Pálóczi, K.; Szabó-Taylor, K.É.; Vukman, K.V.; Kittel, Á.; Turiák, L.; Wiener, Z.; et al. Antibiotic-Induced Release of Small Extracellular Vesicles (Exosomes) with Surface-Associated DNA. Sci. Rep. 2017, 7, 8202. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Cornils, K.; Speiseder, T.; Badbaran, A.; Reimer, R.; Indenbirken, D.; Grundhoff, A.; Brunswig-Spickenheier, B.; Alawi, M.; Lange, C. Indication of Horizontal DNA Gene Transfer by Extracellular Vesicles. PLoS ONE 2016, 11, e0163665. [Google Scholar] [CrossRef]
- Shelke, G.; Jang, S.C.; Yin, Y.; Lässer, C.; Lötvall, J. Human Mast Cells Release Extracellular Vesicle-Associated DNA. Matters Zür. 2016, 2, e201602000034. [Google Scholar] [CrossRef]
- Papini, E.; Tavano, R.; Mancin, F. Opsonins and Dysopsonins of Nanoparticles: Facts, Concepts, and Methodological Guidelines. Front. Immunol. 2020, 11, 567365. [Google Scholar] [CrossRef]
- Meidert, A.S.; Hermann, S.; Brandes, F.; Kirchner, B.; Buschmann, D.; Billaud, J.-N.; Klein, M.; Lindemann, A.; Aue, E.; Schelling, G.; et al. Extracellular Vesicle Associated miRNAs Regulate Signaling Pathways Involved in COVID-19 Pneumonia and the Progression to Severe Acute Respiratory Corona Virus-2 Syndrome. Front. Immunol. 2021, 12, 784028. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Li, Y.-J.; Wang, J.; Hu, X.-B.; Huang, S.; Luo, S.; Xiang, D.-X. Multifunctional Exosome-Mimetics for Targeted Anti-Glioblastoma Therapy by Manipulating Protein Corona. J. Nanobiotechnol. 2021, 19, 405. [Google Scholar] [CrossRef]
- Moreno-Gonzalo, O.; Villarroya-Beltri, C.; Sánchez-Madrid, F. Post-Translational Modifications of Exosomal Proteins. Front. Immunol. 2014, 5, 383. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Bandari, S.K.; Vlodavsky, I. Proteases and Glycosidases on the Surface of Exosomes: Newly Discovered Mechanisms for Extracellular Remodeling. Matrix Biol. 2019, 75–76, 160–169. [Google Scholar] [CrossRef]
- Paolini, L.; Orizio, F.; Busatto, S.; Radeghieri, A.; Bresciani, R.; Bergese, P.; Monti, E. Exosomes Secreted by HeLa Cells Shuttle on Their Surface the Plasma Membrane-Associated Sialidase NEU3. Biochemistry 2017, 56, 6401–6408. [Google Scholar] [CrossRef] [PubMed]
- Sumida, M.; Hane, M.; Yabe, U.; Shimoda, Y.; Pearce, O.M.T.; Kiso, M.; Miyagi, T.; Sawada, M.; Varki, A.; Kitajima, K.; et al. Rapid Trimming of Cell Surface Polysialic Acid (PolySia) by Exovesicular Sialidase Triggers Release of Preexisting Surface Neurotrophin. J. Biol. Chem. 2015, 290, 13202–13214. [Google Scholar] [CrossRef] [PubMed]
- Tundo, G.R.; Sbardella, D.; Ciaccio, C.; Grasso, G.; Gioia, M.; Coletta, A.; Polticelli, F.; Di Pierro, D.; Milardi, D.; Van Endert, P.; et al. Multiple Functions of Insulin-Degrading Enzyme: A Metabolic Crosslight? Crit. Rev. Biochem. Mol. Biol. 2017, 52, 554–582. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, I.Y.; Barth, E.; Christian, L.; Siepmann, M.; Kumar, S.; Singh, S.; Tolksdorf, K.; Heneka, M.T.; Lütjohann, D.; Wunderlich, P.; et al. Statins Promote the Degradation of Extracellular Amyloid β-Peptide by Microglia via Stimulation of Exosome-Associated Insulin-Degrading Enzyme (IDE) Secretion. J. Biol. Chem. 2010, 285, 37405–37414. [Google Scholar] [CrossRef]
- Itoh, Y.; Seiki, M. MT1-MMP: A Potent Modifier of Pericellular Microenvironment. J. Cell. Physiol. 2006, 206, 1–8. [Google Scholar] [CrossRef]
- Hakulinen, J.; Sankkila, L.; Sugiyama, N.; Lehti, K.; Keski-Oja, J. Secretion of Active Membrane Type 1 Matrix Metalloproteinase (MMP-14) into Extracellular Space in Microvesicular Exosomes. J. Cell. Biochem. 2008, 105, 1211–1218. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-Type Matrix Metalloproteinases: Their Functions and Regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Albacete-Albacete, L.; Navarro-Lérida, I.; López, J.A.; Martín-Padura, I.; Astudillo, A.M.; Ferrarini, A.; Van-Der-Heyden, M.; Balsinde, J.; Orend, G.; Vázquez, J.; et al. ECM Deposition Is Driven by Caveolin-1–Dependent Regulation of Exosomal Biogenesis and Cargo Sorting. J. Cell Biol. 2020, 219, e202006178. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, Y.; Zeng, Y.; Yang, D.; Mo, J.; Zheng, Z.; Wang, J.; Zhang, Y.; Zhou, Z.; Zhong, X.; et al. Impaired Angiogenesis in Ageing: The Central Role of the Extracellular Matrix. J. Transl. Med. 2023, 21, 457. [Google Scholar] [CrossRef]
- Adams, J.C. Passing the Post: Roles of Posttranslational Modifications in the Form and Function of Extracellular Matrix. Am. J. Physiol.-Cell Physiol. 2023, 324, C1179–C1197. [Google Scholar] [CrossRef]
- Haucke, E.; Navarrete-Santos, A.; Simm, A.; Silber, R.; Hofmann, B. Glycation of Extracellular Matrix Proteins Impairs Migration of Immune Cells. Wound Repair Regen. 2014, 22, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.P.; Sebinelli, H.G.; Ciancaglini, P.; Rosato, N.; Mebarek, S.; Buchet, R.; Millán, J.L.; Bottini, M. The Functional Role of Soluble Proteins Acquired by Extracellular Vesicles. J. Extracell. Biol. 2022, 1, e34. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; Albert, A.; Shnayder, R.; Gobezie, R.; Nigrovic, P.A.; et al. The Exposure of Autoantigens by Microparticles Underlies the Formation of Potent Inflammatory Components: The Microparticle-associated Immune Complexes. EMBO Mol. Med. 2013, 5, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a Factor That Links Apoptotic Cells to Phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef]
- Véron, P.; Segura, E.; Sugano, G.; Amigorena, S.; Théry, C. Accumulation of MFG-E8/Lactadherin on Exosomes from Immature Dendritic Cells. Blood Cells. Mol. Dis. 2005, 35, 81–88. [Google Scholar] [CrossRef]
- Bradley, A.J.; Brooks, D.E.; Norris-Jones, R.; Devine, D.V. C1q Binding to Liposomes Is Surface Charge Dependent and Is Inhibited by Peptides Consisting of Residues 14–26 of the Human C1qA Chain in a Sequence Independent Manner. Biochim. Biophys. Acta BBA Biomembr. 1999, 1418, 19–30. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and Its Role in Innate and Adaptive Immune Responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Braig, D.; Nero, T.L.; Koch, H.-G.; Kaiser, B.; Wang, X.; Thiele, J.R.; Morton, C.J.; Zeller, J.; Kiefer, J.; Potempa, L.A.; et al. Transitional Changes in the CRP Structure Lead to the Exposure of Proinflammatory Binding Sites. Nat. Commun. 2017, 8, 14188. [Google Scholar] [CrossRef]
- Kato, T.; Fahrmann, J.F.; Hanash, S.M.; Vykoukal, J. Extracellular Vesicles Mediate B Cell Immune Response and Are a Potential Target for Cancer Therapy. Cells 2020, 9, 1518. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Liu, N.; Lv, D.; Chen, Y.; Liu, Z.; Jin, X.; Xiao, M.; Lavillette, D.; Zhong, J.; et al. Extracellular Vesicles from Zika Virus-infected Cells Display Viral E Protein That Binds ZIKV-neutralizing Antibodies to Prevent Infection Enhancement. EMBO J. 2023, 42, e112096. [Google Scholar] [CrossRef]
- Ugalde, C.L.; Gordon, S.E.; Shambrook, M.; Nasiri Kenari, A.; Coleman, B.M.; Perugini, M.A.; Lawson, V.A.; Finkelstein, D.I.; Hill, A.F. An Intact Membrane Is Essential for Small Extracellular Vesicle-induced Modulation of A-synuclein Fibrillization. J. Extracell. Vesicles 2020, 10, e12034. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta BBA Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-Nucleation Clusters as Solute Precursors in Crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Cui, F.Z. Two Types of Mineral-Related Matrix Vesicles in the Bone Mineralization of Zebrafish. Biomed. Mater. 2007, 2, 21–25. [Google Scholar] [CrossRef]
- Thouverey, C.; Malinowska, A.; Balcerzak, M.; Strzelecka-Kiliszek, A.; Buchet, R.; Dadlez, M.; Pikula, S. Proteomic Characterization of Biogenesis and Functions of Matrix Vesicles Released from Mineralizing Human Osteoblast-like Cells. J. Proteom. 2011, 74, 1123–1134. [Google Scholar] [CrossRef]
- Martens, S.; McMahon, H.T. Mechanisms of Membrane Fusion: Disparate Players and Common Principles. Nat. Rev. Mol. Cell Biol. 2008, 9, 543–556. [Google Scholar] [CrossRef]
- Siwaponanan, P.; Keawvichit, R.; Udompunturak, S.; Hunnangkul, S.; Reesukumal, K.; Sukapirom, K.; Pattanapanyasat, K.; Krittayaphong, R. Altered Profile of Circulating Microparticles in Nonvalvular Atrial Fibrillation. Clin. Cardiol. 2019, 42, 425–431. [Google Scholar] [CrossRef]
- Antonova, O.A.; Yakushkin, V.V.; Mazurov, A.V. Coagulation Activity of Membrane Microparticles. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2019, 13, 169–186. [Google Scholar] [CrossRef]
- Pérez-Casal, M.; Downey, C.; Fukudome, K.; Marx, G.; Toh, C.H. Activated Protein C Induces the Release of Microparticle-Associated Endothelial Protein C Receptor. Blood 2005, 105, 1515–1522. [Google Scholar] [CrossRef]
- Lacroix, R.; Dubois, C.; Leroyer, A.S.; Sabatier, F.; Dignat-George, F. Revisited Role of Microparticles in Arterial and Venous Thrombosis. J. Thromb. Haemost. 2013, 11, 24–35. [Google Scholar] [CrossRef]
- Lacroix, R.; Plawinski, L.; Robert, S.; Doeuvre, L.; Sabatier, F.; Martinez De Lizarrondo, S.; Mezzapesa, A.; Anfosso, F.; Leroyer, A.S.; Poullin, P.; et al. Leukocyte- and Endothelial-Derived Microparticles: A Circulating Source for Fibrinolysis. Haematologica 2012, 97, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Brukman, N.G.; Uygur, B.; Podbilewicz, B.; Chernomordik, L.V. How Cells Fuse. J. Cell Biol. 2019, 218, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Mondal Roy, S.; Sarkar, M. Membrane Fusion Induced by Small Molecules and Ions. J. Lipids 2011, 2011, 528784. [Google Scholar] [CrossRef]
- Kumar, S.; Karmacharya, M.; Michael, I.J.; Choi, Y.; Kim, J.; Kim, I.; Cho, Y.-K. Programmed Exosome Fusion for Energy Generation in Living Cells. Nat. Catal. 2021, 4, 763–774. [Google Scholar] [CrossRef]
- Segev, N.; Avinoam, O.; Podbilewicz, B. Fusogens. Curr. Biol. 2018, 28, R378–R380. [Google Scholar] [CrossRef]
- Prada, I.; Meldolesi, J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef]
- Ishikawa, R.; Yoshida, S.; Sawada, S.; Sasaki, Y.; Akiyoshi, K. Development and Single-particle Analysis of Hybrid Extracellular Vesicles Fused with Liposomes Using Viral Fusogenic Proteins. FEBS Open Bio 2022, 12, 1178–1187. [Google Scholar] [CrossRef]
- Zubarev, I.; Vladimirtsev, D.; Vorontsova, M.; Blatov, I.; Shevchenko, K.; Zvereva, S.; Lunev, E.A.; Faizuloev, E.; Barlev, N. Viral Membrane Fusion Proteins and RNA Sorting Mechanisms for the Molecular Delivery by Exosomes. Cells 2021, 10, 3043. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering Hybrid Exosomes by Membrane Fusion with Liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Mazur, F.; Chandrawati, R. Membrane Fusion Models for Bioapplications. ChemNanoMat 2021, 7, 223–237. [Google Scholar] [CrossRef]
- Nolte-‘t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular Vesicles and Viruses: Are They Close Relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef] [PubMed]
- Ñahui Palomino, R.A.; Vanpouille, C.; Laghi, L.; Parolin, C.; Melikov, K.; Backlund, P.; Vitali, B.; Margolis, L. Extracellular Vesicles from Symbiotic Vaginal Lactobacilli Inhibit HIV-1 Infection of Human Tissues. Nat. Commun. 2019, 10, 5656. [Google Scholar] [CrossRef] [PubMed]
- El-Shennawy, L.; Hoffmann, A.D.; Dashzeveg, N.K.; McAndrews, K.M.; Mehl, P.J.; Cornish, D.; Yu, Z.; Tokars, V.L.; Nicolaescu, V.; Tomatsidou, A.; et al. Circulating ACE2-Expressing Extracellular Vesicles Block Broad Strains of SARS-CoV-2. Nat. Commun. 2022, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Broad, K.; Walker, S.A.; Davidovich, I.; Witwer, K.; Talmon, Y.; Wolfram, J. Unraveling Multilayered Extracellular Vesicles: Speculation on Cause. J. Extracell. Vesicles 2023, 12, e12309. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Ehab, S.; Cho, J. Relevance of Multilamellar and Multicompartmental Vesicles in Biological Fluids: Understanding the Significance of Proportional Variations and Disease Correlation. Biomark. Res. 2023, 11, 77. [Google Scholar] [CrossRef]
- Shin, W.; Arpino, G.; Thiyagarajan, S.; Su, R.; Ge, L.; McDargh, Z.; Guo, X.; Wei, L.; Shupliakov, O.; Jin, A.; et al. Vesicle Shrinking and Enlargement Play Opposing Roles in the Release of Exocytotic Contents. Cell Rep. 2020, 30, 421–431.e7. [Google Scholar] [CrossRef]
- Fathali, H.; Dunevall, J.; Majdi, S.; Cans, A.-S. Extracellular Osmotic Stress Reduces the Vesicle Size While Keeping a Constant Neurotransmitter Concentration. ACS Chem. Neurosci. 2017, 8, 368–375. [Google Scholar] [CrossRef]
- Tomlins, P.; Grant, P.; Mikhalovsky, S.; James, S.; Mikhalovska, L. Measurement of Pore Size and Porosity of Tissue Scaffolds. J. ASTM Int. 2004, 1, 1–8. [Google Scholar] [CrossRef]
- Debnath, K.; Las Heras, K.; Rivera, A.; Lenzini, S.; Shin, J.-W. Extracellular Vesicle–Matrix Interactions. Nat. Rev. Mater. 2023, 8, 390–402. [Google Scholar] [CrossRef]
- Hill, A.E.; Shachar-Hill, Y. Are Aquaporins the Missing Transmembrane Osmosensors? J. Membr. Biol. 2015, 248, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Mitra, A.K. Structure and Function of Aquaporin Water Channels. Am. J. Physiol.-Ren. Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; Liu, J.; Vidal, M.; Chasis, J.A.; An, X.; Mohandas, N. The Water Channel Aquaporin-1 Partitions into Exosomes during Reticulocyte Maturation: Implication for the Regulation of Cell Volume. Blood 2009, 114, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.L.; Cho, W.J.; Jeremic, A.; Abu-Hamdah, R.; Jena, B.P. Vesicle Swelling Regulates Content Expulsion during Secretion. Cell Biol. Int. 2004, 28, 709–716. [Google Scholar] [CrossRef]
- Clarke-Bland, C.E.; Bill, R.M.; Devitt, A. Emerging Roles for AQP in Mammalian Extracellular Vesicles. Biochim. Biophys. Acta BBA Biomembr. 2022, 1864, 183826. [Google Scholar] [CrossRef]
- Jeremic, A.; Cho, W.J.; Jena, B.P. Involvement of Water Channels in Synaptic Vesicle Swelling. Exp. Biol. Med. 2005, 230, 674–680. [Google Scholar] [CrossRef]
- Shin, L.; Basi, N.; Jeremic, A.; Lee, J.; Cho, W.J.; Chen, Z.; Abu-Hamdah, R.; Oupicky, D.; Jena, B.P. Involvement of vH+ -ATPase in Synaptic Vesicle Swelling. J. Neurosci. Res. 2010, 88, 95–101. [Google Scholar] [CrossRef]
- Matsuki, M.; Hashimoto, S.; Shimono, M.; Murakami, M.; Fujita-Yoshigaki, J.; Furuyama, S.; Sugiya, H. Involvement of Aquaporin-5 Water Channel in Osmoregulation in Parotid Secretory Granules. J. Membr. Biol. 2005, 203, 119–126. [Google Scholar] [CrossRef]
- Sugiya, H.; Matsuki, M. AQPs and Control of Vesicle Volume in Secretory Cells. J. Membr. Biol. 2006, 210, 155–159. [Google Scholar] [CrossRef]
- Jiang, Z.; De Messieres, M.; Lee, J.C. Membrane Remodeling by α-Synuclein and Effects on Amyloid Formation. J. Am. Chem. Soc. 2013, 135, 15970–15973. [Google Scholar] [CrossRef]
- Andersson, A.; Fornasier, M.; Makasewicz, K.; Pálmadóttir, T.; Linse, S.; Sparr, E.; Jönsson, P. Single-Vesicle Intensity and Colocalization Fluorescence Microscopy to Study Lipid Vesicle Fusion, Fission, and Lipid Exchange. Front. Mol. Neurosci. 2022, 15, 1007699. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Idini, I.; Carlström, G.; Linse, S.; Sparr, E. Transient Lipid-Protein Structures and Selective Ganglioside Uptake During α-Synuclein-Lipid Co-Aggregation. Front. Cell Dev. Biol. 2021, 9, 622764. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Dempsey, C.; Parker, C.; Mironov, A.; Bradley, H.; Saha, V. Acute Lymphoblastic Leukaemia Cells Produce Large Extracellular Vesicles Containing Organelles and an Active Cytoskeleton. J. Extracell. Vesicles 2017, 6, 1294339. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-Dependent Cellular Uptake of Exosomes. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1011–1020. [Google Scholar] [CrossRef]
- Johnson, S.M.; Dempsey, C.; Chadwick, A.; Harrison, S.; Liu, J.; Di, Y.; McGinn, O.J.; Fiorillo, M.; Sotgia, F.; Lisanti, M.P.; et al. Metabolic Reprogramming of Bone Marrow Stromal Cells by Leukemic Extracellular Vesicles in Acute Lymphoblastic Leukemia. Blood 2016, 128, 453–456. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J. Diversity of Extracellular Vesicles in Human Ejaculates Revealed by Cryo-electron Microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef]
- Zabeo, D.; Cvjetkovic, A.; Lässer, C.; Schorb, M.; Lötvall, J.; Höög, J.L. Exosomes Purified from a Single Cell Type Have Diverse Morphology. J. Extracell. Vesicles 2017, 6, 1329476. [Google Scholar] [CrossRef]
- Rilla, K. Diverse Plasma Membrane Protrusions Act as Platforms for Extracellular Vesicle Shedding. J. Extracell. Vesicles 2021, 10, e12148. [Google Scholar] [CrossRef]
- Rilla, K.; Mustonen, A.-M.; Arasu, U.T.; Härkönen, K.; Matilainen, J.; Nieminen, P. Extracellular Vesicles Are Integral and Functional Components of the Extracellular Matrix. Matrix Biol. 2019, 75–76, 201–219. [Google Scholar] [CrossRef]
- Lenzini, S.; Debnath, K.; Joshi, J.C.; Wong, S.W.; Srivastava, K.; Geng, X.; Cho, I.S.; Song, A.; Bargi, R.; Lee, J.C.; et al. Cell–Matrix Interactions Regulate Functional Extracellular Vesicle Secretion from Mesenchymal Stromal Cells. ACS Nano 2021, 15, 17439–17452. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Buzás, E.I.; Tóth, E.Á.; Sódar, B.W.; Szabó-Taylor, K.É. Molecular Interactions at the Surface of Extracellular Vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Clément, V.; Roy, V.; Paré, B.; Goulet, C.R.; Deschênes, L.T.; Berthod, F.; Bolduc, S.; Gros-Louis, F. Tridimensional Cell Culture of Dermal Fibroblasts Promotes Exosome-Mediated Secretion of Extracellular Matrix Proteins. Sci. Rep. 2022, 12, 19786. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Electron Microscopic Studies of Induced Cartilage Development and Calcification. J. Cell Biol. 1967, 35, 81–101. [Google Scholar] [CrossRef]
- Bonucci, E.; Dearden, L.C. Matrix Vesicles in Aging Cartilage. Fed. Proc. 1976, 35, 163–168. [Google Scholar]
- Golub, E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta BBA Gen. Subj. 2009, 1790, 1592–1598. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Iyer, B.S.; Cui, Y. Effects of Ascorbic Acid on Collagen Matrix Formation and Osteoblast Differentiation in Murine MC3T3-E1 Cells. J. Bone Miner. Res. 1994, 9, 843–854. [Google Scholar] [CrossRef]
- Bolean, M.; Izzi, B.; Van Kerckhoven, S.; Bottini, M.; Ramos, A.P.; Millán, J.L.; Hoylaerts, M.F.; Ciancaglini, P. Matrix Vesicle Biomimetics Harboring Annexin A5 and Alkaline Phosphatase Bind to the Native Collagen Matrix Produced by Mineralizing Vascular Smooth Muscle Cells. Biochim. Biophys. Acta BBA Gen. Subj. 2020, 1864, 129629. [Google Scholar] [CrossRef]
- Huleihel, L.; Hussey, G.S.; Naranjo, J.D.; Zhang, L.; Dziki, J.L.; Turner, N.J.; Stolz, D.B.; Badylak, S.F. Matrix-Bound Nanovesicles within ECM Bioscaffolds. Sci. Adv. 2016, 2, e1600502. [Google Scholar] [CrossRef]
- Al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the Surface of Myeloma Cell-Derived Exosomes Mediates Exosome-Cell Interactions. J. Biol. Chem. 2016, 291, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Atai, N.A.; Balaj, L.; Van Veen, H.; Breakefield, X.O.; Jarzyna, P.A.; Van Noorden, C.J.F.; Skog, J.; Maguire, C.A. Heparin Blocks Transfer of Extracellular Vesicles between Donor and Recipient Cells. J. Neurooncol. 2013, 115, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Rana, S.; Zöller, M. Host Matrix Modulation by Tumor Exosomes Promotes Motility and Invasiveness. Neoplasia 2013, 15, 875-IN4. [Google Scholar] [CrossRef] [PubMed]
- Dolo, V.; D’Ascenzo, S.; Violini, S.; Pompucci, L.; Festuccia, C.; Ginestra, A.; Vittorelli, M.L.; Canevari, S.; Pavan, A. Matrix-Degrading Proteinases Are Shed in Membrane Vesicles by Ovarian Cancer Cells in Vivo and in Vitro. Clin. Exp. Metastasis 1999, 17, 131–140. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-Free Exosome-Laden Scaffolds for Tissue Repair. Nanoscale 2021, 13, 8740–8750. [Google Scholar] [CrossRef]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the Extracellular Vesicle Surface for Clinical Molecular Biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef]
- McCaughey, J.; Stephens, D.J. ER-to-Golgi Transport: A Sizeable Problem. Trends Cell Biol. 2019, 29, 940–953. [Google Scholar] [CrossRef]
- Malhotra, V.; Erlmann, P. The Pathway of Collagen Secretion. Annu. Rev. Cell Dev. Biol. 2015, 31, 109–124. [Google Scholar] [CrossRef]
- Zhu, M.; Tao, J.; Vasievich, M.P.; Wei, W.; Zhu, G.; Khoriaty, R.N.; Zhang, B. Neural Tube Opening and Abnormal Extraembryonic Membrane Development in SEC23A Deficient Mice. Sci. Rep. 2015, 5, 15471. [Google Scholar] [CrossRef]
- Sarmah, S.; Barrallo-Gimeno, A.; Melville, D.B.; Topczewski, J.; Solnica-Krezel, L.; Knapik, E.W. Sec24D-Dependent Transport of Extracellular Matrix Proteins Is Required for Zebrafish Skeletal Morphogenesis. PLoS ONE 2010, 5, e10367. [Google Scholar] [CrossRef]
- Ji, H.; Greening, D.W.; Barnes, T.W.; Lim, J.W.; Tauro, B.J.; Rai, A.; Xu, R.; Adda, C.; Mathivanan, S.; Zhao, W.; et al. Proteome Profiling of Exosomes Derived from Human Primary and Metastatic Colorectal Cancer Cells Reveal Differential Expression of Key Metastatic Factors and Signal Transduction Components. Proteomics 2013, 13, 1672–1686. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hernandez, J.M.; Doussot, A.; Bojmar, L.; Zambirinis, C.P.; Costa-Silva, B.; Van Beek, E.J.A.H.; Mark, M.T.; Molina, H.; Askan, G.; et al. Extracellular Matrix Proteins and Carcinoembryonic Antigen-Related Cell Adhesion Molecules Characterize Pancreatic Duct Fluid Exosomes in Patients with Pancreatic Cancer. HPB 2018, 20, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, E. Fine Structure and Histochemistry of “Calcifying Globules” In Epiphyseal Cartilage. Z. Zellforsch. Mikrosk. Anat. 1970, 103, 192–217. [Google Scholar] [CrossRef] [PubMed]
- Mebarek, S.; Abousalham, A.; Magne, D.; Do, L.; Bandorowicz-Pikula, J.; Pikula, S.; Buchet, R. Phospholipases of Mineralization Competent Cells and Matrix Vesicles: Roles in Physiological and Pathological Mineralizations. Int. J. Mol. Sci. 2013, 14, 5036–5129. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the Migrasome, an Organelle Mediating Release of Cytoplasmic Contents during Cell Migration. Cell Res. 2015, 25, 24–38. [Google Scholar] [CrossRef]
- Yu, S.; Yu, L. Migrasome Biogenesis and Functions. FEBS J. 2022, 289, 7246–7254. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Bi, M.; Liu, W.; Zhou, L.; Liu, H.; Yan, F.; Guan, L.; Zhang, J.; Xu, J. Migrasomes: From Biogenesis, Release, Uptake, Rupture to Homeostasis and Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 4525778. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Wan, W.; Zhu, Z.; Sho, T.; Zheng, Y.; Zhang, X.; Dou, L.; Ding, Q.; Yu, L.; et al. Engineered Migrasomes: A Robust, Thermally Stable Vaccination Platform. eLife 2024, 13, RP97621. [Google Scholar] [CrossRef]
- Hormozi, A.; Hasanzadeh, S.; Ebrahimi, F.; Daei, N.; Hajimortezayi, Z.; Mehdizadeh, A.; Zamani, M. Treatment with Exosomes Derived from Mesenchymal Stem Cells: A NewWindow of Healing Science in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2024, 19, 879–893. [Google Scholar] [CrossRef]
- Yudintceva, N.; Mikhailova, N.; Fedorov, V.; Samochernych, K.; Vinogradova, T.; Muraviov, A.; Shevtsov, M. Mesenchymal Stem Cells and MSCs-Derived Extracellular Vesicles in Infectious Diseases: From Basic Research to Clinical Practice. Bioengineering 2022, 9, 662. [Google Scholar] [CrossRef]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-Derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shi, Y.; Song, Z.; Wu, B.; Zhou, C.; Liu, W.; Gao, W. Regulatory Effect of Rat Bone Marrow Mesenchymal Stem Cells on Treg/Th17 Immune Balance in vitro. Mol. Med. Rep. 2020, 21, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, S.; Wu, S.; Qi, J.; Li, W.; Liu, S.; Cong, Y.; Chen, H.; Lu, L.; Shi, S.; et al. Clearance of Apoptotic Cells by Mesenchymal Stem Cells Contributes to Immunosuppression via PGE2. EBioMedicine 2019, 45, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.G.; Pink, R.C.; Erdbrügger, U.; Siljander, P.R.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In Sickness and in Health: The Functional Role of Extracellular Vesicles in Physiology and Pathology in Vivo: Part II: Pathology. J. Extracell. Vesicles 2022, 11, e12190. [Google Scholar] [CrossRef]
- Rieck, B. Unexpected Durability of PKH 26 Staining on Rat Adipocytes. Cell Biol. Int. 2003, 27, 445–447. [Google Scholar] [CrossRef]
- Rodriguez, B.V.; Wen, Y.; Shirk, E.N.; Vazquez, S.; Gololobova, O.; Maxwell, A.; Plunkard, J.; Castell, N.; Carlson, B.; Queen, S.E.; et al. An Ex Vivo Model of Interactions between Extracellular Vesicles and Peripheral Mononuclear Blood Cells in Whole Blood. J. Extracell. Vesicles 2023, 12, 12368. [Google Scholar] [CrossRef]
- Russell, A.E.; Sneider, A.; Witwer, K.W.; Bergese, P.; Bhattacharyya, S.N.; Cocks, A.; Cocucci, E.; Erdbrügger, U.; Falcon-Perez, J.M.; Freeman, D.W.; et al. Biological Membranes in EV Biogenesis, Stability, Uptake, and Cargo Transfer: An ISEV Position Paper Arising from the ISEV Membranes and EVs Workshop. J. Extracell. Vesicles 2019, 8, 1684862. [Google Scholar] [CrossRef]
- Witwer, K.W.; Goberdhan, D.C.; O’Driscoll, L.; Théry, C.; Welsh, J.A.; Blenkiron, C.; Buzás, E.I.; Di Vizio, D.; Erdbrügger, U.; Falcón-Pérez, J.M.; et al. Updating MISEV: Evolving the Minimal Requirements for Studies of Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12182. [Google Scholar] [CrossRef]
- Liam-Or, R.; Faruqu, F.N.; Walters, A.; Han, S.; Xu, L.; Wang, J.T.-W.; Oberlaender, J.; Sanchez-Fueyo, A.; Lombardi, G.; Dazzi, F.; et al. Cellular Uptake and in Vivo Distribution of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Are Protein Corona Dependent. Nat. Nanotechnol. 2024, 19, 846–855. [Google Scholar] [CrossRef]
- Dietz, L.; Oberländer, J.; Mateos-Maroto, A.; Schunke, J.; Fichter, M.; Krämer-Albers, E.; Landfester, K.; Mailänder, V. Uptake of Extracellular Vesicles into Immune Cells Is Enhanced by the Protein Corona. J. Extracell. Vesicles 2023, 12, e12399. [Google Scholar] [CrossRef]
- Gomes, F.G.; Andrade, A.C.; Wolf, M.; Hochmann, S.; Krisch, L.; Maeding, N.; Regl, C.; Poupardin, R.; Ebner-Peking, P.; Huber, C.G.; et al. Synergy of Human Platelet-Derived Extracellular Vesicles with Secretome Proteins Promotes Regenerative Functions. Biomedicines 2022, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; LeClaire, M.; Wohlschlegel, J.; Gimzewski, J. Impact of Isolation Methods on the Biophysical Heterogeneity of Single Extracellular Vesicles. Sci. Rep. 2020, 10, 13327. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Bernardo, L.; Sánchez, C.; Morato, E.; Solana, J.C.; Carrillo, E. Comparing the Proteomic Profiles of Extracellular Vesicles Isolated Using Different Methods from Long-Term Stored Plasma Samples. Biol. Proced. Online 2024, 26, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Skog, J.; Hsu, C.-H.; Lessard, R.T.; Balaj, L.; Wurdinger, T.; Carter, B.S.; Breakefield, X.O.; Toner, M.; Irimia, D. Microfluidic Isolation and Transcriptome Analysis of Serum Microvesicles. Lab Chip 2010, 10, 505–511. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous Particle Separation in Spiral Microchannels Using Dean Flows and Differential Migration. Lab Chip 2008, 8, 1906. [Google Scholar] [CrossRef]
- Di Santo, R.; Niccolini, B.; Romanò, S.; Vaccaro, M.; Di Giacinto, F.; De Spirito, M.; Ciasca, G. Advancements in Mid-Infrared Spectroscopy of Extracellular Vesicles. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2024, 305, 123346. [Google Scholar] [CrossRef]
- Verweij, F.J.; Balaj, L.; Boulanger, C.M.; Carter, D.R.F.; Compeer, E.B.; D’Angelo, G.; El Andaloussi, S.; Goetz, J.G.; Gross, J.C.; Hyenne, V.; et al. The Power of Imaging to Understand Extracellular Vesicle Biology In Vivo. Nat. Methods 2021, 18, 1013–1026. [Google Scholar] [CrossRef]


| Subtype | Size | Surface Marker | Biogenesis | Ref. |
|---|---|---|---|---|
| Exosomes | 50–150 nm | Classical panel: CD9, CD63, TSG101, Alix, Hsp70 Potential specific markers: LAMP1, LAMP2, Rab27, Rab27b | Inward budding of a late endosome, also known as a multivesicular body (MVB) with subsequent fusion with the cell surface and realese of exosomes into the extracellular space | [7,8,9] |
| Microvesicles/Ectosomes | 100–500 nm | MT1-MMP, GP1b, GPIIb/GPIIa, P-selectin, Integrin, Mac-1 | Direct budding out of a cell’s plasma membrane and shedding into the extracellular space | [10] |
| Apoptotic bodies | 1000–5000 nm | Annexin V, Thrombospondin, C3b | Remnants of apoptotic cells | [11] |
| Migrasomes | 500–3000 nm | TSPAN4, NDST1, PIGK, CPQ, EOG, IntegrinA5 | Formation of primary swelling on the retraction follicles of migrating cells and filling with migrasomes with the formation of a pomegranate like structure (PLS) | [6,12] |
| EV’s Membrane Protein | EV’s Lipid Membrane | |
|---|---|---|
| Covalent binding to matrix |  |  |
| Non-covalent interaction of EVs with the matrix | 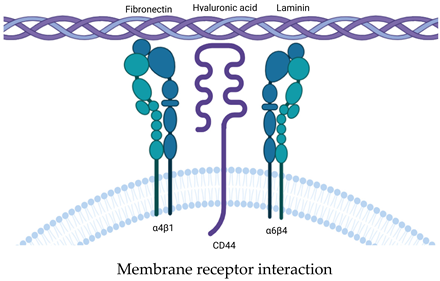 | 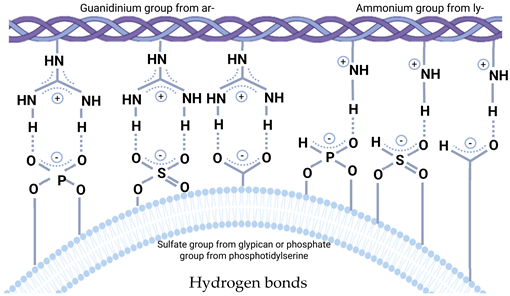 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ten, A.; Yudintceva, N.; Samochernykh, K.; Combs, S.E.; Jha, H.C.; Gao, H.; Shevtsov, M. Post-Secretion Processes and Modification of Extracellular Vesicles. Cells 2025, 14, 408. https://doi.org/10.3390/cells14060408
Ten A, Yudintceva N, Samochernykh K, Combs SE, Jha HC, Gao H, Shevtsov M. Post-Secretion Processes and Modification of Extracellular Vesicles. Cells. 2025; 14(6):408. https://doi.org/10.3390/cells14060408
Chicago/Turabian StyleTen, Artem, Natalia Yudintceva, Konstantin Samochernykh, Stephanie E. Combs, Hem Chandra Jha, Huile Gao, and Maxim Shevtsov. 2025. "Post-Secretion Processes and Modification of Extracellular Vesicles" Cells 14, no. 6: 408. https://doi.org/10.3390/cells14060408
APA StyleTen, A., Yudintceva, N., Samochernykh, K., Combs, S. E., Jha, H. C., Gao, H., & Shevtsov, M. (2025). Post-Secretion Processes and Modification of Extracellular Vesicles. Cells, 14(6), 408. https://doi.org/10.3390/cells14060408









