Imbalance in MICOS Proteins in Rat Liver Mitochondria in an Induced Hyperthyroidism Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Total Carbonyl Colorimetric Assay
2.3. Isolation of Rat Liver Mitochondria
2.4. Assay of the Expression of MICOS Genes Using Quantitative Real-Time PCR
2.4.1. Extraction of RNA
2.4.2. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.5. Sample Preparation, Electrophoresis and Immunoblotting Analysis
2.6. Electron Microscopy
2.7. High-Resolution Respirometry
- V4(0), basal substrate respiration;
- V3, active respiration following ADP addition;
- V4, metabolic state upon depletion of all ADP;
- VDNP (state of uncoupled respiration), maximum uncoupled respiration;
- Volig, respiration in the presence of oligomycin;
- tph, the time required for ADP phosphorylation.
2.8. Measurement of Mitochondrial Membrane Potential
2.9. Registration of Ca2+ Retention Capacity by Mitochondria
2.10. Cardiolipin Assay
2.11. Statistical Analysis
3. Results
3.1. Assessment of the Hyperthyroidism Model
3.2. Analysis of Genes Responsible for Mitochondrial Cristae Biogenesis in a Rat Model of Hyperthyroidism
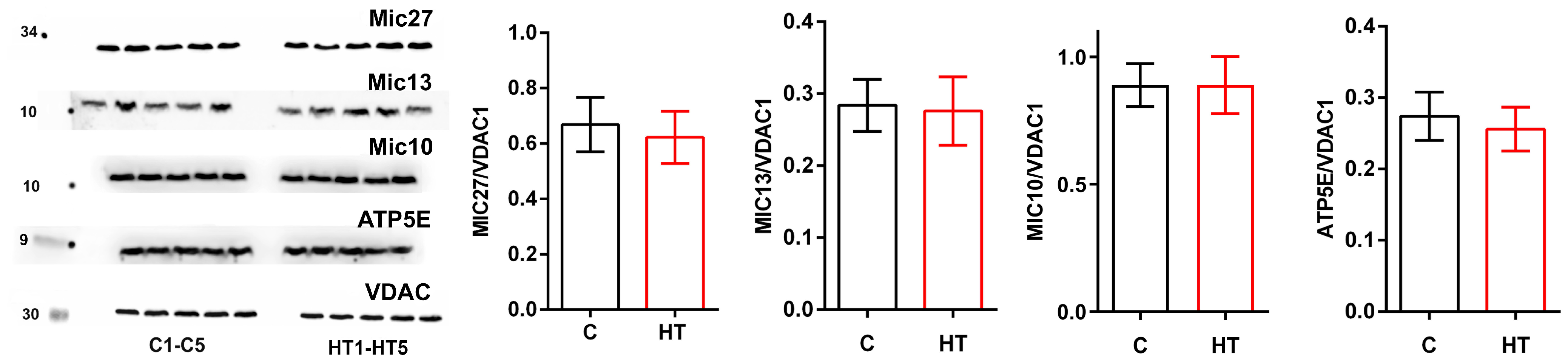
3.3. Changes in MICOS Proteins in the Liver of Rats in a Hyperthyroidism Model
3.4. Hyperthyroidism—Induced Changes in MICOS Balance Affect the Ultrastructure of Mitochondria in the Liver of Hyperthyroid Rats
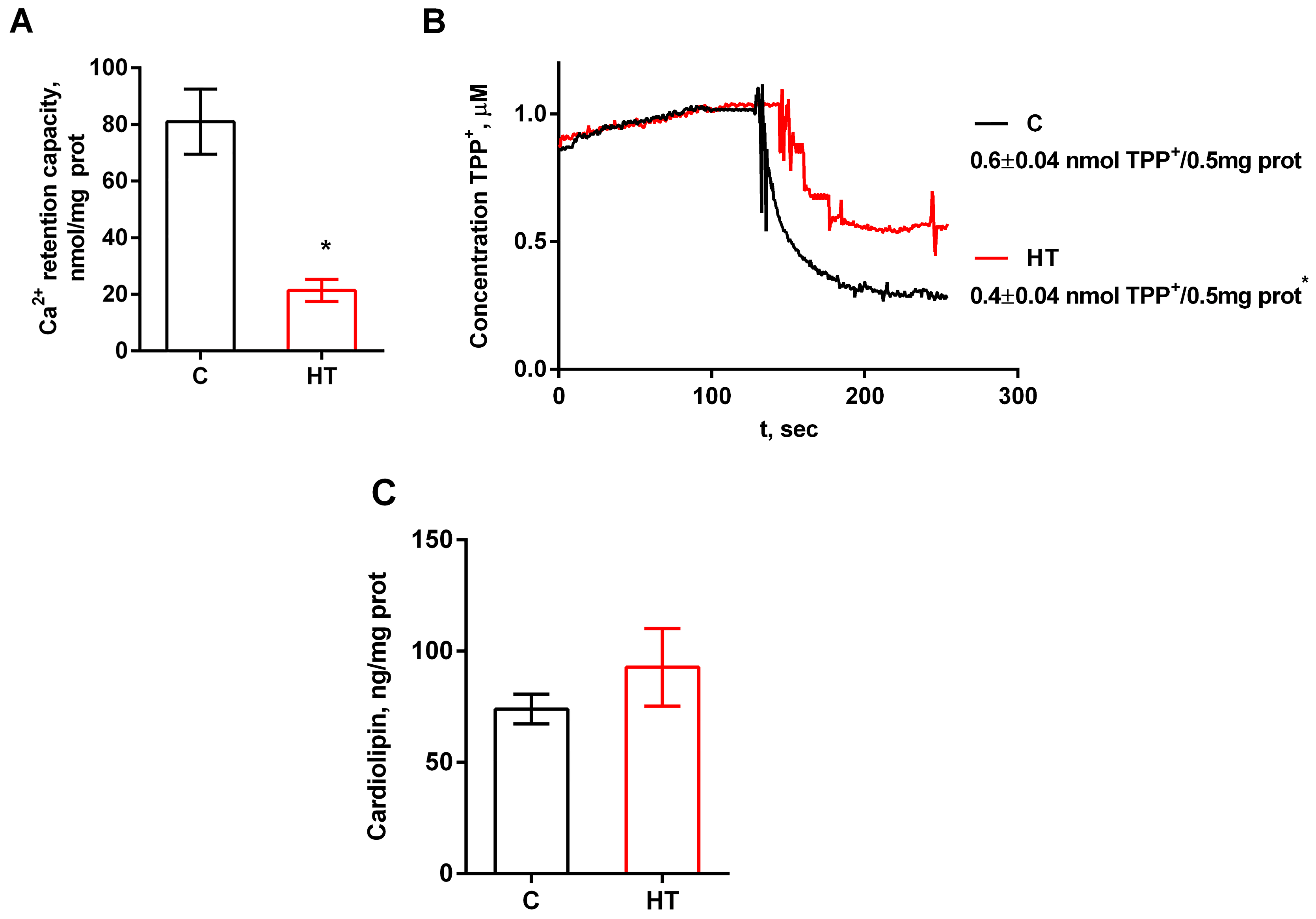
3.5. Effect of MICOS Protein Changes in Hyperthyroidism on the Functional State of Liver Mitochondria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in Human Health, Ageing and Disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Mayorov, V.I.; Dikalov, S. Metabolic Syndrome and β-Oxidation of Long-Chain Fatty Acids in the Brain, Heart, and Kidney Mitochondria. Int. J. Mol. Sci. 2022, 23, 4047. [Google Scholar] [CrossRef]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Venediktova, N.I.; Mashchenko, O.V.; Talanov, E.Y.; Belosludtseva, N.V.; Mironova, G.D. Energy Metabolism and Oxidative Status of Rat Liver Mitochondria in Conditions of Experimentally Induced Hyperthyroidism. Mitochondrion 2020, 52, 190–196. [Google Scholar] [CrossRef]
- Venediktova, N.; Solomadin, I.; Starinets, V.; Mironova, G. Structural and Dynamic Features of Liver Mitochondria and Mitophagy in Rats with Hyperthyroidism. Int. J. Mol. Sci. 2022, 23, 14327. [Google Scholar] [CrossRef]
- Venediktova, N.; Solomadin, I.; Nikiforova, A.; Starinets, V.; Mironova, G. Functional State of Rat Heart Mitochondria in Experimental Hyperthyroidism. Int. J. Mol. Sci. 2021, 22, 11744. [Google Scholar] [CrossRef]
- Bennett, C.F.; Latorre-Muro, P.; Puigserver, P. Mechanisms of Mitochondrial Respiratory Adaptation. Nat. Rev. Mol. Cell Biol. 2022, 23, 817–835. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Smeitink, J.A.M.; Rodenburg, R.J.T. Mitochondrial ATP Synthase: Architecture, Function and Pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. Available online: https://onlinelibrary.wiley.com/doi/10.1007/s10545-011-9382-9 (accessed on 20 October 2025). [CrossRef]
- Anand, R.; Reichert, A.S.; Kondadi, A.K. Emerging Roles of the MICOS Complex in Cristae Dynamics and Biogenesis. Biology 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Venediktova, N.; Khmil, N.; Pavlik, L.; Mikheeva, I.; Mironova, G. Pathological Changes in Liver Mitochondria of Rats with Experimentally Induced Hyperthyroidism and Their Correction with Uridine. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. Respiratory Enzymes in Oxidative Phosphorylation. III. The Steady State. J. Biol. Chem. 1955, 217, 409–427. [Google Scholar] [CrossRef]
- Van Laar, V.S.; Berman, S.B.; Hastings, T.G. Mic60/Mitofilin Overexpression Alters Mitochondrial Dynamics and Attenuates Vulnerability of Dopaminergic Cells to Dopamine and Rotenone. Neurobiol. Dis. 2016, 91, 247–261. [Google Scholar] [CrossRef]
- Milone, M.; Wong, L.-J. Diagnosis of Mitochondrial Myopathies. Mol. Genet. Metab. 2013, 110, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Galloway, C.A.; Yoon, Y. Mitochondrial Dynamics in Diabetic Cardiomyopathy. Antioxid. Redox Signal. 2015, 22, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
- Zick, M.; Rabl, R.; Reichert, A.S. Cristae Formation-Linking Ultrastructure and Function of Mitochondria. Biochim. Biophys. Acta 2009, 1793, 5–19. [Google Scholar] [CrossRef]
- Mannella, C.A. The Relevance of Mitochondrial Membrane Topology to Mitochondrial Function. Biochim. Biophys. Acta 2006, 1762, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hackenbrock, C.R. Ultrastructural Bases for Metabolically Linked Mechanical Activity in Mitochondria. I. Reversible Ultrastructural Changes with Change in Metabolic Steady State in Isolated Liver Mitochondria. J. Cell Biol. 1966, 30, 269–297. [Google Scholar] [CrossRef]
- Hackenbrock, C.R. Chemical and Physical Fixation of Isolated Mitochondria in Low-Energy and High-Energy States. Proc. Natl. Acad. Sci. USA 1968, 61, 598–605. [Google Scholar] [CrossRef]
- Dlasková, A.; Špaček, T.; Engstová, H.; Špačková, J.; Schröfel, A.; Holendová, B.; Smolková, K.; Plecitá-Hlavatá, L.; Ježek, P. Mitochondrial Cristae Narrowing upon Higher 2-Oxoglutarate Load. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 659–678. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Mehrotra, A.; Rigoni, G.; Soriano, M.E. Who and How in the Regulation of Mitochondrial Cristae Shape and Function. Biochem. Biophys. Res. Commun. 2018, 500, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Huynen, M.A.; Mühlmeister, M.; Gotthardt, K.; Guerrero-Castillo, S.; Brandt, U. Evolution and Structural Organization of the Mitochondrial Contact Site (MICOS) Complex and the Mitochondrial Intermembrane Space Bridging (MIB) Complex. Biochim. Biophys. Acta 2016, 1863, 91–101. [Google Scholar] [CrossRef]
- Eramo, M.J.; Lisnyak, V.; Formosa, L.E.; Ryan, M.T. The “mitochondrial Contact Site and Cristae Organising System” (MICOS) in Health and Human Disease. J. Biochem. 2020, 167, 243–255. [Google Scholar] [CrossRef]
- Mukherjee, I.; Ghosh, M.; Meinecke, M. MICOS and the Mitochondrial Inner Membrane Morphology—When Things Get out of Shape. FEBS Lett. 2021, 595, 1159–1183. [Google Scholar] [CrossRef]
- Harper, M.-E.; Seifert, E.L. Thyroid Hormone Effects on Mitochondrial Energetics. Thyroid Off. J. Am. Thyroid Assoc. 2008, 18, 145–156. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver Enzyme Alteration: A Guide for Clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, R.A.; Pais, S.; Reynolds, T.B.; Kaplowitz, N. Serum Alanine Aminotransferase in Skeletal Muscle Diseases. Hepatology 2005, 41, 380–382. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Shi, J.; He, Q.; Lui, K.; Liu, Y.; Huang, Y.; Sheikh, M.S. CHCM1/CHCHD6, Novel Mitochondrial Protein Linked to Regulation of Mitofilin and Mitochondrial Cristae Morphology. J. Biol. Chem. 2012, 287, 7411–7426. [Google Scholar] [CrossRef]
- Darshi, M.; Mendiola, V.L.; Mackey, M.R.; Murphy, A.N.; Koller, A.; Perkins, G.A.; Ellisman, M.H.; Taylor, S.S. ChChd3, an Inner Mitochondrial Membrane Protein, Is Essential for Maintaining Crista Integrity and Mitochondrial Function. J. Biol. Chem. 2011, 286, 2918–2932. [Google Scholar] [CrossRef]
- Ott, C.; Dorsch, E.; Fraunholz, M.; Straub, S.; Kozjak-Pavlovic, V. Detailed Analysis of the Human Mitochondrial Contact Site Complex Indicate a Hierarchy of Subunits. PLoS ONE 2015, 10, e0120213. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, K.; Dong, J.; Yan, C.; Hu, C.; Ji, H.; Chen, L.; Chen, S.; Zhao, H.; Song, Z. Sam50-Mic19-Mic60 Axis Determines Mitochondrial Cristae Architecture by Mediating Mitochondrial Outer and Inner Membrane Contact. Cell Death Differ. 2020, 27, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Colina-Tenorio, L.; Horten, P.; Pfanner, N.; Rampelt, H. Shaping the Mitochondrial Inner Membrane in Health and Disease. J. Intern. Med. 2020, 287, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Rabl, R.; Soubannier, V.; Scholz, R.; Vogel, F.; Mendl, N.; Vasiljev-Neumeyer, A.; Körner, C.; Jagasia, R.; Keil, T.; Baumeister, W.; et al. Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. J. Cell Biol. 2009, 185, 1047–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Luo, Y.-X.; An, X.-Z.; Zhang, R.; Liu, G.; Li, H.; Chen, H.-Z.; Liu, D.-P. Overexpression of mitofilin in the mouse heart promotes cardiac hypertrophy in response to hypertrophic stimuli. Antioxid. Redox Signal. 2014, 21, 1693–1707. [Google Scholar] [CrossRef]
- Feng, Y.; Ngonidzashe, M.; Jean, B. Mitochondrial inner membrane protein, Mic60/mitofilin in mammalian organ protection. J. Cell Physiol. 2018, 234, 3383–3393. [Google Scholar] [CrossRef]
- Suárez-Arroyo, I.J.; Feliz-Mosquea, Y.R.; Pérez-Laspiur, J.; Arju, R.; Giashuddin, S.; Maldonado-Martínez, G.; Cubano, L.A.; Schneider, R.J.; Martínez-Montemayor, M.M. The Proteome Signature of the Inflammatory Breast Cancer Plasma Membrane Identifies Novel Molecular Markers of Disease. Am. J. Cancer Res. 2016, 6, 1720–1740. [Google Scholar] [PubMed]
- Guo, Y.; Darshi, M.; Ma, Y.; Perkins, G.A.; Shen, Z.; Haushalter, K.J.; Saito, R.; Chen, A.; Lee, Y.S.; Patel, H.H.; et al. Quantitative Proteomic and Functional Analysis of Liver Mitochondria from High Fat Diet (HFD) Diabetic Mice. Mol. Cell. Proteom. MCP 2013, 12, 3744–3758. [Google Scholar] [CrossRef]
- Perks, K.L.; Ferreira, N.; Richman, T.R.; Ermer, J.A.; Kuznetsova, I.; Shearwood, A.-M.J.; Lee, R.G.; Viola, H.M.; Johnstone, V.P.A.; Matthews, V.; et al. Adult-Onset Obesity Is Triggered by Impaired Mitochondrial Gene Expression. Sci. Adv. 2017, 3, e1700677. [Google Scholar] [CrossRef]
- Chella Krishnan, K.; Kurt, Z.; Barrere-Cain, R.; Sabir, S.; Das, A.; Floyd, R.; Vergnes, L.; Zhao, Y.; Che, N.; Charugundla, S.; et al. Integration of Multi-Omics Data from Mouse Diversity Panel Highlights Mitochondrial Dysfunction in Non-Alcoholic Fatty Liver Disease. Cell Syst. 2018, 6, 103–115.e7. [Google Scholar] [CrossRef]
- Paumard, P.; Vaillier, J.; Coulary, B.; Schaeffer, J.; Soubannier, V.; Mueller, D.M.; Brèthes, D.; di Rago, J.-P.; Velours, J. The ATP Synthase Is Involved in Generating Mitochondrial Cristae Morphology. EMBO J. 2002, 21, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bornhövd, C.; Vogel, F.; Neupert, W.; Reichert, A.S. Mitochondrial Membrane Potential Is Dependent on the Oligomeric State of F1F0-ATP Synthase Supracomplexes. J. Biol. Chem. 2006, 281, 13990–13998. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Cabrera, R.; Quirin, C.; Glytsou, C.; Corrado, M.; Urbani, A.; Pellattiero, A.; Calvo, E.; Vázquez, J.; Enríquez, J.A.; Gerle, C.; et al. The Cristae Modulator Optic Atrophy 1 Requires Mitochondrial ATP Synthase Oligomers to Safeguard Mitochondrial Function. Nat. Commun. 2018, 9, 3399. [Google Scholar] [CrossRef]
- Olichon, A.; Baricault, L.; Gas, N.; Guillou, E.; Valette, A.; Belenguer, P.; Lenaers, G. Loss of OPA1 Perturbates the Mitochondrial Inner Membrane Structure and Integrity, Leading to Cytochrome c Release and Apoptosis. J. Biol. Chem. 2003, 278, 7743–7746. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Martins de Brito, O.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. OPA1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef]
- Stephan, T.; Brüser, C.; Deckers, M.; Steyer, A.M.; Balzarotti, F.; Barbot, M.; Behr, T.S.; Heim, G.; Hübner, W.; Ilgen, P.; et al. MICOS Assembly Controls Mitochondrial Inner Membrane Remodeling and Crista Junction Redistribution to Mediate Cristae Formation. EMBO J. 2020, 39, e104105. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.; Koob, S.; Dikov, D.; Vogel, F.; Reichert, A.S. OPA1 Functionally Interacts with MIC60 but Is Dispensable for Crista Junction Formation. FEBS Lett. 2016, 590, 3309–3322. [Google Scholar] [CrossRef]
- Clarke, S.L.; Bowron, A.; Gonzalez, I.L.; Groves, S.J.; Newbury-Ecob, R.; Clayton, N.; Martin, R.P.; Tsai-Goodman, B.; Garratt, V.; Ashworth, M.; et al. Barth Syndrome. Orphanet. J. Rare Dis. 2013, 8, 23. [Google Scholar] [CrossRef]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria Supply Membranes for Autophagosome Biogenesis during Starvation. Cell 2010, 141, 656–667. [Google Scholar] [CrossRef]
- Cioffi, F.; Giacco, A.; Goglia, F.; Silvestri, E. Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones. Cells 2022, 11, 997. [Google Scholar] [CrossRef]
- Bonekamp, N.A.; Jiang, M.; Motori, E.; Garcia Villegas, R.; Koolmeister, C.; Atanassov, I.; Mesaros, A.; Park, C.B.; Larsson, N.-G. High Levels of TFAM Repress Mammalian Mitochondrial DNA Transcription in Vivo. Life Sci. Alliance 2021, 4, e202101034. [Google Scholar] [CrossRef]
- Gottschalk, B.; Klec, C.; Leitinger, G.; Bernhart, E.; Rost, R.; Bischof, H.; Madreiter-Sokolowski, C.T.; Radulović, S.; Eroglu, E.; Sattler, W.; et al. MICU1 Controls Cristae Junction and Spatially Anchors Mitochondrial Ca2+ Uniporter Complex. Nat. Commun. 2019, 10, 3732. [Google Scholar] [CrossRef] [PubMed]
- Belosludtseva, N.V.; Talanov, E.Y.; Venediktova, N.I.; Sharapov, M.G.; Mironova, G.D.; Belosludtsev, K.N. Structural and Functional Features of Ca2+ Transport Systems in Liver Mitochondria of Rats with Experimental Hyperthyroidism. Bull. Exp. Biol. Med. 2020, 169, 224–228. [Google Scholar] [CrossRef]
- Upadhyay, G.; Singh, R.; Kumar, A.; Kumar, S.; Kapoor, A.; Godbole, M.M. Severe Hyperthyroidism Induces Mitochondria-Mediated Apoptosis in Rat Liver. Hepatology 2004, 39, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Hu, Z.; Ma, W.; Zang, L.; Tian, Z.; Hou, Q. Quercetin Alleviates Hyperthyroidism-Induced Liver Damage via Nrf2 Signaling Pathway. BioFactors 2020, 46, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.; McKenty, T.; Ali, S.; Sonntag, D.; Ravipaty, S.; Cui, Y.; Slate, D.; Lin, Q.; Christiansen, A.; Jacobson, S.; et al. Antisense Oligonucleotide STK-002 Increases OPA1 in Retina and Improves Mitochondrial Function in Autosomal Dominant Optic Atrophy Cells. Nucleic Acid Ther. 2024, 34, 221–233. [Google Scholar] [CrossRef]





| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| Micos10 | GACGCGTTAGTGAAGCTAGG | TGCTGACAGTTGGAGTAGGC |
| Atp5f1e | ACAGGCTGGACTCAGCTAC | TCTCAGCGTTCGCTTTGAAC |
| Micos13 | AGCAATGTACCAGTTCAGCC | GACCGAGATGATGCCTGAGT |
| Micos 19 (Chchd3) | CAAAGGTGAAGCATCTGGCTC | CATACCGCCTGAACTTGGAC |
| Micos 25 (Chchd6) | GTCAGATCGCCTAACCAGGG | TCTCTTGGATGCGTTCCTGC |
| Micos 27 (Apool) | CCAAAGGAAGAAACCAAGGAAGG | CATGAGCTTGGGGTCAGGTAT |
| Micos 60 (Immt) | TGGTCCAAGCAAGGGATGAC | AGTGGAGAGTGTGCCAGCT |
| Samm50 | GCCTTGCTCAAAGTCAACCA | GACGTGGAGAACACCGAATC |
| OPA1 | GCAGAAGACAGCTTGAGGGT | TGCGTCCCACTGTTGCTTAT |
| Tafazzin | GCGCTTCAAATGGGGAATTG | GTAGGGTGGACTGTTAGGGA |
| Rplp2 | CATCGCTCAGGGTGTTGG | AACAGGCCAAATCCCATGTC |
| PPIA | GAGCACTGGGGAGAAAGGATT | GTTTGGTCCAGCATTTGCCA |
| Antibodies | Source |
|---|---|
| TFAM | AF0531, Affinity Biosciences, Zhenjiang, China |
| NRF1 | AF5298, Affinity Biosciences |
| OPA1 | ab119685, Abcam, Cambridge, UK |
| Mic 60 (Mitofilin) | DF7074, Affinity Biosciences |
| Samm 50 | DF12729, Affinity Biosciences |
| Mic 27 (ApoOL) | AF9010, Affinity Biosciences |
| Mic 25 (CHCHD6) | DF12255, Affinity Biosciences |
| Mic 19 (CHCHD3) | DF12254, Affinity Biosciences |
| Mic 10 (MINOS1) | DF14761, Affinity Biosciences |
| ATP5E | DF9238, Affinity Biosciences |
| Mic 13 (Qil1) | PA5-69966, Invitrogen, Burlington, ON, Canada |
| GAPDH | ab181602, Abcam, Cambridge, UK |
| VDAC | ab154856, Abcam, Cambridge, UK |
| C | HT | |
|---|---|---|
| T3 free, pmol/L | 6.5 ± 0.3 | 12.8 ± 0.8 ** |
| T4 free, pmol/L | 27.5 ± 1.3 | 78.5 ± 1.5 ** |
| Body weight, g | 263 ± 3 | 243 ± 3 ** |
| Liver weight, g | 11.8 ± 0.2 | 9.6 ± 0.2 ** |
| Body weight gain, g | 39 ± 1.8 | 12 ± 1.1 ** |
| ALT, µmol/min·L | 34.2 ± 1.6 | 54.8 ± 3.3 ** |
| AST, µmol/min·L | 57.5 ± 2.3 | 111.4 ± 5.4 ** |
| LDH, µmol/min·L | 374 ± 12 | 389 ± 16 |
| Total carbonyl, serum, µg carbonyl group/mg protein | 4.7 ± 0.3 | 7 ± 0.6 * |
| Total carbonyl, tissue, µg carbonyl group/mg protein | 9.9 ± 0.6 | 14.3 ± 0.7 * |
| C | HT | |
|---|---|---|
| Number of mitochondria per image (60 µm2) | 27 ± 0.8 | 40 ± 1.2 * |
| Percentage of swollen mitochondria | 2 | 14 |
| Percentage of mitochondrial damage | 3 | 11.3 |
| Average area of mitochondria, µm2 | 0.37 ± 0.01 | 0.50 ± 0.02 ** |
| Average perimeter of mitochondria, µm | 2.30 ± 0.04 | 2.80 ± 0.05 ** |
| Aspect ratio (major axis/minor axis) | 1.7 ± 0.04 | 2.1 ± 0.05 ** |
| Number of lamellar regular cristae/area MX | 215.1 ± 20.5 | 168.4 ± 16 ** |
| Width of regular lamellar cristae, nm | 14.6 ± 0.54 | 16.5 ± 0.28 * |
| C | HT | |
|---|---|---|
| V4(0) | 3.9 ± 0.3 | 5.2 ± 0.6 * |
| V3 | 41.8 ± 1.8 | 52.8 ± 3.1 * |
| V4 | 4.4 ± 0.2 | 7.2± 0.3 * |
| Volig | 4.0± 0.1 | 5.2± 0.3 * |
| VDNP | 51.7± 1.3 | 47.9± 2.9 |
| RCR (V3/V4) | 9.5± 0.3 | 7.3± 0.2 * |
| tph, s | 0.87± 0.03 | 0.81± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venediktova, N.; Solomadin, I.; Nikiforova, A.; Bessonova, T. Imbalance in MICOS Proteins in Rat Liver Mitochondria in an Induced Hyperthyroidism Model. Cells 2025, 14, 1877. https://doi.org/10.3390/cells14231877
Venediktova N, Solomadin I, Nikiforova A, Bessonova T. Imbalance in MICOS Proteins in Rat Liver Mitochondria in an Induced Hyperthyroidism Model. Cells. 2025; 14(23):1877. https://doi.org/10.3390/cells14231877
Chicago/Turabian StyleVenediktova, Natalya, Ilya Solomadin, Anna Nikiforova, and Tatiana Bessonova. 2025. "Imbalance in MICOS Proteins in Rat Liver Mitochondria in an Induced Hyperthyroidism Model" Cells 14, no. 23: 1877. https://doi.org/10.3390/cells14231877
APA StyleVenediktova, N., Solomadin, I., Nikiforova, A., & Bessonova, T. (2025). Imbalance in MICOS Proteins in Rat Liver Mitochondria in an Induced Hyperthyroidism Model. Cells, 14(23), 1877. https://doi.org/10.3390/cells14231877





