Psychedelics in Multiple Sclerosis: Mechanisms, Challenges, and Prospects for Neuroimmune Modulation and Repair
Abstract
1. Introduction
2. Comparative Summary of Psychedelic Classes and Rationale
3. Search Strategy and Selection Criteria
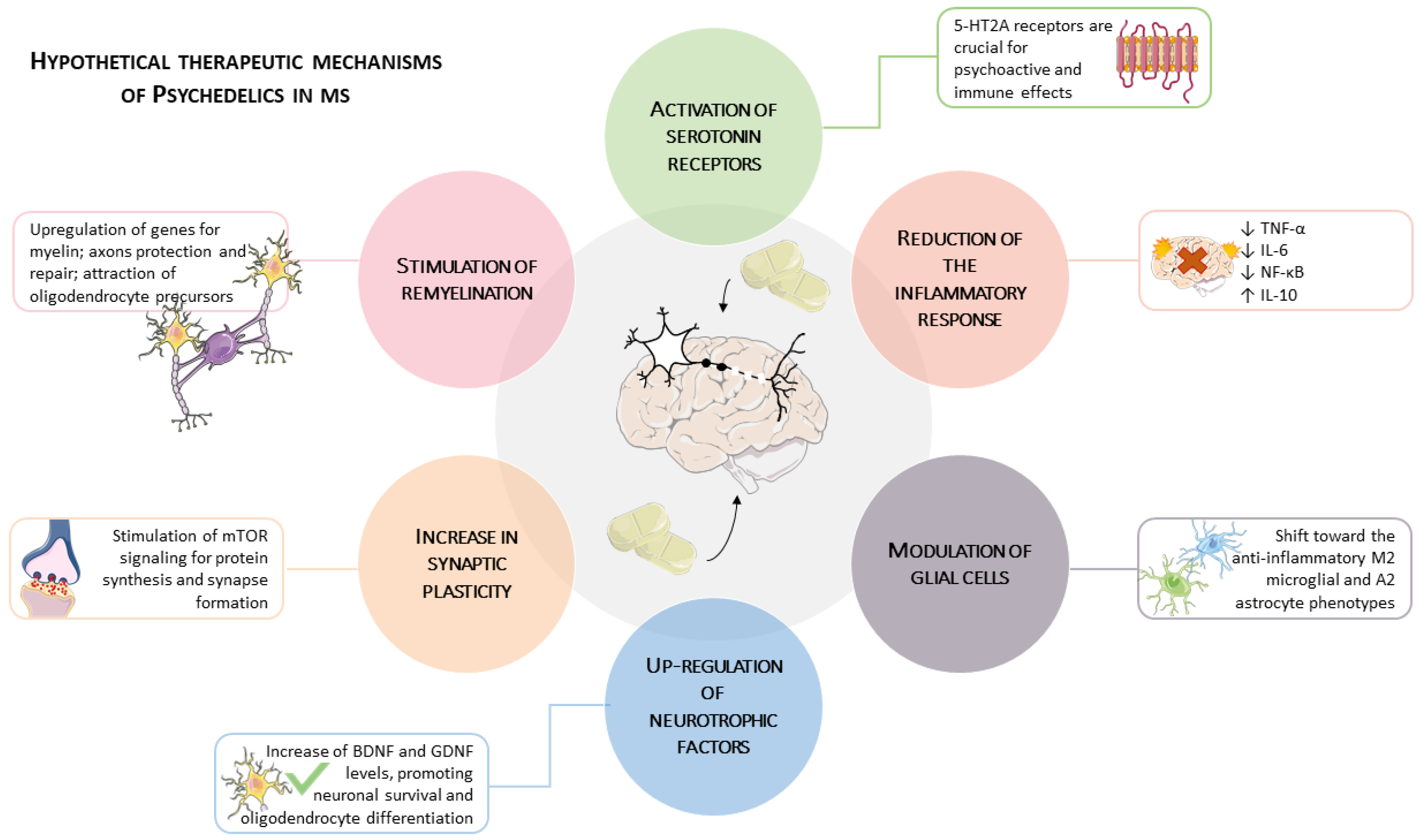
4. Mechanisms of Psychedelic-Induced Neuroimmune Modulation
4.1. Inflammation and Cytokine Regulation
4.1.1. Evidence from Animal Models
4.1.2. Evidence from Human Studies
4.1.3. Receptor-Specific Mechanisms
4.2. Neurotrophic Factors, Plasticity, and Cellular Repair
4.3. Remyelination and Oligodendrocyte Support
4.4. An Integrative Model: Synergies in Neuro-Reparative Pathways
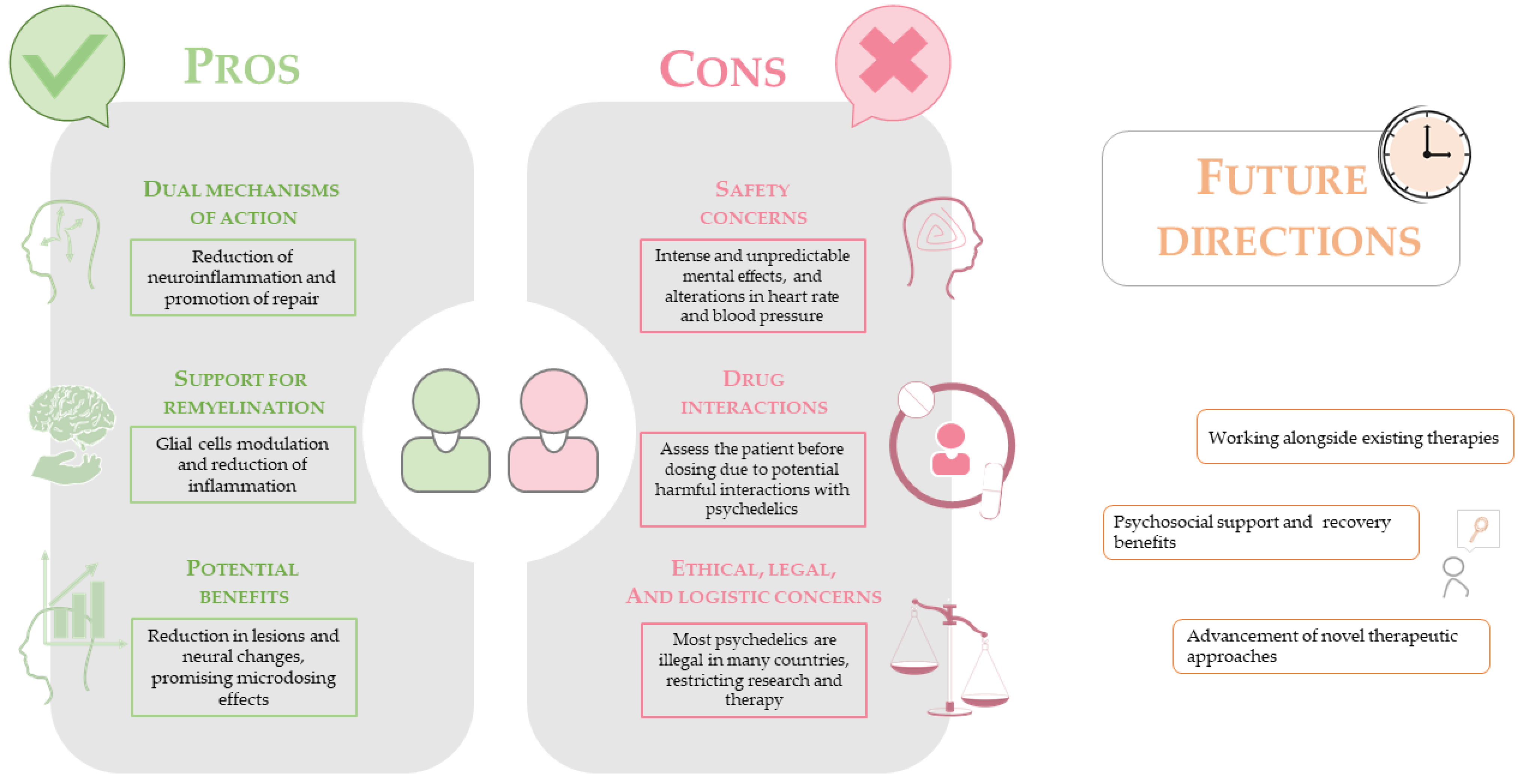
5. Challenges in Translating PSYs to MS Therapy
5.1. Safety and Neuropsychiatric Considerations
5.1.1. Cardiovascular Risks and Systemic Toxicity
5.1.2. Neuropsychiatric Vulnerability in MS
5.1.3. Pharmacological Interactions with DMTs
5.2. Legal, Ethical, and Logistical Barriers
6. Prospects for Clinical Use and Future Directions
6.1. Early Evidence and Dosing Strategies
6.2. Methodologies for Future Research: Biomarkers and Neuroimaging
6.3. Integration with Existing Therapies and Dual Benefits
6.4. Development of Novel Psychedelic-Derived Therapies
6.5. Roadmap for Clinical Translation: Challenges and Outstanding Questions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine (Serotonin) |
| 5-HT2A | 5-hydroxytryptamine receptor 2A |
| 5-MeO-DMT | 5-methoxy-N,N-dimethyltryptamine |
| AHSCT | Autologous Hematopoietic Stem Cell Transplantation |
| AI | Artificial Intelligence |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| Arg1 | Arginase 1 |
| Ayahuasca | Psychoactive brew containing DMT and MAOIs |
| BDNF | Brain-Derived Neurotrophic Factor |
| BP | Blood Pressure |
| CNS | Central Nervous System |
| CNP/CNPase | 2′,3′-cyclic nucleotide 3′-phosphodiesterase |
| COX-2 | Cyclooxygenase-2 |
| CRP | C-reactive protein |
| DMF | Dimethyl fumarate |
| DMT | N,N-Dimethyltryptamine |
| DMTs | Disease-Modifying Therapies |
| DOI | (R)-2,5-Dimethoxy-4-iodoamphetamine |
| ECG | Electrocardiogram |
| ECT | Electroconvulsive Therapy |
| FDA | Food and Drug Administration |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial Fibrillary Acidic Protein |
| GR | Glutathione reductase |
| hiPSCs | human induced Pluripotent Stem Cells |
| HPPD | Hallucinogen Persisting Perception Disorder |
| HR | Heart Rate |
| IL-6 | Interleukin-6 |
| iNOS | inducible nitric oxide synthase |
| i.p. | intra-peritoneal |
| Ketamine | 2-(2-chlorophenyl)-2-(methylamino)cyclohexanone |
| LPS | Lipopolysaccharide |
| LSD | Lysergic acid diethylamide |
| MAOI | Monoamine oxidase inhibitor |
| MBP | Myelin basic protein |
| MDD | Major Depressive Disorder |
| MDMA | 3,4-Methylenedioxymethamphetamine |
| moDC | Monocyte-derived Dendritic Cells |
| moMAC | Monocyte-derived Macrophages |
| MS | Multiple Sclerosis |
| mTOR | mammalian target of rapamycin |
| MTR | magnetization transfer ratio |
| NF-κB | nuclear factor kappa B |
| NfL | Neurofilament light chain |
| NKCC | Natural Killer (NK) cells |
| NMDA | N-methyl-D-aspartate |
| NOX | NADPH oxidase |
| PISD | Psychedelic Iatrogenic Structural Dissociation |
| Psilocybin | 4-phosphoryloxy-N,N-dimethyltryptamine |
| PSYs | Psychedelics |
| PTSD | post-traumatic stress disorder |
| PV+ | parvalbumin-positive |
| ROS | Reactive oxygen species |
| S1R | Sigma-1 |
| SOD | superoxide dismutase 1 |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| SV2A | synaptic vesicle protein 2A |
| TBG | Tabernanthalog |
| TNF-α | tumor necrosis factor-alpha |
| TrkB | Tropomyosin receptor kinase B |
| VHD | valvular heart disease |
| y/o | year-old |
References
- Kiss, M.G.; Mindur, J.E.; Yates, A.G.; Lee, D.; Fullard, J.F.; Anzai, A.; Poller, W.C.; Christie, K.A.; Iwamoto, Y.; Roudko, V.; et al. Interleukin-3 Coordinates Glial-Peripheral Immune Crosstalk to Incite Multiple Sclerosis. Immunity 2023, 56, 1502–1514.e8. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Rempe, T.; Whitmire, N.; Dunn-Pirio, A.; Graves, J.S. Therapeutic Advances in Multiple Sclerosis. Front. Neurol. 2022, 13, 824926. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, C.; Pugliatti, M.; Vermersch, P.; Grigoriadis, N.; Alkhawajah, M.; Airas, L.; Oreja-Guevara, C. Diagnosis and Treatment of Progressive Multiple Sclerosis: A Position Paper. Eur. J. Neurol. 2023, 30, 9–21. [Google Scholar] [CrossRef]
- Mansilla, M.J.; Presas-Rodríguez, S.; Teniente-Serra, A.; González-Larreategui, I.; Quirant-Sánchez, B.; Fondelli, F.; Djedovic, N.; Iwaszkiewicz-Grześ, D.; Chwojnicki, K.; Miljković, Đ.; et al. Paving the Way towards an Effective Treatment for Multiple Sclerosis: Advances in Cell Therapy. Cell. Mol. Immunol. 2021, 18, 1353–1374. [Google Scholar] [CrossRef]
- Auletta, J.J.; Bartholomew, A.M.; Maziarz, R.T.; Deans, R.J.; Miller, R.H.; Lazarus, H.M.; Cohen, J.A. The Potential of Mesenchymal Stromal Cells as a Novel Cellular Therapy for Multiple Sclerosis. Immunotherapy 2012, 4, 529–547. [Google Scholar] [CrossRef]
- Szabo, A. Psychedelics and Immunomodulation: Novel Approaches and Therapeutic Opportunities. Front. Immunol. 2015, 6, 358. [Google Scholar] [CrossRef]
- Chi, T.; Gold, J.A. A Review of Emerging Therapeutic Potential of Psychedelic Drugs in the Treatment of Psychiatric Illnesses. J. Neurol. Sci. 2020, 411, 116715. [Google Scholar] [CrossRef]
- de Vos, C.M.H.; Mason, N.L.; Kuypers, K.P.C. Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics. Front. Psychiatry 2021, 12, 724606. [Google Scholar] [CrossRef]
- Lima da Cruz, R.V.; Moulin, T.C.; Petiz, L.L.; Leão, R.N. Corrigendum: A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus. Front. Mol. Neurosci. 2018, 11, 312, Erratum in Front. Mol. Neurosci. 2019, 12, 312. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, N.; Yang, C.; Li, X.M.; Zhou, Z.Q.; Yang, J.J. Ketamine-Induced Antidepressant Effects Are Associated with AMPA Receptors-Mediated Upregulation of MTOR and BDNF in Rat Hippocampus and Prefrontal Cortex. Eur. Psychiatry 2014, 29, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.N.; Meshkat, S.; Benitah, K.; Lipsitz, O.; Gill, H.; Lui, L.M.W.; Teopiz, K.M.; McIntyre, R.S.; Rosenblat, J.D. Registered Clinical Studies Investigating Psychedelic Drugs for Psychiatric Disorders. J. Psychiatr. Res. 2021, 139, 71–81. [Google Scholar] [CrossRef]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a Convergent Mechanism of Ketamine and Classical Psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef]
- Khan, S.M.; Carter, G.T.; Aggarwal, S.K.; Holland, J. Psychedelics for Brain Injury: A Mini-Review. Front. Neurol. 2021, 12, 772692. [Google Scholar] [CrossRef] [PubMed]
- de Deus, J.L.; Maia, J.M.; Soriano, R.N.; Amorim, M.R.; Branco, L.G.S. Psychedelics in neuroinflammation: Mechanisms and therapeutic potential. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 137, 111278. [Google Scholar] [CrossRef] [PubMed]
- Inserra, A.; De Gregorio, D.; Gobbi, G. Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Pharmacol. Rev. 2021, 73, 202–277. [Google Scholar] [CrossRef]
- Weiss, F.; Magnesa, A.; Gambini, M.; Gurrieri, R.; Annuzzi, E.; Elefante, C.; Perugi, G.; Marazziti, D. Psychedelic-Induced Neural Plasticity: A Comprehensive Review and a Discussion of Clinical Implications. Brain Sci. 2025, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, U.; Nichols, C.; Wiatr, K.; Figiel, M. From Psychiatry to Neurology: Psychedelics as Prospective Therapeutics for Neurodegenerative Disorders. J. Neurochem. 2022, 162, 89–108. [Google Scholar] [CrossRef]
- Banks, M.I.; Zahid, Z.; Jones, N.T.; Sultan, Z.W.; Wenthur, C.J. Catalysts for Change: The Cellular Neurobiology of Psychedelics. Mol. Biol. Cell 2021, 32, 1135–1144. [Google Scholar] [CrossRef]
- Winkelman, M.J.; Szabo, A.; Frecska, E. The Potential of Psychedelics for the Treatment of Alzheimer’s Disease and Related Dementias. Eur. Neuropsychopharmacol. 2023, 76, 3–16. [Google Scholar] [CrossRef]
- Chen, D.Q.; Inzunza Domínguez, J.A.; Valle Uzeta, J.M.; Pushparaj, A.P.; Dickinson, J.E. Case Report: Significant Lesion Reduction and Neural Structural Changes Following Ibogaine Treatments for Multiple Sclerosis. Front. Immunol. 2025, 16, 1535782. [Google Scholar] [CrossRef]
- Behera, H.K.; Joga, R.; Yerram, S.; Karnati, P.; Mergu, T.; Gandhi, K.; Sowndharya, M.; Nathiya, D.; Singh, R.P.; Srivastava, S.; et al. Exploring the Regulatory Framework of Psychedelics in the US & Europe. Asian J. Psychiatr. 2024, 102, 104242. [Google Scholar] [PubMed]
- Mitchell, J.M.; Ot’alora G, M.; van der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedo, S.; Balliett, B.; et al. MDMA-Assisted Therapy for Moderate to Severe PTSD: A Randomized, Placebo-Controlled Phase 3 Trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Che, T.; Panova, O.; DiBerto, J.F.; Lyu, J.; Krumm, B.E.; Wacker, D.; Robertson, M.J.; Seven, A.B.; Nichols, D.E.; et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 2020, 182, 1574–1588.e19. [Google Scholar] [CrossRef] [PubMed]
- Zota, I.; Chanoumidou, K.; Gravanis, A.; Charalampopoulos, I. Stimulating Myelin Restoration with BDNF: A Promising Therapeutic Approach for Alzheimer’s Disease. Front. Cell Neurosci. 2024, 18, 1422130. [Google Scholar] [CrossRef]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics Promote Plasticity by Directly Binding to BDNF Receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef]
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, N.F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835. [Google Scholar] [CrossRef]
- Heal, D.J.; Gosden, J.; Smith, S.L.; Atterwill, C.K. Experimental Strategies to Discover and Develop the next Generation of Psychedelics and Entactogens as Medicines. Neuropharmacology 2023, 225, 109375. [Google Scholar] [CrossRef]
- Mandal, G.; Kirkpatrick, M.; Alboni, S.; Mariani, N.; Pariante, C.M.; Borsini, A. Ketamine Prevents Inflammation-Induced Reduction of Human Hippocampal Neurogenesis via Inhibiting the Production of Neurotoxic Metabolites of the Kynurenine Pathway. Int. J. Neuropsychopharmacol. 2024, 27, pyae041. [Google Scholar] [CrossRef]
- Yang, S.; Xu, K.; Xu, X.; Zhu, J.; Jin, Y.; Liu, Q.; Xu, R.; Gu, X.; Liu, Y.; Huang, Y.; et al. S-Ketamine Pretreatment Alleviates Anxiety-Like Behaviors and Mechanical Allodynia and Blocks the Pro-Inflammatory Response in Striatum and Periaqueductal Gray From a Post-Traumatic Stress Disorder Model. Front. Behav. Neurosci. 2022, 16, 848232. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Wolin, I.A.V.; Kaster, M.P.; Rodrigues, A.L.S. The Resilient Phenotype Elicited by Ketamine against Inflammatory Stressors-Induced Depressive-like Behavior Is Associated with NLRP3-Driven Signaling Pathway. J. Psychiatr. Res. 2021, 144, 118–128. [Google Scholar] [CrossRef]
- Tian, J.; Xie, Y.; Ye, S.; Hu, Y.; Feng, J.; Li, Y.; Lou, Z.; Ruan, L.; Wang, Z. S-Ketamine Ameliorates Post-Stroke Depression in Mice via Attenuation of Neuroinflammation, Synaptic Restoration, and BDNF Pathway Activation. Biochem. Biophys. Res. Commun. 2025, 769, 151965. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Niwa, H.; Seya, K.; Kawaguchi, J.; Kushikata, T.; Hirota, K. Ketamine Does Not Change Natural Killer Cell Cytotoxicity in Patients Undergoing Cancer Surgery: Basic Experiment and Clinical Trial. J. Oncol. 2022, 2022, 8946269. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Savalia, N.K.; Kwan, A.C. Ketamine for a Boost of Neural Plasticity: How, but Also When? Biol. Psychiatry 2021, 89, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef]
- Wu, W.; Gong, X.; Qin, Z.; Wang, Y. Molecular Mechanisms of Excitotoxicity and Their Relevance to the Pathogenesis of Neurodegenerative Diseases—An Update. Acta Pharmacol. Sin. 2025, 46, 3129–3142. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-Ketamine Ameliorates Demyelination and Facilitates Remyelination in Cuprizone-Treated Mice: A Role of Gut–Microbiota–Brain Axis. Neurobiol. Dis. 2022, 165, 105635. [Google Scholar] [CrossRef]
- Lullau, A.P.M.; Haga, E.M.W.; Ronold, E.H.; Dwyer, G.E. Antidepressant Mechanisms of Ketamine: A Review of Actions with Relevance to Treatment-Resistance and Neuroprogression. Front. Neurosci. 2023, 17, 1223145. [Google Scholar] [CrossRef]
- Lutfy, K.; Pechnick, R.N.; Darmani, N.A. Editorial: Pharmacology of New Psychoactive Substances. Front. Pharmacol. 2023, 14, 1208957. [Google Scholar] [CrossRef]
- Egger, K.; Aicher, H.D.; Cumming, P.; Scheidegger, M. Neurobiological Research on N,N-Dimethyltryptamine (DMT) and Its Potentiation by Monoamine Oxidase (MAO) Inhibition: From Ayahuasca to Synthetic Combinations of DMT and MAO Inhibitors. Cell. Mol. Life Sci. 2024, 81, 395. [Google Scholar] [CrossRef]
- Deliganis, A.V.; Pierce, P.A.; Peroutka, S.J. Differential Interactions of Dimethyltryptamine (DMT) with 5-HT1A and 5-HT2 Receptors. Biochem. Pharmacol. 1991, 41, 1739–1744. [Google Scholar] [CrossRef]
- Yu, B.; Becnel, J.; Zerfaoui, M.; Rohatgi, R.; Boulares, A.H.; Nichols, C.D. Serotonin 5-Hydroxytryptamine2A Receptor Activation Suppresses Tumor Necrosis Factor-α-Induced Inflammation with Extraordinary Potency. J. Pharmacol. Exp. Ther. 2008, 327, 316–323. [Google Scholar] [CrossRef]
- Nau, F.; Yu, B.; Martin, D.; Nichols, C.D. Serotonin 5-HT2A Receptor Activation Blocks TNF-α Mediated Inflammation In Vivo. PLoS ONE 2013, 8, e75426. [Google Scholar] [CrossRef]
- Krupp, K.T.; Yaeger, J.D.W.; Ledesma, L.J.; Withanage, M.H.H.; Gale, J.J.; Howe, C.B.; Allen, T.J.; Sathyanesan, M.; Newton, S.S.; Summers, C.H. Single Administration of a Psychedelic [(R)-DOI] Influences Coping Strategies to an Escapable Social Stress. Neuropharmacology 2024, 252, 109949. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Foster, T.P.; Galbato, T.E.; Lum, P.Y.; Louie, B.; Song, G.; Halberstadt, A.L.; Billac, G.B.; Nichols, C.D. Sero-tonin-2 Receptor Agonists Produce Anti-Inflammatory Effects Through Functionally Selective Mechanisms That Involve the Suppression of Disease-Induced Arginase 1 Expression. ACS Pharmacol. Transl. Sci. 2024, 7, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, T.W.; Billac, G.B.; Landry, A.N.; Sebastian, M.N.; Cormier, S.A.; Nichols, C.D. Structure-Activity Relationship Analysis of Psychedelics in a Rat Model of Asthma Reveals the Anti-Inflammatory Pharmacophore. ACS Pharmacol. Transl. Sci. 2021, 4, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Wiens, K.R.; Brooks, N.A.H.; Riar, I.; Greuel, B.K.; Lindhout, I.A.; Klegeris, A. Psilocin, the Psychoactive Metabolite of Psilocybin, Modulates Select Neuroimmune Functions of Microglial Cells in a 5-HT2 Receptor-Dependent Manner. Molecules 2024, 29, 5084. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Kondo, M.; Shimada, S.; Kaneda, K. IGF-1 Release in the Medial Prefrontal Cortex Mediates the Rapid and Sustained Antidepressant-like Actions of Ketamine. Transl. Psychiatry 2022, 12, 178. [Google Scholar] [CrossRef]
- Shao, L.-X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin Induces Rapid and Persistent Growth of Dendritic Spines in Frontal Cortex In Vivo. Neuron 2021, 109, 2535–2544. [Google Scholar] [CrossRef]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A Non-Hallucinogenic Psychedelic Analogue with Therapeutic Potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef]
- Robinson, G.I.; Gerasymchuk, M.; Zanikov, T.; Gojani, E.G.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cruz, C.; et al. LPS-Induced Liver Inflammation Is Inhibited by Psilocybin and Eugenol in Mice. Pharmaceuticals 2025, 18, 451. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics Promote Neuroplasticity through the Activation of Intracellular 5-HT2A Receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The Endogenous Hallucinogen and Trace Amine N,N-Dimethyltryptamine (DMT) Displays Potent Protective Effects Against Hypoxia via Sigma-1 Receptor Activation in Human Primary IPSC-Derived Cortical Neurons and Microglia-like Immune Cells. Front. Neurosci. 2016, 10, 423. [Google Scholar] [CrossRef]
- Govender, D.; Moloko, L.; Papathanasopoulos, M.; Tumba, N.; Owen, G.; Calvey, T. Ibogaine Administration Following Repeated Morphine Administration Upregulates Myelination Markers 2′, 3′-Cyclic Nucleotide 3′-Phosphodiesterase (CNP) and Myelin Basic Protein (MBP) MRNA and Protein Expression in the Internal Capsule of Sprague Dawley Rats. Front. Neurosci. 2024, 18, 1378841. [Google Scholar] [CrossRef]
- Vidonja Uzelac, T.; Tatalović, N.; Mijović, M.; Miler, M.; Grahovac, T.; Oreščanin Dušić, Z.; Nikolić-Kokić, A.; Blagojević, D. Ibogaine Induces Cardiotoxic Necrosis in Rats—The Role of Redox Processes. Int. J. Mol. Sci. 2024, 25, 6527. [Google Scholar] [CrossRef] [PubMed]
- Rubi, L.; Eckert, D.; Boehm, S.; Hilber, K.; Koenig, X. Anti-Addiction Drug Ibogaine Prolongs the Action Potential in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cardiovasc. Toxicol. 2017, 17, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Wu, K.J.; Wang, Y.S.; Bae, E.; Chianelli, F.; Bambakidis, N.; Wang, Y. Neuroprotective Effects of Psilocybin in a Rat Model of Stroke. BMC Neurosci. 2024, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Vella, Y.; Syrová, K.; Petrušková, A.; Koutrouli, I.; Kútna, V.; Pala, J.; Šíchová, K.; Nikolič, M.; Mazoch, V.; Jurok, R.; et al. Effects of Serotonergic Psychedelics on Synaptogenesis and Immediate Early Genes Expression—Comparison with Ketamine, Fluoxetine and Lithium. J. Psychopharmacol. 2025, 39, 1023–1030. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Anti-Inflammatory Effects of Four Psilocybin-Containing Magic Mushroom Water Extracts In Vitro on 15-Lipoxygenase Activity and on Lipopolysaccharide-Induced Cyclooxygenase-2 and Inflammatory Cytokines in Human U937 Macrophage Cells. J. Inflamm. Res. 2021, 14, 3729–3738. [Google Scholar] [CrossRef]
- Parekh, S.V.; Adams, L.O.; Barkell, G.A.; Lysle, D.T. MDMA Administration Attenuates Hippocampal IL-β Immunoreactivity and Subsequent Stress-Enhanced Fear Learning: An Animal Model of PTSD. Brain Behav. Immun. Health 2022, 26, 100542. [Google Scholar] [CrossRef]
- Mason, N.L.; Szabo, A.; Kuypers, K.P.C.; Mallaroni, P.A.; de la Torre Fornell, R.; Reckweg, J.T.; Tse, D.H.Y.; Hutten, N.R.P.W.; Feilding, A.; Ramaekers, J.G. Psilocybin Induces Acute and Persisting Alterations in Immune Status in Healthy Volunteers: An Experimental, Placebo-Controlled Study. Brain Behav. Immun. 2023, 114, 299–310. [Google Scholar] [CrossRef]
- Mestre, D.; Paula, A.; Gil, F.P.; Vaz, J. Multiple Episodes of Cardiac Arrest Induced by Treatment with Ibogaine: A Case Report. Cureus 2024, 16, e63487. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Deyell, M.W. Cardiac Arrest after Ibogaine Intoxication. J. Arrhythm. 2018, 34, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Wießner, I.; Olivieri, R.; Falchi, M.; Palhano-Fontes, F.; Oliveira Maia, L.; Feilding, A.; Araujo, D.B.; Ribeiro, S.; Tófoli, L.F. LSD, Afterglow and Hangover: Increased Episodic Memory and Verbal Fluency, Decreased Cognitive Flexibility. Eur. Neuropsychopharmacol. 2022, 58, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Baker, M.R.; O’Shea, C.I. Drug-Induced Psychosis Following Use of Ayahuasca: A Presentation to Forensic Psychiatric Services. BMJ Case Rep. 2024, 17, e260648. [Google Scholar] [CrossRef]
- Litenski, M.N.; O’Reardon, A.B.; Pabon, N.; Hernandez, M.; Niyazov, Y.; Cruz, J. Navigating Treatment Challenges: A Case Study on Refractory Psychosis in a Chronic MDMA (3,4-Methylenedioxymethamphetamine) User. Cureus 2024, 16, e59641. [Google Scholar] [CrossRef]
- Chung, A.N.; Huang, M.C.; Liu, T.H.; Chang, H.M.; Chen, P.Y.; Liu, Y.L.; Bavato, F. Ketamine-Dependent Patients with Persistent Psychosis Have Higher Neurofilament Light Chain Levels than Patients with Schizophrenia. Asian J. Psychiatr. 2024, 100, 104167. [Google Scholar] [CrossRef]
- Rothberg, R.L.; Azhari, N.; Haug, N.A.; Dakwar, E. Mystical-Type Experiences Occasioned by Ketamine Mediate Its Impact on at-Risk Drinking: Results from a Randomized, Controlled Trial. J. Psychopharmacol. 2021, 35, 150–158. [Google Scholar] [CrossRef]
- Husain, M.I.; Ledwos, N.; Fellows, E.; Baer, J.; Rosenblat, J.D.; Blumberger, D.M.; Mulsant, B.H.; Castle, D.J. Serotonergic Psychedelics for Depression: What Do We Know about Neurobiological Mechanisms of Action? Front. Psychiatry 2023, 13, 1076459. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Preller, K.H. Psychedelic Drugs: Neurobiology and Potential for Treatment of Psychiatric Disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef]
- Xiang, C.; Hong, S.M.; Zhao, B.; Pi, H.; Du, F.; Lu, X.; Tang, Y.; Shen, N.; Yang, C.; Wang, R. Fibroblast Expression of Neurotransmitter Receptor HTR2A Associates with Inflammation in Rheumatoid Arthritis Joint. Clin. Exp. Med. 2024, 24, 84. [Google Scholar] [CrossRef]
- Leó N-Ponte, M.; Ahern, G.P.; O’connell, P.J. Serotonin Provides an Accessory Signal to Enhance T-Cell Activation by Signaling through the 5-HT 7 Receptor. Blood 2007, 109, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Idova, G.V.; Alperina, E.L.; Cheido, M.A. Contribution of Brain Dopamine, Serotonin and Opioid Receptors in the Mechanisms of Neuroimmunomodulation: Evidence from Pharmacological Analysis. Int. Immunopharmacol. 2012, 12, 618–625. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Sousa, D.; de Azevedo, M.; Santos, M.L.; Borges, T.K.D.S.; de Oliveira, D.M.; Caldas, E.D. Immunomodulatory and Behavioral Effects of Ayahuasca and N, N-Dimethyltryptamine in a Rat Model of Lipopolysaccharide-Induced Depression. Metab. Brain Dis. 2025, 40, 220. [Google Scholar] [CrossRef] [PubMed]
- Floris, G.; Dabrowski, K.R.; Zanda, M.T.; Daws, S.E. Psilocybin Reduces Heroin Seeking Behavior and Modulates Inflammatory Gene Expression in the Nucleus Accumbens and Prefrontal Cortex of Male Rats. Mol. Psychiatry 2025, 30, 1801–1816. [Google Scholar] [CrossRef]
- Burmester, D.R.; Madsen, M.K.; Szabo, A.; Aripaka, S.S.; Stenbæk, D.S.; Frokjaer, V.G.; Elfving, B.; Mikkelsen, J.D.; Knudsen, G.M.; Fisher, P.M.D. Subacute Effects of a Single Dose of Psilocybin on Biomarkers of Inflammation in Healthy Humans: An Open-Label Preliminary Investigation. Compr. Psychoneuroendocrinol. 2023, 13, 100163. [Google Scholar] [CrossRef]
- Idell, R.D.; Florova, G.; Komissarov, A.A.; Shetty, S.; Girard, R.B.S.; Idell, S. The Fibrinolytic System: A New Target for Treatment of Depression with Psychedelics. Med. Hypotheses 2017, 100, 46–53. [Google Scholar] [CrossRef]
- Graça, S.C.; Bustelli, I.B.; dos Santos, É.V.; Fernandes, C.G.; Lanaro, R.; Stilhano, R.S.; Linardi, A.; Caetano, A.L. Banisteriopsis Caapi Extract: Implications for Neuroinflammatory Pathways in Locus Coeruleus Lesion Rodent Model. J. Ethnopharmacol. 2025, 337, 118775. [Google Scholar] [CrossRef]
- Rudin, D.; Areesanan, A.; Liechti, M.E.; Gründemann, C. Classic Psychedelics Do Not Affect T Cell and Monocyte Immune Responses. Front. Psychiatry 2023, 14, 1042440. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Wan, X.; Shan, J.; Qu, Y.; Hashimoto, K. (R)-Ketamine Attenuates LPS-Induced Endotoxin-Derived Delirium through Inhibition of Neuroinflammation. Psychopharmacology 2021, 238, 2743–2753. [Google Scholar] [CrossRef]
- Nau, F., Jr.; Miller, J.; Saravia, J.; Ahlert, T.; Yu, B.; Happel, K.I.; Cormier, S.A.; Nichols, C.D. Serotonin 5-HT 2 Receptor Activation Prevents Allergic Asthma in a Mouse Model. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, 191–198. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of Pro-Inflammatory Cytokines Released from Microglia in Neurodegenerative Diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Gruchot, J.; Weyers, V.; Göttle, P.; Förster, M.; Kremer, D.; Hartung, H.P.; Küry, P. The Molecular Basis for Remyelination Failure in Multiple Sclerosis. Cells 2019, 8, 825. [Google Scholar] [CrossRef]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.P.; et al. A Lymphocyte–Microglia–Astrocyte Axis in Chronic Active Multiple Sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Laabi, S.; LeMmon, C.; Vogel, C.; Chacon, M.; Jimenez, V.M. Psilocybin and Psilocin Regulate Microglial Immunomodulation and Support Neuroplasticity via Serotonergic and AhR Signaling. Int. Immunopharmacol. 2025, 159, 114940. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.X.B.; Ng, W.S.; Lim, E.S.Y.; Goh, B.H.; Kumari, Y. The Immunomodulatory Effects of Classical Psychedelics: A Systematic Review of Preclinical Studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 136, 111139. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Casili, G.; Lanza, M.; Filippone, A.; Cucinotta, L.; Paterniti, I.; Repici, A.; Capra, A.P.; Cuzzocrea, S.; Esposito, E.; Campolo, M. Dimethyl Fumarate (DMF) Alleviated Post-Operative (PO) Pain through the N-Methyl-d-Aspartate (NMDA) Receptors. Antioxidants 2022, 11, 1774. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive Multiple Sclerosis: From Pathophysiology to Therapeutic Strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Erkizia-Santamaría, I.; Horrillo, I.; Martínez-Álvarez, N.; Pérez-Martínez, D.; Rivero, G.; Erdozain, A.M.; Meana, J.J.; Ortega, J.E. Evaluation of Behavioural and Neurochemical Effects of Psilocybin in Mice Subjected to Chronic Unpredictable Mild Stress. Transl. Psychiatry 2025, 15, 201. [Google Scholar] [CrossRef]
- Brunello, C.A.; Cannarozzo, C.; Castrén, E. Rethinking the Role of TRKB in the Action of Antidepressants and Psychedelics. Trends Neurosci. 2024, 47, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ben-Tal, T.; Pogodin, I.; Botvinnik, A.; Lifschytz, T.; Heresco-Levy, U.; Lerer, B. Synergistic Behavioral and Neuroplastic Effects of Psilocybin-NMDAR Modulator Administration. Transl. Psychiatry 2025, 15, 200. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, I.M.; Cini, F.A.; Wießner, I.; Marcos, E.; Araújo, D.B.; Goto-Silva, L.; Nascimento, J.; Silva, S.R.B.; Costa, M.N.; Falchi, M.; et al. Nootropic Effects of LSD: Behavioral, Molecular and Computational Evidence. Exp. Neurol. 2022, 356, 114148. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.N.; Goto-Silva, L.; Nascimento, J.M.; Domith, I.; Karmirian, K.; Feilding, A.; Trindade, P.; Martins-De-Souza, D.; Rehen, S.K. LSD Modulates Proteins Involved in Cell Proteostasis, Energy Metabolism and Neuroplasticity in Human Cerebral Organoids. ACS Omega 2024, 9, 36553–36568. [Google Scholar] [CrossRef]
- Lipton, J.O.; Sahin, M. The Neurology of MTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef]
- Martín-Guerrero, S.M.; Alonso, P.; Iglesias, A.; Cimadevila, M.; Brea, J.; Loza, M.I.; Casado, P.; Martín-Oliva, D.; Cutillas, P.R.; González-Maeso, J.; et al. His452Tyr Polymorphism in the Human 5-HT2A Receptor Affects Clozapine-Induced Signaling Networks Revealed by Quantitative Phosphoproteomics. Biochem. Pharmacol. 2021, 185, 114440. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Bannon, R.; Calabresi, P.A. Breaking the Barriers to Remyelination in Multiple Sclerosis. Curr. Opin. Pharmacol. 2022, 63, 102194. [Google Scholar] [CrossRef]
- Gautier, H.O.B.; Evans, K.A.; Volbracht, K.; James, R.; Sitnikov, S.; Lundgaard, I.; James, F.; Lao-Peregrin, C.; Reynolds, R.; Franklin, R.J.M.; et al. Neuronal Activity Regulates Remyelination via Glutamate Signalling to Oligodendrocyte Progenitors. Nat. Commun. 2015, 6, 8518. [Google Scholar] [CrossRef]
- Funk, D.; Araujo, J.; Slassi, M.; Lanthier, J.; Atkinson, J.; Feng, D.; Lau, W.; Lê, A.; Higgins, G.A. Effect of a Single Psilocybin Treatment on Fos Protein Expression in Male Rat Brain. Neuroscience 2024, 539, 1–11. [Google Scholar] [CrossRef]
- EI Waly, B.; Macchi, M.; Cayre, M.; Durbec, P. Oligodendrogenesis in the Normal and Pathological Central Nervous System. Front. Neurosci. 2014, 8, 145. [Google Scholar] [CrossRef]
- Irvine, K.A.; Blakemore, W.F. Remyelination Protects Axons from Demyelination-Associated Axon Degeneration. Brain 2008, 131, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Grieco, S.F.; Castrén, E.; Knudsen, G.M.; Kwan, A.C.; Olson, D.E.; Zuo, Y.; Holmes, T.C.; Xu, X. Psychedelics and Neural Plasticity: Therapeutic Implications. J. Neurosci. Soc. Neurosci. 2022, 42, 8439–8449. [Google Scholar] [CrossRef] [PubMed]
- Maas, D.A.; Angulo, M.C. Can Enhancing Neuronal Activity Improve Myelin Repair in Multiple Sclerosis? Front. Cell Neurosci. 2021, 15, 645240. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Griffiths, R.R.; Hendricks, P.S.; Henningfield, J.E. The Abuse Potential of Medical Psilocybin According to the 8 Factors of the Controlled Substances Act. Neuropharmacology 2018, 142, 143–166. [Google Scholar] [CrossRef]
- Romeo, B.; Kervadec, E.; Fauvel, B.; Strika-Bruneau, L.; Amirouche, A.; Verroust, V.; Piolino, P.; Benyamina, A. Safety and Risk Assessment of Psychedelic Psychotherapy: A Meta-Analysis and Systematic Review. Psychiatry Res. 2024, 335, 115880. [Google Scholar] [CrossRef]
- Bremler, R.; Katati, N.; Shergill, P.; Erritzoe, D.; Carhart-Harris, R.L. Case Analysis of Long-Term Negative Psychological Responses to Psychedelics. Sci. Rep. 2023, 13, 15998. [Google Scholar] [CrossRef]
- Marrie, R.A.; Fisk, J.; Tremlett, H.; Wolfson, C.; Warren, S.; Blanchard, J.; Patten, S.B. Neurology® Clinical Practice Differing Trends in the Incidence of Vascular Comorbidity in MS and the General Population. Neurol. Clin. Pract. 2016, 6, 120–128. [Google Scholar] [CrossRef]
- Nahlawi, A.; Ptaszek, L.M.; Ruskin, J.N. Cardiovascular Effects and Safety of Classic Psychedelics. Nat. Cardiovasc. Res. 2025, 4, 131–144. [Google Scholar] [CrossRef]
- Rouaud, A.; Calder, A.E.; Hasler, G. Microdosing Psychedelics and the Risk of Cardiac Fibrosis and Valvulopathy: Comparison to Known Cardiotoxins. J. Psychopharmacol. 2024, 38, 217–224. [Google Scholar] [CrossRef]
- Tagen, M.; Mantuani, D.; van Heerden, L.; Holstein, A.; Klumpers, L.E.; Knowles, R. The Risk of Chronic Psychedelic and MDMA Microdosing for Valvular Heart Disease. J. Psychopharmacol. 2023, 37, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Downey, A.E.; Chaphekar, A.V.; Woolley, J.; Raymond-Flesch, M. Psilocybin Therapy and Anorexia Nervosa: A Narrative Review of Safety Considerations for Researchers and Clinicians. J. Eat. Disord. 2024, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Depression and Anxiety in Multiple Sclerosis. Review of a Fatal Combination. J. Neural Transm. 2024, 131, 847–869. [Google Scholar] [CrossRef] [PubMed]
- Mustač, F.; Pašić, H.; Medić, F.; Bjedov, B.; Vujević, L.; Alfirević, M.; Vidrih, B.; Tudor, K.I.; Bošnjak Pašić, M. Anxiety and Depression as Comorbidities of Multiple Sclerosis. Psychiatr. Danub. 2021, 33, 480–485. [Google Scholar]
- Marrocu, A.; Kettner, H.; Weiss, B.; Zeifman, R.J.; Erritzoe, D.; Carhart-Harris, R.L. Psychiatric Risks for Worsened Mental Health after Psychedelic Use. J. Psychopharmacol. 2024, 38, 225–235. [Google Scholar] [CrossRef]
- Simonsson, O.; Goldberg, S.B.; Chambers, R.; Osika, W.; Simonsson, C.; Hendricks, P.S. Psychedelic Use and Psychiatric Risks. Psychopharmacology 2023, 242, 1577–1583. [Google Scholar] [CrossRef]
- Elfrink, S.; Bergin, L. Psychedelic Iatrogenic Structural Dissociation: An Exploratory Hypothesis on Dissociative Risks in Psychedelic Use. Front. Psychol. 2025, 16, 1528253. [Google Scholar] [CrossRef]
- Borkel, L.F.; Rojas-Hernández, J.; Quintana-Hernández, D.J.; Henríquez-Hernández, L.A. Therapeutic Benefit versus Epistemic Risk: Need for Empirical Research in Psychedelic Epistemology. J. Psychiatr. Res. 2025, 188, 117–125. [Google Scholar] [CrossRef]
- Caporuscio, C.; Fink, S.B. Epistemic Risk Reduction in Psychedelic-Assisted Therapy; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–14. [Google Scholar]
- Pacilio, R.M.; Geller, J.A. Persistent Psychosis Associated with Intravenous Ketamine in a Patient Using Cannabis: A Case Report and Literature Review. J. Clin. Psychopharmacol. 2025, 45, 290–291. [Google Scholar] [CrossRef]
- Bavato, F.; Quednow, B.B. Ketamine Addiction in Europe: Any Risks on the Horizons? Eur. Neuropsychopharmacol. 2025, 94, 39–40. [Google Scholar] [CrossRef]
- Morton, E.; Sakai, K.; Ashtari, A.; Pleet, M.; Michalak, E.E.; Woolley, J. Risks and Benefits of Psilocybin Use in People with Bipolar Disorder: An International Web-Based Survey on Experiences of ‘Magic Mushroom’ Consumption. J. Psychopharmacol. 2023, 37, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.P.; González, R.S.; Lázaro, E.B. Treatment of Mood Disorders in Multiple Sclerosis. Curr. Treat. Options Neurol. 2015, 17, 323. [Google Scholar] [CrossRef] [PubMed]
- Soto-Angona, Ó.; Fortea, A.; Fortea, L.; Martínez-Ramírez, M.; Santamarina, E.; López, F.J.G.; Knudsen, G.M.; Ona, G. Do Classic Psychedelics Increase the Risk of Seizures? A Scoping Review. Eur. Neuropsychopharmacol. 2024, 85, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.; Sarris, J.; Rossell, S.; Bonomo, Y.; Forbes, D.; Davey, C.; Hoyer, D.; Loo, C.; Murray, G.; Hood, S.; et al. Medicinal Psychedelics for Mental Health and Addiction: Advancing Research of an Emerging Paradigm. Aust. N. Z. J. Psychiatry 2021, 55, 1127–1133. [Google Scholar] [CrossRef]
- Jones, N.T.; Zahid, Z.; Grady, S.M.; Sultan, Z.W.; Zheng, Z.; Razidlo, J.; Banks, M.I.; Wenthur, C.J. Transient Elevation of Plasma Glucocorticoids Supports Psilocybin-Induced Anxiolysis in Mice. ACS Pharmacol. Transl. Sci. 2023, 6, 1221–1231. [Google Scholar] [CrossRef]
- Rucker, J.J.H.; Iliff, J.; Nutt, D.J. Psychiatry & the Psychedelic Drugs. Past, Present & Future. Neuropharmacology 2018, 142, 200–218. [Google Scholar] [CrossRef]
- Schonholz, S.M.; Appel, J.M.; Bursztajn, H.J.; Nair, M.; MacIntyre, M.R. Legal and Ethics Concerns of Psilocybin as Medicine. J. Am. Acad. Psychiatry Law 2024, 52, 477–485. [Google Scholar]
- Siegel, J.S.; Daily, J.E.; Perry, D.A.; Nicol, G.E. Psychedelic Drug Legislative Reform and Legalization in the US. JAMA Psychiatry 2023, 80, 77–83. [Google Scholar] [CrossRef]
- Barnett, B.S.; Anand, A.; Dewey, E.N.; Smith, D.; Nayak, S.M.; Bruckman, D.; Weleff, J. Perceived Risk of Trying Lysergic Acid Diethylamide in the United States from 2015 to 2019: Are Americans Assessing Lysergic Acid Diethylamide’s Risk Profile More Favorably? Psychedelic Med. 2024, 2, 74–86. [Google Scholar] [CrossRef]
- Xenakis, S.N.; Shannon, S.M. What Is Needed for the Roll-out of Psychedelic Treatments? Curr. Opin. Psychiatry 2024, 37, 277–281. [Google Scholar] [CrossRef]
- Sheppard, B. A Trip Through Employment Law: Protecting Therapeutic Psilocybin Users in the Workplace. J. Law. Health 2021, 35, 146–180. [Google Scholar]
- Aday, J.S.; Barnett, B.S.; Grossman, D.; Murnane, K.S.; Nichols, C.D.; Hendricks, P.S. Psychedelic Commercialization: A Wide-Spanning Overview of the Emerging Psychedelic Industry. Psychedelic Med. 2023, 1, 150–165. [Google Scholar] [CrossRef]
- Li, S.; Kurtzweil, T.; Shams, S.; Pratt, A.; Rudi, S. Intellectual Property of Psychedelics for Addiction Treatment: Enabling Access and Protecting Innovation Opportunities Through Preserving the Public Domain. J. Stud. Alcohol. Drugs 2024, 85, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Belouin, S.J.; Averill, L.A.; Henningfield, J.E.; Xenakis, S.N.; Donato, I.; Grob, C.S.; Berger, A.; Magar, V.; Danforth, A.L.; Anderson, B.T. Policy Considerations That Support Equitable Access to Responsible, Accountable, Safe, and Ethical Uses of Psychedelic Medicines. Neuropharmacology 2022, 219, 109214. [Google Scholar] [CrossRef] [PubMed]
- Oates, E. Spectrum of Appearance of Hyperostosis Frontalis Interna on In-111 Leukocyte Scans. Clin. Nucl. Med. 1988, 13, 922–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Mathai, D.S.; Gukasyan, N.; Nayak, S.; Garcia-Romeu, A. Knowledge, Attitudes, and Concerns about Psilocybin and MDMA as Novel Therapies among U.S. Healthcare Professionals. Sci. Rep. 2024, 14, 28022. [Google Scholar] [CrossRef]
- Pierre, M.S.; Standing, L.; Herman, Y.; Haden, M.; Walsh, Z. Patients’ Experiences Discussing Psychedelics for Therapeutic Purposes with Physicians and Other Health Care Providers. Psychedelic Med. 2024, 2, 161–165. [Google Scholar] [CrossRef]
- Boehnke, K.F.; Cox, K.; Weston, C.; Herberholz, M.; Glynos, N.; Kolbman, N.; Fields, C.W.; Barron, J.; Kruger, D.J. Slouching towards Engagement: Interactions between People Using Psychedelics Naturalistically and Their Healthcare Providers. Front. Psychiatry 2023, 14, 1224551. [Google Scholar] [CrossRef]
- Celidwen, Y.; Redvers, N.; Githaiga, C.; Calambás, J.; Añaños, K.; Evanjuanoy Chindoy, M.; Vitale, R.; Nelson Rojas, J.; Mondragón, D.; Vázquez Rosalío, Y.; et al. Ethical Principles of Traditional Indigenous Medicine to Guide Western Psychedelic Research and Practice. Lancet Reg. Health Am. 2022, 18, 100410. [Google Scholar] [CrossRef]
- Spriggs, M.J.; Murphy-Beiner, A.; Murphy, R.; Bornemann, J.; Thurgur, H.; Schlag, A.K. ARC: A Framework for Access, Reciprocity and Conduct in Psychedelic Therapies. Front. Psychol. 2023, 14, 1119115. [Google Scholar] [CrossRef]
- Back, A.L.; Freeman-Young, T.K.; Morgan, L.; Sethi, T.; Baker, K.K.; Myers, S.; McGregor, B.A.; Harvey, K.; Tai, M.; Kollefrath, A.; et al. Psilocybin Therapy for Clinicians with Symptoms of Depression from Frontline Care During the COVID-19 Pandemic: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2449026. [Google Scholar] [CrossRef]
- Cavarra, M.; Falzone, A.; Ramaekers, J.G.; Kuypers, K.P.C.; Mento, C. Psychedelic-Assisted Psychotherapy—A Systematic Review of Associated Psychological Interventions. Front. Psychol. 2022, 13, 887255. [Google Scholar] [CrossRef]
- Mintz, K.T.; Gammer, B.; Khan, A.J.; Shaub, G.; Levine, S.; Sisti, D. Physical Disability and Psychedelic Therapies: An Agenda for Inclusive Research and Practice. Front. Psychiatry 2022, 13, 914458. [Google Scholar] [CrossRef] [PubMed]
- Villiger, D. Personal Psychedelic Experience of Psychedelic Therapists During Training: Should It Be Required, Optional, or Prohibited? Int. Rev. Psychiatry 2024, 36, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.R.; Faber, S.C.; Zare, M.; Fontaine, M.; Williams, M.T. Wolves Among Sheep: Sexual Violations in Psychedelic-Assisted Therapy. Am. J. Bioeth. 2025, 25, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A.; Nayak, S.M.; Siegel, J.S.; Hellerstein, D.J.; Ercal, B.C.; Lenze, E.J. The Role of Touch in Psychedelic Therapy: Perspectives from a Survey of Practitioners in Research Settings. Am. J. Psychother. 2025. In press. [Google Scholar] [CrossRef]
- Buchman, D.; Rosenbaum, D. Psychedelics in PERIL: The Commercial Determinants of Health, Financial Entanglements and Population Health Ethics. Public Health Ethics 2024, 17, 24–39. [Google Scholar] [CrossRef]
- Olson, D.E. Biochemical Mechanisms Underlying Psychedelic-Induced Neuroplasticity. Biochemistry 2022, 61, 127–136. [Google Scholar] [CrossRef]
- Sellers, E.M.; Romach, M.K. Psychedelics: Science Sabotaged by Social Media. Neuropharmacology 2023, 227, 109426. [Google Scholar] [CrossRef]
- Bellman, V. Review of Psilocybin Use for Depression among Cancer Patients After Approval in Oregon. Cancers 2024, 16, 1702. [Google Scholar] [CrossRef]
- Korthuis, P.T.; Hoffman, K.; Wilson-Poe, A.R.; Luoma, J.B.; Bazinet, A.; Pertl, K.; Morgan, D.L.; Cook, R.R.; Bielavitz, S.; Myers, R.; et al. Developing the Open Psychedelic Evaluation Nexus Consensus Measures for Assessment of Supervised Psilocybin Services: An e-Delphi Study. J. Psychopharmacol. 2024, 38, 761–768. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Nichols, C.D. Psychedelics and Anti-Inflammatory Activity in Animal Models. In Disruptive Psychopharmacology; Barrett, F.S., Preller, K.H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 229–245. ISBN 978-3-031-12184-5. [Google Scholar]
- Polito, V.; Stevenson, R.J. A Systematic Study of Microdosing Psychedelics. PLoS ONE 2019, 14, e0211023. [Google Scholar] [CrossRef]
- Anderson, T.; Petranker, R.; Christopher, A.; Rosenbaum, D.; Weissman, C.; Dinh-Williams, L.A.; Hui, K.; Hapke, E. Psychedelic Microdosing Benefits and Challenges: An Empirical Codebook. Harm Reduct. J. 2019, 16, 43. [Google Scholar] [CrossRef]
- Bavato, F.; Stamatakos, S.; Yde Ohki, C.M.; Seifritz, E.; Romualdi, P.; Grünblatt, E.; Quednow, B.B. Brain-Derived Neurotrophic Factor Protects Serotonergic Neurons against 3,4-Methylenedioxymethamphetamine (“Ecstasy”) Induced Cytoskeletal Damage. J. Neural Transm. 2022, 129, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Ffrench-Constant, C. Remyelination in the CNS: From Biology to Therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Rudick, R.A.; Larocca, N.; Hudson, L.D. Multiple Sclerosis Outcome Assessments Consortium: Genesis and Initial Project Plan. Mult. Scler. J. 2013, 20, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.A.; Mariottini, A.; Greco, R.; Burman, J.; Iacobaeus, E.; Inglese, M.; Snowden, J.A.; Alexander, T.; Amato, M.P.; Bø, L.; et al. Autologous Haematopoietic Stem Cell Transplantation for Treatment of Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder—Recommendations from ECTRIMS and the EBMT. Nat. Rev. Neurol. 2025, 21, 140–158. [Google Scholar] [CrossRef]
- Dhib-Jalbut, S.; Marks, S. Interferon-β Mechanisms of Action in Multiple Sclerosis. Neurology 2010, 74, S17–S24. [Google Scholar] [CrossRef]
- Aharoni, R. The Mechanism of Action of Glatiramer Acetate in Multiple Sclerosis and Beyond. Autoimmun. Rev. 2013, 12, 543–553. [Google Scholar] [CrossRef]
- Banks, W.A.; Rhea, E.M.; Reed, M.J.; Erickson, M.A. The Penetration of Therapeutics across the Blood-Brain Barrier: Classic Case Studies and Clinical Implications. Cell Rep. Med. 2024, 5, 101760. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for Structural Modification of Small Molecules to Improve Blood-Brain Barrier Penetration: A Recent Perspective. J. Med. Chem. 2021, 64, 13152–13173. [Google Scholar] [CrossRef]
- Evens, R.; Schmidt, M.E.; Majić, T.; Schmidt, T.T. The Psychedelic Afterglow Phenomenon: A Systematic Review of Subacute Effects of Classic Serotonergic Psychedelics. Ther. Adv. Psychopharmacol. 2023, 13, 20451253231172254. [Google Scholar] [CrossRef]
- Henner, R.L.; Keshavan, M.S.; Hill, K.P. Review of Potential Psychedelic Treatments for PTSD. J. Neurol. Sci. 2022, 439, 120302. [Google Scholar] [CrossRef] [PubMed]
- Valdez, T.; Patel, V.; Senesombath, N.; Hatahet-Donovan, Z.; Hornick, M. Therapeutic Potential of Psychedelic Compounds for Substance Use Disorders. Pharmaceuticals 2024, 17, 1484. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.; Cao, A.B.; Calkins, M.M.; Heim, A.J.; Lanham, J.K.; Bonniwell, E.M.; Hennessey, J.J.; Bock, H.A.; Anderson, E.I.; Sherwood, A.M.; et al. Identification of 5-HT2A Receptor Signaling Pathways Associated with Psychedelic Potential. Nat. Commun. 2023, 14, 8221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kodidela, S.; Godse, S.; Thomas-Gooch, S.; Kumar, A.; Raji, B.; Zhi, K.; Kochat, H.; Kumar, S. Targeted Drug Delivery to the Central Nervous System Using Extracellular Vesicles. Pharmaceuticals 2022, 15, 358. [Google Scholar] [CrossRef]
- Lu, J.; Tjia, M.; Mullen, B.; Cao, B.; Lukasiewicz, K.; Shah-Morales, S.; Weiser, S.; Cameron, L.P.; Olson, D.E.; Chen, L.; et al. An Analog of Psychedelics Restores Functional Neural Circuits Disrupted by Unpredictable Stress. Mol. Psychiatry 2021, 26, 6237–6252. [Google Scholar] [CrossRef]
- Caspani, G.; Ruffell, S.G.D.; Tsang, W.F.; Netzband, N.; Rohani-Shukla, C.; Swann, J.R.; Jefferies, W.A. Mind over Matter: The Microbial Mindscapes of Psychedelics and the Gut-Brain Axis. Pharmacol. Res. 2024, 207, 107338. [Google Scholar] [CrossRef]
- Szabo, A.; Frecska, E. Dimethyltryptamine (DMT): A Biochemical Swiss Army Knife in Neuroinflammation and Neuroprotection? Neural Regen. Res. 2016, 11, 396–397. [Google Scholar] [CrossRef]
| Substance | Model/Study Design | Key Mechanistic Finding | Relevant Outcome | Relevance for MS and Key Limitations | Ref. |
|---|---|---|---|---|---|
| Pre-Clinical Studies | |||||
| (R)-DOI | In vitro, RASMCs stimulated with TNF-α. | 5-HT2A activation (confirmed with selective antagonists) inhibits nuclear translocation of NF-κB via a PKC-dependent pathway. | Suppression of pro-inflammatory gene expression (ICAM-1, VCAM-1, IL-6) and NOS activity with extraordinary potency (IC50 ~10–20 pM). | Relevance: Establishes the 5-HT2A/NF-κB mechanism and a picomolar potency, suggesting anti-inflammatory action is possible at sub-psychedelic doses. Limitations: In vitro model using vascular cells, not immune or CNS cells. | [41] |
| (R)-DOI | In vivo, murine model of systemic inflammation induced by TNF-α. | 5-HT2A receptor activation (confirmed with the selective antagonist M100907). | Blockade of pro-inflammatory gene expression (Icam-1, Mcp-1, etc.) and circulating IL-6 at sub-behavioral doses. Effect is tissue-specific. | Relevance: Translates the potent anti-inflammatory effect to an animal model, confirming in vivo efficacy at non-psychotropic doses. Limitations: Acute induced-inflammation model, not a chronic autoimmune disease. The effect is tissue-specific, not global. | [42] |
| (R)-DOI | In vivo, mouse model of escapable social stress (Stress Alternatives Model-SAM). | Presumed 5-HT2A-mediated anti-inflammatory action. | A single low dose (0.015 mg/kg) promoted active coping strategies and reduced stress-induced TNF-α levels in plasma and limbic brain regions (BLA, PFC). | Relevance: Links anxiolytic/antidepressant-like behavioral effects with anti-inflammatory (TNF-α reduction) actions at sub-hallucinogenic doses in vivo. Limitations: Social stress model, not an autoimmune/demyelinating disease model. | [43] |
| (R)-DOI, (R)-DOTFM | In vivo, murine model of acute allergic asthma (OVA sensitization). | Demonstrates functional selectivity at the 5-HT2A receptor. The anti-inflammatory effect is linked to the suppression of arginase 1 (Arg1) expression. | (R)-DOI prevented airway inflammation, and this correlated with Arg1 suppression. The similar agonist (R)-DOTFM failed to suppress Arg1 and had no anti-inflammatory effect. | Relevance: Provides a specific molecular mechanism (Arg1 suppression) and highlights that not all 5-HT2A agonists are equal, crucial for designing new therapies. Limitations: Asthma is a peripheral, Th2-driven model, not a CNS autoimmune disease. | [44] |
| Various Psychedelic Analogs | In vivo, rat model of allergic asthma (OVA sensitization). | A systematic structure-activity relationship (SAR) study identified a common “anti-inflammatory pharmacophore” for 5-HT2A agonists distinct from the hallucinogenic pharmacophore. | The anti-inflammatory effect requires a specific chemical structure (e.g., 2,5-dimethoxy substitution) but is tolerant of changes at a different position (4-position) often associated with hallucinogenic potency. | Relevance: Identifies the 2C-H pharmacophore to decouple anti-inflammatory efficacy from hallucinogenic activity. Validates a chemical roadmap for non-psychotropic therapeutics. Limitations: Peripheral Th2-driven asthma model. Efficacy in neuroinflammatory conditions (e.g., MS) is theoretically inferred but not directly tested. | [45] |
| Psilocin | In vitro, murine (BV-2) and human (HL-60) microglial cell lines. | 5-HT2 receptor activation (demonstrated using antagonists like cyproheptadine and risperidone). | Significant inhibition of reactive oxygen species (ROS), nitric oxide (NO) production, and phagocytic activity in LPS-activated microglia. No effect on TNF secretion. | Relevance: Provides direct evidence for a 5-HT2R-dependent mechanism by which psilocin modulates microglial effector functions (ROS, NO) central to neurodegenerative diseases and depression. Limitations: In vitro study on immortalized cell lines; requires validation in primary human microglia, human iPSC-derived brain organoids, and in vivo models. | [46] |
| Ketamine | In vivo, healthy rats (Forced Swimming Test - FST behavioral model). | Downstream activation of AMPA receptors, leading to increased mTOR phosphorylation and BDNF expression. | Rapid antidepressant-like effects (reduced immobility) associated with increased p-mTOR and BDNF levels in the hippocampus and prefrontal cortex. | Relevance: Elucidates the signaling cascade (AMPA→mTOR/BDNF) driving pro-plastic effects, a key mechanism for fast-acting antidepressant effects. Limitations: Behavioral model in healthy animals (Forced Swimming Test), restricted to acute stress response rather than a chronic depression model | [10] |
| Ketamine | In vivo, rat model of PTSD (Single-Prolonged Stress). | S-Ketamine pre-treatment attenuated pro-inflammatory responses. | Reduced stress-induced increases in TNF-α and IL-1β levels in key brain regions (striatum, periaqueductal gray). | Relevance: Links ketamine’s anxiolytic effects directly to the reduction of pro-inflammatory cytokines within the CNS, supporting its potential as a neuro-immunomodulator. Limitations: Lack of specific microglia inhibitors to confirm causality. Only prophylactic administration was tested. | [29] |
| Ketamine | In vivo, mouse model of inflammation-induced depression (LPS/TNF-α). | Ketamine’s prophylactic effect is mediated by the inhibition of the NLRP3 inflammasome signaling pathway specifically in the ventral hippocampus. | Ketamine pre-treatment prevented depressive-like behaviors induced by inflammatory stressors through suppression of NLRP3 activation. | Relevance: Identifies the NLRP3 inflammasome in the ventral hippocampus as a key target for ketamine’s pro-resilience effects against inflammation Limitations: Study limited to acute inflammatory stressors rather than chronic stress models; efficacy is strictly dose-dependent | [30] |
| Ketamine | In vivo, mouse model of post-stroke depression. | S-Ketamine’s effects are mediated by activation of the BDNF/TrkB pathway and modulation of synaptic proteins (PSD-95, SYP). | S-Ketamine ameliorated depressive-like behaviors, attenuated neuroinflammation (reduced Iba1, GFAP), and restored dendritic spine density post-stroke. | Relevance: Demonstrates that ketamine can promote synaptic restoration and reduce glial activation in a CNS injury model, providing strong evidence for its dual neuro-restorative and anti-inflammatory action. Limitations: Stroke is an acute ischemic injury, different from the chronic autoimmune demyelination of MS. | [31] |
| Ketamine | In vivo (mice) and in vitro (primary neurons), studies on antidepressant mechanisms. | IGF-1, in addition to BDNF, is an essential mediator for the rapid and sustained antidepressant effects of ketamine. | Ketamine increased extracellular IGF-1 levels in the mPFC. Blocking IGF-1 signaling (with a neutralizing antibody) abolished both the long-term and the immediate, antidepressant-like behavioral effects. | Relevance: Refines the model of ketamine’s action, showing that multiple neurotrophic factors (BDNF and IGF-1 for both rapid and sustained effects) are involved, which may be relevant for long-term repair in MS. Limitations: Study mainly in healthy animals, but confirmed in a disease model (LPS). Conducted only in male mice. | [47] |
| Ketamine | In vitro, human hippocampal progenitor cells (HPCs) treated with the pro-inflammatory cytokine IL-1β. | Ketamine prevents the inflammation-induced shift in the kynurenine pathway, reducing the production of the neurotoxic metabolite kynurenine (KYN) | IL-1β reduced neurogenesis and increased KYN production in HPCs. Co-treatment with ketamine rescued neurogenesis and normalized the kynurenine pathway. | Relevance: Provides a specific molecular mechanism linking inflammation to neurogenesis deficits and shows how ketamine can directly counter this, a highly relevant pathway for depression where inflammation impairs repair. Limitations: In vitro model using progenitor cells; needs in vivo validation in an depression model. | [28] |
| Psilocybin | In vivo (mice), chronic 2-photon microscopy of medial frontal cortex. | Structural remodeling (increased spine formation rate) is associated with 5-HT2AR activation but persists despite partial antagonism with ketanserin. | Rapid (~24 h) and persistent (>1 month) increases in dendritic spine density (~10%) and size. Increased mEPSC frequency. | Relevance: Provides direct in vivo evidence for rapid and long-lasting structural plasticity in the cortex, supporting a potential mechanism for sustained CNS repair. Limitations: Study in healthy animals, not a disease model. The exact contribution of 5-HT2AR vs. other targets remains debatable. | [48] |
| LSD, Psilocin | In vivo (mutant mice) and in vitro (HEK293T cells, neuronal cultures), biochemical/biophysical binding assays. | Proposed direct, high-affinity binding to the transmembrane domain of the BDNF receptor, TrkB. | Pro-plasticity and antidepressant-like effects are TrkB-dependent and 5-HT2A-independent. Hallucinogenic-like effects are 5-HT2A-dependent and TrkB-independent. | Relevance: Paradigm-shifting finding suggesting a direct link to the TrkB repair pathway, potentially separating therapeutic plasticity from hallucinosis. Limitations: Novel and debated mechanism that requires independent replication. The precise interplay between TrkB and 5-HT2A in vivo is still unclear. | [25] |
| Tabernanthalog (TBG) | In vitro (rat cortical neurons) and in vivo (rodent models of addiction/depression). | Designed as a 5-HT2A agonist that promotes structural plasticity (dendritic growth) in a ketanserin-sensitive manner. | Promotes neuroplasticity without inducing hallucinogenic-like effects (HTR). Shows antidepressant and anti-addictive effects with an improved safety profile (low cardiotoxicity) compared to ibogaine. | Relevance: Proof-of-concept for “non-hallucinogenic psychoplastogens,” a key future direction for developing tolerable MS therapies. Limitations: Neuroinflammatory effects are uncharacterized. Not tested in demyelination or autoimmunity models. | [49] |
| Psilocybin | In vivo, C57BL/6J mice; LPS-induced acute liver inflammation (pre- and post-treatment). | Inhibits macrophage pro-inflammatory cascade; specifically downregulates IL-12p70 protein (a key driver of Th1 and cytotoxic T-cell responses). | mRNA: Significant downregulation of IL-1β, IL-6, TNF-α, and COX-2 2. Protein: Significant reduction of IL-12p70 (Th1 driver), unlike other cytokines where changes were non-significant Histology: Reversed nuclear circularity defects (marker of cellular stress/apoptosis) and reduced inflammatory infiltration4 | Relevance: IL-12p70 inhibition blocks Th1 differentiation, a critical pathway in MS autoimmunity. Limitations: Peripheral data (liver tissue), not CNS; acute inflammation model (not EAE/demyelinating). | [50] |
| DMT, Psilocin, 5-HT | In vitro (rat cortical neurons) and in vivo (mice with virally-expressed SERT in PFC). | Lipophilicity (membrane permeability) allows psychedelics to activate an intracellular pool of 5-HT2ARs, which mediates plasticity. | Lipophilic psychedelics (DMT, psilocin) promote plasticity; polar serotonin does not, unless given intracellular access (e.g., via SERT expression). | Relevance: Provides a fundamental explanation (location bias) for why psychedelics are effective psychoplastogens while endogenous serotonin is not. Limitations: Primarily an in vitro mechanistic study; the in vivo component uses an artificial system. Relevance in a disease state is unknown. | [51] |
| DMT | In vitro, human iPSC-derived cortical neurons and monocyte-derived microglia-like cells under hypoxia. | Activation of the Sigma-1 Receptor (Sig-1R), an intracellular chaperone protein. | Increased cell survival under severe hypoxic stress (0.5% O2). This protective effect was associated with downregulation of the hypoxia-inducible factor (HIF-1α). | Relevance: Identifies a neuroprotective mechanism (Sig-1R) independent of 5-HT receptors that could be relevant for mitigating hypoxic/ischemic damage within MS lesions. Limitations: In vitro model only; relevance of this anti-hypoxic mechanism to MS pathophysiology requires in vivo validation. | [52] |
| Ibogaine | In vivo, Sprague Dawley rats with a 10-day escalating morphine regimen. | Multi-receptor modulation (opioid, NMDA). Effect is an interaction between prior morphine exposure and ibogaine administration. | Administration after repeated morphine exposure significantly upregulated myelin markers CNP and MBP at mRNA (24 h) and protein (72 h) levels in the internal capsule. | Relevance: Provides rare preclinical evidence that ibogaine can upregulate key myelin proteins (CNP, MBP), supporting its hypothetical pro-remyelination potential. Limitations: The effect was only significant following morphine pre-treatment. Not a demyelinating disease model. Severe cardiotoxicity limits translation. | [53] |
| Ibogaine | In vivo, healthy male and female Wistar rats given a single oral dose (1 or 20 mg/kg). | Ibogaine induces myocardial necrosis without inflammatory infiltrate. The mechanism’s link to oxidative stress markers was inconsistent. | Dose-dependent, non-inflammatory myocardial necrosis observed 6 h and 24 h post-treatment in both sexes. | Relevance: Provides direct histopathological evidence of ibogaine’s severe cardiotoxicity (myocardial necrosis), a critical barrier to its clinical use for MS. Limitations: Did not directly link necrosis to arrhythmia; leaves the precise molecular pathway open to question. | [54] |
| Ibogaine, Noribogaine | In vitro, human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). | Inhibition of repolarizing hERG potassium channels. | Therapeutic concentrations (3 µM) of both ibogaine and its long-lasting metabolite noribogaine significantly delay repolarization and prolong the action potential duration. | Relevance: Provides direct experimental proof in human cardiomyocytes for the cellular mechanism that underpins QT interval prolongation and arrhythmia risk. Limitations: In vitro model; does not capture systemic effects in a whole organism. | [55] |
| Psilocybin | In vitro (rat cortical neurons, glutamate excitotoxicity) and in vivo (rat model of stroke-MCAO). | Neuroprotection is mediated by the BDNF/TrkB pathway, as effects were blocked by the TrkB inhibitor ANA12. | Reduced glutamate-induced neuronal loss in vitro. Pre- and post-stroke treatment reduced brain infarction, improved locomotor behavior, upregulated MAP2/synaptophysin, and reduced microglial activation (IBA1). | Relevance: First demonstration of psilocybin’s neuroprotective and anti-inflammatory effects in a relevant CNS injury model (stroke). Directly links the therapeutic effect to the BDNF pathway. Limitations: Stroke is an acute ischemic injury, different from the chronic autoimmune demyelination of MS. | [56] |
| Psilocybin | In vivo, healthy domestic pigs; autoradiography at 1 and 7 days post-injection. | Agonist stimulation of 5-HT2AR leads to downstream synaptogenesis and transient receptor downregulation. | Increased SV2A density (synaptic marker) in the hippocampus (+4.4% at 1 d, +9.2% at 7 d) and PFC (+6.1% at 7 d). Transient downregulation of 5-HT2AR density at 1 d, which normalized by 7 d. | Relevance: Provides robust evidence for psilocybin-induced synaptogenesis in a large animal model with a gyrencephalic brain, increasing translational confidence. Limitations: Study in healthy animals, not a disease model. SV2A is a presynaptic marker; post-synaptic changes were not measured. | [26] |
| Psilocin, Ketamine | In vitro, primary rat cortical neurons. | Psychedelics and ketamine act on synaptogenesis via 5-HT2AR and TrkB signaling pathways. | Psilocin and ketamine both induced a comparable, rapid increase in the number and size of PSD-95 clusters (a postsynaptic marker), indicating synaptogenesis. | Relevance: Directly compares psilocin and ketamine, showing they promote synaptogenesis with similar efficacy and reinforcing the “psychoplastogen” concept for CNS repair. Limitations: In vitro study on healthy neurons. Does not model a disease state or neuroinflammation. | [57] |
| Hot-water extracts of 4 Psilocybe and Panaeolus mushroom species (containing psilocybin) | In vitro, Human U937 macrophages induced with LPS (1 μg/mL3) | Significant inhibition of TNF-α, IL-1β, IL-6, and COX-2 | Potent suppression of inflammation with high safety profile (increased cell viability). | Relevance: argets key cytokines (TNF-α, IL-1β) involved in MS, but data is specific to osteoarthritis/general inflammation. Limitations: Uses whole extracts, not pure psilocybin. Effects likely driven by synergistic compounds (flavonoids, saponins). | [58] |
| MDMA | In vivo, rats exposed to a severe foot shock stress (SEFL) model. | MDMA administration attenuated the stress-induced increase of IL-1β and rescued microglial reduction (IBA-1) in the dorsal hippocampus. | MDMA administration significantly attenuated stress-enhanced fear learning | Relevance: Demonstrates anti-neuroinflammatory effects (specifically IL-1β) supporting the hypothesis that these compounds attenuate fear memory via immunosuppression. Limitations: Study used only male rats | [59] |
| Clinical Studies | |||||
| Psilocybin | Human (Healthy Volunteers)Randomized, double-blind, placebo-controlled trial (N = 60) | Acute reduction in plasma TNF-α significantly correlated with reduced glutamate concentrations in the hippocampus. Glial Status: No significant changes in myo-inositol (marker of glial activation). | Acute: Rapid suppression of TNF-α. Persisting (7 days): Sustained reduction of IL-6 and CRP (C-Reactive Protein) | Relevance: Targets two core MS pathologies: systemic inflammation (IL-6/CRP are drivers of autoimmunity) and excitotoxicity (glutamate-mediated axonal damage). Limitations: Study performed in healthy subjects, not patients with active neuroinflammation. Lack of effect on glial marker (myo-inositol) may weaken the rationale for targeting microglial activation directly. | [60] |
| Ibogaine | Case report (n = 1 human male with opioid dependence). | Acquired long QT syndrome leading to torsade de pointes. | Multiple episodes of cardiac arrest requiring defibrillation after a single, low dose (2.6 mg/kg) of ibogaine. QTc interval was massively prolonged (636 ms). | Relevance: Clinically demonstrates that even low, supposedly “safe” doses of ibogaine can be lethal, highlighting an extremely narrow and unpredictable therapeutic window. Limitations: An n = 1 case report; cannot establish causality or incidence rates. | [61] |
| Ibogaine | Case report (n = 1 human male). | Acquired long QT syndrome leading to life-threatening ventricular arrhythmia. | Cardiac arrest after a very high dose (5.6 g, ~65–70 mg/kg) of ibogaine. QTc was massively prolonged, and recovery took 7 days. | Relevance: Clinically demonstrates the severe, dose-related cardiotoxic risk of ibogaine. The long recovery time underscores the danger of its long-lasting metabolite, noribogaine. Limitations: An n = 1 case report. Hypokalemia was a co-trigger. | [62] |
| LSD (low dose) | Randomized, double-blind, placebo-controlled crossover trial (n = 24 healthy volunteers). | Not directly tested; discusses potential 5-HT2A involvement. | 24 h post-dose, LSD improved visuospatial memory (ROCF, OLMT) and phonological verbal fluency, but impaired cognitive flexibility (increased perseverative errors on WCST). | Relevance: Provides human evidence for sub-acute pro-cognitive effects (memory/language). However, it also highlights a significant negative effect on executive function (cognitive rigidity), a key translational challenge. Limitations: Healthy volunteers, not MS patients. Low dose (50 µg). | [63] |
| Psilocybin | Randomized, waiting list-controlled clinical trial (n = 24 patients with MDD). | Not a mechanistic study; focused on clinical efficacy. | Two sessions of psilocybin with psychotherapy produced large, rapid, and sustained antidepressant effects (~71% response, ~54% remission at week 4). | Relevance: Establishes psilocybin as a powerful therapeutic tool for depression, a major comorbidity in MS. Provides a clinical framework and safety data for psychedelic-assisted therapy. Limitations: Not in MS patients. Waiting list control does not account for expectancy effects. Small sample size. | [64] |
| MDMA | Randomized, placebo-controlled Phase 3 trial (n = 104 patients with moderate to severe PTSD). | Not a mechanistic study; focused on clinical efficacy. Proposed mechanisms include increased monoamine release and reduced fear response. | MDMA-assisted therapy significantly reduced PTSD symptoms and functional impairment compared to placebo with therapy. Generally well-tolerated. | Relevance: Provides a regulatory roadmap (Phase 3 trial) for bringing psychedelic-like compounds to market. Establishes a model for combining a psychoactive substance with therapy for a CNS disorder. Limitations: Not in MS patients. MDMA is an entactogen with different primary mechanisms than classic psychedelics. Blinding is a significant challenge. | [22] |
| Ayahuasca | Case report (n = 1 human male). | Not applicable (clinical observation). | First-episode, enduring psychosis with significant violence requiring forensic psychiatric admission after on three occasions Ayahuasca ceremony in a patient with no prior risk factors. | Relevance: Highlights the severe and unpredictable psychiatric risks of psychedelics, even in individuals without pre-existing vulnerabilities, reinforcing the need for rigorous screening. Limitations: An n = 1 case report; cannot establish causality or incidence rates. Composition of the brew was unknown. | [65] |
| MDMA (chronic use) | Case report (n = 1 human female). | Not applicable (clinical observation). | Severe, treatment-refractory psychosis with catatonic features that failed to respond to conventional antipsychotics and required electroconvulsive therapy (ECT). | Relevance: Demonstrates potential for severe, persistent psychiatric syndromes with chronic use, a key concern for any proposed long-term MS therapy. Limitations: An n = 1 case report from a chronic, recreational use scenario, which may not be representative of controlled therapeutic use. | [66] |
| Ketamine (chronic use) | Clinical study, comparing ketamine-dependent patients with persistent psychosis to patients with schizophrenia and healthy controls. | Not applicable (biomarker study). | Patients with ketamine-induced persistent psychosis had significantly higher levels of neurofilament light chain (NfL), a marker of neuroaxonal damage, than both schizophrenia patients and healthy controls. | Relevance: Provides crucial clinical evidence that chronic ketamine use can be associated with measurable neuroaxonal injury, raising significant safety concerns for its long-term application in MS. Limitations: Cross-sectional study in a substance use population; does not establish a causal link between ketamine and neurotoxicity. | [67] |
| Ketamine | Randomized, controlled trial (n = 40 alcohol-dependent adults). | Mediation by psychoactive effects (mystical-type experience) | The “mystical-type experience” (measured by the Hood Mysticism Scale). | Relevance: Provides clinical evidence supporting the hypothesis that subjective, psychoactive effects are critical for efficacy. Limitations: Secondary analysis. | [68] |
| PSYs | Cardiovascular Risks | Neuropsychiatric Risks | Other Risks | Ref |
|---|---|---|---|---|
| Ibogaine | High cardiotoxicity risk, dose-dependent myocardial necrosis, cardiac arrest, QT prolongation, arrhythmias. | Hallucinations | Requires continuous ECG monitoring. | [54] |
| LSD | Transient increase in blood pressure and heart rate. Chronic use: VHD risk (5-HT2B agonism). | May increase anxiety, HPPD, psychosis | Serotonergic toxicity, especially with SSRIs. | [15,105,110,116] |
| Psilocybin | Transient increase in blood pressure (systolic and diastolic) and heart rate. | Can worsen pre-existing psychiatric conditions, particularly bipolar disorder and psychosis. | Risk of serotonin syndrome, especially with concurrent use of other serotonergic medications. | [106,122] |
| Ketamine | Dissociation, hallucinations, abuse/addiction risk, persistent psychosis (NfL↑). | Bladder toxicity with chronic use | [67,68,121] | |
| DMT (Ayahuasca) | Increases BP/HR, especially with MAOIs (Ayahuasca). | Intense hallucinations, risk of psychosis even without prior history. | Risk of serotonin syndrome (with SSRIs/MAOIs) | [39,65] |
| Analogs (e.g., DOI) | Activates 5-HT2A receptors, which can modulate vasoconstriction | Produces hallucinogenic effects at specific doses and is classified as a psychedelic. | At higher doses, risks include vasoconstriction. The drug’s mechanism, excessive mTOR stimulation, has been associated with other disorders | [34,41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anchesi, I.; Astorino, M.F.; Raffaele, I.; Donato, D.S.; Silvestro, S.; Minuti, A.; Calabrò, M.; Scuruchi, M.; Cipriano, G.L. Psychedelics in Multiple Sclerosis: Mechanisms, Challenges, and Prospects for Neuroimmune Modulation and Repair. Cells 2025, 14, 1872. https://doi.org/10.3390/cells14231872
Anchesi I, Astorino MF, Raffaele I, Donato DS, Silvestro S, Minuti A, Calabrò M, Scuruchi M, Cipriano GL. Psychedelics in Multiple Sclerosis: Mechanisms, Challenges, and Prospects for Neuroimmune Modulation and Repair. Cells. 2025; 14(23):1872. https://doi.org/10.3390/cells14231872
Chicago/Turabian StyleAnchesi, Ivan, Maria Francesca Astorino, Ivana Raffaele, Deborah Stefania Donato, Serena Silvestro, Aurelio Minuti, Marco Calabrò, Michele Scuruchi, and Giovanni Luca Cipriano. 2025. "Psychedelics in Multiple Sclerosis: Mechanisms, Challenges, and Prospects for Neuroimmune Modulation and Repair" Cells 14, no. 23: 1872. https://doi.org/10.3390/cells14231872
APA StyleAnchesi, I., Astorino, M. F., Raffaele, I., Donato, D. S., Silvestro, S., Minuti, A., Calabrò, M., Scuruchi, M., & Cipriano, G. L. (2025). Psychedelics in Multiple Sclerosis: Mechanisms, Challenges, and Prospects for Neuroimmune Modulation and Repair. Cells, 14(23), 1872. https://doi.org/10.3390/cells14231872








