MerTK and the Role of Phagoptosis in Neonatal Hypoxia-Ischemia
Highlights
- MerTK and other key phagoptosis-related molecules are upregulated in the neonatal brain after hypoxia-ischemia.
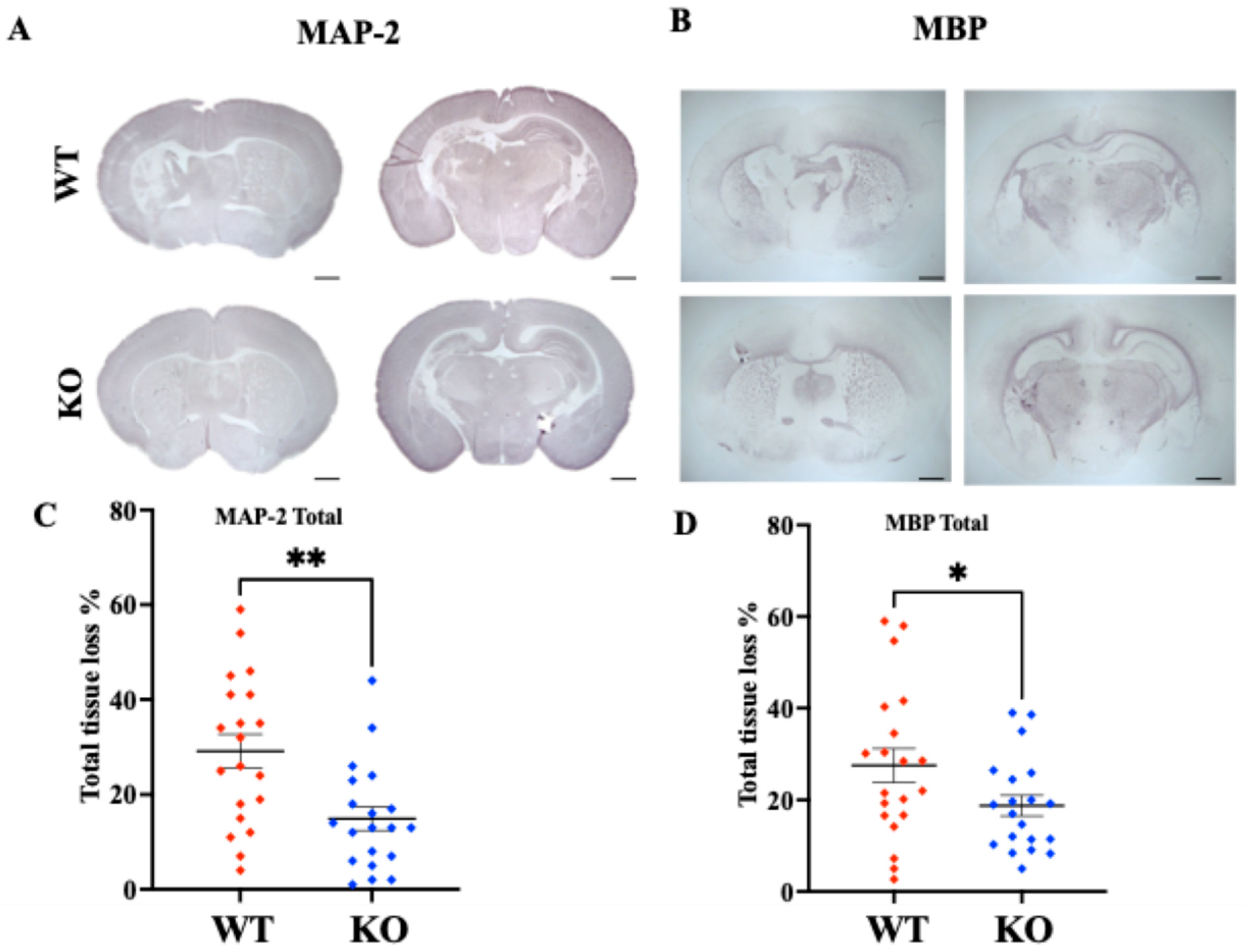
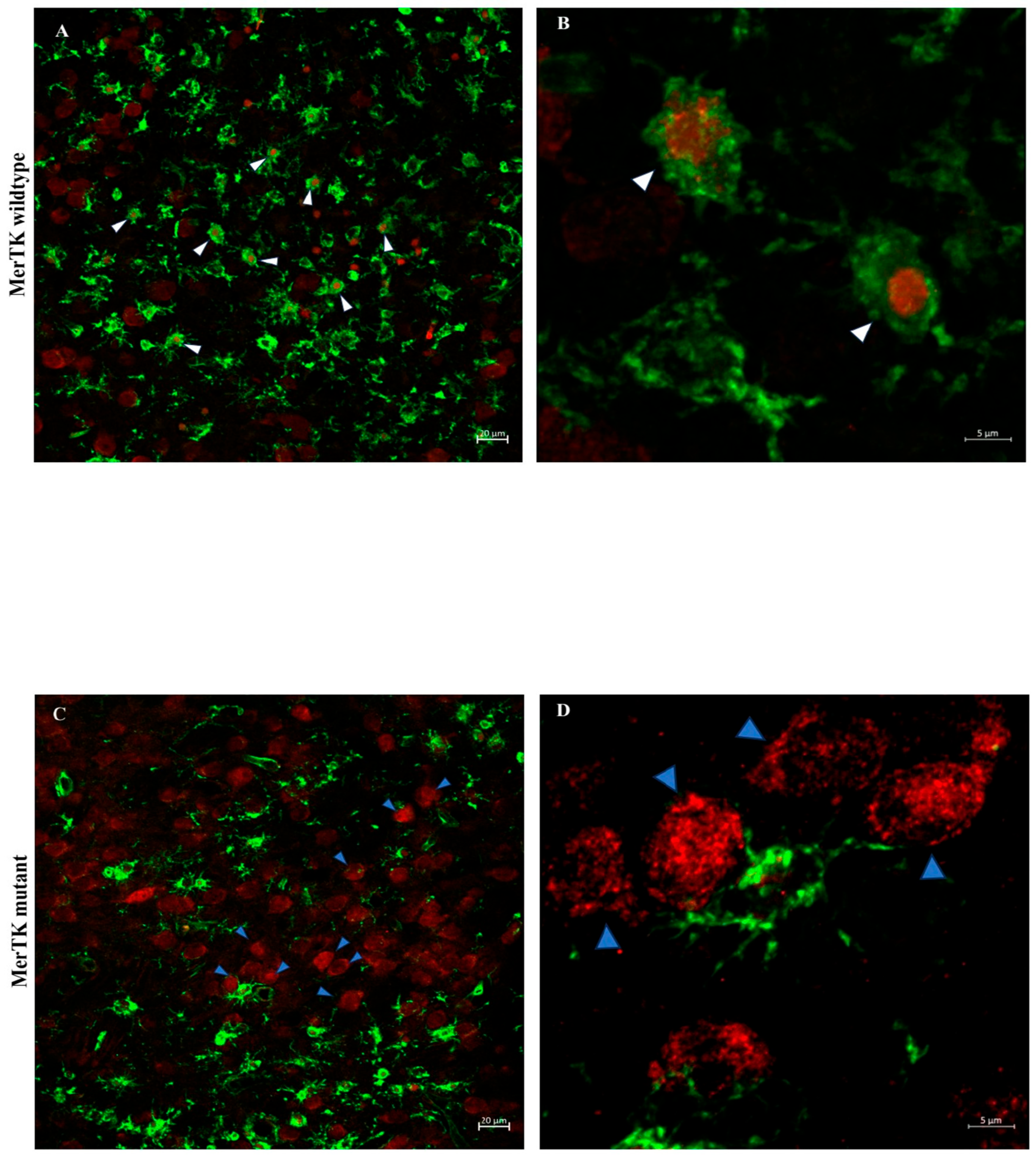
- Deletion of MerTK reduces microglial engulfment of neurons and significantly attenuates gray and white matter injury.
- Our findings indicate a potential contribution of phagoptosis to neuronal loss following neonatal hypoxia-ischemia.
- Targeting MerTK-mediated phagocytosis may represent a potential therapeutic strategy for neuroprotection in neonatal brain injury.
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Hypoxia-Ischemia
2.3. RNA Sequencing
2.4. Immunohistochemistry
Tissue Preparation
2.5. Brain Injury Evaluation
2.6. NeuN/Isolectin
2.7. Confocal Microscopy
2.8. Protein Samples Preparation
2.9. ELISA
2.10. Western Blot
2.11. Bio-Plex
2.12. Caspase-3 Activity Measurement
2.13. Statistical Analysis
3. Ethics
4. Results
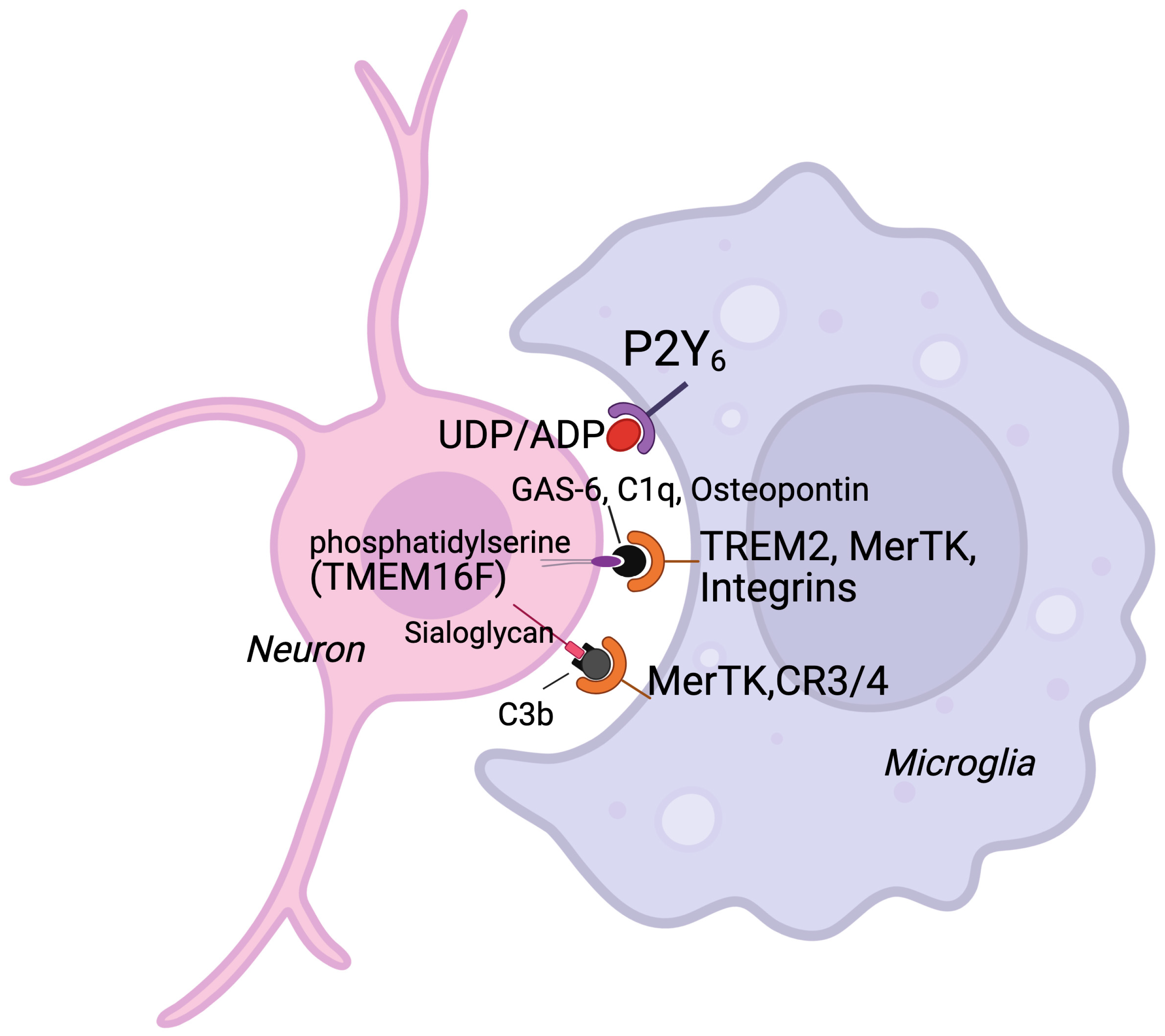
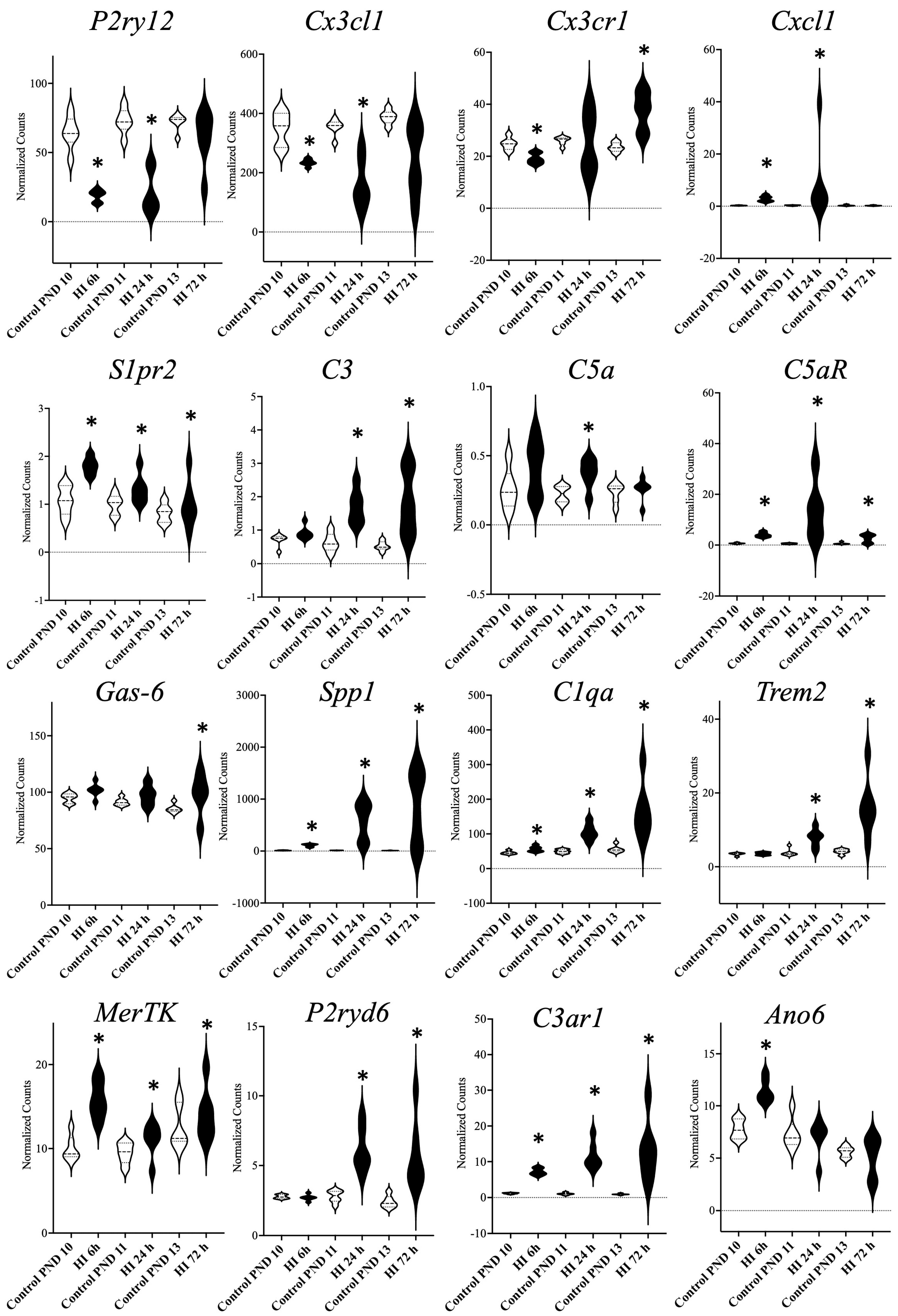
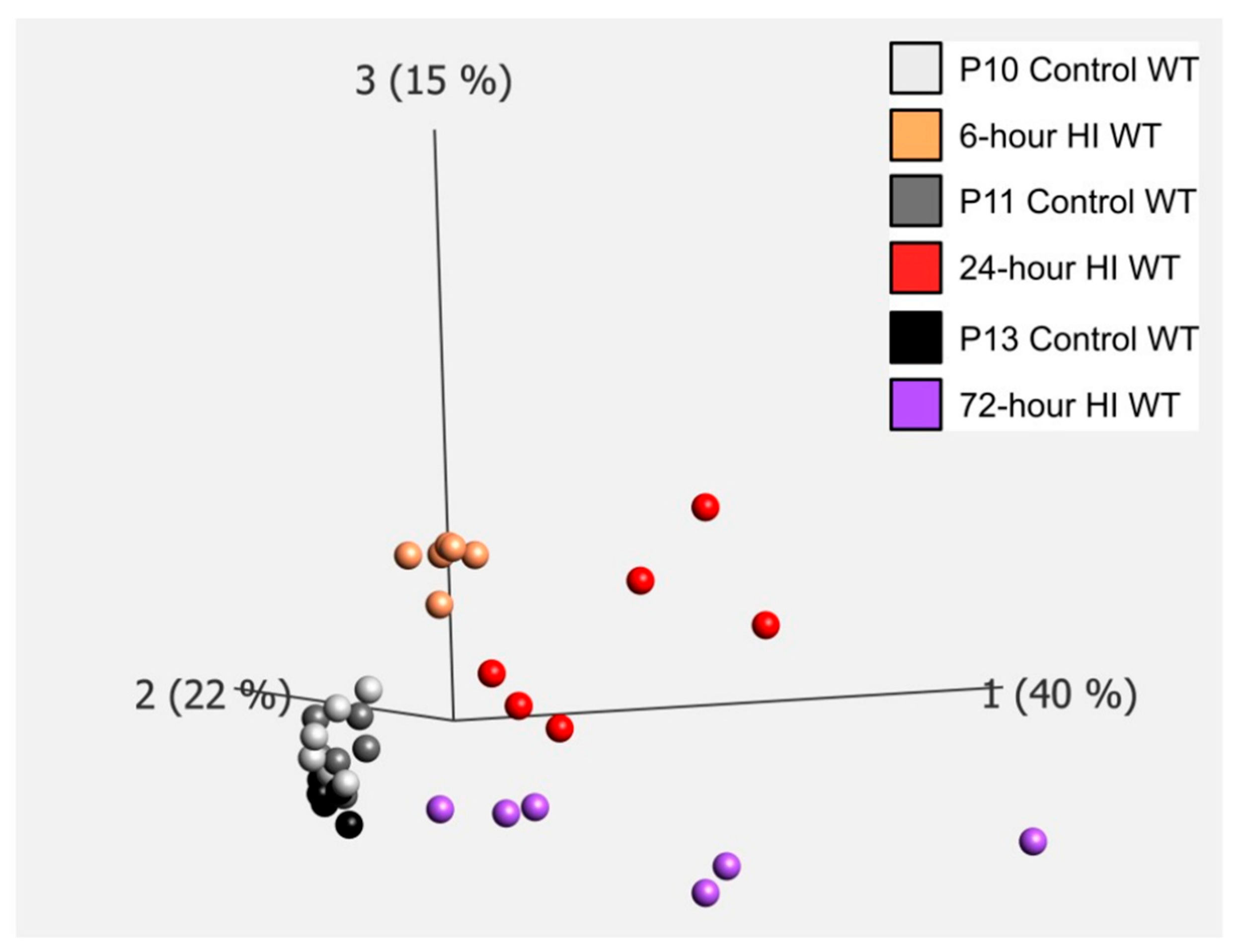
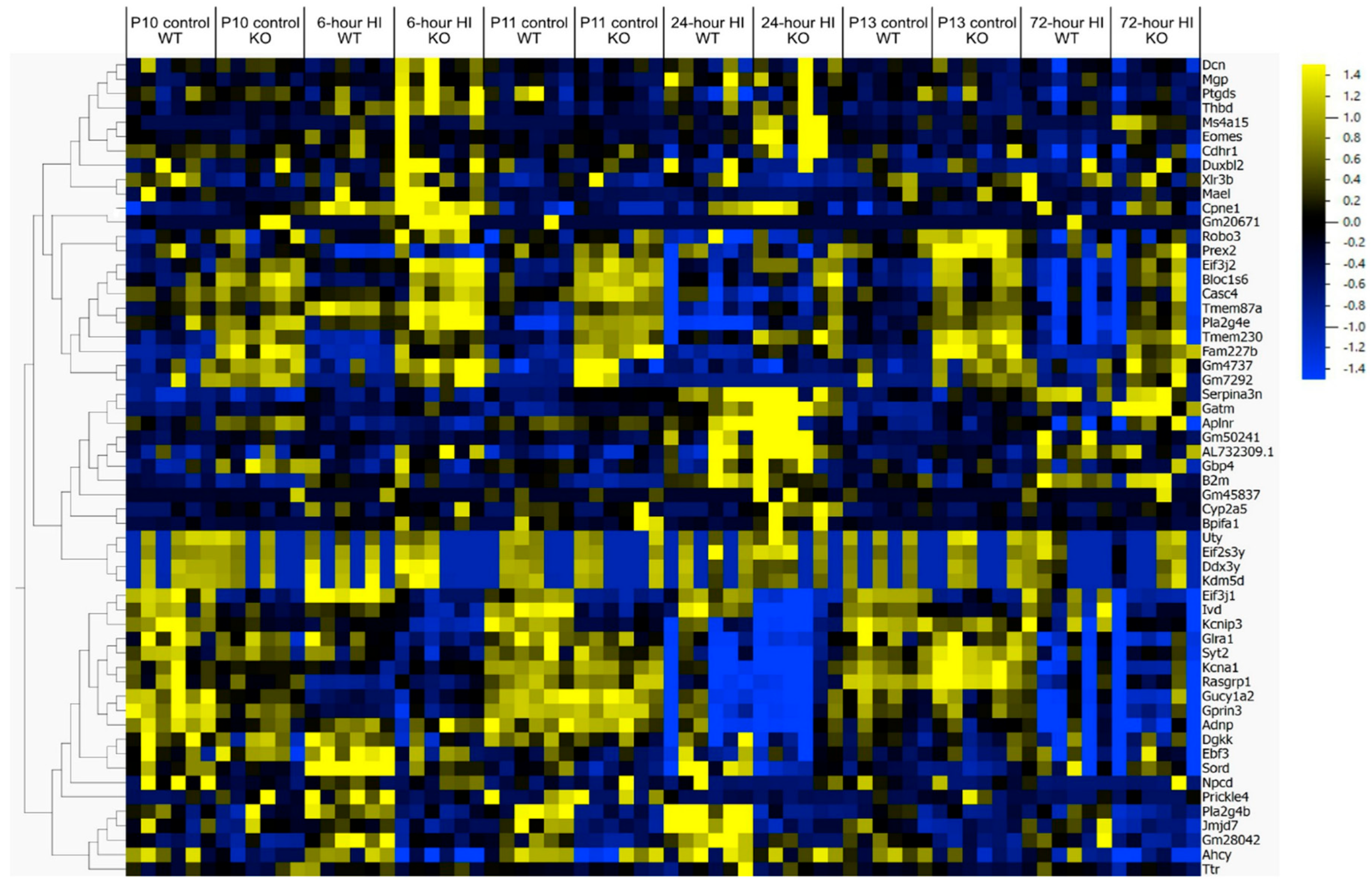
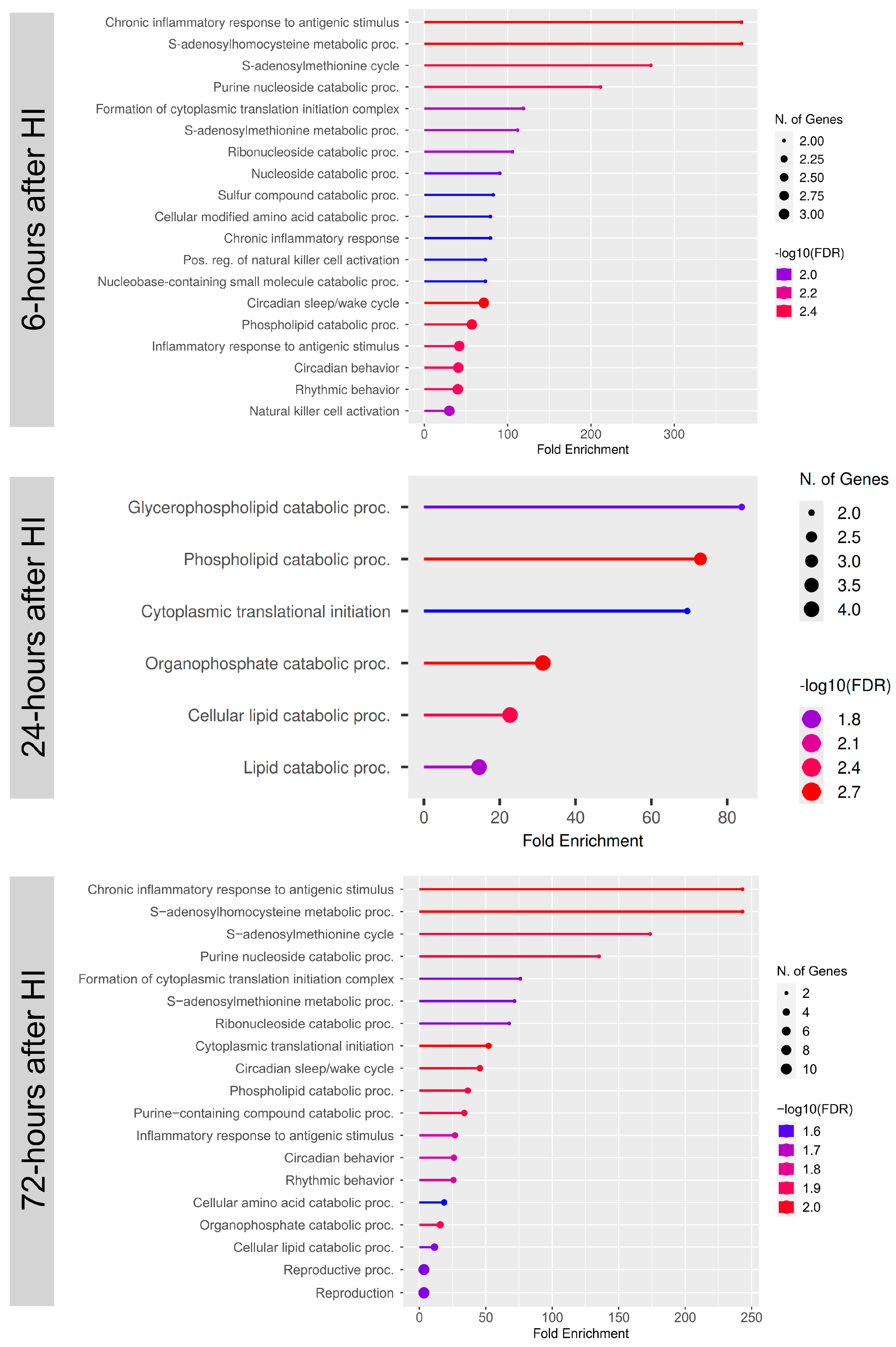
4.1. Gene Expression Related to Microglial Phagoptosis
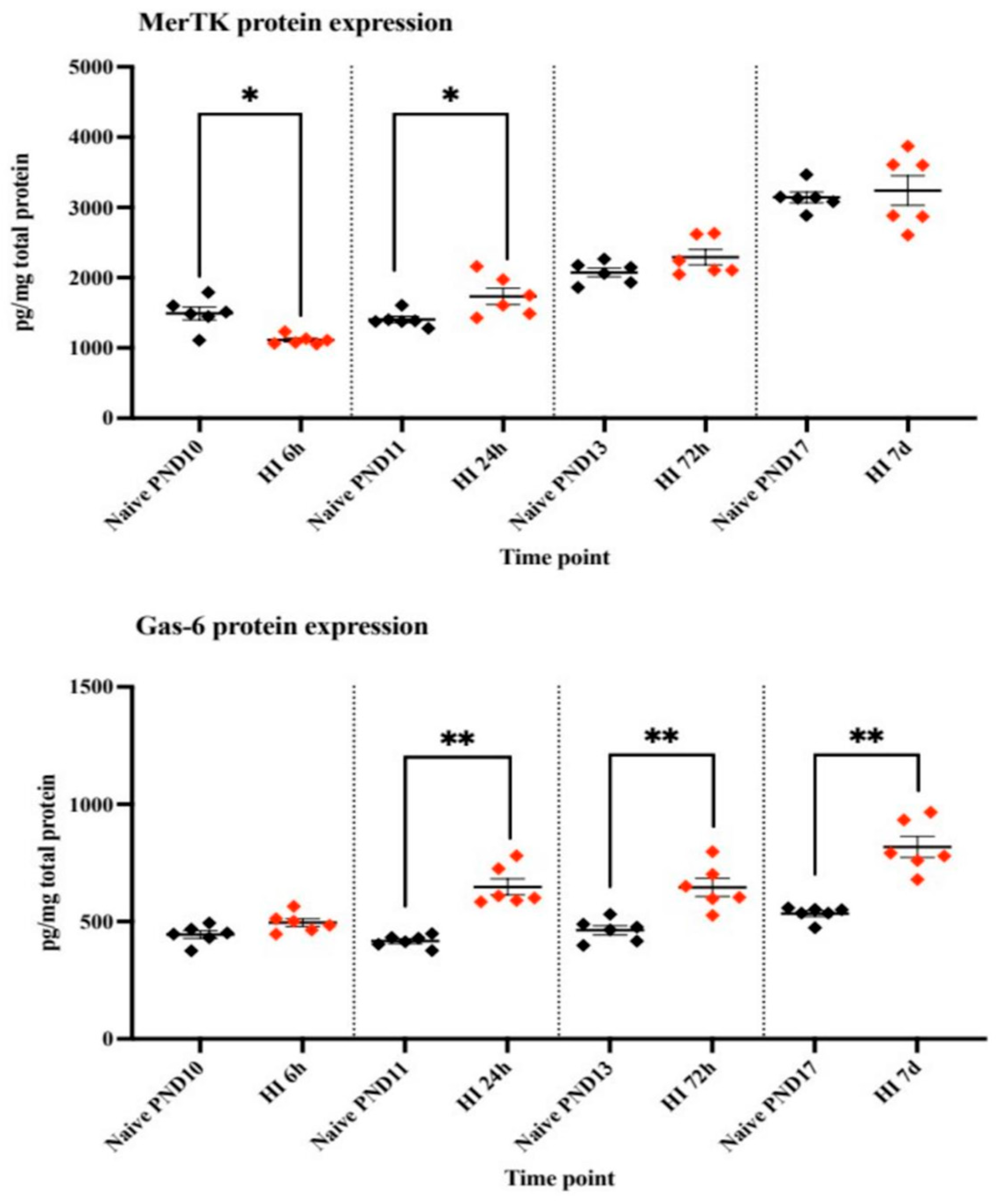
4.2. Protein Expression of MerTK and Gas-6
4.3. Brain Injury in MerTK KO and WT Animals After HI
4.4. Analysis of Microglial Phagocytosis of Neurons After HI
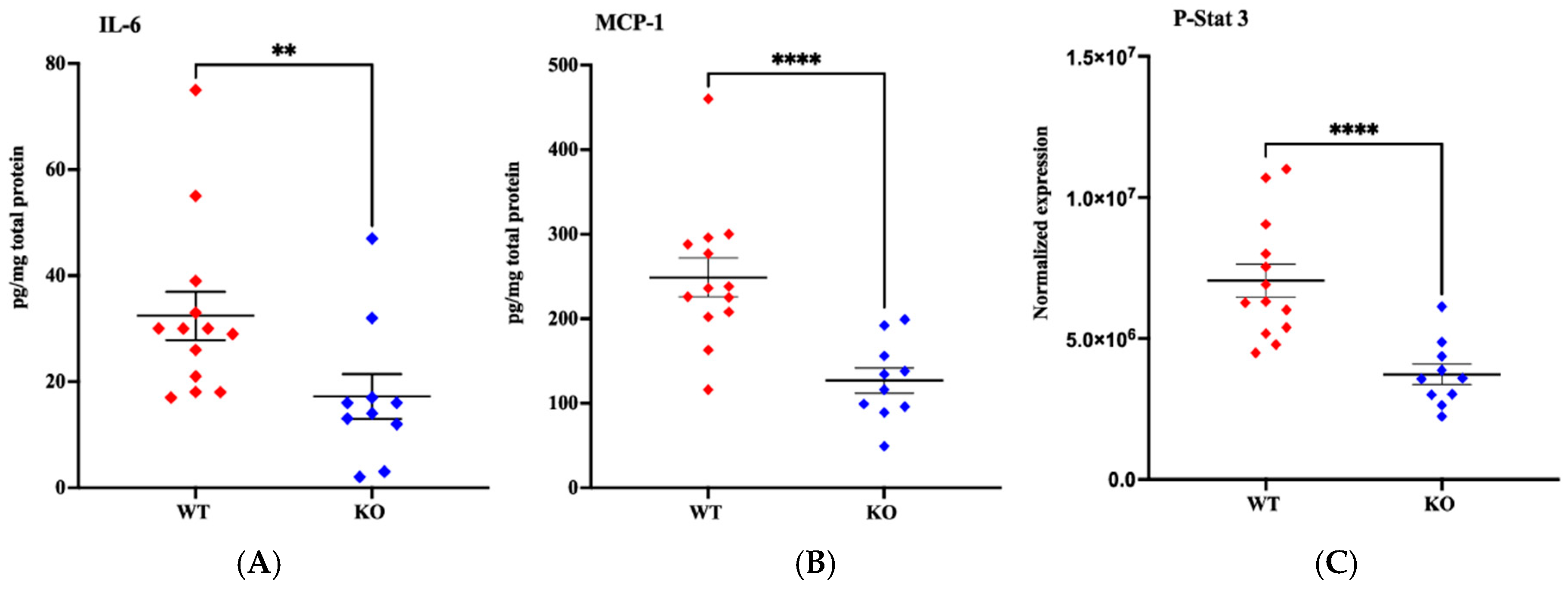
4.5. Gene and Protein Expression After Brain HI in MerTK KO and WT Mice
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hagberg, H.; David Edwards, A.; Groenendaal, F. Perinatal brain damage: The term infant. Neurobiol. Dis. 2016, 92 Pt A, 102–112. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef]
- Thayyil, S.; Pant, S.; Montaldo, P.; Shukla, D.; Oliveira, V.; Ivain, P.; Bassett, P.; Swamy, R.; Mendoza, J.; Moreno-Morales, M.; et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): A randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 2021, 9, e1273–e1285. [Google Scholar] [CrossRef]
- Shao, R.; Sun, D.; Hu, Y.; Cui, D. White matter injury in the neonatal hypoxic-ischemic brain and potential therapies targeting microglia. J. Neurosci. Res. 2021, 99, 991–1008. [Google Scholar] [CrossRef]
- Hedtjärn, M.; Leverin, A.L.; Eriksson, K.; Blomgren, K.; Mallard, C.; Hagberg, H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J. Neurosci. 2002, 22, 5910–5919. [Google Scholar] [CrossRef] [PubMed]
- McRae, A.; Gilland, E.; Bona, E.; Hagberg, H. Microglia activation after neonatal hypoxic-ischemia. Brain Res. Dev. Brain Res. 1995, 84, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Gilland, E.; Bona, E.; Hanson, L.A.; Hahin-Zoric, M.; Blennow, M.; Holst, M.; McRae, A.; Söder, O. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr. Res. 1996, 40, 603–609. [Google Scholar] [CrossRef]
- Brown, G.C. Neuronal Loss after Stroke Due to Microglial Phagocytosis of Stressed Neurons. Int. J. Mol. Sci. 2021, 22, 13442. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol. 2014, 49, 1422–1434. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef]
- Brown, G.C. Cell death by phagocytosis. Nat. Rev. Immunol. 2024, 24, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Emmrich, J.V.; Fricker, M.; Mander, P.K.; Théry, C.; Brown, G.C. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, E4098–E4107. [Google Scholar] [CrossRef]
- Milde, S.; van Tartwijk, F.W.; Vilalta, A.; Hornik, T.C.; Dundee, J.M.; Puigdellívol, M.; Brown, G.C. Inflammatory neuronal loss in the substantia nigra induced by systemic lipopolysaccharide is prevented by knockout of the P2Y(6) receptor in mice. J. Neuroinflamm. 2021, 18, 225. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem. Sci. 2012, 37, 325–332. [Google Scholar] [CrossRef]
- Pickett, L.A.; VanRyzin, J.W.; Marquardt, A.E.; McCarthy, M.M. Microglia phagocytosis mediates the volume and function of the rat sexually dimorphic nucleus of the preoptic area. Proc. Natl. Acad. Sci. USA 2023, 120, e2212646120. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Webster, C.M.; Hokari, M.; McManus, A.; Tang, X.N.; Ma, H.; Kacimi, R.; Yenari, M.A. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS ONE 2013, 8, e70927. [Google Scholar] [CrossRef]
- Soriano, S.G.; Amaravadi, L.S.; Wang, Y.F.; Zhou, H.; Yu, G.X.; Tonra, J.R.; Fairchild-Huntress, V.; Fang, Q.; Dunmore, J.H.; Huszar, D.; et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J. Neuroimmunol. 2002, 125, 59–65. [Google Scholar] [CrossRef]
- Cockram, T.O.J.; Dundee, J.M.; Popescu, A.S.; Brown, G.C. The Phagocytic Code Regulating Phagocytosis of Mammalian Cells. Front. Immunol. 2021, 12, 629979. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Gaire, B.P.; Kang, M.G.; Choi, J.W. S1P(2) contributes to microglial activation and M1 polarization following cerebral ischemia through ERK1/2 and JNK. Sci. Rep. 2019, 9, 12106. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Pan, J.; Mamtilahun, M.; Zhu, Y.; Wang, L.; Venkatesh, A.; Shi, R.; Tu, X.; Jin, K.; Wang, Y.; et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020, 10, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Kim, H.L.; Choi, J.S.; Choi, J.Y.; Cha, J.H.; Lee, M.Y. Osteopontin: Correlation with phagocytosis by brain macrophages in a rat model of stroke. Glia 2011, 59, 413–423. [Google Scholar] [CrossRef]
- Linnartz, B.; Kopatz, J.; Tenner, A.J.; Neumann, H. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J. Neurosci. 2012, 32, 946–952. [Google Scholar] [CrossRef]
- Kurisu, K.; Zheng, Z.; Kim, J.Y.; Shi, J.; Kanoke, A.; Liu, J.; Hsieh, C.L.; Yenari, M.A. Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Neniskyte, U.; Hornik, T.; Brown, G.C. Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo. Glia 2014, 62, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Li, X.; Wu, J.; Xue, T.; Wu, J.; Shen, H.; Shen, M.; Chen, G. TMEM16F Aggravates Neuronal Loss by Mediating Microglial Phagocytosis of Neurons in a Rat Experimental Cerebral Ischemia and Reperfusion Model. Front. Immunol. 2020, 11, 1144. [Google Scholar] [CrossRef]
- Lyu, J.; Xie, D.; Bhatia, T.N.; Leak, R.K.; Hu, X.; Jiang, X. Microglial/Macrophage polarization and function in brain injury and repair after stroke. CNS Neurosci. Ther. 2021, 27, 515–527. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, B.; Lu, X.X.; Liu, Y.L.; Li, M.; Xu, L.X.; Feng, C.-X.; Ding, X.; Feng, X. The role of microglia mediated pyroptosis in neonatal hypoxic-ischemic brain damage. Biochem. Biophys. Res. Commun. 2020, 521, 933–938. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Rousset, C.I.; Thornton, C. Mitochondria: Hub of injury responses in the developing brain. Lancet Neurol. 2014, 13, 217–232. [Google Scholar] [CrossRef]
- Thoresen, M.; Satas, S.; Puka-Sundvall, M.; Whitelaw, A.; Hallström, A.; Løberg, E.M.; Ungerstedt, U.; Steen, P.A.; Hagberg, H. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997, 8, 3359–3362. [Google Scholar] [CrossRef]
- Ferriero, D.M. Oxidant mechanisms in neonatal hypoxia-ischemia. Dev. Neurosci. 2001, 23, 198–202. [Google Scholar] [CrossRef]
- Gustavsson, M.; Wilson, M.A.; Mallard, C.; Rousset, C.; Johnston, M.V.; Hagberg, H. Global gene expression in the developing rat brain after hypoxic preconditioning: Involvement of apoptotic mechanisms? Pediatr. Res. 2007, 61, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Bernaudin, M.; Tang, Y.; Reilly, M.; Petit, E.; Sharp, F.R. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J. Biol. Chem. 2002, 277, 39728–39738. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.C.; Nakamura, M.C.; Hsieh, C.L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflamm. 2016, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc. Natl. Acad. Sci. USA 2005, 102, 8686–8691. [Google Scholar] [CrossRef]
- He, G.L.; Luo, Z.; Shen, T.T.; Li, P.; Yang, J.; Luo, X.; Chen, C.-H.; Gao, P.; Yang, X.-S. Inhibition of STAT3- and MAPK-dependent PGE(2) synthesis ameliorates phagocytosis of fibrillar β-amyloid peptide (1-42) via EP2 receptor in EMF-stimulated N9 microglial cells. J. Neuroinflamm. 2016, 13, 296. [Google Scholar] [CrossRef]
- Van Steenwinckel, J.; Schang, A.L.; Krishnan, M.L.; Degos, V.; Delahaye-Duriez, A.; Bokobza, C.; Csaba, Z.; Verdonk, F.; Montané, A.; Sigaut, S.; et al. Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain 2019, 142, 3806–3833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonsdotter, A.; Hagberg, H.; Leverin, A.-L.; Ek, J.; Ebefors, K.; Rocha-Ferreira, E.; Carlsson, Y. MerTK and the Role of Phagoptosis in Neonatal Hypoxia-Ischemia. Cells 2025, 14, 1862. https://doi.org/10.3390/cells14231862
Jonsdotter A, Hagberg H, Leverin A-L, Ek J, Ebefors K, Rocha-Ferreira E, Carlsson Y. MerTK and the Role of Phagoptosis in Neonatal Hypoxia-Ischemia. Cells. 2025; 14(23):1862. https://doi.org/10.3390/cells14231862
Chicago/Turabian StyleJonsdotter, Andrea, Henrik Hagberg, Anna-Lena Leverin, Joakim Ek, Kerstin Ebefors, Eridan Rocha-Ferreira, and Ylva Carlsson. 2025. "MerTK and the Role of Phagoptosis in Neonatal Hypoxia-Ischemia" Cells 14, no. 23: 1862. https://doi.org/10.3390/cells14231862
APA StyleJonsdotter, A., Hagberg, H., Leverin, A.-L., Ek, J., Ebefors, K., Rocha-Ferreira, E., & Carlsson, Y. (2025). MerTK and the Role of Phagoptosis in Neonatal Hypoxia-Ischemia. Cells, 14(23), 1862. https://doi.org/10.3390/cells14231862





