β-Cell Mitochondrial Dysfunction: Underlying Mechanisms and Potential Therapeutic Strategies
Abstract
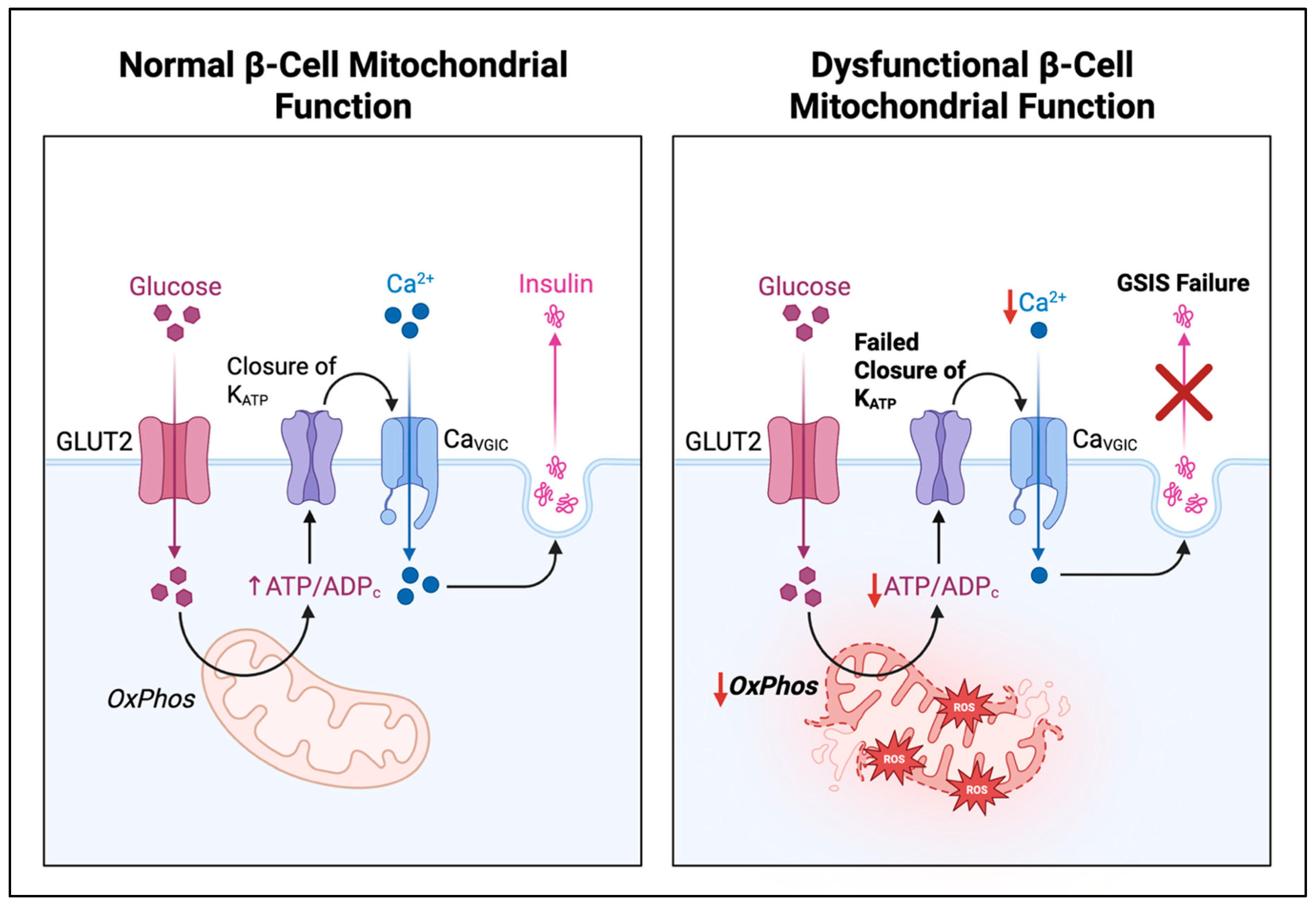
1. Introduction
2. Mitochondrial Dynamics, Quality Control, and Mitophagy
3. Genetic and Transcriptional Regulators of β-Cell Mitochondria
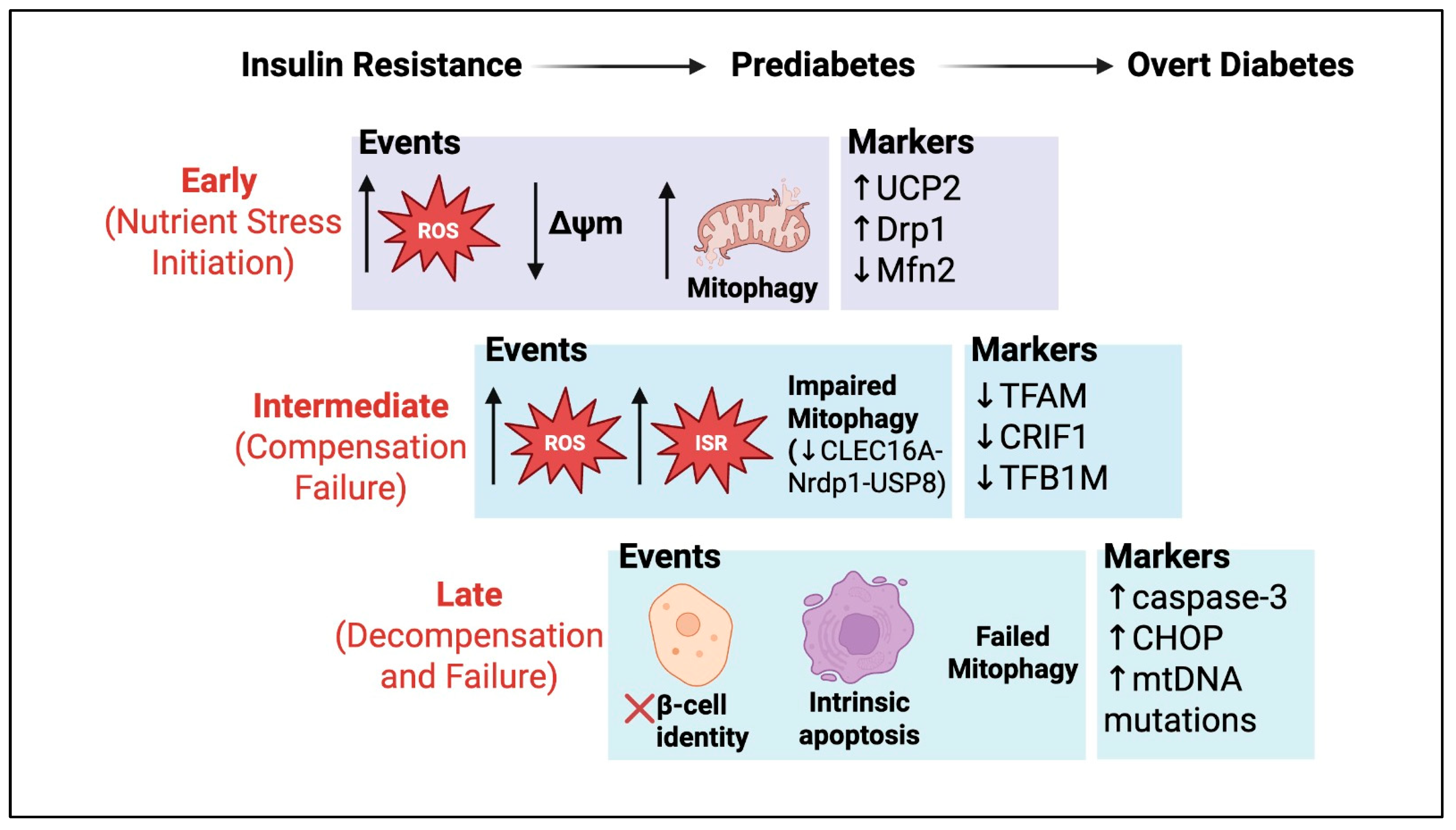
4. Oxidative Stress, Nutrient Excess, and Consequences for β-Cell Failure
5. Therapeutics
6. Future Perspectives
6.1. Mitochondrial Heterogeneity and Spatial Organization
6.2. Mitochondria-Derived Vesicles as Signaling Mediators
6.3. Rewiring the Integrated Stress Response
6.4. Precision Mitophagy Enhancement
6.5. Mitochondrial-Derived Peptides
6.6. Multi-Lineage Mitochondrial Dynamics in Intact Islets
6.7. Circadian Regulation of Mitochondrial Function
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rivera Nieves, A.M.; Wauford, B.M.; Fu, A. Mitochondrial bioenergetics, metabolism, and beyond in pancreatic beta-cells and diabetes. Front. Mol. Biosci. 2024, 11, 1354199. [Google Scholar] [CrossRef]
- Ishihara, H.; Wang, H.; Drewes, L.R.; Wollheim, C.B. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells. J. Clin. Investig. 1999, 104, 1621–1629. [Google Scholar] [CrossRef]
- Wiederkehr, A.; Park, K.S.; Dupont, O.; Demaurex, N.; Pozzan, T.; Cline, G.W.; Wollheim, C.B. Matrix alkalinization: A novel mitochondrial signal for sustained pancreatic beta-cell activation. EMBO J. 2009, 28, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Hiriart, M.; Aguilar-Bryan, L. Channel regulation of glucose sensing in the pancreatic beta-cell. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1298–E1306. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, A.; Wollheim, C.B. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium 2008, 44, 64–76. [Google Scholar] [CrossRef]
- Haataja, L.; Arunagiri, A.; Hassan, A.; Regan, K.; Tsai, B.; Dhayalan, B.; Weiss, M.A.; Liu, M.; Arvan, P. Distinct states of proinsulin misfolding in MIDY. Cell Mol. Life Sci. 2021, 78, 6017–6031. [Google Scholar] [CrossRef]
- Hudson, D.A.; Gannon, S.A.; Thorpe, C. Oxidative protein folding: From thiol-disulfide exchange reactions to the redox poise of the endoplasmic reticulum. Free Radic. Biol. Med. 2015, 80, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Rohli, K.E.; Stubbe, N.J.; Walker, E.M.; Pearson, G.L.; Soleimanpour, S.A.; Stephens, S.B. A metabolic redox relay supports ER proinsulin export in pancreatic islet beta cells. JCI Insight 2024, 9, e178725. [Google Scholar] [CrossRef]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Montemurro, C.; Vadrevu, S.; Gurlo, T.; Butler, A.E.; Vongbunyong, K.E.; Petcherski, A.; Shirihai, O.S.; Satin, L.S.; Braas, D.; Butler, P.C.; et al. Cell cycle-related metabolism and mitochondrial dynamics in a replication-competent pancreatic beta-cell line. Cell Cycle 2017, 16, 2086–2099. [Google Scholar] [CrossRef]
- Lewandowski, S.L.; Cardone, R.L.; Foster, H.R.; Ho, T.; Potapenko, E.; Poudel, C.; VanDeusen, H.R.; Sdao, S.M.; Alves, T.C.; Zhao, X.; et al. Pyruvate Kinase Controls Signal Strength in the Insulin Secretory Pathway. Cell Metab. 2020, 32, 736–750.e5. [Google Scholar] [CrossRef]
- Foster, H.R.; Ho, T.; Potapenko, E.; Sdao, S.M.; Huang, S.M.; Lewandowski, S.L.; VanDeusen, H.R.; Davidson, S.M.; Cardone, R.L.; Prentki, M.; et al. beta-cell deletion of the PKm1 and PKm2 isoforms of pyruvate kinase in mice reveals their essential role as nutrient sensors for the K(ATP) channel. eLife 2022, 11, e79422. [Google Scholar] [CrossRef]
- Ho, T.; Potapenko, E.; Davis, D.B.; Merrins, M.J. A plasma membrane-associated glycolytic metabolon is functionally coupled to K(ATP) channels in pancreatic alpha and beta cells from humans and mice. Cell Rep. 2023, 42, 112394. [Google Scholar] [CrossRef]
- Wiederkehr, A.; Wollheim, C.B. Mitochondrial signals drive insulin secretion in the pancreatic beta-cell. Mol. Cell. Endocrinol. 2012, 353, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 2012, 942, 3–37. [Google Scholar] [CrossRef] [PubMed]
- Haythorne, E.; Lloyd, M.; Walsby-Tickle, J.; Tarasov, A.I.; Sandbrink, J.; Portillo, I.; Exposito, R.T.; Sachse, G.; Cyranka, M.; Rohm, M.; et al. Altered glycolysis triggers impaired mitochondrial metabolism and mTORC1 activation in diabetic beta-cells. Nat. Commun. 2022, 13, 6754. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Dinh, H.V.; Cruz, E.R.; Chen, Z.; Bartman, C.R.; Xiao, T.; Call, C.M.; Ryseck, R.P.; Pratas, J.; Weilandt, D.; et al. Mitochondrial ATP generation is more proteome efficient than glycolysis. Nat. Chem. Biol. 2024, 20, 1123–1132. [Google Scholar] [CrossRef]
- Lobas, M.A.; Tao, R.; Nagai, J.; Kronschläger, M.T.; Borden, P.M.; Marvin, J.S.; Looger, L.L.; Khakh, B.S. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat. Commun. 2019, 10, 711. [Google Scholar] [CrossRef]
- Yoshida, T.; Kakizuka, A.; Imamura, H. BTeam, a Novel BRET-Based Biosensor for the Accurate Quantification of ATP Concentration Within Living Cells. Sci. Rep. 2016, 6, 39618. [Google Scholar] [CrossRef]
- Anastasiou, D.; Yu, Y.; Israelsen, W.J.; Jiang, J.K.; Boxer, M.B.; Hong, B.S.; Tempel, W.; Dimov, S.; Shen, M.; Jha, A.; et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012, 8, 839–847. [Google Scholar] [CrossRef]
- Lam, S.S.; Martell, J.D.; Kamer, K.J.; Deerinck, T.J.; Ellisman, M.H.; Mootha, V.K.; Ting, A.Y. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 2015, 12, 51–54. [Google Scholar] [CrossRef]
- Haythorne, E.; Rohm, M.; van de Bunt, M.; Brereton, M.F.; Tarasov, A.I.; Blacker, T.S.; Sachse, G.; Silva Dos Santos, M.; Terron Exposito, R.; Davis, S.; et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells. Nat. Commun. 2019, 10, 2474. [Google Scholar] [CrossRef]
- Bai, D.; Peng, J.; Yi, C. Advances in single-cell multi-omics profiling. RSC Chem. Biol. 2021, 2, 441–449. [Google Scholar] [CrossRef]
- Zaher, A.; Stephens, S.B. Breaking the Feedback Loop of Beta-Cell Failure: Insight into the Pancreatic Beta-Cell’s ER-Mitochondria Redox Balance. Cells 2025, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Tseng, W.W.; Chu, C.H.; Lee, Y.J.; Zhao, S.; Chang, C.; Ho, Y.P.; Wei, A.C. Metabolic regulation of mitochondrial morphologies in pancreatic beta cells: Coupling of bioenergetics and mitochondrial dynamics. Commun. Biol. 2024, 7, 1267. [Google Scholar] [CrossRef]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Gan, K.X.; Wang, C.; Chen, J.H.; Zhu, C.J.; Song, G.Y. Mitofusin-2 ameliorates high-fat diet-induced insulin resistance in liver of rats. World J. Gastroenterol. 2013, 19, 1572–1581. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Men, X.; Zhang, W.; Wang, H.; Xu, S.; Xu, M.; Xu, Y.; Yang, W.; Lou, J. Dynamin-related protein 1 is implicated in endoplasmic reticulum stress-induced pancreatic beta-cell apoptosis. Int. J. Mol. Med. 2011, 28, 161–169. [Google Scholar] [CrossRef]
- Men, X.; Wang, H.; Li, M.; Cai, H.; Xu, S.; Zhang, W.; Xu, Y.; Ye, L.; Yang, W.; Wollheim, C.B.; et al. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int. J. Biochem. Cell Biol. 2009, 41, 879–890. [Google Scholar] [CrossRef]
- Sidarala, V.; Pearson, G.L.; Parekh, V.S.; Thompson, B.; Christen, L.; Gingerich, M.A.; Zhu, J.; Stromer, T.; Ren, J.; Reck, E.C.; et al. Mitophagy protects beta cells from inflammatory damage in diabetes. JCI Insight 2020, 5, e141138. [Google Scholar] [CrossRef]
- Hennings, T.G.; Chopra, D.G.; DeLeon, E.R.; VanDeusen, H.R.; Sesaki, H.; Merrins, M.J.; Ku, G.M. In Vivo Deletion of Beta-Cell Drp1 Impairs Insulin Secretion Without Affecting Islet Oxygen Consumption. Endocrinology 2018, 159, 3245–3256. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Holmstrom, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Truban, D.; Hou, X.; Caulfield, T.R.; Fiesel, F.C.; Springer, W. PINK1, Parkin, and Mitochondrial Quality Control: What can we Learn about Parkinson’s Disease Pathobiology? J. Parkinsons Dis. 2017, 7, 13–29. [Google Scholar] [CrossRef]
- Pearson, G.; Chai, B.; Vozheiko, T.; Liu, X.; Kandarpa, M.; Piper, R.C.; Soleimanpour, S.A. Clec16a, Nrdp1, and USP8 Form a Ubiquitin-Dependent Tripartite Complex That Regulates Beta-Cell Mitophagy. Diabetes 2018, 67, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Chung, K.W.; Won Kim, J.; Kim, J.; Komatsu, M.; Tanaka, K.; Nguyen, Y.H.; Kang, T.M.; Yoon, K.H.; Kim, J.W.; et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008, 8, 318–324. [Google Scholar] [CrossRef]
- Marasco, M.R.; Linnemann, A.K. Beta-Cell Autophagy in Diabetes Pathogenesis. Endocrinology 2018, 159, 2127–2141. [Google Scholar] [CrossRef]
- Kronke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Molina, A.J.; Wikstrom, J.D.; Stiles, L.; Las, G.; Mohamed, H.; Elorza, A.; Walzer, G.; Twig, G.; Katz, S.; Corkey, B.E.; et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 2009, 58, 2303–2315. [Google Scholar] [CrossRef]
- Poitout, V.; Robertson, R.P. Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocr. Rev. 2008, 29, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.M.; Pearson, G.L.; Lawlor, N.; Stendahl, A.M.; Lietzke, A.; Sidarala, V.; Zhu, J.; Stromer, T.; Reck, E.C.; Li, J.; et al. Retrograde mitochondrial signaling governs the identity and maturity of metabolic tissues. Science 2025, 388, eadf2034. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, S.A.; Ferrari, A.M.; Raum, J.C.; Groff, D.N.; Yang, J.; Kaufman, B.A.; Stoffers, D.A. Diabetes Susceptibility Genes Pdx1 and Clec16a Function in a Pathway Regulating Mitophagy in beta-Cells. Diabetes 2015, 64, 3475–3484. [Google Scholar] [CrossRef]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef]
- Jerome, M.S.; Kuthethur, R.; Kabekkodu, S.P.; Chakrabarty, S. Regulation of mitochondrial function by forkhead transcription factors. Biochimie 2022, 198, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Wei, Z.; Lin, C.S.; Fang, S.; Ahmadian, M.; Kida, Y.; Tseng, T.; Dai, Y.; Yu, R.T.; Liddle, C.; et al. ERRgamma Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive beta Cells. Cell Metab. 2016, 23, 622–634. [Google Scholar] [CrossRef]
- Nakae, J.; Cao, Y.; Oki, M.; Orba, Y.; Sawa, H.; Kiyonari, H.; Iskandar, K.; Suga, K.; Lombes, M.; Hayashi, Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes 2008, 57, 563–576. [Google Scholar] [CrossRef]
- Tikhanovich, I.; Cox, J.; Weinman, S.A. Forkhead box class O transcription factors in liver function and disease. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 125–131. [Google Scholar] [CrossRef]
- Oropeza, D.; Jouvet, N.; Bouyakdan, K.; Perron, G.; Ringuette, L.J.; Philipson, L.H.; Kiss, R.S.; Poitout, V.; Alquier, T.; Estall, J.L. PGC-1 coactivators in beta-cells regulate lipid metabolism and are essential for insulin secretion coupled to fatty acids. Mol. Metab. 2015, 4, 811–822. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, B.R.; Wiederkehr, A.; Baquie, M.; Dai, C.; Powers, A.C.; Kerr-Conte, J.; Pattou, F.; MacDonald, R.J.; Ferrer, J.; Wollheim, C.B. PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab. 2009, 10, 110–118. [Google Scholar] [CrossRef]
- Liu, X.; Shen, S.; Wu, P.; Li, F.; Liu, X.; Wang, C.; Gong, Q.; Wu, J.; Yao, X.; Zhang, H.; et al. Structural insights into dimethylation of 12S rRNA by TFB1M: Indispensable role in translation of mitochondrial genes and mitochondrial function. Nucleic Acids Res. 2019, 47, 7648–7665. [Google Scholar] [CrossRef] [PubMed]
- Sharoyko, V.V.; Abels, M.; Sun, J.; Nicholas, L.M.; Mollet, I.G.; Stamenkovic, J.A.; Gohring, I.; Malmgren, S.; Storm, P.; Fadista, J.; et al. Loss of TFB1M results in mitochondrial dysfunction that leads to impaired insulin secretion and diabetes. Hum. Mol. Genet. 2014, 23, 5733–5749. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef] [PubMed]
- van Stijn, C.M.; Kim, J.; Lusis, A.J.; Barish, G.D.; Tangirala, R.K. Macrophage polarization phenotype regulates adiponectin receptor expression and adiponectin anti-inflammatory response. FASEB J. 2015, 29, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Koeck, T.; Olsson, A.H.; Nitert, M.D.; Sharoyko, V.V.; Ladenvall, C.; Kotova, O.; Reiling, E.; Ronn, T.; Parikh, H.; Taneera, J.; et al. A common variant in TFB1M is associated with reduced insulin secretion and increased future risk of type 2 diabetes. Cell Metab. 2011, 13, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.H.J.; Ghosh, S.; Bok, C.M.; Ching, C.; Low, B.S.J.; Chen, J.T.; Lim, E.; Miserendino, M.C.; Tan, Y.S.; Hoon, S.; et al. HNF4A and HNF1A exhibit tissue specific target gene regulation in pancreatic beta cells and hepatocytes. Nat. Commun. 2024, 15, 4288. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef]
- Pinney, S.E.; Simmons, R.A. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr. Diabetes Rep. 2012, 12, 67–74. [Google Scholar] [CrossRef]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef]
- TeSlaa, T.; Chaikovsky, A.C.; Lipchina, I.; Escobar, S.L.; Hochedlinger, K.; Huang, J.; Graeber, T.G.; Braas, D.; Teitell, M.A. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab. 2016, 24, 485–493. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Dematteo, R.G.; Venneti, S.; Finley, L.W.; Lu, C.; Judkins, A.R.; Rustenburg, A.S.; Grinaway, P.B.; Chodera, J.D.; Cross, J.R.; et al. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell Metab. 2015, 22, 304–311. [Google Scholar] [CrossRef]
- Sylvester, J.E.; Fischel-Ghodsian, N.; Mougey, E.B.; O’Brien, T.W. Mitochondrial ribosomal proteins: Candidate genes for mitochondrial disease. Genet. Med. 2004, 6, 73–80. [Google Scholar] [CrossRef]
- Hong, H.J.; Joung, K.H.; Kim, Y.K.; Choi, M.J.; Kang, S.G.; Kim, J.T.; Kang, Y.E.; Chang, J.Y.; Moon, J.H.; Jun, S.; et al. Mitoribosome insufficiency in beta cells is associated with type 2 diabetes-like islet failure. Exp. Mol. Med. 2022, 54, 932–945. [Google Scholar] [CrossRef]
- Greber, B.J.; Bieri, P.; Leibundgut, M.; Leitner, A.; Aebersold, R.; Boehringer, D.; Ban, N. The complete structure of the 55S mammalian mitochondrial ribosome. Science 2015, 348, 303–308. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwon, M.C.; Ryu, M.J.; Chung, H.K.; Tadi, S.; Kim, Y.K.; Kim, J.M.; Lee, S.H.; Park, J.H.; Kweon, G.R.; et al. CRIF1 is essential for the synthesis and insertion of oxidative phosphorylation polypeptides in the mammalian mitochondrial membrane. Cell Metab. 2012, 16, 274–283. [Google Scholar] [CrossRef]
- t Hart, L.M.; Hansen, T.; Rietveld, I.; Dekker, J.M.; Nijpels, G.; Janssen, G.M.; Arp, P.A.; Uitterlinden, A.G.; Jorgensen, T.; Borch-Johnsen, K.; et al. Evidence that the mitochondrial leucyl tRNA synthetase (LARS2) gene represents a novel type 2 diabetes susceptibility gene. Diabetes 2005, 54, 1892–1895. [Google Scholar] [CrossRef][Green Version]
- Reiling, E.; Jafar-Mohammadi, B.; van ‘t Riet, E.; Weedon, M.N.; van Vliet-Ostaptchouk, J.V.; Hansen, T.; Saxena, R.; van Haeften, T.W.; Arp, P.A.; Das, S.; et al. Genetic association analysis of LARS2 with type 2 diabetes. Diabetologia 2010, 53, 103–110. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Baffy, G.; Perret, P.; Krauss, S.; Peroni, O.; Grujic, D.; Hagen, T.; Vidal-Puig, A.J.; Boss, O.; Kim, Y.B.; et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 2001, 105, 745–755. [Google Scholar] [CrossRef]
- Bordone, L.; Motta, M.C.; Picard, F.; Robinson, A.; Jhala, U.S.; Apfeld, J.; McDonagh, T.; Lemieux, M.; McBurney, M.; Szilvasi, A.; et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006, 4, e31, Erratum in PLoS Biol. 2015, 13, e1002346. [Google Scholar] [CrossRef]
- Joseph, J.W.; Koshkin, V.; Zhang, C.Y.; Wang, J.; Lowell, B.B.; Chan, C.B.; Wheeler, M.B. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes 2002, 51, 3211–3219. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chu, W.S.; Lu, T.; Hasstedt, S.J.; Kern, P.A.; Elbein, S.C. Uncoupling protein-2 polymorphisms in type 2 diabetes, obesity, and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, A.; Ludovico, O.; Coco, A.; Di Paola, R.; Quattrone, A.; Carella, M.; Pellegrini, F.; Prudente, S.; Trischitta, V. The common -866G/A polymorphism in the promoter region of the UCP-2 gene is associated with reduced risk of type 2 diabetes in Caucasians from Italy. J. Clin. Endocrinol. Metab. 2005, 90, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Tsuno, T.; Togashi, Y.; Okuyama, T.; Sato, A.; Nishiyama, K.; Kyohara, M.; Li, J.; Fukushima, S.; Kin, T.; et al. Uncoupling protein 2 and aldolase B impact insulin release by modulating mitochondrial function and Ca2+ release from the ER. iScience 2022, 25, 104603. [Google Scholar] [CrossRef] [PubMed]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Fu, A.; Robson-Doucette, C.; Allister, E.M.; Wheeler, M.B.; Screaton, R.; Harper, M.E. Glutathionylation state of uncoupling protein-2 and the control of glucose-stimulated insulin secretion. J. Biol. Chem. 2012, 287, 39673–39685. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef]
- Diao, J.; Allister, E.M.; Koshkin, V.; Lee, S.C.; Bhattacharjee, A.; Tang, C.; Giacca, A.; Chan, C.B.; Wheeler, M.B. UCP2 is highly expressed in pancreatic alpha-cells and influences secretion and survival. Proc. Natl. Acad. Sci. USA 2008, 105, 12057–12062. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Zhang, C.Y.; Lowell, B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005, 6, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.T.; Porco, J.A., Jr.; Lowell, B.B. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006, 3, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Skorpen, F.; Egeberg, K.; Jørgensen, I.H.; Grill, V. Induction of uncoupling protein 2 mRNA in beta-cells is stimulated by oxidation of fatty acids but not by nutrient oversupply. Endocrinology 2002, 143, 1371–1377. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Ling, C.; Poulsen, P.; Carlsson, E.; Ridderstrale, M.; Almgren, P.; Wojtaszewski, J.; Beck-Nielsen, H.; Groop, L.; Vaag, A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J. Clin. Investig. 2004, 114, 1518–1526. [Google Scholar] [CrossRef]
- Ling, C.; Del Guerra, S.; Lupi, R.; Ronn, T.; Granhall, C.; Luthman, H.; Masiello, P.; Marchetti, P.; Groop, L.; Del Prato, S. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 2008, 51, 615–622. [Google Scholar] [CrossRef]
- Ek, J.; Andersen, G.; Urhammer, S.A.; Gaede, P.H.; Drivsholm, T.; Borch-Johnsen, K.; Hansen, T.; Pedersen, O. Mutation analysis of peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) and relationships of identified amino acid polymorphisms to Type II diabetes mellitus. Diabetologia 2001, 44, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Lacquemant, C.; Chikri, M.; Boutin, P.; Samson, C.; Froguel, P. No association between the G482S polymorphism of the proliferator-activated receptor-gamma coactivator-1 (PGC-1) gene and Type II diabetes in French Caucasians. Diabetologia 2002, 45, 602–603. [Google Scholar] [CrossRef]
- Muller, Y.L.; Bogardus, C.; Pedersen, O.; Baier, L. A Gly482Ser missense mutation in the peroxisome proliferator-activated receptor gamma coactivator-1 is associated with altered lipid oxidation and early insulin secretion in Pima Indians. Diabetes 2003, 52, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Oberkofler, H.; Linnemayr, V.; Weitgasser, R.; Klein, K.; Xie, M.; Iglseder, B.; Krempler, F.; Paulweber, B.; Patsch, W. Complex haplotypes of the PGC-1alpha gene are associated with carbohydrate metabolism and type 2 diabetes. Diabetes 2004, 53, 1385–1393. [Google Scholar] [CrossRef][Green Version]
- Vimaleswaran, K.S.; Radha, V.; Ghosh, S.; Majumder, P.P.; Deepa, R.; Babu, H.N.; Rao, M.R.; Mohan, V. Peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1alpha) gene polymorphisms and their relationship to Type 2 diabetes in Asian Indians. Diabet. Med. 2005, 22, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Barroso, I.; Luan, J.; Sandhu, M.S.; Franks, P.W.; Crowley, V.; Schafer, A.J.; O’Rahilly, S.; Wareham, N.J. Meta-analysis of the Gly482Ser variant in PPARGC1A in type 2 diabetes and related phenotypes. Diabetologia 2006, 49, 501–505. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Z.; Jin, F.; Zhu, X.Q.; Qu, Y.C.; Shi, X.H.; Wang, L. The Gly482Ser variant of the PPARGC1 gene is associated with Type 2 diabetes mellitus in northern Chinese, especially men. Diabet. Med. 2006, 23, 1085–1092. [Google Scholar] [CrossRef]
- Silva, J.P.; Kohler, M.; Graff, C.; Oldfors, A.; Magnuson, M.A.; Berggren, P.O.; Larsson, N.G. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat. Genet. 2000, 26, 336–340. [Google Scholar] [CrossRef]
- Nicholas, L.M.; Valtat, B.; Medina, A.; Andersson, L.; Abels, M.; Mollet, I.G.; Jain, D.; Eliasson, L.; Wierup, N.; Fex, M.; et al. Mitochondrial transcription factor B2 is essential for mitochondrial and cellular function in pancreatic beta-cells. Mol. Metab. 2017, 6, 651–663. [Google Scholar] [CrossRef]
- Finck, B.N.; Kelly, D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006, 116, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Shin, H.D.; Park, B.L.; Kim, J.H.; Park, K.S.; Kim, S.Y.; Lee, H.K. Association between polymorphisms in the nuclear respiratory factor 1 gene and type 2 diabetes mellitus in the Korean population. Diabetologia 2005, 48, 2033–2038. [Google Scholar] [CrossRef][Green Version]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T.; et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef]
- Clark, E.; Johnson, J.; Dong, Y.N.; Mercado-Ayon, E.; Warren, N.; Zhai, M.; McMillan, E.; Salovin, A.; Lin, H.; Lynch, D.R. Role of frataxin protein deficiency and metabolic dysfunction in Friedreich ataxia, an autosomal recessive mitochondrial disease. Neuronal Signal. 2018, 2, NS20180060. [Google Scholar] [CrossRef]
- Supale, S.; Thorel, F.; Merkwirth, C.; Gjinovci, A.; Herrera, P.L.; Scorrano, L.; Meda, P.; Langer, T.; Maechler, P. Loss of prohibitin induces mitochondrial damages altering beta-cell function and survival and is responsible for gradual diabetes development. Diabetes 2013, 62, 3488–3499. [Google Scholar] [CrossRef]
- Ngo, H.B.; Kaiser, J.T.; Chan, D.C. The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA. Nat. Struct. Mol. Biol. 2011, 18, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Amunts, A.; Bai, X.C.; Sugimoto, Y.; Edwards, P.C.; Murshudov, G.; Scheres, S.H.W.; Ramakrishnan, V. Structure of the large ribosomal subunit from human mitochondria. Science 2014, 346, 718–722. [Google Scholar] [CrossRef]
- Berardi, M.J.; Shih, W.M.; Harrison, S.C.; Chou, J.J. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature 2011, 476, 109–113. [Google Scholar] [CrossRef]
- Schubert, A.F.; Gladkova, C.; Pardon, E.; Wagstaff, J.L.; Freund, S.M.V.; Steyaert, J.; Maslen, S.L.; Komander, D. Structure of PINK1 in complex with its substrate ubiquitin. Nature 2017, 552, 51–56. [Google Scholar] [CrossRef]
- Gladkova, C.; Maslen, S.L.; Skehel, J.M.; Komander, D. Mechanism of parkin activation by PINK1. Nature 2018, 559, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.; Guanga, G.P.; Rose, R.B. Structural basis for induced fit mechanisms in DNA recognition by the Pdx1 homeodomain. Biochemistry 2007, 46, 2948–2957. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Herlein, J.A.; Fink, B.D.; Sivitz, W.I. Superoxide production by mitochondria of insulin-sensitive tissues: Mechanistic differences and effect of early diabetes. Metabolism 2010, 59, 247–257. [Google Scholar] [CrossRef][Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Sako, Y.; Grill, V.E. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology 1990, 127, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Elks, M.L. Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology 1993, 133, 208–214. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Grill, V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J. Clin. Endocrinol. Metab. 1995, 80, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chuang, L.M. The role of oxidative stress in the pathogenesis of type 2 diabetes: From molecular mechanism to clinical implication. Am. J. Transl. Res. 2010, 2, 316–331. [Google Scholar] [PubMed]
- Srinivasan, M.; Choi, C.S.; Ghoshal, P.; Pliss, L.; Pandya, J.D.; Hill, D.; Cline, G.; Patel, M.S. ss-Cell-specific pyruvate dehydrogenase deficiency impairs glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E910–E917. [Google Scholar] [CrossRef]
- Kaneto, H.; Xu, G.; Fujii, N.; Kim, S.; Bonner-Weir, S.; Weir, G.C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 2002, 277, 30010–30018. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Torii, S.; Saito, N.; Hosaka, M.; Takeuchi, T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology 2008, 149, 1654–1665. [Google Scholar] [CrossRef]
- Lanuza-Masdeu, J.; Arevalo, M.I.; Vila, C.; Barbera, A.; Gomis, R.; Caelles, C. In vivo JNK activation in pancreatic beta-cells leads to glucose intolerance caused by insulin resistance in pancreas. Diabetes 2013, 62, 2308–2317. [Google Scholar] [CrossRef]
- Kawamori, D.; Kajimoto, Y.; Kaneto, H.; Umayahara, Y.; Fujitani, Y.; Miyatsuka, T.; Watada, H.; Leibiger, I.B.; Yamasaki, Y.; Hori, M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 2003, 52, 2896–2904. [Google Scholar] [CrossRef]
- Humphrey, R.K.; Yu, S.M.; Flores, L.E.; Jhala, U.S. Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J. Biol. Chem. 2010, 285, 3406–3416. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, J.W.; Gao, B.; Jung, M.H. Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: Apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell. Signal. 2005, 17, 1516–1532. [Google Scholar] [CrossRef]
- Ammendrup, A.; Maillard, A.; Nielsen, K.; Aabenhus Andersen, N.; Serup, P.; Dragsbaek Madsen, O.; Mandrup-Poulsen, T.; Bonny, C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 2000, 49, 1468–1476. [Google Scholar] [CrossRef]
- Ashcroft, F.M. KATP Channels and the Metabolic Regulation of Insulin Secretion in Health and Disease: The 2022 Banting Medal for Scientific Achievement Award Lecture. Diabetes 2023, 72, 693–702. [Google Scholar] [CrossRef]
- Sha, W.; Hu, F.; Bu, S. Mitochondrial dysfunction and pancreatic islet beta-cell failure (Review). Exp. Ther. Med. 2020, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Iheagwam, F.N.; Joseph, A.J.; Adedoyin, E.D.; Iheagwam, O.T.; Ejoh, S.A. Mitochondrial Dysfunction in Diabetes: Shedding Light on a Widespread Oversight. Pathophysiology 2025, 32, 9. [Google Scholar] [CrossRef] [PubMed]
- Cree, L.M.; Patel, S.K.; Pyle, A.; Lynn, S.; Turnbull, D.M.; Chinnery, P.F.; Walker, M. Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia 2008, 51, 1440–1443. [Google Scholar] [CrossRef]
- Packer, M.A.; Murphy, M.P. Peroxynitrite formed by simultaneous nitric oxide and superoxide generation causes cyclosporin-A-sensitive mitochondrial calcium efflux and depolarisation. Eur. J. Biochem. 1995, 234, 231–239. [Google Scholar] [CrossRef]
- Suresh, V.; Reddy, A. Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impedement. J. Diabetes Metab. Disord. 2021, 20, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Sussel, L.; Davidson, H.W. Inherent Beta Cell Dysfunction Contributes to Autoimmune Susceptibility. Biomolecules 2021, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wei, Y.H. Roles of Mitochondrial Sirtuins in Mitochondrial Function, Redox Homeostasis, Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 5266. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Fang, X.; Zhang, Y.; Wei, J.; Zhang, Y.; Tian, J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: Mechanisms and therapeutic opportunities. Cell Death Dis. 2023, 14, 186. [Google Scholar] [CrossRef]
- Georgiadou, E.; Rutter, G.A. Control by Ca2+ of mitochondrial structure and function in pancreatic beta-cells. Cell Calcium 2020, 91, 102282. [Google Scholar] [CrossRef]
- Wollheim, C.B.; Maechler, P. Beta-cell mitochondria and insulin secretion: Messenger role of nucleotides and metabolites. Diabetes 2002, 51 (Suppl. S1), S37–S42. [Google Scholar] [CrossRef]
- Olsson, A.H.; Yang, B.T.; Hall, E.; Taneera, J.; Salehi, A.; Nitert, M.D.; Ling, C. Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes. Eur. J. Endocrinol. 2011, 165, 589–595. [Google Scholar] [CrossRef]
- Mulder, H. Transcribing beta-cell mitochondria in health and disease. Mol. Metab. 2017, 6, 1040–1051. [Google Scholar] [CrossRef]
- Moon, D.O. NADPH Dynamics: Linking Insulin Resistance and Beta-Cells Ferroptosis in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 25, 342. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Wutthisathapornchai, A.; Wallace, J.C.; MacDonald, M.J. Regulation of insulin secretion: Role of mitochondrial signalling. Diabetologia 2010, 53, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Bayir, H.; Kagan, V.E. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis--there is nothing more practical than a good theory. Crit. Care 2008, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Kubli, D.A.; Gustafsson, A.B. Mitochondria and mitophagy: The yin and yang of cell death control. Circ. Res. 2012, 111, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.S.; Dhawan, S. Senescence: A double-edged sword in beta-cell health and failure? Front. Endocrinol. 2023, 14, 1196460. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Liu, W.; Lu, Y.; Cheng, J.; Chen, Y. Beta cell aging and age-related diabetes. Aging 2021, 13, 7691–7706. [Google Scholar] [CrossRef]
- Lu, H.; Koshkin, V.; Allister, E.M.; Gyulkhandanyan, A.V.; Wheeler, M.B. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 2010, 59, 448–459. [Google Scholar] [CrossRef]
- Carroll, D.T.; Miller, A.; Fuhr, J.; Elsakr, J.M.; Ricciardi, V.; Del Bene, A.N.; Stephens, S.; Krystofiak, E.; Lindsley, S.R.; Kirigiti, M.; et al. Analysis of beta-cell maturity and mitochondrial morphology in juvenile non-human primates exposed to maternal Western-style diet during development. Front. Endocrinol. 2024, 15, 1417437. [Google Scholar] [CrossRef]
- Oleson, B.J.; Corbett, J.A. Dual Role of Nitric Oxide in Regulating the Response of Beta Cells to DNA Damage. Antioxid. Redox Signal. 2018, 29, 1432–1445. [Google Scholar] [CrossRef]
- Sokolova, M.; Sahraoui, A.; Hoyem, M.; Ogaard, J.; Lien, E.; Aukrust, P.; Yndestad, A.; Ranheim, T.; Scholz, H. NLRP3 inflammasome mediates oxidative stress-induced pancreatic islet dysfunction. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E912–E923. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, S.; Sun, R.; Zhang, X.; Wang, D. The NLRP3 inflammasome: Role in metabolic disorders and regulation by metabolic pathways. Cancer Lett. 2018, 419, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Robson-Doucette, C.A.; Sultan, S.; Allister, E.M.; Wikstrom, J.D.; Koshkin, V.; Bhattacharjee, A.; Prentice, K.J.; Sereda, S.B.; Shirihai, O.S.; Wheeler, M.B. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes 2011, 60, 2710–2719, Erratum in Diabetes 2011, 60, 3307. [Google Scholar] [CrossRef]
- Gansemer, E.R.; McCommis, K.S.; Martino, M.; King-McAlpin, A.Q.; Potthoff, M.J.; Finck, B.N.; Taylor, E.B.; Rutkowski, D.T. NADPH and Glutathione Redox Link TCA Cycle Activity to Endoplasmic Reticulum Homeostasis. iScience 2020, 23, 101116. [Google Scholar] [CrossRef]
- American Diabetes Association Primary Care Advisory Group; ElSayed, N.; Baig, A.; Balapattabi, K.; Bradley, S.; Capelson, R.; D’sOuza, J.; Gonzalez, J.; Haynes, A.; Kim, M.; et al. Standards of Care in Diabetes-2025 Abridged for Primary Care. Clin. Diabetes 2025, 43, 182. [Google Scholar] [CrossRef]
- de Marañón, A.M.; Díaz-Pozo, P.; Canet, F.; Díaz-Morales, N.; Abad-Jiménez, Z.; López-Domènech, S.; Vezza, T.; Apostolova, N.; Morillas, C.; Rocha, M.; et al. Metformin modulates mitochondrial function and mitophagy in peripheral blood mononuclear cells from type 2 diabetic patients. Redox Biol. 2022, 53, 102342. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef]
- Larsen, S.; Rabol, R.; Hansen, C.N.; Madsbad, S.; Helge, J.W.; Dela, F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 2012, 55, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rovira-Llopis, S.; Banuls, C.; Diaz-Morales, N.; Castello, R.; Falcon, R.; Gomez, M.; Rocha, M.; Hernandez-Mijares, A. Effects of metformin on mitochondrial function of leukocytes from polycystic ovary syndrome patients with insulin resistance. Eur. J. Endocrinol. 2015, 173, 683–691. [Google Scholar] [CrossRef]
- Sun, G.; Tarasov, A.I.; McGinty, J.; McDonald, A.; da Silva Xavier, G.; Gorman, T.; Marley, A.; French, P.M.; Parker, H.; Gribble, F.; et al. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia 2010, 53, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Park, S.H.; Sohn, J.W.; Jeon, J.H.; Park, J.H.; Song, D.K.; Lee, S.H.; Ho, W.K. Glucose deprivation regulates KATP channel trafficking via AMP-activated protein kinase in pancreatic beta-cells. Diabetes 2009, 58, 2813–2819. [Google Scholar] [CrossRef]
- Tsuboi, T.; da Silva Xavier, G.; Leclerc, I.; Rutter, G.A. 5’-AMP-activated protein kinase controls insulin-containing secretory vesicle dynamics. J. Biol. Chem. 2003, 278, 52042–52051. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.; Fogarty, S.; Leclerc, I.; Hill, E.V.; Hardie, D.G.; Rutter, G.A. Control of insulin granule dynamics by AMPK dependent KLC1 phosphorylation. Islets 2009, 1, 198–209. [Google Scholar] [CrossRef]
- Leclerc, I.; Woltersdorf, W.W.; da Silva Xavier, G.; Rowe, R.L.; Cross, S.E.; Korbutt, G.S.; Rajotte, R.V.; Smith, R.; Rutter, G.A. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: Impact on glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E1023–E1031. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chung, A.C.K.; Fan, R.; Lee, H.M.; Xu, G.; Tomlinson, B.; Chan, J.C.N.; Kong, A.P.S. Sirt3 Deficiency Increased the Vulnerability of Pancreatic Beta Cells to Oxidative Stress-Induced Dysfunction. Antioxid. Redox Signal. 2017, 27, 962–976. [Google Scholar] [CrossRef]
- Akasaka, H.; Nakagami, H.; Sugimoto, K.; Yasunobe, Y.; Minami, T.; Fujimoto, T.; Yamamoto, K.; Hara, C.; Shiraki, A.; Nishida, K.; et al. Effects of nicotinamide mononucleotide on older patients with diabetes and impaired physical performance: A prospective, placebo-controlled, double-blind study. Geriatr. Gerontol. Int. 2023, 23, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Dollerup, O.L.; Trammell, S.A.J.; Hartmann, B.; Holst, J.J.; Christensen, B.; Møller, N.; Gillum, M.P.; Treebak, J.T.; Jessen, N. Effects of Nicotinamide Riboside on Endocrine Pancreatic Function and Incretin Hormones in Nondiabetic Men with Obesity. J. Clin. Endocrinol. Metab. 2019, 104, 5703–5714. [Google Scholar] [CrossRef]
- Yi, L.; Maier, A.B.; Tao, R.; Lin, Z.; Vaidya, A.; Pendse, S.; Thasma, S.; Andhalkar, N.; Avhad, G.; Kumbhar, V. The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: A randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. Geroscience 2023, 45, 29–43. [Google Scholar] [CrossRef]
- Roos, J.; Zinngrebe, J.; Fischer-Posovszky, P. Nicotinamide mononucleotide: A potential effective natural compound against insulin resistance. Signal Transduct. Target. Ther. 2021, 6, 310. [Google Scholar] [CrossRef]
- Chang, N.; Li, J.; Lin, S.; Zhang, J.; Zeng, W.; Ma, G.; Wang, Y. Emerging roles of SIRT1 activator, SRT2104, in disease treatment. Sci. Rep. 2024, 14, 5521. [Google Scholar] [CrossRef]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Luo, M.; Xu, C.; Luo, Y.; Wang, G.; Wu, J.; Wan, Q. Circulating miR-103 family as potential biomarkers for type 2 diabetes through targeting CAV-1 and SFRP4. Acta Diabetol. 2020, 57, 309–322. [Google Scholar] [CrossRef]
- Krishnan, P.; Branco, R.C.S.; Weaver, S.A.; Chang, G.; Lee, C.C.; Syed, F.; Evans-Molina, C. miR-146a-5p mediates inflammation-induced beta cell mitochondrial dysfunction and apoptosis. J. Biol. Chem. 2024, 300, 107827. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Rackham, C.L.; Hubber, E.L.; Czajka, A.; Malik, A.N.; King, A.J.F.; Jones, P.M. Optimizing beta cell function through mesenchymal stromal cell-mediated mitochondria transfer. Stem Cells 2020, 38, 574–584. [Google Scholar] [CrossRef]
- Hubber, E.L.; Rackham, C.L.; Jones, P.M. Protecting islet functional viability using mesenchymal stromal cells. Stem Cells Transl. Med. 2021, 10, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Adachi, T.; Kin, T.; Ono, S.; Sakai, Y.; Adachi, T.; Soyama, A.; Hidaka, M.; Takatsuki, M.; Shapiro, A.M.J.; et al. An engineered cell sheet composed of human islets and human fibroblast, bone marrow-derived mesenchymal stem cells, or adipose-derived mesenchymal stem cells: An in vitro comparison study. Islets 2018, 10, e1445948. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, G.; Olivi, E.; Bianchi, F.; Neri, F.; Foroni, L.; Valente, S.; La Manna, G.; Nardo, B.; Stefoni, S.; Ventura, C. Mesenchymal stem cells and islet cotransplantation in diabetic rats: Improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant. 2012, 21, 2771–2781. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef]
- Lee, H.J.; Chae, C.W.; Han, H.J. Enhancing the therapeutic efficacy of mesenchymal stem cell transplantation in diabetes: Amelioration of mitochondrial dysfunction-induced senescence. Biomed. Pharmacother. 2023, 168, 115759. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Sadr Hashemi Nejad, A.; Moazenchi, M.; Masoumi, S.; Rabbani, A.; Kompani, F.; Hedayati Asl, A.A.; Abbasi Kakroodi, F.; Jaroughi, N.; Mohseni Meybodi, M.A.; et al. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: A phase I/II randomized placebo-controlled clinical trial. Stem Cell Res. Ther. 2022, 13, 264. [Google Scholar] [CrossRef]
- Yaochite, J.N.; de Lima, K.W.; Caliari-Oliveira, C.; Palma, P.V.; Couri, C.E.; Simões, B.P.; Covas, D.T.; Voltarelli, J.C.; Oliveira, M.C.; Donadi, E.A.; et al. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res. Ther. 2016, 7, 14. [Google Scholar] [CrossRef]
- Yaochite, J.N.; Caliari-Oliveira, C.; de Souza, L.E.; Neto, L.S.; Palma, P.V.; Covas, D.T.; Malmegrim, K.C.; Voltarelli, J.C.; Donadi, E.A. Therapeutic efficacy and biodistribution of allogeneic mesenchymal stem cells delivered by intrasplenic and intrapancreatic routes in streptozotocin-induced diabetic mice. Stem Cell Res. Ther. 2015, 6, 31. [Google Scholar] [CrossRef]
- Tsuboi, T.; da Silva Xavier, G.; Holz, G.G.; Jouaville, L.S.; Thomas, A.P.; Rutter, G.A. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem. J. 2003, 369, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.Y.; Oh, T.J.; Cho, Y.M. Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells. Endocrinol. Metab. 2015, 30, 216–220. [Google Scholar] [CrossRef]
- Yusta, B.; Baggio, L.L.; Estall, J.L.; Koehler, J.A.; Holland, D.P.; Li, H.; Pipeleers, D.; Ling, Z.; Drucker, D.J. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006, 4, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, D.; Vander Mierde, D.; Song, B.; Flamez, D.; Creemers, J.W.; Tsukamoto, K.; Ribick, M.; Schuit, F.C.; Kaufman, R.J. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat. Med. 2005, 11, 757–764. [Google Scholar] [CrossRef]
- Petcherski, A.; Trudeau, K.M.; Wolf, D.M.; Segawa, M.; Lee, J.; Taddeo, E.P.; Deeney, J.T.; Liesa, M. Elamipretide Promotes Mitophagosome Formation and Prevents Its Reduction Induced by Nutrient Excess in INS1 beta-cells. J. Mol. Biol. 2018, 430, 4823–4833. [Google Scholar] [CrossRef]
- Cerqueira, F.M.; Kozer, N.; Petcherski, A.; Baranovski, B.M.; Wolf, D.; Assali, E.A.; Roth, Y.; Gazit, R.; Barr, H.; Lewis, E.C.; et al. MitoTimer-based high-content screen identifies two chemically-related benzothiophene derivatives that enhance basal mitophagy. Biochem. J. 2020, 477, 461–475. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Felix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.F.; Toth, R.; James, J.; Ganley, I.G. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 2013, 14, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zhang, Y.; Zeng, X.; Shulman, G.I.; Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014, 20, 1263–1269. [Google Scholar] [CrossRef]
- Tai, Y.; Li, L.; Peng, X.; Zhu, J.; Mao, X.; Qin, N.; Ma, M.; Huo, R.; Bai, Y.; Dong, D. Mitochondrial uncoupler BAM15 inhibits artery constriction and potently activates AMPK in vascular smooth muscle cells. Acta Pharm. Sin. B 2018, 8, 909–918. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Harbauer, A.B.; Schneider, A.; Wohlleber, D. Analysis of Mitochondria by Single-Organelle Resolution. Annu. Rev. Anal. Chem. 2022, 15, 1–16. [Google Scholar] [CrossRef]
- Schneider, A.; Kurz, S.; Manske, K.; Janas, M.; Heikenwalder, M.; Misgeld, T.; Aichler, M.; Weissmann, S.F.; Zischka, H.; Knolle, P.; et al. Single organelle analysis to characterize mitochondrial function and crosstalk during viral infection. Sci. Rep. 2019, 9, 8492. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhu, S.; Tian, Y.; Zhang, W.; Wang, S.; Chen, C.; Wu, L.; Yan, X. Label-Free Analysis of Single Viruses with a Resolution Comparable to That of Electron Microscopy and the Throughput of Flow Cytometry. Angew. Chem. Int. Ed. Engl. 2016, 55, 10239–10243. [Google Scholar] [CrossRef]
- Degtyarev, M.; Reichelt, M.; Lin, K. Novel quantitative autophagy analysis by organelle flow cytometry after cell sonication. PLoS ONE 2014, 9, e87707. [Google Scholar] [CrossRef]
- Wang, Z.; Gurlo, T.; Satin, L.S.; Fraser, S.E.; Butler, P.C. Subcellular Compartmentalization of Glucose Mediated Insulin Secretion. Cells 2025, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Ichim, G.; Lopez, J.; Ahmed, S.U.; Muthalagu, N.; Giampazolias, E.; Delgado, M.E.; Haller, M.; Riley, J.S.; Mason, S.M.; Athineos, D.; et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 2015, 57, 860–872. [Google Scholar] [CrossRef]
- Goldstein, J.C.; Waterhouse, N.J.; Juin, P.; Evan, G.I.; Green, D.R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2000, 2, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Parsons, M.J.; Llambi, F.; Bouchier-Hayes, L.; Connell, S.; Munoz-Pinedo, C.; Green, D.R. Resistance to caspase-independent cell death requires persistence of intact mitochondria. Dev. Cell 2010, 18, 802–813. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Picard, M.; Taivassalo, T.; Ritchie, D.; Wright, K.J.; Thomas, M.M.; Romestaing, C.; Hepple, R.T. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS ONE 2011, 6, e18317. [Google Scholar] [CrossRef]
- Schneider, J.R.; Evans, C.S. Fluorescent pulse-chase labeling to monitor long-term mitochondrial degradation in primary hippocampal neurons. STAR Protoc. 2022, 3, 101822. [Google Scholar] [CrossRef] [PubMed]
- Csordas, G.; Weaver, D.; Hajnoczky, G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, J.; Zhang, J.; Li, P.; Bai, H.; Fang, B.; Fang, H.; Huang, K.; Wang, G.; Nowell, C.J.; et al. Mitochondrial segmentation and function prediction in live-cell images with deep learning. Nat. Commun. 2025, 16, 743. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Liu, Y.; Li, Y.; Li, D.; Mei, Z.; Deng, Y. Mitochondrial quality control in diabetes mellitus and complications: Molecular mechanisms and therapeutic strategies. Cell Death Dis. 2025, 16, 652. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.; Tanaka, A.; Suen, D.F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Suen, D.F.; Narendra, D.P.; Tanaka, A.; Manfredi, G.; Youle, R.J. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11835–11840. [Google Scholar] [CrossRef]
- Gao, Y.; Mi, N.; Wu, W.; Zhao, Y.; Fan, F.; Liao, W.; Ming, Y.; Guan, W.; Bai, C. Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis. J. Extracell. Vesicles 2024, 13, e12410. [Google Scholar] [CrossRef]
- Quiros, P.M.; Prado, M.A.; Zamboni, N.; D’Amico, D.; Williams, R.W.; Finley, D.; Gygi, S.P.; Auwerx, J. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol. 2017, 216, 2027–2045. [Google Scholar] [CrossRef]
- Shan, J.; Fu, L.; Balasubramanian, M.N.; Anthony, T.; Kilberg, M.S. ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. J. Biol. Chem. 2012, 287, 36393–36403. [Google Scholar] [CrossRef]
- Halliday, M.; Radford, H.; Sekine, Y.; Moreno, J.; Verity, N.; le Quesne, J.; Ortori, C.A.; Barrett, D.A.; Fromont, C.; Fischer, P.M.; et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 2015, 6, e1672. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.L.; Buckner, N.; Fernandez-Albert, F.; Pedone, E.; Postiglione, L.; Shi, G.; Allen, N.; Wong, L.F.; Magini, L.; Marucci, L.; et al. A dual druggable genome-wide siRNA and compound library screening approach identifies modulators of parkin recruitment to mitochondria. J. Biol. Chem. 2020, 295, 3285–3300, Erratum in J. Biol. Chem. 2020, 295, 5835. [Google Scholar] [CrossRef] [PubMed]
- Bekes, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Baker, Z.N.; Forny, P.; Pagliarini, D.J. Mitochondrial proteome research: The road ahead. Nat. Rev. Mol. Cell Biol. 2024, 25, 65–82. [Google Scholar] [CrossRef]
- Cobb, L.J.; Lee, C.; Xiao, J.; Yen, K.; Wong, R.G.; Nakamura, H.K.; Mehta, H.H.; Gao, Q.; Ashur, C.; Huffman, D.M.; et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging 2016, 8, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhai, D.; Cabezas, E.; Welsh, K.; Nouraini, S.; Satterthwait, A.C.; Reed, J.C. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 2003, 423, 456–461. [Google Scholar] [CrossRef]
- Muzumdar, R.H.; Huffman, D.M.; Atzmon, G.; Buettner, C.; Cobb, L.J.; Fishman, S.; Budagov, T.; Cui, L.; Einstein, F.H.; Poduval, A.; et al. Humanin: A novel central regulator of peripheral insulin action. PLoS ONE 2009, 4, e6334. [Google Scholar] [CrossRef]
- Hoang, P.T.; Park, P.; Cobb, L.J.; Paharkova-Vatchkova, V.; Hakimi, M.; Cohen, P.; Lee, K.W. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism 2010, 59, 343–349. [Google Scholar] [CrossRef]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef]
- Kim, K.H.; Son, J.M.; Benayoun, B.A.; Lee, C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metab. 2018, 28, 516–524.e7. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Bettahi, I.; Jerobin, J.; Chandra, P.; Abi Khalil, C.; Skarulis, M.; Atkin, S.L.; Abou-Samra, A.B. Mitochondrial-Derived Peptides Are Down Regulated in Diabetes Subjects. Front. Endocrinol. 2019, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.T.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 2021, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Mehta, H.H.; Wan, J.; Kuehnemann, C.; Chen, J.; Hu, J.F.; Hoffman, A.R.; Cohen, P. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging 2018, 10, 1239–1256. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Chiariello, A.; Franceschi, C.; Salvioli, S. Mitochondria, immunosenescence and inflammaging: A role for mitokines? Semin. Immunopathol. 2020, 42, 607–617. [Google Scholar] [CrossRef]
- Steiner, D.J.; Kim, A.; Miller, K.; Hara, M. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2010, 2, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Roscioni, S.S.; Migliorini, A.; Gegg, M.; Lickert, H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 2016, 12, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Brissova, M.; Haliyur, R.; Saunders, D.; Shrestha, S.; Dai, C.; Blodgett, D.M.; Bottino, R.; Campbell-Thompson, M.; Aramandla, R.; Poffenberger, G.; et al. α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Cell Rep. 2018, 22, 2667–2676. [Google Scholar] [CrossRef]
- Panzer, J.K.; Tamayo, A.; Caicedo, A. Restoring glutamate receptor signaling in pancreatic alpha cells rescues glucagon responses in type 1 diabetes. Cell Rep. 2022, 41, 111792. [Google Scholar] [CrossRef]
- Quoix, N.; Cheng-Xue, R.; Mattart, L.; Zeinoun, Z.; Guiot, Y.; Beauvois, M.C.; Henquin, J.C.; Gilon, P. Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse alpha-cells. Diabetes 2009, 58, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Briant, L.J.B.; Reinbothe, T.M.; Spiliotis, I.; Miranda, C.; Rodriguez, B.; Rorsman, P. δ-cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. J. Physiol. 2018, 596, 197–215. [Google Scholar] [CrossRef]
- Hauge-Evans, A.C.; King, A.J.; Carmignac, D.; Richardson, C.C.; Robinson, I.C.; Low, M.J.; Christie, M.R.; Persaud, S.J.; Jones, P.M. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 2009, 58, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Arrojo, E.D.R.; Jacob, S.; García-Prieto, C.F.; Zheng, X.; Fukuda, M.; Nhu, H.T.T.; Stelmashenko, O.; Peçanha, F.L.M.; Rodriguez-Diaz, R.; Bushong, E.; et al. Structural basis for delta cell paracrine regulation in pancreatic islets. Nat. Commun. 2019, 10, 3700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gerdes, H.H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015, 22, 1181–1191. [Google Scholar] [CrossRef]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Giesen, C.; Wang, H.A.; Schapiro, D.; Zivanovic, N.; Jacobs, A.; Hattendorf, B.; Schüffler, P.J.; Grolimund, D.; Buhmann, J.M.; Brandt, S.; et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 2014, 11, 417–422. [Google Scholar] [CrossRef]
- Damond, N.; Engler, S.; Zanotelli, V.R.T.; Schapiro, D.; Wasserfall, C.H.; Kusmartseva, I.; Nick, H.S.; Thorel, F.; Herrera, P.L.; Atkinson, M.A.; et al. A Map of Human Type 1 Diabetes Progression by Imaging Mass Cytometry. Cell Metab. 2019, 29, 755–768.e5. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.; Pachter, L. Museum of spatial transcriptomics. Nat. Methods 2022, 19, 534–546. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Bambah-Mukku, D.; Eichhorn, S.W.; Vaughn, E.; Shekhar, K.; Perez, J.D.; Rubinstein, N.D.; Hao, J.; Regev, A.; Dulac, C.; et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018, 362, eaau5324. [Google Scholar] [CrossRef]
- Richardson, D.S.; Lichtman, J.W. Clarifying Tissue Clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef]
- Susaki, E.A.; Shimizu, C.; Kuno, A.; Tainaka, K.; Li, X.; Nishi, K.; Morishima, K.; Ono, H.; Ode, K.L.; Saeki, Y.; et al. Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues. Nat. Commun. 2020, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wu, H.; Xu, W. Deletion of Bmal1 Impairs Pancreatic beta-Cell Function via Mitochondrial Signaling Pathway. Biomed. Res. Int. 2020, 2020, 9803024. [Google Scholar] [CrossRef] [PubMed]
- Cortassa, S.; Aon, M.A.; Winslow, R.L.; O’Rourke, B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys. J. 2004, 87, 2060–2073. [Google Scholar] [CrossRef]
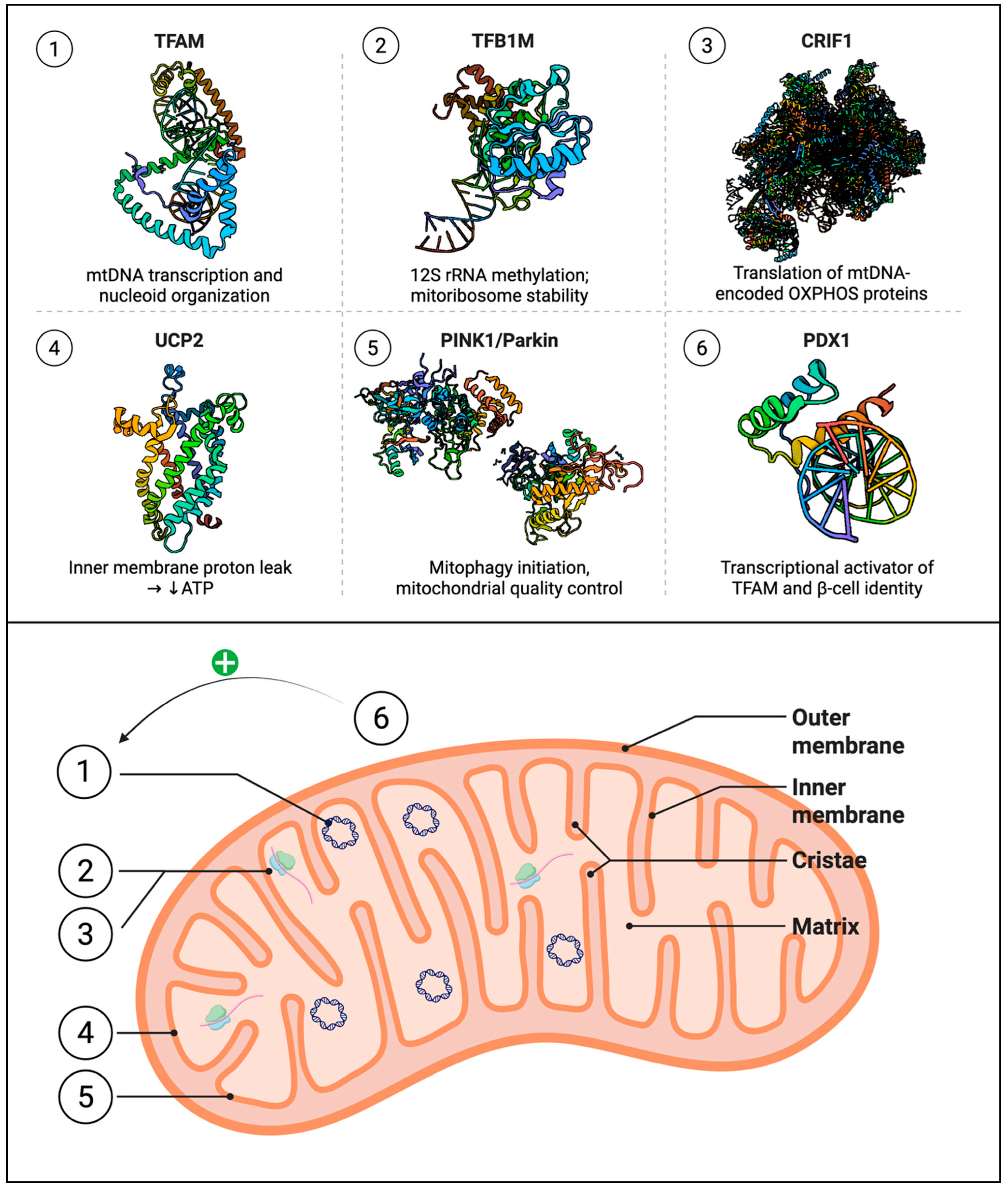
| Gene Symbol | Function | Relevance to β-Cells/T2D | Reference |
|---|---|---|---|
| TFAM | Initiates and regulates mtDNA transcription | β-cell-specific knockout impairs insulin secretion and induces diabetes | [99] |
| TFB1M | Methylates mitochondrial 12S rRNA to stabilize ribosomes | Risk allele (rs950994) linked to reduced insulin secretion and mitochondrial dysfunction | [59] |
| TFB2M | Regulates transcription of mtDNA | Knockout causes reduced mtDNA, impaired ATP production, β-cell apoptosis | [100] |
| PGC-1α | Coactivates NRF1/2 for mitochondrial biogenesis | Overexpression suppresses insulin secretion; polymorphisms linked to β-cell dysfunction | [54,101] |
| NRF1 | Regulates respiratory chain genes and TFAM | Polymorphisms associated with T2DM in Korean population | [102] |
| PDX1 | Maintains β-cell identity and insulin expression | Controls mitophagy; reduced in TFB2M-deficient β-cells | [103] |
| LARS2 | Catalyzes tRNA leucylation for mitochondrial translation | Genetic variation associated with T2DM susceptibility | [73] |
| UCP2 | Modulates mitochondrial coupling and ATP production | Overexpression impairs insulin secretion; mildly elevated in T2DM islets | [80] |
| FXN | Facilitates iron-sulfur cluster assembly | Deficiency leads to β-cell apoptosis and mitochondrial ROS buildup | [104] |
| PHB2 | Maintains mitochondrial cristae integrity | Knockdown impairs GSIS and causes β-cell loss | [105] |
| Process/Factor | Dysfunction in Diabetes | Consequences for β-Cell | Example/Ref. |
|---|---|---|---|
| mtDNA maintenance (TFAM) | Downregulation or knockout of TFAM, mtDNA depletion | Collapse of OXPHOS, failed ATP production, impaired Ca2+ signalling, reduced GSIS; progressive β-cell loss | Tfam β-cell KO mice [57,99] |
| Mitoribosomes (CRIF1, TFB1M) | Reduced expression or mutation of CRIF1 (MRPL59) or TFB1M in β-cells | Impaired mitochondrial translation, lower ATP/O2 consumption, reduced first-phase insulin release; β-cell failure under stress | Crif1β+/− mice [70]; Tfb1mβ−/− mice [59] |
| Mitophagy (PINK1/Parkin, CLEC16A) | Impaired ubiquitin signalling (e.g., CLEC16A deficiency) or mitophagy blockade | Accumulation of damaged mitochondria, decreased respiration, increased apoptosis [33] | CLEC16A-NRDP1-USP8 complex inactivation [40] |
| Oxidative phosphorylation (respiratory chain) | Inhibition by ROS or nutrient stress (e.g., UCP2 upregulation, calcium overload) | Reduced ATP synthesis, depolarized mitochondria, blunted insulin secretion; eventual β-cell apoptosis [99] | UCP2 upregulation, ROS damage [75,83,152] |
| ER–mitochondria coupling | Chronic ER stress, disrupted ER Ca2+ handling | NADPH imbalance, disrupted protein folding, feed-forward mitochondrial damage; impaired insulin biosynthesis | ER stress in T2DM β-cells [8,153] |
| Strategy/Agent | Mechanism | Evidence/Effect | Stage of Therapeutic Approach | References |
|---|---|---|---|---|
| Metformin | Activates AMPK, enhances mitophagy, reduces mitochondrial ROS | Improves mitochondrial fitness and insulin secretion; increased mitophagy markers | In vitro | [160,161,162] |
| GLP-1 receptor agonists | Enhance cAMP/PKA signalling; upregulate biogenesis and survival | Promote β-cell proliferation/survival and possibly mitochondrial biogenesis | In vitro | [184,185] |
| Gene therapy (TFAM, TFB1M) | Restore expression of mitochondrial transcription factors | TFAM re-expression rescues insulin secretion in PDX1-deficient islets | In vitro and in vivo | [57] |
| Mitophagy activators | Stimulate PINK1-Parkin pathway or CLEC16A complex | Increased clearance of damaged mitochondria; improves β-cell survival (preclinical) | In vitro and in vivo | [33] |
| Lifestyle (diet, exercise) | Reduce metabolic stress; induce biogenesis | Improves whole-body insulin sensitivity; may enhance β-cell mitochondrial function indirectly (via lower glucose/weight) | Clinical guideline | [194] |
| Focus Area | Key Knowledge Gap/Question | Emerging Tools & Approaches | Proposed Future Directions/Therapeutic Potential |
|---|---|---|---|
| Mitochondrial Heterogeneity | Current models treat β-cell mitochondria as uniform, despite evidence of structural and functional diversity. | High-sensitivity flow cytometry, spectral analyzers, organelle-targeted fluorescent reporters, live-cell imaging, mito-targeted dyes (TMRE, Rhodamine 123), deep learning image analysis. | Characterize mitochondrial subpopulations linked to insulin secretion phases. Build β-cell mitochondrial atlases integrating imaging, transcriptomics, and metabolomics. Identify functionally resilient mitochondrial subtypes resistant to metabolic stress. |
| Organelle Communication via Mitochondria-Derived Vesicles (MDVs) | Role of MDVs in β-cells is virtually unexplored. | MDV formation and secretion assays, extracellular vesicle profiling. | Define whether MDVs act as adaptive or pathological signals. Explore MDVs as biomarkers of mitochondrial stress or therapeutic targets. Assess MDVs as non-invasive indicators of β-cell mitochondrial health. |
| Integrated Stress Response (ISR) Modulation | Unclear how to selectively shift ISR from maladaptive to adaptive signaling in β-cells. | Single-cell multiomics, ISR modulators (e.g., ATF4 inducers, ISRIB). | Dissect ISR branch-specific effects (adaptive vs. apoptotic).—Develop ISR-targeted therapies preserving β-cell identity and stress tolerance. |
| Precision Mitophagy Enhancement | Current mitophagy activators lack selectivity between healthy and damaged mitochondria. | Synthetic Parkin recruiters, PROTAC-like mitophagy tags, voltage-sensitive degradation sensors. | Design mitochondria-specific mitophagy enhancers that act only under defined redox/metabolic states. Aim for precision mitochondrial pruning to maintain functional networks. |
| Mitochondria-Derived Peptides (MDPs) | Poorly characterized in β-cells despite known roles in other tissues. | Ribosome profiling, mass spectrometry-based sORF discovery, CRISPR functional screens. | Annotate β-cell mitoproteome to identify novel MDPs. Explore MDPs as regulators of insulin secretion, oxidative stress, and UPR. Develop MDP-based therapeutic peptides. |
| Multi-Lineage Mitochondrial Interactions in Islets | Focus has been primarily on β-cells, neglecting α- and δ-cell mitochondrial adaptations. | Spatial transcriptomics, imaging mass cytometry, tissue clearing with mitochondrial dyes. | Map mitochondrial dynamics across all islet cell types. Investigate paracrine mitochondrial resilience mechanisms. |
| Circadian Regulation of Mitochondrial Function | Temporal control of mitochondrial dynamics in β-cells is understudied. | Mitochondria-on-a-chip systems, circadian models, chrono-metabolic assays. | Examine mitochondrial behavior across circadian cycles. Assess impact of circadian disruption on β-cell mitochondrial health. Explore chronotherapy and time-restricted feeding to restore function. |
| Integrative Framework for β-Cell Mitochondrial Research | Lack of unified view connecting mitochondrial quality, signaling, and turnover to β-cell health. | Single-organelle and spatial imaging integrated with omics data. | Combine mechanistic and therapeutic studies. Test interventions targeting mitochondrial quality control and signaling to preserve β-cell function in diabetes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, R.; Alcibahy, Y.; Abu-Sharia, G.; Butler, A.E. β-Cell Mitochondrial Dysfunction: Underlying Mechanisms and Potential Therapeutic Strategies. Cells 2025, 14, 1861. https://doi.org/10.3390/cells14231861
Darwish R, Alcibahy Y, Abu-Sharia G, Butler AE. β-Cell Mitochondrial Dysfunction: Underlying Mechanisms and Potential Therapeutic Strategies. Cells. 2025; 14(23):1861. https://doi.org/10.3390/cells14231861
Chicago/Turabian StyleDarwish, Radwan, Yasmine Alcibahy, Ghena Abu-Sharia, and Alexandra E. Butler. 2025. "β-Cell Mitochondrial Dysfunction: Underlying Mechanisms and Potential Therapeutic Strategies" Cells 14, no. 23: 1861. https://doi.org/10.3390/cells14231861
APA StyleDarwish, R., Alcibahy, Y., Abu-Sharia, G., & Butler, A. E. (2025). β-Cell Mitochondrial Dysfunction: Underlying Mechanisms and Potential Therapeutic Strategies. Cells, 14(23), 1861. https://doi.org/10.3390/cells14231861







