The KIF18A Inhibitor ATX020 Induces Mitotic Arrest and DNA Damage in Chromosomally Instable High-Grade Serous Ovarian Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Murine Models of HGSOC
2.3. Cell Growth Assays
2.4. Clonogenic Assays
2.5. Flow Cytometry
2.6. DepMap Analysis
2.7. Mitotic Arrest Assays
2.8. Matrigel Invasion Assays
2.9. Immunoblotting
2.10. Immunofluorescence Microscopy
2.11. Quantification and Statistical Analysis
3. Results
3.1. High Ploidy and as Are Associated with Sensitivity to KIF18A Inhibition in HGSOC
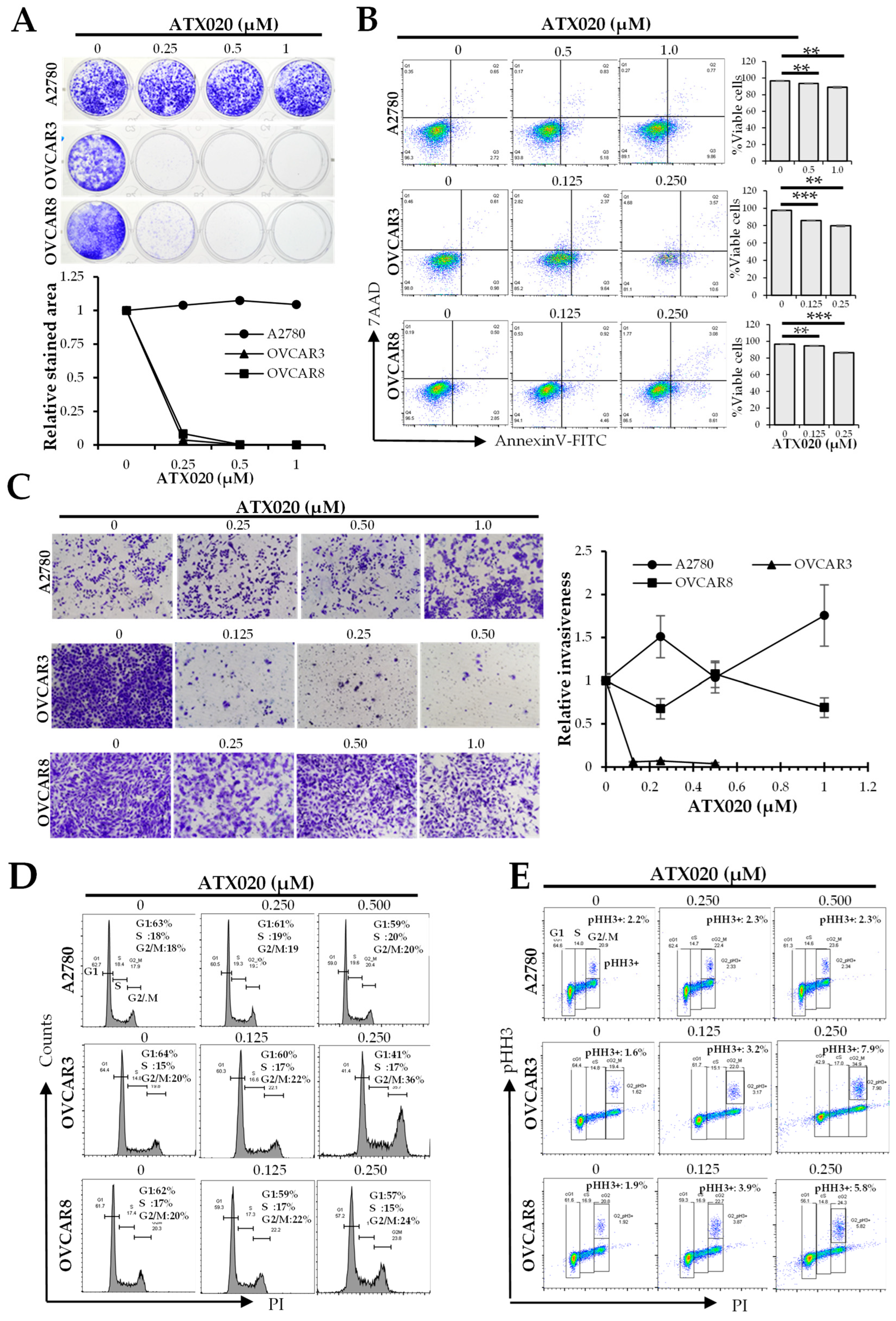
3.2. Growth Inhibition by ATX020 Was Primarily Caused by Its Effect on Tumorigenicity and Proliferation
3.3. Effect of ATX020 on KIF18A Motor Function
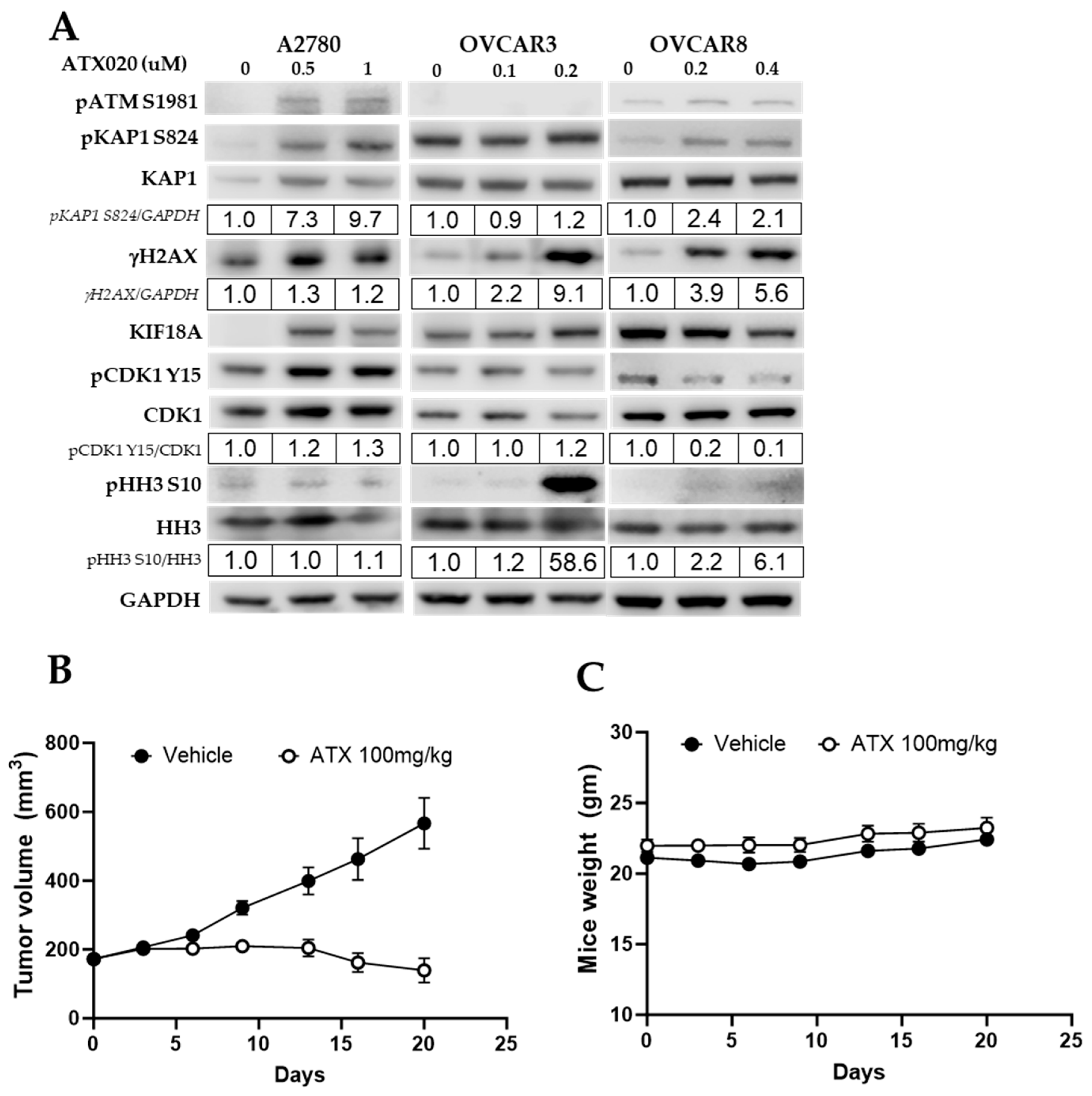
3.4. Crosstalk Between KIF18A Regulators and the DNA Damage Response
3.5. In Vivo Activity of ATX020 in HGSOC Murine Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HGSOC | High-grade serous ovarian cancer |
| CIN | Chromosomally instable |
| AS | Aneuploidy score |
| KIF18A | Kinesin Family Member 18A |
| DDR | DNA damage repair |
| PARP | Poly(ADP-Ribose) Polymerase |
| FIGO | International Federation of Gynecology and Obstetrics |
| NCI | National Cancer Institute |
| NIH | National Institutes of Health |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| FI | Fluorescence intensity |
| DSB | Double-strand break |
| DNA | Deoxyribo nucleic acid |
| XTT | 2,3-Bis-(2-Methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide |
| RNase | Ribonuclease |
| FBS | Fetal bovine serum |
| DAPI | 4′,6-diamidino-2-phenylindole |
| GOF | Gain of function |
| LOF | Loss of function |
References
- Kurman, R.J.; Shih Ie, M. Pathogenesis of ovarian cancer: Lessons from morphology and molecular biology and their clinical implications. Int. J. Gynecol. Pathol. 2008, 27, 151–160. [Google Scholar] [CrossRef]
- King, M.C.; Marks, J.H.; Mandell, J.B.; New York Breast Cancer Study, G. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef]
- Brill, E.; Yokoyama, T.; Nair, J.; Yu, M.; Ahn, Y.R.; Lee, J.M. Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer. Oncotarget 2017, 8, 111026–111040. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Lee, J.M.; Ledermann, J.A.; Kohn, E.C. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann. Oncol. 2014, 25, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Schiewek, J.; Schumacher, U.; Lange, T.; Joosse, S.A.; Wikman, H.; Pantel, K.; Mikhaylova, M.; Kneussel, M.; Linder, S.; Schmalfeldt, B.; et al. Clinical relevance of cytoskeleton associated proteins for ovarian cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2195–2205. [Google Scholar] [CrossRef]
- Mayr, M.I.; Hummer, S.; Bormann, J.; Gruner, T.; Adio, S.; Woehlke, G.; Mayer, T.U. The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Curr. Biol. 2007, 17, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.N.; Ems-McClung, S.C.; Stout, J.R.; LeBlanc, C.; Shaw, S.L.; Gardner, M.K.; Walczak, C.E. Kif18A uses a microtubule binding site in the tail for plus-end localization and spindle length regulation. Curr. Biol. 2011, 21, 1500–1506. [Google Scholar] [CrossRef]
- Liu, G.; Cai, G.; He, X.; Huang, D.; Zhu, G.; Chen, C.; Zhang, X. KIF18A promotes head and neck squamous cell carcinoma invasion and migration via activation of Akt signaling pathway. Transl. Cancer. Res. 2019, 8, 2252–2263. [Google Scholar] [CrossRef]
- Qian, L.X.; Cao, X.; Du, M.Y.; Ma, C.X.; Zhu, H.M.; Peng, Y.; Hu, X.Y.; He, X.; Yin, L. KIF18A knockdown reduces proliferation, migration, invasion and enhances radiosensitivity of esophageal cancer. Biochem. Biophys. Res. Commun. 2021, 557, 192–198. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, C.; Chen, H.; Li, L.; Guo, L.; Jiang, W.; Lu, S.H. Kif18A is involved in human breast carcinogenesis. Carcinogenesis 2010, 31, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, T.; Zhang, Z.; Li, Y.; Pan, Z. Expression of KIF18A Is Associated with Increased Tumor Stage and Cell Proliferation in Prostate Cancer. Med. Sci. Monit. 2019, 25, 6418–6428. [Google Scholar] [CrossRef] [PubMed]
- Geigl, J.B.; Obenauf, A.C.; Schwarzbraun, T.; Speicher, M.R. Defining ‘chromosomal instability’. Trends. Genet. 2008, 24, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic instabilities in human cancers. Nature 1998, 396, 643–649. [Google Scholar] [CrossRef]
- Fukasawa, K. Oncogenes and tumour suppressors take on centrosomes. Nat. reviews. Cancer 2007, 7, 911–924. [Google Scholar] [CrossRef]
- Payton, M.; Belmontes, B.; Hanestad, K.; Moriguchi, J.; Chen, K.; McCarter, J.D.; Chung, G.; Ninniri, M.S.; Sun, J.; Manoukian, R.; et al. Small-molecule inhibition of kinesin KIF18A reveals a mitotic vulnerability enriched in chromosomally unstable cancers. Nat. Cancer 2024, 5, 66–84. [Google Scholar] [CrossRef]
- Morden, C.R.; Farrell, A.C.; Sliwowski, M.; Lichtensztejn, Z.; Altman, A.D.; Nachtigal, M.W.; McManus, K.J. Chromosome instability is prevalent and dynamic in high-grade serous ovarian cancer patient samples. Gynecol. Oncol. 2021, 161, 769–778. [Google Scholar] [CrossRef]
- Marquis, C.; Fonseca, C.L.; Queen, K.A.; Wood, L.; Vandal, S.E.; Malaby, H.L.H.; Clayton, J.E.; Stumpff, J. Chromosomally unstable tumor cells specifically require KIF18A for proliferation. Nat. Commun. 2021, 12, 1213. [Google Scholar] [CrossRef]
- Sparling, B.A.; Lee, H.; Zablocki, M.-M.; Lynes, M.M.; Grigoriu, S.; Shehaj, L.; Lockbaum, G.J.; Khan, S.K.; Hotz, T.; Lee, Y.-T.; et al. Discovery of Kinesin KIF18A Inhibitor ATX020: Tactical Application of Silicon Atom Replacement. ACS Med. Chem. Lett. 2025. [Google Scholar] [CrossRef]
- Adams, K.M.; Wendt, J.-R.; Wood, J.; Olson, S.; Moreno, R.; Jin, Z.; Gopalan, S.; Lang, J.D. Cell-intrinsic platinum response and associated genetic and gene expression signatures in ovarian cancer. Cancer Gene Ther. 2025, 32, 985–996. [Google Scholar] [CrossRef]
- Behrens, B.C.; Hamilton, T.C.; Masuda, H.; Grotzinger, K.R.; Whang-Peng, J.; Louie, K.G.; Knutsen, T.; McKoy, W.M.; Young, R.C.; Ozols, R.F. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987, 47, 414–418. [Google Scholar]
- Bradbury, A.; O’Donnell, R.; Drew, Y.; Curtin, N.J.; Sharma Saha, S. Characterisation of Ovarian Cancer Cell Line NIH-OVCAR3 and Implications of Genomic, Transcriptomic, Proteomic and Functional DNA Damage Response Biomarkers for Therapeutic Targeting. Cancers 2020, 12, 1939. [Google Scholar] [CrossRef]
- Fedier, A.; Maggi, N.; Tozzi, A.; Disler, M.; Coelho, R.; Jacob, F.; Heinzelmann-Schwarz, V. Exposure to escalating olaparib does not induce acquired resistance to PARPi and to other chemotherapeutic compounds in ovarian cancer cell lines. Int. J. Oncol. 2022, 61. [Google Scholar] [CrossRef]
- Hoare, J.I.; Hockings, H.; Saxena, J.; Silva, V.L.; Haughey, M.J.; Wood, G.E.; Nicolini, F.; Mirza, H.; McNeish, I.A.; Huang, W.; et al. A novel cell line panel reveals non-genetic mediators of platinum resistance and phenotypic diversity in high grade serous ovarian cancer. Gynecol. Oncol. 2022, 167, 96–106. [Google Scholar] [CrossRef]
- Xiang, J.; Zhou, L.; He, Y.; Wu, S. LDH-A inhibitors as remedies to enhance the anticancer effects of PARP inhibitors in ovarian cancer cells. Aging 2021, 13, 25920–25930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, X.; Bai, G.; Huang, X.; Hu, S.; Mao, H.; Liu, P. Identification of Three Potential Prognostic Genes in Platinum-Resistant Ovarian Cancer via Integrated Bioinformatics Analysis. Cancer Manag. Res. 2021, 13, 8629–8646. [Google Scholar] [CrossRef] [PubMed]
- Provencher, D.M.; Lounis, H.; Champoux, L.; Tetrault, M.; Manderson, E.N.; Wang, J.C.; Eydoux, P.; Savoie, R.; Tonin, P.N.; Mes-Masson, A.M. Characterization of four novel epithelial ovarian cancer cell lines. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.; Huang, T.T.; Murai, J.; Haynes, B.; Steeg, P.S.; Pommier, Y.; Lee, J.M. Resistance to the CHK1 inhibitor prexasertib involves functionally distinct CHK1 activities in BRCA wild-type ovarian cancer. Oncogene 2020, 39, 5520–5535. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Juan, G. DNA content measurement for DNA ploidy and cell cycle analysis. Curr. Protoc. Cytom. 2001. [Google Scholar] [CrossRef]
- Nair, J.R.; Huang, T.-T.; Sunkara, A.; Pruitt, M.R.; Ibanez, K.R.; Chiang, C.-Y.; Cheng, K.C.-C.; Wilson, K.; Cardillo, T.M.; Hofsess, S.; et al. Distinct effects of sacituzumab govitecan and berzosertib on DNA damage response in ovarian cancer. iScience 2024, 27, 111283. [Google Scholar] [CrossRef]
- Du, M.; Zhang, S.; Liu, X.; Xu, C.; Zhang, X. Ploidy Status of Ovarian Cancer Cell Lines and Their Association with Gene Expression Profiles. Biomolecules 2023, 13, 92. [Google Scholar] [CrossRef]
- Cohen-Sharir, Y.; McFarland, J.M.; Abdusamad, M.; Marquis, C.; Bernhard, S.V.; Kazachkova, M.; Tang, H.; Ippolito, M.R.; Laue, K.; Zerbib, J.; et al. Aneuploidy renders cancer cells vulnerable to mitotic checkpoint inhibition. Nature 2021, 590, 486–491. [Google Scholar] [CrossRef]
- Huang, T.T.; Burkett, S.S.; Tandon, M.; Yamamoto, T.M.; Gupta, N.; Bitler, B.G.; Lee, J.M.; Nair, J.R. Distinct roles of treatment schemes and BRCA2 on the restoration of homologous recombination DNA repair and PARP inhibitor resistance in ovarian cancer. Oncogene 2022, 41, 5020–5031. [Google Scholar] [CrossRef]
- Gliech, C.R.; Yeow, Z.Y.; Tapias-Gomez, D.; Yang, Y.; Huang, Z.; Tijhuis, A.E.; Spierings, D.C.; Foijer, F.; Chung, G.; Tamayo, N.; et al. Weakened APC/C activity at mitotic exit drives cancer vulnerability to KIF18A inhibition. EMBO J. 2024, 43, 666–694. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.; Mayr, M.I.; Mockel, M.M.; Mayer, T.U. Pre-anaphase chromosome oscillations are regulated by the antagonistic activities of Cdk1 and PP1 on Kif18A. Nat. Commun. 2014, 5, 4397. [Google Scholar] [CrossRef]
- Serpico, A.F.; Pisauro, C.; Trano, A.; Grieco, D. Chromosome alignment and Kif18A action rely on spindle-localized control of Cdk1 activity. Front. Cell. Dev. Biol. 2024, 12, 1490781. [Google Scholar] [CrossRef]
- Lawrence, C.J.; Dawe, R.K.; Christie, K.R.; Cleveland, D.W.; Dawson, S.C.; Endow, S.A.; Goldstein, L.S.; Goodson, H.V.; Hirokawa, N.; Howard, J.; et al. A standardized kinesin nomenclature. J. Cell Biol. 2004, 167, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wei, Y.L.; She, Z.Y. Kinesin-8 motors: Regulation of microtubule dynamics and chromosome movements. Chromosoma 2020, 129, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, M.; Zhang, B.; Zhao, W. KIF18A improves migration and invasion of colorectal cancer (CRC) cells through inhibiting PTEN signaling. Aging 2023, 15, 9182–9192. [Google Scholar] [CrossRef]
- Sabnis, R.W. Novel KIF18A Inhibitors for Treating Cancer. ACS Med. Chem. Lett. 2020, 11, 2079–2080. [Google Scholar] [CrossRef]
- Amin, A.S.M.; Eastwood, S.; Pilcher, C.; Truong, J.Q.; Foitzik, R.; Boag, J.; Gorringe, K.L.; Holien, J.K. KIF18A inhibition: The next big player in the search for cancer therapeutics. Cancer Metastasis Rev. 2024, 44, 1–12. [Google Scholar] [CrossRef]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010, 108, 73–112. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Rafalska-Metcalf, I.U.; Ivanov, A.V.; Corsinotti, A.; Peng, H.; Lee, S.C.; Trono, D.; Janicki, S.M.; Rauscher, F.J., 3rd. The ATM substrate KAP1 controls DNA repair in heterochromatin: Regulation by HP1 proteins and serine 473/824 phosphorylation. Mol. Cancer Res. 2012, 10, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.; Murai, J.; Lee, J.M. Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition. Cancer Treat. Rev. 2018, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Czechanski, A.; Kim, H.; Byers, C.; Greenstein, I.; Stumpff, J.; Reinholdt, L.G. Kif18a is specifically required for mitotic progression during germ line development. Dev. Biol. 2015, 402, 253–262. [Google Scholar] [CrossRef]


| Sensitivity to ATX020 | Cell Line | IC50 (µM) | % Viable @max C | Ploidy | AS $ | TP53 Status | CNV | BRCA Mutation | Olaparib Sensitivity | Cisplatin Sensitivity |
|---|---|---|---|---|---|---|---|---|---|---|
| Resistant | PEO4 | ND | >80% | 1.9 | 12 # | LOF | - | BRCA2 GOF | R | R |
| A2780 | ND | >80% | 2 | 2 | Wt | L | Wt | S | S | |
| OV90 | ND | >80% | 2.0 | 13 | LOF | L | Wt | S | S | |
| Moderate | TOV21G | ND | >50% | 2 | 2 | Wt | L | Wt | S | S |
| SKOV3 | ND | >50% | 3.4 | 5 | Null | L | Wt | R | R | |
| OVSAHO | ND | >50% | 2.7 | 31 | LOF | H | BRCA2 LOF | S | S | |
| High | PEO1 | 0.64 | <50% | 3.0 | 37 # | LOF | - | BRCA2 LOF | S | S |
| JHOS4 | 0.65 | <50% | 3.2 | 22 * | ? | H | BRCA1 LOF | S | S | |
| OVCAR8 | 0.46 | <50% | 2.5 | 28 | LOF | H | Wt | R | R | |
| OVCAR3 | 0.22 | <90% | 3.0 | 26 | LOF | H | Wt | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, J.; Huang, T.-T.; Lynes, M.; Khan, S.; Silver, S.; Lee, J.-M. The KIF18A Inhibitor ATX020 Induces Mitotic Arrest and DNA Damage in Chromosomally Instable High-Grade Serous Ovarian Cancer Cells. Cells 2025, 14, 1863. https://doi.org/10.3390/cells14231863
Nair J, Huang T-T, Lynes M, Khan S, Silver S, Lee J-M. The KIF18A Inhibitor ATX020 Induces Mitotic Arrest and DNA Damage in Chromosomally Instable High-Grade Serous Ovarian Cancer Cells. Cells. 2025; 14(23):1863. https://doi.org/10.3390/cells14231863
Chicago/Turabian StyleNair, Jayakumar, Tzu-Ting Huang, Maureen Lynes, Sanjoy Khan, Serena Silver, and Jung-Min Lee. 2025. "The KIF18A Inhibitor ATX020 Induces Mitotic Arrest and DNA Damage in Chromosomally Instable High-Grade Serous Ovarian Cancer Cells" Cells 14, no. 23: 1863. https://doi.org/10.3390/cells14231863
APA StyleNair, J., Huang, T.-T., Lynes, M., Khan, S., Silver, S., & Lee, J.-M. (2025). The KIF18A Inhibitor ATX020 Induces Mitotic Arrest and DNA Damage in Chromosomally Instable High-Grade Serous Ovarian Cancer Cells. Cells, 14(23), 1863. https://doi.org/10.3390/cells14231863







