Strategies for the Development of NK Cell-Based Therapies for Cancer Treatment
Highlights
- For the generation of CAR-NK cells, the most promising approach is to use the CD56brightCD16dimCD57neg NK cell population.
- The design strategy for CARs is based on either creating a highly specific CAR-NK (e.g., anti-CD19 CAR-NK), or a broadly acting CAR-NK with the sequence of a conventional activating NK receptor (e.g., NKG2D CAR-NK).
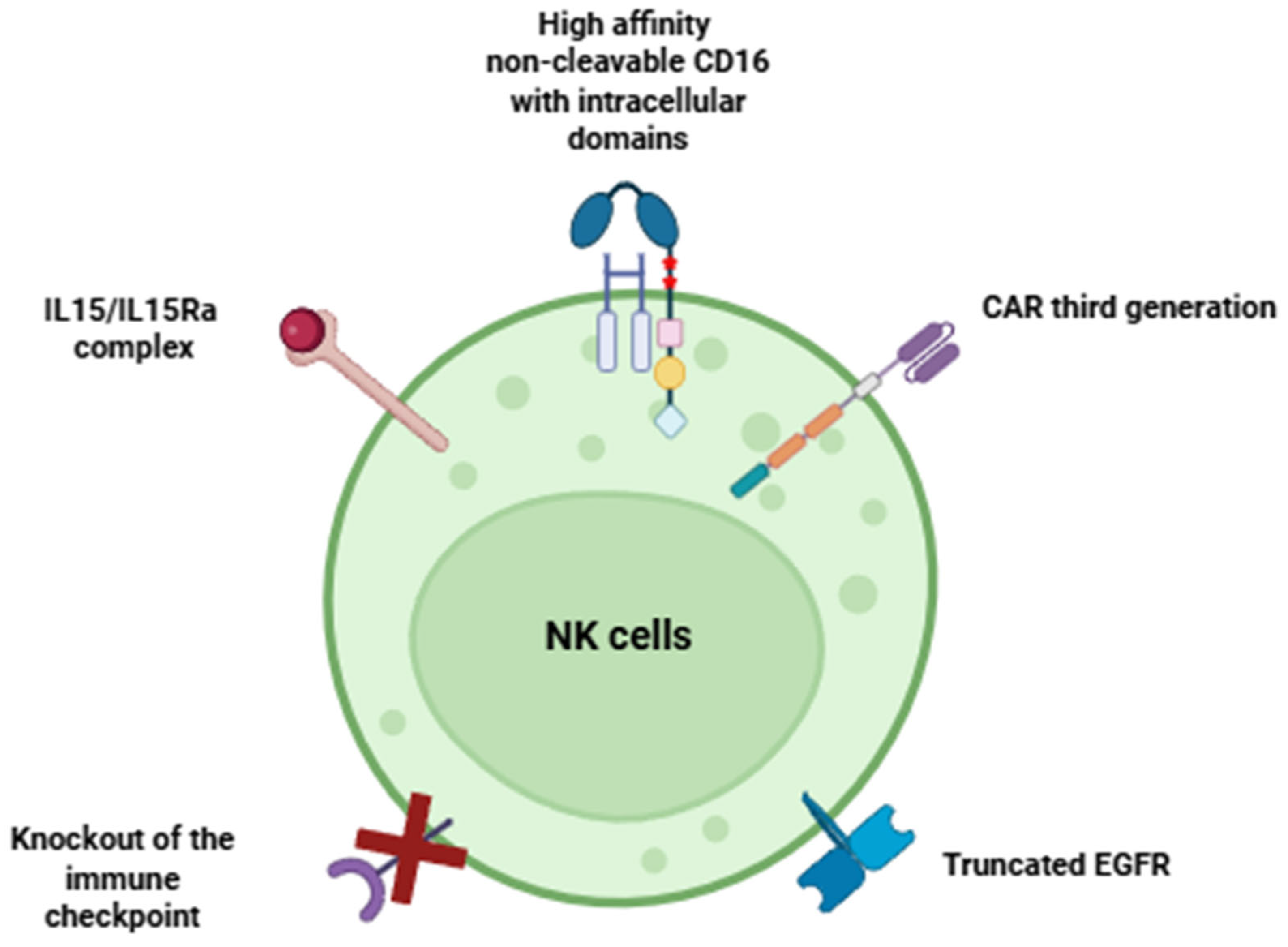
- To enhance the cytotoxicity of NK cells, it is possible to modify them with high-affinity non-cleavable CD16 and intracellular activation domains.
- To overcome tumor immune suppression, it is feasible to knock out immune checkpoints in NK cells.
- To maintain long-term proliferative activity, NK cells can be engineered to express IL15 and IL15Rα.
- To ensure the safety of CAR-NK cells for humans, they can be modified by safety switch.
- Clinical trials have confirmed the efficacy of CAR-NK cells and the genetic modifications listed above.
- The strategy for creating a next-generation CAR-NK cell product should adhere to the following principles simultaneously: increased antitumor cytotoxicity, increased proliferative activity, and continued safety for patients.
Abstract
1. Introduction
2. NK Cell Biology and Cancer Progression
3. Genetic Modifications in CAR Cell Therapy
3.1. Strategies for Modifying the Chimeric Antigen Receptor
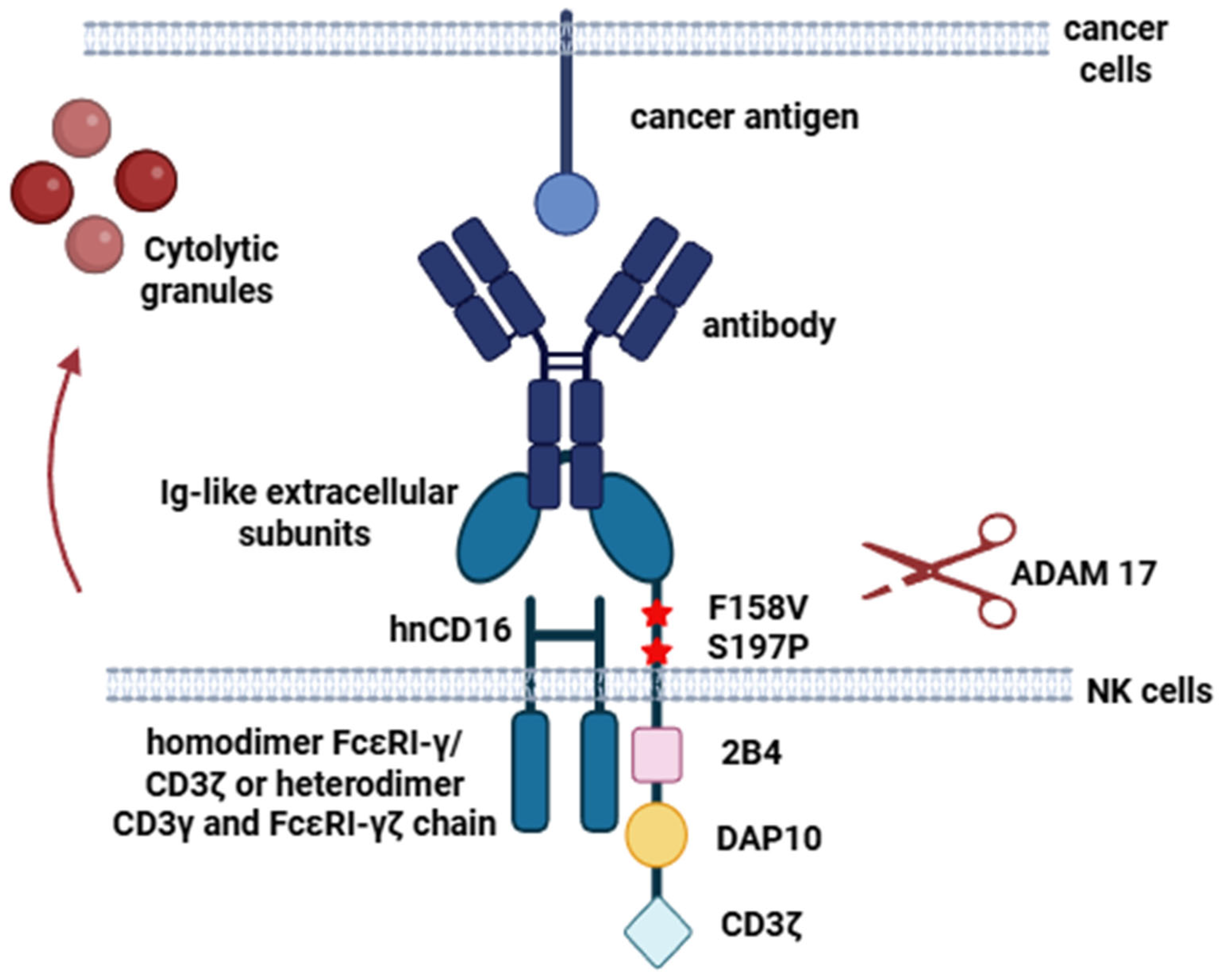
3.2. Enhancing NK Cell Cytotoxicity Using the High-Affinity, Non-Cleavable CD16 Receptor with Enhanced Functional Activity
3.3. Membrane-Bound IL-15/IL-15Rα Complex (IL-15RF)—A Strategy for Improved NK Cell Survival
3.4. Immune Checkpoint Inhibition as a Strategy to Increase Cytotoxicity and NK Cell Survival
- increasing antitumor cytotoxicity;
- increasing the lifespan of cells and the time of their persistence in the patient’s body;
- increasing their safety for the patient.
3.5. Safety Switches
4. Modern Clinical Trials of CAR-NK Cell Products
- -
- Firstly, choosing the right tumor target. Ideally, this target should not be expressed by normal cells. However, in reality, the best option would be a target that is characterized by low expression in healthy cells and high expression in tumor cells.
- -
- Secondly, the correct design of the CAR, the choice of optimal intracellular domains, depends on the tasks set.
- -
- Thirdly, the introduction of high-affinity, non-cleavable CD16 molecules with intracellular domains into CAR-NK cells will allow the use of therapeutic antibodies in combination with CAR-NK therapy for the treatment of patients. This will provide antitumor activity through ADCC (antibody-dependent cell-mediated cytotoxicity) due to the strong intracellular signal generated by the CD16 molecule (Figure 3).
5. Optimization of Antitumor CAR-NK Therapy
6. Conclusions
- (1)
- Powerful and versatile antitumor activity
- (2)
- Long-lasting presence in the body
- (3)
- Impeccable safety profile.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| ADR | alloimmune defense receptor |
| AHR | aryl hydrocarbon receptor |
| BCMA | B-cell maturation antigen |
| CAR | Chimeric Antigen Receptor |
| CARD | Caspase activation and recruitment domains |
| CISH | Cytokine-inducible SH2-containing protein |
| DC | dendritic cell |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HIF | Hypoxia-Inducible Factor |
| HIV | human immunodeficiency viruses |
| HLA/MHC | Human Leukocyte Antigen/Major Histocompatibility Complex |
| hnCD16 | highly affinity and non-cleavable CD16 |
| HPA | human protein atlas |
| HSV-TK | Thymidine kinase from herpesvirus |
| iCasp9 | Inducible Caspase9 |
| ITAM | immunoreceptor tyrosine-based activation motif |
| ITIM | immunoreceptor tyrosine-based inhibitory motif |
| ITSM | immunoreceptor tyrosine-based switch motif |
| ITT-like motif | immunoreceptor tyrosine tail (ITT)-like motif |
| LAG3 | Lymphocyte-activation gene |
| mb | membrane bind |
| MDSC | Myeloid-derived suppressor cells |
| MICA | MHC class I polypeptide–related sequence A |
| MICB | MHC class I polypeptide–related sequence B |
| MSLN | Mesothelin |
| MUC | mucin |
| NK | natural killer |
| PD | Programmed cell death |
| RF | fusion with receptor |
| ROR1 | Tyrosine-protein kinase transmembrane receptor |
| scFv | single-chain variable fragment |
| SREBP | Sterol regulatory element-binding proteins |
| TCR | T-cell receptor |
| tEGFR | truncated epidermal growth factor receptor |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| TIM3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TIR | Toll-interleukin receptor |
| TM | transmembrane domain |
| TME | tumor microenvironment |
| TRAF | TNF receptor-associated factors |
| ULBP | UL16 binding protein |
References
- Ahuja, S.; Zaheer, S. The Evolution of Cancer Immunotherapy: A Comprehensive Review of Its History and Current Perspectives. Korean J. Clin. Oncol. 2024, 20, 51–73. [Google Scholar] [CrossRef]
- Mitra, A.; Kumar, A.; Amdare, N.P.; Pathak, R. Current Landscape of Cancer Immunotherapy: Harnessing the Immune Arsenal to Overcome Immune Evasion. Biology 2024, 13, 307. [Google Scholar] [CrossRef]
- Center for Biologics Evaluation and Research. Approved Cellular and Gene Therapy Products; Food and Drug Administration: Silver Spring, MD, USA, 2025.
- Joy, R.; Phair, K.; O’Hara, R.; Brady, D. Recent Advances and Current Challenges in CAR-T Cell Therapy. Biotechnol. Lett. 2024, 46, 115–126. [Google Scholar] [CrossRef]
- Dabas, P.; Danda, A. Revolutionizing Cancer Treatment: A Comprehensive Review of CAR-T Cell Therapy. Med. Oncol. 2023, 40, 275. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Woo, J.S.; Nguyen, K.; Liu, L.; Krishnan, A.; Siddiqi, T.; Borogovac, A. Mobilizing CARs: Benefits, Drawbacks, and Directions for Outpatient CAR T-Cell Therapy. Semin. Hematol. 2024, 61, 273–283. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhang, X.; Aziz, A.U.R.; Wang, D. CAR-NK Cell Therapy: A Transformative Approach to Overcoming Oncological Challenges. Biomolecules 2024, 14, 1035. [Google Scholar] [CrossRef]
- Balkhi, S.; Zuccolotto, G.; Di Spirito, A.; Rosato, A.; Mortara, L. CAR-NK Cell Therapy: Promise and Challenges in Solid Tumors. Front. Immunol. 2025, 16, 1574742. [Google Scholar] [CrossRef]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as Cellular Cancer Immunotherapy for Solid Tumors. Cell. Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [CrossRef]
- Bachanova, V.; Deol, A.; Al-Juhaishi, T.M.S.; Lulla, P.D.; Byrne, M.T.; Wong, C.; Bickers, C.; Greene, T.; Wong, L.; Villa, B.; et al. Safety and Efficacy of FT522, a First-in-Class, Multi-Antigen Targeted, Off-the-Shelf, iPSC-Derived CD19 CAR NK Cell Therapy with Alloimmune Defense Receptor (ADR) in Relapsed/Refractory B-Cell Lymphoma. Blood 2024, 144, 6543. [Google Scholar] [CrossRef]
- Jiang, P.; Jing, S.; Sheng, G.; Jia, F. The Basic Biology of NK Cells and Its Application in Tumor Immunotherapy. Front. Immunol. 2024, 15, 1420205. [Google Scholar] [CrossRef]
- Vivier, E.; Rebuffet, L.; Narni-Mancinelli, E.; Cornen, S.; Igarashi, R.Y.; Fantin, V.R. Natural Killer Cell Therapies. Nature 2024, 626, 727–736. [Google Scholar] [CrossRef]
- Rishabh, K.; Matosevic, S. The Diversity of Natural Killer Cell Functional and Phenotypic States in Cancer. Cancer Metastasis Rev. 2025, 44, 26. [Google Scholar] [CrossRef]
- Khanal, S.; Bhattarai, N. A Scalable Protocol for Ex Vivo Production of CAR-Engineered Human NK Cells. Methods Protoc. 2025, 8, 102. [Google Scholar] [CrossRef]
- Bisht, K.; Merino, A.; Igarashi, R.; Gauthier, L.; Chiron, M.; Desjonqueres, A.; Smith, E.; Briercheck, E.; Romee, R.; Alici, E.; et al. Natural Killer Cell Biology and Therapy in Multiple Myeloma: Challenges and Opportunities. Exp. Hematol. Oncol. 2024, 13, 114. [Google Scholar] [CrossRef]
- Cantoni, C.; Huergo-Zapico, L.; Parodi, M.; Pedrazzi, M.; Mingari, M.C.; Moretta, A.; Sparatore, B.; Gonzalez, S.; Olive, D.; Bottino, C.; et al. NK Cells, Tumor Cell Transition, and Tumor Progression in Solid Malignancies: New Hints for NK-Based Immunotherapy? J. Immunol. Res. 2016, 2016, 4684268. [Google Scholar] [CrossRef]
- Sánchez-Paulete, A.R.; Mateus-Tique, J.; Mollaoglu, G.; Nielsen, S.R.; Marks, A.; Lakshmi, A.; Khan, J.A.; Wilk, C.M.; Pia, L.; Baccarini, A.; et al. Targeting Macrophages with CAR T Cells Delays Solid Tumor Progression and Enhances Antitumor Immunity. Cancer Immunol. Res. 2022, 10, 1354–1369. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Molto, L.; Rayman, P.; Paszkiewicz-Kozik, E.; Thornton, M.; Reese, L.; Thomas, J.C.; Das, T.; Kudo, D.; Bukowski, R.; Finke, J.; et al. The Bcl-2 Transgene Protects T Cells from Renal Cell Carcinoma-Mediated Apoptosis1. Clin. Cancer Res. 2003, 9, 4060–4068. [Google Scholar]
- Gastman, B.R.; Johnson, D.E.; Whiteside, T.L.; Rabinowich, H. Tumor-Induced Apoptosis of T Lymphocytes: Elucidation of Intracellular Apoptotic Events. Blood 2000, 95, 2015–2023. [Google Scholar] [CrossRef]
- Bashiri Dezfouli, A.; Yazdi, M.; Pockley, A.G.; Khosravi, M.; Kobold, S.; Wagner, E.; Multhoff, G. NK Cells Armed with Chimeric Antigen Receptors (CAR): Roadblocks to Successful Development. Cells 2021, 10, 3390. [Google Scholar] [CrossRef]
- Wang, D.; Shao, Y.; Zhang, X.; Lu, G.; Liu, B. IL-23 and PSMA-Targeted Duo-CAR T Cells in Prostate Cancer Eradication in a Preclinical Model. J. Transl. Med. 2020, 18, 23. [Google Scholar] [CrossRef]
- Kulemzin, S.; Kuznetsova, V.; Mamonkin, M.; Taranin, A.; Gorchakov, A. Engineering Chimeric Antigen Receptors. Available online: https://cyberleninka.ru/article/n/osnovy-dizayna-himernyh-antigennyh-retseptorov/viewer (accessed on 28 September 2025).
- Yang, K.; Zhao, Y.; Sun, G.; Zhang, X.; Cao, J.; Shao, M.; Liang, X.; Wang, L. Clinical Application and Prospect of Immune Checkpoint Inhibitors for CAR-NK Cell in Tumor Immunotherapy. Front. Immunol. 2023, 13, 1081546. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 12 October 2025).
- Huang, S.; Qin, Z.; Wang, F.; Kang, Y.; Ren, B. A Potential Mechanism of Tumor Immune Escape: Regulation and Application of Soluble Natural Killer Group 2 Member D Ligands (Review). Oncol. Rep. 2024, 52, 137. [Google Scholar] [CrossRef]
- Tan, G.; Spillane, K.M.; Maher, J. The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer. Biology 2023, 12, 1079. [Google Scholar] [CrossRef]
- Gong, Y.; Klein Wolterink, R.G.J.; Wang, J.; Bos, G.M.J.; Germeraad, W.T.V. Chimeric Antigen Receptor Natural Killer (CAR-NK) Cell Design and Engineering for Cancer Therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef]
- Whitlow, M.; Bell, B.A.; Feng, S.L.; Filpula, D.; Hardman, K.D.; Hubert, S.L.; Rollence, M.L.; Wood, J.F.; Schott, M.E.; Milenic, D.E. An Improved Linker for Single-Chain Fv with Reduced Aggregation and Enhanced Proteolytic Stability. Protein Eng. 1993, 6, 989–995. [Google Scholar] [CrossRef]
- Yao, P.; Liu, Y.-G.; Huang, G.; Hao, L.; Wang, R. The Development and Application of Chimeric Antigen Receptor Natural Killer (CAR-NK) Cells for Cancer Therapy: Current State, Challenges and Emerging Therapeutic Advances. Exp. Hematol. Oncol. 2024, 13, 118. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, M.; Zhang, W.; Liu, N.; Wang, D.; Jing, L.; Xu, N.; Yang, N.; Ren, T. Chimeric Antigen Receptor-Based Natural Killer Cell Immunotherapy in Cancer: From Bench to Bedside. Cell Death Dis. 2024, 15, 50. [Google Scholar] [CrossRef]
- Qin, H.; Ramakrishna, S.; Nguyen, S.; Fountaine, T.J.; Ponduri, A.; Stetler-Stevenson, M.; Yuan, C.M.; Haso, W.; Shern, J.F.; Shah, N.N.; et al. Preclinical Development of Bivalent Chimeric Antigen Receptors Targeting Both CD19 and CD22. Mol. Ther. Oncolytics 2018, 11, 127–137. [Google Scholar] [CrossRef]
- Park, E.; Mun, H.; Seo, E.; Hwang, S.; Lee, J.H.; Song, S.; Sung, H.; Kim, H.-Y.; Kwon, M.-J. CAR NK92 Cells Targeting BCMA Can Effectively Kill Multiple Myeloma Cells Both In Vitro and In Vivo. Biomedicines 2024, 12, 248. [Google Scholar] [CrossRef]
- Ferguson, I.D.; Patiño-Escobar, B.; Tuomivaara, S.T.; Lin, Y.-H.T.; Nix, M.A.; Leung, K.K.; Kasap, C.; Ramos, E.; Nieves Vasquez, W.; Talbot, A.; et al. The Surfaceome of Multiple Myeloma Cells Suggests Potential Immunotherapeutic Strategies and Protein Markers of Drug Resistance. Nat. Commun. 2022, 13, 4121. [Google Scholar] [CrossRef]
- Bernard, G.; Evgin, L. Non-Signaling but All Important: How the Linker, Hinge, and Transmembrane Domains in the CAR Hold It All Together. Front. Immunol. 2025, 16, 1664403. [Google Scholar] [CrossRef]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T Cell Receptor (TCR) Signaling in Health and Disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Q.; Zhong, M.; Wang, Z.; Chen, Z.; Zhang, Y.; Xing, H.; Tian, Z.; Tang, K.; Liao, X.; et al. 2B4 Costimulatory Domain Enhancing Cytotoxic Ability of Anti-CD5 Chimeric Antigen Receptor Engineered Natural Killer Cells against T Cell Malignancies. J. Hematol. Oncol. 2019, 12, 49. [Google Scholar] [CrossRef]
- Fu, Z.; Zhou, J.; Chen, R.; Jin, Y.; Ni, T.; Qian, L.; Xiao, C. Cluster of Differentiation 19 Chimeric Antigen Receptor T-Cell Therapy in Pediatric Acute Lymphoblastic Leukemia. Oncol. Lett. 2020, 20, 36. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, J.; Huang, B.; Zhang, Z.; Wang, S.; Tang, F.; Teng, M.; Li, Y. Special Chimeric Antigen Receptor (CAR) Modifications of T Cells: A Review. Front. Oncol. 2022, 12, 832765. [Google Scholar] [CrossRef]
- Ku, K.S.; Tang, J.; Chen, Y.; Shi, Y. Current Advancements in Anti-Cancer Chimeric Antigen Receptor T Cell Immunotherapy and How Nanotechnology May Change the Game. Int. J. Mol. Sci. 2024, 25, 5361. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, J.; Liu, T.; Xu, Q.; Song, X.; Zeng, J. DNAM1 and 2B4 Costimulatory Domains Enhance the Cytotoxicity of Anti-GPC3 Chimeric Antigen Receptor-Modified Natural Killer Cells Against Hepatocellular Cancer Cells In Vitro. Cancer Manag. Res. 2020, 12, 3247–3255. [Google Scholar] [CrossRef]
- Yi, E.; Lee, E.; Park, H.J.; Lee, H.H.; Yun, S.H.; Kim, H.S. A Chimeric Antigen Receptor Tailored to Integrate Complementary Activation Signals Potentiates the Antitumor Activity of NK Cells. J. Exp. Clin. Cancer Res. 2025, 44, 86. [Google Scholar] [CrossRef]
- Zhi, L.; Zhang, Z.; Gao, Q.; Shang, C.; He, W.; Wang, Y.; Guo, C.; Niu, Z.; Zhu, W. CAR-NK Cells with Dual Targeting of PD-L1 and MICA/B in Lung Cancer Tumor Models. BMC Cancer 2025, 25, 337. [Google Scholar] [CrossRef]
- Wang, C.; Liu, T.; Wang, Q.; Gong, Y.; Gao, F.; Zhou, F.; Cao, Z. Abstract 6122: Off-the-Shelf BCMA/GPRC5D Dual Targeted CAR-NK Cell Therapy Combined with Daratumumab in Treating Multiple Myeloma. Cancer Res. 2025, 85, 6122. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Jin, Y.; Dai, L.; Yue, Y.; Hu, J.; Liu, X.; Pang, K.; Ye, S.; Chen, Y.; et al. An iPSC-Derived CD19/BCMA CAR-NK Therapy in a Patient with Systemic Sclerosis. Cell 2025, 188, 4225–4238.e12. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Q.; Zhu, Y.; Ou, R.; Peng, L.; Wang, B.; Shen, H.; Liu, Z.; Lu, L.; Zhang, P.; et al. Engineering Novel CD19/CD22 Dual-Target CAR-T Cells for Improved Anti-Tumor Activity. Cancer Investig. 2022, 40, 282–292. [Google Scholar] [CrossRef]
- Li, Y.; Basar, R.; Wang, G.; Liu, E.; Moyes, J.S.; Li, L.; Kerbauy, L.N.; Uprety, N.; Fathi, M.; Rezvan, A.; et al. KIR-Based Inhibitory CARs Overcome CAR-NK Cell Trogocytosis-Mediated Fratricide and Tumor Escape. Nat. Med. 2022, 28, 2133–2144. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Xie, J.; Zhou, Y.; Wu, Q.; Lu, B.; Zhou, S.; Zhao, X.; Li, Y. Leveraging CD16 Fusion Receptors to Remodel the Immune Response for Enhancing Anti-Tumor Immunotherapy in iPSC-Derived NK Cells. J. Hematol. Oncol. 2023, 16, 62. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive Snapshots of Natural Killer Cells Functions, Signaling, Molecular Mechanisms and Clinical Utilization. Signal Transduct. Target. Ther. 2024, 9, 302. [Google Scholar] [CrossRef]
- Wu, J.; Mishra, H.K.; Walcheck, B. Role of ADAM17 as a Regulatory Checkpoint of CD16A in NK Cells and as a Potential Target for Cancer Immunotherapy. J. Leukoc. Biol. 2019, 105, 1297–1303. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.Y.; Kim, H.G.; Kwak, M.W.; Kang, T.H. Fc Receptor Variants and Disease: A Crucial Factor to Consider in the Antibody Therapeutics in Clinic. Int. J. Mol. Sci. 2021, 22, 9489. [Google Scholar] [CrossRef]
- Deng, M.; Du, S.; Hou, H.; Xiao, J. Structural Insights into the High-Affinity IgE Receptor FcεRI Complex. Nature 2024, 633, 952–959. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.-G.; Long, E.O. Activation, Coactivation, and Costimulation of Resting Human Natural Killer Cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef]
- Gaud, G.; Achar, S.; Bourassa, F.X.P.; Davies, J.; Hatzihristidis, T.; Choi, S.; Kondo, T.; Gossa, S.; Lee, J.; Juneau, P.; et al. CD3ζ ITAMs Enable Ligand Discrimination and Antagonism by Inhibiting TCR Signaling in Response to Low-Affinity Peptides. Nat. Immunol. 2023, 24, 2121–2134. [Google Scholar] [CrossRef]
- Aguilar, O.A.; Fong, L.-K.; Ishiyama, K.; DeGrado, W.F.; Lanier, L.L. The CD3ζ Adaptor Structure Determines Functional Differences Between Human and Mouse CD16 Fc Receptor Signaling. J. Exp. Med. 2022, 219, e20220022. [Google Scholar] [CrossRef]
- Zhu, H.; Blum, R.H.; Bjordahl, R.; Gaidarova, S.; Rogers, P.; Lee, T.T.; Abujarour, R.; Bonello, G.B.; Wu, J.; Tsai, P.-F.; et al. Pluripotent Stem Cell-Derived NK Cells with High-Affinity Noncleavable CD16a Mediate Improved Antitumor Activity. Blood 2020, 135, 399–410. [Google Scholar] [CrossRef]
- Mahaweni, N.M.; Olieslagers, T.I.; Rivas, I.O.; Molenbroeck, S.J.J.; Groeneweg, M.; Bos, G.M.J.; Tilanus, M.G.J.; Voorter, C.E.M.; Wieten, L. A Comprehensive Overview of FCGR3A Gene Variability by Full-Length Gene Sequencing Including the Identification of V158F Polymorphism. Sci. Rep. 2018, 8, 15983. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Foster, C.B.; Zhu, S.; Leitman, S.F.; Goldin, L.R.; Huppi, K.; Chanock, S.J. Variant Genotypes of the Low-Affinity Fcγ Receptors in Two Control Populations and a Review of Low-Affinity Fcγ Receptor Polymorphisms in Control and Disease Populations. Blood 1999, 94, 4220–4232. [Google Scholar] [CrossRef] [PubMed]
- Arriga, R.; Caratelli, S.; Lanzilli, G.; Ottaviani, A.; Cenciarelli, C.; Sconocchia, T.; Spagnoli, G.C.; Iezzi, G.; Roselli, M.; Lauro, D.; et al. CD16-158-Valine Chimeric Receptor T Cells Overcome the Resistance of KRAS-Mutated Colorectal Carcinoma Cells to Cetuximab. Int. J. Cancer 2020, 146, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- van Hauten, P.M.M.; Hooijmaijers, L.; Vidal-Manrique, M.; van der Waart, A.B.; Hobo, W.; Wu, J.; Blijlevens, N.M.A.; Jansen, J.H.; Walcheck, B.; Schaap, N.P.M.; et al. Engineering of CD34+ Progenitor-Derived Natural Killer Cells with Higher-Affinity CD16a for Enhanced Antibody-Dependent Cellular Cytotoxicity. Cytotherapy 2024, 26, 252–260. [Google Scholar] [CrossRef]
- Rataj, F.; Jacobi, S.J.; Stoiber, S.; Asang, F.; Ogonek, J.; Tokarew, N.; Cadilha, B.L.; van Puijenbroek, E.; Heise, C.; Duewell, P.; et al. High-Affinity CD16-Polymorphism and Fc-Engineered Antibodies Enable Activity of CD16-Chimeric Antigen Receptor-Modified T Cells for Cancer Therapy. Br. J. Cancer 2019, 120, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Rodriguez Benavente, M.C.; Lorenz, W.W.; Mace, E.M.; Barb, A.W. Fc γ Receptor IIIa/CD16a Processing Correlates with the Expression of Glycan-Related Genes in Human Natural Killer Cells. J. Biol. Chem. 2021, 296, 100183. [Google Scholar] [CrossRef]
- Jing, Y.; Ni, Z.; Wu, J.; Higgins, L.; Markowski, T.W.; Kaufman, D.S.; Walcheck, B. Identification of an ADAM17 Cleavage Region in Human CD16 (FcγRIII) and the Engineering of a Non-Cleavable Version of the Receptor in NK Cells. PLoS ONE 2015, 10, e0121788. [Google Scholar] [CrossRef]
- Daly, J.; Carlsten, M.; O’Dwyer, M. Sugar Free: Novel Immunotherapeutic Approaches Targeting Siglecs and Sialic Acids to Enhance Natural Killer Cell Cytotoxicity Against Cancer. Front. Immunol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Tarannum, M.; Ding, X.; Barisa, M.; Hu, S.; Anderson, J.; Romee, R.; Zhang, J. Engineering Innate Immune Cells for Cancer Immunotherapy. Nat. Biotechnol. 2025, 43, 516–533. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Shen, L.; Teng, X.; Xiao, Y.; Yu, B.; Lu, Z. TCR Extracellular Domain Genetically Linked to CD28, 2B4/41BB and DAP10/CD3ζ -Engineered NK Cells Mediates Antitumor Effects. Cancer Immunol. Immunother. 2023, 72, 769–774. [Google Scholar] [CrossRef]
- Capuano, C.; Pighi, C.; Battella, S.; Pulcinelli, F.; Santoro, C.; Ferretti, A.; Turriziani, O.; De Federicis, D.; Fionda, C.; Sciumè, G.; et al. (Auto)Antibody Responses Shape Memory NK Cell Pool Size and Composition. Biomedicines 2022, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luan, L.; Patil, N.K.; Sherwood, E.R. Immunobiology of the IL-15/IL-15Rα Complex as an Antitumor and Antiviral Agent. Cytokine Growth Factor Rev. 2017, 38, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Budagova, T.; Efremova, A.; Usman, N.; Mokrousova, D.; Goldshtein, D. Differentiating Induced Pluripotent Stem Cells into Natural Killer Cells for Adoptive Cell Immunotherapies—Comparative Characterization of Current Protocols. Int. J. Mol. Sci. 2025, 26, 1107. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, R.N.; Eitler, J.; de Azevedo, J.T.C.; Tirapelle, M.C.; Fantacini, D.M.C.; de Souza, L.E.B.; Swiech, K.; Covas, D.T.; Calado, R.T.; Montero, P.O.; et al. Engineering NK-CAR.19 Cells with the IL-15/IL-15Rα Complex Improved Proliferation and Anti-Tumor Effect In Vivo. Front. Immunol. 2023, 14, 1226518. [Google Scholar] [CrossRef] [PubMed]
- Mortier, E.; Quéméner, A.; Vusio, P.; Lorenzen, I.; Boublik, Y.; Grötzinger, J.; Plet, A.; Jacques, Y. Soluble Interleukin-15 Receptor Alpha (IL-15R Alpha)-Sushi as a Selective and Potent Agonist of IL-15 Action through IL-15R Beta/Gamma. Hyperagonist IL-15 x IL-15R Alpha Fusion Proteins. J. Biol. Chem. 2006, 281, 1612–1619. [Google Scholar] [CrossRef]
- Moui, A.; Klein, M.; Hassoun, D.; Dijoux, E.; Cheminant, M.-A.; Magnan, A.; Bouchaud, G. The IL-15/sIL-15Rα Complex Modulates Immunity Without Effect on Asthma Features in Mouse. Respir. Res. 2020, 21, 33. [Google Scholar] [CrossRef]
- Skariah, N.; James, O.; Swamy, M. Signalling Mechanisms Driving Homeostatic and Inflammatory Effects of Interleukin-15 on Tissue Lymphocytes. Discov. Immunol. 2024, 3, kyae002. [Google Scholar] [CrossRef]
- Zanoni, I.; Spreafico, R.; Bodio, C.; Di Gioia, M.; Cigni, C.; Broggi, A.; Gorletta, T.; Caccia, M.; Chirico, G.; Sironi, L.; et al. IL-15 Cis Presentation Is Required for Optimal NK Cell Activation in Lipopolysaccharide-Mediated Inflammatory Conditions. Cell Rep. 2013, 4, 1235–1249. [Google Scholar] [CrossRef]
- Chirifu, M.; Hayashi, C.; Nakamura, T.; Toma, S.; Shuto, T.; Kai, H.; Yamagata, Y.; Davis, S.J.; Ikemizu, S. Crystal Structure of the IL-15-IL-15Ralpha Complex, a Cytokine-Receptor Unit Presented in Trans. Nat. Immunol. 2007, 8, 1001–1007. [Google Scholar] [CrossRef]
- Jabri, B.; Abadie, V. IL-15 Functions as a Danger Signal to Regulate Tissue-Resident T Cells and Tissue Destruction. Nat. Rev. Immunol. 2015, 15, 771–783. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; He, Z.; Li, L.; Liu, S.; Jiang, M.; Zhao, B.; Deng, M.; Wang, W.; Mi, X.; et al. Breakthrough of Solid Tumor Treatment: CAR-NK Immunotherapy. Cell Death Discov. 2024, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Hu, L.-J.; Miller, J.S.; Huang, X.-J.; Zhao, X.-Y. CAR-NK Cell Therapy: A Potential Antiviral Platform. Sci. Bull. 2025, 70, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, I.; Dingley, A.J.; Jacques, Y.; Grötzinger, J. The Structure of the Interleukin-15 Alpha Receptor and Its Implications for Ligand Binding. J. Biol. Chem. 2006, 281, 6642–6647. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Kumaki, S.; Ahdieh, M.; Bertles, J.; Tometsko, M.; Loomis, A.; Giri, J.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A. Functional Characterization of the Human Interleukin-15 Receptor Alpha Chain and Close Linkage of IL15RA and IL2RA Genes. J. Biol. Chem. 1995, 270, 29862–29869. [Google Scholar] [CrossRef]
- Hurton, L.V.; Singh, H.; Najjar, A.M.; Switzer, K.C.; Mi, T.; Maiti, S.; Olivares, S.; Rabinovich, B.; Huls, H.; Forget, M.-A.; et al. Tethered IL-15 Augments Antitumor Activity and Promotes a Stem-Cell Memory Subset in Tumor-Specific T Cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7788–E7797. [Google Scholar] [CrossRef]
- Ye, J.; Liu, Q.; He, Y.; Song, Z.; Lin, B.; Hu, Z.; Hu, J.; Ning, Y.; Cai, C.; Li, Y. Combined Therapy of CAR-IL-15/IL-15Rα-T Cells and GLIPR1 Knockdown in Cancer Cells Enhanced Anti-Tumor Effect against Gastric Cancer. J. Transl. Med. 2024, 22, 171. [Google Scholar] [CrossRef] [PubMed]
- Bexte, T.; Albinger, N.; Al Ajami, A.; Wendel, P.; Buchinger, L.; Gessner, A.; Alzubi, J.; Särchen, V.; Vogler, M.; Rasheed, H.M.; et al. CRISPR/Cas9 Editing of NKG2A Improves the Efficacy of Primary CD33-Directed Chimeric Antigen Receptor Natural Killer Cells. Nat. Commun. 2024, 15, 8439. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Voorhees, P.M.; Schjesvold, F.; Cohen, Y.C.; Hungria, V.; Sandhu, I.; Lindsay, J.; Baker, R.I.; Suzuki, K.; Kosugi, H.; et al. Daratumumab or Active Monitoring for High-Risk Smoldering Multiple Myeloma. N. Engl. J. Med. 2025, 392, 1777–1788. [Google Scholar] [CrossRef]
- Sanchez, L.; Wang, Y.; Siegel, D.S.; Wang, M.L. Daratumumab: A First-in-Class CD38 Monoclonal Antibody for the Treatment of Multiple Myeloma. J. Hematol. Oncol. 2016, 9, 51. [Google Scholar] [CrossRef]
- Clara, J.A.; Levy, E.R.; Reger, R.; Barisic, S.; Chen, L.; Cherkasova, E.; Chakraborty, M.; Allan, D.S.J.; Childs, R. High-Affinity CD16 Integration into a CRISPR/Cas9-Edited CD38 Locus Augments CD38-Directed Antitumor Activity of Primary Human Natural Killer Cells. J. Immunother. Cancer 2022, 10, e003804. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An Endogenous Tumour-Promoting Ligand of the Human Aryl Hydrocarbon Receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Trikha, P.; Moseman, J.E.; Thakkar, A.; Campbell, A.R.; Elmas, E.; Foltz, J.A.; Chakravarti, N.; Fitch, J.R.; Mardis, E.R.; Lee, D.A. Defining the AHR-Regulated Transcriptome in NK Cells Reveals Gene Expression Programs Relevant to Development and Function. Blood Adv. 2021, 5, 4605–4618. [Google Scholar] [CrossRef]
- Wang, M.; Krueger, J.B.; Gilkey, A.K.; Stelljes, E.M.; Kluesner, M.G.; Pomeroy, E.J.; Skeate, J.G.; Slipek, N.J.; Lahr, W.S.; Vázquez, P.N.C.; et al. Precision Enhancement of CAR-NK Cells through Non-Viral Engineering and Highly Multiplexed Base Editing. J. Immunother. Cancer 2025, 13, e009560. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P.-L.; Delconte, R.; Pastor, S.; Laletin, V.; Costa Da Silva, C.; Goubard, A.; Josselin, E.; Castellano, R.; Krug, A.; Vernerey, J.; et al. Targeting CISH Enhances Natural Cytotoxicity Receptor Signaling and Reduces NK Cell Exhaustion to Improve Solid Tumor Immunity. J. Immunother. Cancer 2022, 10, e004244. [Google Scholar] [CrossRef]
- Maymand, S.; Lakkavaram, A.L.; Naser, W.; Rasighaemi, P.; Dlugolenski, D.; Liongue, C.; Stambas, J.; de Koning-Ward, T.F.; Ward, A.C. Role of Cytokine-Inducible SH2 Domain-Containing (CISH) Protein in the Regulation of Erythropoiesis. Biomolecules 2023, 13, 1510. [Google Scholar] [CrossRef]
- Acosta, J.C.; Bahr, J.M.; Basu, S.; O’Donnell, J.T.; Barua, A. Expression of CISH, an Inhibitor of NK Cell Function, Increases in Association with Ovarian Cancer Development and Progression. Biomedicines 2023, 11, 299. [Google Scholar] [CrossRef]
- Blake, S.J.; Stannard, K.; Liu, J.; Allen, S.; Yong, M.C.R.; Mittal, D.; Aguilera, A.R.; Miles, J.J.; Lutzky, V.P.; de Andrade, L.F.; et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov. 2016, 6, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Molfetta, R.; Zitti, B.; Lecce, M.; Milito, N.D.; Stabile, H.; Fionda, C.; Cippitelli, M.; Gismondi, A.; Santoni, A.; Paolini, R. CD155: A Multi-Functional Molecule in Tumor Progression. Int. J. Mol. Sci. 2020, 21, 922. [Google Scholar] [CrossRef]
- Harjunpää, H.; Blake, S.J.; Ahern, E.; Allen, S.; Liu, J.; Yan, J.; Lutzky, V.; Takeda, K.; Aguilera, A.R.; Guillerey, C.; et al. Deficiency of Host CD96 and PD-1 or TIGIT Enhances Tumor Immunity without Significantly Compromising Immune Homeostasis. Oncoimmunology 2018, 7, e1445949. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, S.; Wu, Q.; Liu, L.; Wang, Y.; Mo, Y.; Zeng, Z.; Zu, X.; Xiong, W.; Wang, F. CD155 and Its Receptors in Cancer Immune Escape and Immunotherapy. Cancer Lett. 2023, 573, 216381. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Guillerey, C. TIGIT as an Emerging Immune Checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef]
- Feng, S.; Isayev, O.; Werner, J.; Bazhin, A.V. CD96 as a Potential Immune Regulator in Cancers. Int. J. Mol. Sci. 2023, 24, 1303. [Google Scholar] [CrossRef]
- Mohammadian Gol, T.; Kim, M.; Sinn, R.; Ureña-Bailén, G.; Stegmeyer, S.; Gratz, P.G.; Zahedipour, F.; Roig-Merino, A.; Antony, J.S.; Mezger, M. CRISPR-Cas9-Based Gene Knockout of Immune Checkpoints in Expanded NK Cells. Int. J. Mol. Sci. 2023, 24, 16065. [Google Scholar] [CrossRef]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking Expression of Inhibitory Receptor NKG2A Overcomes Tumor Resistance to NK Cells. J. Clin. Investig. 2019, 129, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Kaulfuss, M.; Mietz, J.; Fabri, A.; Vom Berg, J.; Münz, C.; Chijioke, O. The NK Cell Checkpoint NKG2A Maintains Expansion Capacity of Human NK Cells. Sci. Rep. 2023, 13, 10555. [Google Scholar] [CrossRef]
- Ruggeri, L.; Urbani, E.; André, P.; Mancusi, A.; Tosti, A.; Topini, F.; Bléry, M.; Animobono, L.; Romagné, F.; Wagtmann, N.; et al. Effects of Anti-NKG2A Antibody Administration on Leukemia and Normal Hematopoietic Cells. Haematologica 2016, 101, 626–633. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune Checkpoint Molecules in Natural Killer Cells as Potential Targets for Cancer Immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.; Croft, W.; Parry, H.M.; Verma, K.; Kinsella, F.A.M.; Xu, J.; Bone, D.; McSkeane, T.; Paneesha, S.; Pratt, G.; et al. PD-1 Expression Contributes to Functional Impairment of NK Cells in Patients with B-CLL. Leukemia 2024, 38, 1813–1817. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, S.; Guo, G.; Fei, L.; Guo, S.; Yang, C.; Fu, X.; Wu, Y. Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection. PLoS Pathog. 2011, 7, e1001347. [Google Scholar] [CrossRef]
- Hasim, M.S.; Marotel, M.; Hodgins, J.J.; Vulpis, E.; Makinson, O.J.; Asif, S.; Shih, H.-Y.; Scheer, A.K.; MacMillan, O.; Alonso, F.G.; et al. When Killers Become Thieves: Trogocytosed PD-1 Inhibits NK Cells in Cancer. Sci. Adv. 2022, 8, eabj3286. [Google Scholar] [CrossRef]
- Benson, D.M.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 Axis Modulates the Natural Killer Cell Versus Multiple Myeloma Effect: A Therapeutic Target for CT-011, a Novel Monoclonal Anti-PD-1 Antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.-C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK Cells to Immunotherapy Mediated by PD-1/PD-L1 Blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, Office of the Commissioner. Available online: https://www.fda.gov/about-fda/fda-organization/office-commissioner (accessed on 12 November 2024).
- Ge, H.; Guo, N.; Liu, Y.; Lang, B.; Yin, X.; Yu, X.; Zhang, Z.; Fu, Y.; Ding, H.; Hu, Q.; et al. The Inhibitory Receptor LAG3 Affects NK Cell IFN-γ Production through Glycolysis and the PSAT1/STAT1/IFNG Pathway. mBio 2025, 16, e0023025. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Tian, J.; Zhou, Y.; Shen, Y.; Wang, M.; Tang, L.; Liu, C.; Zhang, X.; Shen, F.; et al. Clinical Significance of Soluble LAG-3 (sLAG-3) in Patients with Cervical Cancer Determined via Enzyme-Linked Immunosorbent Assay with Monoclonal Antibodies. Technol. Cancer Res. Treat. 2023, 22, 15330338231202650. [Google Scholar] [CrossRef]
- Mariuzza, R.A.; Shahid, S.; Karade, S.S. The Immune Checkpoint Receptor LAG3: Structure, Function, and Target for Cancer Immunotherapy. J. Biol. Chem. 2024, 300, 107241. [Google Scholar] [CrossRef]
- Jing, W.; Gershan, J.A.; Weber, J.; Tlomak, D.; McOlash, L.; Sabatos-Peyton, C.; Johnson, B.D. Combined Immune Checkpoint Protein Blockade and Low Dose Whole Body Irradiation as Immunotherapy for Myeloma. J. Immunother. Cancer 2015, 3, 2. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Gulhati, P.; Schalck, A.; Jiang, S.; Shang, X.; Wu, C.-J.; Hou, P.; Ruiz, S.H.; Soto, L.S.; Parra, E.; Ying, H.; et al. Targeting T Cell Checkpoints 41BB and LAG3 and Myeloid Cell CXCR1/CXCR2 Results in Antitumor Immunity and Durable Response in Pancreatic Cancer. Nat. Cancer 2023, 4, 62–80. [Google Scholar] [CrossRef]
- Lu, C.; Tan, Y. Promising Immunotherapy Targets: TIM3, LAG3, and TIGIT Joined the Party. Mol. Ther. Oncol. 2024, 32, 200773. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Lesokhin, A.M.; Paul, B.; Kaufman, J.L.; Pianko, M.; Biran, N.; Vij, R.; Doxie, D.B.; Azeem, M.I.; Martillo, M.; et al. Clinical Response and Pathway-Specific Correlates Following TIGIT-LAG3 Blockade in Myeloma: The MyCheckpoint Randomized Clinical Trial. Nat. Cancer 2024, 5, 1459–1464. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Klyushova, L.S.; Perepechaeva, M.L. AhR and Wnt/β-Catenin Signaling Pathways and Their Interplay. Curr. Issues Mol. Biol. 2023, 45, 3848–3876. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The Aryl Hydrocarbon Receptor: An Environmental Sensor Integrating Immune Responses in Health and Disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Xu, L.; Deng, D.; Lu, L.; Tian, J.; Zhou, D.; Rui, K. New Insight into the Role of SOCS Family in Immune Regulation and Autoimmune Pathogenesis. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Yoon, T.; Lee, Y.; Kim, J.; Park, Y.-B. ADSC Secretome Constrains NK Cell Activity by Attenuating IL-2-Mediated JAK-STAT and AKT Signaling Pathway via Upregulation of CIS and DUSP4. Stem Cell Res. Ther. 2023, 14, 329. [Google Scholar] [CrossRef]
- Zhu, H.; Blum, R.H.; Bernareggi, D.; Ask, E.H.; Wu, Z.; Hoel, H.J.; Meng, Z.; Wu, C.; Guan, K.-L.; Malmberg, K.-J.; et al. Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-Tumor Activity. Cell Stem Cell 2020, 27, 224–237. [Google Scholar] [CrossRef]
- Tang, W.; Chen, J.; Ji, T.; Cong, X. TIGIT, a Novel Immune Checkpoint Therapy for Melanoma. Cell Death Dis. 2023, 14, 466. [Google Scholar] [CrossRef]
- Yue, C.; Gao, S.; Li, S.; Xing, Z.; Qian, H.; Hu, Y.; Wang, W.; Hua, C. TIGIT as a Promising Therapeutic Target in Autoimmune Diseases. Front. Immunol. 2022, 13, 911919. [Google Scholar] [CrossRef]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, H.; Ning, Z. Implications of NKG2A in Immunity and Immune-Mediated Diseases. Front. Immunol. 2022, 13, 960852. [Google Scholar] [CrossRef]
- Cazzetta, V.; Depierreux, D.; Colucci, F.; Mikulak, J.; Mavilio, D. NKG2A Immune Checkpoint in Vδ2 T Cells: Emerging Application in Cancer Immunotherapy. Cancers 2023, 15, 1264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Tang, Y.; Zhuang, X.; Feng, W.; Boor, P.P.C.; Buschow, S.; Sprengers, D.; Zhou, G. Unlocking the Therapeutic Potential of the NKG2A-HLA-E Immune Checkpoint Pathway in T Cells and NK Cells for Cancer Immunotherapy. J. Immunother. Cancer 2024, 12, e009934. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Wang, Y.; Wang, Y. Palmitic Acid Upregulates CD96 Expression to Mediate Maternal–Foetal Interface Immune Tolerance by Inhibiting Cytotoxic Activity and Promoting Adhesion Function in Human Decidual Natural Killer Cells. Bioengineering 2023, 10, 1008. [Google Scholar] [CrossRef]
- Chan, C.J.; Martinet, L.; Gilfillan, S.; Souza-Fonseca-Guimaraes, F.; Chow, M.T.; Town, L.; Ritchie, D.S.; Colonna, M.; Andrews, D.M.; Smyth, M.J. The Receptors CD96 and CD226 Oppose Each Other in the Regulation of Natural Killer Cell Functions. Nat. Immunol. 2014, 15, 431–438. [Google Scholar] [CrossRef]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New Checkpoint Receptor Targets for Cancer Immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, A.; Huang, Y.; Li, H.; Cui, J.; Yang, H.; Si, L.; Jiao, T.; Ren, Z.; Zhang, Z.; et al. Ligand-Induced Ubiquitination Unleashes LAG3 Immune Checkpoint Function by Hindering Membrane Sequestration of Signaling Motifs. Cell 2025, 188, 2354–2371. [Google Scholar] [CrossRef] [PubMed]
- Graydon, C.G.; Mohideen, S.; Fowke, K.R. LAG3’s Enigmatic Mechanism of Action. Front. Immunol. 2020, 11, 615317. [Google Scholar] [CrossRef]
- Huo, J.-L.; Wang, Y.-T.; Fu, W.-J.; Lu, N.; Liu, Z.-S. The Promising Immune Checkpoint LAG-3 in Cancer Immunotherapy: From Basic Research to Clinical Application. Front. Immunol. 2022, 13, 956090. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.; Adhikary, P.; Li, G.; Cheng, K. The TIM3/Gal9 Signaling Pathway: An Emerging Target for Cancer Immunotherapy. Cancer Lett. 2021, 510, 67–78. [Google Scholar] [CrossRef]
- Yu, X.; Lang, B.; Chen, X.; Tian, Y.; Qian, S.; Zhang, Z.; Fu, Y.; Xu, J.; Han, X.; Ding, H.; et al. The Inhibitory Receptor Tim-3 Fails to Suppress IFN-γ Production via the NFAT Pathway in NK-Cell, Unlike That in CD4+ T Cells. BMC Immunol. 2021, 22, 25. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Wu, Q.; Wang, Z.; Hu, B.; Bo, L. The Role of TIM-3 in Sepsis: A Promising Target for Immunotherapy? Front. Immunol. 2024, 15, 1328667. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 Comes of Age as an Inhibitory Receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Mariotti, F.R.; Supino, D.; Landolina, N.; Garlanda, C.; Mantovani, A.; Moretta, L.; Maggi, E. IL-1R8: A Molecular Brake of Anti-Tumor and Anti-Viral Activity of NK Cells and ILC. Semin. Immunol. 2023, 66, 101712. [Google Scholar] [CrossRef]
- Supino, D.; Minute, L.; Mariancini, A.; Riva, F.; Magrini, E.; Garlanda, C. Negative Regulation of the IL-1 System by IL-1R2 and IL-1R8: Relevance in Pathophysiology and Disease. Front. Immunol. 2022, 13, 804641. [Google Scholar] [CrossRef]
- Landolina, N.; Mariotti, F.R.; Ingegnere, T.; Alicata, C.; Ricci, B.; Pelosi, A.; Veneziani, I.; Azzarone, B.G.; Garlanda, C.; Mantovani, A.; et al. IL-1R8 Silencing Improves the Anti-Tumor Function of Freshly Isolated Human NK Cells. J. Immunother. Cancer 2022, 10, e003858. [Google Scholar] [CrossRef] [PubMed]
- Molgora, M.; Bonavita, E.; Ponzetta, A.; Riva, F.; Barbagallo, M.; Jaillon, S.; Popović, B.; Bernardini, G.; Magrini, E.; Gianni, F.; et al. IL-1R8 Is a Checkpoint in NK Cells Regulating Anti-Tumour and Anti-Viral Activity. Nature 2017, 551, 110–114. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C. The Rise of NK Cell Checkpoints as Promising Therapeutic Targets in Cancer Immunotherapy. Front. Immunol. 2019, 10, 2354. [Google Scholar] [CrossRef]
- Bordoloi, D.; Kulkarni, A.J.; Adeniji, O.S.; Pampena, M.B.; Bhojnagarwala, P.S.; Zhao, S.; Ionescu, C.; Perales-Puchalt, A.; Parzych, E.M.; Zhu, X.; et al. Siglec-7 Glyco-Immune Binding mAbs or NK Cell Engager Biologics Induce Potent Antitumor Immunity Against Ovarian Cancers. Sci. Adv. 2023, 9, eadh4379. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Christensen, E.B.; Barnkob, M.B.; Barington, T. The Clinical Landscape of CAR NK Cells. Exp. Hematol. Oncol. 2025, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Głowacki, P.; Rieske, P. Application and Design of Switches Used in CAR. Cells 2022, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, Mechanisms and Clinical Application. Oncotarget 2017, 8, 23996–24008. [Google Scholar] [CrossRef]
- Gargett, T.; Brown, M.P. The Inducible Caspase-9 Suicide Gene System as a “Safety Switch” to Limit on-Target, off-Tumor Toxicities of Chimeric Antigen Receptor T Cells. Front. Pharmacol. 2014, 5, 235. [Google Scholar] [CrossRef]
- Shabaneh, T.B.; Moffett, H.F.; Stull, S.M.; Derezes, T.; Tait, L.J.; Park, S.; Riddell, S.R.; Lajoie, M.J. Safety Switch Optimization Enhances Antibody-Mediated Elimination of CAR T Cells. Front. Mol. Med. 2022, 2, 1026474. [Google Scholar] [CrossRef]
- Sahillioglu, A.C.; Schumacher, T.N. Safety Switches for Adoptive Cell Therapy. Curr. Opin. Immunol. 2022, 74, 190–198. [Google Scholar] [CrossRef]
- Celichowski, P.; Turi, M.; Charvátová, S.; Radhakrishnan, D.; Feizi, N.; Chyra, Z.; Šimíček, M.; Jelínek, T.; Bago, J.R.; Hájek, R.; et al. Tuning CARs: Recent Advances in Modulating Chimeric Antigen Receptor (CAR) T Cell Activity for Improved Safety, Efficacy, and Flexibility. J. Transl. Med. 2023, 21, 197. [Google Scholar] [CrossRef]
- Lu, L.; Xie, M.; Yang, B.; Zhao, W.-B.; Cao, J. Enhancing the Safety of CAR-T Cell Therapy: Synthetic Genetic Switch for Spatiotemporal Control. Sci. Adv. 2024, 10, eadj6251. [Google Scholar] [CrossRef]
- Bessede, A.; Peyraud, F.; Besse, B.; Cousin, S.; Cabart, M.; Chomy, F.; Rey, C.; Lara, O.; Odin, O.; Nafia, I.; et al. TROP2 Is Associated with Primary Resistance to Immune Checkpoint Inhibition in Patients with Advanced Non–Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 779–785. [Google Scholar] [CrossRef]
- El Achi, H.; Dupont, E.; Paul, S.; Khoury, J.D. CD123 as a Biomarker in Hematolymphoid Malignancies: Principles of Detection and Targeted Therapies. Cancers 2020, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, E.; Castelli, G.; Testa, U. CD123 a Therapeutic Target for Acute Myeloid Leukemia and Blastic Plasmocytoid Dendritic Neoplasm. Int. J. Mol. Sci. 2023, 24, 2718. [Google Scholar] [CrossRef] [PubMed]
- Sermer, D.; Elavalakanar, P.; Abramson, J.S.; Palomba, M.L.; Salles, G.; Arnason, J. Targeting CD19 for Diffuse Large B Cell Lymphoma in the Era of CARs: Other Modes of Transportation. Blood Rev. 2023, 57, 101002. [Google Scholar] [CrossRef]
- Dogan, A.; Siegel, D.; Tran, N.; Fu, A.; Fowler, J.; Belani, R.; Landgren, O. B-Cell Maturation Antigen Expression across Hematologic Cancers: A Systematic Literature Review. Blood Cancer J. 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Sauer, T.; Parikh, K.; Sharma, S.; Omer, B.; Sedloev, D.; Chen, Q.; Angenendt, L.; Schliemann, C.; Schmitt, M.; Müller-Tidow, C.; et al. CD70-Specific CAR T Cells Have Potent Activity against Acute Myeloid Leukemia Without HSC Toxicity. Blood 2021, 138, 318–330. [Google Scholar] [CrossRef]
- Rudin, C.M.; Reck, M.; Johnson, M.L.; Blackhall, F.; Hann, C.L.; Yang, J.C.-H.; Bailis, J.M.; Bebb, G.; Goldrick, A.; Umejiego, J.; et al. Emerging Therapies Targeting the Delta-like Ligand 3 (DLL3) in Small Cell Lung Cancer. J. Hematol. Oncol. 2023, 16, 66. [Google Scholar] [CrossRef]
- Su, P.-L.; Chakravarthy, K.; Furuya, N.; Brownstein, J.; Yu, J.; Long, M.; Carbone, D.; Li, Z.; He, K. DLL3-Guided Therapies in Small-Cell Lung Cancer: From Antibody-Drug Conjugate to Precision Immunotherapy and Radioimmunotherapy. Mol. Cancer 2024, 23, 97. [Google Scholar] [CrossRef]
- Qing, L.; Li, Q.; Dong, Z. MUC1: An Emerging Target in Cancer Treatment and Diagnosis. Bull. Cancer 2022, 109, 1202–1216. [Google Scholar] [CrossRef]
- Stern, P.L.; Harrop, R. 5T4 Oncofoetal Antigen: An Attractive Target for Immune Intervention in Cancer. Cancer Immunol. Immunother. 2016, 66, 415–426. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Wang, X.; Sheng, G.; Wang, F.; Wu, K.; Huang, X.; Yang, L.; Li, Y.; Weng, S.; et al. Preclinical Studies of an Innovative 5T4-ADC ACR246 with the Potential to Better Treat 5T4-Positive Solid Tumors. J. Clin. Oncol. 2024, 42, e15005. [Google Scholar] [CrossRef]
- Harrop, R.; Blount, D.G.; Khan, N.; Soyombo, M.; Moyce, L.; Drayson, M.T.; Down, J.; Lawson, M.A.; O’Connor, D.; Nimmo, R.; et al. Targeting Tumor Antigen 5T4 Using CAR T Cells for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2025, 24, 93–104. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; Lei, Y.; Wang, G.; Liu, M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 824208. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, H.; Zheng, J.; Liu, Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J. Cancer 2020, 11, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.; Stikvoort, A.; Nolan, E.; Kirkham-McCarthy, L.; Khoruzhenko, S.; Shivakumar, R.; Zweegman, S.; Van de Donk, N.W.C.J.; Mutis, T.; Szegezdi, E.; et al. CD38 Knockout Natural Killer Cells Expressing an Affinity Optimized CD38 Chimeric Antigen Receptor Successfully Target Acute Myeloid Leukemia with Reduced Effector Cell Fratricide. Haematologica 2022, 107, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Mandai, M.; Hamanishi, J.; Matsumura, N.; Suzuki, A.; Yagi, H.; Yamaguchi, K.; Baba, T.; Fujii, S.; Konishi, I. Clinical Significance of the NKG2D Ligands, MICA/B and ULBP2 in Ovarian Cancer: High Expression of ULBP2 Is an Indicator of Poor Prognosis. Cancer Immunol. Immunother. 2008, 58, 641–652. [Google Scholar] [CrossRef]
- Ghadially, H.; Brown, L.; Lloyd, C.; Lewis, L.; Lewis, A.; Dillon, J.; Sainson, R.; Jovanovic, J.; Tigue, N.J.; Bannister, D.; et al. MHC Class I Chain-Related Protein A and B (MICA and MICB) Are Predominantly Expressed Intracellularly in Tumour and Normal Tissue. Br. J. Cancer 2017, 116, 1208–1217. [Google Scholar] [CrossRef]
- Jiang, Z.; Liang, G.; Xiao, Y.; Qin, T.; Chen, X.; Wu, E.; Ma, Q.; Wang, Z. Targeting the SLIT/ROBO Pathway in Tumor Progression: Molecular Mechanisms and Therapeutic Perspectives. Ther. Adv. Med. Oncol. 2019, 11, 1758835919855238. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, T.-J.; Shin, D.; Hur, K.J.; Hong, S.-H.; Lee, J.Y.; Ha, U.-S. ROBO1 Protein Expression Is Independently Associated with Biochemical Recurrence in Prostate Cancer Patients Who Underwent Radical Prostatectomy in Asian Patients. Gland. Surg. 2021, 10, 2956–2965. [Google Scholar] [CrossRef]
- Walter, R.B. The Role of CD33 as Therapeutic Target in Acute Myeloid Leukemia. Expert Opin. Ther. Targets 2014, 18, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, C.; Lv, X.; Cui, B.; Yan, J.; Li, Y.; Li, K.; Hua, F.; Zhang, X.; Yu, J.; et al. The Chemokine CCL1 Triggers an AMFR-SPRY1 Pathway That Promotes Differentiation of Lung Fibroblasts into Myofibroblasts and Drives Pulmonary Fibrosis. Immunity 2021, 54, 2042–2056. [Google Scholar] [CrossRef]
- Rodriguez-Otero, P.; van de Donk, N.W.C.J.; Pillarisetti, K.; Cornax, I.; Vishwamitra, D.; Gray, K.; Hilder, B.; Tolbert, J.; Renaud, T.; Masterson, T.; et al. GPRC5D as a Novel Target for the Treatment of Multiple Myeloma: A Narrative Review. Blood Cancer J. 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ Biology in Cancer Progression and Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Yan, M.; Wang, J.; Wang, H.; Zhou, J.; Qi, H.; Naji, Y.; Zhao, L.; Tang, Y.; Dai, Y. Knockdown of NR3C1 Inhibits the Proliferation and Migration of Clear Cell Renal Cell Carcinoma through Activating Endoplasmic Reticulum Stress–Mitophagy. J. Transl. Med. 2023, 21, 701. [Google Scholar] [CrossRef]
- Bush, K.A.; Krukowski, K.; Eddy, J.L.; Janusek, L.W.; Mathews, H.L. Glucocorticoid Receptor Mediated Suppression of Natural Killer Cell Activity: Identification of Associated Deacetylase and Corepressor Molecules. Cell Immunol. 2012, 275, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.; Martinez-Turtos, A.; Verhoeyen, E. Importance of T, NK, CAR T and CAR NK Cell Metabolic Fitness for Effective Anti-Cancer Therapy: A Continuous Learning Process Allowing the Optimization of T, NK and CAR-Based Anti-Cancer Therapies. Cancers 2021, 14, 183. [Google Scholar] [CrossRef]
- Mah, A.Y.; Rashidi, A.; Keppel, M.P.; Saucier, N.; Moore, E.K.; Alinger, J.B.; Tripathy, S.K.; Agarwal, S.K.; Jeng, E.K.; Wong, H.C.; et al. Glycolytic Requirement for NK Cell Cytotoxicity and Cytomegalovirus Control. JCI Insight 2017, 2, e95128. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Badeti, S.; Tseng, H.; Ma, M.T.; Liu, T.; Jiang, J.-G.; Liu, C.; Liu, D. Superior Expansion and Cytotoxicity of Human Primary NK and CAR-NK Cells from Various Sources via Enriched Metabolic Pathways. Mol. Ther. Methods Clin. Dev. 2020, 18, 428–445. [Google Scholar] [CrossRef]
- Yang, Y.; Nilsson, M.B.; Yu, X.; Poteete, A.; Jiang, H.; Huang, Q.; He, J.; Heeke, S.; Heymach, J.V. The Activity of EGFR CAR NK and CAR T Cells Against EGFR Inhibitor–Resistant NSCLC and Drug-Tolerant Persister Cells. Clin. Cancer Res. 2025, 31, 4745–4762. [Google Scholar] [CrossRef]
- Chu, Y.; Tian, M.; Saini, U.; Ayala-Cuesta, J.; Klose, K.; Mendelowitz, A.S.; Foley, K.; Ozkaynak, M.F.; Luo, W.; Cripe, T.P.; et al. Combinatorial Immunotherapy with Anti-ROR1 CAR NK Cells and an IL-21 Secreting Oncolytic Virus Against Neuroblastoma. Mol. Ther. Oncol. 2025, 33, 200927. [Google Scholar] [CrossRef]
- Da, Y.; Liu, Y.; Hu, Y.; Liu, W.; Ma, J.; Lu, N.; Zhang, C.; Zhang, C. STING Agonist cGAMP Enhances Anti-Tumor Activity of CAR-NK Cells Against Pancreatic Cancer. OncoImmunology 2022, 11, 2054105. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, L.; Yin, L.; Shi, H.; Gu, Y.; Xing, N. Combined Treatment with anti-PSMA CAR NK-92 Cell and Anti-PD-L1 Monoclonal Antibody Enhances the Antitumour Efficacy Against Castration-Resistant Prostate Cancer. Clin. Transl. Med. 2022, 12, e901. [Google Scholar] [CrossRef]
- Dong, W.; Wu, X.; Ma, S.; Wang, Y.; Nalin, A.P.; Zhu, Z.; Zhang, J.; Benson, D.M.; He, K.; Caligiuri, M.A.; et al. The Mechanism of Anti-PD-L1 Antibody Efficacy Against PD-L1 Negative Tumors Identifies NK Cells Expressing PD-L1 as a Cytolytic Effector. Cancer Discov. 2019, 9, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shen, M.; Xu, L.J.; Yang, X.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O. Enhancing NK Cell-Mediated Cytotoxicity to Cisplatin-Resistant Lung Cancer Cells via MEK/Erk Signaling Inhibition. Sci. Rep. 2017, 7, 7958. [Google Scholar] [CrossRef]
- Wang, D.; Li, B.; Shen, G.; Zhang, H.; Gao, Y.; Du, Z.; Dai, X.; Bao, X.; Zhu, X.; Tong, Z.; et al. NKG2D CAR-NK Adoptive Cellular Immunotherapy Combined with or without PD-1 Blockade in the Treatment of Patients with Metastatic Colorectal Cancer: An Exploratory Study. Cancer Immunol. Immunother. 2025, 74, 341. [Google Scholar] [CrossRef]
- Eivary, S.H.A.; Kheder, R.K.; Najmaldin, S.K.; Kheradmand, N.; Esmaeili, S.-A.; Hajavi, J. Implications of IL-21 in Solid Tumor Therapy. Med. Oncol. 2023, 40, 191. [Google Scholar] [CrossRef] [PubMed]

| CAR-T Product | Approval Year * | Target | Intracellular Domains | Tumor Type |
|---|---|---|---|---|
| Tisagenlecleucel | 2018 | CD19 | 4-1BB + CD3ζ | Acute lymphoblastic leukemia, large B-cell lymphoma, follicular lymphoma |
| Brexucabtagene autoleucel | 2020 ** | CD19 | CD28 + CD3ζ | Acute lymphoblastic leukemia, mantle cell lymphoma |
| Idecabtagene vicleucel | 2021 | BCMA | 4-1BB + CD3ζ | Multiple myeloma |
| Axicabtagene ciloleucel | 2022 | CD19 | CD28 + CD3ζ | Large B-cell lymphoma |
| Lisocabtagene maraleucel | 2022 *** | CD19 | 4-1BB + CD3ζ | Large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia, small lymphocytic lymphoma, mantle cell lymphoma |
| Ciltacabtagene autoleucel | 2022 | BCMA | 4-1BB + CD3ζ | Multiple myeloma |
| Obecabtagene autoleucel | 2024 | CD19 | 4-1BB + CD3ζ | Acute lymphoblastic leukemia |
| Immune Checkpoint | Cell Specificity (in Descending Order of Gene Expression Levels) * | Intracellular Signaling Pathway | Function | Clinical Trials (NCT) |
|---|---|---|---|---|
| AHR | Dendritic cells, monocytes, granulocytes, NK cells, T lymphocytes, B lymphocytes | AHR is a transcription factor that is found in the cytoplasm in an inactive state, bound to other proteins. When AHR binds to specific ligands, the complex dissociates, allowing AHR to become activated. Once activated, AHR moves to the nucleus where it interacts with ARNT to form a heterodimer that can affect gene expression. In addition, other proteins may bind to the AHR/ARNT complex through a non-canonical pathway [123,124]. | Under normal conditions, it affects the expression of genes involved in cell proliferation and promotes the differentiation of NK cells in a specific direction. In the tumor microenvironment, altered tryptophan metabolism products, such as kynurenine and kynurenic acid, act as ligands for the AHR and suppress the antitumor activity of NK cells by activating genes that lead to oxidative stress, such as CYP1A, genes for NADPH oxidases, and cyclooxygenases, as well as by suppressing genes that express antioxidants, such as NQO1 and TXN [90,125]. | 04069026 (2024); 04999202 (2025); 06874257 (2025) |
| CISH | T lymphocytes, granulocytes, monocytes, dendritic cells, NK cells, B lymphocytes | Binds to phosphorylated proteins JAK1, JAK3, and STAT5 (JAK-STAT signaling pathway). Binds to the IL-2Rb subunit [126,127]. | Inhibits the signals from IL-15 and IL-2 directly. Directs the proteins of the JAK/STAT signaling pathway that are bound to it to proteasomal degradation, attracting an E3 ubiquitin ligase. Inhibition of the JAK/STAT pathway leads to a decrease in the survival of mature NK cells, a disruption in their maturation, and a disruption of the production of perforin and granzyme. The perception of signals from other cytokines is also disrupted, resulting in decreased cytotoxicity [126,127,128]. | 05566223 (2022); 04426669 (2025) |
| TIGIT | T lymphocytes, NK cells | TIGIT binds to its main ligand, CD155. Then, the ITT-like domain and ITIM domain become phosphorylated. They bind to the GRB2 protein and phosphatase SHIP1 becomes involved. This leads to the inhibition of the PI3K/AKT and MAPK signaling pathways. Phosphorylation of the ITT-like domain also attracts β-arrestin 2, which binds to the ITT-like domain and also attracts phosphatase SHIP1. SHIP1 disrupts the autoubiquitination of TRAF6, leading to NF-κB suppression [129,130,131]. | PI3K/AKT pathway inhibition results in impaired sensitivity of NK cells not only to IL-15, but also to other cytokines. This leads to decreased proliferation of NK cells, suppression of IFNγ and granzyme B synthesis, impaired signal transmission from activating receptors, and impaired polarization of lytic granules. Glycolysis is inhibited. Collectively, the cytotoxicity of NK cells decreases. Inhibition of the MAPK pathway primarily affects the proliferation and differentiation of NK cells. It also disrupts the distribution of perforin B and granzymes in the immunological synapse. Inhibition of the NF-κB signaling pathway disrupts rapid synthesis of pro-inflammatory cytokines such as IFNγ, TNF-α, IL-6, IL-1β, as well as the synthesis of anti-apoptotic proteins. This, in turn, disrupts proliferation of NK cells [129,130,131]. | 04354246 (2020); 04995523 (2021); 05607563 (2022); 05537051 (2023); 06003621 (2023); 05645692 (2023); 06784947 (2025); 06754501 (2025); 06713798 (2025); 06250036 (2025); |
| NKG2A/CD94 | NK cells T lymphocytes, | It contains two ITIM domains. When the receptor interacts with the HLA-E ligand, tyrosine phosphorylation of the ITIM domain occurs due to the action of SRC and BTK kinases. This triggers the recruitment of phosphatases SHP1 and SHP2, which disrupts the intracellular signaling pathways PI3K/AKT, MAPK, and NF-κB [132,133,134]. | The effects of inhibiting the PI3K/AKT, MAPK, and NF-κB pathways are discussed above. | 02557516 (2015); 04590963 (2020); 05162755 (2021); 06892223 (2021); 06094296 (2023); 06152523 (2023); 06162572 (2024); 06116136 (2024); 06662669 (2024); 06952010 (2025); |
| PD-1 | T lymphocytes, B lymphocytes. NK-only with the development of certain types of tumors | Contains the ITIM and ITSM domains. Upon binding to the ligand, tyrosine phosphorylation occurs in these domains. This process is associated with the involvement of phosphatases SHP1 and SHP2, as well as the disruption of intracellular signaling pathways, including PI3K/AKT and MAPK, as well as NF-κB [108,135]. | The effects of inhibiting the PI3K/AKT, MAPK, and NF-κB pathways are discussed above. | 06751901 (2024); 06620822 (2024); 06952010 (2025); 07110103 (2025); 07090707 (2025); 07068763 (2025); 07062484 (2025); 07132528 (2025); |
| CD96 | T lymphocytes, NK cells, B lymphocytes, granulocytes | It contains the ITIM inhibitory domain, which has an inhibitory effect on the cell through the mechanism described above. It also contains the Tyr-XX-MET motif, which increases the functional activity of NK cells after tyrosine phosphorylation, leading to its recognition by the protein subunit p85 of the PI3K/AKT signaling pathway [100,136,137,138]. | Modern data is still limited, but it has been shown that CD96, expressed by NK cells, can reduce the production of IFNγ and granzyme B and has a negative effect on NK cell proliferation. This leads to an increase in the expression of anti-inflammatory cytokine IL-10. It is likely that inhibition of NK cells via the ITIM CD96 motif is responsible for these effects. Experimental data confirms the best antitumor activity of NK cells when CD96 is inhibited, suggesting it as a potential therapeutic target for cancer treatment. On the other hand, the presence of the Tyr-XX-MET motif can activate the PI3K/AKT intracellular signaling pathway, favoring both the cytotoxic activity and proliferation of NK cells [100,136,137,138]. | 03739710 (2019); 04446351 (2020); |
| LAG3 | T lymphocytes, NK cells ** | It contains intracellular motifs RRFSALE, KIEELE, EX/EP. The features of LAG3 signal transmission are still poorly characterized. On the one hand, the inhibitory function of LAG3 occurs when the receptor interacts with the ligand and releases its RRFSALE motif from the cell membrane. Next, the cytoplasmic tail is ubiquitinated by ligases of the Cbl family, which leads to an increase in the functional activity of the receptor. Activation of the receptor leads to inhibition of the PI3K/AKT and JAK/STAT signaling pathways. The consequences of inhibiting these molecular pathways are described above [115,139,140,141] | Modern data is still limited. LAG3 has been shown to inhibit the PI3K/AKT and Jak/STAT signaling pathways, with the effects described above. The functional activation of LAG3 results in a decrease in the expression of the cell proliferation activity marker Ki67 [115,139,140,141]. | 01968109 (2013); 02460224 (2015); 03005782 (2016); 03311412 (2017); 03489369 (2018); 03470922 (2018); 04140500 (2019); 04641871 (2020); 03538028 (2020); 05002569 (2021); |
| TIM3 | Dendritic cells, NK cells, monocytes, T-lymphocytes, granulocytes | It has various effects on NK cells and T lymphocytes. In NK cells, it can promote their activation, while in T lymphocytes, it exhibits a more prominent inhibitory function. The cytoplasmic tail of the protein contains five tyrosine motifs. In the absence of interaction with a ligand, these tyrosine motifs are bound to the Bat3 protein. This activates the catalytic tyrosine kinase LCK, which can lead to the activation of the immune cell. When TIM3 binds to a ligand, such as galectin 9, Bat3 is cleaved and the tyrosine motifs on TIM3 are phosphorylated. It regulates the signaling pathways of NF-κB and MAPK [142,143,144,145]. | It has both activating and inhibitory effects on cells, especially NK cells. It disrupts the functioning of the immune synapse. In T lymphocytes, it leads to a decrease in the production of IFNγ, TNF-α, IL-2. In NK cells, it inhibits the CD107a protein, a marker of NK cell degranulation, but it does not affect IFNγ synthesis. The data is varied. In one study, it was proved that despite the fact that TIM3 does not directly affect the synthesis of IFNγ, its inhibition indirectly leads to a more increased synthesis of IFNγ. In another study, if NK cells had been previously treated with cytokines, TIM3 had a stimulating effect on them [142,143,144,145]. | 02608268 (2015); 02817633 (2016); 03066648 (2017); 03099109 (2017); 03652077 (2018); 04370704 (2020); 04931654 (2021); 04812548 (2021); 05216835 (2022); 05287113 (2022); |
| IL-1R8 | T lymphocytes, NK cells, granulocytes, monocytes, dendritic cells, B-lymphocytes | It contains an intracellular TIR domain with amino acid substitutions that confer inhibitory properties, in contrast to the native TIR. When it binds to the native TIR on interleukin receptors (ILRs, such as IL-1R) and Toll-like receptors (TLRs), the signal from these receptors is inhibited. This is because TIR/IL-1R8 prevents the recruitment of signaling adapter proteins, such as MyD88, TRAM, SARM, TRIF, etc. These proteins negatively regulate the signaling of the NF-κB and STAT/JNK pathways [132,133,134,135]. | As for NK cells, it has been found that the combined inhibition of IL-1R8 and exposure to IL-15 enhances the functional activity of these cells through the activation of the Jak/STAT and PI3K/AKT signaling pathways. This activation leads to an increase in the synthesis and secretion of IFNγ, GM-CSF, CCLS, CXCL8, as well as granzyme B. As a result, the antitumor activity of NK cells is significantly increased [146,147,148,149]. | - |
| KIR (inhibitory receptors) | NK cells | The cytoplasmic tail contains several intracellular inhibitory ITIM domains. The inhibitory signal from these domains is carried out through a mechanism that involves phosphorylation of the ITIM domain, as well as the involvement of the phosphatases SHP1 and SHP2. These phosphatases disrupt the functional activity of molecular pathways such as PI3K/AKT and MAPK, as well as NF-kB [51,105,150]. | The effects of inhibiting the PI3K/AKT, MAPK, and NF-κB pathways are discussed above. | 00552396 (2007); 01256073 (2007); 00552396 (2007); 00999830 (2009); 01248455 (2010); 01222286 (2010); 01217203 (2010); |
| Siglec-7 | Granulocytes, monocytes, NK cells, dendritic cells, T lymphocytes | It contains an intracellular ITIM domain on the cytoplasmic tail, which transmits an inhibitory signal through a mechanism involving phosphorylation of the ITIM domain and the participation of phosphatases SHP1 and SHP2. This disrupts the functional activity of molecular pathways such as PI3K/AKT and MAPK, and NF-κB [66,151]. | The effects of inhibiting the PI3K/AKT, MAPK, and NF-κB pathways are discussed above. | - |
| Group Name | ID | Disease | Genetic Modifications | Target | Modification Properties |
|---|---|---|---|---|---|
| “Classic” CAR-NK cells, targeted at specific tumor antigen | NCT06454890 (2024–to the present) | Non-small cell lung cancer | antiTROP2-CAR-NK | TROP2 | TROP2 is a transmembrane protein that is expressed in various types of epithelial tumors. It activates the MAPK and PI3K/AKT signaling pathways, which are involved in cell growth, survival, and metastasis. antiTROP2-CAR-NK cells eliminate TROP2-expressing cells [160]. |
| NCT06201247 (2023–to the present) | Acute myeloid leukemia | antiCD123-CAR-NK | CD123 | CD123 is expressed at moderate levels on CD34+ hematopoietic precursor cells, and its expression significantly increases with the development of hematolymphoid neoplasms, including acute myeloid leukemia. antiCD123-CAR-NK cells eliminate CD123-positive cells [161,162] | |
| NCT05645601 (2022–2024) | Refractory B-cell hematological malignancies | antiCD19-CAR-NK | CD19 | CD19 is ubiquitously expressed by B cells at all stages of their differentiation. Its expression significantly increases with the development of B-cell malignancies. Therefore, antiCD19-CAR-NK cells specifically target and eliminate CD19-positive cells [163]. | |
| NCT06045091 (2023–to the present) | Relapsed/refractory multiple myeloma and plasma cell leukemia | antiBCMA-CAR-NK | BCMA | BCMA is involved in the proliferation and differentiation of B cells. Its expression can increase with the development of malignant blood diseases, especially with the development of multiple myeloma. Due to this, BCMA has been considered one of the promising markers for the treatment of this disease. antiBCMA-CAR-NK cells are designed to specifically eliminate BCMA-positive cells [164]. | |
| NCT06696846 (2024–to the present) | Acute myeloid leukemia | antiCD70-CAR-NK | CD70 | CD70 is a transmembrane protein belonging to the TNF family that is expressed on acute myeloid leukemia blast cells. Unlike CD123, it is not expressed in normal tissues, making it a promising target for immunotherapy. antiCD70-CD70 CAR-NK cells can specifically eliminate CD70-positive cells [165]. | |
| NCT05507593 (2022–2023) | Small-cell lung cancer | antiDLL3-CAR-NK | DLL3 | DLL3 is overexpressed during the development of small-cell lung cancer at various stages and promotes cell proliferation, modulates the microenvironment, and contributes to resistance to the immune response. It inhibits Notch signaling pathway and is activated by ASCL1 transcription factor. In normal cells, its expression is low. antiDLL3-CAR-NK cells are designed to specifically eliminate DLL3-positive cells [166,167]. | |
| NCT02839954 (2016–2018) | Solid tumors, such as MUC1+ (Mucin short variant S1, or Mucin 1) malignant glioma of the brain, colorectal carcinoma, gastric carcinoma, hepatocellular carcinoma, non-small cell lung cancer, pancreatic carcinoma, and breast carcinoma. | antiMUC1-CAR-NK | MUC1 | MUC1 (mucin) is a transmembrane protein that is highly glycosylated and normally forms a protective layer on the surface of epithelial cells. However, in certain types of tumors, its expression significantly increases and it changes its localization within cells. Additionally, its glycosylation becomes incomplete. MUC1 plays a role in tumor metastasis, apoptosis regulation, and formation of resistance to the immune response. antiMUC1-CAR-NK cells are designed to specifically eliminate MUC1-positive cells [168]. | |
| NCT05194709 (2021–2022) | Solid tumors | anti5T4-CAR-NK | 5T4 | 5T4 (trophoblast glycoprotein) is a transmembrane protein that is normally expressed in placental cells and plays an important role in fetal survival. However, it is almost never expressed in normal adult tissues. Its overexpression has been linked to the formation of various types of solid tumors. Evidence is also emerging that 5T4 may be overexpressed in the development of certain hematological malignancies. anti5T4-CAR-NK cells are designed to specifically target and eliminate 5T4-positive cells [169,170,171]. | |
| CAR-NK cells of “universal action” | NCT05247957 (2021–2022) | Acute myeloid leukemia | CAR-NK-NKG2D | NKG2DL (MICA, MICB, ULBP1-6) | NKG2D is an activating receptor of NK cells. It is expressed in more than 80% of tumor types, but it is practically not expressed in normal cells. Therefore, CAR-NK-NKG2D can be used to treat a wide range of tumors [29]. |
| NCT03415100 (2018–2019) | Metastatic solid tumors | ||||
| NCT05213195 (2021–to the present) | Refractory metastatic colorectal cancer | ||||
| NCT06478459 (2024–to the present) | Pancreatic cancer | ||||
| NCT05776355 (2023–2024) | Ovarian cancer | ||||
| “biCAR”-NK cells that target two antigens at once, or CAR-NK cells with multiple genetic modifications | NCT06652243 (2024–to the present) | Hepatocellular carcinoma | antiGPC3-CAR-NK + secreted IL15 | GPC3 | GPC3 (glypican 3) is a protein that is associated with the cell membrane and belongs to the GPC family. There are 6 types of glypicans in this family, including GPC1-GPC6. GPC3 is expressed in the ovaries and embryo cells, but its expression has not been observed in other tissues. During the development of liver cancer, however, the expression of GPC3 increases significantly, making it a promising target for immunotherapy. antiGPC3-CAR-NK cells are designed to specifically eliminate GPC3-positive cells [172,173]. IL-15 plays a critical role in the development of NK cells, from early precursors to mature NK cells. In mature NK cells, the transition from the CD56bright to CD56dim phenotype is facilitated by the interaction with IL-2 and IL-15. Therefore, IL-15 secreted by CAR-NK cells can further activate the antitumor activity of NK cells in the patient’s body, as well as support the CAR-NK cells that are injected into the patient [71]. |
| NCT06342986 (2024–to the present) | Ovarian, fallopian tube, and primary peritoneal cancer | antiMICA/B-CAR-NK + CD38 knockout, high-affinity, non-cleavable CD16 (hnCD16), IL-15 with IL-15R expression (IL15/IL15R) | MICA, MICB | MICA and MICB are ligands for the NK cell-activating receptor NKG2D. They are expressed in more than 80% of various types of malignant neoplasms. It has also been noted that MICA and MICB are expressed by ovarian cancer tumor cells, but they are not expressed in normal tissues except for epithelial cells. antiMICA/B-CAR-NK cells specifically eliminate MICA- and MICB-positive cells [174]. The CD38 receptor is expressed on immune system cells, particularly NK cells. To protect NK cells from therapeutic antibodies targeting CD38, a knockout can be introduced into the CD38 gene, avoiding a “fratricidal” reaction between NK cells. A genetically modified version of the CD16 receptor, hnCD16, has increased affinity for the Fc fragment of antibodies and resistance to ADAM17 metalloproteinase. This is achieved by modifying the molecular structure of the protein by replacing phenylalanine with valine at position 158 and serine with proline at position 197 [175,176]. | |
| NCT05182073 (2021–to the present) | Multiple myeloma | antiBCMA-CAR-NK + CD38 knockout, hnCD16 expression, IL15/IL15R expression | BCMA | BCMA, CD38 and CD16 receptors—see above in the table. | |
| NCT05987696 (2023–to the present) | Acute myeloid leukemia | antiCD33-CAR-NK + CLL1 secretion | CD33 | CD33 is a transmembrane protein that is expressed on the surface of myeloid lineage cells. It plays a role in cell adhesion and the transmission of intercellular signals. It can be found on blast cells in acute myeloid leukemia. antiCD33-CAR-NKT purposefully eliminate CD33-positive cells [164,177,178]. CLL1 is a chemokine that interacts with the CCR8 receptor to attract immune cells to the inflammation site and activate them. Thus, the expression of CLL1 chemokine by antiCD33-CAR-NK cells can further attract the patient’s own immune cells to the tumor site. This can enhance the cytotoxic effect against cancer cells [179,180]. | |
| NCT06594211 (2024–to the present) | Multiple myeloma | antiBCMA/GPRC5D-CAR-NK (biCAR) | BCMA and GPRC5D | GPRC5D is a transmembrane protein that is typically expressed in plasma and epithelial cells. It is highly expressed in multiple myeloma cells, and its function is not yet fully understood [181]. However, this protein has shown promise as a potential target for treatment of multiple myeloma. antiBCMA/GPRC5D-CAR-NK cells can specifically eliminate BCMA- and GPRC5D-positive cells [164]. Bispecific CARs may increase the cytotoxicity of these CAR-NK cells against tumor cells and improve their selectivity for target cells. | |
| NK cells with other genetic modifications | NCT04991870 (2023–to the present) | Glioblastoma | NK cells with TGF-βR2 and NR3C1 knockout | Glioblastoma cells | TGF-β is a cytokine secreted by cancer cells that has immunosuppressive properties. It helps reduce the body’s antitumor response while simultaneously forming the tumor microenvironment and promoting metastasis. The inhibition of TGF-β signaling through the knockout of its receptor is a promising immunotherapy strategy [182]. NR3C1 encodes the glucocorticoid receptor, and it has been found that the binding of glucocorticoids to this receptor leads to a significant decrease in the functional activity of NK cells. The knockout of this gene may lead to increased antitumor cytotoxicity in CAR-NK cells [183,184]. |
| Disease | Target | Clinical Trials |
|---|---|---|
| B-Cell Non-Hodgkin Lymphoma | CD19 | NCT06707259, NCT06334991, NCT05842707, NCT05739227, NCT06464861, NCT05020678 NCT03824964 |
| CD22 | NCT03824964, NCT03692767 | |
| CD70 | NCT05842707, NCT05092451 | |
| B-lymphoblastic leukemia (B-ALL) | CD19 | NCT06631040, NCT05739227, NCT05563545, NCT05020678 |
| Blastic plasmacytoid dendritic cell neoplasm | CD123 | NCT06690827, NCT06006403 |
| Hepatocellular carcinoma | GPC3 (glypican 3) | NCT06652243, |
| CD70 | NCT05703854 | |
| MUC1 (mucin 1) | NCT02839954 | |
| Glioma | MUC1 (mucin 1) | NCT02839954 |
| Breast carcinoma | MUC1 (mucin 1) | NCT02839954 |
| Endometrial cancer | Claudin 6 | NCT05410717 |
| GPC3 | NCT05410717 | |
| Mesothelin | NCT05410717 | |
| Castration-resistant prostate cancer | PSMA | NCT03692663 |
| Colorectal cancer | TROP2 | NCT06358430 |
| MUC1 (mucin 1) | NCT02839954 | |
| Mantle cell lymphoma (MCL) | CD5 | NCT05110742 |
| CD19 | NCT06464861, NCT05020678 | |
| Hodgkin’s lymphoma | CD70 | NCT05092451 |
| Central Nervous System Lymphoma | CD19 | NCT06827782 |
| Mesonephric-like adenocarcinoma | TROP2 | NCT05922930 |
| Mesothelioma | CD70 | NCT05703854 |
| Myelodysplastic syndrome | CD33 | NCT06325748 |
| CD70 | NCT05092451 | |
| FLT3 | NCT06325748 | |
| Small-cell lung cancer | DLL3 | NCT05507593 |
| Multiple myeloma | CD70 | NCT05092451 |
| BCMA | NCT06594211, NCT06242249, NCT06045091, NCT05652530, NCT05182073 | |
| GPRC5D | NCT06594211 | |
| Non-small cell lung cancer | TROP2 | NCT06454890 |
| MUC1 (mucin 1) | NCT02839954 | |
| Osteosarcoma | CD70 | NCT05703854 |
| Acute myeloid leukemia (AML) | CD7 | NCT02742727 |
| CD33 | NCT06325748, NCT05987696, NCT05215015, NCT05008575 | |
| CD70 | NCT06696846, NCT05092451 | |
| CD123 | NCT06690827, NCT06201247, NCT06006403, NCT05574608 | |
| FLT3 | NCT06325748 | |
| Primary mediastinal B-cell lymphoma (PMBCL) | CD19 | NCT06464861 |
| Peritoneal carcinomatosis | MICA, MICB | NCT06342986 |
| Plasma cell leukemia | CD70 | NCT05092451 |
| BCMA | NCT06045091 | |
| Gastric cancer | Claudin18.2 | NCT06464965 |
| MUC1 (mucin 1) | NCT02839954 | |
| Pancreatic cancer | Claudin18.2 | NCT06464965 |
| TROP2 | NCT05922930 | |
| ROBO1 | NCT03941457 | |
| MUC1 (mucin 1) | NCT02839954 | |
| Fallopian tube cancer | MICA, MICB | NCT06342986 |
| Ovarian carcinoma | MICA, MICB | NCT06342986 |
| TROP2 | NCT05922930 | |
| Claudin 6 | NCT05410717 | |
| GPC3 | NCT05410717 | |
| Mesothelin | NCT05410717, NCT03692637 | |
| Adult T-cell leukemia/lymphoma (ATLL) | CD5 | NCT06909474, NCT05110742 |
| CD7 | NCT06849401, NCT02742727 | |
| CD19 | NCT05563545 | |
| CD70 | NCT06696846, NCT05092451 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budagova, T.; Efremova, A.; Maiak, M.; Goldshtein, D. Strategies for the Development of NK Cell-Based Therapies for Cancer Treatment. Cells 2025, 14, 1858. https://doi.org/10.3390/cells14231858
Budagova T, Efremova A, Maiak M, Goldshtein D. Strategies for the Development of NK Cell-Based Therapies for Cancer Treatment. Cells. 2025; 14(23):1858. https://doi.org/10.3390/cells14231858
Chicago/Turabian StyleBudagova, Tatiana, Anna Efremova, Margarita Maiak, and Dmitry Goldshtein. 2025. "Strategies for the Development of NK Cell-Based Therapies for Cancer Treatment" Cells 14, no. 23: 1858. https://doi.org/10.3390/cells14231858
APA StyleBudagova, T., Efremova, A., Maiak, M., & Goldshtein, D. (2025). Strategies for the Development of NK Cell-Based Therapies for Cancer Treatment. Cells, 14(23), 1858. https://doi.org/10.3390/cells14231858








