Extracellular Vesicle microRNAs in the Crosstalk Between Cancer Cells and Natural Killer (NK) Cells
Abstract
1. Introduction
2. Natural Killer Cells, Extracellular Vesicles, and microRNAs
2.1. Natural Killer (NK) Cell Classification, Maturation, and Cytotoxicity Mechanisms
2.2. Extracellular Vesicles (EVs) and microRNAs (miRs)
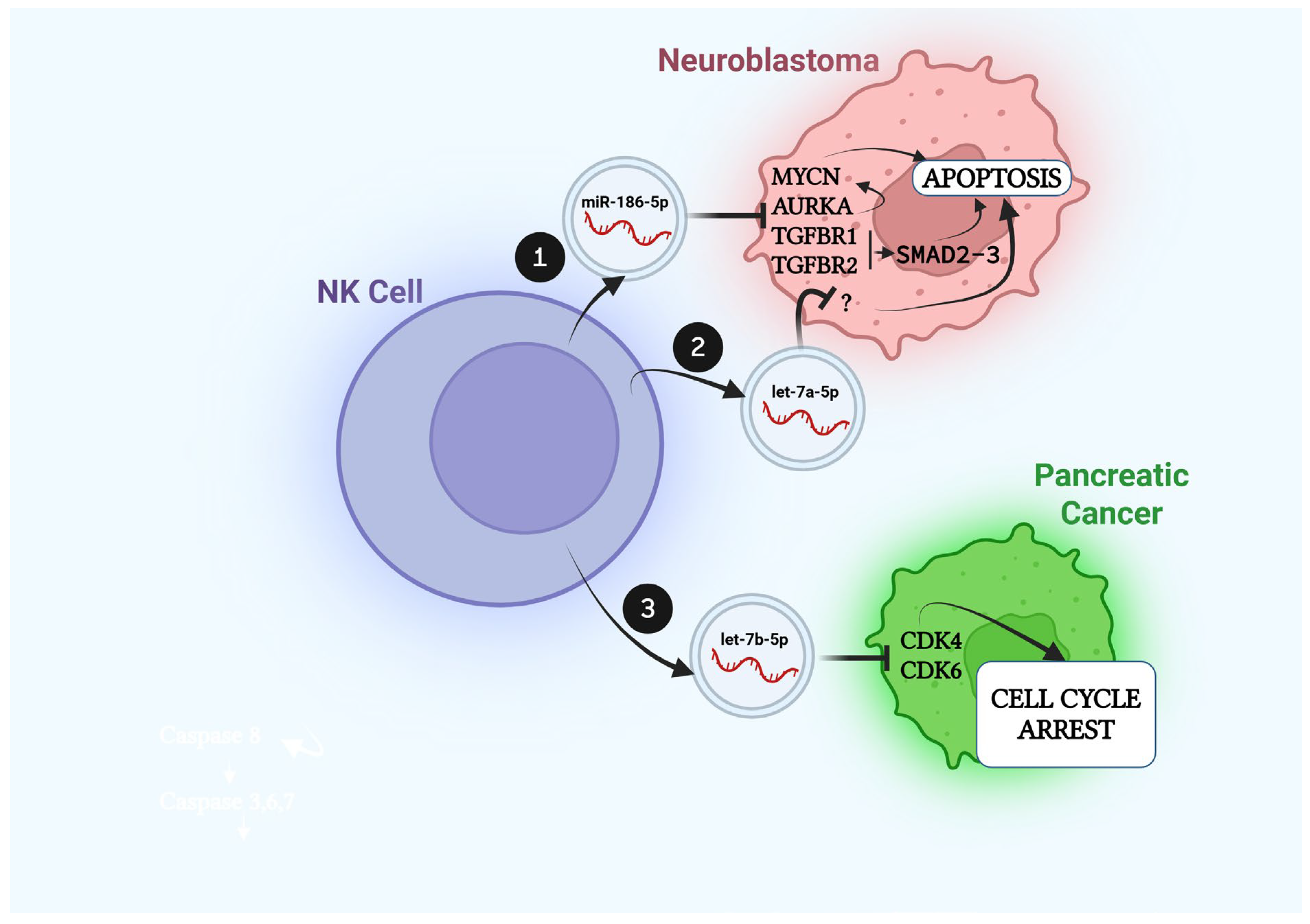
3. Effects of NK-Derived Extracellular Vesicular miRs on Cancer Cells
3.1. NK-Derived Vesicular miRs in Neuroblastoma
3.2. NK-Derived Vesicular miRs in Pancreatic Cancer
3.3. NK-Derived Vesicular Mechanisms of Cytotoxicity in Other Types of Cancers
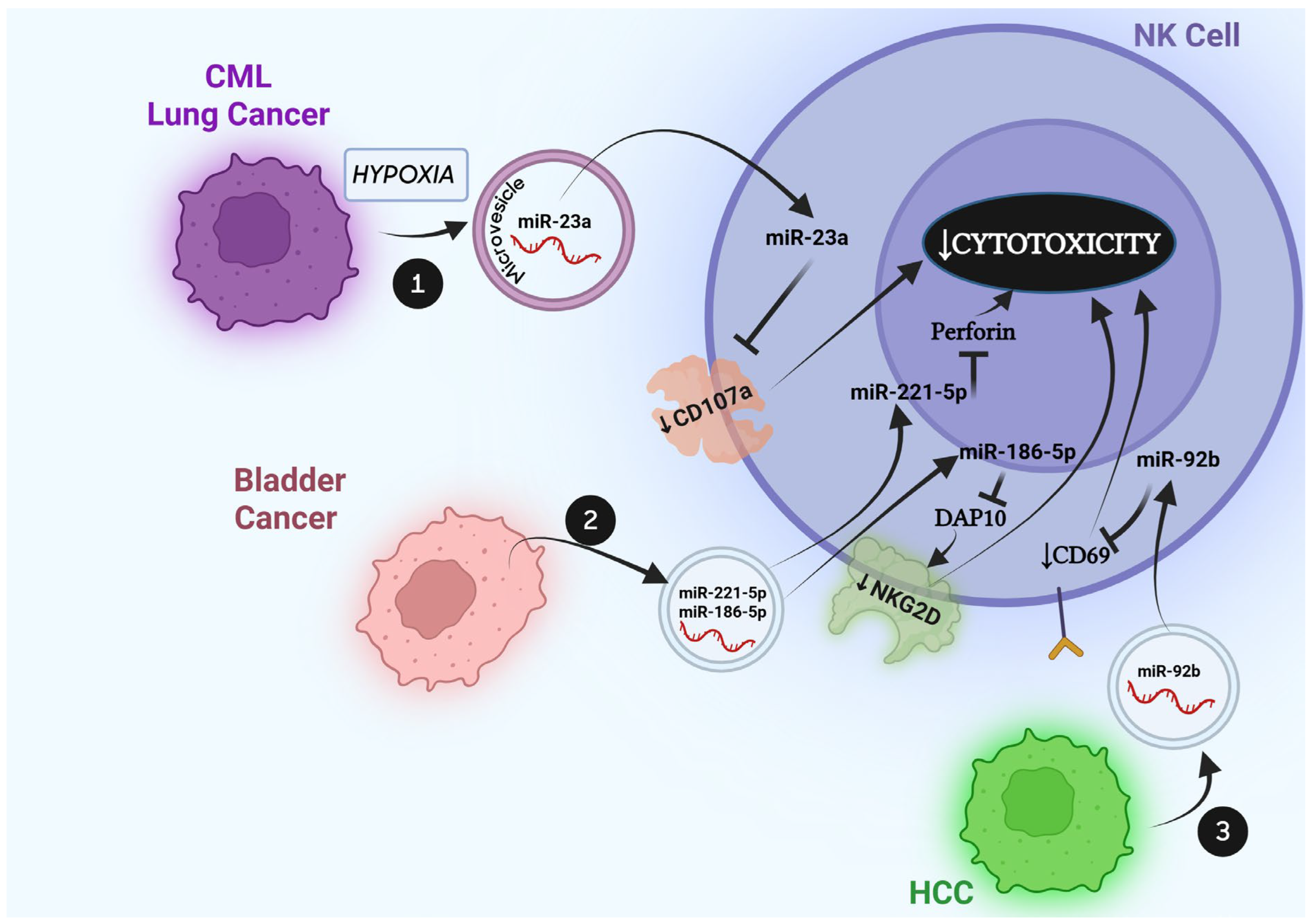
4. Effects of Tumor-Derived Extracellular Vesicle miRs on NK Cells
| NK-EVs on Cancer Cells | Source | EV Cargo Content | Targets | Type of Cancer | Reference |
| NK from PBMCs | miR-186-5p | MYCN, AURKA, TGFBR1, TGFBR2 | Neuroblastoma | [39] | |
| Biomimetics NPs from NK from PBMCs | let-7a-5p | N/A | Neuroblastoma | [43] | |
| NK from PBMCs | let-7b-5p | CDK6, CDK4 | Pancreatic | [46] | |
| NK from PBMCs | FASL, PFN | FAS | Jurkat (T-cell leukemia), K562 (CML), Daudi (Burkitt lymphoma) | [48] | |
| NK from PBMCs | PFN, GNLY, GZMA, GZMB | CASP3 CASP7 CASP9 | CHLA-255, CHLA-136 (neuroblastoma), SupB15, NALM6 (ALL), MCF7 (breast) | [44] | |
| NK-92MI | FASL | FAS | B16F10 (melanoma), SNU484 (stomach), HCT-15 (colon) | [49] | |
| Tumor-Derived EVs on NK Cells | Cancer Source of EVs | EV Cargo/Surface Agent | Targets | Effect | Reference |
| AML | TGFβ (surface) | NKG2D (downregulation) | Reduced cytotoxicity by primary NK | [50] | |
| HeLa (cervical cancer) | MICA*008 | NKG2D | Reduced cytotoxicity by primary NK | [51] | |
| IGR-Heu (lung cancer), K562 (CML) under hypoxic conditions | TGFβ, miR-210, miR-23a | CD107a (targeted by miR-23a), IFNγ (downregulation), GZMBB (downregulation) | Reduced cytotoxicity by primary NK | [52] | |
| HCC | miR-92b | CD69 | Reduced cytotoxicity by NK92 cells | [53] | |
| T24 (bladder cancer) | miR-221-5p miR-186-5p | NKG2D (downregulated through DAP10 targeting by miR-186-5p), NKp30 (downregulation), CD226 (downregulation), PFN (downregulation by direct targeting by miR-221-5p) | Reduced cytotoxicity by primary NK | [54] |
5. miR-186-5p as a Key Player in NK–Cancer Cell Interaction
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.M.; Isnard, P.; Dorfman, J.R.; Coles, M.C.; Raulet, D.H. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J. Immunol. 2004, 172, 864–870. [Google Scholar] [CrossRef]
- Zhang, Y.; Wallace, D.L.; de Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.L.; et al. In vivo kinetics of human natural killer cells: The effects of ageing and acute and chronic viral infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef]
- Borger, J.G.; Lau, M.; Hibbs, M.L. The Influence of Innate Lymphoid Cells and Unconventional T Cells in Chronic Inflammatory Lung Disease. Front. Immunol. 2019, 10, 1597. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Sliz, A.; Locker, K.C.S.; Lampe, K.; Godarova, A.; Plas, D.R.; Janssen, E.M.; Jones, H.; Herr, A.B.; Hoebe, K. Gab3 is required for IL-2- and IL-15-induced NK cell expansion and limits trophoblast invasion during pregnancy. Sci. Immunol. 2019, 4, eaav3866. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Miller, M.; Stojanovic, A.; Garbi, N.; Cerwenka, A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J. Exp. Med. 2012, 209, 2351–2365. [Google Scholar] [CrossRef]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef]
- Bottino, C.; Picant, V.; Vivier, E.; Castriconi, R. Natural killer cells and engagers: Powerful weapons against cancer. Immunol. Rev. 2024, 328, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Venstrom, J.M.; Pittari, G.; Gooley, T.A.; Chewning, J.H.; Spellman, S.; Haagenson, M.; Gallagher, M.M.; Malkki, M.; Petersdorf, E.; Dupont, B.; et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N. Engl. J. Med. 2012, 367, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, J.; Chen, S.; Yang, H.; Dong, Z. Synergized regulation of NK cell education by NKG2A and specific Ly49 family members. Nat. Commun. 2019, 10, 5010. [Google Scholar] [CrossRef]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.J.; Andrews, D.M.; McLaughlin, N.M.; Yagita, H.; Gilfillan, S.; Colonna, M.; Smyth, M.J. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J. Immunol. 2010, 184, 902–911. [Google Scholar] [CrossRef]
- Narni-Mancinelli, E.; Gauthier, L.; Baratin, M.; Guia, S.; Fenis, A.; Deghmane, A.-E.; Rossi, B.; Fourquet, P.; Escalière, B.; Kerdiles, Y.M.; et al. Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci. Immunol. 2017, 2, eaam9628. [Google Scholar] [CrossRef]
- Siewiera, J.; Gouilly, J.; Hocine, H.-R.; Cartron, G.; Levy, C.; Al-Daccak, R.; Jabrane-Ferrat, N. Natural cytotoxicity receptor splice variants orchestrate the distinct functions of human natural killer cell subtypes. Nat. Commun. 2015, 6, 10183. [Google Scholar] [CrossRef]
- Textor, S.; Bossler, F.; Henrich, K.-O.; Gartlgruber, M.; Pollmann, J.; Fiegler, N.; Arnold, A.; Westermann, F.; Waldburger, N.; Breuhahn, K.; et al. The proto-oncogene Myc drives expression of the NK cell-activating NKp30 ligand B7-H6 in tumor cells. Oncoimmunology 2016, 5, e1116674. [Google Scholar] [CrossRef]
- Boudreau, J.E.; Giglio, F.; Gooley, T.A.; Stevenson, P.A.; Le Luduec, J.-B.; Shaffer, B.C.; Rajalingam, R.; Hou, L.; Hurley, C.K.; Noreen, H.; et al. KIR3DL1/HLA-B Subtypes Govern Acute Myelogenous Leukemia Relapse After Hematopoietic Cell Transplantation. J. Clin. Oncol. 2017, 35, 2268–2278. [Google Scholar] [CrossRef]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2017, 6, e1264562. [Google Scholar] [CrossRef]
- Nowak, I.; Wilczyńska, K.; Wilczyński, J.R.; Malinowski, A.; Radwan, P.; Radwan, M.; Kuśnierczyk, P. KIR, LILRB and their Ligands’ Genes as Potential Biomarkers in Recurrent Implantation Failure. Arch. Immunol. Ther. Exp. 2017, 65, 391–399. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Yamaji, T.; Teranishi, T.; Alphey, M.S.; Crocker, P.R.; Hashimoto, Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to α2,8-disialyl and branched α2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 2002, 277, 6324–6332. [Google Scholar] [CrossRef]
- Ito, M.; Maruyama, T.; Saito, N.; Koganei, S.; Yamamoto, K.; Matsumoto, N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J. Exp. Med. 2006, 203, 289–295. [Google Scholar] [CrossRef]
- Schmitz, I.; Kirchhoff, S.; Krammer, P.H. Regulation of death receptor-mediated apoptosis pathways. Int. J. Biochem. Cell Biol. 2000, 32, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404, Erratum in J. Extracell. Vesicles 2024, 13, e12451. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell 2003, 113, 673–676. [Google Scholar] [CrossRef]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef]
- Challagundla, K.B.; Wise, P.M.; Neviani, P.; Chava, H.; Murtadha, M.; Xu, T.; Kennedy, R.; Ivan, C.; Zhang, X.; Vannini, I.; et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J. Natl. Cancer Inst. 2015, 107, djv135. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef]
- Prochownik, E.V.; Vogt, P.K. Therapeutic Targeting of Myc. Genes Cancer 2010, 1, 650–659. [Google Scholar] [CrossRef]
- Wolpaw, A.J.; Bayliss, R.; Büchel, G.; Dang, C.V.; Eilers, M.; Gustafson, W.C.; Hansen, G.H.; Jura, N.; Knapp, S.; Lemmon, M.A.; et al. Drugging the “Undruggable” MYCN Oncogenic Transcription Factor: Overcoming Previous Obstacles to Impact Childhood Cancers. Cancer Res. 2021, 81, 1627–1632. [Google Scholar] [CrossRef]
- Neviani, P.; Wise, P.M.; Murtadha, M.; Liu, C.W.; Wu, C.-H.; Jong, A.Y.; Seeger, R.C.; Fabbri, M. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res. 2019, 79, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Trotta, R.; Dal Col, J.; Yu, J.; Ciarlariello, D.; Thomas, B.; Zhang, X.; Allard, J.; Wei, M.; Mao, H.; Byrd, J.C.; et al. TGF-β utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J. Immunol. 2008, 181, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef]
- Liao, Y.-M.; Hung, T.-H.; Tung, J.K.; Yu, J.; Hsu, Y.-L.; Hung, J.-T.; Yu, A.L. Low Expression of IL-15 and NKT in Tumor Microenvironment Predicts Poor Outcome of MYCN-Non-Amplified Neuroblastoma. J. Pers. Med. 2021, 11, 122. [Google Scholar] [CrossRef]
- Wang, G.; Hu, W.; Chen, H.; Shou, X.; Ye, T.; Xu, Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers 2019, 11, 1560. [Google Scholar] [CrossRef]
- Jong, A.Y.; Wu, C.-H.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles 2017, 6, 1294368. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Di Pace, A.L.; Pelosi, A.; Fiore, P.F.; Tumino, N.; Besi, F.; Quatrini, L.; Santopolo, S.; Vacca, P.; Moretta, L. MicroRNA analysis of Natural Killer cell-derived exosomes: The microRNA let-7b-5p is enriched in exosomes and participates in their anti-tumor effects against pancreatic cancer cells. Oncoimmunology 2023, 12, 2221081. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Li, P.; Jenkins, L.M.; Anastasakis, D.; Lyons, G.C.; Hafner, M.; Leonard, W.J. Cytokine-enhanced cytolytic activity of exosomes from NK Cells. Cancer Gene Ther. 2022, 29, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012, 189, 2833–2842. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, M.J.; Szajnik, M.; Welsh, A.; Whiteside, T.L.; Boyiadzis, M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-β1. Haematologica 2011, 96, 1302–1309. [Google Scholar] [CrossRef]
- Ashiru, O.; Boutet, P.; Fernández-Messina, L.; Agüera-González, S.; Skepper, J.N.; Valés-Gómez, M.; Reyburn, H.T. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010, 70, 481–489. [Google Scholar] [CrossRef]
- Berchem, G.; Noman, M.Z.; Bosseler, M.; Paggetti, J.; Baconnais, S.; Le Cam, E.; Nanbakhsh, A.; Moussay, E.; Mami-Chouaib, F.; Janji, B.; et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology 2016, 5, e1062968. [Google Scholar] [CrossRef]
- Nakano, T.; Chen, I.-H.; Wang, C.-C.; Chen, P.-J.; Tseng, H.-P.; Huang, K.-T.; Hu, T.-H.; Li, L.-C.; Goto, S.; Cheng, Y.-F.; et al. Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am. J. Transplant. 2019, 19, 3250–3262. [Google Scholar] [CrossRef]
- Huyan, T.; Gao, L.; Gao, N.; Wang, C.; Guo, W.; Zhou, X.; Li, Q. miR-221-5p and miR-186-5p Are the Critical Bladder Cancer Derived Exosomal miRNAs in Natural Killer Cell Dysfunction. Int. J. Mol. Sci. 2022, 23, 15177. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Ivan, M.; Cimmino, A.; Negrini, M.; Calin, G.A. Regulatory mechanisms of microRNAs involvement in cancer. Expert Opin. Biol. Ther. 2007, 7, 1009–1019. [Google Scholar] [CrossRef]
- Calin, G.A.; Cimmino, A.; Fabbri, M.; Ferracin, M.; Wojcik, S.E.; Shimizu, M.; Taccioli, C.; Zanesi, N.; Garzon, R.; Aqeilan, R.I.; et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 5166–5171. [Google Scholar] [CrossRef] [PubMed]
- Dosil, S.G.; Lopez-Cobo, S.; Rodriguez-Galan, A.; Fernandez-Delgado, I.; Ramirez-Huesca, M.; Milan-Rois, P.; Castellanos, M.; Somoza, A.; Gómez, M.J.; Reyburn, H.T.; et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. eLife 2022, 11, e76319. [Google Scholar] [CrossRef] [PubMed]
- Humbert, C.; Cordier, C.; Drut, I.; Hamrick, M.; Wong, J.; Bellamy, V.; Flaire, J.; Bakshy, K.; Dingli, F.; Loew, D.; et al. GMP-Compliant Process for the Manufacturing of an Extracellular Vesicles-Enriched Secretome Product Derived From Cardiovascular Progenitor Cells Suitable for a Phase I Clinical Trial. J. Extracell. Vesicles 2025, 14, e70145. [Google Scholar] [CrossRef]
- Hong, C.M.; Gangadaran, P.; Oh, J.M.; Rajendran, R.L.; Gopal, A.; Zhu, L.; Ahn, B.-C. Radioiodine labeling and in vivo trafficking of extracellular vesicles. Sci. Rep. 2021, 11, 5041. [Google Scholar] [CrossRef]
- Chan, A.M.L.; Cheah, J.M.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Natural Killer Cell-Derived Extracellular Vesicles as a Promising Immunotherapeutic Strategy for Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4026. [Google Scholar] [CrossRef]
- Palade, J.; Alsop, E.; Tang, N.; Antone, J.; Paredes, D.M.; Halder, T.G.; Soldi, R.; Bargenquast, T.; Schwartz, G.; Finholt, J.; et al. NK cell-derived extracellular vesicles enhance cytotoxicity and immune cell recruitment in non-small cell lung cancer. Front. Immunol. 2025, 16, 1633010. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lai, H.; Chen, T. Boosting Natural Killer Cell-Based Cancer Immunotherapy with Selenocystine/Transforming Growth Factor-Beta Inhibitor-Encapsulated Nanoemulsion. ACS Nano 2020, 14, 11067–11082. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Gao, J.; Ma, X. Natural killer cell-derived exosome-based cancer therapy: From biological roles to clinical significance and implications. Mol. Cancer 2024, 23, 134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toldo, N.; Wu, Y.; Fabbri, M. Extracellular Vesicle microRNAs in the Crosstalk Between Cancer Cells and Natural Killer (NK) Cells. Cells 2025, 14, 1697. https://doi.org/10.3390/cells14211697
Toldo N, Wu Y, Fabbri M. Extracellular Vesicle microRNAs in the Crosstalk Between Cancer Cells and Natural Killer (NK) Cells. Cells. 2025; 14(21):1697. https://doi.org/10.3390/cells14211697
Chicago/Turabian StyleToldo, Nicolo, Yunjie Wu, and Muller Fabbri. 2025. "Extracellular Vesicle microRNAs in the Crosstalk Between Cancer Cells and Natural Killer (NK) Cells" Cells 14, no. 21: 1697. https://doi.org/10.3390/cells14211697
APA StyleToldo, N., Wu, Y., & Fabbri, M. (2025). Extracellular Vesicle microRNAs in the Crosstalk Between Cancer Cells and Natural Killer (NK) Cells. Cells, 14(21), 1697. https://doi.org/10.3390/cells14211697







