Negative Allosteric Modulation of Agonist-Induced M2 Muscarinic Receptor/β-Arrestin Interaction by Serum Autoantibodies from Patients with Chronic Chagas Disease
Highlights
- Circulating autoantibodies to M2 muscarinic receptors from patients with chronic Chagas disease can inhibit the interaction of agonist-induced arrestin-2, but not arrestin-3.
- These antibodies can act as negative allosteric modulators of agonist efficacy.
- Allosteric inhibition of agonist-induced M2 receptor activation by autoantibodies could play a pathogenic role in cardiac parasympathetic dysfunction secondary to Chagas disease. These results support the potential therapeutic use of allosteric ligands to treat pathogenic effects of functional autoantibodies to M2 muscarinic receptors in patients with chronic Chagas disease and other pathological conditions.
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Reagents and Antibodies
2.3. ELISA
2.4. Purification of the IgG Fraction from Human Serum
2.5. Plasmids, Cell Culture, and Transfection
2.6. BRET Assays
2.6.1. Preparation of Cells Expressing BRET Constructs
2.6.2. Assessment of Total Fluorescence, Total Luminescence, and Basal BRET Ratio
2.6.3. BRET Protocols
2.7. Statistical Analysis
3. Results
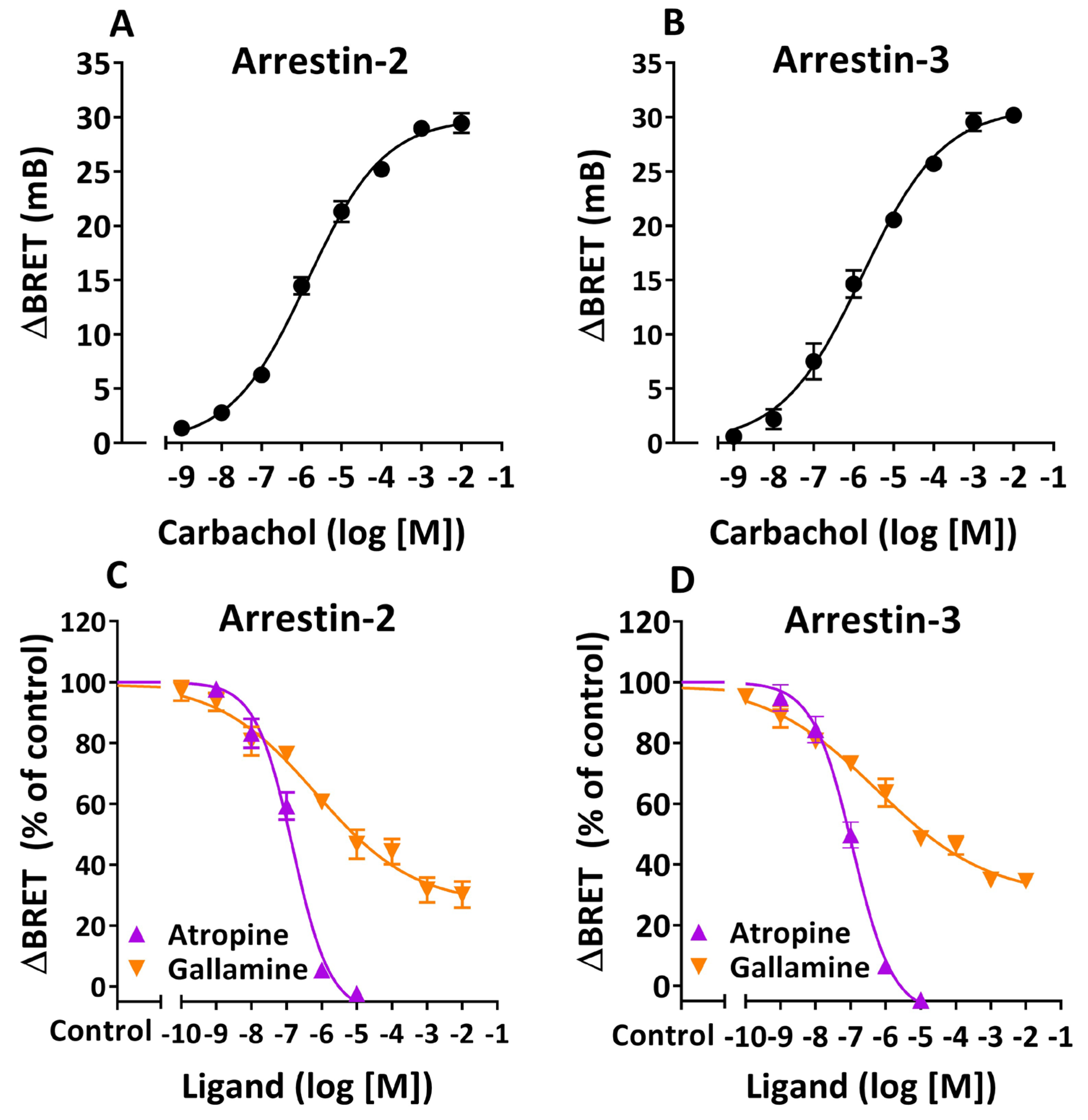
3.1. Agonist-Induced β-Arrestin Translocation in HEK 293T Cells. Orthosteric and Allosteric Modulation
3.2. Effect of Serum M2 Muscarinic Receptor Autoantibodies on β-Arrestin Recruitment
3.3. Allosteric Modulation of Agonist-Induced β-Arrestin Recruitment by M2 Muscarinic Autoantibodies
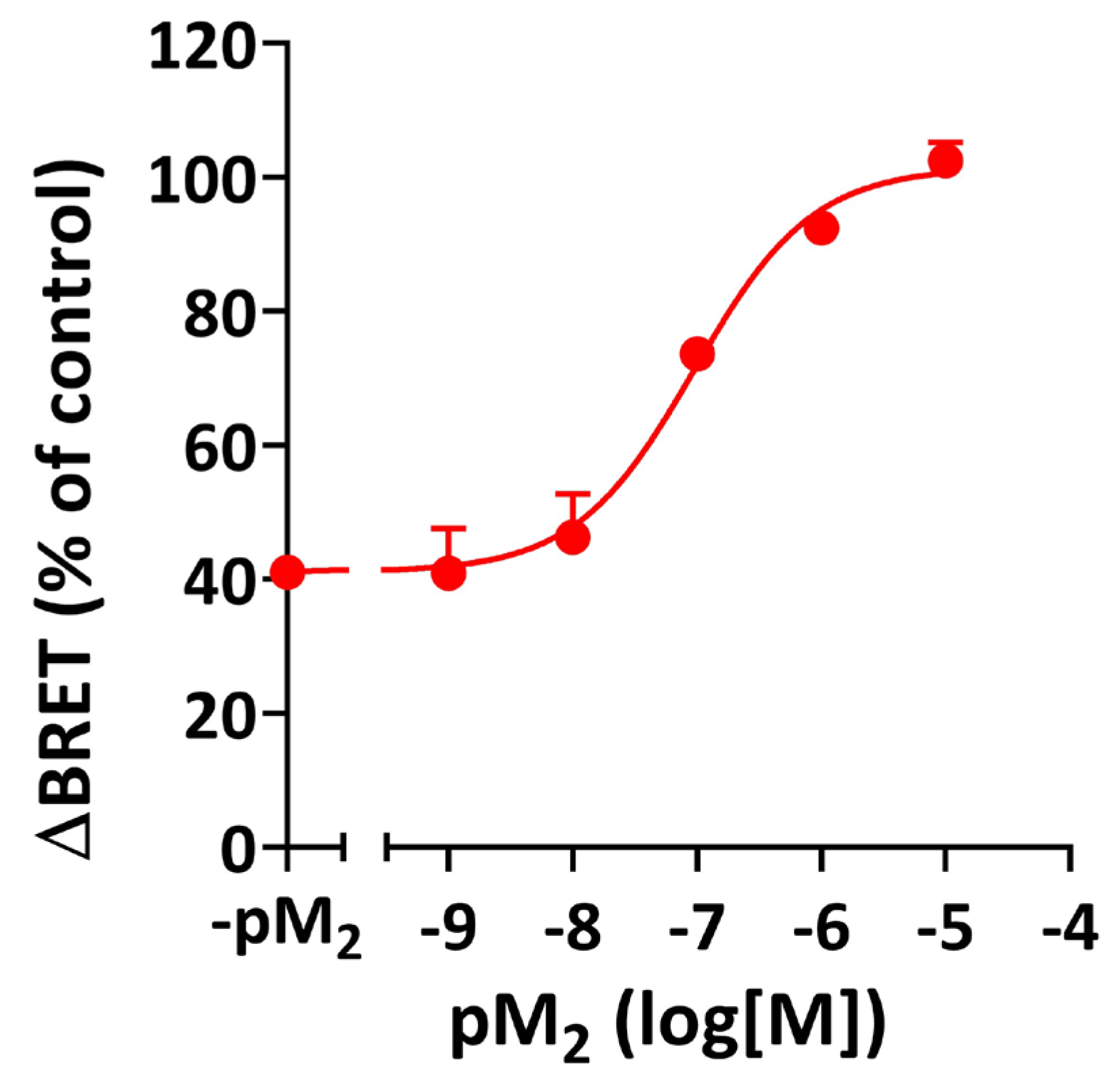
3.4. Epitope Specificity of the Negative Allosteric Modulation by M2 Muscarinic Autoantibodies
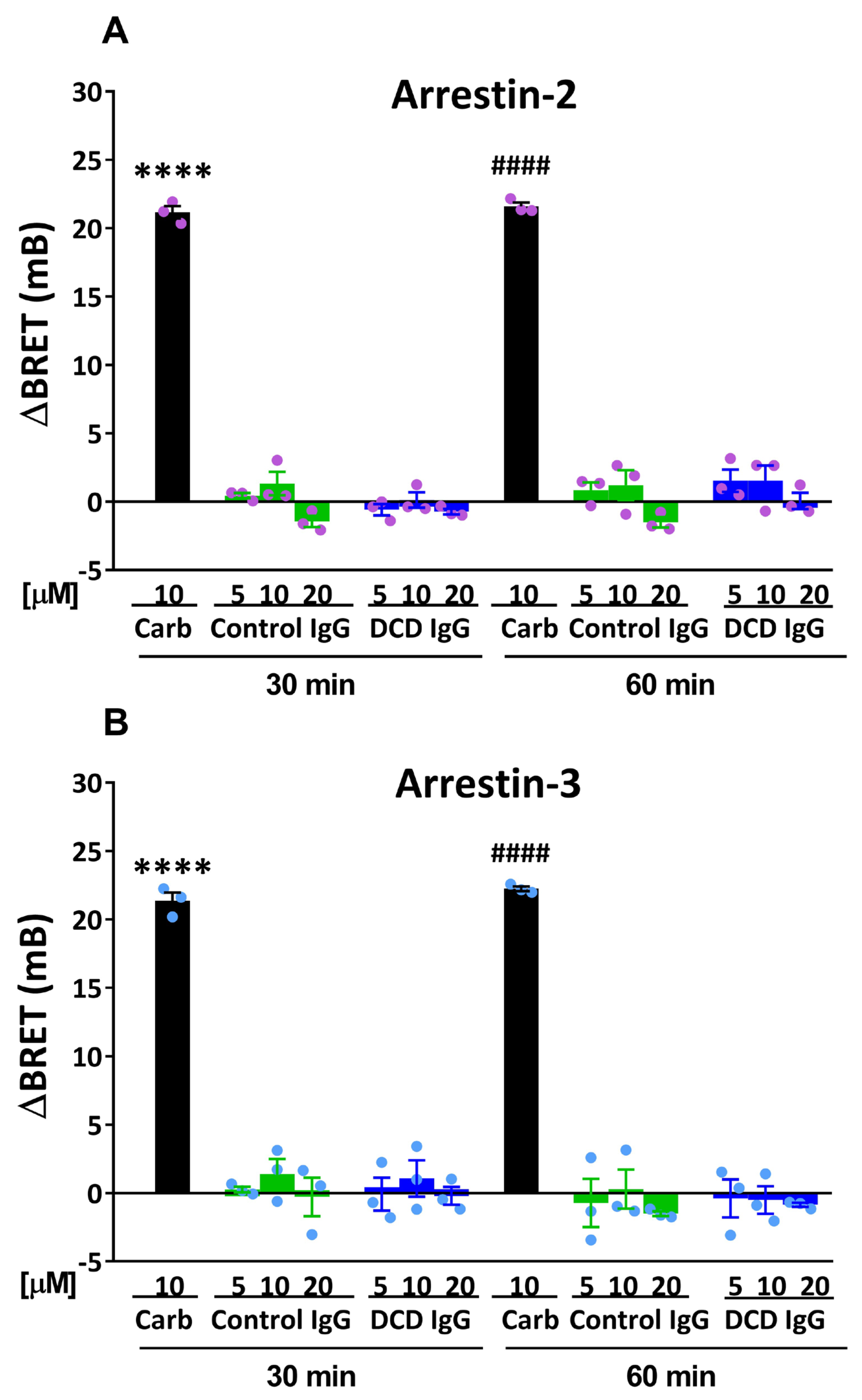
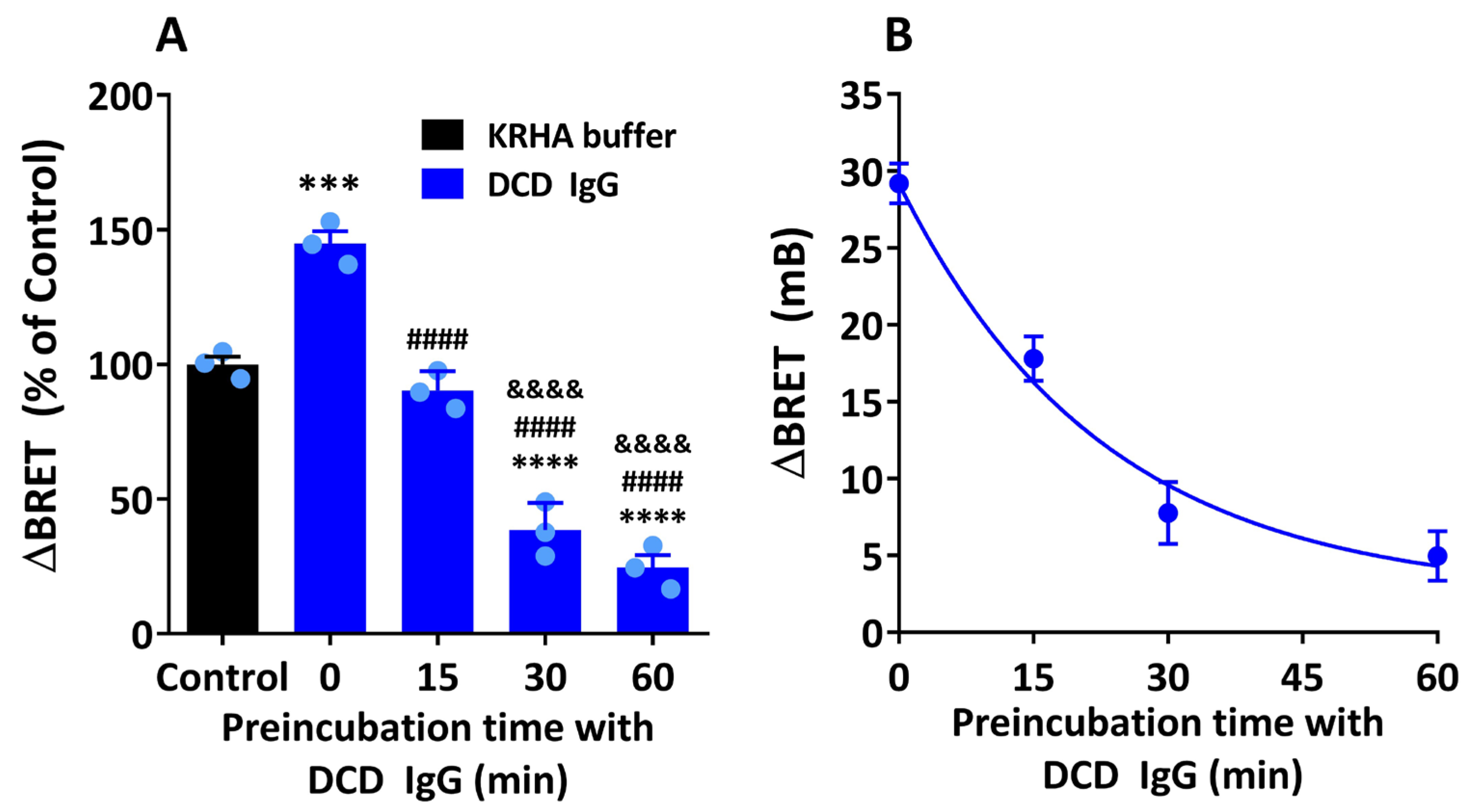
3.5. Allosteric Modulation by M2 Muscarinic Autoantibodies: Dependence on the Timing and Duration of Exposure of M2 Muscarinic Receptors to Specific Autoantibodies
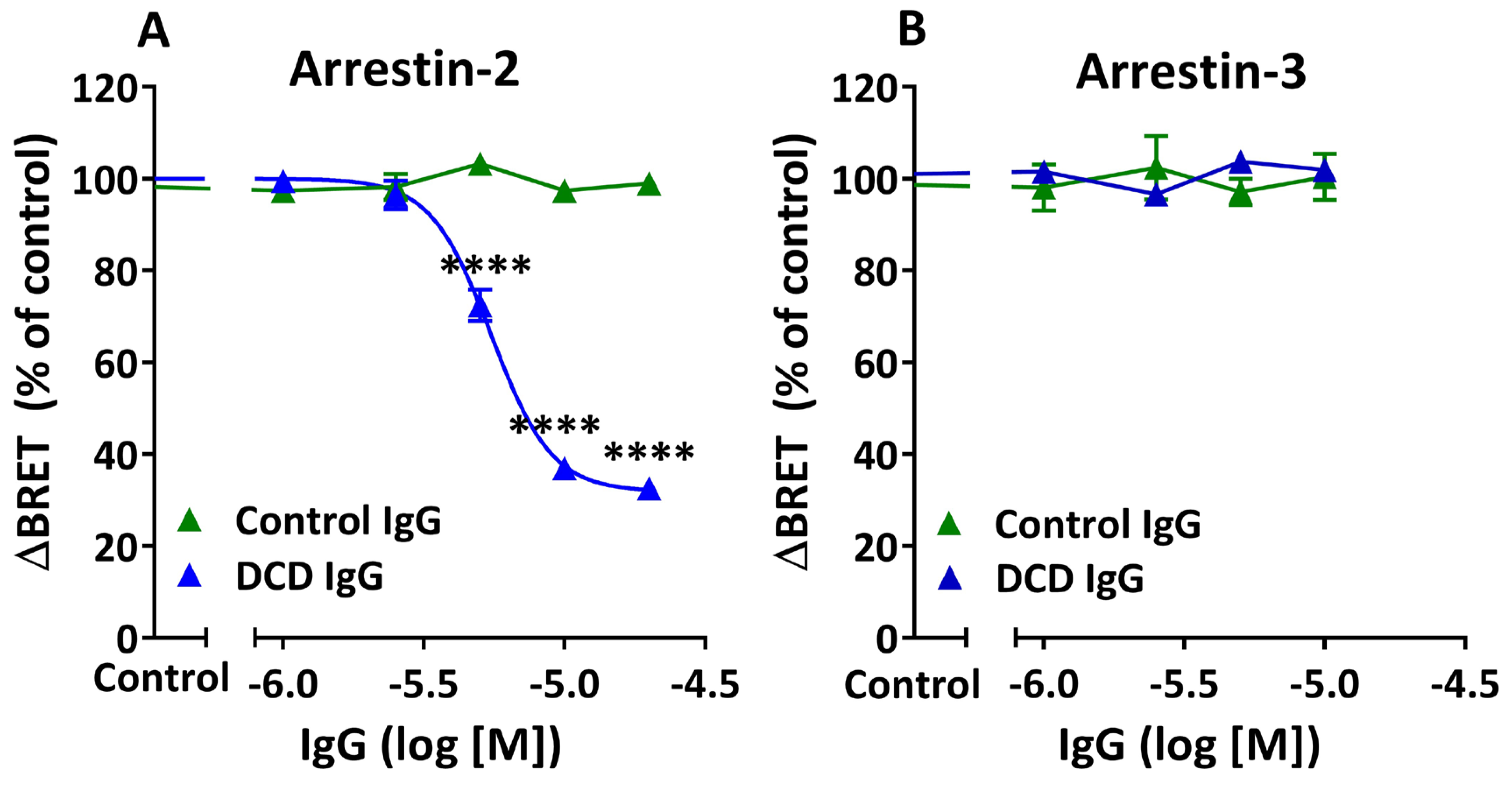
3.6. Inhibition of Agonist-Induced β-Arrestin Recruitment by M2 Muscarinic Autoantibodies: Allosteric Mechanism
4. Discussion
4.1. Intrinsic Activity of M2 Muscarinic Receptor Autoantibodies
4.2. Arr-2-Biased Modulation of β-Arrestin Recruitment by M2 Muscarinic Receptor Autoantibodies
4.3. M2 Muscarinic Receptor Autoantibodies as Negative Allosteric Modulators
4.4. Crosslinking-Mediated Negative Allosteric Modulation by M2R Autoantibodies
4.5. Time-Dependent Direction of Allosteric Modulation by M2 Muscarinic Autoantibodies
4.6. Implications of the Present Findings in the Pathophysiology and Therapeutics of Chagas Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| II-ECL | second extracellular loop |
| α1AR | α1 adrenergic receptor |
| βAR, β1AR, or β2AR | β, β1, or β2 adrenergic receptor(s) |
| AAb | autoantibody(ies) |
| ACh | acetylcholine |
| Anti-α1AR AAb | AAb against α1AR |
| Anti-βAR AAb, anti-β1AR AAb, or anti-β2AR AAb | AAb against βAR, β1AR, or β2AR |
| Anti-M2R AAb/M2R AAb | AAb against M2R |
| Arr-2 or Arr-3 | arrestin-2 or arrestin-3 |
| Arr-2-YFP or Arr-3-YFP | Arr-2 or Arr-3 fused to enhanced yellow fluorescence protein |
| BRET | bioluminescence resonance energy transfer |
| BSA | bovine serum albumin |
| CD | Chagas disease |
| C-R | concentration–response |
| Control IgG | IgG(s) from (a) noninfected individual(s) |
| DCD IgG | IgG(s) from (a) patient(s) with DCD |
| DCD | dysautonomia secondary to CD |
| ECG | electrocardiogram |
| ERI | ELISA reactivity index |
| FBS | fetal bovine serum |
| GPCR | G protein-coupled receptor |
| GRK2 | G protein-coupled receptor kinase 2 |
| KHRA | Krebs-HEPES-Ringer buffer supplemented with BSA |
| M2R | M2 muscarinic receptor(s) |
| M2R-RLuc | M2R fused to Renilla luciferase |
| NAM | negative allosteric modulator(s) |
| PAM | positive allosteric modulator(s) |
| PBS | phosphate-buffered saline |
| POTS | postural orthostatic tachycardia syndrome |
| RFU | relative fluorescence units |
| RLU | relative luminescence units |
| SD | standard deviation |
| T. cruzi | Trypanosoma cruzi |
References
- Cucunuba, Z.M.; Gutierrez-Romero, S.A.; Ramirez, J.D.; Velasquez-Ortiz, N.; Ceccarelli, S.; Parra-Henao, G.; Henao-Martinez, A.F.; Rabinovich, J.; Basanez, M.G.; Nouvellet, P.; et al. The epidemiology of Chagas disease in the Americas. Lancet Reg. Health Am. 2024, 37, 100881. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverria, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef] [PubMed]
- Borda, E.; Pascual, J.; Cossio, P.; De La Vega, M.; Arana, R.; Sterin-Borda, L. A circulating IgG in Chagas’ disease which binds to beta-adrenoceptors of myocardium and modulates their activity. Clin. Exp. Immunol. 1984, 57, 679–686. [Google Scholar]
- Sterin-Borda, L.; Perez Leiros, C.; Wald, M.; Cremaschi, G.; Borda, E. Antibodies to beta 1 and beta 2 adrenoreceptors in Chagas’ disease. Clin. Exp. Immunol. 1988, 74, 349–354. [Google Scholar] [PubMed]
- Goin, J.C.; Borda, E.; Leiros, C.P.; Storino, R.; Sterin-Borda, L. Identification of antibodies with muscarinic cholinergic activity in human Chagas’ disease: Pathological implications. J. Auton. Nerv. Syst. 1994, 47, 45–52. [Google Scholar] [CrossRef]
- Ferrari, I.; Levin, M.J.; Wallukat, G.; Elies, R.; Lebesgue, D.; Chiale, P.; Elizari, M.; Rosenbaum, M.; Hoebeke, J. Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human beta 1-adrenergic receptor. J. Exp. Med. 1995, 182, 59–65. [Google Scholar] [CrossRef]
- Elies, R.; Ferrari, I.; Wallukat, G.; Lebesgue, D.; Chiale, P.; Elizari, M.; Rosenbaum, M.; Hoebeke, J.; Levin, M.J. Structural and functional analysis of the B cell epitopes recognized by anti-receptor autoantibodies in patients with Chagas’ disease. J. Immunol. 1996, 157, 4203–4211. [Google Scholar] [CrossRef]
- Mahler, E.; Hoebeke, J.; Levin, M.J. Structural and functional complexity of the humoral response against the Trypanosoma cruzi ribosomal P2 beta protein in patients with chronic Chagas’ heart disease. Clin. Exp. Immunol. 2004, 136, 527–534. [Google Scholar] [CrossRef]
- Chiale, P.A.; Ferrari, I.; Mahler, E.; Vallazza, M.A.; Elizari, M.V.; Rosenbaum, M.B.; Levin, M.J. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation 2001, 103, 1765–1771. [Google Scholar] [CrossRef]
- Goin, J.C.; Sterin-Borda, L.; Bilder, C.R.; Varrica, L.M.; Iantorno, G.; Rios, M.C.; Borda, E. Functional implications of circulating muscarinic cholinergic receptor autoantibodies in chagasic patients with achalasia. Gastroenterology 1999, 117, 798–805. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Goin, J.C.; Bilder, C.R.; Iantorno, G.; Hernando, A.C.; Borda, E. Interaction of human chagasic IgG with human colon muscarinic acetylcholine receptor: Molecular and functional evidence. Gut 2001, 49, 699–705. [Google Scholar] [CrossRef]
- Medei, E.; Pedrosa, R.C.; Benchimol Barbosa, P.R.; Costa, P.C.; Hernandez, C.C.; Chaves, E.A.; Linhares, V.; Masuda, M.O.; Nascimento, J.H.; Campos de Carvalho, A.C. Human antibodies with muscarinic activity modulate ventricular repolarization: Basis for electrical disturbance. Int. J. Cardiol. 2007, 115, 373–380. [Google Scholar] [CrossRef]
- Goin, J.C.; Borda, E.S.; Auger, S.; Storino, R.; Sterin-Borda, L. Cardiac M(2) muscarinic cholinoceptor activation by human chagasic autoantibodies: Association with bradycardia. Heart 1999, 82, 273–278. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Gimenez, L.E.; Hernandez, C.C.; de Carvalho, A.C.; Teixeira, M.M.; Guedes, V.C.; Barros, M.V.; Lombardi, F.; Rocha, M.O. Early occurrence of anti-muscarinic autoantibodies and abnormal vagal modulation in Chagas disease. Int. J. Cardiol. 2007, 117, 59–63. [Google Scholar] [CrossRef]
- Beltrame, S.P.; Carrera Paez, L.C.; Auger, S.R.; Sabra, A.H.; Bilder, C.R.; Waldner, C.I.; Goin, J.C. Impairment of agonist-induced M2 muscarinic receptor activation by autoantibodies from chagasic patients with cardiovascular dysautonomia. Clin. Immunol. 2020, 212, 108346. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.F.; Pedrosa, R.C.; Nascimento, J.H.; Campos de Carvalho, A.C.; Masuda, M.O. Sera from chronic chagasic patients with complex cardiac arrhythmias depress electrogenesis and conduction in isolated rabbit hearts. Circulation 1997, 96, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.C.; Nascimento, J.H.; Chaves, E.A.; Costa, P.C.; Masuda, M.O.; Kurtenbach, E.; Campos, D.E.C.A.C.; Gimenez, L.E. Autoantibodies enhance agonist action and binding to cardiac muscarinic receptors in chronic Chagas’ disease. J. Recept. Signal Transduct. Res. 2008, 28, 375–401. [Google Scholar] [CrossRef] [PubMed]
- Leppik, R.A.; Miller, R.C.; Eck, M.; Paquet, J.L. Role of acidic amino acids in the allosteric modulation by gallamine of antagonist binding at the m2 muscarinic acetylcholine receptor. Mol. Pharmacol. 1994, 45, 983–990. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Barcellos, L.C.; Gimenez, L.E.; Cabarcas, R.A.; Garcia, S.; Pedrosa, R.C.; Nascimento, J.H.; Kurtenbach, E.; Masuda, M.O.; Campos de Carvalho, A.C. Human chagasic IgGs bind to cardiac muscarinic receptors and impair L-type Ca2+ currents. Cardiovasc. Res. 2003, 58, 55–65. [Google Scholar] [CrossRef]
- Beltrame, S.P.; Auger, S.R.; Bilder, C.R.; Waldner, C.I.; Goin, J.C. Modulation of M(2) muscarinic receptor-receptor interaction by immunoglobulin G antibodies from Chagas’ disease patients. Clin. Exp. Immunol. 2011, 164, 170–179. [Google Scholar] [CrossRef]
- Pan American Health Organization. Guidelines for the Diagnosis and Treatment of Chagas Disease; PAHO: Washington, DC, USA, 2019; Available online: https://iris.paho.org/bitstream/handle/10665.2/49653/9789275120439_eng.pdf (accessed on 18 November 2025).
- Iosa, D.; Dequattro, V.; Lee, D.D.; Elkayam, U.; Caeiro, T.; Palmero, H. Pathogenesis of cardiac neuro-myopathy in Chagas’ disease and the role of the autonomic nervous system. J. Auton. Nerv. Syst. 1990, 30, S83–S87. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Laux, G.; Dannehl, K.; Spuler, M.; Muhlen, H.; Mayer, P.; Gries, F.A. Assessment of cardiovascular autonomic function: Age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet. Med. 1992, 9, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Goin, J.C.; Leiros, C.P.; Borda, E.; Sterin-Borda, L. Interaction of human chagasic IgG with the second extracellular loop of the human heart muscarinic acetylcholine receptor: Functional and pathological implications. FASEB J. 1997, 11, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.X.; Magnusson, Y.; Bergh, C.H.; Liljeqvist, J.A.; Waagstein, F.; Hjalmarson, A.; Hoebeke, J. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J. Clin. Investig. 1993, 91, 1964–1968. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mellon, P.; Parker, V.; Gluzman, Y.; Maniatis, T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell 1981, 27, 279–288. [Google Scholar] [CrossRef]
- Carrera Páez, L.C. Interacción del Receptor Muscarínico M2 con Arrestinas no Visuales y su Modulación por Anticuerpos Séricos de Pacientes Chagásicos Crónicos. Master’s Thesis, Universidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Buenos Aires, Argentina, 13 October 2021. Available online: http://repositoriouba.sisbi.uba.ar/gsdl/cgi-bin/library.cgi?a=d&c=afamaster&cl=CL1&d=HWA_7958 (accessed on 18 November 2025).
- Herda, L.R.; Felix, S.B.; Boege, F. Drug-like actions of autoantibodies against receptors of the autonomous nervous system and their impact on human heart function. Br. J. Pharmacol. 2012, 166, 847–857. [Google Scholar] [CrossRef]
- Goin, J.C.; Perez Leiros, C.; Borda, E.; Sterin-Borda, L. Modification of cholinergic-mediated cellular transmembrane signals by the interaction of human chagasic IgG with cardiac muscarinic receptors. Neuroimmunomodulation 1994, 1, 284–291. [Google Scholar] [CrossRef]
- Pals-Rylaarsdam, R.; Gurevich, V.V.; Lee, K.B.; Ptasienski, J.A.; Benovic, J.L.; Hosey, M.M. Internalization of the m2 muscarinic acetylcholine receptor. Arrestin-independent and -dependent pathways. J. Biol. Chem. 1997, 272, 23682–23689. [Google Scholar] [CrossRef]
- Wallukat, G.; Fu, H.M.; Matsui, S.; Hjalmarson, A.; Fu, M.L. Autoantibodies against M2 muscarinic receptors in patients with cardiomyopathy display non-desensitized agonist-like effects. Life Sci. 1999, 64, 465–469. [Google Scholar] [CrossRef]
- Jones, K.T.; Echeverry, M.; Mosser, V.A.; Gates, A.; Jackson, D.A. Agonist mediated internalization of M2 mAChR is beta-arrestin-dependent. J. Mol. Signal. 2006, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Silvano, E.; Millan, M.J.; Mannoury la Cour, C.; Han, Y.; Duan, L.; Griffin, S.A.; Luedtke, R.R.; Aloisi, G.; Rossi, M.; Zazzeroni, F.; et al. The tetrahydroisoquinoline derivative SB269,652 is an allosteric antagonist at dopamine D3 and D2 receptors. Mol. Pharmacol. 2010, 78, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.P. Allosteric Modulation. In A Pharmacology Primer: Techniques for More Effective and Strategic Drug Discovery, 5th ed.; Elsevier/Academic Press: London, UK, 2019; pp. 173–205. [Google Scholar]
- Pisterzi, L.F.; Jansma, D.B.; Georgiou, J.; Woodside, M.J.; Chou, J.T.; Angers, S.; Raicu, V.; Wells, J.W. Oligomeric size of the m2 muscarinic receptor in live cells as determined by quantitative fluorescence resonance energy transfer. J. Biol. Chem. 2010, 285, 16723–16738. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.A.; Kruse, A.C. Autoantibodies as Endogenous Modulators of GPCR Signaling. Trends Pharmacol. Sci. 2021, 42, 135–150. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Joensen, L.; Bayo-Hanza, C.; Esteva, M.; Borda, E. Therapeutic use of muscarinic acetylcholine receptor peptide to prevent mice chagasic cardiac dysfunction. J. Mol. Cell. Cardiol. 2002, 34, 1645–1654. [Google Scholar] [CrossRef]
- Wallukat, G.; Muller, J.; Haberland, A.; Berg, S.; Schulz, A.; Freyse, E.J.; Vetter, R.; Salzsieder, E.; Kreutz, R.; Schimke, I. Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G-protein coupled receptors: A vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies. Atherosclerosis 2016, 244, 44–47. [Google Scholar] [CrossRef]
- Haberland, A.; Holtzhauer, M.; Schlichtiger, A.; Bartel, S.; Schimke, I.; Muller, J.; Dandel, M.; Luppa, P.B.; Wallukat, G. Aptamer BC 007—A broad spectrum neutralizer of pathogenic autoantibodies against G-protein-coupled receptors. Eur. J. Pharmacol. 2016, 789, 37–45. [Google Scholar] [CrossRef]
- Singh, K.D.; Jara, Z.P.; Harford, T.; Saha, P.P.; Pardhi, T.R.; Desnoyer, R.; Karnik, S.S. Novel allosteric ligands of the angiotensin receptor AT1R as autoantibody blockers. Proc. Natl. Acad. Sci. USA 2021, 118, e2019126118. [Google Scholar] [CrossRef]
- Smulski, C.; Labovsky, V.; Levy, G.; Hontebeyrie, M.; Hoebeke, J.; Levin, M.J. Structural basis of the cross-reaction between an antibody to the Trypanosoma cruzi ribosomal P2beta protein and the human beta1 adrenergic receptor. FASEB J. 2006, 20, 1396–1406. [Google Scholar] [CrossRef]
- Pizarro, J.C.; Boulot, G.; Bentley, G.A.; Gomez, K.A.; Hoebeke, J.; Hontebeyrie, M.; Levin, M.J.; Smulski, C.R. Crystal structure of the complex mAb 17.2 and the C-terminal region of Trypanosoma cruzi P2beta protein: Implications in cross-reactivity. PLoS Negl. Trop. Dis. 2011, 5, e1375. [Google Scholar] [CrossRef]
- Korczynska, M.; Clark, M.J.; Valant, C.; Xu, J.; Moo, E.V.; Albold, S.; Weiss, D.R.; Torosyan, H.; Huang, W.; Kruse, A.C.; et al. Structure-based discovery of selective positive allosteric modulators of antagonists for the M2 muscarinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 2018, 115, E2419–E2428. [Google Scholar] [CrossRef]
- Chakraborty, P.; Farhat, K.; Morris, L.; Whyte, S.; Yu, X.; Stavrakis, S. Non-invasive Vagus Nerve Simulation in Postural Orthostatic Tachycardia Syndrome. Arrhythm. Electrophysiol. Rev. 2023, 12, e31. [Google Scholar] [CrossRef]
- Adler, B.L.; Chung, T.; Rowe, P.C.; Aucott, J. Dysautonomia following Lyme disease: A key component of post-treatment Lyme disease syndrome? Front. Neurol. 2024, 15, 1344862. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Liles, C.; Khan, M.; Vanderlinde-Wood, M.; Galloway, A.; Zillner, C.; Benbrook, A.; Reim, S.; Collier, D.; et al. Autoimmune basis for postural tachycardia syndrome. J. Am. Heart Assoc. 2014, 3, e000755. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Forsythe, E.; Okamoto, L.E.; Yu, X. Implications of Antimuscarinic Autoantibodies in Postural Tachycardia Syndrome. J. Cardiovasc. Transl. Res. 2022, 15, 438–440. [Google Scholar] [CrossRef] [PubMed]
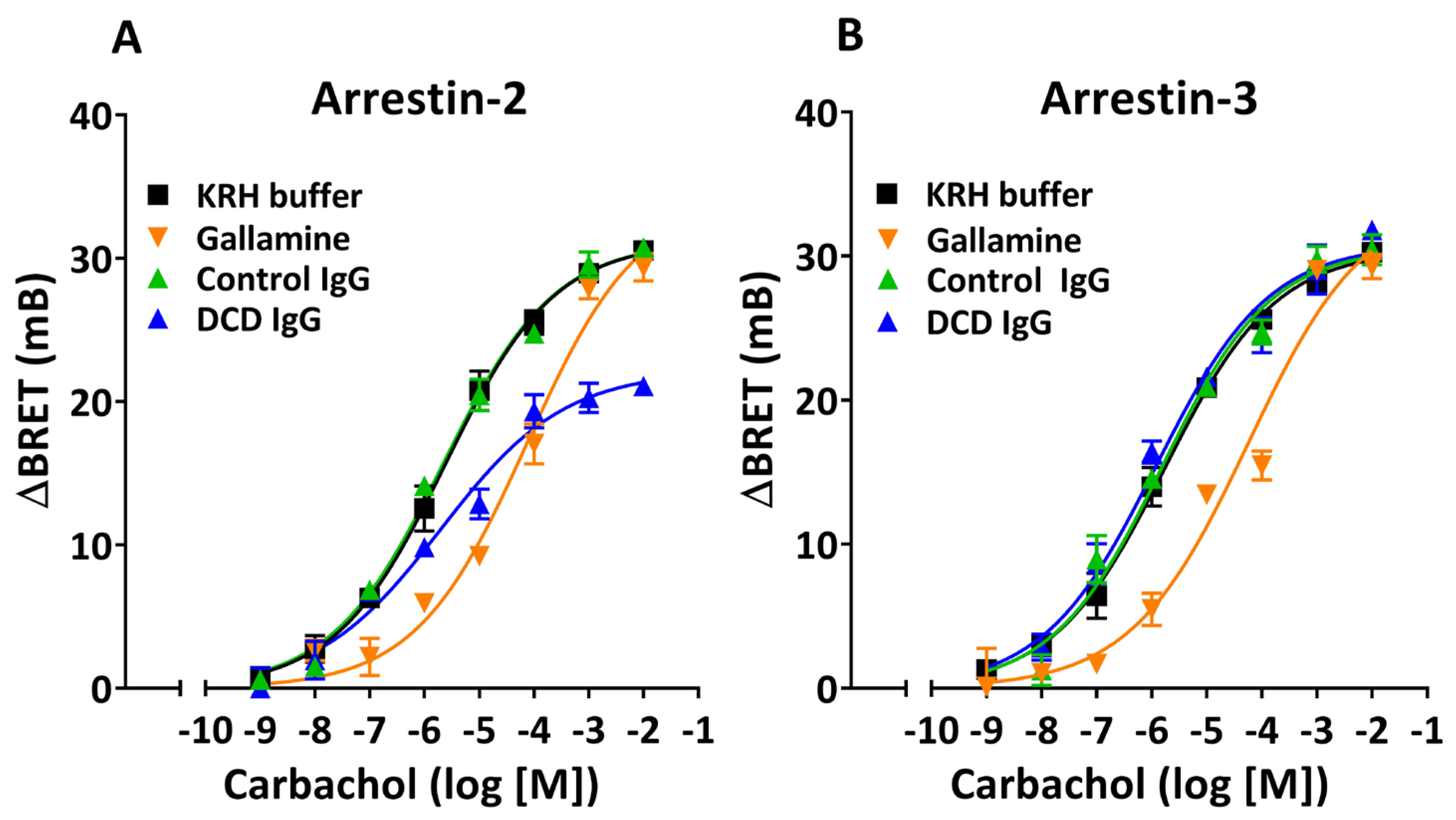
| Arrestin-2 | Arrestin-3 | |||||
|---|---|---|---|---|---|---|
| Ligand | pIC50 | Imax (%) | Hill Slope | pIC50 | Imax (%) | Hill Slope |
| Atropine | 6.83 ± 0.10 * | 108.5 ± 5.1 ** | −0.82 ± 0.12 **** | 6.94 ± 0.09 * | 109.2 ± 4.4 *** | −0.76 ± 0.09 **** |
| Gallamine | 6.16 ± 0.29 | 73.2 ± 4.9 | −0.31 ± 0.05 | 6.24 ± 0.26 | 70.9 ± 4.0 | −0.27 ± 0.03 |
| Arrestin-2 | Arrestin-3 | |||
|---|---|---|---|---|
| Treatment | pEC50 | Emax (mB) | pEC50 | Emax (mB) |
| KRHA Buffer | 5.63 ± 0.10 | 31.1 ± 0.8 | 5.77 ± 0.10 | 30.5 ± 0.8 |
| Gallamine | 4.13 ± 0.26 **** | 34.2 ± 3.0 | 4.24 ± 0.32 **** | 34.1 ± 3.5 |
| Control IgG | 5.67 ± 0.10 | 31.2 ± 0.8 | 5.81 ± 0.15 | 30.8 ± 1.2 |
| DCD IgG | 5.71 ± 0.22 | 22.1 ± 1.2 &&, &&&& | 5.92 ± 0.17 | 30.8 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera Páez, L.C.; Beltrame, S.P.; Auger, S.R.; Sabra, A.H.; Bilder, C.R.; Irurzun, I.M.; Waldner, C.I.; Goin, J.C. Negative Allosteric Modulation of Agonist-Induced M2 Muscarinic Receptor/β-Arrestin Interaction by Serum Autoantibodies from Patients with Chronic Chagas Disease. Cells 2025, 14, 1857. https://doi.org/10.3390/cells14231857
Carrera Páez LC, Beltrame SP, Auger SR, Sabra AH, Bilder CR, Irurzun IM, Waldner CI, Goin JC. Negative Allosteric Modulation of Agonist-Induced M2 Muscarinic Receptor/β-Arrestin Interaction by Serum Autoantibodies from Patients with Chronic Chagas Disease. Cells. 2025; 14(23):1857. https://doi.org/10.3390/cells14231857
Chicago/Turabian StyleCarrera Páez, Laura C., Sabrina P. Beltrame, Sergio R. Auger, Ahmad H. Sabra, Claudio R. Bilder, Isabel M. Irurzun, Claudia I. Waldner, and Juan C. Goin. 2025. "Negative Allosteric Modulation of Agonist-Induced M2 Muscarinic Receptor/β-Arrestin Interaction by Serum Autoantibodies from Patients with Chronic Chagas Disease" Cells 14, no. 23: 1857. https://doi.org/10.3390/cells14231857
APA StyleCarrera Páez, L. C., Beltrame, S. P., Auger, S. R., Sabra, A. H., Bilder, C. R., Irurzun, I. M., Waldner, C. I., & Goin, J. C. (2025). Negative Allosteric Modulation of Agonist-Induced M2 Muscarinic Receptor/β-Arrestin Interaction by Serum Autoantibodies from Patients with Chronic Chagas Disease. Cells, 14(23), 1857. https://doi.org/10.3390/cells14231857






